Abstract
The neurotoxin of strain 111 (111/NT) associated with type B infant botulism showed antigenic and biological properties different from that (Okra/NT) produced by a food-borne botulism-related strain, Okra. The specific toxicity of 111/NT was found to be about 10 times lower than that of Okra/NT. The monoclonal antibodies recognizing the light chain cross-reacted with both neurotoxins, whereas most of the antibodies recognizing the carboxyl-terminal half of the heavy chain of Okra/NT did not react to 111/NT. Binding experiments with rat brain synaptosomes revealed that 125I-labeled 111/NT bound to a single binding site with a dissociation constant (Kd) of 2.5 nM; the value was rather lower than that (0.42 nM) of 125I-Okra/NT for the high-affinity binding site. In the lipid vesicles reconstituted with ganglioside GT1b, 125I-Okra/NT interacted with the amino-terminal domain of synaptotagmin 1 (Stg1N) or synaptotagmin 2 (Stg2N), fused with the maltose-binding protein, in the same manner as the respective full-length synaptotagmins, and the Kd values accorded with those of the low- and high-affinity binding sites in synaptosomes. However, 125I-111/NT only exhibited a low capacity for binding to the lipid vesicles containing Stg2N, but not Stg1N, in the presence of ganglioside GT1b. Moreover, synaptobrevin-2, an intracellular target protein, was digested to the same extent by the light chains of both neurotoxins in a concentration-dependent manner. These findings indicate that the 111/NT molecule possesses the receptor-recognition site structurally different from Okra/NT, probably causing a decreased specific toxicity.
Since the first case of infant botulism was diagnosed in the United States in 1976 (21, 31), more than 1,000 cases have been reported from around the world. Infant botulism is represented by neuromuscular paralysis due to the toxin produced in the intestines after germination and outgrowth of ingested spores of Clostridium botulinum (1). This disease, which affects children up to 6 months old, with rare exceptions, is characterized by constipation, generalized weakness, and various neurological disorders, although cases represent a spectrum of disease ranging from subclinical infection to the most fulminant form of the disease, which presents unexpected sudden death (5). Most cases have been caused by C. botulinum type A or B (2), although there have been a few exceptions caused by type C, E, or F. The toxigenic organisms causing type E and F infant botulism were not C. botulinum, but were culturally and biochemically identical to Clostridium butyricum and Clostridium baratii, respectively (7, 20). Since the first Japanese case of type A infant botulism reported in 1986 (29), there have been 14 cases, 9 due to type A and 1 due to type C (30). The types of toxin in the other four cases were not described. In 1995, there was a 6-month-old patient with infant botulism identified in Ishikawa Prefecture; this was found to be the first reported case of type B infant botulism in Japan (9).
The properties of the neurotoxin produced by the organism associated with infant botulism have extensively been examined physicochemically, immunochemically, and genetically. The neurotoxin from type A isolates associated with infant botulism in Japan was antigenically similar but not identical to that produced by food-borne botulism-related type A strains (33). In addition, such antigenic difference was restricted to the heavy chain (∼100 kDa), which is comprised in the neurotoxin together with the light chain (∼50 kDa) (15). The antigenic dissimilarity was also found between the neurotoxins produced by type E organisms and those produced by toxigenic C. butyricum (17). These observations were supported by the notion that the nucleotide sequence of the infant neurotoxin gene was clearly related but not identical to that of the authentic neurotoxin gene (22). These findings evoke the question of whether type B neurotoxin associated with infant botulism in Japan possesses properties different from those of the authentic neurotoxin. In the present study, we found that this infant botulism-related neurotoxin shows toxicity lower than that of the previously known neurotoxin, which is probably due to a low capacity for binding to the toxin receptor.
MATERIALS AND METHODS
Strains and neurotoxins.
Strain 111 was isolated from the feces of a patient with type B infant botulism (9). Strain Okra, associated with food-borne botulism, was used for preparation of the authentic type B neurotoxin. The progenitor toxins of both strains 111 and Okra were purified according to a method previously described (18). After dialysis against 10 mM phosphate buffer (pH 7.5), the progenitor toxin was loaded onto a DEAE-Sepharose Fast Flow column (Pharmacia, Uppsala, Sweden) equilibrated with the same buffer. The neurotoxin was eluted with an NaCl linear gradient from 0 to 0.3 M, concentrated with YM-10 membrane (Amicon, Inc., Beverly, Mass.), and then converted to the nicked form by treatment with N-tosyl-l-phenylalanine chloromethyl ketone-treated trypsin (Sigma Chemical Co., St. Louis, Mo.) at a toxin/enzyme ratio of 100:1 for 15 min at pH 7.5 and 37°C. The purified neurotoxins were referred to as 111/NT and Okra/NT, respectively. Toxicity was assayed by the time-to-death method by intravenous injection into mice (10). Samples were also titrated by intraperitoneal injection into mice with serial twofold dilutions to obtain a mean 50% lethal dose (LD50) by the Reed and Muench calculation (32).
