Abstract
Background
Management of Helicobacter pylori (H. pylori) infection requires co‐treatment with proton pump inhibitors (PPIs) and the use of antibiotics to achieve successful eradication.
Aim
To evaluate the role of dosage of PPIs and the duration of therapy in the effectiveness of H. pylori eradication treatments based on the ‘European Registry on Helicobacter pylori management’ (Hp‐EuReg).
Methods
Hp‐EuReg is a multicentre, prospective, non‐interventionist, international registry on the routine clinical practice of H. pylori management by European gastroenterologists. All infected adult patients were systematically registered from 2013 to 2022.
Results
Overall, 36,579 patients from five countries with more than 1000 patients were analysed. Optimal (≥90%) first‐line‐modified intention‐to‐treat effectiveness was achieved with the following treatments: (1) 14‐day therapies with clarithromycin‐amoxicillin‐bismuth and metronidazole‐tetracycline‐bismuth, both independently of the PPI dose prescribed; (2) All 10‐day (except 10‐day standard triple therapy) and 14‐day therapies with high‐dose PPIs; and (3) 10‐day quadruple therapies with clarithromycin‐amoxicillin‐bismuth, metronidazole‐tetracycline‐bismuth, and clarithromycin‐amoxicillin‐metronidazole (sequential), all with standard‐dose PPIs. In first‐line treatment, optimal effectiveness was obtained with high‐dose PPIs in all 14‐day treatments, in 10‐ and 14‐day bismuth quadruple therapies and in 10‐day sequential with standard‐dose PPIs. Optimal second‐line effectiveness was achieved with (1) metronidazole‐tetracycline‐bismuth quadruple therapy for 14‐ and 10 days with standard and high‐dose PPIs, respectively; and (2) levofloxacin‐amoxicillin triple therapy for 14 days with high‐dose PPIs. None of the 7‐day therapies in both treatment lines achieved optimal effectiveness.
Conclusions
We recommend, in first‐line treatment, the use of high‐dose PPIs in 14‐day triple therapy and in 10‐or 14‐day quadruple concomitant therapy in first‐line treatment, while standard‐dose PPIs would be sufficient in 10‐day bismuth quadruple therapies. On the other hand, in second‐line treatment, high‐dose PPIs would be more beneficial in 14‐day triple therapy with levofloxacin and amoxicillin or in 10‐day bismuth quadruple therapy either as a three‐in‐one single capsule or in the traditional scheme.
Keywords: amoxicillin, bismuth, clarithromycin, Helicobacter pylori, levofloxacin, metronidazole, proton pump inhibitor, tetracycline, tinidazole, treatment

Key summary.
Summarise the established knowledge on this subject
Proton pump inhibitors (PPIs) are essential in the eradication treatment of Helicobacter pylori (H. pylori), enhancing the effectiveness of antibiotics.
There is currently no unanimity regarding the dose of PPIs for H. pylori eradication.
What are the significant and/or new findings of this study
None of the 7‐day therapies achieved optimal effectiveness.
In first‐line treatment ≥90% effectiveness is obtained with high‐dose PPIs in all 14‐day treatments, in 10‐ and 14‐day bismuth quadruple therapies and in 10‐day sequential with standard‐dose PPIs.
In second‐line treatment, the highest effectiveness is obtained with 14‐day triple therapy with levofloxacin and with bismuth quadruple therapy with high‐dose PPIs.
There is evidence of differences in the management of H. Pylori among countries in the European continent.
Bismuth quadruple therapy with PPIs, metronidazole or tinidazole, tetracycline, bismuth, henceforth referred to as PPI‐M/T + Tc + B, is used mainly in Spain and Italy, with only testimonial use in Russia, Lithuania and Slovenia.
INTRODUCTION
Helicobacter pylori (H. pylori) is a gram‐negative bacterium that affects billions of people worldwide. 1 It is the leading cause of gastritis, peptic ulcer disease and gastric cancer. 2 , 3 However, even after more than 30 years of experience, there is no unanimity on the treatment of H. pylori infection. 4 , 5 , 6
Proton pump inhibitors (PPIs) are essential in the eradication treatment of H. pylori, enhancing the effectiveness of antibiotics. This benefit, related to the increase in gastric pH, seems to be more relevant with double daily doses than with single doses, as shown with classic triple therapies containing amoxicillin and clarithromycin or metronidazole. 7 , 8 The influence of PPIs, on the other hand, is not well established with currently recommended quadruple treatments, either in first‐ or second‐ treatment lines. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22
The ‘European Registry on Helicobacter pylori management’ (Hp‐EuReg) brings together information on the real clinical practice in a majority of European countries, including thousands of patients. The Registry represents a good mapping overview of the current situation regarding H. pylori management, allowing not only continuous assessment of the integration of clinical recommendations agreed on medical consensus but also of the possible strategies for improvement. 23
The objective of the present study was to obtain information from the data of the Hp‐EuReg to optimise the dose of PPI in the most commonly used eradication treatments in the routine clinical practice. We analysed the role of PPI dosage in the effectiveness of eradication treatments, stratifying the analysis by prescription duration.
METHODS
This analysis focused on the ‘European Registry on H. pylori Management’ (Hp‐EuReg), an international (27 countries) multicentre (300 investigators) prospective non‐interventionist registry that started in 2013 and was promoted by the European Helicobacter and Microbiota Study Group (www.helicobacter.org).
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee. The study was classified by the Spanish Agency for Medicines and Health Products, and prospectively registered in ClinicalTrials.gov (NCT02328131), and was approved in 2012 by the Ethics Committee of the Hospital Universitario de La Princesa (Madrid, Spain), which acted as the reference Institutional Review Board.
Participants
Criteria for country selection, national coordinators, and gastroenterologist recruiting investigators are shown in the protocol publication. 21 Main criteria for the eligible investigators were that they had to be gastroenterologists managing patients infected with H. pylori and a valid confirmatory testing method had to be available in their centre.
Data were recorded in an Electronic Case Report Form (e‐CRF) using the collaborative research platform REDCap hosted at ‘Asociación Española de Gastroenterología’ (AEG; www.aegastro.es), a non‐profit Scientific and Medical Society focused on Gastroenterology research. 24 Data were anonymised. Written, informed consent was obtained from all patients included in the study.
Data management
After extracting the data and prior to the statistical analysis, the database was reviewed for inconsistencies and subsequent data cleaning. The data quality review process evaluated whether the study selection criteria had been met and whether data were correctly collected, ensuring that the study was conducted according to the highest scientific and ethical standards. Data discordances were resolved by querying the investigators and through group emailing.
Statistical analysis
Variables categorisation and definition
In order to compare the different dosage schedules prescribed with the different types of PPIs (omeprazole, lansoprazole, pantoprazole, rabeprazole, and esomeprazole) a priori collected from the Hp‐EuReg dataset, it was decided to calculate the different PPI dosages by standardising PPI potency, in terms of the duration of intragastric pH > 4/24 h (pH4‐time) to rank PPIs, where relative potency varied from 4.5 mg omeprazole equivalents (20 mg pantoprazole) to 72 mg omeprazole equivalents (40 mg rabeprazole), as reported by Graham et al. 16 and Kirchheiner et al. 17 As described by these authors, such standardisation allows the interchangeable use of PPIs based on relative potency, and so, following this method, the different PPIs schedules and types were grouped into three categories: low dose, if the potency of acid inhibition was between 4.5 and 27 mg omeprazole equivalents when given twice daily; standard dose, between 32 and 40 mg omeprazole equivalents when given twice daily; and high dose, between 54 and 128 mg omeprazole equivalents when given twice daily (Table S1).
Also, in order to synthetise the different treatment durations encompassed in the Hp‐EuReg, the therapy length was categorised into three levels, corresponding to the most frequent treatment durations: 7, 10 and 14 days.
Adequate compliance with treatment was defined as having taken at least 90% of the prescribed drugs.
The first‐line treatment was classified into six therapeutic groups, which were established according to the most frequently prescribed treatments: (1) Triple therapy with a PPI, clarithromycin, amoxicillin or metronidazole, henceforth reported as PPI‐C + A/M; (2) Quadruple sequential therapy with a PPI, clarithromycin, amoxicillin, metronidazole or tinidazole, henceforth reported as PPI‐C + A + M/T; (3) Quadruple concomitant therapy with a PPI, clarithromycin, amoxicillin, metronidazole or tinidazole, henceforth reported as PPI‐C + A + M/T; (4) Bismuth quadruple therapy with a PPI, metronidazole or tinidazole, tetracycline, bismuth, henceforth reported as PPI‐M/T + Tc + B, either as three‐in‐one single capsule or in the traditional scheme; (5) Quadruple therapy with a PPI, clarithromycin, amoxicillin and bismuth, henceforth reported as PPI‐C + A + B; finally, (6) the ‘other’ group contained the dual and hybrid therapies.
