Abstract
The secreted broad-range phosphatidylcholine (PC)-preferring phospholipase C (PC-PLC) of Listeria monocytogenes plays a role in the bacterium’s ability to escape from phagosomes and spread from cell to cell. Based on comparisons with two orthologs, Clostridium perfringens α-toxin and Bacillus cereus PLC (PLCBc), we generated PC-PLC mutants with altered enzymatic activities and substrate specificities and analyzed them for biological function in tissue culture and mouse models of infection. Two of the conserved active-site zinc-coordinating histidines were confirmed by single amino acid substitutions H69G and H118G, which resulted in proteins inactive in broth culture and unstable intracellularly. Substitutions D4E and H56Y remodeled the PC-PLC active site to more closely resemble the PLCBc active site, while a gene replacement resulted in L. monocytogenes secreting PLCBc. All of these mutants yielded similar amounts of active enzyme as wild-type PC-PLC both in broth culture and intracellularly. D4E increased activity on and specificity for PC, while H56Y and D4E H56Y showed higher activity on both PC and sphingomyelin, with reduced specificity for PC. As expected, PLCBc expressed by L. monocytogenes was highly specific for PC. During early intracellular growth in human epithelial cells, the D4E mutant and the PLCBc-expressing strain performed significantly better than the wild type, while the H56Y and D4E H56Y mutants showed a significant defect. In assays for cell-to-cell spread, the H56Y and D4E mutants had close to wild-type characteristics, while the spreading efficiency of PLCBc was significantly lower. These studies emphasize the species-specific features of PC-PLC important for growth in mammalian cells.
Listeria monocytogenes, a gram-positive, facultative intracellular bacterial pathogen, is capable of infecting a variety of mammalian cells both in vivo and in vitro (8, 13, 23, 31, 46). Following internalization, the bacteria escape from the phagosomal vacuole, proliferate rapidly in the cytosol, and exploit host actin-based motility to spread to adjacent cells by means of filopodium-like projections. At this stage of infection, the bacteria are transiently confined in double-membrane vacuoles, from which they escape to repeat the cycle (10, 42, 54, 55).
L. monocytogenes secretes three virulence factors that interact with host cell membranes. Lysis of the host cell vacuole is mainly mediated by the thiol-activated pore-forming hemolysin listeriolysin O (LLO; encoded by hly) (2, 13, 40, 55). Nonhemolytic mutants are incapable of intracellular growth in most cell types and are avirulent in the mouse model of infection (9, 13, 14, 29, 41, 46). The two other secreted proteins are phospholipase C (PLC) enzymes: phosphatidylinositol (PI)-specific PLC (PI-PLC; encoded by plcA) is highly specific for PI (6, 18, 32, 38) and has weak activity on glycosyl-PI anchors (15). Phosphatidylcholine (PC)-preferring PLC (PC-PLC; plcB) is active on a broad range of phospholipids (PC > phosphatidylethanolamine > phosphatidylserine > sphingomyelin [SM] ≫ PI), hydrolyzing SM approximately one-fourth as rapidly as PC (16, 17, 57). Mutants lacking PI-PLC have been shown to escape less efficiently from the primary phagocytic vacuole (7), while PC-PLC-deficient mutants were seen to accumulate in the secondary double-membrane vacuoles (57). Studies on single and double mutants revealed that the two phospholipases have overlapping roles in escape from primary and secondary vacuoles (50). In Henle 407 human epithelial cells, PC-PLC is able to mediate escape from the primary vacuole in the absence of LLO (35).
PC-PLC is zinc dependent and secreted as an inactive proenzyme. Activation requires cleavage of an amino-terminal propeptide by either a listerial metalloprotease (Mpl) or other proteases, presumably of host origin (12, 16, 36, 39, 44, 48, 49). Zinc phospholipases homologous to the listerial PC-PLC have been identified in other gram-positive bacteria, including the extracellular pathogens Bacillus cereus (PLCBc) and Clostridium perfringens (α-toxin) (56, 57). C. perfringens α-toxin, a cytolysin, is active on both PC and SM, and a series of point mutations have defined active-site residues involved in zinc binding and catalysis (19, 43). PLCBc is nonhemolytic and, compared to PC-PLC, exhibits approximately 5- to 10-fold-higher activity on PC (17). PLCBc has generally been considered to have little or no sphingomyelinase (SMase) activity (34). Indeed, B. cereus expresses a separate SMase, which is encoded immediately downstream of the plc gene. Recent studies with recombinant enzyme indicate that PLCBc itself is capable of hydrolyzing SM at a rate 2% of that on PC (52). Furthermore, PLCBc, but not PC-PLC, is inhibited by halides, including chloride at physiological concentrations (1, 17).
In amino acid alignments, PC-PLC shows 38.7 and 22.0% identity with PLCBc and α-toxin, respectively (57). The crystal structure of PLCBc has been determined, and the nine zinc-coordinating residues identified (W1, H14, D55, H69, H118, D122, H128, H142, and E146) are conserved among the three proteins (5, 21, 25). Sequence alignments, however, suggest that some of the other active-site residues (PLCBc residues S2, A3, E4, Y56, S64, F66, F70, Y79, I80, T133, A134, L135, and S143) differ between the species. In PC-PLC, residues E4, Y56, I80, L135, and S143 of PLCBc are replaced by D4, H56, L80, I135, and C143, respectively.
Our goal in this study was to construct PC-PLC mutants with altered enzyme activities and substrate specificities and investigate the effects of these mutations on listerial intracellular growth and cell-to-cell spread. Furthermore, we examined if PLCBc could complement PC-PLC in tissue culture and animal models of infection.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains and relevant genotypes are listed in Table 1. The strains used and constructed in this study were based on two wild-type L. monocytogenes strains, 10403S (3) and SLCC 5764 (33). Strain 10403S and isogenic mutants were used for all cell infection assays. Strain SLCC 5764 and isogenic mutants were used for detection of PC-PLC and mutants thereof in broth culture. All strains were grown in brain heart infusion broth (BHI; Difco) and maintained on BHI agar. Stock cultures were stored at −80°C. Derivatives of pKSV7 were maintained in Escherichia coli host strain DH5α (Life Technologies) or XL1-Blue (Stratagene) by growth in liquid or on solid Luria-Bertani (LB) medium in the presence of 100 μg of ampicillin per ml. B. cereus ATCC 10987 was grown in BHI.
TABLE 1.