MAbs and ELISA.
Monoclonal antibodies (MAbs) against 111/NT were obtained by a method described elsewhere (16). Five cell lines were established in this study. MAbs were purified from ascitic fluid by Affi-Gel-protein A (Bio-Rad Laboratories, Richmond, Calif.) chromatography. The subclass and light chain of each MAb were determined by the method described previously (12). In addition, 13 MAbs against Okra/NT prepared by the procedures described previously (16) were used to determine their reactivities to 111/NT: 4 MAbs reacted with the amino-terminal half of the heavy chain, another 5 reacted with the carboxyl-terminal half of the heavy chain, and the other 4 reacted with the light chain. The reactivities of MAbs to neurotoxin were examined by enzyme-linked immunosorbent assay (ELISA) according to the previously described method (16) and immunoblotting as described below.
Binding of 125I-labeled neurotoxin to synaptosomes.
The neurotoxin was radioiodinated with Na 125I (Dupont, NEN Research Products, Boston, Mass.) by the chloramine-T method as described previously (11). The specific activities of 125I-111/NT and 125I-Okra/NT were 5.1 to 7.5 mCi/mg of protein (28 to 41 Mbq/nmol), and the residual toxicities were higher than 80% of that of the unlabeled toxin. Synaptosomes were prepared from rat brain (26) and suspended in 3 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES)-NaOH buffer (pH 7.0) containing 120 mM NaCl, 2.5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, and 0.1% bovine serum albumin (HBS-BSA). Synaptosomes were incubated for 30 min at 37°C with 125I-labeled neurotoxin in 0.2 ml of HBS-BSA. To test the inhibition of binding of the 125I-labeled neurotoxin, synaptosomes were preincubated for 10 min at 37°C with unlabeled neurotoxin at various concentrations. The 125I-labeled neurotoxin having bound to synaptosomes was separated by filtration through a Millititer-HA plate well (Millipore Corp., Bedford, Mass.). The filters were washed five times each with 0.25 ml of chilled HBS-BSA. The radioactivity retained on the filter was determined in a gamma counter. Specific binding was determined as the difference between means of triplicate assays in the presence or absence of 200-fold excess unlabeled toxin. Scatchard plot analysis was performed with the computer program SP123 (8).
Expression of recombinant amino-terminal domains of synaptotagmins 1 and 2 and their toxin-binding activities.
The PCR products of rat synaptotagmins 1 and 2 were cloned into pET-3a vector (Novagen Inc., Madison, Wis.) as described before (26, 28) and used as templates to generate the synaptotagmin-truncation mutants denoted Stg1N (amino acids 1 to 78) and Stg2N (amino acids 1 to 87), respectively. The cDNA fragments encoding Stg1N and Stg2N were amplified by PCR with the following primers: forward, 5′-AGGCGCCATGGTGAGTGCCAGTCATCCTGAGGCCCTG-3′ (Stg1N) and 5′-AGGCGCCATGAGAAACATCTTCAAGAGG-3′ (Stg2N); reverse, 5′-GCTCTAGATTAACAAAAGCAGCAGGTTACGAC-3′ (Stg1N) and 5′-GCTCTAGACTATTAACAGATGCAGAAGCAGCAGGTGAG-3′ (Stg2N), where EheI and XbaI sites were included at the ends of the forward and reverse primers, respectively. After digestion with EheI and XbaI, the PCR fragment was ligated into XmnI- and XbaI-digested pMAL-c2 vector (New England Biolabs, Beverly, Mass.) and verified by DNA sequencing.
Expression of recombinant synaptotagmin mutants with the pMAL-c2 fusion protein expression system (New England Biolabs) was performed by the manufacturer’s protocol. Overnight cultures of Escherichia coli DH5 or TB1 cells containing the recombinant plasmid were grown in rich medium (tryptone, 10 g; yeast extract, 5 g; NaCl, 5 g; glucose, 2 g per liter) containing 100 μg of ampicillin per ml at 37°C, with shaking, to an A600 of 0.5. Isopropyl-β-d-thiogalactopyranoside (IPTG; Wako Pure Chemicals, Osaka, Japan) was added to 0.3 mM (final concentration), and incubation was continued for additional 2 h. The cells were harvested by centrifugation at 4°C and suspended in buffer A (20 mM Tris, 0.2 M NaCl, 1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride [PMSF; Sigma], 60 μM leupeptin [Peptide Institute, Inc., Osaka, Japan], and 40 μM pepstatin A [Peptide Institute, Inc.] [pH 7.4]) at 0.1 volume of the original culture and lysed on ice by sonication with two pulses of 15 s each. Lysates were centrifuged at 8,000 × g for 20 min at 4°C. The clear supernatant was loaded onto a column of amylose resin, equilibrated with buffer A. After the column had been washed with buffer A, bound proteins were eluted with buffer A containing 10 mM maltose.
