Abstract
Background
Earlier studies on the possible association between eosinophilic esophagitis (EoE) and inflammatory bowel disease (IBD) have been contradictory.
Methods
Patients with biopsy‐verified EoE diagnosed between 1990 and 2017 in Sweden (n = 1587) were age‐ and sex‐matched with up to five general population reference individuals (n = 7808). EoE was defined using pathology reports from all 28 pathology centers in Sweden (the ESPRESSO study). Multivariate Cox regression then estimated hazard ratios for future IBD. IBD was defined based on the international classification of disease codes and histopathology codes. In secondary analyses, sibling comparators were used to further reduce potential familial confounding. Additionally, we performed logistic regression examining earlier IBD in EoE.
Results
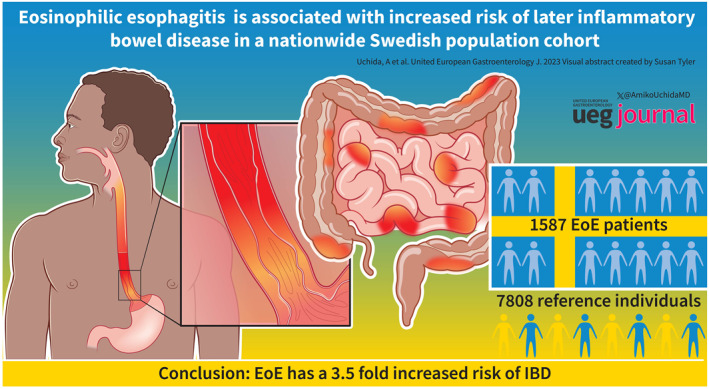
During follow‐up until 2020, 16 (0.01%) EoE patients and 21 (0.003%) general population reference individuals diagnosed with IBD, corresponding to a 3.5‐fold increased risk of future IBD (aHR = 3.56; 95% CI 1.79–7.11). EoE was linked to Crohn's disease (aHR = 3.39 [95% CI 1.02–9.60]) but not to ulcerative colitis (aHR = 1.37; 95% CI 0.38–4.86). Compared to their siblings, patients with EoE were at a 2.48‐fold increased risk of IBD (aHR = 2.48; 95% CI 0.92–6.70). Earlier IBD was 15 times more likely in EoE patients than in matched reference individuals (odds ratio, 15.39; 95% CI 7.68–33.59).
Conclusion
In this nationwide cohort study, EoE was associated with a 3.5‐fold increased risk of later IBD diagnosis. This risk increase may be due to shared genetic or early environmental risk factors, but also surveillance bias could play a role.
Keywords: allergy, autoimmunity, Crohn's disease, EoE, eosinophilic esophagitis, epidemiology, IBD, immune dysregulation, inflammatory bowel disease, risk factor
Key summary.
What is known
Eosinophilic esophagitis (EoE) and inflammatory bowel disease (IBD) are examples of immune dysregulation within the gastrointestinal tract.
Studies have shown mixed results for the risk of EoE co‐occurring when having a prior diagnosis of IBD, and the inverse relationship of IBD before EoE is not well studied.
What is new here
EoE was significantly associated with a 3.5‐fold increased risk of later IBD diagnosis, particularly with Crohn's disease.
Having IBD was associated with a 15‐fold increased odds of later EoE.
INTRODUCTION
Allergic and autoinflammatory gastrointestinal (GI) diseases such as eosinophilic esophagitis (EoE) and inflammatory bowel diseases (IBD), respectively, are diseases that exhibit loss of immune tolerance. EoE is a chronic, allergic disease of the esophagus with rising incidence that affects all ages, sexes, and races. 1 , 2 , 3 , 4 , 5 , 6 , 7 It is defined as having symptoms of esophageal dysfunction combined with histologic presence of esophageal intraepithelial eosinophils ≥15 per high power field. 8 The loss of tolerance seen in EoE arises from developing a hypersensitivity toward predominantly food antigens and a type 2 inflammatory response. 9 , 10 However, the factors that influence this loss of tolerance, such as the microbiome and other environmental factors, are incompletely understood. It is hypothesized that environmental factors, as opposed to genetics, are more likely to account for the rapid increase in incidence and prevalence of EoE. 11 As such, we and others recently reported that antecedent environmental factors such as infection—particularly GI and respiratory sources—as well as antibiotic exposure are associated with increased odds of later EoE diagnosis. 12 , 13 These data indirectly suggest immunologic and/or microbial disruption underpinnings to EoE.
Similar to EoE, IBD exhibits loss of immune tolerance to the gut microbiome with associated chronic inflammation of the GI tract. The two major types of IBD are Crohn's disease (CD) and ulcerative colitis (UC). Additionally, the occurrence of IBD has also been associated with prior infection and antibiotic use. 14 , 15 There is mounting evidence of an increased co‐occurrence of EoE and IBD. Most previous studies examining this relationship utilized nationwide or local claims databases, medical code databases, or regional patient cohorts. 16 , 17 , 18 These studies found that on average having IBD translated to roughly 2–5 times increased risk of later EoE diagnosis. Interestingly, when Sonnenberg et al. utilized a United States‐based histopathologic database of patients undergoing same day upper and lower endoscopy and compared to general population controls, they unexpectedly found EoE was less common in IBD patients. 19 However, to date, research has primarily examined IBD and later EoE, rather than EoE and later IBD diagnosis.