Likewise, the second‐line treatment was classified into five therapeutic groups, which were established according to the most frequently prescribed treatments: (1) Triple therapy with a PPI, levofloxacin and amoxicillin, henceforth reported as PPI‐L + A; (2) Quadruple sequential and concomitant unified therapies with a PPI, clarithromycin, amoxicillin and metronidazole or tinidazole, henceforth reported as PPI ‐C + A + M/T; (3) Bismuth quadruple therapy with a PPI, metronidazole or tinidazole, tetracycline, bismuth, henceforth reported as PPI‐M/T + Tc + B, either as three‐in‐one single capsule or in the traditional scheme; (4) Quadruple therapy with PPIs, levofloxacin, amoxicillin and bismuth, henceforth reported as PPI‐L + A + B; finally, (5) the ‘other’ group contained the dual and hybrid therapies.
Continuous variables are presented as the arithmetic mean and the respective standard deviation (SD). Qualitative variables were presented as percentages and absolute frequencies, and 95% confidence intervals (CI) were provided. The significance level was established at a p‐value p < 0.05.
Data analysis
Univariate sub‐analyses were performed according to the treatment duration (7, 10 and 14 days), PPI doses (low, standard, high) and line of treatment. Differences between groups were analysed using the Chi‐square test.
The analysis was also stratified geographically, by country, assessing the effect of the different PPI doses (low, standard or high) used in therapy. Only those countries with more than 1000 patients included, were analysed.
Additionally, the trends in prescriptions according to the PPI dosage were also studied, as well as the treatment effectiveness patterns.
The effectiveness analysis was performed in three sets of patients: (1) the intention‐to‐treat (ITT) analysis, including all patients who had been registered up to February 2022 to allow at least a 6‐month follow‐up, and where lost to follow‐up cases were considered treatment failures; (2) the per‐protocol (PP) analysis, including all cases that had completed follow‐up, that is those cases with a result (either success or failure) of the confirmatory test after the eradication treatment, and that had taken at least 90% of the treatment drugs (that is, those compliant with treatment); and (3) the modified ITT (mITT) analysis, including all cases that had completed follow up, regardless of compliance (that is, both compliant and non‐compliant patients were accounted).
In the current study, the mITT analysis was reported within the text of the manuscript as representing the closest effectiveness analysis to that obtained in real clinical practice. The remaining analyses (ITT and PP) were also reported within the summary tables.
The effectiveness of the most common first‐ and second‐line treatments was also analysed according to PPI doses and the different therapy durations, using the categories described above.
Multivariate analysis was performed using a logistic regression model by the stepwise likelihood method. The eradication rate in the mITT population was set as the dependent variable, and the independent variables were the following: gender (female [reference category] vs. male), treatment duration (7 [reference category] vs. 10 or 14 days), dose of PPI (low [reference category], vs. standard or high), compliance (yes: ≥90% of drug intake [reference category] vs. no: <90%), and country of origin (Spain [reference category], Russia, Italy, Slovenia and Lithuania), and the prescribed first‐ or second‐line eradication treatment separately, using the categories previously described above.
The odds ratio (OR) and 95% CI for eradication in each variable were calculated relative to a reference category; therefore, the OR was considered treatment success within each independent variable studied when associated to a higher modified intention‐to‐treat (mITT) rate, at a p‐value of <0.05.
RESULTS
Baseline characteristics
In total, 45,778 cases from 27 countries were registered, and a final dataset including those countries recruiting more than 1000 patients was analysed. The highest recruiters were Spain (18,513 cases, 50.6%), Russia (7476 cases, 20.4%), Italy (4652 cases, 12.7%), Slovenia (3726 cases, 10.2%) and Lithuania (2212 cases, 6.0%). In total, 36,579 patients were analysed encompassing these five countries, and representing 80% of the total cases registered in the Hp‐EuReg (Figure 1).
FIGURE 1.
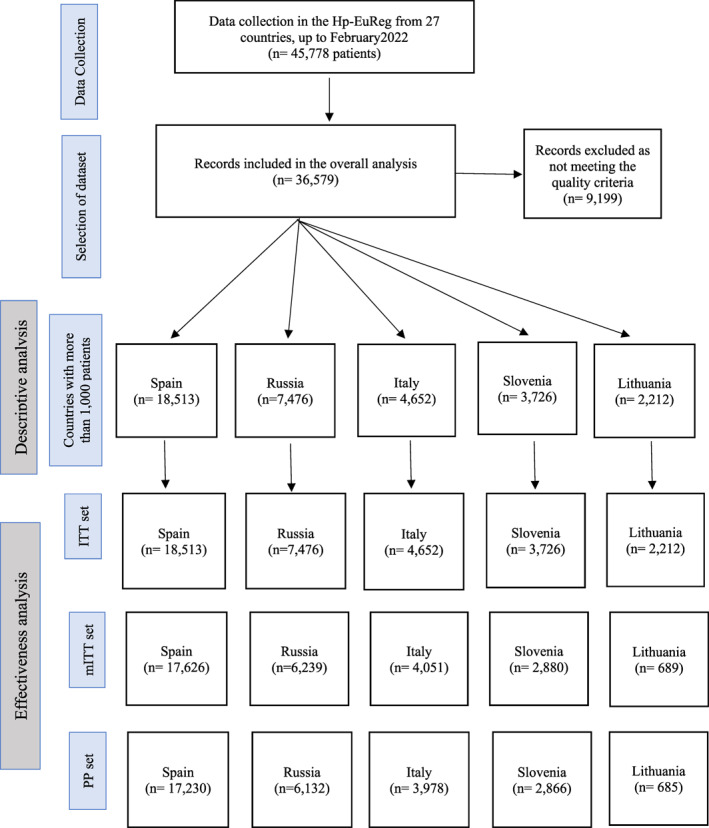
Study flow chart. ITT, intention to treat; mITT, modified intention to treat; PP, per protocol.
The mean (SD) age of patients was 50 (±15) years, 61% were female, 1.2% of cases were allergic to penicillin, and dyspepsia was the indication for eradication in 84% of cases. Adequate compliance with treatment was reported in 89% of patients.
Table 1 presents the overall demographic characteristics and the first‐ and second‐line treatment differences by geographic area. Regarding the duration of treatment, 91% of patients were prescribed with 10‐ or 14‐day regimens. In 26% and 35% of cases, standard‐ or high‐dose PPIs were used. Significant statistical differences (p < 0.0001) were observed in both the PPI dosage and the duration of treatment when comparing the data by country.
TABLE 1.
Baseline characteristics of Helicobacter pylori (H. pylori) infected patients by country.
| Variable | Overall | Spain | Russia | Italy | Slovenia | Lithuania |
|---|---|---|---|---|---|---|
| Number of patients | 36,579 | 18,513 | 7476 | 4652 | 3726 | 2212 |
| Female, N (%) | 22,626 (61.9) | 11,428 (61.8) | 4696 (62.9) | 2968 (63.8) | 2244 (60.2) | 1290 (58.3) |
| Age, mean (SD) | 49.9 (15.0) | 50.2 (14.8) | 46.6 (14.9) | 51.8 (14.9) | 52.7 (14.8) | 50.2 (15.5) |
| Penicillin allergy, N (%) | 1256 (3.4) | 873 (4.7) | 123 (1.2) | 68 (1.5) | 166 (4.5) | 26 (1.2) |
| Indication* | ||||||
| Dyspepsia | 30,802 (84.3) | 15,611 (84.4) | 5704 (76.4) | 4452 (96.0) | 3252 (87.4) | 1785 (80.7) |
| Ulcer disease | 5727 (15.7) | 2886 (15.6) | 1760 (23.6) | 187 (4.0) | 467 (12.6) | 427 (19.3) |
| Treatment length, N (%)* | ||||||
| 7 days | 3138 (8.8) | 201 (1.1) | 368 (5.1) | 101 (2.5) | 1717 (47.4) | 707 (32.5) |
| 10 days | 19,079 (53.5) | 11,347 (61.6) | 2893 (39.7) | 3684 (90.4) | 247 (6.7) | 908 (41.7) |
| 14 days | 13,456 (37.7) | 6880 (37.3) | 4021 (55.2) | 289 (7.1) | 1705 (45.9) | 501 (25.8) |
| PPI dose, N (%)* | ||||||