L. monocytogenes strains used in this study
| Strain | Genotype (PC-PLC phenotype) | Reference |
|---|---|---|
| 10403S | Wild-type isolate | 46 |
| SLCC 5764 | Hypersecreting wild-type isolate | 33 |
| DP-L1552 | 10403S ΔplcA | 7 |
| DP-L1553 | SLCC 5764 ΔplcA | 7 |
| DP-L1935 | 10403S ΔplcB | 50 |
| DP-L1936 | 10403S ΔplcA ΔplcB | 50 |
| DP-L1938 | SLCC 5764 ΔplcA ΔplcB | 36 |
| DP-L2161 | 10403S Δhly | 27 |
| DP-L2318 | 10403S Δhly ΔplcB | 35 |
| DP-L3176 | 10403S plcB (H56Y) | This study |
| DP-L3177 | 10403S plcB (H69G) | This study |
| DP-L3178 | 10403S plcB (H118G) | This study |
| DP-L3179 | 10403S ΔplcA plcB (H56Y) | This study |
| DP-L3180 | 10403S ΔplcA plcB (H69G) | This study |
| DP-L3181 | 10403S ΔplcA plcB (H118G) | This study |
| DP-L3182 | SLCC 5764 ΔplcA plcB (H56Y) | This study |
| DP-L3183 | SLCC 5764 ΔplcA plcB (H69G) | This study |
| DP-L3184 | SLCC 5764 ΔplcA plcB (H118G) | This study |
| DP-L3185 | 10403S Δhly plcB (H56Y) | This study |
| DP-L3186 | 10403S Δhly plcB (H69G) | This study |
| DP-L3187 | 10403S Δhly plcB (H118G) | This study |
| DP-L3525 | 10403S Φ(plcB′-plc+)a | This study |
| DP-L3526 | 10403S ΔplcA Φ(plcB′-plc+)a | This study |
| DP-L3527 | SLCC 5764 ΔplcA Φ(plcB′-plc+)a | This study |
| DP-L3528 | 10403S Δhly Φ(plcB′-plc+)a | This study |
| DP-L3532 | 10403S plcB (D4E) | This study |
| DP-L3533 | 10403S ΔplcA plcB (D4E) | This study |
| DP-L3534 | SLCC 5764 ΔplcA plcB (D4E) | This study |
| DP-L3535 | 10403S Δhly plcB (D4E) | This study |
| DP-L3536 | 10403S plcB (D4E H56Y) | This study |
| DP-L3537 | 10403S ΔplcA plcB (D4E H56Y) | This study |
| DP-L3538 | SLCC 5764 ΔplcA plcB (D4E H56Y) | This study |
| DP-L3539 | 10403S Δhly plcB (D4E H56Y) | This study |
Gene fusion of L. monocytogenes 10403S prepro-PC-PLC plcB sequence and B. cereus ATCC 10987 mature PLC plc sequence (see Materials and Methods and Table 2), leading to secretion of wild-type PLCBc instead of PC-PLC.
Construction of L. monocytogenes PC-PLC mutant strains. (i) PC-PLC amino acid substitutions.
All PC-PLC amino acid substitution mutants were constructed by PCR sequence overlap extension (24) using Pwo proofreading thermostable DNA polymerase (Boehringer Mannheim) and overlapping, complementary oligonucleotide primers (Gibco BRL) listed in Table 2. The template for all reactions was purified chromosomal L. monocytogenes 10403S DNA. The plcB mutational primers contained nucleotide changes leading to (i) the desired amino acid substitution and (ii) the creation of a translationally silent, proximal restriction endonuclease recognition site. Flanking primers were positioned at least 400 bp up- or downstream of the mutation. The resulting primary PCR products were spliced together in a secondary PCR. The final PCR products were cloned into the pKSV7 shuttle vector. Allelic exchange was performed as described earlier (7). PCR products, pKSV7 clones, and listerial clones after allelic exchange were screened for the mutations by endonuclease digestion of PCR products with the respective enzymes listed in Table 2. Correct sequences were confirmed by automated cycle sequencing of the exchanged alleles on both strands (ABI 377 and 373A Stretch sequencers, Taq FS dye terminator chemistry; DNA Sequencing Facility, University of Pennsylvania).
TABLE 2.
Oligonucleotide primers used in this study
| Name | Nucleotide sequence (5′-3′)a | Characteristicsb |
|---|---|---|
| 3168 | GGCTGCAGAGTGAGGTGAATGATA | 5′ plcB PstI site fwd |
| 3169 | GGGAATTCGAGTGGATAAGAATGT | 3′ plcB EcoRI site rev |
| 3170 | ATATGGATTCTTATAATCCGCATC | H56Y HinfI rev |
| 3171 | GATGCGGATTATAAGAATCCATAT | H56Y HinfI fwd |
| 3172 | CTCTATCAGGATTATAGAATCCAGATAAAAATGTA | H69G HinfI rev |
| 3173 | TACATTTTTATCTGGATTCTATAATCCTGATAGAG | H69G HinfI fwd |
| 3174 | ATCCGTATAGTACCCGATTGCTAG | H118G RsaI rev |
| 3175 | CTAGCAATCGGGTACTATACGGAT | H118G RsaI fwd |
| 3519 | GGCTGCAGAAATGGTAGAGGAAAG | 5′ plcB PstI fwd |
| 3520 | TTTATGTTTATCTTCAGCAGACCA | plcB-plc junction rev |
| ACTAAGTTTATGTGGTAATTTGCT | ||
| 3521 | AGCAAATTACCACATAAACTTAGT | plcB-plc junction fwd |
| TGGTCTGCTGAAGATAAACATAAA | ||
| 3522 | ATAAGAATGTATTCCTAAATATTG | plc-3′ plcB junction rev |
| TTAACGATCTCCGTACGTATCAAA | ||
| 3523 | TTTGATACGTACGGAGATCGTTAA | plc-3′ plcB junction fwd |
| CAATATTTAGGAATACATTCTTAT | ||
| 3524 | GGGGATCCACACGAAAAAGTCACA | 3′ plcB BamHI rev |
| 3529 | GGCTGCAGTAGGGAAAATAAAACA | 5′ plcB PstI fwd |
| 3530 | GGTCCGCGGAGAATCCGACAAATA | D4E HinfI fwd |
| 3531 | TATTTGTCGGATTCTCCGCGGACC | D4E HinfI rev |
Introduced nucleotide changes are underlined, the resulting amino acid codons are in bold, and the created translationally silent restriction sites are italicized.
Amino acid changes and introduced restriction sites (BamHI [G′GATCC], EcoRI [G′AATTC], HinfI [G′ANTC], PstI [CTGCA′G], and RsaI [GT′AC]) are indicated. rev, reverse primer; fwd, forward primer.
H69G and H118G were constructed using primer pairs 3172-3173 and 3174-3175, respectively. Flanking primers were 3168 and 3169. D4E was constructed by using primer pair 3530-3531 and flanking primers 3529 and 3169. H56Y was constructed by using primer pair 3170-3171 and flanking primers 3168 and 3169. The H56Y D4E double mutant was constructed by using primer pairs 3530-3531 and 3170-3171, with flanking primers 3529 and 3169.
(ii) PLCBc-expressing L. monocytogenes.
Chromosomal PLCBc-expressing L. monocytogenes strains were constructed as described above. To avoid potential problems in secretion and activation, we fused the PC-PLC signal and propeptide coding sequence to that coding for the mature PLCBc enzyme. Since the first three amino acids of the mature PC-PLC and PLCBc are identical (WSA), we hypothesized that activation of the hybrid should be analogous to the wild-type PC-PLC. Chromosomal DNA of L. monocytogenes 10403S and B. cereus ATCC 10987 were used as templates. Primer pairs used were 3520-3521 and 3522-3523, with flanking primers 3519 and 3524. Mutation screening was performed as described above, using a plc-specific SphI site as evidence. The correct sequences were corroborated by DNA sequencing on both strands.
SDS-PAGE, Western immunoblotting, and egg yolk overlay.
Secreted L. monocytogenes proteins were prepared from strain DP-L1553 and isogenic mutants as previously described (7, 36). Briefly, secreted polypeptides were obtained from BHI culture supernatants by precipitation in 10% trichloroacetic acid and resuspended in 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer–0.2 N NaOH to 1% the original volume. Proteins were fractionated by electrophoresis on an SDS–10% polyacrylamide gel. For Coomassie blue staining, 25 μl (corresponding to 2.5 ml of bacterial culture supernatant) of the preparation was loaded per well, whereas 10 μl (equivalent of 1.0 ml of supernatant) was loaded for Western immunoblotting and egg yolk overlay assay.