We thus aimed to better understand the temporal relationship between EoE and IBD by using a validated nationwide histopathologic database and compared patients with EoE to matched reference individuals from the general population. Given the shared development of mucosal immune dysregulation, we hypothesized that EoE conferred an increased risk for later IBD diagnosis. In secondary analyses, we compared individuals with EoE to their siblings to address potential genetic and shared early environmental factors. We also assessed the reverse relationship for the patients who had IBD diagnosed before EoE.
METHODS
Patient cohort
Ascertainment of EoE cases
We utilized the ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden) cohort, which contains data from over 6 million GI biopsies registered according to the SNOMED clinical term system collected from 1965 to 2017 from all 28 pathology departments in Sweden. 20 All Swedes are assigned a Personal Identity Number, which is a unique number that allows large‐scale linkages and epidemiological research. 21 We linked data on all EoE cases in the ESPRESSO cohort (Topography T62, Morphology M47150) to nationwide Swedish healthcare registers including the National Patient Register. 20 , 22 , 23 , 24 A major component of the diagnosis of EoE is histological with ≥15 intraepithelial esophageal eosinophils/high power field, and upon validation, the EoE cohort derived from ESPRESSO was found to have a positive predictive value of 89%. 20 For this study, we examined individuals with EoE diagnosed between 1 January 1990 and 31 December 2017. Follow‐up for our outcome of IBD ended on 31 December 2019. We chose to limit the years of inclusion for EoE diagnosis to 1990 or later because of significant heterogeneity in diagnostic criteria and generally low awareness of EoE before this. 24 For the main analysis, EoE individuals were excluded if they had a prior diagnosis of IBD, or if they emigrated prior to EoE diagnosis/matching date since this would prohibit a complete follow‐up prior to EoE (and an earlier IBD diagnosis may have been missed). EoE medications administered before and after EoE diagnosis were assessed using the National Prescribed Drug Register, which began record keeping 1 July 2005, and utilizes anatomical therapeutic chemical (ATC) codes. The ATC code A02BC was used for proton pump inhibitors (PPI), while A07EA and H02AB were used for topical corticosteroids (steroids) and assessed beginning 1 Jan 2006. These data were also obtained in reference individuals.
General population reference individuals
We matched all individuals with EoE with up to five reference individuals from the general population according to age, sex, calendar year (year of biopsy), county of residency and education from the Swedish Total Population Register. 25 County of residence was utilized in attempts to reflect healthcare access and balance the risk of surveillance bias as practices may vary. Reference individuals did not have a prior diagnosis of EoE or IBD at the time of matching. If a reference individual had a later EoE diagnosis, the individual was censored on that date and stopped contributing follow‐up to the reference cohort.
Sibling comparators
The siblings of individuals with EoE were identified through the Swedish Multigeneration Register, a sub‐section of the Total Population Register. Sibling data were available on all individuals born after 1932 and who were registered as residents of Sweden in 1961 or later. EoE sibling comparators were utilized to minimize intrafamilial confounders (shared genetic and some early environmental factors) that may have potential to influence development, awareness, or surveillance/detection for IBD.
Outcome measures
Our primary outcome measurement was the diagnosis of IBD (i.e., later IBD) according to the Swedish Patient Register and GI biopsy data. The patient register includes individual‐level data on inpatient and outpatient encounters at a nationwide level since 1987 (with regional reporting since 1964). 25 IBD was defined as having two records of IBD, or one International Classification of Diseases (ICD) codes for IBD plus one histopathology SNOMED code consistent with IBD (Table S1). 26 Endoscopic codes are shown in Table S2. Subtyping of IBD (into UC and CD) was performed according to the approach by Forss et al. 26 While IBD overall included IBD‐U (unclassified), we performed no analyses for this subtype due to lack of statistical power. The date of diagnosis of IBD (equivalent to end of follow‐up) was determined by either the histopathology code or the ICD code.
Using this method of both ICD and histopathology coding to ascertain diagnoses of IBD has been previously validated with an accuracy of 95%. 15 Follow‐up ended with the development of IBD, death, emigration, or the end of the study period (31 December 2019).
Secondary analyses
We examined patients who had a diagnosis of IBD in the ESPRESSO cohort prior to their EoE diagnosis. We compared these individuals deemed “earlier IBD” who later were diagnosed with EoE to general population reference individuals using a case‐control analysis to assess the relationship of earlier IBD to risk of EoE diagnosis.
Statistical analyses
All analyses were performed with R 4.0.5 primarily using the survival package. 27 , 28 For the main analysis, multivariate Cox regression was utilized to determine hazard ratios (HRs) for later diagnosis of IBD or IBD subtype. Follow‐up began after the index EoE diagnosis date. Adjusted models were conditioned for age, sex, calendar period, and county, autoimmune status (yes/no), and education level (see Table S3 for list of autoimmune conditions and codes). A sensitivity analysis adjusted for the number of non‐primary care visits between 6 and 24 months prior to EoE diagnosis and the date of matching was conducted. In addition, a sensitivity analysis using EoE siblings as comparators were run using a stratified Cox regression adjusting for age, sex, calendar period, county, autoimmune status (yes/no), and education level.