| Low | 13,941 (39.0) | 6968 (38.1) | 2479 (34.1) | 2202 (51.6) | 1481 (40.1) | 811 (37.1) |
| Standard | 9221 (25.8) | 4381 (23.9) | 3996 (55.0) | 85 (2.0) | 91 (2.5) | 668 (30.5) |
| High | 12,562 (35.2) | 6957 (38.0) | 796 (10.9) | 1977 (46.4) | 2123 (57.5) | 709 (32.4) |
| PPIs used* | ||||||
| Omeprazole | 12, 976 (36.1) | 9889 (53.8) | 1787 (24.4) | 353 (8.2) | 77 (2.1) | 871 (39.5) |
| Lansoprazole | 821 (2.3) | 170 (0.9) | 90 (1.2) | 73 (1.7) | 475 (12.8) | 13 (0.6) |
| Pantoprazole | 5195 (14.4) | 1016 (5.5) | 1163 (15.8 | 1830 (42.3) | 951 (25.6) | 235 (10.7) |
| Esomeprazole | 13,880 (38.6) | 7172 (39.0) | 1663 (22.7) | 2012 (46.5) | 2207 (59.4) | 826 (37.5) |
| Rabeprazole | 3012 (8.3) | 101 (0.6) | 2608 (35.5) | 56 (1.3) | 1 (0.01) | 246 (11.2) |
| Other coadjuvant | 42 (0.11) | 9 (0.01) | 24 (0.3) | 2 (0.01) | 2 (0.01) | 5 (0.4) |
| Compliance, N (%)* | ||||||
| No (<90% drug intake) | 1185 (3.2) | 535 (2.9) | 195 (2.6) | 353 (7.6) | 90 (2.4) | 12 (0.5) |
| Yes (≥90% drug intake) | 32,401 (88.6) | 17,451 (94.3) | 7221 (96.6) | 4078 (87.7) | 2953 (79.3) | 698 (31.6) |
| Unknown | 2993 (8.2) | 527 (2.8) | 60 (0.8) | 221 (4.8) | 683 (18.3) | 1502 (67.9) |
| Most frequent first‐line treatments, N (%)* | ||||||
| PPI‐C + A/M | 10,402 (35.5) | 3277 (25.6) | 2473 (39.7) | 145 (4.9) | 2945 (96.8) | 1562 (92.2) |
| SEQ PPI‐C + A + M/T | 1971 (6.7) | 234 (1.8) | 22 (0.4) | 1684 (57.4) | 29 (1.0) | 2 (0.1) |
| CONC PPI‐C + A + M/T | 5965 (20.4) | 4951 (37.1) | 92 (1.5) | 301 (10.3) | 19 (0.6) | 1 (0.1) |
| PPI‐M/T + Tc + B | 5364 (18.3) | 3263 (25.5) | 28 (0.5) | 650 (22.2) | 0 (0.0) | 1 (0.1) |
| PPI‐C + A + B | 3027 (10.3) | 1163 (8.7) | 1800 (28.9) | 0 (0.0) | 0 (0.0) | 64 (3.7) |
| Other | 2537 (8.7) | 455 (3.4) | 1815 (29.1) | 154 (5.2) | 49 (1.6) | 64 (3.7) |
| Most frequent second‐line treatments, N (%)* | ||||||
| PPI‐L + A | 1580 (33.4) | 983 (31.8) | 51 (10.5) | 202 (36.3) | 229 (66.8) | 115 (44.6) |
| PPI‐C + A + M/T | 354 (7.5) | 240 (7.7) | 4 (0.8) | 101 (18.2) | 6 (1.7) | 3 (1.2) |
| PPI‐M/T + Tc + B | 1179 (24.9) | 832 (27.0) | 125 (25.6) | 199 (35.8) | 23 (6.7) | 0 (0.0) |
| PPI‐L + A + B | 657 (13.9) | 539 (17.4) | 43 (8.8) | 0 (4.0) | 10 (2.9) | 65 (25.2) |
| Others | 966 (20.4) | 497 (16.1) | 265 (54.3) | 54 (9.7) | 75 (21.9) | 75 (29.1) |
| First‐line of treatment with doses of PPI, N (%)* | ||||||
| Low | 11,450 (34.1) | 5533 (39.9) | 2236 (34.3) | 1586 (51.2) | 1365 (42.0) | 730 (39.5) |
| Standard | 7491 (22.3) | 3452 (24.9) | 3361 (55.3) | 43 (1.4) | 82 (2.5) | 553 (30.0) |
| High | 9413 (28.0) | 4899 (35.3) | 679 (10.4) | 1466 (47.4) | 1806 (55.5) | 563 (30.5) |
| Second‐line of treatment with doses of PPI, N (%)* | ||||||
| Low | 861 (2.6) | 1057 (33.1) | 226 (32.8) | 358 (50.4) | 98 (27.8) | 74 (25.3) |
| Standard | 1136 (3.4) | 657 (20.6) | 353 (51.2) | 24 (3.4) | 5 (1.4) | 97 (33.2) |
| High | 2292 (6.8) | 1483 (46.4) | 110 (16.0) | 329 (46.3) | 249 (70.7) | 121 (41.4) |
Note: Low dose PPI: 4.5–27 mg omeprazole equivalents, two times per day (i.e., 20 mg omeprazole equivalents, two times per day), standard dose PPI: 32–40 mg omeprazole equivalents, two times per day (i.e., 40 mg omeprazole equivalents, two times per day), high dose PPI: 54–128 mg omeprazole equivalents, two times per day (i.e., 80 mg omeprazole equivalents, two times per day). The treatments labelled as ‘other’ correspond to dual and hybrid therapy.
Abbreviations: A, amoxicillin; B, bismuth salts; C, clarithromycin; Conc, concomitant; L, levofloxacin; M, metronidazole; N, sample size; PPI, proton pump inhibitor; PPI‐M/T + Tc + B, prescribed either in the classical form or as three‐in‐one single capsule, marketed as Pylera®; Seq, sequential; T, tinidazole; Tc, tetracycline hydrochloride.
*Significant differences were observed in all the study variables with a p‐value of ≤0.0001.
Spain and Lithuania were the countries with the most homogeneous distribution of PPI doses. Low‐, standard‐, or high‐dose PPIs were used in 38%, 24% and 38% of cases in Spain, and in 37%, 31% and 32% of cases in Lithuania. In Russia, 89% of cases used low‐ or standard‐doses PPIs. In Italy, 98% of cases used low‐ or high‐dose PPIs, and in Slovenia, 98% of cases also used low‐ or high‐dose PPIs.
In first‐line treatments, low‐, standard‐, or high‐dose PPIs were used in 25%, 15% and 60% of cases in the 14‐day quadruple concomitant therapy, and in 58%, 23% and 19% of cases in the 10‐day quadruple concomitant therapy. Low‐, standard‐, or high‐dose PPIs were used in 42%, 23% and 35% of cases in the 10‐day bismuth quadruple therapy (PPI‐M/T + Tc + B), either as a three‐in‐one single capsule or in the traditional scheme (Table 2).
TABLE 2.
Effectiveness of first‐line treatment according to the proton pump inhibitor dose and therapy length.
| mITT | PP | |||||||
|---|---|---|---|---|---|---|---|---|
| Duration of treatment | Low | Standard | High | Low | Standard | High | ||
| 7 days (10.3%**) | PPIC‐C + A/M | Success (n) | 1273 | 112 | 295 | 1270 | 112 | 295 |
| Overall (N) | 1549 | 143 | 338 | 1542 | 142 | 337 | ||
| Eradication rate % | 82.2 | 78.3 | 87.3 | 82.4 | 78.8 | 87.5 | ||
| p‐value/effect size | 0.028/0.059* | 0.029/0.059* | ||||||
| CONC PPI‐C + A + M/T | Success (n) | 6 | 14 | 1 | 6 | 13 | 1 | |
| Overall (N) | 7 | 16 | 1 | 7 | 14 | 1 | ||
| Eradication rate % | 85.7 | 87.5 | 100 | 85.7 | 92.9 | 100 | ||
| p‐value/effect size | 0.922/0.082 | 0.822/0.134 | ||||||
| PPI‐C + A + B | Success (n) | 9 | 6 | ‐‐‐ | 9 | 6 | ‐‐‐ | |
| Overall (N) | 13 | 7 | ‐‐‐ | 13 | 6 | ‐‐‐ | ||
| Eradication rate % | 69.2 | 85.7 | ‐‐‐ | 69.2 | 100 | ‐‐‐ | ||
| p‐value/effect size | 0.417/0.182 | 0.126/0.351 | ||||||
| PPI‐M/T + Tc + B | Success (n) | 4 | 2 | ‐‐‐ | 4 | 2 | ‐‐‐ | |
| Overall (N) | 6 | 2 | ‐‐‐ | 5 | 2 | ‐‐‐ | ||
| Eradication rate % | 66.7 | 100 | ‐‐‐ | 80.0 | 100 | ‐‐‐ | ||
| p‐value/effect size | 0.346/0.333 | 0.495/0.258 | ||||||
| 10 days (51.5%**) | PPI‐C + A/M | Success (n) | 1277 | 1088 | 417 | 1274 | 1077 | 411 |
| Overall (N) | 1634 | 1261 | 466 | 1613 | 1245 | 457 | ||
| Eradication rate % | 78.2 | 86.3 | 89.5 | 79.0 | 86.5 | 89.9 | ||
| p‐value/effect size | ≤0.0001/0.122* | ≤0.0001/0.117* | ||||||
| SEQ PPI‐C + A + M/T | Success (n) | 981 | 48 | 603 | 962 | 48 | 598 | |
| Overall (N) | 1112 | 51 | 647 | 1081 | 51 | 635 | ||
| Eradication rate % | 88.2 | 94.1 | 93.2 | 89.0 | 94.1 | 94.2 | ||
| p‐value/effect size | 0.002/0.083* | ≤0.001/0.088* | ||||||
