Abstract
Helicobacter pylori exhibits chemotactic responses to urea, flurofamide, acetohydroxamic acid, and sodium bicarbonate. In buffer, the chemotactic activities of a urease-positive strain were higher than those of the isogenic urease-negative strain. Moreover, the chemotactic activities of the urease-positive strain were increased in a viscous solution containing 3% polyvinylpyrrolidone, whereas those of the urease-negative mutant were not. These results are in accordance with the fact that the mutant strain did not show swarming in motility agar regardless of having flagella. Incubation of the wild-type strain with flurofamide resulted in partial inhibition of the chemotactic activities in the viscous solution. In addition, incubation with acetohydroxamic acid, a low-molecular-weight, diffusible urease inhibitor, resulted in complete loss of chemotactic activity in the viscous solution. The inhibition of the chemotactic activity by urease inhibitors paralleled the inhibition of urease. The chemotactic activity of H. pylori was also inhibited by the proton carrier carbonyl cyanide m-chlorophenylhydrazone, showing that H. pylori utilizes proton motive force for motility. These results indicate that cytoplasmic urease plays an important role in the chemotactic motility of H. pylori under a condition that mimics the ecological niche of the bacterium, the gastric mucous layer.
Helicobacter pylori is a gram-negative microaerophilic bacterium which was first isolated from a human gastric biopsy specimen in 1983 (10, 29). The spiral body of this microorganism, with its unipolar flagella, is well adapted for motility in a viscous environment (11). H. pylori colonizes the mucous layer of the gastric epithelium and is thought to cause gastritis, peptic ulcer disease, gastric adenocarcinoma (4, 9), and gastric lymphoma (22, 30). Therefore, it is important to understand the mechanism by which H. pylori colonizes and persists in the mucous layer of the human stomach.
Urease and motility by flagella are essential factors for H. pylori colonization of the stomach. The urease of H. pylori is located within the cytoplasm in freshly cultured bacteria and then appears on the outer membrane in older preparations (12, 23). It has been demonstrated that a mutant with a ureB disruption does not cause gastritis in nude mice due to difficulty in colonization (28). In addition, a mutant that has a disruption of ureG does not colonize the stomach in normochlorhydric or achlorhydric piglets or in normochlorhydric piglets coinoculated with urease-positive bacteria (8). These results suggest that the role of urease in bacterial colonization is not limited to the neutralization of gastric acid. It is unlikely that urease plays a direct role in adhesion, since the adherence of the bacteria to gastric cells is not affected by ureB disruption (5).
Concerning bacterial motility, a flagellated strain has been shown to colonize the stomach in gnotobiotic piglets, whereas an aflagellated strain colonizes the stomach less frequently (7). In the stomachs of infected patients, the bacteria reside mainly in the surface mucous gel layer (15, 24). Because the gel layer has a rapid turnover (19), bacteria proliferating in the mucous layer should have the ability to move toward the epithelial cell surface, against the mucous flow toward the duodenum. Some of us hypothesized that the chemotaxis of H. pylori by flagellar locomotion must be crucial for bacterial colonization and have found that H. pylori is attracted to urea, flurofamide (a urease inhibitor), sodium bicarbonate, and sodium chloride when each is in a fluid solution (20). Interestingly, a ureB disruption mutant also showed a chemotactic response to these substances. In a preliminary study, however, we noted that a urease-negative strain did not exhibit swarming on motility agar and was less motile than the wild-type strain in a viscous environment.
In this study, we investigated the chemotactic activity of H. pylori in the absence or presence of 3% polyvinylpyrrolidone (PVP) and found that the chemotactic motility of urease-positive bacteria, but not that of urease-negative bacteria, was increased markedly in the viscous environment. Furthermore, we observed a prominent decrease in chemotactic responses produced by treatment with urease inhibitors, with a concomitant decrease in urease activity. This is the first report to show that urease is essential for the motility of H. pylori colonizing the gastric mucous layer.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. pylori CPY3401 and its ureB disruption mutant HPT73 (20, 28) were used in this study. Bacteria were grown on brucella broth supplemented with 5% horse serum (brucella-serum broth) or on agar plates with brucella-serum broth solidified with 1.5% agar (brucella-serum agar) and incubated under microaerobic conditions (N2, 85%; O2, 5%; and CO2, 10%) at 37°C (20). The number of CFU was determined by duplicate plating on brucella-serum agar after appropriate dilution of cultures with saline.