A logistic regression, using the same adjustments as in the Cox model, calculating odds ratios (ORs) was performed to examine risk for EoE when IBD was diagnosed before the index EoE date (“earlier IBD”).
Ethics
This study was approved by the Stockholm Ethics Board. Informed consent was waived since the study was strictly register‐based. 29
RESULTS
Study cohort
Between 1990 and 2017, we identified 1587 patients with histologically verified EoE and 7808 matched general population reference individuals (Table 1). In keeping with other population studies, the proportion of male to female individuals with EoE was approximately 3:1 (M = 1194: F = 393). The mean age at follow‐up for both groups was 37 years. The age distribution of the EoE cohort was generally distributed across the lifespan, with pediatric patients accounting for 354 individuals (22%), 18–39‐year‐olds accounting for 464 (29%), 40–59‐year‐olds had 514 EoE individuals (32%), and those ≥60 had the fewest at 255 individuals (16%). On average, EoE patients underwent 1.07 endoscopies during follow‐up, compared with 0.09 in matched reference individuals. PPI and steroid use were more common in individuals with EoE than in reference individuals (Table S4).
TABLE 1.
Characteristics of EoE patients and reference individuals.
| Reference individuals | EoE individuals | |
|---|---|---|
| N [%] | N [%] | |
| Total | 7808 [100.00] | 1587 [100.00] |
| Sex | ||
| Male | 5862 [75.08] | 1194 [75.24] |
| Female | 1946 [24.92] | 393 [24.76] |
| Age at start follow‐up (years) | ||
| Mean [SD] | 37.39 [20.18] | 37.77 [20.37] |
| Median [IQR] | 38.00 [19.00–53.00] | 39.00 [19.00–53.00] |
| Range, min‐max | 0–94 | 0–94 |
| <18 | 1761 [22.55] | 354 [22.31] |
| 18 < 40 | 2314 [29.64] | 464 [29.24] |
| 40 < 60 | 2532 [32.43] | 514 [32.39] |
| ≥60 | 1201 [15.38] | 255 [16.07] |
| Country of birth | ||
| Nordic | 6550 [83.89] | 1508 [95.02] |
| Other | 1257 [16.10] | 79 [4.98] |
| Not available | 1 [0.01] | 0 [0.00] |
| Education | ||
| Compulsory school (= 9 years) | 1498 [19.19] | 251 [15.82] |
| Upper secondary school (10–12 years) | 2786 [35.68] | 567 [35.73] |
| College or university (= 13 years) | 2041 [26.14] | 493 [31.06] |
| Missing education | 1483 [18.99] | 276 [17.39] |
| Start of follow‐up | ||
| 2002–2009 | 909 [11.64] | 185 [11.66] |
| 2010–2017 | 6899 [88.36] | 1402 [88.34] |
| Years of follow‐up | ||
| Mean [SD] | 6.47 [2.62] | 6.48 [2.57] |
| Median [IQR] | 6.04 [4.59–7.96] | 6.05 [4.61–7.99] |
| Range, min‐max | 0.01–17.74 | 0.03–17.41 |
| <1 | 77 [0.99] | 7 [0.44] |
| 1 < 5 | 2415 [30.93] | 492 [31.00] |
| 5 < 10 | 4466 [57.20] | 920 [57.97] |
| ≥10 | 850 [10.89] | 168 [10.59] |
| Endoscopies (>30 days after EoE diagnosis) a | ||
| Mean (SD) | 1.07 [1.78] | 0.09 [0.45] |
| Autoimmune comorbidity at start of follow‐up | ||
| Autoimmunity present (yes) | 104 [6.55] | 249 [3.19] |
Abbreviations: EoE, eosinophilic esophagitis; IBD, inflammatory bowel disease.
We used >30 days so as not to include endoscopies that were part of the EoE diagnostic work‐up. Note that no IBD case was diagnosed in the first 30 days after EoE diagnosis.
The mean number of years of follow‐up was 6.4 for both EoE and reference population control groups. All cases of IBD were identified in the first 10 years after index EoE diagnosis, with 31% (n = 5) diagnosed in the first year after EoE diagnosis. In reference individuals, some 15% (n = 3/20) of IBD cases were diagnosed in the first year after matching. Excluded patients were few, with one excluded due to migration, and two that were unable to be matched (Table S5).
EoE and later IBD
Sixteen (0.01%) EoE patients and 21 (0.003%) general population reference individuals were diagnosed with IBD in our cohort, corresponding to a nearly 4‐fold increased risk of later IBD (crude HR [HR] = 3.73; 95% CI 1.95–7.15 and adjusted [aHR] = 3.90; 95% CI 2.02–7.52) in patients with EoE (Table 2, Figure 1a). Given the concern of high healthcare utilization by EoE patients just before their diagnosis and the potential for surveillance bias, we adjusted for non‐primary care visits that occurred between 6 and 24 months prior to EoE diagnosis. Risk estimates remained significant with an aHR of 3.56 (95% CI = 1.79–7.11) for EoE patients compared to reference individuals. In subgroup analyses, we found the highest risk estimates seen in CD with an aHR of 3.39 (95% CI 1.2–9.60) compared to UC (aHR 1.37; 95% CI 0.38–4.86) (Figure 1b,c). Due to low numbers of individuals, no conclusions could be meaningfully reported regarding IBD‐U (Figure 1d). Notably, most IBD diagnoses (n = 15/16) occurred more than 1 month after EoE diagnosis (Table S6), supporting that these diagnoses are distinct.
