Abstract
Although intravenous bevacizumab (IVBEV) is the most promising treatment for cerebral radiation necrosis (CRN), there is no conclusion on the optimal dosage. Our retrospective study aimed to compare the efficacy and safety of high‐dose with low‐dose IVBEV in treating CRN associated with radiotherapy for brain metastases (BMs). This paper describes 75 patients who were diagnosed with CRN secondary to radiotherapy for BMs, treated with low‐dose or high‐dose IVBEV and followed up for a minimum of 6 months. The clinical data collected for this study include changes in brain MRI, clinical symptoms, and corticosteroid usage before, during, and after IVBEV treatment. At the 3‐month mark following administration of IVBEV, a comparison of two groups revealed that the median percentage decreases in CRN volume on T2‐weighted fluid‐attenuated inversion recovery and T1‐weighted gadolinium contrast‐enhanced image (T1CE), as well as the signal ratio reduction on T1CE, were 65.8% versus 64.8% (p = 0.860), 41.2% versus 51.9% (p = 0.396), and 37.4% versus 35.1% (p = 0.271), respectively. Similarly, at 6 months post‐IVBEV, the median percentage reductions of the aforementioned parameters were 59.5% versus 62.0% (p = 0.757), 39.1% versus 31.3% (p = 0.851), and 35.4% versus 28.2% (p = 0.083), respectively. Notably, the incidence of grade ≥3 adverse events was higher in the high‐dose group (n = 4, 9.8%) than in the low‐dose group (n = 0). Among patients with CRN secondary to radiotherapy for BMs, the administration of high‐dose IVBEV did not demonstrate superiority over low‐dose IVBEV. Moreover, the use of high‐dose IVBEV was associated with a higher incidence of grade ≥3 adverse events compared with low‐dose IVBEV.
Keywords: brain metastases, cerebral radiation necrosis, dose, intravenous bevacizumab, toxicity
In this study, it was found that there were no discernible additional benefits in utilizing high‐dose intravenous bevacizumab (IVBEV) compared with low‐dose IVBEV for treating CRN. In fact, the high‐dose IVBEV group appeared to be associated with a higher incidence of adverse events.
Abbreviations
- AE
Adverse events
- BED
Biological effective dose
- BMs
Brain metastases
- CK
Cyberknife
- CRN
Cerebral radiation necrosis
- DBCRNAB
Duration between CRN diagnosis and BEV treatment
- DBRAB
Duration between radiotherapy and BEV treatment
- DBRACRN
Duration between radiotherapy and CRN diagnosis
- ESMO
European Society for Medical Oncology
- IQRs
Interquartile ranges
- IVBEV
Intravenous bevacizumab
- NCI‐CTCAE
National Cancer Institute's Common Terminology Criteria
- OS
Overall survival
- PD
Progressive disease
- PET/CT
Positron‐emission tomography /computed tomography
- PFS
Progression‐free survival
- SRS
Stereotactic radiosurgery
- T1CE
T1‐weighted gadolinium contrast‐enhanced image
- T2FLAIR
T2‐weighted fluid‐attenuated inversion recovery
- VEGF
Vascular endothelial growth factor
- WBRT
Whole brain radiation therapy
1. INTRODUCTION
Brain metastases represent the most prevalent tumors of the central nervous system and present an ever‐growing challenge to modern oncology. 1 In recent decades, significant strides have been made toward managing BMs, largely thanks to continual advancements in medical oncology treatments (e.g., targeted therapies, immunotherapy and anti‐angiogenesis therapy), neurosurgery, neuroimaging, and radiotherapy. 2 As radiation therapy technology continues to progress, the use of precision radiotherapy—embodied by SRS—has become increasingly widespread. While SRS can result in long‐term local control and an extended survival period by providing high local radical doses to the affected area while sparing the surrounding normal tissue, it may also increase the risk of local CRN with an incidence of 20%. This necrosis can lead to progressive neurological deficits. 3 , 4 , 5 , 6
The etiology of CRN still remains inconclusive. Endothelial dysfunction has been widely acknowledged as the responsible mechanism for this condition. It is well established that radiation can cause injury to the endothelial cells, leading to a gradual onset of tissue hypoxia. This state then triggers the production of VEGF, which is known to augment the permeability of the blood–brain barrier. 7 Research conducted on animal models of CRN has long since demonstrated the involvement of hypoxia in the induction of VEGF. 8 , 9 , 10
Bevacizumab (Avastin; Genentech), a humanized monoclonal antibody that inhibits VEGF‐A, has been demonstrated to effectively alleviate CRN in a randomized, double‐blinded, placebo‐controlled clinical trial (7.5 mg/kg administrated intravenously every 3 weeks). 11 Since then, an increasing number of retrospective and prospective studies have illustrated the effectiveness of IVBEV in treating CRN. Most research centers have used dosages of 5 mg/kg every 2 weeks, 7.5 mg/kg every 3 weeks, or 10 mg/kg every 2 weeks, with few using doses of 2.5 mg/kg every 2 weeks, 15 mg/kg every 3 weeks, or 4 weeks. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Two prospective studies conducted in China have successively demonstrated that IVBEV (5 mg/kg every 2 weeks) is superior to corticosteroids in treating CRN, and that ultra‐low‐dose IVBEV (1 mg/kg every 3 weeks) remains efficacious. 23 , 24 Despite the therapeutic effects of different doses of IVBEV for CRN demonstrated in previous studies, the risk of treatment‐related AEs appears to increase with dose escalation, thus increasing the financial burden for patients. Therefore, this study aimed to assess the efficacy and safety of high‐dose and low‐dose IVBEV for treating CRN secondary to BM radiotherapy and to determine the optimal dose of IVBEV for CRN treatment.
2. MATERIALS AND METHODS
2.1. Patients
The local ethics committee granted approval for the retrospective analysis of patient data (bc2022184). The study collected a total of 75 CRN patients with central nervous system metastases who received brain radiotherapy at the hospital between January 2012 and February 2021. Inclusion criteria for the study required that the patient: (1) had received brain radiotherapy at least 3 months prior to IVBEV, (2) age >18 years, (3) had intracranial metastases, (4) had been diagnosed with CRN through pathology or radiographic imaging, (5) had no history of intracranial hemorrhage prior to enrollment, and (6) had undergone MRI within 1 week prior to the first IVBEV, as well as 3 months (±30 days) and 6 months (±30 days) after the first IVBEV, with measurable lesions visible on the MRI. Exclusion criteria included: (1) primary intracranial tumor, (2) intracranial metastasis that recurred in situ, (3) concurrent use of other angiogenic agents, (4) various nervous system diseases such as neurovascular diseases and demyelinating diseases, and (5) incomplete or lost follow‐up imaging data.