| CONC PPI‐C + A + M/T | Success (n) | 1262 | 507 | 440 | 1230 | 502 | 434 | |
| Overall (N) | 1437 | 580 | 476 | 1390 | 569 | 466 | ||
| Eradication rate % | 87.8 | 87.4 | 92.4 | 88.5 | 88.2 | 93.1 | ||
| p‐value/effect size | 0.013/0.059* | 0.012/0.060* | ||||||
| PPI‐C + A + B | Success (n) | 200 | 213 | 93 | 200 | 211 | 93 | |
| Overall (N) | 233 | 235 | 101 | 229 | 233 | 100 | ||
| Eradication rate % | 85.8 | 90.6 | 93.0 | 87.3 | 90.6 | 93.0 | ||
| p‐value/effect size | 0.137/0.084 | 0.254/0.070 | ||||||
| PPI‐M/T + Tc + B | Success (n) | 1533 | 865 | 1300 | 1511 | 857 | 1288 | |
| Overall (N) | 1683 | 917 | 1371 | 1645 | 904 | 1346 | ||
| Eradication rate % | 91.1 | 94.3 | 94.8 | 91.9 | 94.8 | 95.7 | ||
| p‐value/effect size | ≤0.0001/0.269* | ≤0.0001/0.273* | ||||||
| 14 days (36.8%**) | PPI‐C + A/M | Success (n) | 426 | 758 | 1273 | 349 | 392 | 99 |
| Overall (N) | 528 | 850 | 1391 | 442 | 421 | 108 | ||
| Eradication rate % | 80.7 | 89.2 | 91.5 | 78.9 | 93.1 | 91.7 | ||
| p‐value/effect size | ≤0.0001/0.128* | ≤0.0001/0.133* | ||||||
| CONC PPI‐C + A + M/T | Success (n) | 597 | 385 | 1573 | 581 | 381 | 1544 | |
| Overall (N) | 702 | 429 | 1685 | 679 | 422 | 1648 | ||
| Eradication rate % | 85.0 | 89.7 | 93.4 | 85.6 | 90.3 | 93.7 | ||
| p‐value/effect size | ≤0.0001/0.121* | ≤0.0001/0.120* | ||||||
| PPI‐C + A + B | Success (n) | 165 | 797 | 969 | 163 | 791 | 958 | |
| Overall (N) | 180 | 859 | 1067 | 178 | 847 | 1053 | ||
| Eradication rate % | 91.6 | 92.8 | 90.8 | 91.6 | 93.4 | 90.1 | ||
| p‐value/effect size | 0.299/0.034 | 0.153/0.043 | ||||||
| PPI‐M/T + Tc + B | Success (n) | 18 | 55 | 11 | 18 | 55 | 11 | |
| Overall (N) | 20 | 59 | 12 | 20 | 59 | 11 | ||
| Eradication rate % | 90.0 | 93.2 | 91.6 | 90.0 | 93.2 | 100 | ||
| p‐value/effect size | 0.893/0.050 | 0.564/0.113 | ||||||
Note: Low dose PPI: 4.5–27 mg omeprazole equivalents, two times per day (i.e., 20 mg omeprazole equivalents, two times per day), standard dose PPI: 32–40 mg omeprazole equivalents, two times per day (i.e., 40 mg omeprazole equivalents, two times per day), high dose PPI: 54–128 mg omeprazole equivalents, two times per day (i.e., 80 mg omeprazole equivalents, two times per day).
Abbreviations: A, amoxicillin; B, bismuth salts; C, clarithromycin; Conc, concomitant; M, metronidazole; PPI‐M/T + Tc + B, prescribed either in the classical form or as three‐in‐one single capsule, marketed as Pylera®; Seq, sequential; T, tinidazole; Tc, tetracycline hydrochloride; V, cramer's v (effect size).
*The p‐value of those statistically significant (≤0.0001) comparisons is marked in bold.
**Percentage of total first‐line prescriptions by duration of treatment.
In second‐line treatments, low‐, standard‐, or high‐dose PPIs were used in 39%, 21% and 39% of cases in the 10‐ and 14 day triple therapy (PPI‐L + A) and in 45%, 18% and 37% of cases in the 10‐day bismuth quadruple therapy (PPI‐M/T + Tc + B), either as three‐in‐one single capsule or in the traditional scheme (Table 3).
TABLE 3.
Effectiveness of second‐line treatment according to the proton pump inhibitor dose and therapy length.
| mITT | PP | |||||||
|---|---|---|---|---|---|---|---|---|
| Duration of treatment | Low | Standard | High | Low | Standard | High | ||
| 7 days (2.5%**) | PPI‐A + L | Success (n) | 8 | 1 | 4 | 8 | 1 | 4 |
| Overall (N) | 9 | 2 | 6 | 9 | 2 | 6 | ||
| Eradication rate % | 88.9 | 50.0 | 66.7 | 88.9 | 50.0 | 66.7 | ||
| p‐value/effect size | 0.392/0.332 | 0.392/0.332 | ||||||
| PPI‐M/T + Tc + B | Success (n) | 3 | 0 | 0 | 3 | 0 | 0 | |
| Overall (N) | 4 | 1 | 1 | 4 | 1 | 1 | ||
| Eradication rate % | 75.0 | 0.0 | 0.0 | 75.0 | 0.0 | 0.0 | ||
| p‐value/effect size | 0.233/0.707 | 0.233/0.707 | ||||||
| 10 days (54.9%**) | PPI‐A + L | v | 366 | 199 | 178 | 363 | 197 | 177 |
| Overall (N) | 497 | 256 | 213 | 491 | 250 | 212 | ||
| Eradication rate % | 84.4 | 77.7 | 83.6 | 73.9 | 78.8 | 83.5 | ||
| p‐value/effect size | 0.015/0.093* | 0.017/0.092* | ||||||
| PPI‐C + A + M/T | Success (n) | 58 | 15 | 54 | 57 | 15 | 53 | |
| Overall (N) | 79 | 18 | 69 | 78 | 18 | 68 | ||
| Eradication rate % | 73.4 | 83.3 | 78.3 | 73.1 | 83.3 | 77.9 | ||
| p‐value/effect size | 0.605/0.078 | 0.586/0.080 | ||||||
| PPI‐L + A + B | Success (n) | 12 | 7 | 3 | 12 | 7 | 3 | |
| Overall (N) | 14 | 9 | 3 | 14 | 9 | 3 | ||
| Eradication rate % | 85.7 | 77.8 | 100 | 85.7 | 77.8 | 100 | ||
| p‐value/effect size | 0.643/0.184 | 0.643/0.184 | ||||||
| PPI‐M/T + Tc + B | Success (n) | 404 | 169 | 357 | 398 | 166 | 352 | |
| Overall (N) | 467 | 191 | 387 | 453 | 187 | 382 | ||
| Eradication rate % | 86.5 | 88.5 | 92.2 | 87.9 | 88.8 | 92.1 | ||
| p‐value/effect size | 0.028/0.083* | 0.118/0.065 | ||||||
| 14 days (38.1%**) | PPI‐A + L | Success (n) | 45 | 35 | 318 | 45 | 35 | 316 |
| Overall (N) | 57 | 43 | 341 | 55 | 43 | 338 | ||
| Eradication rate % | 78.9 | 81.4 | 93.3 | 81.8 | 81.4 | 93.5 | ||
| p‐value/effect size | ≤0.0001/0.188* | 0.002/0.172* | ||||||
| PPI‐C + A + M/T | Success (n) | 36 | 46 | 73 | 35 | 45 | 71 | |
| Overall (N) | 54 | 51 | 83 | 52 | 49 | 81 | ||
| Eradication rate % | 66.7 | 90.2 | 88.0 | 67.3 | 91.8 | 87.7 | ||
| p‐value/effect size | ≤0.0001/0.264* | ≤0.0001/0.267* | ||||||
| PPI‐L + A + B | Success (n) | 36 | 43 | 416 | 36 | 42 | 404 | |
| Overall (N) | 50 | 49 | 462 | 50 | 48 | 446 | ||
| Eradication rate % | 72.0 | 87.8 | 82.4 | 72.0 | 87.5 | 90.6 | ||
| p‐value/effect size | ≤0.0001/0.159* | ≤0.0001/0.168* | ||||||
| PPI‐M/T + Tc + B | Success (n) | 14 | 43 | 33 | 13 | 41 | 33 | |
| Overall (N) | 19 | 45 | 34 | 18 | 43 | 34 | ||
| Eradication rate % | 73.7 | 95.6 | 97.1 | 72.2 | 95.3 | 97.1 | ||
| p‐value/effect size | 0.005/0.326* | 0.004/0.338* | ||||||
Note: Low dose PPI: 4.5–27 mg omeprazole equivalents, two times per day (i.e., 20 mg omeprazole equivalents, two times per day), standard dose PPI: 32–40 mg omeprazole equivalents, two times per day (i.e., 40 mg omeprazole equivalents, two times per day), high dose PPI: 54–128 mg omeprazole equivalents, two times per day (i.e., 80 mg omeprazole equivalents, two times per day).
Abbreviations: A, amoxicillin; B, bismuth salts; C, clarithromycin; Conc, concomitant; M, metronidazole; PPI‐M/T + Tc + B, prescribed either in the classical form or as three‐in‐one single capsule, marketed as Pylera®; Seq, sequential; T, tinidazole; Tc, tetracycline hydrochloride; V, cramer's v (effect size).