For Western immunoblots, proteins were electrotransferred (Bio-Rad semidry transfer cell) to Immunoblot P membranes (Millipore) and reacted with anti-PC-PLC or anti-PLCBc antibodies. Alkaline phosphatase-conjugated affinity-purified goat antibody to rabbit immunoglobulin G (Kirkegaard & Perry Laboratories) was used as the secondary antibody. Colorimetric detection was performed with a stabilized alkaline phosphatase substrate solution (Promega).
For the egg yolk overlay assay (30), the gel was washed immediately after electrophoresis once in 25% isopropanol and twice in phosphate-buffered saline (PBS; Ca2+ and Mg2+ free) for 30 min each time. The gel was then overlaid with egg yolk soft agar (0.7% agarose, 2.5% egg yolk, 50 μM ZnCl2, 0.05 μM CaCl2, 1 mM dithiothreitol, and 25 μg of gentamicin per ml in 1× PBS) and incubated at 37°C. PC-PLC activity was detected by the formation of zones of opacity.
In vitro enzyme assay.
BHI culture supernatants obtained as described above were assayed for activity on PC and SM as previously described (17), with the following modifications. [Choline-methyl-3H]PC, 1,2-dipalmitoyl (0.125 μCi; NEN), and PC (144 μg; Sigma) were sonicated in 50 μl of morpholinepropanesulfonic acid buffer (pH 7.2)–1.84% Triton X-100 per assay. The mixed micelle suspension was diluted to 100 μl by adding salts, water, and culture supernatants (final concentrations of 0.025% bovine serum albumin, 0.2 M NaCl, and 0.1 mM ZnSO4). For SM assays, the mixed micelles contained 0.06 μCi of [choline-methyl-14C]SM (NEN) and 147 μg of SM (Sigma). Hydrolysis was measured as described previously (17) after a 5-min incubation at 37°C.
Tissue culture growth assays in Henle 407 and J774 cells.
Growth in Henle 407 human epithelial cells was measured as described earlier (50). Briefly, Henle 407 cells were seeded on glass coverslips and infected (t = 0) with 2 × 106 bacteria/ml, which resulted in infection of approximately 5% of the cells. At t = 1 h, cells were washed with PBS, and at t = 1.5 h, gentamicin was added at a final concentration of 50 μg/ml. Cytochalasin D (Sigma) was added at a final concentration of 0.25 μg/ml where indicated. At each subsequent time point (2.5, 5.5, 8.5, and, where indicated, 11.5 h postinfection), the number of viable bacteria was determined by lysing cells on individual coverslips in water and plating them on LB agar as described previously (46).
Intracellular growth in J774 cells was determined as previously described (27, 46). Gentamicin (25 μg/ml) was added 1 h after infection. For the growth curve without gentamicin, the J774 cells were washed with PBS at 2 h postinfection, and fresh medium prewarmed to 37°C was added. Viable bacteria at each time point were determined as described above.
Escape from the primary vacuole.
The percentage of bacteria which had escaped into the host cell cytoplasm in Henle 407 cells 2 h after infection was determined by immunofluorescence microscopy as described previously (27), with some modifications. Briefly, the total number of bacteria was obtained by permeabilizing the cells and labeling bacteria with fluorescein isothiocyanate-conjugated polyclonal anti-L. monocytogenes antibody (Difco). The number of intracytoplasmic, and thereby F-actin-coated (10), bacteria was then inferred by colabeling with tetramethyl rhodamine isothiocyanate-phalloidin (Molecular Probes). Extracellular bacteria were detected by labeling with polyclonal anti-Listeria O antibody (Difco) and then with coumarin-conjugated donkey anti-rabbit immunoglobulin G (Molecular Probes) prior to cell permeabilization.
Plaque formation and virulence assay.
The plaquing assay was performed as previously described (51). Plaques formed in monolayers of mouse fibroblast L2 cells were visualized by staining the cells with neutral red at 4 days after infection. The mean plaque diameter formed by each strain was compared with the mean plaque diameter of strain 10403S. The relative plaque size is reported as a percentage of wild-type plaque size. Fifty percent lethal doses (LD50) were determined in BALB/c mice by tail vein injection of bacteria as described previously (46).
Affinity purification of antibody.
Antiserum against recombinant B. cereus PLCBc protein (gift from M. Roberts, Boston College, Boston, Mass.) (52) was raised in rabbits (Cocalico Biologicals). Specific PLCBc and PC-PLC antibodies were affinity purified by using the respective rabbit polyclonal antisera and the purified proteins as previously described (36, 45). Antibody preparations were titrated to determine the optimal required volume for immunoprecipitation of PLCBc and PC-PLC from a lysate of 106 J774 cells infected with DP-L3525 or wild-type L. monocytogenes, respectively.
Immunoprecipitation of PC-PLC and PLCBc from infected J774 cells.
Immunoprecipitation of PC-PLC and PLCBc from infected J774 cells was performed as previously described (4, 36). Briefly, J774 cells were infected to achieve an initial infection of one to two bacteria per cell. Infected cells were washed after 30 min, and gentamicin was added to a final concentration of 50 μg/ml at 1 h postinfection. Cells were starved for methionine 30 min prior to pulse-labeling. Cells were pulsed for 30 min with [35S]methionine (Express 35S protein labeling mix; NEN Research Laboratories) at 4 h postinfection and then lysed in radioimmunoprecipitation buffer. Where indicated, chloramphenicol (20 μg/ml, final concentration) or anisomycin (30 μg/ml) and cycloheximide (22.5 μg/ml) were present during labeling. PC-PLC and PLCBc were immunoprecipitated with the respective affinity-purified antibodies, fractionated by SDS-PAGE, and detected by fluorography. Bacterial counts were determined in parallel dishes.
RESULTS
Construction of L. monocytogenes PC-PLC mutant strains.
Three classes of L. monocytogenes PC-PLC mutant strains were constructed by PCR-mediated mutagenesis using the oligonucleotide primers listed in Table 2: strains expressing PC-PLC with mutations in (i) two zinc-coordinating residues and (ii) two active-site residues involved in substrate binding (separate or combined), and (iii) L. monocytogenes secreting B. cereus PLCBc instead of PC-PLC. The six mutant phospholipase constructs were introduced into the chromosomes of L. monocytogenes strains with four different genetic backgrounds: DP-L1553 (PI-PLC− SLCC 5764 hypersecreting isolate), 10403S (wild type), DP-L1552 (PI-PLC− 10403S) and DP-L2161 (LLO− 10403S) (Table 1).
Zinc-coordinating residue mutations H69G and H118G.