TABLE 2.
Risk of developing IBD in individuals with EoE.
| Outcome | Sample size and statistical test | Reference individuals | EoE individuals |
|---|---|---|---|
| IBD (any) | N total | 7808 | 1587 |
| N events | 21 | 16 | |
| IR [95% CI] | 0.42 [0.26–0.64] | 1.56 [0.89–2.53] | |
| IRD [95% CI] | 1.14 [0.36–1.92] | ||
| HR [95% CI] | 3.73 [1.95–7.15] | ||
| aHR [95% CI] | 3.56 [1.79–7.11] | ||
| CD | N total | 7808 | 1587 |
| N events | 9 | 6 | |
| IR [95% CI] | 0.18 [0.08–0.34] | 0.58 [0.21–1.27] | |
| IRD [95% CI] | 0.4 [−0.08 to 0.89] | ||
| HR [95% CI] | 3.27 [1.16–9.18] | ||
| aHR [95% CI] | 3.39 [1.20–9.60] | ||
| UC | N total | 7808 | 1587 |
| N events | 12 | 3 | |
| IR [95% CI] | 0.24 [0.12–0.42] | 0.29 [0.06–0.85] | |
| IRD [95% CI] | 0.05 [−0.3 to 0.41] | ||
| HR [95% CI] | 1.22 [0.35–4.34] | ||
| aHR [95% CI] | 1.37 [0.38–4.86] | ||
| IBD‐U | N total | 7808 | 1587 |
| N events | 0 | 7 | |
| IR [95% CI] | 0.00 [0.00–0.06] | 0.59 [0.24–1.22] | |
| IRD [95% CI] | 0.59 [0.15–1.03] | ||
| HR [95% CI] | N/A | ||
| aHR [95% CI] | N/A |
Note: N/A values could not be calculated due to insufficient data.
Abbreviations: aHR, adjusted for age at EoE, sex, calendar year, county, education, and presence of autoimmune disease (see Table S2 for list of diseases); CD, Crohn’s disease; EoE, eosinophilic esophagitis; HR, crude hazard ratio; IBD, inflammatory bowel disease; IBD‐U, IBD unclassified; IR, incidence rate; IRD, incidence rate difference; UC, ulcerative colitis.
FIGURE 1.
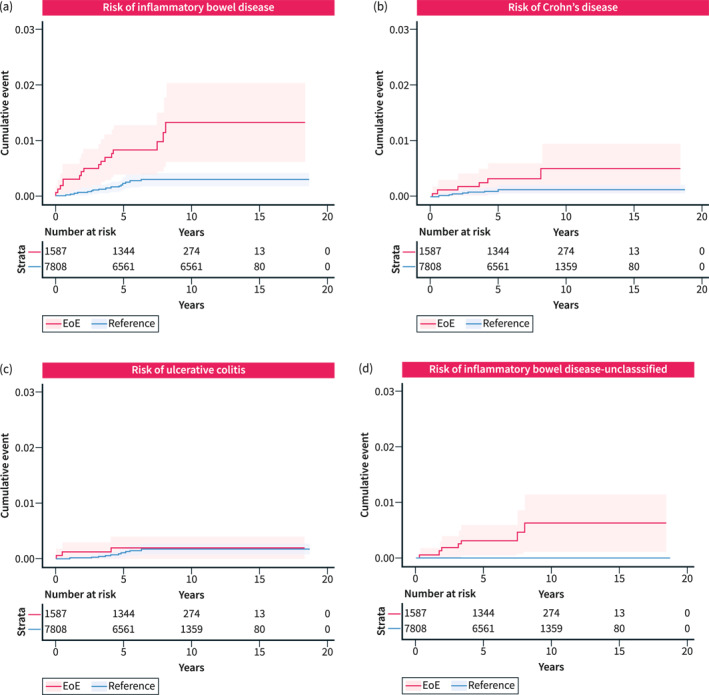
Kaplan–Meier curves of cumulative IBD diagnostic events among EoE and general population reference individuals. Any IBD (a) and subtypes of IBD including Crohn's disease (b), ulcerative colitis (c), and IBD‐unclassified (d) are shown. Shaded areas represent 95% confidence intervals. EoE, eosinophilic esophagitis; IBD, inflammatory bowel disease.
In sibling analyses, we identified 1209 individuals with EoE and 2054 siblings as reference individuals (Table 3). We found that 12 patients with EoE later were diagnosed with IBD compared to 11 siblings. This corresponded to an aHR of 2.48 (95% CI 0.92–6.70).
TABLE 3.