The purified recombinant truncation mutants were incorporated into phosphatidylcholine lipid vesicles together with ganglioside GT1b (Wako) by the acetone-precipitation method as described previously (27). The binding of 125I-111/NT or 125I-Okra/NT to the reconstituted lipid vesicles was measured by the filtration assay as described above.
Expression of rat VAMP-2 (synaptobrevin-2).
A cDNA fragment encoding vesicle-associated membrane protein 2 (VAMP-2 [synaptobrevin]) was amplified by PCR with rat brain cDNA (6) and the following primers: forward, 5′-AGACATAATGTCGGCTACCGCTGCCACCGTC-3′; reverse, 5′-CTTGGATCCTATTAAGTGCTGAAGTAAACGATGAT-3′. NdeI and BamHI sites were included at the ends of the forward and reverse primers, respectively. The amplified product was cleaved with NdeI and BamHI and ligated with NdeI- and BamHI-digested pET-16b vector (Novagen). After confirmation of the integrity of the coding region by sequencing, the recombinant plasmid was introduced into E. coli BL21(DE3)pLysS.
Expression of recombinant VAMP-2 was performed according to the pET System manual (Novagen). Cultures were grown at 37°C in Luria-Bertani broth containing 50 μg of carbenicillin per ml and 34 μg of chloramphenicol per ml, with shaking, to an A600 of 0.5 at 600 nm. After addition of 30 μM IPTG (final concentration), incubation was continued for an additional 2 h. The cells were collected by centrifugation and suspended in buffer B (20 mM Tris, 0.5 M NaCl, 5 mM imidazole, 0.1 mM PMSF, 60 μM leupeptin, and 40 μM pepstatin A [pH 7.9]) at 0.02 volume of the original culture. After treatment with 0.1 mg of lysozyme per ml for 15 min at 30°C, the cells were lysed on ice by sonication with three pulses of 10 s each. The lysates were centrifuged at 80,000 × g for 1 h at 4°C. The supernatant was discarded, and the pellet was dissolved in buffer B containing 1% n-octyl-β-glucoside (Dojindo Laboratories, Kumamoto, Japan). After centrifugation at 80,000 × g for 1 h at 4°C, the clear supernatant was loaded onto a column of His · Bond resin (Novagen), equilibrated with buffer B. After the column had been washed, bound proteins were eluted with buffer B containing 1 M imidazole. The eluate was concentrated with YM-10 membrane (Amicon) and dialyzed against 5 mM 3-morpholinopropanesulfonic acid (MOPS [Dojindo])–0.3 M glycine buffer (pH 6.5).
In vitro cleavage of VAMP-2.
The neurotoxin (150 nM) was reduced for 30 min at 37°C in 5 mM MOPS–0.3 M glycine buffer (pH 6.5) containing 10 mM dithiothreitol (DTT). The reduced neurotoxin was then incubated with the recombinant VAMP-2 at a final concentration of 5 μM. After 1 h at 37°C, the reaction was terminated by boiling for 3 min with 1% sodium dodecyl sulfate (SDS), and the mixture underwent SDS-polyacrylamide gel electrophoresis (PAGE) as described below. After electrophoresis, the gel was stained with Coomassie brilliant blue, and the intensity of stained band was quantified by scanning with a dual-wavelength densitometer (CS-9000 [Shimadzu, Kyoto, Japan]).
Other methods.
SDS-PAGE was performed in a 10 or 15% gel by the method of Laemmli (19). For immunoblotting, the protein was transferred to nitrocellulose (TM-2 [Tokyo Roshi, Tokyo, Japan]). The nitrocellulose paper was then incubated for 30 min with the respective MAbs. The immunoreactive bands were visualized with the ProtBlot Western AP system (Promega Corp., Madison, Wis.). Polyclonal rabbit antiserum against Okra/NT was prepared as described previously (18). Protein contents were determined by the method of Bradford with bovine gamma globulin as a standard (3). The quantity of ganglioside was defined as the amount of N-acetylneuraminic acid (NeuAc) determined by the method of Svennerholm (37).
RESULTS
Properties of 111/NT.