2.2. Data collection
Demographic and clinical data were retrieved from electronic medical records, including age, sex, body weight, KPS, primary tumor type, location of intracranial CRN lesions, underlying disease, CRN‐associated symptoms, diagnostic methods for CRN, systemic therapy at the time of CRN diagnosis, total radiation dose for CRN lesions, CRN lesion volumes at different periods on MRI, radiation approaches, CK parameters, BED of CRN lesions, DBRACRN, DBCRNAB, DBRAB, cycles of IVBEV, interval time of IVBEV, BEV‐related AE, and changes in symptoms and corticosteroid use during IVBEV treatment.
In this study, we ascertained the presence of CRN based on the following clinical evaluation and radiological characteristics. First, the patient must have a history of radiotherapy in the lesion area. Furthermore, CRN generally occurs 3–12 months or even several years after radiation therapy. 25 Third, the radiographic characteristics of the lesion should demonstrate consistency with the presence of CRN, as opposed to a recurrent tumor. 26 This is based on traditional anatomical MRI imaging features, such as a ring‐enhancing lesion accompanied by perilesional edema, 26 and functional MRI imaging features, including an elevated lipid‐lactate peak on magnetic resonance spectroscopy 27 , 28 , 29 and a decreased relative cerebral blood volume on perfusion‐weighted imaging (PWI). 30 , 31 There is a low uptake of fluorodeoxyglucose (FDG) and amino acid on PET/CT involving the intravenous administration of either the conventional nonspecific tracer (FDG) or more specific amino acid tracers for CRN such as carbon‐11 methionine (MET), fluoro‐l‐thymidine (FLT), and fluoroethyltyrosine (FET). 32 , 33 , 34 , 35 The final imaging diagnosis was determined by three independent investigators based on the analysis of imaging findings. The lesion can be surgically excised and histologically confirmed if the patient presents with severe symptoms and meets the criteria for surgery. 27 Based on the BEV dose, patients were divided into a high‐dose group (≥5 mg/kg) and a low‐dose group (<5 mg/kg) using a cutoff of 5 mg/kg.
2.3. Bevacizumab treatment
Overall, bevacizumab treatment for CRN in our center primarily focused on antitumor dosages (≥5 mg/kg) prior to 2018. However, since 2018, there has been a shift toward utilizing low dosages (<5 mg/kg) of bevacizumab for CRN treatment due to emerging evidence from retrospective studies and clinical trials.
2.4. Radiological and clinical measurements
The baseline (follow‐up 0, F0) was defined as within 1 week before the administration of IVBEV. The first follow‐up (follow‐up 1, F1) was scheduled for 3 months (±30 days) after IVBEV administration, while the second follow‐up (follow‐up 2, F2) was scheduled for 6 months (±30 days) after administration.
2.5. MRI scan
MRI images of 64 patients at F0, F1, and F2 were collected from the Department of Radiology at our hospital. The contour of the lesion is meticulously delineated layer by layer by experienced neuroradiologists, and the corresponding volume is automatically calculated by Carestream's Picture Archiving and Communication System (PACS) v11.0 software (Carestream Health, Rochester, NY, USA). The specific method involved calculating the CRN‐enhanced volume (Figure S1A,B) by subtracting the inner diameter volume from the outer diameter volume of the CRN‐enhanced area (volume B minus volume A). The CRN edema volume was measured on T2FLAIR images (Figure S1C). Signal changes in the CRN‐enhanced region were measured on T1CE images, and three regions of interest (ROIs) with a diameter of 1 mm were randomly selected on the CRN‐enhanced area to measure the signal values and calculate the average values (Figure S1D). The signal values were compared with the ipsilateral white matter mean signal value of the same MRI (Figure S1E) to eliminate the influence of different degrees of enhancement. The ratio was used to measure the signal changes in the CRN‐enhanced area before and after treatment. For patients with follow‐up radiological images in other hospitals, DICOM format files of follow‐up MRI were imported into MIM Maestro software (7.0.5 US), and the same delineating principles and methods were used to obtain imaging data by manual and semi‐automatic methods. The radiological response rate of IVBEV was defined as the percentage decrease in CRN edema volume on T2FLAIR, CRN‐enhanced volume on T1CE, and signal ratio reduction on T1CE at F1 or F2, compared with that of images at F0. The effective response was defined as a reduction in CRN volume and signal ratio of ≥25% at F1 and F2 compared with F0.
We defined CRN PD as either: (1) >10% increase in the CRN edema volume on T2FLAIR, (2) new CRN lesion/site findings on MRI, or (3) clinically observable deterioration. The duration between the initiation of IVBEV therapy and the onset of CRN progression was defined as PFS. In calculating OS, the period was measured from the beginning of IVBEV treatment to the time of death, with patients who were still alive at the last follow‐up date being censored.
2.6. Improvement of clinical symptoms
Defining the clinical response directly proved to be a challenging task due to the intricate clinical conditions of the patients. Therefore, the patients were approximately categorized based on the National Cancer Institute's Common Terminology Criteria (version 5.0; NCI‐CTCAE 5.0) for CRN into the following severity levels: 0 (no symptoms), 1 (mild symptoms), 2 (moderate symptoms), 3 (severe symptoms), 4 (life‐threatening symptoms), and 5 (fatal symptoms). We considered symptom improvement as the reduction or disappearance of the severity of CRN‐related clinical symptoms, symptom stability as the unchanged severity of CRN‐related clinical symptoms, and symptom progression as the increase in the severity of CRN‐related clinical symptoms.
2.7. Increase or decrease of corticosteroid use
The CRN patients were classified into four distinct groups based on their glucocorticoid use, which included a non‐using group, a low‐dose group (prednisolone: <7.5 mg/day), a moderate‐dose group (prednisolone: 7.5–30 mg/day), and a high‐dose group (prednisolone: 30–100 mg/day). The criteria used to define glucocorticoid improvement was based on a reduction in the severity of steroid dosage. To facilitate the analysis, an equivalent dose conversion scale was used, which established: 0.75 mg dexamethasone = 5 mg prednisone = 4 mg methylprednisolone = 20 mg hydrocortisone.
2.8. Safety assessment
As part of the safety evaluation, AEs were assessed based on their frequency and severity, in compliance with the NCI‐CTCAE 5.0 guidelines.
2.9. Statistical analysis
The categorical variables were summarized using proportions, while the continuous variables were presented as medians with IQRs. For normally distributed variables, between‐group differences were assessed using Student's t‐test, while the Mann–Whitney U‐test was used for non‐parametric variables. Categorical variables were evaluated using either the chi‐squared or Fisher's exact test. A competing risk analysis (Fine and Gray method) by R software (version 4.1.1) was used to model the cumulative incidence function of CRN progression, taking into account the competing risk of death caused by non‐CRN progression.