*The p‐value of those statistically significant (≤0.0001) comparisons is marked in bold.
**Percentage of total second‐line prescriptions by duration of treatment.
Treatment prescriptions
As for the first‐line treatments triple therapy PPI‐C + A/M was the most commonly used (36%), followed by quadruple concomitant therapy PPI‐C + A + M/T (20%) and bismuth quadruple therapy PPI‐M/T + Tc + B, either as a three‐in‐one single capsule or in the traditional scheme (18%). In the 7‐day treatments, PPI doses were low in 77%, standard in 8%, and high in 15%. In the 10‐day treatments, PPI doses were low in 50%, standard in 25%, and high in 25%. In the 14‐day treatments, PPI doses were low in 18%, standard in 28%, and high in 53% of cases (Table 2).
In second‐line treatments, triple therapy PPI‐L + A was the most used therapy (33%) followed by bismuth quadruple therapy PPI + M/T + Tc + B, either as a three‐in‐one‐single capsule or in the traditional scheme (25%) or dual and hybrid therapies (20%). In the 7‐day treatments, PPI doses were low in 57%, standard in 13%, and high in 30%. In the 10‐day treatments, PPI doses were low in 48%, standard in 22%, and high in 30%. In the 14‐day treatments, PPI doses were low in 14%, standard in 14%, and high in 72% of cases (Table 3).
Effectiveness in first‐line treatment
Optimal first‐line effectiveness (∼90%) was achieved with the following treatments: Quadruple therapy PPI‐C + A + B and bismuth quadruple therapy PPI‐M/T + Tc + B for 14 days, irrespective of the PPI dose used; in all 10‐ or 14‐day treatments combined with high‐dose PPI (except with standard 10‐day triple therapy PPI‐C + A + M/T); in 10‐day quadruple sequential therapy, PPI‐C + A + M/T, quadruple PPI‐C + A + B and bismuth quadruple therapy PPI‐M/T + Tc + B, either as three‐in‐one‐single capsule or in the traditional scheme, with standard‐dose PPI. None of the 7‐day therapies achieved optimal cure rates (>90%) regardless of the PPI dose used (Table 2).
In 7‐day regimens, no significant differences with PPI doses were observed, except with triple therapy PPI‐C + A/M showing greater effectiveness (87%) when combined with high‐dose PPI. An increase in the eradication rate with high‐dose PPI and 10‐day treatments was observed in triple therapy PPI‐C + A/M (89%) and quadruple concomitant therapy C + A + M/T (92%). In PPI‐C + A + B and PPI‐M/T + Tc + B quadruple therapies, no advantage was shown with the use of higher acid inhibition. Similarly, with 14‐day regimens, an improvement in effectiveness was observed with high‐dose PPI in all treatments except with PPI‐C + A + B and PPI‐M/T + Tc + B quadruple therapies.
In summary, the PPI dose was significantly associated with an increase in effectiveness in 7‐day triple therapy with PPI‐C + A/M (87%), in 10‐ and 14‐day quadruple concomitant therapy, PPI‐C + A + M/T (92% and 93%, respectively), in 10‐day quadruple sequential therapy, PPI‐C + A + M/T (94%) and in 10‐day bismuth quadruple therapy, PPI‐M/T + Tc + B, either as three‐in‐one‐single capsule or in the traditional scheme (95%) (Table 2).
First‐line effectiveness by geographic area
In Spain, a higher eradication rate was observed in 14‐day regimens such as triple therapy, PPI‐C + A/M, prescribed with high‐dose PPI (91%), in quadruple therapy, C + A + B, with either standard‐ (94%) or high‐dose PPI (91%) and in quadruple concomitant therapy, PPI‐C + A + M/T (93%), with high‐dose PPI. Those treatments lasting 10‐day provided high cure rates when the quadruple concomitant therapy, PPI‐C + A + M/T, was prescribed with high‐dose PPI (92%), in quadruple sequential therapy with standard‐dose PPI (92%) and in bismuth quadruple therapy, PPI‐M/T + Tc + B, either as three‐in‐one single capsule or in the traditional scheme, with standard‐ (94%) or high‐dose PPI (95%).
In Russia, a higher eradication rate was observed in 14 ‐day treatments with high‐dose PPI in triple therapy, PPI‐C + A/M (98%), in bismuth quadruple therapy, PPI‐M/T + Tc + B, with standard‐dose PPI,PPI‐M/T + Tc + B (93%), and in quadruple therapy, PPI‐C + A + B, with either low‐ (92%), standard‐ (92%) and high‐dose PPI (91%). In 10‐day treatments was observed in triple therapy with high‐dose PPI, C + A/M (91%), in quadruple therapy, C + A + B, with standard‐ (91%) and high‐dose (94%) PPI, and in quadruple therapy with bismuth, PPI‐M/T + Tc + B, either as three‐in‐one single capsule or in the traditional scheme, with low‐ (93%) or standard‐dose (100%) PPI.
In Italy, a higher eradication rate was observed in 14‐day treatment with high‐dose PPI in quadruple concomitant therapy, PPI‐C + A + M/T (96%), and in 10‐day treatments with standard‐dose PPI (100%) in quadruple sequential therapy PPI‐,C + A + M/T, and with high‐dose PPI (94%) in bismuth quadruple therapy, PPI‐M/T + Tc + B, either as three‐in‐one single capsule or in the traditional scheme, with low‐ (96%) or high‐dose (95%) PPI.
In Slovenia, a higher eradication rate was observed in 14‐day treatment with low‐ (100%) and high‐dose (91%) PPI in triple therapy, PPI‐C + A/M, and in 10‐day treatment with standard‐dose PPI in quadruple sequential therapy,PPI‐C + A + M/T (100%), and in 7‐day treatment with standard‐dose PPI in quadruple concomitant therapy,PPI‐C + A + M/T (100%).
In Lithuania, a higher eradication rate was observed in triple therapy,PPI‐C + A + M, with standard‐dose PPI in 7‐ (91%) and 10‐day (96%) treatments, and with low‐ (93%), standard‐ (100%) and high‐dose PPI (92%) in 14‐day treatment.
Details about the effectiveness of first‐line treatment in relation to the PPI dose and the treatment duration by geographic area are reported in Supplementary Material S1 (Table S4).
Effectiveness in second‐line treatment
Optimal second‐line effectiveness (∼90%) was achieved with the following treatments: Bismuth quadruple therapy, PPI‐M/T + Tc + B for 10 days, either as three‐in‐one single capsule or in the traditional scheme or only when combined with high‐dose PPI (92%); in 14‐day treatments with triple therapy PPI‐L + A with high‐dose PPI (93%) and with bismuth quadruple therapy, PPI‐M/T/+Tc + B with standard‐ (96%) and high‐dose PPI (97%), either as three‐in‐one single capsule or in the traditional scheme. None of the 7‐day therapies achieved optimal cure rates regardless of the dose of PPI used.
No significant differences were observed in any of the 7‐day treatments' schemes effectiveness regardless of the PPI dose prescribed. In 10‐ and 14‐day regimens, a higher eradication rate was observed with high‐dose PPI in the triple therapy, L + A (84% and 93%) and in the bismuth quadruple therapy, PPI‐M/T + Tc + B (92% and 97%), either as a three‐in‐one single capsule or in the traditional scheme (Table 3).
Second‐line effectiveness by geographic area
In Spain, a higher eradication rate was observed during 14‐day treatment in triple therapy, PPI‐L + A, and high‐dose PPI (97%) and in quadruple therapies with standard‐and high‐dose PPI:PPI‐C + A + M/T (90% and 88%),PPI‐L + A + B (88% and 90%) and PPI‐M/T + Tc + B (100%). The highest eradication rate during 10‐day treatments was observed with bismuth quadruple therapy,PPI‐M/T + Tc + B (91%) either as a three‐in‐one single capsule or in the traditional scheme, or standard‐ or high‐dose PPI.
In Russia, the eradication rate was higher in triple therapy, PPI‐L + A (93%) and quadruple therapy, PPI‐L + A + B (100%) during 10‐day‐treatment and high‐dose PPI. The highest eradication rate during 14‐day treatments was observed in triple therapy, PPI‐L + A (100%), irrespective of the PPI dose used, and in bismuth quadruple therapy, PPI‐M/T + Tc + B, and standard‐ (95%) or high‐dose PPIs (94%), either as three‐in‐one single capsule or in the traditional scheme.
In Italy, a high eradication rate was observed in triple therapy, L + A, during 10‐day treatment with standard‐dose PPI (90%) and with 10‐day bismuth quadruple therapy, PPI‐M/T + Tc + B, combined with low‐ (93%) or high‐dose (95%) PPIs, either as a three‐in‐one single capsule or in the traditional scheme.
In Slovenia, optimal eradication rates were observed with high‐dose PPI in triple therapy, PPI‐L + A, during 10‐ and 14‐day treatments (100% and 89%), and in bismuth quadruple therapy, PPI‐M/T + TC + B, either as a three‐in‐one single capsule or in the traditional scheme, during 10‐day treatment with high‐dose PPI (100%).
