Dear Editors,
Recent studies have shed light on the significant connection of SARS‐CoV‐2 infection with a heightened risk of experiencing a wide range of newly emerging autoimmune conditions. 1 Among others, SARS‐CoV‐2 has been associated with exacerbation of acute pancreatitis 2 and new‐onset diabetes, in line with viral infection of both endocrine and exocrine pancreatic cells. 3 To what extent vaccines can either cause or trigger disease activity in rare autoimmune pancreatitis (AIP) remains unclear, albeit single observational reports on post‐vaccination cases of acute pancreatitis revealed no evidence for a significant association. 2 Moreover, AIP patients represent a cohort at risk of a severe course of COVID‐19 as they commonly use immunosuppressive medication along with frequent comorbidities. Here we report on infection‐ or vaccination‐associated complications in an at least twice SARS‐CoV‐2 vaccinated AIP cohort and compare the results with a healthy control group.
In this cross‐sectional study (Ethical Review Board Ulm University, number 259/22 and 118/21), 30 AIP patients (16 AIP type 1/IgG4‐related disease (IgG4‐RD), 14 type 2) were prospectively enrolled in a questionnaire‐based (Supplementary Information S7) post‐vaccination interview. Baseline characteristics are shown in Table 1 and Table S1. All patients received at least two vaccinations, while 90% (n = 27) and 43% (n = 13) received a third and fourth boost, respectively. Eighty percent (n = 24) received homologous mRNA vaccine immunization, whereas six patients (20%) had a heterologous regimen with at least one conventional SARS‐CoV‐2 vaccine. Vaccinations started in January 2021 and ended in October 2022 and thus covered a relevant SARS‐CoV‐2 variant spectrum.
TABLE 1.
Baseline characteristics.
| Age, years median (Range) | 54.2 (22–84) |
| Autoimmune pancreatitis (AIP) type 1 | 63 (33–84) |
| AIP type 2 | 38 (22–79) |
| Sex (n) | |
| Male | 21 |
| Female | 9 |
| Autoimmune pancreatitis (n) | |
| Type 1/IgG4‐RD | 16 |
| >1 organ involvement | 12 |
| Type 2 | 14 |
| Accompanying ulcerative colitis | 8 |
| AIP specific therapy at first vaccination (n) | |
| Prednisolone | 9 |
| Rituximab | 1 |
| Prednisolone + rituximab | 2 |
| Prednisolone + azathioprine | 1 |
| None | 15 |
| Unknown | 2 |
| Alcohol use (n) | |
| Daily | 5 |
| Sporadic | 23 |
| None | 2 |
| Nicotine use (n) | |
| Daily | 16 |
| Sporadic | 6 |
| None | 8 |
| SARS‐Cov‐2 vaccine, first‐vaccination (n) | |
| Comirnaty® | 23 |
| Vaxzevria® | 4 |
| Jcovden® | 2 |
| Spikevax® | 1 |
| Second‐vaccination (n) | |
| Comirnaty® | 26 |
| Vaxzevria® | 2 |
| Spikevax® | 2 |
| Third‐vaccination (n) | |
| Comirnaty® | 20 |
| Spikevax® | 6 |
| Fourth‐vaccination (n) | |
| Comirnaty® | 13 |
Most patients (n = 20, 66.7%) reported no vaccine‐related side effects, while only a few (n = 4, 13.3%) reported local symptoms defined as redness and/or swelling at the injection site. At the same time, six patients (20%) complained of systemic side effects such as cough, fever, headache, aching limbs, or insomnia after the first vaccination. Comparable symptoms were noted following the second (n = 30) and the third vaccination (n = 27), with 53.3% and 52% of individuals without adverse events. Meanwhile, 20% and 26% reported local symptoms, and 26.7% and 22% reported systemic symptoms. Next, we generated an age‐matched cohort of 159 healthy volunteers (Table S2) from previously published data 4 who willingly filled a questionnaire related to SARS‐CoV‐2 vaccine‐related side effects. Interestingly, in this twice vaccinated cohort, systemic side effects occurred slightly more often than in the AIP patients, with 31.4% after the first vaccination and 44.7% after the second vaccination. Moreover, the incidence of local reactions was notably higher, 68.6% and 58.5% after the first and second vaccinations. Among the smaller group of AIP patients who received a fourth vaccination (n = 13), side effects were even rarer, 69.2% (n = 9) had no, 23.1% (n = 3) experienced local, and only one patient reported systemic symptoms. At the timepoints of first and second vaccination, 46.7% (14/30) had received immunosuppressive therapy for AIP (Table S3). This was even higher at the time of the third (64%, 16/25) and fourth (84.6%, 11/13) vaccinations. These findings suggest that vaccine‐related side effects in AIP patients are comparable to those observed in healthy individuals (Figure 1a).
FIGURE 1.