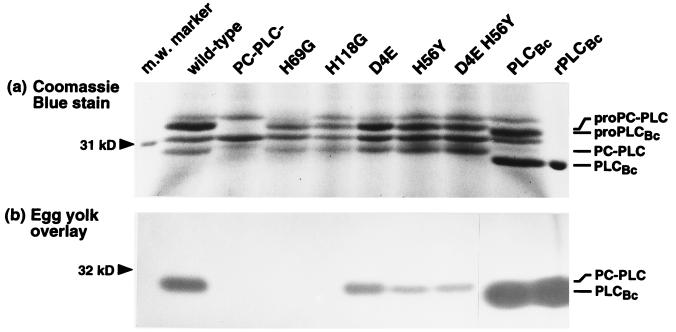
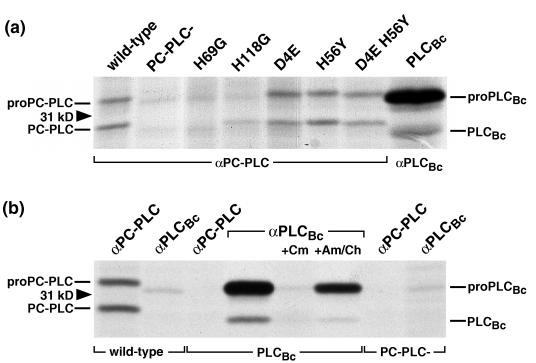
Amino acid alignments indicate that all zinc-coordinating residues identified in the active site of PLCBc are conserved among the three phospholipases (57). In studies with the C. perfringens α-toxin, substitutions of two of these residues, H68 and H126, by G resulted in an inactive enzyme and one with only 1% of the wild-type activity, respectively (43). In another recent study, substitutions of H68 and H126 by S produced similar results (19). All substitutions yielded stably produced and secreted α-toxin. To generate a PC-PLC with no or very low activity and study its effect on biological function, we separately changed the corresponding residues H69 and H118 to G. Both mutations led to stable secreted proteins in culture supernatants (Fig. 1a), but no activity on either PC or SM could be detected by in vitro enzyme assays (58) or by egg yolk overlay (Fig. 1b). In tissue culture models of infection, including LLO-independent growth of L. monocytogenes in Henle 407 human epithelial cells and plaquing in L2 mouse fibroblasts (35, 46), H69G and H118G mutants paralleled an in-frame plcB deletion mutant (58). However, immunoprecipitation of the mutant enzymes from infected J774 macrophage-like cells indicated that both H69G and H118G were unstable when expressed inside a mammalian cell (Fig. 2a). It is likely that the elimination of zinc-coordinating residues leads to protein misfolding and increased susceptibility to proteolytic degradation. The phenotypes observed in tissue culture models of infection are therefore a combination of inactive and unstable proteins.
FIG. 1.
Detection of PC-PLC and PLCBc in the supernatant of L. monocytogenes broth cultures. For this experiment, strain DP-L1553 and isogenic mutants were used. The same volume of concentrated bacterial secreted proteins was loaded per lane. Secreted phospholipase phenotypes are indicated above the lanes. The position of the 31-kDa molecular weight (m.w.) marker is shown at the left. Pro and mature forms of PC-PLC and PLCBc (hybrid pro-PLCBc; see text) were identified by Western blotting with affinity-purified antibodies and are indicated to the right. (a) Coomassie blue staining of secreted proteins resolved by SDS-PAGE. Recombinant purified mature form PLCBc (rPLCBc) was run as a size and activity control. (b) Egg yolk overlay assay. An empty gel lane between D4E H56Y and PLCBc, originally introduced to prevent confluence of zones of opacity, was removed so that panels a and b could be aligned without changing the vertical position.
FIG. 2.
Detection of PC-PLC and PLCBc from lysates of infected cells. J774 cells were infected with strain 10403S and isogenic mutants. At 4 h after infection, cells were pulse-labeled for 30 min with [35S]methionine. Immediately after the pulse, the cells were lysed and PC-PLC or PLCBc was immunoprecipitated with the respective affinity-purified antibodies, followed by fractionation by SDS-PAGE and detection of radiolabeled proteins by fluorography. (a) Expressed phospholipase phenotypes are indicated above the lanes; positions of the 31-kDa protein size marker and pro and mature forms of the enzymes are marked on the sides. (b) Affinity-purified antibodies used for immunoprecipitation and expressed phospholipase phenotypes are shown at the top and bottom, respectively. Where indicated, prokaryotic (chloramphenicol [Cm]) and eukaryotic (anisomycin/cycloheximide [Am/Ch]) protein synthesis inhibitors were present during labeling.
Active-site mutations D4E and H56Y.
A comparison of the two sequences reveals that PLCBc active-site residues E4, Y56, I80, L135, and S143 are substituted by D4, H56, L80, I135, and C143, respectively, in PC-PLC. We surmised that the distinct properties of PLCBc and PC-PLC might at least in part be due to the differences in these active-site residues. To generate a PC-PLC enzyme with activities and substrate specificities more similar to those of PLCBc, we changed D4 to E and H56 to Y, since among the variant residues, they are most proximal to the substrate in PLCBc (11).
Two single mutants and one double mutant were generated. All three mutants secrete equivalent amounts of mature protein when grown in BHI (Fig. 1a). D4E shows a slight increase in activity on PC (113% compared to the wild-type PC-PLC) and a decrease in SMase activity (77%), leading to an increased specificity for PC, while H56Y shows increased activity on both PC and SM (177 and 269%, respectively), making the enzyme less specific for PC. The D4E H56Y double mutant has an intermediate phenotype (156 and 223%), with values closer to those for the H56Y mutant, suggesting that substitutions D4E and H56Y have additive but antagonistic effects (Table 3). In summary, H56Y significantly affects the overall activity, while D4E has a more subtle effect on specificity.
TABLE 3.
Biochemical characteristics of phospholipase mutants
| PC-PLC phenotypea | Mean in vitro enzymatic activity (nmol of substrate hydrolyzed/min/ml) ± SDb
|
Specificity (SM/PC) | |
|---|---|---|---|
| PC | SM | ||
| Wild type | 52 ± 18 (8) | 13 ± 3 (10) | 0.26 |
| D4E | 59 ± 27 (6) | 10 ± 3* (9) | 0.17 |
| H56Y | 92 ± 25** (8) | 35 ± 9*** (10) | 0.39 |
| D4E H56Y | 81 ± 29* (6) | 29 ± 6*** (9) | 0.36 |
| PLCBc | 570 ± 133*** (6) | 3 ± 3*** (9) | 0.004 |
All assays were performed in DP-L1553 (hypersecreting L. monocytogenes SLCC 5764, PI-PLC−) background (see also text).
L. monocytogenes culture supernatants were assayed for hydrolysis of isotope-labeled phospholipids in Triton X-100 mixed micelles (see Materials and Methods) at pH 7.2 and 37°C. Numbers of separate experiments are indicated in parentheses. Two-tailed P values of unpaired t tests are classified as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to values obtained with wild-type PC-PLC.
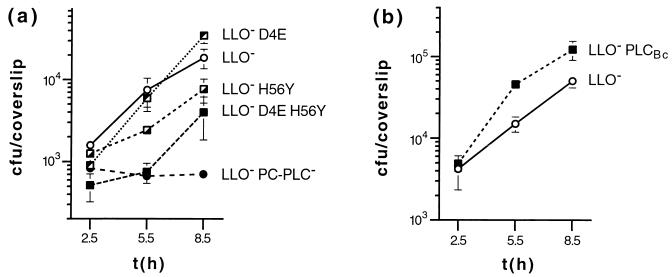
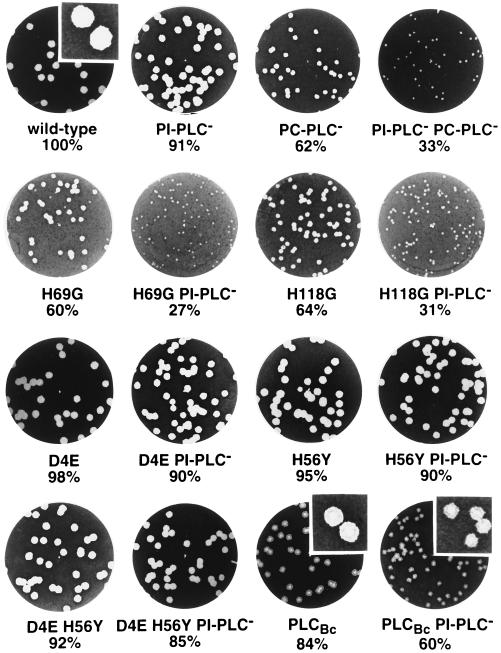
Escape from a primary vacuole was assessed by using an LLO− strain, as it is known that PC-PLC mediates LLO-independent escape of L. monocytogenes from a primary vacuole in human epithelial cells (35). Compared to L. monocytogenes expressing wild-type PC-PLC, bacteria expressing H56Y show a delayed escape from the primary vacuole of Henle 407 epithelial cells, as evidenced by a lower percentage of intracytoplasmic bacteria at 2 h postinfection and indirectly by a higher apparent doubling time between 2.5 and 5.5 h postinfection. D4E, on the other hand, leads to higher efficiency in phagosomal escape, the D4E H56Y double mutant again showing intermediate values closer to those for H56Y. In later stages of intracellular growth and cell-to-cell spread, the mutants display phenotypes closer to that of the wild type: apparent doubling times between 5.5 and 8.5 h postinfection are essentially identical (Table 4 and Fig. 3a). Plaque sizes, indicative of ability to spread from cell to cell, are only minimally affected, most pronounced in the D4E H56Y and H56Y mutants (8 and 5% decreases in plaque size, respectively [Fig. 4 and Table 4]). All three mutant proteins were immunoprecipitated from infected J774 cells in amounts similar to those for wild-type PC-PLC (Fig. 2a).