Overall survival was compared using Kaplan–Meier survival curves and log‐rank tests. Statistical analysis was carried out using IBM SPSS 24.0 software, while the survival curve was plotted using GraphPad Prism 9.0. A significance level of p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Demographic and clinical characteristics
In Figure S2, a patient flowchart is presented. The two groups demonstrated no significant differences in demographics and clinical characteristics, with the exception of gender (Table 1). Out of 75 CRN patients treated with IVBEV, 33 (44%) were identified as male and 42 (56%) as female. Notably, parameters such as age, KPS, primary tumor type, underlying disease, DBRACRN, DBCRNAB, DBRAB, prior BEV history, steroid therapy history for CRN, history of targeted therapy, and WBRT history demonstrated no statistically significant difference between the two groups (p > 0.05). Moreover, Table 1 presents the pathological types of non–small‐cell lung cancer, the clinical symptoms related to CRN before BEV treatment, and the types of targeted drugs observed between the two groups.
TABLE 1.
Baseline characteristics of patient in each group.
| Items | Total (n = 75) | Low‐dose group (n = 34) | High‐dose group (n = 41) | p‐value |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age, years | 58 (41–64) | 59 (51.2–63.8) | 57 (50–64) | 0.301 |
| Sex | 0.019 | |||
| Male | 33 (44.0) | 20 (58.8) | 13 (31.7) | |
| Female | 42 (56.0) | 14 (41.2) | 28 (68.3) | |
| KPS | 0.944 | |||
| ≥80 | 46 (61.3) | 21 (61.8) | 25 (61.0) | |
| <80 | 29 (38.7) | 13 (38.2) | 16 (39.0) | |
| Primary tumor | 0.909 | |||
| NSCLC | 57 (76.0) | 27 (79.5) | 30 (73.2) | |
| Adenocarcinoma | 47 (62.7) | 23 (67.6) | 24 (58.4) | |
| Squamous cell carcinoma | 4 (5.3) | 1 (2.9) | 3 (7.4) | |
| Others | 6 (8.0) | 3 (8.8) | 3 (7.4) | |
| SCLC | 6 (8.0) | 3 (8.8) | 3 (7.4) | |
| Breast cancer | 5 (6.3) | 2 (5.9) | 3 (7.4) | |
| Thymus cancer | 2 (2.9) | 1 (2.9) | 1 (2.4) | |
| Stomach cancer | 2 (2.9) | 1 (2.9) | 1 (2.4) | |
| Endometrial cancer | 1 (1.3) | 0 (0.0) | 1 (2.4) | |
| Ovarian cancer | 1 (1.3) | 0 (0.0) | 1 (2.4) | |
| Rectal cancer | 1 (1.3) | 0 (0.0) | 1 (2.4) | |
| Underlying disease | 0.773 | |||
| Yes | 23 (30.7) | 11 (32.3) | 12 (29.3) | |
| Hypertension | 18 (24.0) | 8 (23.5) | 10 (24.4) | |
| Diabetes | 5 (6.7) | 3 (8.8) | 2 (4.9) | |
| DBRACRN, months | 9.8 (5.3–17.9) | 10.8 (5.7–19.3) | 9.5 (4.5–15.0) | 0.223 |
| DBRAB, months | 10.5 (5.6–18.9) | 11.3 (6.4–20.5) | 10.1 (4.7–16.4) | 0.203 |
| DBCRNAB, days | 8 (4–16) | 8.5 (3–16.5) | 8 (4–15) | 0.974 |
| CRN‐associated symptoms | 0.781 | |||
| None | 12 (16.0) | 5 (14.7) | 7 (17.1) | |
| Yes | 63 (84.0) | 29 (85.3) | 34 (82.9) | |
| Headache | 28 (37.3) | 13 (38.2) | 15 (36.6) | |
| Dizziness | 23 (30.7) | 7 (20.6) | 16 (39.0) | |
| Nausea and vomiting | 3 (4.0) | 1 (2.9) | 2 (4.9) | |
| Reduced muscle strength | 14 (18.7) | 7 (20.6) | 7 (17.1) | |
| Ataxia | 6 (8.0) | 4 (11.8) | 2 (4.9) | |
| Epilepsy | 8 (10.7) | 4 (11.8) | 4 (9.8) | |
| Memory loss | 4 (5.3) | 2 (5.9) | 2 (4.9) | |
| Movement disorder | 10 (13.3) | 4 (11.8) | 6 (14.6) | |
| Dysarthria | 8 (10.7) | 3 (8.8) | 5 (12.2) | |
| Visual field defect | 1 (1.3) | 0 (0.0) | 1 (2.4) | |
| Auditory or visual hallucinations | 1 (1.3) | 1 (2.9) | 0 (0.0) | |
| Prior BEV history | 10 (13.3) | 5 (14.7) | 5 (12.2) | 0.750 |
| Steroid therapy history for CRN | 35 (46.7) | 16 (47.1) | 19 (46.3) | 0.951 |
| History of targeted therapy | 0.460 | |||
| No | 41 (54.7) | 17 (50.0) | 24 (58.5) | |
| Yes | 34 (45.3) | 17 (50.0) | 17 (41.5) | |
| EGFR‐targeted drugs | 24 (32.0) | 14 (41.2) | 10 (24.4) | |
| ALK‐targeted drugs | 2 (2.7) | 1 (2.9) | 1 (2.4) | |
| Other targeted drugs | 8 (10.7) | 2 (5.9) | 6 (14.6) | |
| WBRT history | ||||
| Yes | 34 (45.3) | 16 (47.1) | 18 (43.9) | 0.785 |
Note: Data are shown as numbers (%) or medians (IQRs). No difference was found between the low‐dose group and high‐dose group regarding either the clinical characteristics or the follow‐up data (p = 0.087–1.000), except sex (p = 0.019).
Abbreviations: ALK, anaplastic lymphoma kinase; CRN, cerebral radiation necrosis; DBCRNAB, duration between CRN diagnosis and BEV treatment; DBRAB, duration between radiotherapy and BEV treatment; DBRACRN, duration between radiotherapy and CRN diagnosis; EGFR, epidermal growth factor receptor; KPS, Karnofsky Performance Status scores; NSCLC, non–small‐cell lung cancer; SCLC, small‐cell lung cancer; WBRT, whole brain radiotherapy.