In Lithuania, a higher eradication rate was observed with triple therapy,PPI‐L + A, and low‐ (100%) and standard‐ dose (93%) PPIs and with quadruple therapy, PPI‐L + A + B, irrespective of the PPI dose used (100%).
Details on the effectiveness of second‐line treatment in relation to the PPI dose and the treatment duration by geographic area are reported in Supplementary Material S1 (Table S5).
Multivariate analysis
Final model
Results of the multivariate logistic regression analysis showed that in first‐line treatment, the use of bismuth quadruple therapy, PPI‐M/T + Tc + B, and quadruple sequential therapy, PPI‐C + A + M/T (OR 2.06; 95% CI 1.82–2.32; and OR 1.71; 95% CI: 1.45–2.02), the use of standard‐ (OR 1.66; 95% CI 1.51–1.82) or high‐dose PPI (OR 1.71; 95% CI 1.63–1.95), and adequate compliance (OR 6.20; 95% CI 5.20–7.40) were reported as significant independent predictors of eradication success. The model had an adequate goodness‐of‐fit (Hosmer‐Lemeshow test: 0.203) and a Nagelkerke's R2 of 6.5% (Table 4).
TABLE 4.
Multivariate logistic regression: Final model.
| Independent variables | Odds ratio | 95% confidence interval | p‐value | |
|---|---|---|---|---|
| Lower | Upper | |||
| First line (ref. PPI‐C + A/M) | ||||
| PPI‐C + A/M | 1 | <0.001 | ||
| SEQ PPI‐C + A + M/T | 1.718 | 1.456 | 2.027 | <0.001 |
| CONC PPI‐C + A + M/T | 1.408 | 1.261 | 1.572 | <0.001 |
| PPI‐M/T + TC + B | 2.063 | 1.829 | 2.327 | <0.001 |
| PPI‐C + A + B | 1.395 | 1.202 | 1.620 | <0.001 |
| Others | 0.804 | 0.729 | 0.887 | <0.001 |
| Sex (ref. female) | ||||
| Male | 1.177 | 1.095 | 1.266 | <0.001 |
| PPI dose (ref. low dose PPI) | ||||
| Low | 1 | <0.001 | ||
| Standard | 1.663 | 1.517 | 1.821 | <0.001 |
| High | 1.791 | 1.639 | 1.958 | <0.001 |
| Length (ref. 7 days) | ||||
| 7 days | 1 | <0.001 | ||
| 10 days | 1.047 | 0.920 | 1.192 | 0.033 |
| 14 days | 1.286 | 1.119 | 1.479 | <0.001 |
| Compliance (ref. no <90% drug intake) | ||||
| Yes (≥90% drug intake) | 6.204 | 5.198 | 7.405 | <0.001 |
| Second‐line (ref. PPI‐L + A) | ||||
| PPI‐L + A | 1 | <0.001 | ||
| PPI‐C + A + M/T | 2.202 | 1.824 | 2.660 | <0.001 |
| PPI‐M/T + TC + B | 2.663 | 2.289 | 3.099 | <0.001 |
| Others | 1.440 | 1.266 | 1.639 | <0.001 |
| Sex (ref. female) | ||||
| Male | 1.179 | 1.096 | 1.267 | <0.001 |
| PPI dose (low dose PPI) | ||||
| Low | 1 | <0.001 | ||
| Standard | 1.597 | 1.459 | 1.748 | <0.001 |
| High | 1.822 | 1.667 | 1.992 | <0.001 |
| Length (ref. 7 days) | ||||
| 7 days | 1 | <0.001 | ||
| 10 days | 1.174 | 1.038 | 1.328 | <0.001 |
| 14 days | 1.462 | 1.284 | 1.665 | <0.001 |
| Adherence (ref. no <90% drug intake) | ||||
| Yes (≥90% drug intake) | 6.021 | 5.048 | 7.181 | <0.001 |
Note: Dependent variable: Effectiveness (by modified intention‐to‐treat) Low dose PPI: 4.5–27 mg omeprazole equivalents, two times per day (i.e., 20 mg omeprazole equivalents, two times per day); Standard dose PPI: 32–40 mg omeprazole equivalents, two times per day (i.e., 40 mg omeprazole equivalents, two times per day); High dose PPI: 54–128 mg omeprazole equivalents, two times per day (i.e., 80 mg omeprazole equivalents, two times per day). The treatments called ‘other’ correspond to those dual and hybrid therapies.
Abbreviations: A, amoxicillin; B, bismuth salts; C, clarithromycin; Conc, concomitant; M, metronidazole; mITT, modified intention to treat; N, total sample; n, successful treatment sample; PP, per protocol; PPI, proton pump inhibitor; PPI‐M/T + Tc + B, prescribed either in the classical form or as three‐in‐one single capsule, marketed as Pylera®; Seq, sequential; T, tinidazole; Tc, tetracycline hydrochloride.
The p‐value of those statistically significant (p < 0.001) comparisons is marked in bold.
In second‐line treatment, the use of bismuth quadruple therapy, PPI‐M/T + Tc + B, and quadruple therapy, PPI‐C + A + M/T (OR 1.85; 95% CI: 1.65–2.03 and OR 1.56; 95% CI: 1.12–1.52), the use of standard‐ (OR 1.60; 95% CI 1.45–1.74) or high‐dose PPI (OR 1.82; 95% CI 1.66–1.99) and adequate compliance (OR 6.02; 95% CI 5.04–7.18) were reported as significant independent predictors of eradication success. The model had an adequate goodness of fit (Hosmer‐Lemeshow test: 0.350) and a Nagelkerke's R2 of 5.5% (Table 4).
First‐line treatment
Results of the multivariate logistic regression analysis (dependent variable mITT) showed that the effectiveness of the following first‐line treatments had a statistically significant association with high‐dose PPI versus standard‐dose PPI: Quadruple sequential therapy, PPI‐C + A + M/T (OR 1.71; 95% CI 1.45–2.02); quadruple concomitant therapy (OR 1.40; 95% CI 1.26–1.57); bismuth quadruple therapy, either as single capsule or in its traditional scheme, PPI‐M/T + Tc + B (OR 2.06; 95% CI 1.82–2.32) and PPI‐C + A + B (OR 1.40; 95% CI 1.20–1.62).
Results of the analysis of the first‐line treatments are presented in detail in Supplementary File S2, and Tables S6A and S6B. In this analysis, the variable country was added to evaluate the model with respect to geographical area.
Second‐line treatment
Results of the multivariate logistic regression analysis (dependent variable mITT) showed that the effectiveness of the following second‐line treatments had a statistically significant association with high‐dose PPI versus standard‐dose PPI: Quadruple therapy, PPI‐C + A + M/T (OR 2.02,95% CI 1.82–2.66); bismuth quadruple therapy, either as single‐capsule or in its traditional scheme, PPI‐M/T + Tc + B (OR 1.64; 95% CI 1.31–2.05). Non‐significant treatments (PPI‐L + A + B) are removed from the final model.
Results of the analysis of the second‐line treatments are presented in detail in Supplementary File S3, and Tables S7A and S7B. In this analysis, the variable country was added to evaluate the model with respect to geographical area.
DISCUSSION
The appropriateness of including a PPI in H. pylori eradication treatments is undisputed. In the most influential consensus documents such as Maastricht, Toronto or the American College of Gastroenterology, PPIs have been included in all recommended eradication treatments. 2 , 5 , 6 , 9 , 25
The benefit of PPI treatment was initially confirmed in the MACH 2 study when it was observed that the combination of omeprazole either with amoxicillin and clarithromycin or with metronidazole and clarithromycin resulted in a much higher eradication rate than when antibiotics were used alone. 26 Several clinical studies published between 1993 and 1996 reported a high eradication rate with triple therapies including a PPI, clarithromycin, amoxicillin or metronidazole/tinidazole, the most widely used eradication treatments initially. 9 , 27 , 28 , 29
In the first Maastricht Consensus document (1997), 5 triple therapy with a PPI, twice daily, at standard doses (omeprazole 20 mg, lansoprazole 30 mg or pantoprazole 40 mg) associated with two antibiotics (amoxicillin, clarithromycin or a nitroimidazole) was recommended as first‐line eradication treatment. Vallve et al. 30 confirmed, in a meta‐analysis, that triple therapies with single PPI doses were less effective than double doses. Furthermore, in a systematic review, Villoria et al. 7 reported that standard PPI doses (omeprazole 20 mg, lansoprazole 30 mg, pantoprazole 40 mg, rabeprazole 20 mg and esomeprazole 20 mg) twice a day were less effective than high‐dose PPIs, where previous doses were doubled. Finally, McNicholl et al. 8 reported, in a meta‐analysis, a greater efficacy of eradication treatment with triple therapies with esomeprazole and rabeprazole than with the so‐called first‐generation PPIs, probably due to their higher antisecretory potency and the lesser influence of CYP2C19 polymorphisms. 31
Currently, an eradication treatment is recommended when its efficacy is of approximately 90%, threshold currently achieved with quadruple therapies containing a PPI, metronidazole, tetracycline and bismuth, or with a PPI, clarithromycin, amoxicillin and metronidazole. This situation occurs in most European countries. This efficacy is more likely to be obtained with 14‐day treatments and greater inhibition of gastric acid is achieved with high‐dose PPIs. While the benefit of the latter strategy is well defined in triple therapy, the same is not true for the currently recommended quadruple therapies (both bismuth and non‐bismuth‐based regimens). 32 , 33 , 34 , 35 Thus, up to now, it remains unclear whether higher doses of PPIs can improve the effectiveness of these latter treatments. 2
In our study, in first‐line treatment, over 90% effectiveness was obtained in all 14‐day treatments with high‐dose PPIs, and in bismuth quadruple therapies with standard‐dose PPIs. This effectiveness was also observed in 10‐day treatments with quadruple sequential therapy and bismuth quadruple therapies with either standard‐ or high‐dose PPIs.