Risk of developing IBD in individuals with EoE compared to siblings.
| Outcome IBD | Reference individuals | EoE |
|---|---|---|
| N total | 2054 | 1209 |
| N events | 11 | 12 |
| IR [95% CI] | 0.81 [0.41–1.46] | 1.52 [0.79–2.66] |
| IRD [95% CI] | 0.71 [−0.28 to 1.69] | |
| HR [95% CI] | 2.19 [0.92–5.21] | |
| aHR [95% CI] | 2.48 [0.92–6.70] |
Note: N/A values could not be calculated due to insufficient data.
Abbreviations: aHR, adjusted for age at EoE, sex, calendar year, county, education, and presence of autoimmune disease (see Table S2 for list of diseases); EoE, eosinophilic esophagitis; HR, crude hazard ratio; IBD, inflammatory bowel disease; IR, incidence rate; IRD, incidence rate difference.
Results of stratified analyses by sex, years of follow‐up, area of residence, level of education and presence of autoimmunity are presented in Table 4. Results were similar in subgroups (Table 4).
TABLE 4.
Stratified analyses of IBD risk in EoE individuals.
| Reference individuals (events/N) | EoE (events/N, aHR [95% CI]) | |
|---|---|---|
| Sex | ||
| Males | 18/5862 | 11/1194, 3.35 [1.58–7.11] |
| Females | 3/1946 | 5/393, 7.72 [1.72–34.60] |
| Age at start of follow‐up (years) | ||
| <18 | 9/1761 | 7/354, 3.81 [1.38–10.53] |
| 18 < 40 | 4/2314 | 3/464, 3.53 [0.78–15.89] |
| 40 < 60 | 5/2532 | 4/514, 4.42 [1.17–16.77] |
| ≥60 | 3/1201 | 2/255, 3.24 [0.52–20.20] |
| Follow‐up (years) | ||
| <1 | 3/77 | 5/7, 8.18 [1.92–34.97] |
| 1 < 5 | 14/2415 | 8/492, 2.87 [1.19–6.90] |
| 5 < 10 | 4/4466 | 3/920, 4.26 [0.94–19.24] |
| ≥10 | 0/850 | 0/168, N/A [N/A–N/A] |
| Start of follow‐up | ||
| 2002–2009 | 7/909 | 3/185, 3.05 [0.75–12.44] |
| 2010–2017 | 14/6899 | 13/1402, 4.66 [2.17–10.01] |
| Country of birth | ||
| Nordic | 18/6550 | 16/1508, 3.86 [1.96–7.62] |
| Other | 3/1257 | 0/79, N/A [N/A–N/A] |
| Education (years) | ||
| Compulsory school (≤9) | 6/1498 | 2/251, 2.27 [0.38–13.60] |
| Upper secondary school (10–12) | 8/2786 | 5/567, 1.87 [0.50–7.05] |
| College or university (≥13) | 0/2041 | 3/493, N/A [N/A–N/A] |
| NA | 7/1483 | 6/276, 4.14 [1.33–12.83] |
| Comorbidity | ||
| Autoimmunity present | 1/249 | 1/104, N/A [N/A–N/A] |
| No autoimmunity | 20/7559 | 15/1483, 3.79 [1.94–7.41] |
Note: N/A values could not be calculated due to insufficient data. See Table S2 for list of autoimmune diseases.
Abbreviations: EoE, eosinophilic esophagitis; IBD, inflammatory bowel disease.
EoE and earlier IBD
In a case‐control fashion, we also examined the relationship between earlier IBD and later EoE diagnosis. These analyses revealed that of the 44 patients with IBD that were diagnosed before EoE, 27 (90%) of these individuals were diagnosed more than 1 month prior to being diagnosed with EoE, again supporting these as separate entities (Table S6). We found that individuals with IBD had 15 times the odds of later EoE diagnosis compared to general population controls (OR = 15.39, 95% CI = 7.68–33.59) (Table 5). This pattern was seen in all forms of IBD with CD and UC linked to a 22.86‐fold (95% CI = 5.83–150.71) and 21.54‐fold (95% CI = 6.82–94.99) increased odds respectively. Odds ratios for IBD‐U was 7.80 (95% CI = 2.56–26.18).
TABLE 5.
EoE and earlier IBD.
| EoE | Reference individuals | Odds ratio [95% CI] | |
|---|---|---|---|
| N = 1631 (events) | N = 8033 (events) | ||
| Exposure | |||
| IBD (any) | 30 | 10 | 15.39 [7.68–33.59] |
| CD | 9 | 2 | 22.86 [5.83–150.71] |
| UC | 13 | 3 | 21.54 [6.82–94.99] |
| IBD‐U | 8 | 5 | 7.80 [2.56–26.18] |
Abbreviations: CD, Crohn’s disease; EoE, eosinophilic esophagitis; IBD, inflammatory bowel disease; IBD‐U, IBD unclassified; UC, ulcerative colitis.