Motility assay.
Bacterial cells grown microaerobically at 37°C for 5 days on brucella-serum agar were stabbed with toothpicks into a motility agar containing 0.35% refined agar (Kyokuto, Tokyo, Japan) in brucella-serum broth.
Electron microscopy.
Cells grown in brucella-serum broth (A560 of 1.0) were harvested, washed once with 5 volumes of 10% glycerol, and suspended in 5 volumes of saline. The samples were dried onto a collodion-carbon-coated grid, negatively stained with 2% uranyl acetate, and observed with a JEM-200CX (JEOL) transmission electron microscope operating at 80 kV.
Chemotaxis assay.
Cells grown in brucella-serum agar were suspended in 10 mM potassium phosphate buffer, pH 7.0 (chemotaxis buffer), or in chemotaxis buffer supplemented with 3% PVP (PVP-chemotaxis buffer). PVP-360 (K value [intrinsic viscosity], 90; Sigma, St. Louis, Mo.) was dissolved in distilled water to make a 10% (wt/vol) solution, dialyzed against distilled water at 4°C overnight, autoclaved, and diluted as appropriate in 10 mM potassium phosphate buffer, pH 7.0. Bacterial concentrations were adjusted to 3 × 108 cells per ml (A560 of 0.4) as described previously (20). Chemotaxis was assayed by a modification of Adler’s procedure (1, 20). In brief, a small chamber made from a V-shaped sealed micropipette on a slide glass covered with a cover glass was filled with 200 μl of bacterial suspension in chemotaxis buffer or PVP-chemotaxis buffer. The viscosity of the PVP-chemotaxis buffer was determined at 28°C by using an Ostwald viscometer (Sibata, Tokyo, Japan) according to the manufacturer’s instructions. Three 1-μl pipettes were filled with attractants that had been dissolved in chemotaxis buffer or PVP-chemotaxis buffer and were inserted into the chamber filled with the bacterial suspension. N-(Diaminophosphinyl)-4-fluorobenzamide (fluorofamide) (Tocris Cookson Ltd., Bristol, United Kingdom) and acetohydroxamic acid (Nacalai Tesque Inc., Kyoto, Japan) were used as urease inhibitors. After incubation at 28°C for 10, 30, and 60 min, bacteria incorporated into the tube were spread over a known area (0.125 cm2) on a slide glass, Gram stained, and counted. Data are expressed as the means and standard errors for three determinations. In some experiments, bacterial suspensions mixed with a solution of flurofamide, acetohydroxamic acid, potassium bicarbonate, or carbonyl cyanide m-chlorophenylhydrazone (CCCP; Sigma) were placed in the chemotaxis chamber and incubated at 28°C for 10 min prior to carrying out the chemotaxis assay.
Urease assay.
Urease activity in H. pylori was determined by measuring the release of ammonia by a modification of the Berthelot reaction (6). Cells grown in brucella-serum broth (A560 of 1.0) were incubated without or with flurofamide or acetohydroxamic acid for 10 min at 28°C, centrifuged at 4°C (4,000 × g, 5 min), and resuspended in 0.5 volume of ice-cold 0.1 M sodium phosphate buffer (pH 7.3) containing 10 mM EDTA. Cells were disrupted by sonication, and the supernatant obtained after centrifugation at 4°C (12,000 × g, 5 min) was used for the urease assay as well as for the determination of protein amount with a protein assay and standard kit (Bio-Rad, Hercules, Calif.). The reaction mixture contained 50 mM urea, 100 mM sodium phosphate buffer (pH 7.3), and an aliquot of the crude extract in a total volume of 1.0 ml. After incubation for 10 min at 37°C, the reaction was terminated by addition of 2 ml of 0.5% phenol–0.0025% sodium nitroprusside solution. After 2 ml of 0.25% sodium hydroxide–0.21% sodium hypochlorite solution was added, the mixture was incubated for 6 min at 56°C for color development, and the absorbance at 625 nm was determined. Blanks without crude extract or urea were treated similarly. The amount of ammonia produced was calculated by referring to a standard curve made for known concentrations of ammonium chloride. The production of 2 mol of ammonia is equivalent to the hydrolysis of 1 mol of urea. Urease activity was expressed in micromoles of urea hydrolyzed per minute per milligram of protein in the crude extract.