TABLE 4.
Tissue culture model and in vivo characteristics of phospholipase mutants
| PC-PLC phenotype | Value (mean ± SD) for tissue culture model of infectiona
|
In vivo LD50e | ||||
|---|---|---|---|---|---|---|
| Escape primary vacuoleb (%) | Apparent doubling timec (min)
|
Plaque size (%)d
|
||||
| 2.5–5.5 h | 5.5–8.5 h | Wild type | PI-PLC− | |||
| Wild type | 36 ± 4 (6) | 80 ± 24 (12) | 99 ± 14 (12) | 100 (20) | 90.6 ± 3.6 (10) | (1–3) × 104 |
| PC-PLC− | 0 (4) | ND | ND | 62.3 ± 6.4¶¶¶ (20) | 32.7 ± 4.8*** (19) | 2 × 105 |
| D4E | 63 ± 2*** (4) | 65 ± 25 (3) | 94 ± 38 (3) | 97.9 ± 2.7 (5) | 90.2 ± 3.0 (5) | ND |
| H56Y | 11 ± 3*** (4) | 183 ± 71*** (7) | 98 ± 36 (7) | 95.3 ± 4.0¶¶ (16) | 89.9 ± 2.9 (16) | ND |
| D4E H56Y | 21 ± 2*** (4) | 238 ± 104*** (3) | 99 ± 42 (3) | 91.8 ± 2.6¶¶¶†† (5) | 85.4 ± 4.2*† (5) | ND |
| PLCBc | 56 ± 12** (6) | 64 ± 9 (7) | 93 ± 24 (7) | 84.2 ± 4.5¶¶¶§§§ (10) | 60.4 ± 5.9***§§§ (10) | (7–8.5) × 104 |
Numbers of separate experiments are indicated in parentheses. ND, not determined. Two-tailed P values of unpaired t tests are classified as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to values obtained with wild-type PC-PLC; †, P < 0.05; ††, P < 0.01 compared to H56Y mutant; §§§, P < 0.001 compared PC-PLC− mutant. Two-tailed P values of paired t tests are classified as follows: ¶¶, P < 0.01; ¶¶¶, P < 0.001 compared to plaque measurements (in millimeters) obtained with the wild type (see text).
Percent total intracellular bacteria staining with rhodamine-phalloidin at 2 h after infection of Henle 407 human epithelial cells with mutants in an LLO− (DP-L2161) background (see text).
Calculated from slopes of growth curves from 2.5 to 5.5 h and 5.5 to 8.5 h, respectively, after infection of Henle 407 human epithelial cells with mutants in an LLO− (DP-L2161) background (see text).
Plaque size in L2 mouse fibroblast monolayers 4 days after infection with mutants in wild-type and PI-PLC− (DP-L1552) background compared to wild-type plaques (100% by definition).
FIG. 3.
Intracellular growth of L. monocytogenes in Henle 407 human epithelial cells. Henle 407 cells were infected with DP-L2161 (LLO−) and isogenic mutant strains. The data shown are from representative experiments (see also Table 4). Phospholipase phenotypes are indicated. (a) Active-site mutants; (b) B. cereus plc gene replacement. Each datum point and error bar represents the means ± standard deviations of viable bacteria recovered from three coverslips.
FIG. 4.
Plaque formation in infected L2 mouse fibroblasts. L2 cells were infected with strain 10403S (wild type) or DP-L1552 (ΔplcA PI-PLC−) and isogenic mutants thereof. Cell monolayers were stained with neutral red at 4 days after infection, highlighting clear areas of dead cells resulting from L. monocytogenes intracellular growth and cell-to-cell spread. The mean plaque diameters were calculated from several individual experiments (Table 4) and are indicated below each well. Inserts are magnified sections of the shown wells, showing the altered plaque morphology obtained with strains expressing B. cereus PLCBc.
Gene replacement with B. cereus plc.
An important issue to be addressed by this study was whether the PLC of an extracellular pathogen, B. cereus, could complement the listerial PC-PLC and how its significantly different activities would affect distinct steps in L. monocytogenes pathogenesis or overall virulence. Accordingly, we constructed L. monocytogenes strains expressing PLCBc instead of PC-PLC. To avoid conceivable problems with secretion and proteolytic activation, we constructed a hybrid proenzyme, consisting of the listerial PC-PLC signal and propeptide fused to the B. cereus PLCBc mature protein. Thus, the immediate region surrounding the proteolytic activation site should be similar to that in PC-PLC, since the first three amino acids (WSA) of mature PC-PLC and PLCBc are identical.
In BHI supernatants, the hybrid proenzyme is secreted in amounts similar to those for wild-type PC-PLC, and activation seems to be equally efficient, as shown in Fig. 1a. Interestingly, the hybrid proenzyme (calculated molecular mass of 31.1 kDa) as well as the mature PLCBc (28.4 kDa) migrate as expected, but their listerial counterparts (30.4 and 27.7 kDa, respectively) display abnormal electrophoretic mobility. The enzymatic activity of PLCBc was compared to that of PC-PLC in two types of assays. In the egg yolk overlay assay, a larger zone of opacity was obtained with PLCBc secreted by L. monocytogenes (Fig. 1b). This result is corroborated by enzyme essays on culture supernatants, in which PLCBc displays an 11-fold-higher efficiency in cleaving PC (Table 3). SM, on the other hand, is a very poor substrate for PLCBc secreted by L. monocytogenes. These data are consistent with measurements on purified native as well as recombinant PLCBc (52).