In second‐line treatment, optimal effectiveness was obtained in 14‐day treatments with triple therapy with levofloxacin with high‐dose PPIs, and with bismuth quadruple therapy with standard‐ and high‐dose PPIs. In 10‐day treatments, we obtained optimal effectiveness with high‐dose PPIs combined with bismuth quadruple therapy only, either as a three‐in‐one single capsule or in the traditional scheme.
When analysing the results of the most common first‐line treatments, we observed that in triple therapy and in quadruple concomitant therapy, high‐doses were more effective than standard‐doses of PPIs, while the Pylera®/bismuth quadruple therapy could not benefit from higher doses of PPIs in terms of effectiveness; thus, standard PPI doses were sufficient to reach optimal cure rates with this latter therapy.
Our study has some limitations. The heterogeneity in the management of H. pylori infection among the different countries makes it difficult to discern possible confounding variables. The treatments analysed were empirical. Antimicrobial susceptibility testing was not performed. In addition, the second‐line treatment had a small sample size and therefore further data would be needed for a better assessment. An additional limitation could also be that the effectiveness was not assessed by the different types of PPIs (omeprazole, lansoprazole, pantoprazole, esomeprazole, or rabeprazole) but only by the omeprazole equivalent dosage. Thus, for instance, the CYP2C19 polymorphisms, together with the ethnic variability, might influence the metabolism and effectiveness of eradication treatments when combined with omeprazole and lansoprazole.
On the other hand, the study's strengths include, first of all, the establishment of open inclusion criteria in order to represent the real clinical practice of European gastroenterologists, allowing a wide range of therapeutic options. Furthermore, the sample size, including more than 35,000 patients, and its multicentre design allowed us to have a wide knowledge about the subject of study and to carry out a detailed multivariate analysis. To our knowledge, this is the largest study carried out to date worldwide trying to clarify the influence of the acid inhibition on currently recommended H. pylori eradication treatments.
In summary, in our study, the variables that most strongly influenced the H. pylori treatment effectiveness were the treatment duration, the adequate compliance, and the prescribed PPI dose. As per our data, we recommend in first‐line treatment the use of high‐dose PPIs in 14‐day triple therapy and in 10‐or 14‐day quadruple concomitant therapy, while standard‐dose PPIs would be sufficient in 10‐day bismuth quadruple therapies. On the other hand, in second‐line treatment, high‐dose PPIs would be more beneficial in 14‐day triple therapy with levofloxacin and amoxicillin or in 10‐day bismuth quadruple therapy either as a three‐in‐one single capsule or in the traditional scheme.
HP‐EUREG INVESTIGATORS (TO BE INDEXED TO THE PAPER AND IN PUBMED AS “COLLABORATORS”)
Javier Tejedor‐Tejada, Gastroenterology, Hospital Universitario de Cabueñes, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Galina Tarasova, Department of Gastroenterology, Rostov State Medical University, Rostov‐on‐Don, RUSSIA Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Natalia Nikolaevna Dekhnich, Smolensk State Medical University, Smolensk, RUSSIA. Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Alfredo Di Leo, Section of Gastroenterology, Department of Precision and Regenerative Medicine and Ionian Area, University of Bari, 70124 Bari, ITALY Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Giuseppe Losurdo, Section of Gastroenterology, Department Precision and Regenerative and Ionian Area, University of Bari, 70124 Bari, ITALY Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Miguel Fernández‐Bermejo, Digestive Service, Clínica San Francisco, Cáceres, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Eduardo Iyo, Digestive Service, Hospital Comarcal de Inca, Mallorca, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Fernando Bermejo, Digestive Service, Hospital Universitario de Fuenlabrada, idiPAZ, Madrid, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Alicia Algaba, Hospital Universitario de Fuenlabrada, idiPAZ, Madrid, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Ludmila Grigorieva, I. N. Ulianov Chuvash State University, Cheboksary, RUSSIA Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Luis Javier Lamuela Calvo, Gastroenterology Department, Hospital Universitario Miguel Servet, Instituto de Investigación Sanitaria Aragón (IISA), Zaragoza, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Itxaso Jiménez, Gastroenterology Department, Hospital Universitario de Galdakao‐Usansolo, Galdakao, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Boris D Starostin, Saint‐Petersburg State Budgetary Institution Healthcare City Policlinic 38, Saint‐Petersburg, RUSSIA Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Natalia Baryshnikova, Internal disease department of stomatology faculty, Pavlov First Saint Petersburg State Medical University, Saint‐Petersburg, RUSSIA Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Luisa C de la Peña‐Negro, Gastroenterology Department, Viladecans Hospital, Viladecans, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Montserrat Planella, Hospital Universitari Arnau de Vilanova, Lleida, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Consuelo Ramirez, Hospital Universitari Arnau de Vilanova, Lleida, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Natalia V Bakanova, Medical Center Mediceya, Izhevsk, RUSSIA Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Fabio Farinati, Department of Surgery, Oncology and Gastroenterology, University of Padua, Padua, ITALY Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Matteo Ghisa, Department of Surgery, Oncology and Gastroenterology, University of Padua, Padua, ITALY Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Teresa Angueira, Department of Gastroenterology, Hospital General de Tomelloso, Tomelloso, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Alla Kononova, Tver State Madical University, Tver, RUSSIA Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Ana Campillo, Hospital Reina Sofía, 31500 Tudela, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Ramón Pajares Villarroya, Gastroenterology Section, Hospital Universitario Infanta Sofía, Universidad Europea de Madrid, San Sebastián de los Reyes, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Barbara Gomez, Gastroenterology Unit, Hospital de Mataró, Barcelona, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Liya Nikolaevna Belousova, I.I. Mechnikov North‐Western State Medical University, Saint Petersburg, RUSSIA Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Debora Compare, Gastroenterology Unit, Department of Clinical Medicine and Surgery, University Federico II of Naples, Naples, ITALY Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Javier Alcedo, Department of Gastroenterology, Hospital Universitario Miguel Servet, Zaragoza, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Diego Burgos‐Santamaría, Department of Gastroenterology and Hepatology, Hospital Universitario Ramón y Cajal, Madrid SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Francisco‐José Rancel‐Medina, Servicio de Aparato Digestivo, Complejo Asistencial Universitario de Palencia, Palencia, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Isabel Pérez‐Martínez, Departamento de Gastroenterología, Hospital Universitario Central de Asturias, Oviedo, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Igor Maev, A.I. Yevdokimov Moscow State University of Medicine and Dentistry, Moscow, RUSSIA Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Dmitrii Andreev, A.I. Yevdokimov Moscow State University of Medicine and Dentistry, Moscow, RUSSIA Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Jesús M González‐Santiago, Department of Gastroenterology and Hepatology, Salamanca University Clinic Hospital, IBSAL, CIBERehd, Salamanca, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. José Xavier Segarra Ortega, Department of Gastroenterology and Hepatology, Salamanca University Clinic Hospital, Salamanca, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Virginia Flores, Department of Gastroenterology, Hospital General Universitario Gregorio Marañón, Madrid, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Antonio Cuadrado‐Lavín, Department of Gastroenterology and Hepatology, Marqués de Valdecilla University Hospital, University of Cantabria and IDIVAL, Santander, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript. Luis Hernández, Gastroenterology Unit, Hospital Santos Reyes, Aranda de Duero, SPAIN Acquired data, critically reviewed the manuscript draft, and approved the submitted manuscript.
CONFLICT OF INTEREST STATEMENT
Dr. Javier P. Gisbert has served as a speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen. Dr. Olga P. Nyssen has received research funding from Mayoly and Allergan. The remaining authors declare no conflicts of interest.
ETHICS APPROVAL
The Hp‐EuReg protocol was approved by the Ethics Committee of La Princesa University Hospital (Madrid, Spain), which acted as a reference Institutional Review Board (20 December 2012), and was conducted according to the guidelines of the Declaration of Helsinki, was classified by the Spanish Drug and Health Product Agency, and was prospectively registered at Clinical Trials.gov under the code NCT02328131.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
This project was promoted and funded by the European Helicobacter and Microbiota Study Group (EHMSG) and received support from the Spanish Association of Gastroenterology (AEG) and the Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd).