DISCUSSION
EoE and IBD are examples of GI diseases with underlying immune dysregulation. Using a validated nationwide cohort study and general population reference individuals, we show for the first time that EoE is associated with a nearly 4‐fold increased risk of later IBD diagnosis. Overall, similar magnitudes of associations were observed when comparing EoE individuals to their siblings suggesting shared genetics, early environmental factors, or healthcare seeking behaviors. Lastly, in patients with earlier IBD, we found 15‐fold increased odds of later EoE diagnosis.
Interestingly, the increased risk of a later IBD diagnosis was driven predominantly by CD rather than UC. This was mechanistically unexpected since EoE is a type 2 inflammatory, allergic condition, and at least in historic data, UC has been shown to associate with a type 2 inflammatory phenotype. 30 , 31 , 32 However, a recent report by Gonzales Acera et al. suggests that type 2 inflammation is present in both UC and CD, and instead may be associated with a distinct state of intestinal inflammation rather than a disease‐specific mechanism. 33 It is also important to consider the potential for surveillance bias, particularly given the risk association with CD and not UC or IBD‐U and that most IBD diagnoses were made within 5 years of EoE diagnosis. Since CD can affect anywhere in the GI tract, diagnosis by upper endoscopy, which patients with EoE undergo frequently, is possible. However, we note that the non‐primary care healthcare utilization for EoE patients compared to reference individuals was significantly higher even in the 6–24 months prior to EoE diagnosis, yet CD was not diagnosed during that time. Of note, the association between EoE and later IBD (adjusted HR = 3.90) was almost identical to that of celiac disease and later IBD from the same ESPRESSO cohort. 34 However, of note, the study by Mårild et al. found a strong association between celiac disease and UC, something not found in the current study. 34
When comparing EoE individuals to their siblings, the estimated risk decreased to roughly 2.5 with largely overlapping CIs that fell short of significance likely due to limited statistical power. The slightly lower HR in the sibling analysis could indicate that some of the association between EoE and IBD is attributable to shared genetics/environmental factors or similar healthcare seeking behaviors among siblings. It is worth noting that while sibling analyses effectively control for familial factors that are identical amongst siblings, these analyses can also increase confounding factors unique to each sibling. 35 Associations with IBD diagnosis were not materially different when analyses were stratified for sex, age, years of follow‐up, country of birth, education, or presence of other autoimmune diseases with overlapping 95% CIs.
Finally, we demonstrated a bidirectional association between EoE and IBD EoE and IBD since patients with EoE had a markedly higher odds of previously having been diagnosed with IBD than matched controls. These data support the findings of prior studies that utilized case‐controls at tertiary pediatric centers or USA claims databases, which found a 2.5‐fold increased risk of EoE in IBD patients and found IBD to be 5‐fold more prevalent in patients with EoE, respectively. 17 , 18 Importantly, patients with IBD, particularly pediatric IBD, in many practices undergo upper and lower endoscopy indiscriminately. This practice is likely to increase the surveillance bias for detecting a new EoE diagnosis. We found that the vast majority of IBD was diagnosed ≥30 days after an EoE diagnosis, and the fact that upper and lower endoscopy are not often performed in EoE at the same time suggests that EoE and IBD diagnoses arose as different encounters separated by time and clinical inquiry. However, we acknowledge that detection bias is likely to play an important role in our findings since 31% of all later IBD cases were diagnosed in the first year after EoE diagnosis. In reference individuals, 15% of IBD cases were diagnosed in the first year after matching. We also recognize that EoE patients underwent more endoscopies than reference individuals given the nature of the disease. Our findings otherwise pair nicely with most prior studies that investigated the relationship between these diseases. 16 , 17 , 18 Our findings are however incongruous with the only other histopathologic database that utilized general population controls where investigators did not find an increased co‐occurrence of EoE and IBD. 19
We note that our study has several strengths including being a validated nationwide EoE cohort and having the ability to compare our EoE cohort to their siblings in order to control for potential intrafamilial and some environmental confounders. This approach allowed us to minimize biases such as selection bias as well as those that may be associated with practices present at a given medical center. Additionally, since Sweden has largely universal, tax‐funded healthcare with well‐established national healthcare registers, loss to follow up and selection bias due to socioeconomic status is of low concern. 36
Among these strengths, we also acknowledge important limitations of our study. As with all cohorts retrieved from routine healthcare, it is possible that our EoE cohort contains false‐positive cases, particularly since EoE is a clinico‐pathologic disease and therefore requires symptom association for diagnosis; false‐negatives such as undetected EoE in reference individuals are also possible. However, since our inclusion is histology‐based, and our validation study had a substantial positive predictive value of 89% we believe our findings are valid. 20 Swedish national healthcare registers do not contain any data on smoking, which could be a confounding risk factor but was unable to be evaluated, nor do they contain primary care visits. We unfortunately did not have the power to investigate disease severity or complications for either disease, nor if medication effects (either protective or harmful) existed for either EoE or IBD diagnoses. We cannot rule out that medications may have confounded the relationship between EoE and IBD. Finally, we cannot rule out that part of the positive association is due to initial misclassification or surveillance bias. Patients with undiagnosed CD may have undergone upper endoscopy and been misclassified as EoE, and similarly, a patient with true EoE may have undergone endoscopy when mild CD was detected. While we attempted to control for EoE diagnostic errors by limiting our time period to 1990 and later, heterogeneity in diagnostic criteria and low awareness of EoE still existed in subsequent years and therefore could potentially impact the accuracy of case ascertainment.