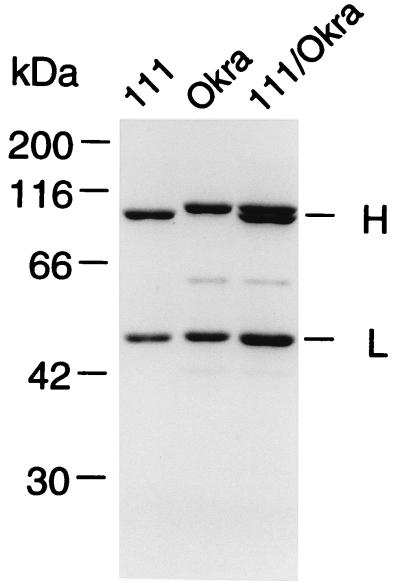
111/NT was purified from the culture in the same way as for Okra/NT. In SDS-PAGE under reducing conditions, 111/NT was separated into heavy and light chains with molecular masses of 94 and 49 kDa, which were, respectively, 5 and 3 kDa smaller than the heavy (99 kDa) and light (51 kDa) chains of Okra/NT (Fig. 1). The toxicity of 111/NT, titrated by intraperitoneal injections to obtain a mean LD50, was 5.4 × 106 LD50/mg of protein, which was about 1/10 that of Okra/NT (6.0 × 107 LD50/mg of protein). These results suggest that 111/NT possesses some characteristics distinguishable, on a molecular basis, from those of Okra/NT.
FIG. 1.
SDS-PAGE of 111/NT and Okra/NT in the presence of DTT. A sample (2 μg of each neurotoxin per lane) was applied to a 10% polyacrylamide gel. The two minor bands in Okra/NT were derivatives of the heavy chain (14). The positions of molecular mass standards are shown on the left. H, heavy chain; L, light chain.
Antigenicity of 111/NT.
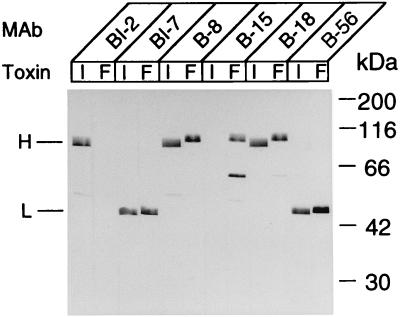
In order to explore the issues discussed above, we first examined the antigenic similarity and dissimilarity between 111/NT and Okra/NT. In the agar gel diffusion test with polyclonal rabbit anti-Okra/NT, each of the two toxins formed a single precipitate line which joined in a line of identity, except for a spur formed by Okra/NT (data not shown), suggesting that the antigenicity of 111/NT was similar but not identical to that of Okra/NT. We then examined the cross-reactivities between 111/NT and Okra/NT by using ELISA and immunoblotting with MAbs against the two neurotoxins (Table 1 and Fig. 2). Of the 13 MAbs against Okra/NT, all recognizing the amino-terminal half of the heavy chain (B-8, B-10, B-12, and B-13) and the light chain (B-56, B-90, B-96, and B-101) were found to cross-react with 111/NT, whereas those recognizing the carboxyl-terminal half of the heavy chain (B-14, B-15, B-16, and B-17), except one (B-18), showed no reactivity. Of the five MAbs against 111/NT, two (BI-4 and BI-7) reacting to the light chain bound to Okra/NT, but one (BI-2) recognizing the heavy chain did not. These results suggest that 111/NT possesses antigenic sites different from that of Okra/NT, which may be located on the carboxyl-terminal half of the heavy chain. Moreover, the remaining two MAbs (BI-3 and BI-6) reacted specifically to 111/NT in ELISA but not to any band in immunoblotting, which may indicate the existence of particular epitopes derived from the conformation of the 111/NT molecule.
TABLE 1.
Properties of MAbs against Okra/NT and 111/NT
| MAba | Isotype | Fragment recognized by immunoblottingb
|
ELISA value (OD450) in well coated with toxinc
|
||
|---|---|---|---|---|---|
| Okra | 111 | Okra | 111 | ||
| B-8 | G2b | HN | H | 1.731 | >2.000 |
| B-10 | G1 | HN | H | 1.388 | 1.696 |
| B-12 | G1 | HN | H | 1.266 | 1.596 |
| B-13 | G1 | HN | H | 1.346 | 1.682 |
| B-14 | G2b | HC | ND | >2.000 | 0.150 |
| B-15 | G2b | HC | ND | 1.822 | 0.233 |
| B-16 | G1 | HC | ND | 1.806 | 0.227 |
| B-17 | G1 | HC | ND | 1.956 | 0.167 |
| B-18 | G1 | HC | H | 1.108 | 0.870 |
| B-56 | G2a | L | L | 1.955 | 1.801 |
| B-90 | G1 | L | L | 1.928 | 1.836 |
| B-96 | G1 | L | L | 0.578 | 0.454 |
| B-101 | G1 | L | L | 1.382 | 1.014 |
| BI-2 | G1 | ND | H | 0.148 | 1.221 |
| BI-3 | G1 | ND | ND | 0.175 | 1.406 |
| BI-4 | G1 | L | L | 1.789 | 1.573 |
| BI-6 | G1 | ND | ND | 0.157 | 1.395 |
| BI-7 | G1 | L | L | 1.195 | 1.292 |
B and BI indicate MAbs against Okra/NT and 111/NT, respectively.