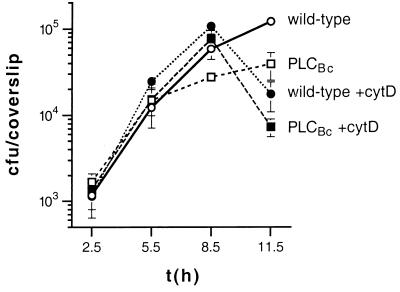
In tissue culture models of infection, PLCBc mediates more efficient escape from the primary vacuole, as evidenced by the higher percentage of intracytosolic bacteria at 2 h postinfection and indirectly by the lower apparent doubling times between 2.5 and 5.5 h postinfection (Fig. 3b and Table 4). In Henle 407 cells, a defect in cell-to-cell spread was identified by a decreased growth rate between 5.5 and 11.5 h postinfection (Fig. 5). As previously shown, for the PC-PLC deletion mutant (50), the intracellular growth rate of L. monocytogenes expressing PLCBc was restored to wild-type growth rate by blocking cell-to-cell spread with cytochalasin D, an inhibitor of actin polymerization. In the presence of cytochalasin D, bacteria multiply in the cytosol of host cells. The abrupt decline in viable bacterial counts of both strains after 8.5 h is attributed to host cell death and permeabilization to gentamicin. The cell-to-cell spread defect of L. monocytogenes expressing PLCBc was also shown by the L2 plaquing assay, in which PLCBc is clearly less effective than PC-PLC, with plaque sizes reduced by 15 to 30% (Table 4 and Fig. 4). Mouse virulence is also adversely affected, with an LD50 approximately four times higher than that obtained with wild-type L. monocytogenes 10403S but lower than the LD50 for a ΔplcB strain (Table 4).
FIG. 5.
Influence of cytochalasin D on growth of PLCBc-expressing L. monocytogenes in Henle 407 cells. Henle 407 cells were infected with 10403S (wild type) and isogenic strains. The data shown are from one of three experiments. Phospholipase phenotypes are indicated. +cytD indicates cytochalasin D addition. Each datum point and error bar represents the means ± standard deviations of viable bacteria recovered from three coverslips.
Interestingly, L. monocytogenes secreting PLCBc produces plaques with an altered, mottled phenotype, showing a central zone of complete lysis of the fibroblast monolayer surrounded by a zone of seemingly incomplete lysis (Fig. 4). To exclude the possibility of cytotoxic properties of the normally extracellularly expressed B. cereus ortholog, we assayed growth in J774 macrophage cells in the presence or absence of gentamicin as described earlier (27). If PLCBc were toxic, it would presumably permeabilize the host cell, allowing extracellular gentamicin to gain access to the cytoplasm and act on the intracytosolic bacteria. We detected no difference between the two curves (58).
PLCBc was immunoprecipitated from infected J774 cells. The active form was present in amounts similar to those for wild-type PC-PLC; however, a band with the size predicted for the proenzyme (approximately 31 kDa) was seen in much larger amounts than pro-PC-PLC (Fig. 2a). To determine if this 31-kDa band represented a host cell protein comigrating with the proenzyme, cells infected with L. monocytogenes expressing PLCBc were treated with chloramphenicol during labeling to block bacterial protein synthesis. This treatment eliminated both the 31-kDa band and mature PLCBc. Inhibition of eukaryotic protein synthesis by addition of anisomycin and cycloheximide, on the other hand, had no effect, indicating that the 31-kDa band was of listerial origin. To check if the anti-PLCBc antibodies cross-reacted with other L. monocytogenes proteins, the antibodies were used for immunoprecipitation from cells infected with wild-type L. monocytogenes. The results showed that no other L. monocytogenes protein precipitated with this antibody. Together, these data indicate that the precursor of PLCBc is present in much larger amounts than pro-PC-PLC in infected J774 cells (Fig. 2b).
DISCUSSION
Since the first demonstration of a bacterial toxin with enzymatic activity (C. perfringens α-toxin), phospholipases have been increasingly acknowledged as important determinants of bacterial pathogenesis. L. monocytogenes employs its PLCs, PI-PLC and PC-PLC, to gain and maintain an intracellular location, thereby evading the host’s humoral immune response. In this study, we further assess the role of PC-PLC in listerial pathogenesis. We constructed mutants with altered levels of enzyme activities and substrate specificities. Well-characterized tissue culture models of infection allowed us to correlate the effects of these changes to the enzyme’s ability to support several crucial steps in the L. monocytogenes intracellular life cycle, either steps immediately after primary cell infection (escape from the single-membrane phagosome) or later events involving the recurring escape from double-membrane vacuoles formed upon cell-to-cell spread. Our study shows that both the specific lecithinase and the SMase activities of PC-PLC are important determinants of listerial pathogenesis.
The first set of PC-PLC mutants, H69G and H118G, confirmed two of the conserved zinc-coordinating histidines. Inactivating the enzyme in vitro, as seen with the analogous C. perfringens α-toxin mutants (19, 43), the partial loss of zinc coordination appears to have a detrimental effect intracellularly, where the presumably misfolded proteins are quickly targeted for degradation by lysosomal proteases and the proteasome (36).
The second set of mutants targeted two active-site residues, D4 and H56. These two positions are filled by E and Y, respectively, in PLCBc. Earlier crystallographic data and molecular modeling indicated that while the overall structure of PLCBc is very rigid (25), the amino-terminal loop containing E4 undergoes structural change upon phosphate binding. Furthermore, the Y56 side chain is disordered in the native enzyme but ordered in the enzyme-substrate complex (20). Recent studies on substrate binding and catalytic mechanism indicate that E4 and Y56 are in close contact with the choline head group (11, 53). Previously, E4 had been implicated in directly initiating the catalytic mechanism by binding and activating the nucleophilic water molecule (5, 37). In the most recent model, however, this role has been taken by D55, one of the conserved active-site residues (11). E4 and Y56 (and their PC-PLC counterparts D4 and H56) are therefore most likely involved in substrate binding, E4 helping to stabilize the positive charge on the choline moiety. We wished to determine if the PC-PLC D4E and H56Y single and double substitutions enhanced PLC characteristics, particularly higher activity on and specificity for PC, and whether these changes would also affect listerial pathogenic potential.
D4E has a subtle yet significant influence on enzyme activity. Modest changes in overall activity could be anticipated, given that D and E vary only in the lengths of their side chains. Yet this mutation increases the specificity for PC by a factor of 1.5, and LLO-deficient L. monocytogenes expressing PC-PLC D4E are 1.7 times more efficient in escaping from the primary vacuole of Henle 407 cells, suggesting a direct correlation of these phenomena. Cell-to-cell spread was not affected with this mutant. H56Y causes a more pronounced mutant phenotype, significantly increasing overall enzymatic activity on both PC and SM, yet in contrast to D4E shifting the SM/PC specificity toward SM. Given the structural information and current model of PLCBc, it is intriguing to conclude that the residue adjacent to the apparently catalytically crucial D55 influences substrate turnover rates. The altered activities of these mutant enzymes need to be confirmed with the pure proteins. During intracellular infection, higher PC and SM hydrolysis rates appear not to benefit the bacterium. With both the H56Y mutant and the D4E H56Y double mutant, escape from the primary vacuole is impaired. Thus, as observed with D4E, specificity for PC seems to be directly linked to efficient escape from the primary vacuole. The single H56Y and double D4E H56Y mutants show small but significant decreases in cell-to-cell spread efficacy, again suggesting that enzyme specificity is more important than overall enzyme activity. In vitro results with D4E echo findings with PC-PLC processed by a presumed host protease, which also showed a shift toward increased PC specificity. This presumably resulted from altered processing site(s) at the N terminus (36).