In summary, our nationwide biopsy‐verified EoE cohort study revealed a 4‐fold increased risk of later IBD diagnosis, which is mostly attributable to CD. The association of these two diseases may be bidirectional and our findings provide further evidence to providers and patients that these diseases are disproportionately comorbid and should be considered in both populations. Among potential explanations are shared genetic or early environmental risk factors, but surveillance bias may also play a role in the associations between EoE and IBD.
AUTHOR CONTRIBUTIONS
Amiko M. Uchida wrote the first draft of the manuscript with contributions from Jonas F. Ludvigsson. All authors read, edited, and agreed with the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
Dr. Uchida is advisor/consultant for Sanofi‐Regeneron and AstraZeneca (unrelated to this study). Dr. Ludvigsson has coordinated an unrelated study on behalf of the Swedish IBD quality register (SWIBREG). That study received funding from Janssen corporation. Dr. Ludvigsson has also received financial support from MSD for developing a paper reviewing national healthcare registers in China. Dr. Ludvigsson is currently discussing potential research collaboration with Takeda.
Dr. Olén has been PI on projects at Karolinska Institutet financed by grants from Janssen, Takeda, AbbVie, Pfizer, and Ferring, and also report grants from Pfizer and Janssen in the context of national safety monitoring programs. None of those studies have any relation to the present study. Karolinska Institutet has also received fees for lectures and participation on advisory boards held by Dr. Olén from Janssen, Ferring, Takeda, Bristol Myer Squibb, Galapagos, and Pfizer regarding topics not related to the present study.
Dr. Halfvarson has served as speaker and/or advisory board member for AbbVie, BMS, Celgene, Celltrion, Ferring, Galapagos, Gilead, Hospira, Janssen, MEDA, Medivir, MSD, Novartis, Olink Proteomics, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, and UCB. He has also received grant support from Janssen, MSD, and Takeda.
ETHICS APPROVAL
This study was approved by the Stockholm Ethics Review Board (2014/1287‐31/4 and 2018/972‐32).
Supporting information
Tables S1–S6
ACKNOWLEDGMENTS
All authors approved the final version of the article, including the authorship list. AMU was supported by the Consortium of Eosinophilic GI Disease Researcher (CEGIR) Training Program. JFL was supported by Karolinska Institutet.
Uchida AM, Garber JJ, Pyne A, Peterson K, Roelstraete B, Olén O, et al. Eosinophilic esophagitis is associated with increased risk of later inflammatory bowel disease in a nationwide Swedish population cohort. United European Gastroenterol J. 2024;12(1):34–43. 10.1002/ueg2.12493
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12(4):589–596.e1. 10.1016/j.cgh.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta‐analysis. Allergy. 2014;69(1):62–75. 10.1111/all.12305 [DOI] [PubMed] [Google Scholar]
- 3. Koplin JJ, Mills EN, Allen KJ. Epidemiology of food allergy and food‐induced anaphylaxis: is there really a Western world epidemic? Curr Opin Allergy Clin Immunol. 2015;15(5):409–416. 10.1097/aci.0000000000000196 [DOI] [PubMed] [Google Scholar]
- 4. Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–e17. 10.1542/peds.2011-0204 [DOI] [PubMed] [Google Scholar]
- 5. Soller L, Ben‐Shoshan M, Harrington DW, Fragapane J, Joseph L, St. Pierre Y, et al. Overall prevalence of self‐reported food allergy in Canada. J Allergy Clin Immunol. 2012;130(4):986–988. 10.1016/j.jaci.2012.06.029 [DOI] [PubMed] [Google Scholar]
- 6. Dunlop JH, Keet CA. Epidemiology of food allergy. Immunol Allergy Clin North Am. 2018;38(1):13–25. 10.1016/j.iac.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 7. Garber JJ, Lochhead PJ, Uchida AM, Roelstraete B, Bergman D, Clements MS, et al. Increasing incidence of eosinophilic esophagitis in Sweden: a nationwide population study. Esophagus. 2022;19(4):535–541. 10.1007/s10388-022-00926-5 [DOI] [PubMed] [Google Scholar]
- 8. Dellon ES, Liacouras CA, Molina‐Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. 2018;155(4):1022–1033.e10. 10.1053/j.gastro.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147(3):602–609. 10.1053/j.gastro.2014.05.036 [DOI] [PubMed] [Google Scholar]
- 10. Wilson JM, Li RC, McGowan EC. The role of food allergy in eosinophilic esophagitis. J Asthma Allergy. 2020;13:679–688. 10.2147/jaa.s238565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spechler SJ. Speculation as to why the frequency of eosinophilic esophagitis is increasing. Curr Gastroenterol Rep. 2018;20(6):26. 10.1007/s11894-018-0633-x [DOI] [PubMed] [Google Scholar]
- 12. Uchida AM, Ro G, Garber JJ, Roelstraete B, Ludvigsson JF. Prior hospital‐based infection and risk of eosinophilic esophagitis in a Swedish nationwide case‐control study. United Eur Gastroenterol J. 2022;10(9):999–1007. 10.1002/ueg2.12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen ET, Kappelman MD, Kim HP, Ringel‐Kulka T, Dellon ES. Early life exposures as risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2013;57(1):67–71. 10.1097/mpg.0b013e318290d15a [DOI] [PubMed] [Google Scholar]