Table S1 reports that the cycles of IVBEV and the interval time for both groups of patients did not demonstrate any statistically significant differences (p > 0.05). Additionally, the study found that, out of the 75 CRN patients, the median dose of IVBEV was 5 mg/kg (1–10 mg/kg), the median cycle was 3 (1–16), and the median interval time was 4 weeks (2–4 weeks). In the low‐dose group, the median dose was 3 mg/kg (1–4.4 mg/kg) and the median cycle was 3 (1–7). In the high‐dose group, the median dose was 5 mg/kg (5–10 mg/kg) and the median cycle was 3 (1–16).
3.2. Baseline characteristics of CRN lesions
There was no statistical difference between the two groups of CRN lesions in terms of location distribution, volume of CRN on T1CE and T2FLAIR, signal ratio of CRN‐enhanced area, cyst, diagnostic method, modality of treatment, and BED (Table S2). The median CRN edema volumes on T2FLAIR were 50.6 cm3 (IQR 23.1–92.4 cm3) and 70.1 cm3 (IQR 25.6–140.3 cm3) (p = 0.303). Similarly, the median CRN‐enhanced volumes on T1CE were 3.3 cm3 (IQR1.9–6.2 cm3) and 3.6 cm3 (IQR 2.1–10.3 cm3) (p = 0.218). Additionally, the median signal ratios of CRN‐enhanced areas on T1CE were 2.1 (IQR 1.9–2.4) and 2.2 (IQR 1.9–2.4) (p = 0.782), respectively.
In the study, in total, 74 CK treatments were administered to 70 patients out of the 75 participants. The dose and fractionation schedule of the treatments were determined based on the location and volume of the BMs (Table S3). The median dose delivered was 26 Gy (12–37 Gy). The volumes of the BMs varied from 0.24 to 32.23 mL. Comprehensive details of the CK‐related radiosurgery treatment parameters used in the study can be found in Tables S3 and S4.
3.3. MRI evaluation of CRN lesions
Table 2 reports the CRN edema volume on T2FLAIR, CRN‐enhanced volume on T1CE, and the signal ratio of CRN‐enhanced area on T1CE for both groups at the F1 and F2. Comparisons with baseline and reduction rates of both groups are also presented. The statistical analysis revealed that there were significant differences in the three imaging features between the two groups at 3 and 6 months of follow‐up (both p < 0.05) compared with the baseline. However, no significant differences between the comparisons of the two groups were found in the edema volume on T2FLAIR, edema volume reduction rate compared with baseline, CRN‐enhanced volume on T1CE, enhanced volume reduction rate compared with baseline, the signal ratio of CRN‐enhanced area, and the signal ratio reduction rate at 3‐month and 6‐month follow‐up compared with baseline (all p > 0.05). To provide a more intuitive comparison of the changes in these three imaging characteristics between the two groups, a waterfall chart was used to visualize the data, as shown in Figure 1.
TABLE 2.
Changes of CRN lesions' MRI features at the different study time points.
| Therapeutic effect | Low‐dose group (35) | High‐dose group (43) | ||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | 3 months | 6 months | P1 | P2 | |
| CRN edema volume on T2FLAIR, ml | 23.4 (6.4–30.4) | 22.6 (7.0–36.2) | 20.1 (4.8–49.1) | 26.5 (5.1–68.2) | 0.655 | 0.404 |
| p for edema volume compared with baseline | <0.001 | <0.001 | <0.001 | <0.001 | — | — |
| % reduction in edema volume compared with baseline | 65.8 (48.5–79.2) | 59.5 (44.9–77.4) | 64.8 (42.5–85.2) | 62.0 (6.7–82.3) | 0.860 | 0.757 |
| CRN‐enhanced volume on T1CE, ml | 2.0 (0.9–3.5) | 2.5 (1.1–4.1) | 2.0 (0.7–5.1) | 2.5 (1.2–6.6) | 0.517 | 0.419 |
| p for enhanced volume compared with baseline | <0.001 | 0.004 | <0.001 | <0.001 | — | — |
| % reduction in enhanced volume compared with baseline | 41.2 (26.3–61.9) | 39.1 (−2.4,54.3) | 51.9 (27.3–66.7) | 31.3 (1.6–60.0) | 0.396 | 0.851 |
| Signal ratio of CRN‐enhanced area on T1CE | 1.3 (1.1–1.4) | 1.3 (1.2–1.6) | 1.3 (1.2–1.6) | 1.5 (1.2–11.9) | 0.154 | 0.091 |
| p for signal ratio compared with baseline | <0.001 | <0.001 | <0.001 | <0.001 | — | — |
| % reduction in signal ratio compared with baseline | 37.4 (31.0–46.5) | 35.4 (22.5–47.4) | 35.1 (25.6–46.5) | 28.2 (8.3–43.9) | 0.271 | 0.083 |
Note: Data are shown as numbers (%) or medians (IQRs). P1, low‐dose group versus high‐dose group at 3 months; P2, low‐dose group versus high‐dose group at 6 months.
Abbreviation: CRN, cerebral radiation necrosis; T2FLAIR, T2‐weighted fluid‐attenuated inversion recovery; T1CE, T1‐weighted gadolinium contrast‐enhanced image.
FIGURE 1.
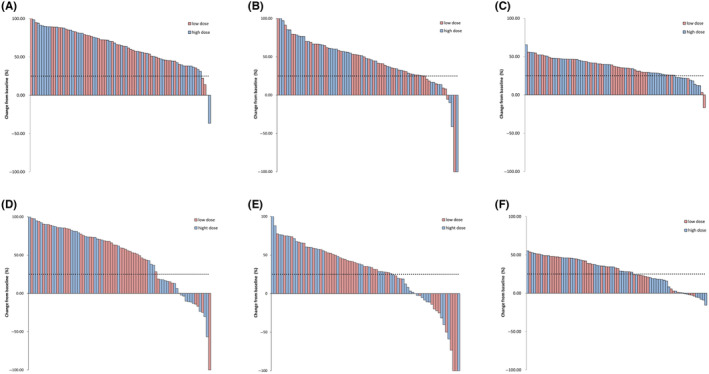
Waterfall plot of changes in MRI characteristics of 78 CRN lesions compared with baseline at different follow‐up time points. (A) CRN edema volume reduction rate on T2FLAIR in 78 evaluable CRN lesions at 3‐month follow‐up. (B) CRN‐enhanced volume reduction rate on T1CE in 78 evaluable CRN lesions at 3‐month follow‐up. (C) Signal ratio reduction rate of CRN‐enhanced area on T1CE in 78 evaluable CRN lesions at 6‐month follow‐up. (D) CRN edema volume reduction rate on T2FLAIR in 78 evaluable CRN lesions at 6‐month follow‐up. (E) T1CE‐enhanced volume reduction rate in 78 evaluable CRN lesions at 6‐month follow‐up. (F) Signal ratio reduction rate of CRN‐enhanced area on T1CE in 78 evaluable CRN lesions at 6‐month follow‐up.