RESULTS
Motility and flagella of urease-positive and urease-negative strains of H. pylori.
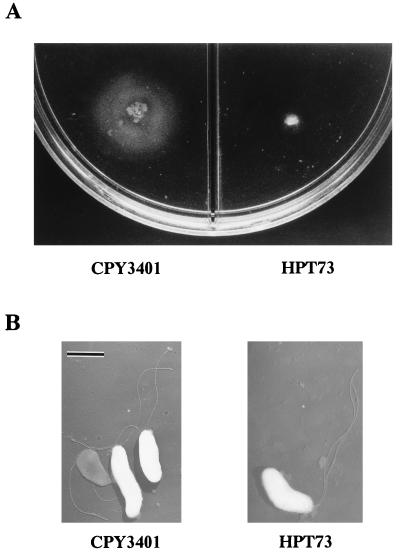
In a previous study some of us observed that not only the wild-type H. pylori CPY3401 but also its isogenic mutant HPT73, which has a disruption of ureB, is attracted to urea, the urease inhibitor flurofamide, sodium bicarbonate, and sodium chloride in the chemotaxis buffer consisting of 10 mM potassium phosphate, pH 7.0 (20). Intriguingly, however, the mutant HPT73 did not show any swarming on brucella-serum motility agar, which was in contrast to the good swarming shown by the wild-type, CPY3401 (Fig. 1A). Therefore, we examined whether the urease-negative mutant has flagella and the ability to swim. As shown in Fig. 1B, each cell of HPT73 was observed to have several sheathed flagella with morphology similar to that for the wild type. In addition, the cells of the mutant strain showed good swimming in the chemotaxis buffer under a dark-field microscope, but the movement of the cells from the same preparation in the viscous PVP-chemotaxis buffer was very much restricted compared to that of the wild-type strain. The apparent correlation between the urease production and swarming motility of H. pylori prompted us to examine the chemotactic motility in a high-viscosity environment.
FIG. 1.
Motility and electron micrographs of H. pylori CPY3401 (Ure+) and HPT73 (Ure−). Each strain was stabbed on brucella-serum motility agar and incubated microaerobically at 37°C for 5 days to observe swarming motility (A). Cells were prepared as described in Materials and Methods and observed by transmission electron microscopy. Bar, 1 μm (B).
Chemotactic motility of H. pylori in viscous and fluid solutions.
To clarify the effects of viscosity on bacterial motility, we determined the chemotactic activity of H. pylori CPY3401 in response to flurofamide in the absence or presence of PVP (Table 1). The average numbers of bacteria attracted in the presence of 3% PVP (5.6 cP) and 5% PVP (16.7 cP) were 7 and 14 times higher, respectively, than those in the absence of PVP. In subsequent experiments, we used PVP-chemotaxis buffer containing 3% PVP instead of 5% PVP for ease of handling on the slide glasses for counting bacteria.
TABLE 1.
Effect of viscosity on chemotaxis of H. pylori toward fluorofamidea
| % PVP-360 in chemotaxis buffer | Viscosityb (cP) | No. of bacteria accumulated at 60 minc |
|---|---|---|
| 0 | 0.9 | 520.3 ± 89.6 |
| 3 | 5.6 | 3,958.0 ± 82.8 |
| 5 | 16.7 | 7,275.7 ± 1,325.8 |
Chemotaxis toward 1 μM flurofamide was determined as described in Materials and Methods.
Viscosity was determined at 28°C by using an Ostwald viscometer.
Values are means ± standard deviations.