HN, amino-terminal half of the heavy chain; H, heavy chain; HC, carboxyl terminal of the heavy chain; L, light chain; ND, not detected.
The values for optical density at 450 nm (OD450) were obtained with each antibody at 1 μg/ml.
FIG. 2.
Immunoblotting analyses of 111/NT (I) and Okra/NT (F) with MAbs. The MAb used is shown on top of the lane. The data presented are representative of MAbs reacting to the same fragment.
Binding of 125I-labeled 111/NT to synaptosomes and synaptotagmin truncation mutants.
125I-111/NT bound to synaptosomes in a saturable manner, and Scatchard analysis indicated a single class of binding site with a dissociation constant (Kd) of 2.5 nM and a maximum binding activity (Bmax) of 0.76 pmol/mg of protein. On the other hand, the binding experiments with 125I-Okra/NT indicated that there were two classes of binding sites with Kd values of 0.42 nM (high affinity) and 11.7 nM (low affinity). The Bmaxs for high- and low-affinity sites were 1.12 and 3.21 pmol/mg of protein, respectively (Fig. 3). Okra/NT completely inhibited the binding of 125I-111/NT to synaptosomes (data not shown), indicating the possibility that both neurotoxins share the same binding site(s) on synaptosomal membranes.
FIG. 3.
Scatchard analyses of 125I-111/NT (A) and 125I-Okra/NT (B) binding to rat brain synaptosomes. Synaptosomes (10 μg of protein) were incubated at 37°C for 30 min with increasing concentrations of 125I-labeled toxin in the absence or presence of excess unlabeled toxin. Specific binding was plotted after correction for nonspecific binding. The data presented are from one experiment and are representative of three experiments with similar results. A binding saturation curve is shown in an inset in each panel.
Our previous data suggested that Okra/NT recognizes the amino-terminal domain of synaptotagmins 1 and 2 as the binding site in the presence of ganglioside GT1b or GD1a (28). In order to investigate the role of synaptotagmin as a receptor protein of 111/NT, we examined the binding activities of Stg1N and Stg2N incorporated into lipid vesicles together with ganglioside GT1b (Stg1N/GT1b and Stg2N/GT1b, respectively). 125I-111/NT bound to Stg2N/GT1b lipid vesicles in a concentration-dependent and saturable manner, while it did not interact with Stg1N/GT1b lipid vesicles (Fig. 4A). Scatchard analysis indicated a single class of binding site with a Kd of 2.4 nM, which was comparable to that obtained with synaptosomes. 125I-Okra/NT bound not only to Stg2N/GT1b lipid vesicles but also to Stg1N/GT1b lipid vesicles, and their Kds were 0.52 and 3.7 nM for Stg2N and Stg1N, respectively (Fig. 4B). These values resembled those obtained with full-length synaptotagmins and synaptosomes (26, 28). The Bmax of 125I-Okra/NT for Stg2N/GT1b lipid vesicles was higher than that of 125I-111/NT.
FIG. 4.
Binding of 125I-labeled toxins to recombinant amino-terminal domain of synaptotagmins 1 (Stg1N) and 2 (Stg2N) associated with ganglioside GT1b. (A) Dose dependence of 125I-labeled 111/NT (solid symbols) and 125I-labeled Okra/NT (open symbols) to Stg1N (triangles) and Stg2N (circles) incorporated into lipid vesicles with ganglioside GT1b. The recombinant synaptotagmin (10 ng of protein) reconstituted into lipid vesicles with ganglioside GT1b (2 ng of NeuAc) was incubated at 37°C for 30 min with increasing concentrations of 125I-labeled toxin. Values are the means ± standard errors from three experiments. Error bars smaller than the symbols were omitted. (B) Scatchard plot of the binding data shown in panel A, except for 125I-labeled 111/NT binding to the lipid vesicles containing Stg1N and ganglioside GT1b, for which the values were too low to show in the panel.
Cleavage of VAMP-2 by 111/NT.