3.4. Clinical efficacy evaluation in CRN patients
Table 3 presents the improvements observed in both patient groups at different time points based on CRN volume, the signal ratio of CRN‐enhanced area, clinical symptoms, and glucocorticoid use. At F1, the effective rate of IVBEV on T2FLAIR was reported to be 94.7% in 75 patients, and radiographic improvement was observed in 32 and 39 patients in the low‐dose and high‐dose groups, respectively (94.1% vs. 95.1%, P1 = 1.000). The treatment response rates of both groups improved based on CRN‐enhanced volume on T1CE (82.4% vs. 82.9%, P1 = 0.948), and the effective rates of both groups improved based on the signal ratio of CRN‐enhanced area on T1CE (91.2% vs. 75.6%, P1 = 0.067). At F2, the response rate of the three imaging features showed no statistical difference between the two groups (P2 = 0.130 for edema volume on T2FLAIR, P2 = 0.549 for enhanced volume on T1CE, and P2 = 0.333 for signal ratio of CRN‐enhanced area on T1CE, all p < 0.05). It was also observed that there was no statistically significant difference in the improvement rate of clinical symptoms and steroid use between the two groups during different follow‐up periods.
TABLE 3.
Efficacy based on MRI, clinical symptoms and steroid usage at the different study time points.
| Therapeutic effect | Lose‐dose group (n = 34) | High‐dose group (n = 41) | ||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | 3 months | 6 months | P1 | P2 | |
| Edema volume effective | 32 (94.1) | 27 (79.4) | 39 (95.1) | 26 (63.4) | 1.000 | 0.130 |
| Enhanced volume effective | 28 (82.4) | 23 (67.6) | 34 (82.9) | 25 (61.0) | 0.948 | 0.549 |
| Signal ratio effective | 31 (91.2) | 22 (64.7) | 31 (75.6) | 22 (53.7) | 0.067 | 0.333 |
| Clinical symptoms' effective | 0.917 | 0.752 | ||||
| Improved | 22 (64.7) | 17 (50) | 27 (65.9) | 19 (46.3) | ||
| Stable | 11 (32.4) | 9 (26.5) | 12 (29.3) | 11 (26.8) | ||
| Progression | 1 (2.9) | 8 (23.5) | 2 (4.9) | 11 (26.8) | ||
| Steroid usage' | Effective | 0.850 | 0.445 | |||
| Improved | 14 (41.2) | 11 (32.4) | 16 (39.0) | 10 (24.4) | ||
| Stable | 20 (58.7) | 18 (52.9) | 23 (56.1) | 22 (53.7) | ||
| Progression | 0 (0.0) | 5 (14.7) | 2 (4.9) | 9 (22.0) | ||
Note: Data are shown as numbers (%). P1, low‐dose group versus high‐dose group at 3 months; P2, low‐dose group versus high‐dose group at 6 months.
3.5. Survival assessment of CRN patients
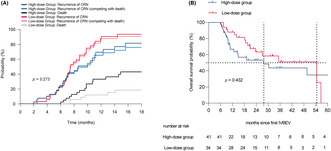
After taking into account the competing risk of death due to non‐CRN progression, the cumulative incidence of CRN progression between the groups was not statistically significant. (p = 0.273; Figure 2A) Regarding the actual 5‐year OS rates, the low‐dose and high‐dose groups had rates of 0% and 7.32%, respectively, but the difference between the groups was not statistically significant (P2 = 0.432; Figure 2B). Furthermore, we collected detailed information regarding the causes of death for two groups of patients with CRN (Table S5). In the high‐dose group, three patients succumbed to intracranial causes: one due to an adverse event related to bevacizumab and two due to untreatable brain edema and cerebral white matter degeneration caused by CRN. Additionally, one patient in the low‐dose group died as a result of recurrent CRN with a significant space‐occupying effect necessitating surgery; however, the family declined surgical intervention, ultimately leading to fatality. The remaining patients succumbed to extracranial progression, fatal traffic accidents, or suicide resulting from depression.
FIGURE 2.
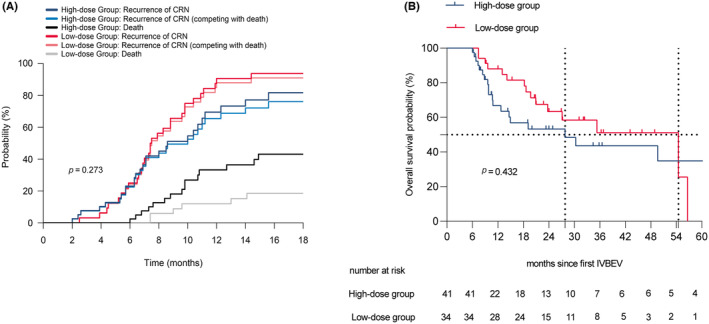
Cumulative incidence of CRN progression (A) and overall survival (B) between the low‐dose group and the high‐dose group.
3.6. Safety assessment
In IVBEV treatment, 34 patients were given low‐dose IVBEV and 41 patients were treated with high‐dose IVBEV, of which 26.5% and 41.5% respectively experienced AEs of any grade. Among the low‐dose IVBEV patients, the most commonly reported AEs were hypertension (6 [17.6%]), diarrhea (1 [2.9%]), hemorrhage (1 [2.9%]), and oral ulcers (1 [2.9%]. No AEs of grade 3 or above were reported in this group. The high‐dose IVBEV patients experienced a higher incidence of AEs, with hypertension (8 [19.5%]), bleeding (3 [7.3%]), diarrhea (2 [4.9%]), fatigue (1 [2.4%]), oral ulcer (1 [2.4%]), lower extremity venous thrombosis (1 [2.4%]), epilepsy (1 [2.4%]) being the most commonly reported. From Table 4, it can be observed that 9.8% of the high‐dose IVBEV patients had grade 3 or above AEs. Of the four patients in this category, one male patient with BMs from small‐cell lung cancer developed life‐threatening hemoptysis after the second IVBEV and had to undergo emergency endovascular embolization to stop the bleeding. Another patient with BMs from rectal cancer developed grand mal seizures with every IVBEV and eventually died of grand mal seizures 5 days after the last IVBEV.
TABLE 4.