Last, in order to be able to compare PLCBc-modeled active-site mutants of PC-PLC with wild-type PLCBc not only in vitro but also in tissue culture models of infection, we set out to construct L. monocytogenes strains secreting PLCBc. Similar gene replacements had been previously performed to elucidate the special features of LLO. Its C. perfringens ortholog, the extracellular cytolysin perfringolysin O, was able to mediate escape of L. monocytogenes from host cell vacuoles. Intracellularly expressed perfringolysin O however, had cytotoxic effects (27, 47) and could only functionally complement LLO when its pH optimum or half-life was altered by single-point mutations (28). Since NIH 3T3 fibroblast cells had been successfully and stably transformed with the gene encoding PLCBc (plc) (26), and salts at physiological concentrations had been shown to inhibit PLCBc (1), we expected that intracellularly expressed PLCBc would have less drastic effects. Data obtained with PLCBc secreted by L. monocytogenes were in agreement with published data on purified native and recombinant PLCBc. In addition, amounts of both forms of PLCBc are similar to those of pro-PC-PLC and PC-PLC in broth-grown bacteria. In infected cells, however, the inactive pro form of PLCBc is present in unexpectedly high amounts compared to pro-PC-PLC. Cytosolic pro-PC-PLC is rapidly degraded (half-life of less than 15 min) via the proteasome (36). The higher level of intracellular proPLCBc is therefore most likely a consequence of the fusion protein’s greater resistance to proteasomal degradation, although we cannot exclude upregulation of gene expression. Important for the interpretation of the L. monocytogenes PLCBc tissue culture data, however, the mature forms of PC-PLC and PLCBc were found in approximately equivalent amounts. These data agree with the current model of proteolytic activation occurring mainly in the limiting environment of the spreading vacuole (36). Alternatively, Mpl or other proteases might be very inefficient in activating PLCBc, or the active form of PLCBc may be very short-lived in eukaryotic cells.
Interestingly, PLCBc confers a phenotype congruent to D4E in early infection in human epithelial cells. In this particular cell type, proteolytically activated PC-PLC can mediate LLO-independent vacuolar escape at approximately 50% of the wild-type, i.e., LLO-dependent, efficiency (35). Intriguingly, both the PLCBc and D4E mutants are able to compensate for the LLO deficiency of the L. monocytogenes strains used in this particular assay, growing similarly to wild-type L. monocytogenes 10403S in human epithelial cells. As anticipated however, PLCBc does not restore the plaquing ability of LLO-deficient strains in fibroblast cells (58). In turn, L. monocytogenes strains expressing PLCBc and LLO show a significant defect in cell-to-cell spread and virulence, with plaque sizes and an LD50 halfway between those for the wild-type and PC-PLC deletion mutants. Our experiments indicate that the mottled L2 fibroblast plaque phenotype is likely due to the spreading defect observed in Henle 407 epithelial cells, although it remains possible that it is the result of a transformed cell phenotype, as observed in NIH 3T3 fibroblasts expressing PLCBc (26).
In an earlier study, PI-PLC and PC-PLC were shown to be essential determinants of L. monocytogenes pathogenicity, cooperating with each other and LLO to promote the bacterium’s access to the host cytoplasm during the initial invasion of host cells and upon subsequent cell-to-cell spread. The lack of both PI-PLC and PC-PLC led to a severe defect in virulence, suggesting additional roles during infection (50). A recent study has shown that the enzymatic activities of the phospholipases directly affect host cell signal transduction pathways by causing IκBβ degradation and thus leading to persistent NF-κB activation (22). In this study, we evaluated the essential features of PC-PLC to further understand its role in the pathogenesis of L. monocytogenes infection. Our data indicate that activities on both PC and SM are essential for virulence. Lecithinase activity seems to be important in the initial escape from the phagolysosome, whereas SMase activity combined with lecithinase activity is more significant for efficient escape from the double-membrane secondary vacuole and cell-to-cell spread. Of interest in this regard is the recent finding that a combination of lecithinase and SMase activities promoted fusion of lipid vesicles consisting of an equimolar mixture of SM, PC, phosphatidylethanolamine, and cholesterol, whereas the addition of lecithinase or SMase alone resulted in no major changes in vesicle architecture (1a). Thus, promotion of fusion of the double-membrane vacuole could be an important component of the escape process. The bacterium does not benefit from the generated shifts in PC-PLC activities and specificities, and an ortholog from an extracellular pathogen, B. cereus PLCBc, cannot fully complement PC-PLC. This finding indicates that PC-PLC is part of a fine-tuned L. monocytogenes intracellular program, which ensures survival in the mammalian host.
ACKNOWLEDGMENTS
We acknowledge the advice and stimulation provided by Dan Portnoy, Trudi Bannam, Darren Higgins, Marlena Moors, Greg Smith, Justin Skoble, Margaret Gedde, and Sîan Jones. We thank Mary Roberts for the gift of recombinant B. cereus PLCBc, John S. Bomalaski for rabbit anti-B. cereus PLCBc serum used in preliminary experiments, and Archie Bouwer and Dave Hinrichs for the animal studies.
This research was supported by grant GM-52797 from the National Institutes of Health.
REFERENCES
- 1.Aakre S-E, Little C. Inhibition of Bacillus cereus phospholipase C by univalent anions. Biochem J. 1982;203:799–801. doi: 10.1042/bj2030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Alonso, A. Personal communication.
- 2.Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 3.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 4.Brundage R A, Smith G A, Camilli A, Theriot J A, Portnoy D A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci USA. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byberg J R, Jørgensen F S, Hansen S, Hough E. Substrate-enzyme interactions and catalytic mechanism in phospholipase C: a molecular modeling study using the GRID program. Proteins Struct Funct Genet. 1992;12:331–338. doi: 10.1002/prot.340120405. [DOI] [PubMed] [Google Scholar]
- 6.Camilli A, Goldfine H, Portnoy D A. Listeria monocytogenes mutants lacking phosphatidylinositol-specific phospholipase C are avirulent. J Exp Med. 1991;173:751–754. doi: 10.1084/jem.173.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cossart P, Mengaud J. Listeria monocytogenes. A model system for the molecular study of intracellular parasitism. Mol Biol Med. 1989;6:463–474. [PubMed] [Google Scholar]
- 9.Cossart P, Vicente M F, Mengaud J, Baquero F, Perez-Diaz J C, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabiri G A, Sanger J M, Portnoy D A, Southwick F S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci USA. 1990;87:6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Graça Thrige D, Byberg Buur J R, Jørgensen F S. Substrate binding and catalytic mechanism in phospholipase C from Bacillus cereus: a molecular mechanism and molecular dynamics study. Biopolymers. 1997;42:319–336. doi: 10.1002/(SICI)1097-0282(199709)42:3<319::AID-BIP5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Domann E, Leimeister-Wächter M, Goebel W, Chakraborty T. Molecular cloning, sequencing, and identification of a metalloprotease gene from Listeria monocytogenes that is species specific and physically linked to the listeriolysin gene. Infect Immun. 1991;59:65–72. doi: 10.1128/iai.59.1.65-72.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard J-L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard J-L, Berche P, Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986;52:50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi A J, Perussia B, Goldfine H. Listeria monocytogenes phosphatidylinositol (PI)-specific phospholipase C has low activity on glycosyl-PI-anchored proteins. J Bacteriol. 1993;175:8014–8017. doi: 10.1128/jb.175.24.8014-8017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geoffroy C, Raveneau J, Beretti J-L, Lecroisey A, Vazquez-Boland J-A, Alouf J E, Berche P. Purification and characterization of an extracellular 29-kilodalton phospholipase C from Listeria monocytogenes. Infect Immun. 1991;59:2382–2388. doi: 10.1128/iai.59.7.2382-2388.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldfine H, Johnston N C, Knob C. Nonspecific phospholipase C of Listeria monocytogenes: activity on phospholipids in Triton X-100-mixed micelles and in biological membranes. J Bacteriol. 1993;175:4298–4306. doi: 10.1128/jb.175.14.4298-4306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldfine H, Knob C. Purification and characterization of Listeria monocytogenes phosphatidylinositol-specific phospholipase C. Infect Immun. 1992;60:4059–4067. doi: 10.1128/iai.60.10.4059-4067.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guillouard I, Garnier T, Cole S T. Use of site-directed mutagenesis to probe structure-function relationships of alpha-toxin from Clostridium perfringens. Infect Immun. 1996;64:2440–2444. doi: 10.1128/iai.64.7.2440-2444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen S, Hansen L K, Hough E. Crystal structures of phosphate, iodide and iodate-inhibited phospholipase C from Bacillus cereus and structural investigations of the binding of reaction products and a substrate analogue. J Mol Biol. 1992;225:543–549. doi: 10.1016/0022-2836(92)90938-g. [DOI] [PubMed] [Google Scholar]