- 14. Axelrad JE, Olen O, Askling J, Lebwohl B, Khalili H, Sachs MC, et al. Gastrointestinal infection increases odds of inflammatory bowel disease in a nationwide case‐control study. Clin Gastroenterol Hepatol. 2019;17(7):1311–1322.e7. 10.1016/j.cgh.2018.09.034 [DOI] [PubMed] [Google Scholar]
- 15. Nguyen LH, Ortqvist AK, Cao Y, Simon TG, Roelstraete B, Song M, et al. Antibiotic use and the development of inflammatory bowel disease: a national case‐control study in Sweden. Lancet Gastroenterol Hepatol. 2020;5(11):986–995. 10.1016/s2468-1253(20)30267-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan YC, Steele D, Kochar B, Arsene D, Long M, Dellon E. Increased prevalence of esophageal eosinophilia in patients with inflammatory bowel disease. Inflamm Intest Dis. 2019;3(4):180–186. 10.1159/000497236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Limketkai BN, Shah SC, Hirano I, Bellaguarda E, Colombel JF. Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective population‐based analysis. Gut. 2019;68(12):2152–2160. 10.1136/gutjnl-2018-318074 [DOI] [PubMed] [Google Scholar]
- 18. Moore H, Wechsler J, Frost C, Whiteside E, Baldassano R, Markowitz J, et al. Comorbid diagnosis of eosinophilic esophagitis and inflammatory bowel disease in the pediatric population. J Pediatr Gastroenterol Nutr. 2021;72(3):398–403. 10.1097/mpg.0000000000003002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sonnenberg A, Turner KO, Genta RM. Comorbid occurrence of eosinophilic esophagitis and inflammatory bowel disease. Clin Gastroenterol Hepatol. 2021;19(3):613–615.e1. 10.1016/j.cgh.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 20. Rojler L, Glimberg I, Walker MM, Garber JJ, Ludvigsson JF. Validation of the diagnosis of eosinophilic esophagitis based on histopathology reports in Sweden. Ups J Med Sci. 2021;126(1). 10.48101/ujms.v127.7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brooke HL, Talback M, Hornblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773. 10.1007/s10654-017-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18(2):143–148. 10.1177/140349489001800209 [DOI] [PubMed] [Google Scholar]
- 24. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Publ Health. 2011;11(1):450. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaëlsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136. 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 26. Forss A, Clements M, Bergman D, Roelstraete B, Kaplan G, Myrelid P, et al. A nationwide cohort study of the incidence of inflammatory bowel disease in Sweden from 1990 to 2014. Aliment Pharmacol Ther. 2022;55(6):691–699. 10.1111/apt.16735 [DOI] [PubMed] [Google Scholar]
- 27. R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing V; 2021. https://www.R‐project.org [Google Scholar]
- 28. Therneau T. _A package for survival analysis in R_. R package version 3.2‐10 U. 2021. https://CRAN.R‐project.org/package=survival
- 29. Ludvigsson JF, Haberg SE, Knudsen GP, LaFolie P, Zoega H, Sarkkola C, et al. Ethical aspects of registry‐based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508. 10.2147/clep.s90589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN‐gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL‐5. J Immunol. 1996;157(3):1261–1270. 10.4049/jimmunol.157.3.1261 [DOI] [PubMed] [Google Scholar]
- 31. Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner‐Feigl S, et al. Nonclassical CD1d‐restricted NK T cells that produce IL‐13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113(10):1490–1497. 10.1172/jci19836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uchida AM, Lenehan PJ, Vimalathas P, Miller KC, Valencia‐Yang M, Qiang L, et al. Tissue eosinophils express the IL‐33 receptor ST2 and type 2 cytokines in patients with eosinophilic esophagitis. Allergy. 2022;77:656–660. 10.1111/all.15127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gonzalez Acera M, Patankar JV, Diemand L, Siegmund B, Neurath MF, Wirtz S, et al. Comparative transcriptomics of IBD patients indicates induction of type 2 immunity irrespective of the disease ideotype. Front Med. 2021;8:664045. 10.3389/fmed.2021.664045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marild K, Soderling J, Lebwohl B, Green PH, Pinto‐Sanchez MI, Halfvarson J, et al. Association of celiac disease and inflammatory bowel disease: a nationwide register‐based cohort study. Am J Gastroenterol. 2022;117(9):1471–1481. 10.14309/ajg.0000000000001834 [DOI] [PubMed] [Google Scholar]
- 35. Frisell T. Invited commentary: sibling‐comparison designs, are they worth the effort? Am J Epidemiol. 2021;190(5):738–741. 10.1093/aje/kwaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wettergren B, Blennow M, Hjern A, Söder O, Ludvigsson JF. Child health systems in Sweden. J Pediatr. 2016;177s:S187–S202. 10.1016/j.jpeds.2016.04.055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.