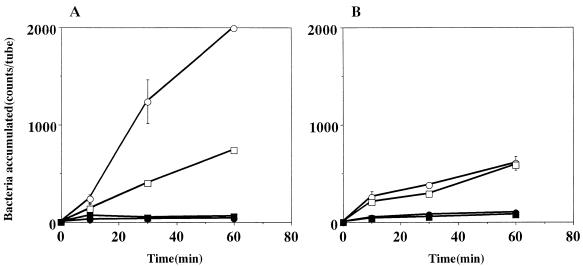
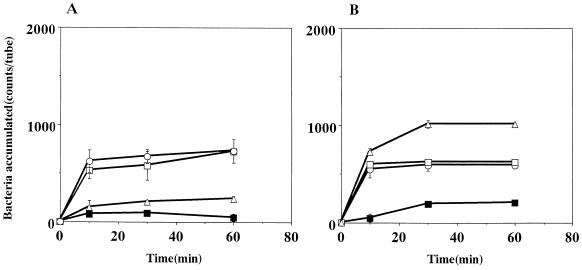
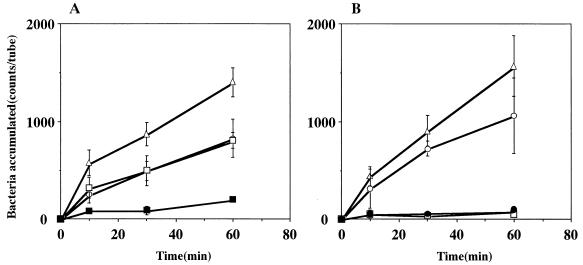
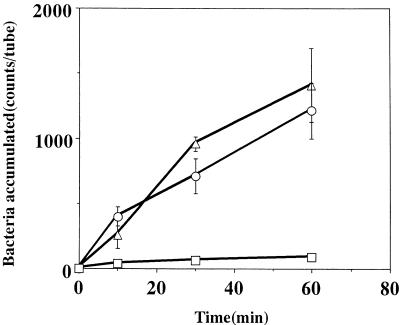
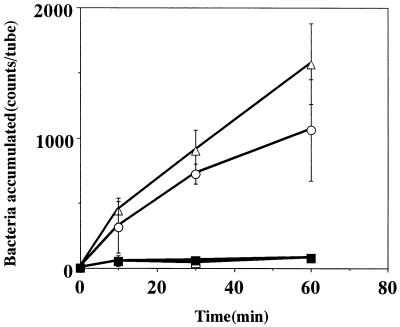
The chemotactic activity of strain CPY3401 in response to urea was high in the chemotaxis buffer and markedly increased by the addition of PVP (Fig. 2A). In contrast, the chemotactic activity of strain HPT73 was low in the chemotaxis buffer and was not increased by PVP (Fig. 2B). Time course experiments on the chemotaxes toward 10 mM sodium bicarbonate (Fig. 3) and toward 10 mM potassium bicarbonate gave similar results (data not shown). In every case, the activity of CPY3401 was higher than that of HPT73, particularly in PVP-chemotaxis buffer. We then tested the chemotactic activity of CPY3401 in response to urease inhibitors. As shown in Fig. 4, the bacteria are attracted by flurofamide and acetohydroxamic acid at the concentrations of 0.1 and 10 μM, respectively. It should be noted that increasing the concentration of acetohydroxamic acid resulted in a decrease in the chemotactic activity (Fig. 4B). In accordance with previous observations (20), CPY3401 and HPT73 did not show any chemotaxis toward potassium chloride in a viscous solution (data not shown).
FIG. 2.
Effects of viscosity on chemotaxis toward urea by H. pylori CPY3401 (Ure+) (A) and HPT73 (Ure−) (B). Chemotactic activities in response to 10 mM urea in PVP-chemotaxis buffer (○) and in chemotaxis buffer (□) were determined. Bacterial accumulation in the absence of attractants was determined in PVP-chemotaxis buffer (•) and in chemotaxis buffer (■). Error bars show standard deviations.
FIG. 3.
Effects of viscosity on chemotaxis toward sodium bicarbonate by H. pylori CPY3401 (Ure+) (A) and HPT73 (Ure−) (B). Chemotactic activities in response to 10 mM NaHCO3 in PVP-chemotaxis buffer (○) and in chemotaxis buffer (□) were determined. Bacterial accumulation without attractants was determined in PVP-chemotaxis buffer (•) and in chemotaxis buffer (■). Error bars show standard deviations.