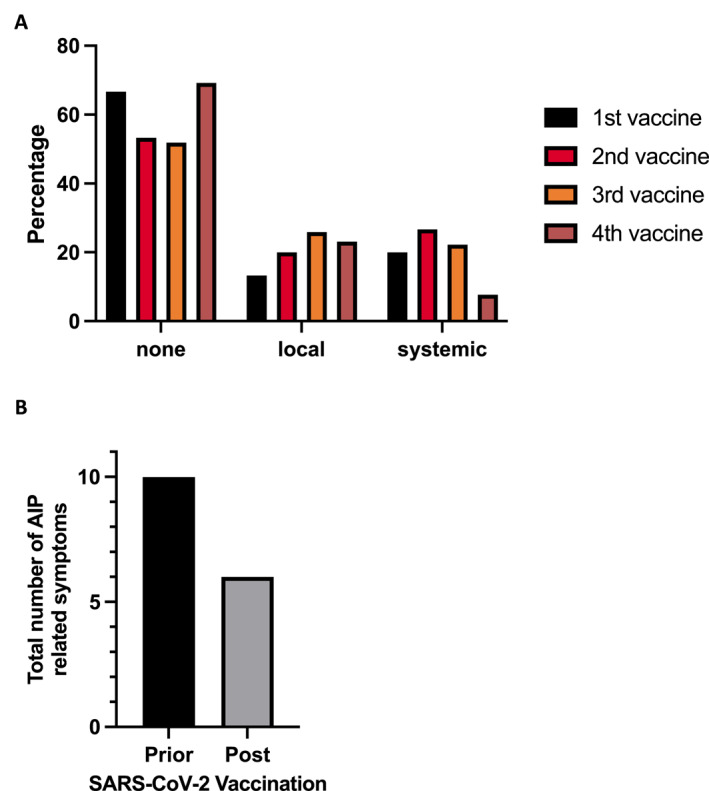
(a) Relative numbers of SARS‐CoV‐2 vaccination related side effects for first (total numbers: none = 20, local = 4, systemic = 6), second (none = 16, local = 6, systemic = 8), third (none = 14, local = 7, systemic = 6) and fourth vaccination (none = 9, local = 3, systemic = 1), (b) and cumulative number of autoimmune pancreatitis (AIP)‐related symptoms prior and post SARS‐CoV‐2 vaccinations.
Only a small number of patients reported AIP‐related symptoms (pain, weight loss, jaundice) prior to their vaccinations, three before the first and four before the second and third vaccination (Figure 1b). Notably, the number of patients experiencing these symptoms did not increase in the weeks following immunization, suggesting no exacerbation of the disease due to vaccination. Overall, none of the patients reported new onset of AIP related symptoms, but two AIP type 2 patients experienced a worsening of their pre‐existing condition. One suffered from abdominal pain and the other one additionally from weight loss, each under medication with prednisolone. In the group of patients who received four vaccine shots, there was no documented deterioration of AIP‐related symptoms (Figure 1b, Tables S4 and S5). Importantly, hospitalization was not required for any of these cases post‐vaccination. In summary, these findings indicate that SARS‐CoV‐2 vaccines do not appear to influence either the number of patients affected by AIP‐related symptoms or the severity of AIP.
Seventeen patients had confirmed SARS‐CoV‐2 infection within a median of 6 months of the last vaccination. None of the patients underwent repetitive infections. One of these patients suffered from a severe course with the need for hospitalization (due to pneumonia). Three patients received COVID‐19 infection‐related therapy. Symptoms were similar (e.g., upper respiratory tract symptoms, fever, see Table S6) to be usually reported in the general population, 5 and lasted for a median of 8 days (range 2–15). In conclusion, vaccinated AIP patients are neither prone to infection nor have a severe disease course upon COVID‐19.
With the next wave of SARS‐CoV‐2 infections on the horizon and the ongoing prevalence of this viral disease, our data demonstrate the safety of mRNA‐based SARS‐CoV‐2 vaccines for the at‐risk population of AIP patients. In our study involving 30 AIP type 1/IgG4‐RD and AIP type 2 patients, we found that SARS‐Cov‐2 vaccination during the recent pandemic did not exacerbate the severity of AIP. Firstly, the specific symptoms following SARS‐CoV‐2 vaccination were not worsened in patients with AIP. In fact, the vaccines' effects in AIP patients closely resembled those in healthy individuals. Secondly, the vaccination against SARS‐CoV‐2 did not lead to an increased disease severity, as the majority of patients experienced no worsening of their condition after vaccination. Thirdly, the susceptibility to acquire a SARS‐CoV‐2 infection was similar between AIP patients and healthy individuals. Collectively, this study indicates that the administration of SARS‐CoV‐2 vaccines is a safe option for AIP patients.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Supporting information
Supporting Information S1
Contributor Information
Lukas Perkhofer, Email: lukas.perkhofer@uniklinik-ulm.de.
Alexander Kleger, Email: Alexander.kleger@uni-ulm.de.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Sharma C, Bayry J. High risk of autoimmune diseases after COVID‐19. Nat Rev Rheumatol. 2023;19(7):399–400. 10.1038/s41584-023-00964-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nayar M, Varghese C, Kanwar A, Siriwardena AK, Haque AR, Awan A, et al. SARS‐CoV‐2 infection is associated with an increased risk of idiopathic acute pancreatitis but not pancreatic exocrine insufficiency or diabetes: long‐term results of the COVIDPAN study. Gut. 2022;71(7):1444–1447. 10.1136/gutjnl-2021-326218 [DOI] [PubMed] [Google Scholar]
- 3. Muller JA, Gross R, Conzelmann C, Kruger J, Merle U, Steinhart J, et al. SARS‐CoV‐2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149–165. 10.1038/s42255-021-00347-1 [DOI] [PubMed] [Google Scholar]
- 4. Hentschel V, Horsch C, Mayer B, Thies A, Qian W, Kroschel J, et al. A systematic evaluation of the SARS‐CoV‐2 vaccine‐induced anti‐S‐RBD‐Ig response in a population of health care workers. Vaccines (Basel). 2023;11(9):1467. 10.3390/vaccines11091467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, Farias de Oliveira T, Campos Alcantara R, Monteiro Arnozo G, et al. Clinical manifestations of COVID‐19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133(7–8):377–382. 10.1007/s00508-020-01760-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