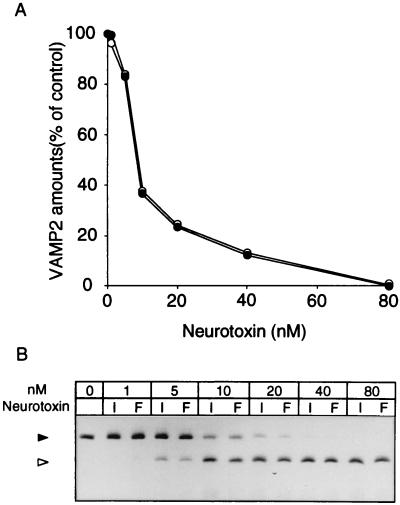
The light chain of type B neurotoxin was found to exhibit a zinc-dependent endopeptidase in response to the synaptic vesicle protein, VAMP-2 (34). After reduction of 111/NT with 10 mM DTT, we examined the proteolytic activity with recombinant VAMP-2 and compared it with that of Okra/NT. As shown in Fig. 5, VAMP-2 was digested to the same extent by reduced 111/NT and Okra/NT in a concentration-dependent manner.
FIG. 5.
Dose dependence of proteolysis of recombinant VAMP-2 by 111/NT and Okra/NT. (A) Samples were treated at 37°C for 1 h with reduced 111/NT (solid circles) or reduced Okra/NT (open circles) at different concentrations and electrophoresed on a 15% polyacrylamide gel. The gel was stained with Coomassie brilliant blue and quantified by densitometry. Results are expressed as a percentage of the initial VAMP-2 content. The data presented are the means of three independent experiments. (B) SDS-PAGE profile of VAMP-2 after incubation with or without reduced 111/NT (I) and reduced Okra/NT (F). The positions of VAMP-2 and the toxin-induced fragment are indicated by solid and open arrowheads, respectively.
DISCUSSION
No attempt has been made to investigate dissimilarity among the neurotoxins produced by proteolytic type B strains, although there has been a report only on the antigenic difference between the neurotoxins produced by proteolytic and nonproteolytic type B strains (14, 23). The present data indicate that 111/NT of the isolate implicated in infant botulism possesses some properties, including toxicity, distinguishable on molecular basis from those of Okra/NT produced by a food-borne botulism-related strain. SDS-PAGE revealed that the heavy and light chains derived from 111/NT have slightly smaller molecular sizes than those of Okra/NT. The ELISA results showed that 111/NT has multiple antigenic sites different from those of Okra/NT. Such differences in antigenicity appeared to be conserved mainly in the carboxyl-terminal half of the heavy chain, but not in the light chain. Since the carboxyl-terminal half of the heavy chain is responsible for the toxin binding to the receptor on the presynaptic membrane (13, 24), these results led us to ask to what extent the antigenic diversity between 111/NT and Okra/NT reflects upon their receptor recognition on the plasma membrane. In fact, binding experiments with synaptosomes showed that 125I-111/NT bound to a single site with a Kd value about one-sixth that of 125I-Okra/NT for the high-affinity binding site, although both neurotoxins appeared to share the same binding site. Furthermore, the light chains of both neurotoxins showed in vitro cleavage of VAMP-2 to the same extent, suggesting that there is no difference in the intracellular action between the two light chains. Perhaps 111/NT shows low toxicity because of its lower capability of binding to the receptor.
We have previously identified synaptotagmins 1 and 2 as the low- and high-affinity protein receptors, respectively, for Okra/NT (28). However, their toxin-binding activities were only observed in the presence of ganglioside GT1b or GD1a, suggesting that synaptotagmins from the toxin-binding site by associating with these gangliosides. Synaptotagmin is an integral membrane protein present on synaptic vesicles and is considered to be involved in their Ca2+-dependent exocytosis at the nerve terminal (36). Synaptotagmin has a single transmembrane region, a short amino-terminal intravesicular domain, and a large cytoplasmic domain (4). After synaptic vesicle exocytosis, the amino-terminal domain is exposed outside the nerve terminal. Therefore, it is likely that the amino-terminal domain consists of the toxin-binding site in association with the specific ganglioside. The present data demonstrated that Stg1N and Stg2N, which represent the amino-terminal domain of synaptotagmin fused with maltose-binding protein, exhibit a 125I-Okra/NT-binding activities equivalent to those of full-length synaptotagmins 1 and 2 (28). These results indicate that the carboxyl-terminal cytoplasmic domain does not contribute to form the toxin recognition site. On the other hand, 125I-111/NT appeared to lose the capacity to bind to Stg1N and had a lower Kd and Bmax for Stg2N, compared with 125I-Okra/NT binding to Stg1N and Stg2N. The Kd value of 125I-111/NT for Stg2N accorded with that of the single binding site on synaptosomes. Since the action of neurotoxin after binding to the receptor involves subsequent internalization and translocation into cytosol, where the light chain reaches a specific target protein (35), functional differences at such subsequent steps between the two neurotoxins still remain to be clarified. The present findings, however, strongly indicate that the receptor recognition site on the 111/NT molecule differs structurally from that of Okra/NT, and this may be supported partly by the results showing that 111/NT contains specific epitopes that recognize the steric conformation. Moreover, synaptotagmin 1 does not seem to play a critical role as the protein receptor for type B neurotoxin.