Treatment‐related adverse events.
| Adverse event, n (%) | Low‐dose group (n = 34) | Low‐dose group (n = 41) | ||||
|---|---|---|---|---|---|---|
| Grades 1–2 | Grade 3 | Grade 4 | Grades 1–2 | Grade 3 | Grade 4 | |
| Total AEs | 9 (26.4) | 0 | 0 | 13 (31.7) | 2 (4.9) | 2 (4.9) |
| Hypertension | 6 (17.6) | 0 | 0 | 7 (17.1) | 1 (2.4) | 0 |
| Diarrhea | 1 (2.9) | 0 | 0 | 2 (4.9) | 0 | 0 |
| Hemorrhage | 1 (2.9) | 0 | 0 | 2 (4.9) | 0 | 1 (2.4) |
| Epilepsy | 0 | 0 | 0 | 0 | 0 | 1 (2.4) |
| Fatigue | 0 | 0 | 0 | 1 (2.4) | 0 | 0 |
| Oral ulcerations | 1 (2.9) | 0 | 0 | 1 (2.4) | 0 | 0 |
| Venous thrombosis | 0 | 0 | 0 | 0 | 1 (2.4) | 0 |
4. DISCUSSION
We retrospectively analyzed the efficacy based on radiological imaging and changes in clinical neurological symptoms and improvement in corticosteroid use at 3‐month and 6‐month follow‐ups among CRN patients after radiotherapy for BMs who received low‐dose or high‐dose IVBEV. To our knowledge, few studies have evaluated the effect of different doses of IVBEV regimens in CRN patients. Based on the three aspects mentioned above, no additional benefits were observed between high‐dose IVBEV and low‐dose IVBEV in terms of response rates. In fact, the high‐dose IVBEV group was associated with a higher incidence of AEs.
The mechanisms underlying the clinical manifestations of CRN remain elusive. Nonetheless, animal and human models of CRN suggest that blood–brain barrier dysfunction is a key factor leading to elevated levels of VEGF. 36 , 37 Nordal et al. 8 conducted a study that demonstrated that laboratory rats that lacked the gene responsible for encoding VEGF displayed a higher resistance to radiation damage. Early blocking of VEGF has been identified as a possible preventive measure for CRN, given that it reduces vascular permeability. Treatment aimed at reversing pathological mechanisms, improving symptoms, and halting further disease progression is now within reach. The representative drug of anti‐angiogenic therapy, BEV, is effective in treating CRN by virtue of its ability to reduce vascular permeability. Over the past 16 years, the effectiveness of IVBEV in treating CRN has been supported by extensive evidence. The potential benefit of IVBEV treatment for CRN was first reported by Gonzalez et al. 38 After treatment with IVBEV at a dosage of 5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks, the average daily dosage of dexamethasone in CRN patients was reduced by 8.6 mg (±3.6), and the average CRN volume reduction on T1CE was 48%, compared with 60% on T2FLAIR.
In this study, the mean lesion reduction rates on T2FLAIR and T1CE at the 3‐month follow‐up were found to be 54.9% and 47.6%, respectively, while at the 6‐month follow‐up these rates were observed to be 55.6% and 40.7%, respectively. Notably, we included the signal ratio of CRN‐enhanced area on T1CE before and after IVBEV, which has not been extensively investigated by other researchers, to provide a more comprehensive evaluation of the anti‐vascular effects of IVBEV in the two groups. High‐dose regimens have been frequently used in previous studies, and our high‐dose group exhibited radiographical changes on T2FLAIR and T1CE that were consistent with previous findings. 11 , 12 , 14 , 18 , 20 , 38 , 39 , 40 While few studies have reported on the effectiveness of low‐dose IVBEV, a previous case report indicated that doses lower than the initial dose (5 or 7.5 mg/kg) can still be effective when used at a dose of 3.27 mg/kg with an interval of 12–16 weeks between each treatment. In addition, a prospective phase II clinical study conducted by Zhuang et al. 24 administered IVBEV at a dosage of 1 mg/kg with an interval of 3 weeks to treat CRN. In comparison with standard‐dose IVBEV, the use of ultra‐low‐dose IVBEV was found to be associated with radiographic response in 95% of patients, a decrease in the severity of symptoms in 90% of patients, a reduction in the intensity of signals of CRN‐enhanced area on T1CE in 20 patients (95.24%), and no AEs more severe than grade 2. In a recent retrospective study conducted by Weng et al., a regimen of low‐dose BEV (3 mg/kg) administered at 2‐week intervals for two cycles resulted in a 45% reduction in the mean volume of CRN lesions on T1CE and a 74% reduction on T2FLAIR. The symptoms and neurological function, as measured by KPS, improved in all patients, and glucocorticoids were discontinued in all cases. No AEs were observed. 41 These findings are consistent with those of a recent meeting abstract presented by Tijtgat et al. at ESMO2021, in which a “low‐dose regimen” of BEV (400 mg loading dose followed by 100 mg Q4W) was investigated in 10 patients diagnosed with CRN. This approach resulted in marked improvement in clinical symptoms and MRI abnormalities, and no severe AEs related to the BEV treatment were observed at the latest follow‐up. 42 Although we used different evaluation criteria, the overall findings are consistent with our data.
Our findings indicate that high‐dose IVBEV does not confer any advantage over low‐dose IVBEV. This observation may be relevant to the mechanism of action of BEV. In clinical settings, high‐dose IVBEV is frequently utilized in antitumor regimens due to its anti‐angiogenic effects against several malignancies including recurrent or metastatic non‐squamous non–small‐cell lung cancer, metastatic colorectal cancer, metastatic renal cell carcinoma, recurrent glioblastoma, and persistent, recurrent, or metastatic cervical cancer. These observations support the hypothesis that anti‐angiogenesis plays a pivotal role in the pathogenesis of cancer (https://www.nccn.org/). However, all the patients in our study had CRN, and VEGF expression in these patients may be lower than that observed in neoplastic diseases. Our results suggest that BEV targets vascular injury caused by new blood vessels in the treatment of CRN, rather than necrosis. Therefore, ischemia and hypoxia persist as long as the pathological basis of necrosis remains unchanged. Upon discontinuation of BEV treatment, HIF‐1α expression in the peri‐necrotic tissue may increase again, renewing the vicious cycle and eventually leading to the recurrence of cerebral necrosis. Considering the vascular mechanism of CRN and the features of anti‐angiogenic therapy, we suggest that the duration of treatment is more critical than blood concentration. 43 Furthermore, a study published in the Journal of Clinical Oncology has reported that excessive vascular pruning by BEV may lead to vascular insufficiency, exacerbating hypoxia and necrosis and resulting in worsening symptoms and severe AEs. 44 Hence, we postulate that this could explain why low‐dose IVBEV was found to be equally effective as high‐dose IVBEV.