The Hp‐EuReg was co‐funded by the European Union programme HORIZON (grant agreement number 101095359) and supported by the UK Research and Innovation (grant agreement number 10058099). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the Health and Digital Executive Agency (HaDEA). Neither the European Union nor the granting authority can be held responsible for them.
The Hp‐EuReg was co‐funded by the European Union programme EU4Health (grant agreement number 101101252).
This study was funded by Diasorin; however, clinical data were not accessible and the company was not involved in any stage of the Hp‐EuReg study (design, data collection, statistical analysis, or manuscript writing). We want to thank Diasorin for their support.
Pabón‐Carrasco M, Keco‐Huerga A, Castro‐Fernández M, Saracino IM, Fiorini G, Vaira D, et al. Role of proton pump inhibitors dosage and duration in Helicobacter pylori eradication treatment: results from the European Registry on H. pylori management. United European Gastroenterol J. 2024;12(1):122–38. 10.1002/ueg2.12476
Contributor Information
Olga P. Nyssen, Email: opn.aegredcap@aegastro.es.
the Hp‐EuReg Investigators:
Javier Tejedor‐Tejada, Galina Tarasova, Natalia Nikolaevna Dekhnich, Alfredo Di Leo, Giuseppe Losurdo, Miguel Fernández‐Bermejo, Eduardo Iyo, Fernando Bermejo, Alicia Algaba, Ludmila Grigorieva, Luis Javier Lamuela Calvo, Itxaso Jiménez, Boris D. Starostin, Natalia Baryshnikova, Luisa C. de la Peña‐Negro, Montserrat Planella, Consuelo Ramirez, Natalia V. Bakanova, Fabio Farinati, Matteo Ghisa, Teresa Angueira, Alla Kononova, Ana Campillo, Ramón Pajares Villarroya, Barbara Gomez, Liya Nikolaevna Belousova, Debora Compare, Javier Alcedo, Diego Burgos‐Santamaría, Francisco‐José Rancel‐Medina, Isabel Pérez‐Martínez, Igor Maev, Dmitrii Andreev, Jesús M. González‐Santiago, José Xavier Segarra Ortega, Virginia Flores, Antonio Cuadrado‐Lavín, and Luis Hernández
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014;20(36):12767–12780. 10.3748/wjg.v20.i36.12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. Management of Helicobacter pylori infection‐the Maastricht VI/Florence Consensus Report. Gut. 2022;71(9):1724–1762. 10.1136/gutjnl-2022-327745 [DOI] [Google Scholar]
- 3. Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: a review. World J Gastroenterol. 2018;24(29):3204–3221. 10.3748/wjg.v24.i29.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Safavi M, Sabourian R, Foroumadi A. Treatment of Helicobacter pylori infection: current and future insights. World J Clin Cases. 2016;4(1):5–19. 10.12998/wjcc.v4.i1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection‐the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. 10.1136/gutjnl-2016-312288 [DOI] [PubMed] [Google Scholar]
- 6. Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151(1):51–69. 10.1053/j.gastro.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 7. Villoria A, García P, Calvet X, Gisbert JP, Vergara M. Meta‐analysis: high‐dose proton pump inhibitors vs. standard dose in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2008;28(7):868–877. 10.1111/j.1365-2036.2008.03807.x [DOI] [PubMed] [Google Scholar]
- 8. McNicholl AG, Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta‐analysis: esomeprazole or rabeprazole vs. firtst‐generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;36(5):414–425. 10.1111/j.1365-2036.2012.05211.x [DOI] [PubMed] [Google Scholar]
- 9. Malfertheiner P, Mégraud F, O'Morain C, Bell D, Porro BG, Deltenre M, et al. Current European concepts in the management of Helicobacter pylori infection‐the Maastricht Consensus Report. The European Helicobacter Pylori Study Group (EHPSG). Eur J Gastroenterol Hepatol. 1997;9:1–2. 10.1097/00042737-199701000-00002 [DOI] [PubMed] [Google Scholar]
- 10. Wani FA, Bashir G, Khan MA, Zargar SA, Rasool Z, Qadri Q. Antibiotic resistance in Helicobacter pylori: a mutational analysis from a tertiary care hospital in Kashmir, India. Indian J Med Microbiol. 2018;36(2):265–272. 10.4103/ijmm.ijmm_18_19 [DOI] [PubMed] [Google Scholar]
- 11. Hu Y, Zhu Y, Lu NH. Novel and effective therapeutic regimens for Helicobacter pylori in an era of increasing antibiotic resistance. Front Cell Infect Microbiol. 2017;7:168. 10.3389/fcimb.2017.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Federico A, Gravina AG, Miranda A, Loguercio C, Romano M. Eradication of Helicobacter pylori infection: which regimen first? World J Gastroenterol. 2014;20(3):665–672. 10.3748/wjg.v20.i3.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239. 10.1038/ajg.2016.563 [DOI] [PubMed] [Google Scholar]
- 14. Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16(2):139–145. 10.1111/j.1523-5378.2011.00828.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, et al. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25(37):5578–5589. 10.3748/wjg.v25.i37.5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graham DY, Lu H, Dore MP. Relative potency of proton‐pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double‐dose PPI. Helicobacter. 2019;24(1):e12554. 10.1111/hel.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton‐pump inhibitors‐comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65(1):19–31. 10.1007/s00228-008-0576-5 [DOI] [PubMed] [Google Scholar]
- 18. Gisbert JP, Calvet X. Review article: the effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment Pharmacol Ther. 2011;34(11‐12):1255–1268. 10.1111/j.1365-2036.2011.04887.x [DOI] [PubMed] [Google Scholar]
- 19. Gisbert JP, McNicholl AG. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter. 2017;22(4):e12392. 10.1111/hel.12392 [DOI] [PubMed] [Google Scholar]
- 20. Megraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374–1384. 10.1136/gut.2003.022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuan Y, Ford AC, Khan KJ, Gisbert JP, Forman D, Leontiadis GI, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;12:CD008337. 10.1002/14651858.cd008337.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calvet X, García N, López T, Gisbert, Gené, Roque. A meta‐analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14(5):603–609. 10.1046/j.1365-2036.2000.00744.x [DOI] [PubMed] [Google Scholar]
- 23. McNicholl AG, O'Morain CA, Megraud F, Gisbert JP. As Scientific Committee of the Hp‐Eureg on Behalf of the National Coordinators. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp‐EuReg). Helicobacter. 2019;24(5):e12630. 10.1111/hel.12630 [DOI] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. 2019;157(1):44–53. 10.1053/j.gastro.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 26. Lind T, Mégraud F, Unge P, Bayerdörffer E, O'Morain C, Spiller R, et al. The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1‐week triple therapies. Gastroenterology. 1999;116(2):248–253. 10.1016/s0016-5085(99)70119-8 [DOI] [PubMed] [Google Scholar]
- 27. Bazzoli F, Zagan RM, Fossi S. Efficacy and tolerability of a short‐term low‐dose triple therapy for eradication of Helicobacter pylori infection. Gastroenterology. 1993;104:A40. [Google Scholar]
- 28. Lamouliatte H, Cayla R, Regland F. Amoxicilin‐clarithromycin‐omeprazole: the best therapy for Helicobacter pylori infection. Acta Gastroenterol Belg. 1993;56:A139. [Google Scholar]
- 29. Lind T, Veldhuyzen van Zanten S, Unge P, Spiller R, Bayerdörffer E, O'Morain C, et al. Eradication of Helicobacter pylori using one‐week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter. 1996;1(3):138–144. 10.1111/j.1523-5378.1996.tb00027.x [DOI] [PubMed] [Google Scholar]
- 30. Vallve M, Vergara M, Gisbert JP, Calvet X. Single vs. double dose of a proton pump inhibitor in triple therapy for Helicobacter pylori eradication: a meta‐analysis. Aliment Pharmacol Ther. 2002;16(6):1149–1156. 10.1046/j.1365-2036.2002.01270.x [DOI] [PubMed] [Google Scholar]
- 31. Kuo CH, Lu CY, Shih HY, Liu CJ, Wu MC, Hu HM, et al. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol. 2014;20(43):16029–16036. 10.3748/wjg.v20.i43.16029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molina‐Infante J, Lucendo AJ, Angueira T, Rodriguez‐Tellez M, Perez‐Aisa A, Balboa A, et al. Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: the OPTRICON study. Aliment Pharmacol Ther. 2015;41(6):581–589. 10.1111/apt.13069 [DOI] [PubMed] [Google Scholar]
- 33. Lerardi E, Losurdo G, La Fortezza RF, Principi M, Barone M, Leo AD. Optimizing proton pump inhibitors in Helicobacter pylori treatment: old and new tricks to improve effectiveness. World J Gastroenterol. 2019;25(34):5097–5104. 10.3748/wjg.v25.i34.5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moayyedi P, Sahay P, Tompkins DS, Axon AT. Efficacy and optimum dose of omeprazole in a new 1‐week triple therapy regimen to eradicate Helicobacter pylori. Eur J Gastroenterol Hepatol. 1995;7(9):835–840. [PubMed] [Google Scholar]
- 35. Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Ikuma M, et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12(4):317–323. 10.1111/j.1523-5378.2007.00508.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