The genes of botulinum neurotoxins have already been cloned, and their nucleotide sequences have been determined (22). These data revealed the nature of the light chain which elicits the proteolytic activity in the cell cytosol to specifically cleave proteins of the exocytotic apparatus, thereby blocking neurotransmitter release (24, 25). In contrast to the function of the light chain, the mechanism of receptor recognition by the neurotoxin has been obscure, being probably due not only to serotype specificity of the toxin receptor but also to heterogeneity of the receptor recognition site on the neurotoxins. Therefore, it is of great value to know the primary structure of the carboxyl-terminal regions of 111/NT and Okra/NT in order to define the receptor-binding site. Studies are now in progress to determine the amino acid sequences of these regions in both neurotoxins.
ACKNOWLEDGMENTS
This work was partly supported by a grant for Scientific Research from the Ministry of Education, Science, Sports, and Culture and by a grant for Health Science Research of Emerging and Reemerging Infectious Diseases from the Ministry of Health and Welfare, Japan.
REFERENCES
- 1.Arnon S S. Infant botulism. Annu Rev Med. 1981;31:541–560. doi: 10.1146/annurev.me.31.020180.002545. [DOI] [PubMed] [Google Scholar]
- 2.Arnon S S. Infant botulism: anticipating the second decade. J Infect Dis. 1986;154:201–205. doi: 10.1093/infdis/154.2.201. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Brose N, Petrenko A G, Südhof T C, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 5.Dodds K L. Worldwide incidence and ecology of infant botulism. In: Hauschild A H W, Dodds K L, editors. Clostridium botulinum: ecology and control in foods. New York, N.Y: Marcel Dekker, Inc.; 1992. pp. 105–117. [Google Scholar]
- 6.Elferink L A, Trimble W S, Scheller R H. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989;264:11061–11064. [PubMed] [Google Scholar]
- 7.Hall J D, McCrosky L M, Pincomb B J, Hatheway C L. Isolation of an organism resembling Clostridium baratii which produces type F botulinal toxin from an infant with botulism. J Clin Microbiol. 1985;21:654–655. doi: 10.1128/jcm.21.4.654-655.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda S, Oka J, Nagao T. Effects of four diltiazem stereoisomers on binding of d-cis-[3H]diltiazem and (+)-[3H]PN200-110 to rabbit T-tubule calcium channels. Eur J Pharmacol. 1991;208:199–205. doi: 10.1016/0922-4106(91)90096-z. [DOI] [PubMed] [Google Scholar]
- 9.Kakinuma H, Maruyama H, Takahashi H, Yamakawa K, Nakamura S. The first case of type B infant botulism in Japan. Acta Paediatr Jpn. 1996;38:541–543. doi: 10.1111/j.1442-200x.1996.tb03542.x. [DOI] [PubMed] [Google Scholar]
- 10.Kondo H, Shimizu T, Kubonoya M, Izumi N, Takahashi M, Sakaguchi G. Titration of botulinum toxins for lethal toxicity by intravenous injection into mice. Jpn J Med Sci Biol. 1984;37:131–135. doi: 10.7883/yoken1952.37.131. [DOI] [PubMed] [Google Scholar]
- 11.Kozaki S. Interaction of botulinum type A, B and E derivative toxins with synaptosomes of rat brain. Naunyn-Schmiedeberg’s Arch Pharmacol. 1979;308:67–70. doi: 10.1007/BF00499721. [DOI] [PubMed] [Google Scholar]
- 12.Kozaki S, Kamata Y, Nagai T, Ogasawara J, Sakaguchi G. The use of monoclonal antibodies to analyze the structure of Clostridium botulinum type E derivative toxin. Infect Immun. 1986;52:786–791. doi: 10.1128/iai.52.3.786-791.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozaki S, Miki A, Kamata Y, Ogasawara J, Sakaguchi G. Immunological characterization of papain-induced fragments of Clostridium botulinum type A neurotoxin and interaction of the fragments with brain synaptosomes. Infect Immun. 1989;57:2634–2639. doi: 10.1128/iai.57.9.2634-2639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozaki S, Miyazaki S, Sakaguchi G. Development of antitoxin with each of two complementary fragments of Clostridium botulinum type B derivative toxin. Infect Immun. 1977;18:761–766. doi: 10.1128/iai.18.3.761-766.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozaki S, Nakaue S, Kamata Y. Immunological characterization of the neurotoxin produced by Clostridium botulinum type A associated with infant botulism in Japan. Microbiol Immunol. 1995;39:767–774. doi: 10.1111/j.1348-0421.1995.