FIG. 4.
Chemotaxis of H. pylori CPY3401 (Ure+) toward flurofamide (A) and toward acetohydroxamic acid (B) in PVP-chemotaxis buffer. The concentrations of the attractant flurofamide were 0 (■), 0.01 (▵), 0.1 (○), and 1 (□) μM, whereas those of acetohydroxamic acid were 0 μM (■), 10 μM (▵), 100 μM (○), and 1 mM (□). Error bars show standard deviations.
The above results are consistent with those of a previous study showing that both urease-positive and -negative strains are able to move toward urea, flurofamide, sodium chloride, and sodium bicarbonate but not toward potassium chloride (20). The results also indicate that the chemotactic activity of the urease-positive strain is higher than that of the urease-negative strain, particularly in a viscous environment.
Effect of urease inhibitors on the chemotaxis of CPY3401 in a viscous environment.
To determine whether urease activity is involved in the chemotactic motility of CPY3401 in a viscous environment, we treated cells with urease inhibitors prior to determining the chemotactic activity in response to sodium bicarbonate in PVP-chemotaxis buffer. Cells treated with 1 μM flurofamide showed a marked decrease in the chemotactic response to 10 mM sodium bicarbonate. Increasing the concentration of flurofamide to 10 μM did not cause further decrease (some residual activity was noted) (Fig. 5A). When the cells were treated with 10 μM acetohydroxamic acid, the chemotactic activity was lost completely (Fig. 5B). These results suggested that the urease inhibitors inhibited the chemotactic activity either by inhibiting urease in the cells or by inhibiting some steps involved in bacterial chemotaxis, e.g., the binding of sodium bicarbonate to a chemotaxis receptor.
FIG. 5.
Effects of flurofamide (A) and acetohydroxamic acid (B) on the chemotaxis of CPY3401 toward sodium bicarbonate in PVP-chemotaxis buffer. Cells of CPY3401 suspended in PVP-chemotaxis buffer were incubated in the absence (▵) or presence of 1 (○) and 10 (□) μM urease inhibitor at 28°C for 10 min, and the chemotactic activities in response to 10 mM NaHCO3 were determined. In panel B, accumulation of cells incubated with 100 μM acetohydroxamic acid prior to the chemotaxis assay (•) is also shown. In panel A, bacterial accumulation in PVP-chemotaxis buffer without attractants (■) is also shown. Error bars show standard deviations.
To determine whether the urease inhibitors inhibited the urease activity in intact cells with consequent reduction in the chemotactic motility, we incubated cells of H. pylori CPY3401 with urease inhibitors for 10 min at 28°C, and urease activities in crude extracts were measured. The activity of the cells treated with flurofamide (17.5 μmol/min/mg) was 5% of the activity of untreated cells (362.5 μmol/min/mg), whereas the cells treated with 1 mM acetohydroxamic acid had no activity. The chemotactic activities in response to urea and flurofamide were not inhibited by potassium bicarbonate (Fig. 6), suggesting that flurofamide does not compete with sodium bicarbonate in the binding to the chemotaxis receptor. The numbers of CFU of CPY3401 were essentially the same following incubations at 28°C for 60 min in the absence and in the presence of 1 μM flurofamide or 1 mM acetohydroxamic acid in PVP-chemotaxis buffer.
FIG. 6.
Effects of potassium bicarbonate treatment on the chemotaxes of CPY3401 toward urea and flurofamide in PVP-chemotaxis buffer. CPY3401 cells suspended in PVP-chemotaxis buffer were incubated with 10 mM KHCO3 at 28°C for 10 min, and the chemotactic activities in the absence (□) or presence of 10 mM urea (○) and 1 μM flurofamide (▵) were determined. Error bars show standard deviations.
Effect of CCCP on ureataxis in a viscous environment.