Consistent efficacy in improving clinical symptoms was observed with different doses of IVBEV. In most retrospective studies, clinical symptoms were assessed using the KPS, whereas some prospective studies used the MIDAS, HIT‐6, LENT/SOMA, MoCA scores, and quality of life (QOL) scale as the evaluation criteria. 11 , 23 , 40 In our current study, we used the CTCAE version 5.0 criteria associated with CRN for the quantification of clinical severity, due to the retrospective nature of the study. Our findings showed that both groups demonstrated significant improvements in clinical symptoms, which is in line with most previous reports. 24 , 45 , 46 However, no significant difference was observed between the two groups in this regard. Interestingly, there were also no significant radiological differences between the two groups. The changes observed in radiographic and clinical appearances in this study are likely to be attributed to BEV's anti‐VEGF properties.
The analysis in this paper demonstrated that 94% of the 35 patients had a total mean reduction or stabilization of steroid treatment (high‐dose IVBEV group: 95.1%; low‐dose IVBEV group: 100%), which was consistent with the findings of two meta‐analyses conducted by Delishaj et al. and Khan et al., 46 , 47 as well as previous studies. 24 As for AEs, the high‐dose group was associated with numerous side effects, including hypertension, proteinuria, thromboembolism, bleeding, and gastrointestinal perforation. In contrast, our study revealed that low‐dose IVBEV was better tolerated and had better safety, with a lower frequency of overall AE reporting and a lower incidence of grade 3 or above AEs. The AE profile was consistent with that of previous reports. 24 Our median recurrence‐free survival (scilicet PFS) was highly similar to that reported in the only large clinical trial conducted historically, which bolsters the reliability of our findings. 40
The study has several limitations that should be considered. First, this was a single‐center retrospective study, and therefore, the findings only suggest that the efficacy of the low‐dose group is not inferior to that of the high‐dose group. Further studies are required to define the optimal dosage of BEV. Second, the retrospective nature of this study may have led to attrition bias, as some records were missing and follow‐up was lost. Third, all the included CRN patients were secondary to radiotherapy for BMs, and the current clinical and radiographic methods are insufficient for the accurate diagnosis of pure CRN.
In summary, it was observed that both high‐dose IVBEV and low‐dose IVBEV were equally efficacious in the treatment of BM patients with CRN. However, it was observed that high‐dose IVBEV was associated with a higher incidence of treatment‐related AEs and increased social and economic burden. To explore the optimal dose of IVBEV for the treatment of CRN, a large‐scale, multicenter, and randomized controlled clinical trial involving multiple diseases is necessary.
AUTHOR CONTRIBUTIONS
Miaomiao Gao: Conceptualization; data curation; formal analysis; investigation; visualization; writing – original draft; writing – review and editing. Xin Wang: Data curation; formal analysis; visualization; writing – original draft; writing – review and editing. Xiaofeng Wang: Data curation; formal analysis; investigation; writing – original draft; writing – review and editing. Gengmin Niu: Data curation; supervision; writing – review and editing. Xiaoye Liu: Resources; supervision; writing – review and editing. Shuzhou Zhao: Resources; supervision; writing – review and editing. Yue Wang: Data curation; supervision; writing – review and editing. Huiwen Yu: Data curation; formal analysis; software. Siyuan Huo: Data curation; formal analysis; investigation. Hui Su: Data curation; formal analysis; software. Yongchun Song: Data curation; resources. Xiaoguang Wang: Data curation; resources. Hong‐Qing Zhuang: Conceptualization; resources; writing – original draft; writing – review and editing. Zhi‐Yong Yuan: Conceptualization; funding acquisition; resources; writing – original draft; writing – review and editing.
FUNDING INFORMATION
This study was funded by the National Natural Science Foundation of China (No. 82172674) and the Natural Science Foundation of Tianjin Municipal Science and Technology Bureau (Grant No. 20JCYBJC00090) to Zhi‐Yong Yuan.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: This study was carried out in accordance with the ethical guidelines of the Declaration of Helsinki and the approval of the research protocol by an Institutional Reviewer Board at our hospital (bc2022184).
Informed Consent: All included patients must be able to understand and give written informed consent and report adverse events.
Registry and the Registration No. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Data S1
ACKNOWLEDGMENTS
We would like to express our gratitude to the Department of Radiation Physics at Tianjin Medical University Cancer Institute and Hospital, for their outstanding technical support.
Gao M, Wang X, Wang X, et al. Can low‐dose intravenous bevacizumab be as effective as high‐dose bevacizumab for cerebral radiation necrosis? Cancer Sci. 2024;115:589‐599. doi: 10.1111/cas.16053
Miaomiao Gao, Xin Wang, and Xiaofeng Wang contribute equally as first author.
Contributor Information
Hong‐Qing Zhuang, Email: hongqingzhuang@163.com.
Zhi‐Yong Yuan, Email: zyuan@tmu.edu.cn.