- 21.Hansen S, Hough E, Svensson L A, Wong Y-L, Martin S F. Crystal structure of phospholipase C from Bacillus cereus complexed with a substrate analog. J Mol Biol. 1993;234:179–187. doi: 10.1006/jmbi.1993.1572. [DOI] [PubMed] [Google Scholar]
- 22.Hauf N, Goebel W, Fiedler F, Sokolovic Z, Kuhn M. Listeria monocytogenes infection of P388D1 macrophages results in a biphasic NF-κB (RelA/p50) activation induced by lipoteichoic acid and bacterial phospholipases and mediated by IκBα and IκBβ degradation. Proc Natl Acad Sci USA. 1997;94:9394–9399. doi: 10.1073/pnas.94.17.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havell E A. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986;54:787–792. doi: 10.1128/iai.54.3.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 25.Hough E, Hansen L K, Birknes B, Jynge K, Hansen S, Hordvik A, Little C, Dodson E, Derewanda Z. High-resolution (1.5Å) crystal structure of phospholipase C from Bacillus cereus. Nature. 1989;338:357–360. doi: 10.1038/338357a0. [DOI] [PubMed] [Google Scholar]
- 26.Johansen T, Bjørkøy G, Øvervatn A, Diaz-Meco M T, Traavik T, Moscat J. NIH 3T3 cells stably transfected with the gene encoding phosphatidylcholine-hydrolyzing phospholipase C from Bacillus cereus acquire a transformed phenotype. Mol Cell Biol. 1994;14:646–654. doi: 10.1128/mcb.14.1.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones S, Portnoy D A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones S, Preiter K, Portnoy D A. Conversion of an extracellular cytolysin into a phagosome-specific lysin which supports the growth of an intracellular pathogen. Mol Microbiol. 1996;21:1219–1225. doi: 10.1046/j.1365-2958.1996.00074.x. [DOI] [PubMed] [Google Scholar]
- 29.Kathariou S, Metz P, Hof H, Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kathariou S, Pine L, George V, Carlone G M, Holloway B P. Nonhemolytic Listeria monocytogenes mutants that are also noninvasive for mammalian cells in culture: evidence for coordinate regulation of virulence. Infect Immun. 1990;58:3988–3995. doi: 10.1128/iai.58.12.3988-3995.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn M, Kathariou S, Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988;56:79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leimeister-Wächter M, Domann E, Chakraborty T. Detection of a gene encoding a phosphatidylinositol-specific phospholipase C that is co-ordinately expressed with listeriolysin in Listeria monocytogenes. Mol Microbiol. 1991;5:361–366. doi: 10.1111/j.1365-2958.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 33.Leimeister-Wächter M, Goebel W, Chakraborty T. Mutations affecting hemolysin production in Listeria monocytogenes located outside of the listeriolysin gene. FEMS Microbiol Lett. 1989;65:23–30. doi: 10.1016/0378-1097(89)90360-1. [DOI] [PubMed] [Google Scholar]
- 34.Little C. Phospholipase C from Bacillus cereus. Methods Enzymol. 1981;71:725–731. [Google Scholar]
- 35.Marquis H, Doshi V, Portnoy D A. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63:4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marquis H, Goldfine H, Portnoy D A. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J Cell Biol. 1997;137:1381–1392. doi: 10.1083/jcb.137.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin S J, Spaller M R, Hergenrother P J. Expression and site-directed mutagenesis of the phosphatidylcholine-preferring phospholipase C of Bacillus cereus: probing the role of the active site Glu146. Biochemistry. 1996;35:12970–12977. doi: 10.1021/bi961316f. [DOI] [PubMed] [Google Scholar]
- 38.Mengaud J, Braun-Breton C, Cossart P. Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol Microbiol. 1991;5:367–372. doi: 10.1111/j.1365-2958.1991.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 39.Mengaud J, Geoffroy C, Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991;59:1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mengaud J, Vicente M F, Chenevert J, Pereira J M, Geoffroy C, Gicquel-Sanzey B, Baquero F, Perez-Diaz J C, Cossart P. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect Immun. 1988;56:766–772. doi: 10.1128/iai.56.4.766-772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michel E, Reich K A, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;4:2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 42.Mounier J, Ryter A, Coquis-Rondon M, Sansonetti P J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990;58:1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagahama M, Okagawa Y, Nakayama T, Nishioka E, Sakurai J. Site-directed mutagenesis of histidine residues in Clostridium perfringens alpha-toxin. J Bacteriol. 1995;177(5):1179–1185. doi: 10.1128/jb.177.5.1179-1185.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niebuhr K, Chakraborty T, Köllner P, Wehland J. Production of monoclonal antibodies to the phosphatidylcholine-specific phospholipase C of Listeria monocytogenes, a virulence factor for this species. Med Microbiol Lett. 1993;2:9–16. [Google Scholar]
- 45.Olmsted J B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981;256:11955–11957. [PubMed] [Google Scholar]
- 46.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Portnoy D A, Tweten R K, Kehoe M, Bielecki J. Capacity of listeriolysin O, streptolysin O, and perfringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect Immun. 1992;60:2710–2717. doi: 10.1128/iai.60.7.2710-2717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poyart C, Abachin E, Razafimanantsoa I, Berche P. The zinc metalloprotease of Listeria monocytogenes is required for maturation of phosphatidylcholine phospholipase C: direct evidence obtained by gene complementation. Infect Immun. 1993;61:1576–1580. doi: 10.1128/iai.61.4.1576-1580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raveneau J, Geoffroy C, Beretti J-L, Gaillard J-L, Alouf J E, Berche P. Reduced virulence of a Listeria monocytogenes phospholipase-deficient mutant obtained by transposon insertion into the zinc metalloprotease gene. Infect Immun. 1992;60:916–921. doi: 10.1128/iai.60.3.916-921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun A N, Camilli A, Portnoy D A. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1990;58:3770–3778. doi: 10.1128/iai.58.11.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan C A, Hehir M J, Roberts M F. Cloning, overexpression, refolding, and purification of the nonspecific phospholipase C from Bacillus cereus. Protein Expr Purif. 1997;10:365–372. doi: 10.1006/prep.1997.0756. [DOI] [PubMed] [Google Scholar]
- 53.Tan C A, Roberts M F. Engineering of the nonspecific phospholipase C from Bacillus cereus: replacement of glutamic acid-4 by alanine results in loss of interfacial catalysis and enhanced phosphomonoesterase activity. Biochemistry. 1998;370:4275–4279. doi: 10.1021/bi972751s. [DOI] [PubMed] [Google Scholar]
- 54.Theriot J A, Mitchison T J, Tilney L G, Portnoy D A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- 55.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Titball R W. Bacterial phospholipases C. Microbiol Rev. 1993;57:347–366. doi: 10.1128/mr.57.2.347-366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vazquez-Boland J-A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zückert, W. R., and H. Goldfine. Unpublished data.