The bacterial flagellum rotates via a motor that converts electrochemical energy into mechanical energy. The energy source for the motor is generally an ion motive force, rather than the force produced by ATP hydrolysis (14, 17). Some bacteria, such as Vibrio parahaemolyticus, use a sodium motive force (2), whereas most bacteria use a proton motive force. In a previous study, some of us showed that the chemotactic activity of H. pylori in response to 1 mM sodium chloride was not inhibited by 5 mM amiloride, a sodium pump inhibitor (20). In this study, we incubated CPY3401 cells in PVP-chemotaxis buffer containing an uncoupler, CCCP which is a proton carrier that abolishes proton electrochemical gradients. As shown in Fig. 7, 10 μM CCCP completely blocked chemotactic activity. These results suggest that H. pylori utilizes a proton motive force for flagellar motility. The numbers of CFU of CPY3401 were essentially the same following incubation in the absence or in the presence of 100 μM CCCP at 28°C for 60 min.
FIG. 7.
Effects of the proton pump inhibitor CCCP on the chemotaxis of CPY3401 toward urea in PVP-chemotaxis buffer. CPY3401 cells suspended in the PVP-chemotaxis buffer were incubated in the absence (▵) or in the presence of 1 (○), 10 (□), and 100 (■) μM CCCP at 28°C for 10 min, and the chemotactic activities in response to 10 mM urea were determined. Error bars show standard deviations.
DISCUSSION
Urea is synthesized in the liver, circulated in the bloodstream, and possibly diffuses to the gastric lumen through capillaries beneath the gastric epithelium. Bicarbonate and sodium ions are secreted by the chloride-bicarbonate exchanger located in parietal cells and by the sodium-proton exchanger distributed in mucous cells, respectively (26). Thus, the concentration gradients of urea and sodium bicarbonate must be formed across the mucous layer. Members of our group previously showed that urea and sodium bicarbonate serve as attractants for H. pylori in buffer (20). In the present study, we determined the chemotactic motility in a high-viscosity environment, a condition that mimics the ecological niche of H. pylori.
The following observations made in this study, indicate that the urease of H. pylori plays an important role in the chemotactic motility under high-viscosity conditions. (i) A urease-negative mutant with a disruption of ureB showed no swarming on motility agar regardless of having apparently normal flagella. (ii) The chemotactic activities of the urease-positive strain in response to all the attractants tested were markedly higher in the presence of PVP than in its absence. (iii) We confirmed the results of a previous study (20) showing that the ureB disruption mutant also has chemotaxis toward the attractants, although the chemotactic motility was low and was not stimulated by increasing the viscosity. (iv) Inhibition of urease activity by urease inhibitors such as flurofamide and acetohydroxamic acid paralleled the decrease of the chemotactic activity in response to sodium bicarbonate under high-viscosity conditions.
The possibility that the ureB disruption mutant HPT73 has acquired some other mutations that reduce motility is unlikely, since the mutant cells were found to have apparently normal flagella and to show good swimming in buffer when observed under a dark-field microscope. We observed that the cells from the same preparation showed restricted motility in PVP-chemotaxis buffer and no swarming on motility agar.
The effects of flurofamide and acetohydroxamic acid on chemotaxis seem to reflect a complex phenomenon. The compounds are urea analogues having similar configurations in parts of their molecules. Therefore, each might serve as an attractant and an inhibitor of urease as well, although this possibility should be tested by further studies. The chemotactic motility toward acetohydroxamic acid was reduced by increasing the concentration (Fig. 4B), possibly because the cells were attracted by the urease inhibitor before entering the capillaries. Treatment of the cells with acetohydroxamic acid prior to chemotaxis assay resulted in a complete loss of the chemotactic activity in response to sodium bicarbonate (Fig. 5B), concomitant with the loss of the urease activity. In contrast, the inhibitory action of flurofamide on the chemotactic (Fig. 5A) and urease activities was partial, and the chemotactic activity in response to the compound was not reduced by increasing its concentration (Fig. 4A). These results may be explained by the difference in the chemical characteristics of these compounds when they encounter the permeability barrier presented by the bacterial membrane. As acetohydroxamic acid is a small molecule (molecular weight, 75.07), it can permeate intact bacterial cells and effectively inhibit the urease activity of H. pylori (21). On the other hand, flurofamide is a rather large hydrophobic molecule (molecular weight, 217.14) which may not easily penetrate the cytoplasm. Therefore, flurofamide may act as a nondiffusible inhibitor and may therefore fail to inhibit cytoplasmic urease (23). Taken together, these results indicate that the cytoplasmic urease is more important than the surface-associated urease for the chemotactic motility of H. pylori under viscous conditions. We could not exclude the possibility, however, that flurofamide and acetohydroxamic acid inhibit chemotaxis by interacting with some other components involved in the chemotactic motility.