REFERENCES
- 1. Upadhyay UM, Tyler B, Patta Y, et al. Intracranial microcapsule chemotherapy delivery for the localized treatment of rodent metastatic breast adenocarcinoma in the brain. Proc Natl Acad Sci USA. 2014;111:16071‐16076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for brain metastases: ASCO‐SNO‐ASTRO guideline. J Clin Oncol. 2022;40:492‐516. [DOI] [PubMed] [Google Scholar]
- 3. Wang H, Liu X, Jiang X, et al. Cystic brain metastases had slower speed of tumor shrinkage but similar prognosis compared with solid tumors that underwent radiosurgery treatment. Cancer Manag Res. 2019;11:1753‐1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiang X, Wang H, Song Y, et al. A second course of stereotactic image‐guided robotic radiosurgery for patients with cerebral metastasis. World Neurosurg. 2019;123:e621‐e628. [DOI] [PubMed] [Google Scholar]
- 5. Kerschbaumer J, Demetz M, Krigers A, Nevinny‐Stickel M, Thomé C, Freyschlag CF. Risk factors for radiation necrosis in patients undergoing cranial stereotactic radiosurgery. Cancers. 2021;13:4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sneed PK, Mendez J, Vemer‐van den Hoek JG, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123:373‐386. [DOI] [PubMed] [Google Scholar]
- 7. Nonoguchi N, Miyatake S, Fukumoto M, et al. The distribution of vascular endothelial growth factor‐producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol. 2011;105:423‐431. [DOI] [PubMed] [Google Scholar]
- 8. Nordal RA, Nagy A, Pintilie M, Wong CS. Hypoxia and hypoxia‐inducible factor‐1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10:3342‐3353. [DOI] [PubMed] [Google Scholar]
- 9. Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS. Hypoxia in radiation‐induced blood‐spinal cord barrier breakdown. Cancer Res. 2001;61:3348‐3354. [PubMed] [Google Scholar]
- 10. Yang R, Duan C, Yuan L, et al. Inhibitors of HIF‐1α and CXCR4 mitigate the development of radiation necrosis in mouse brain. Int J Radiat Oncol Biol Phys. 2018;100:1016‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levin VA, Bidaut L, Hou P, et al. Randomized double‐blind placebo‐controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Pan L, Sheng X, et al. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res. 2012;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duan C, Perez‐Torres CJ, Yuan L, et al. Can anti‐vascular endothelial growth factor antibody reverse radiation necrosis? A preclinical investigation. J Neurooncol. 2017;133:9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15:1257‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alessandretti M, Buzaid AC, Brandão R, Brandão EP. Low‐dose bevacizumab is effective in radiation‐induced necrosis. Case Rep Oncol. 2013;6:598‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deibert CP, Ahluwalia MS, Sheehan JP, et al. Bevacizumab for refractory adverse radiation effects after stereotactic radiosurgery. J Neurooncol. 2013;115:217‐223. [DOI] [PubMed] [Google Scholar]
- 17. Preuss M, Hirsch W, Hoffmann KT, et al. Effectiveness of bevacizumab for radiation‐induced cerebral necrosis in children. Pediatr Neurosurg. 2013;49:81‐85. [DOI] [PubMed] [Google Scholar]
- 18. Yonezawa S, Miwa K, Shinoda J, et al. Bevacizumab treatment leads to observable morphological and metabolic changes in brain radiation necrosis. J Neurooncol. 2014;119:101‐109. [DOI] [PubMed] [Google Scholar]
- 19. Dashti SR, Spalding A, Kadner RJ, et al. Targeted intraarterial anti‐VEGF therapy for medically refractory radiation necrosis in the brain. J Neurosurg Pediatr. 2015;15:20‐25. [DOI] [PubMed] [Google Scholar]
- 20. Sadraei NH, Dahiya S, Chao ST, et al. Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland clinic experience. Am J Clin Oncol. 2015;38:304‐310. [DOI] [PubMed] [Google Scholar]
- 21. Matuschek C, Bolke E, Nawatny J, et al. Bevacizumab as a treatment option for radiation‐induced cerebral necrosis. Strahlenther Onkol. 2011;187:135‐139. [DOI] [PubMed] [Google Scholar]
- 22. Carl CO, Henze M. Reduced radiation‐induced brain necrosis in nasopharyngeal cancer patients with bevacizumab monotherapy. Strahlenther Onkol. 2019;195:277‐280. [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, Rong X, Hu W, et al. Bevacizumab monotherapy reduces radiation‐induced brain necrosis in nasopharyngeal carcinoma patients: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2018;101:1087‐1095. [DOI] [PubMed] [Google Scholar]
- 24. Zhuang H, Zhuang H, Shi S, Wang Y. Ultra‐low‐dose bevacizumab for cerebral radiation necrosis: a prospective phase II clinical study. Onco Targets Ther. 2019;12:8447‐8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vellayappan B, Tan CL, Yong C, et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol. 2018;8:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87:449‐457. [DOI] [PubMed] [Google Scholar]
- 27. Zhuang H, Yuan X, Zheng Y, et al. A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep. 2016;6:24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chan YL, Yeung DK, Leung SF, Cao G. Proton magnetic resonance spectroscopy of late delayed radiation‐induced injury of the brain. J Magn Reson Imaging. 1999;10:130‐137. [DOI] [PubMed] [Google Scholar]
- 29. Shah R, Vattoth S, Jacob R, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32:1343‐1359. [DOI] [PubMed] [Google Scholar]
- 30. Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20:485‐502. [DOI] [PubMed] [Google Scholar]
- 31. Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high‐grade glioma recurrence from posttreatment radiation effect: direct correlation between image‐guided tissue histopathology and localized dynamic susceptibility‐weighted contrast‐enhanced perfusion MR imaging measurements. Am J Neuroradiol. 2009;30:552‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C‐methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49:694‐699. [DOI] [PubMed] [Google Scholar]
- 33. Isselbacher KJ. Sugar and amino acid transport by cells in culture—differences between normal and malignant cells. N Engl J Med. 1972;286:929‐933. [DOI] [PubMed] [Google Scholar]
- 34. Galldiks N, Stoffels G, Filss CP, et al. Role of O‐(2‐(18)F‐fluoroethyl)‐L‐tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med. 2012;53:1367‐1374. [DOI] [PubMed] [Google Scholar]
- 35. Rachinger W, Goetz C, Pöpperl G, et al. Positron emission tomography with O‐(2‐[18F]fluoroethyl)‐l‐tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57:505‐511; discussion 505–511, 511. [DOI] [PubMed] [Google Scholar]
- 36. Kim JH, Chung YG, Kim CY, Kim HK, Lee HK. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci. 2004;19:879‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gerstner ER, Duda DG, di Tomaso E, et al. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat Rev Clin Oncol. 2009;6:229‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323‐326. [DOI] [PubMed] [Google Scholar]
- 39. Torcuator R, Zuniga R, Mohan YS, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94:63‐68. [DOI] [PubMed] [Google Scholar]
- 40. Li H, Rong X, Hu W, et al. Bevacizumab combined with corticosteroids does not improve the clinical outcome of nasopharyngeal carcinoma patients with radiation‐induced brain necrosis. Front Oncol. 2021;11:746941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weng Y, Shen J, Zhang L, et al. Low‐dosage bevacizumab treatment: effect on radiation necrosis after gamma knife radiosurgery for brain metastases. Front Surg. 2021;8:720506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tijtgat J, Schwarze JK, Awada G, Neyns B. 371P low‐dose bevacizumab for the treatment of focal post‐radiation necrosis of the brain. Ann Oncol. 2021;32(S5):S526. [Google Scholar]
- 43. Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 2019;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jeyaretna DS, Curry WT Jr, Batchelor TT, Stemmer‐Rachamimov A, Plotkin SR. Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol. 2011;29:e159‐e162. [DOI] [PubMed] [Google Scholar]
- 45. Furuuchi K, Nishiyama A, Yoshioka H, Yokoyama T, Ishida T. Reenlargement of radiation necrosis after stereotactic radiotherapy for brain metastasis from lung cancer during bevacizumab treatment. Respir Investig. 2017;55:184‐187. [DOI] [PubMed] [Google Scholar]
- 46. Delishaj D, Ursino S, Pasqualetti F, et al. Bevacizumab for the treatment of radiation‐induced cerebral necrosis: a systematic review of the literature. J Clin Med Res. 2017;9:273‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khan M, Zhao Z, Arooj S, Liao G. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta‐analysis. BMC Cancer. 2021;21:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