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 16.Kozaki S, Ogasawara J, Shimote Y, Kamata Y, Sakaguchi G. Antigenic structure of Clostridium botulinum type B neurotoxin and its interaction with gangliosides, cerebroside, and free fatty acids. Infect Immun. 1987;55:3051–3056. doi: 10.1128/iai.55.12.3051-3056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozaki S, Onimaru J, Kamata Y, Sakaguchi G. Immunological characterization of Clostridium butyricum neurotoxin and its trypsin-induced fragments by use of monoclonal antibodies against Clostridium botulinum type E neurotoxin. Infect Immun. 1991;59:457–459. doi: 10.1128/iai.59.1.457-459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozaki S, Sakaguchi G. Antigenicities of fragments of Clostridium botulinum type B derivative toxin. Infect Immun. 1975;11:932–936. doi: 10.1128/iai.11.5.932-936.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.McCroskey L M, Hatheway C L, Fenicia L, Pasolini B, Aureli P. Characterization of an organism that produces type E botulinal toxin but which resembles Clostridium butyricum from the feces of an infant with type E botulism. J Clin Microbiol. 1986;23:201–202. doi: 10.1128/jcm.23.1.201-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Midura T F, Arnon S S. Infant botulism: identification of Clostridium botulinum and its toxin in faeces. Lancet. 1976;ii:934–936. doi: 10.1016/s0140-6736(76)90894-1. [DOI] [PubMed] [Google Scholar]
- 22.Minton N P. Molecular genetics of clostridial neurotoxins. Curr Top Microbiol Immunol. 1995;195:161–194. doi: 10.1007/978-3-642-85173-5_8. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki S, Kozaki S, Sakaguchi S, Sakaguchi G. Comparison of progenitor toxins of nonproteolytic with those of proteolytic Clostridium botulinum type B. Infect Immun. 1976;13:987–989. doi: 10.1128/iai.13.3.987-989.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montecucco C, Schiavo G. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol. 1994;13:1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 25.Niemann H. Sourcebook of bacterial protein toxins. New York, N.Y: Academic Press; 1991. pp. 303–348. [Google Scholar]
- 26.Nishiki T, Kamata Y, Nemoto Y, Omori A, Ito T, Takahashi M, Kozaki S. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J Biol Chem. 1994;269:10498–10503. [PubMed] [Google Scholar]
- 27.Nishiki T, Ogasawara J, Kamata Y, Kozaki S. Solubilization and characterization of the acceptor for Clostridium botulinum type B neurotoxin from rat brain synaptic membranes. Biochim Biophys Acta. 1993;1158:333–338. doi: 10.1016/0304-4165(93)90032-4. [DOI] [PubMed] [Google Scholar]
- 28.Nishiki T, Tokuyama Y, Kamata Y, Nemoto Y, Yoshida A, Sato K, Sekiguchi M, Takahashi M, Kozaki S. The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 1996;378:253–257. doi: 10.1016/0014-5793(95)01471-3. [DOI] [PubMed] [Google Scholar]
- 29.Noda H, Sugita K, Koike A, Nasu T, Takahashi M, Shimizu T, Ooi K, Sakaguchi G. Infant botulism in Asia. Am J Dis Child. 1988;142:125–126. doi: 10.1001/archpedi.1988.02150020019012. [DOI] [PubMed] [Google Scholar]
- 30.Oguma K, Yokota K, Hayashi S, Takeshi K, Kumagai M, Itoh N, Tachi N, Chiba S. Infant botulism due to Clostridium botulinum type C toxin. Lancet. 1990;336:1449–1450. doi: 10.1016/0140-6736(90)93157-k. [DOI] [PubMed] [Google Scholar]
- 31.Pickett J, Berg B, Chaplin E, Brunstetter-Shafer M. Syndrome of botulism in infancy: clinical and electrophysiologic study. New Engl J Med. 1976;295:770–772. doi: 10.1056/NEJM197609302951407. [DOI] [PubMed] [Google Scholar]
- 32.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 33.Sakaguchi G, Sakaguchi S, Kamata Y, Tabita K, Asao T, Kozaki S. Distinct characters of Clostridium botulinum type A strains and their toxin associated with infant botulism in Japan. Int J Food Microbiol. 1990;11:231–242. doi: 10.1016/0168-1605(90)90016-x. [DOI] [PubMed] [Google Scholar]
- 34.Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta B R, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 35.Simpson L L. Molecular pharmacology of botulinum toxin and tetanus toxin. Annu Rev Pharmacol Toxicol. 1986;26:427–453. doi: 10.1146/annurev.pa.26.040186.002235. [DOI] [PubMed] [Google Scholar]
- 36.Südhof T C, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17:379–385. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 37.Svennerholm L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957;24:604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]