Flurofamide and urea appear to have a common ligand-binding chemoreceptor site, since the cells incubated with flurofamide showed lower chemotactic activity in response to urea than to sodium bicarbonate (data not shown). On the other hand, bicarbonate ion appears not to bind to the urea-flurofamide receptor, since bicarbonate did not inhibit the chemotaxes to urea and flurofamide (Fig. 6). The recent sequencing of the entire genome (27) revealed three genes, tlpA, tlpB, and tlpC, each encoding a methyl-accepting chemotaxis protein. To identify the urea- and bicarbonate-specific receptors, we are currently investigating the function of these chemotaxis genes by allelic exchange mutagenesis.
Many bacteria use flagella to move in their environment. The helical flagellar filaments rotate via the action of a motor device located in the proximal portion of the flagellar structure (13, 16). The bacterial flagellar motor is driven by a revolving force (3), the energy of which is usually supplied by proton gradients without consumption of ATP (14, 17). We have shown that CCCP, but not amiloride (20), inhibited the chemotaxis of H. pylori (Fig. 7). Since CCCP is a proton carrier and amiloride is a sodium pump inhibitor, a proton motive force, rather than a sodium motive force, should drive the flagella of H. pylori in the same manner as those of Escherichia coli (16). Two motor proteins, MotA and MotB, and associated switch proteins as well as some chemotaxis proteins have been identified by sequencing of the genome (27).
H. pylori exhibits chemotactic activities in response to urea and bicarbonate even in the absence of urease, suggesting that the proton motive force generated by urease-independent metabolic processes is adequate for swimming in buffer, but it might not be sufficient for motility in highly viscous solution. Ureaplasma urealyticum, a small, wall-less prokaryote associated with urinary infection, also possesses a potent cytoplasmic urease. The urease of this microorganism internally hydrolyzes urea that is incorporated from the environment to produce an ammonium chemical potential and simultaneously, to increase the proton motive force with resultant de novo ATP synthesis (25). In H. pylori, urea may be supplied internally by de novo synthesis in the urea cycle (18) and then hydrolyzed by cytoplasmic urease, possibly resulting in an increase of the proton motive force by a mechanism yet to be clarified. If the de novo synthesis is the sole source of urea to be hydrolyzed internally, the physiological role of H. pylori chemotaxis toward urea might be to obtain urea to be hydrolyzed externally for gastric acid neutralization, possibly by surface urease. Further studies are necessary to clarify how urea is supplied to H. pylori and how urea hydrolysis stimulates chemotaxis of H. pylori in a viscous solution.
The data presented in this report further indicate the importance of urease in H. pylori for colonizing the stomach and thus suggest the potential use of urease inhibitors as therapeutic agents for H. pylori infection.
ACKNOWLEDGMENTS
This work was supported by grants-in-aid from the Ministry of Education, Science, Culture and Sports of Japan (BA08307004, BB08457089).
We are indebted to M. Kimoto for his invaluable technical assistance.
REFERENCES
- 1.Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- 2.Atsumi T, McCarter L, Imae Y. Polar and lateral flagellar motors of marine Vibrio are driven by different ion-motive forces. Nature. 1992;355:182–184. doi: 10.1038/355182a0. [DOI] [PubMed] [Google Scholar]
- 3.Berg H C. Dynamic properties of bacterial flagellar motors. Nature. 1974;249:77–79. doi: 10.1038/249077a0. [DOI] [PubMed] [Google Scholar]
- 4.Blaser M J. Helicobacter pylori: microbiology of a ‘slow’ bacterial infection. Trends Microbiol. 1993;1:255–260. doi: 10.1016/0966-842x(93)90047-u. [DOI] [PubMed] [Google Scholar]
- 5.Clyne M, Drumim B. The urease enzyme of Helicobacter pylori does not function as an adhesin. Infect Immun. 1996;64:2817–2820. doi: 10.1128/iai.64.7.2817-2820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creno R J, Wenk R E, Bohlig P. Automated micromeasurement of urea using urease and the Berthelot reaction. Am J Clin Pathol. 1970;54:828–832. doi: 10.1093/ajcp/54.6.828. [DOI] [PubMed] [Google Scholar]
- 7.Eaton K A, Morgan D R, Krakowka S. Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J Med Microbiol. 1992;37:123–127. doi: 10.1099/00222615-37-2-123. [DOI] [PubMed] [Google Scholar]
- 8.Eaton K A, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62:3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The EUROGAST Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 10.Goodwin C S, Armstrong J A, Chilvers T, Peters M, Collins M D, Sly L, McConnell W, Harper W E S. Transfer of Campylobacter pylori and Campylobacter mustelae to Helicobacter gen. nov. as Helicobacter pylori comb. nov. and Helicobacter mustelae comb. nov., respectively. Int J Syst Bacteriol. 1989;39:397–405. [Google Scholar]
- 11.Hazel S L, Lee A, Brady L, Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986;153:658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- 12.Hu L-T, Mobley H L T. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun. 1990;58:992–998. doi: 10.1128/iai.58.4.992-998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones C J, Aizawa S. The bacterial flagellum and flagellar motor: structure, assembly and function. Adv Microb Physiol. 1991;32:109–172. doi: 10.1016/s0065-2911(08)60007-7. [DOI] [PubMed] [Google Scholar]
- 14.Khan S, Macnab R M. Proton chemical potential, proton electrical potential and bacterial motility. J Mol Biol. 1980;138:599–614. doi: 10.1016/s0022-2836(80)80019-2. [DOI] [PubMed] [Google Scholar]
- 15.Kirschner D E, Blaser M J. The dynamics of Helicobacter pylori infection of the human stomach. J Theor Biol. 1995;176:281–290. doi: 10.1006/jtbi.1995.0198. [DOI] [PubMed] [Google Scholar]
- 16.Macnab R M. Genetics and biogenesis of bacterial flagella. Annu Rev Genet. 1992;26:131–158. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- 17.Manson M D, Tedesco P M, Berg H C. Energetics of flagellar rotation in bacteria. J Mol Biol. 1980;138:541–561. doi: 10.1016/s0022-2836(80)80017-9. [DOI] [PubMed] [Google Scholar]
- 18.Mendz G L, Hazell S L. The urea cycle of Helicobacter pylori. Microbiology. 1996;142:2959–2967. doi: 10.1099/13500872-142-10-2959. [DOI] [PubMed] [Google Scholar]
- 19.Messier B, Leblond C P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960;106:247–265. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- 20.Mizote T, Yoshiyama H, Nakazawa T. Urease-independent chemotactic responses of Helicobacter pylori to urea, urease inhibitors, and sodium bicarbonate. Infect Immun. 1997;65:1519–1521. doi: 10.1128/iai.65.4.1519-1521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mobley H L T, Cortesia M J, Rosenthal L E, Jones B D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988;26:831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 23.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu T, Akamatsu T, Sugiyama A, Ota H, Katsuyama T. Helicobacter pylori and the surface mucous gel layer of the human stomach. Helicobacter. 1996;1:207–218. doi: 10.1111/j.1523-5378.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith D G E, Russell W C, Ingledew W J, Thirkell D. Hydrolysis of urea by Ureaplasma urealyticum generates a transmembrane potential with resultant ATP synthesis. J Bacteriol. 1993;175:3253–3258. doi: 10.1128/jb.175.11.3253-3258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart-Tilley A, Sardet C, Pouyssegur J, Schwartz M A, Brown D, Alper S L. Immunolocalization of anion exchanger AE2 and cation exchanger NHE-1 in distinct adjacent cells of gastric mucosa. Am J Physiol. 1994;266:C559–C568. doi: 10.1152/ajpcell.1994.266.2.C559. [DOI] [PubMed] [Google Scholar]
- 27.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda M, Karita M, Morshed M G, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren J R, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 30.Wotherspoon A C, Ortiz-Hidalgo C, Falzon M R, Isaacson P G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]