Abstract
Tachyzoites (VEG strain) that emerge from host cells infected with Toxoplasma gondii sporozoites proliferate relatively fast and double their number every 6 h. This rate of growth is intrinsic, as neither the number of host cells invaded nor host cell type appears to influence emergent tachyzoite replication. Fast tachyzoite growth was not persistent, and following ∼20 divisions, the population uniformly shifted to slower growth. Parasites 10 days post-sporozoite infection doubled only once every 15 h and, unlike emergent tachyzoites, they grew at this slower rate over several months of continuous cell culture. The spontaneous change in tachyzoite growth rate preceded the expression of the bradyzoite-specific marker, BAG1. Within 24 h of the growth shift, 2% of the population expressed BAG1, and by 15 days post-sporozoite infection, 50% of the parasites were positive for this marker. Spontaneous BAG1 expression was not observed in sporozoites or in tachyzoites during fast growth (through day 6 post-sporozoite inoculation), although these tachyzoites could be induced to express BAG1 earlier by culturing sporozoite-infected cells at pH 8.3. However, alkaline treatment also reduced the replication of emergent tachyzoites to the rate of growth-shifted parasites, supporting a link between reduced parasite growth and bradyzoite differentiation. The shift to slower growth was closely correlated with virulence in mice, as the initially fast-growing emergent tachyzoites were avirulent (100% lethal dose, >104 parasites), while a mutant VEG strain (MS-J) that is unable to growth shift caused 100% mortality in mice inoculated with 10 parasites. Parasites recovered from gamma interferon knockout mice inoculated with emergent tachyzoites grew at a slow rate and expressed BAG1, confirming that the replication switch occurs in animals and in the absence of a protective immune response.
Toxoplasma gondii has a complex life cycle involving two different host types and cycles (reviewed in reference 9). The definitive (sexual) cycle commences when bradyzoites (tissue cysts) infect epithelial cells of the feline intestine and differentiate into the first of a series of merozoite generations (7). Only the bradyzoite stage is capable of initiating the definitive cycle of T. gondii (9) and the equivalent cycles of other cyst-forming coccidians, including Hammondia spp. and Sarcocystis spp. (6). It is intriguing, however, that the interaction of bradyzoite and definitive host, which is exclusive in these closely related coccidians, is nonspecific in T. gondii. T. gondii tissue cysts are infectious for a wide array of other animal hosts, and in these hosts, as well as extraintestinally in the cat, bradyzoites develop into tachyzoites which eventually will reform cysts, thus completing the intermediate host cycle (9). Although the differences between these coccidians are well known, it remains unclear what parasite factors allow T. gondii bradyzoites to initiate a second cycle that in Hammondia and Sarcocystis is solely a function of the sporozoite stage (6).
The unique ability of T. gondii bradyzoites and tachyzoites to interconvert is clinically important, as it is considered to be the underlying cause of Toxoplasma encephalitis in AIDS patients (21). The slow-growing T. gondii bradyzoite is relatively nonpathogenic and not readily eliminated by the host immune system. Thus, in immunodeficient or -compromised hosts, recurrent toxoplasmosis can occur each time latent bradyzoites recrudesce into proliferating tachyzoites. The mechanism that controls T. gondii tachyzoite-to-bradyzoite development is of obvious importance and is currently the subject of some controversy. In vitro, high-temperature or alkaline-pH stress (30) and inhibitors of parasite DNA synthesis or mitochondrial function (2) all induce bradyzoite antigen expression in tachyzoites. Thus, it is proposed that tachyzoite-to-bradyzoite differentiation may be host induced and that host cytokines could mediate induction in vivo (1, 25). If this model is accurate, it remains to be explained why some tachyzoite strains are able to differentiate in culture in the absence of any obvious inductive agents (20, 29).
Like bradyzoites, T. gondii sporozoites are infectious to definitive and intermediate hosts, although in either host type they are restricted to initiating the intermediate host cycle (9, 14). Sporozoites invade host cells, and for 24 h or less they occupy a temporary parasitophorous vacuole where they differentiate into tachyzoites (32–34). Parasite replication begins when the developing tachyzoites move from the transient vacuole into a second parasitophorous vacuole that contains the structures necessary to support parasite growth (32, 34). In this report, we extend our studies of T. gondii sporozoite development by following the growth and stage-specific protein expression of tachyzoites formed by sporozoite infection. Our results show that emergent tachyzoites undergo a rapid but limited expansion that precedes their spontaneous differentiation into bradyzoites.
MATERIALS AND METHODS
Cell culture and parasite strains.
Human foreskin fibroblasts (HFF) were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% (vol/vol) newborn calf serum. Sporulated oocysts from an avirulent strain (VEG) of T. gondii originally isolated from an AIDS patient (23) were purified from cat feces by centrifugal elutriation as described previously (33). Purified oocysts were incubated in 10% (vol/vol) Clorox (in phosphate-buffered saline [PBS]) at room temperature for 30 min, collected by centrifugation, and washed 3 to 4 times in Hanks’ balanced salt solution (GIBCO, Gaithersburg, Md.) to remove residual Clorox. Sporozoites were excysted according to published methods (31), washed in PBS, and in some cases, filtered through 3-μm-pore-size polycarbonate filters (Nucleopore Corp., Pleasanton, Calif.) to remove sporocysts, unbroken oocysts, and oocyst debris. Sporozoites were suspended in culture medium containing 1% fetal bovine serum or newborn calf serum, 50 μg of dihydrostreptomycin per ml, and 50 U of penicillin G per ml, and inoculated into cultured cells. Sporozoite-infected cultures were shifted to alkaline conditions by replacing the standard growth medium with a Tricine (45 mM)-buffered DMEM adjusted to pH 8.3 with KOH and incubating the cultures in a CO2-free environment throughout the experiment.
Tachyzoite strains RH and ME49-P(lk) were maintained in confluent monolayers of HFF cells according to standard protocols (26). Emergent VEG tachyzoites were obtained from cultures of HFF cells inoculated with sporozoites. Mutant VEG strain MS-J arose spontaneously from a sporozoite-infected culture that was continuously passaged in HFF cells for over 4 months. The growth rate and virulence of MS-J tachyzoites have been stable for over a year in culture.
RFLP analysis.
Strains RH, ME49-P(lk), and VEG represent type strains for lineages I, II, and III, respectively (15). Allelic differences were evaluated at the SAG1 and SAG2 loci for these strains and mutant line MS-J by restriction fragment length polymorphisms (RFLPs) in single-copy DNA segments amplified by PCR (for primer designs, see reference 15). Gel-purified PCR product obtained with the SAG1 primers was digested with enzyme DdeI or Sau96I (New England BioLabs, Beverly Mass.), and the fragments were analyzed on 1.2% agarose gels. Similarly, analysis of the SAG2 locus was completed with Sau3A. RFLPs were evaluated by comparison of their size to a standard DNA ladder (Φx174/HaeIII, GIBCO).
Determination of parasite growth rates.
To measure replication rates, HFF monolayers infected with tachyzoites at mid-log growth (4 to 32 parasites per vacuole) were scraped, passed through a 25-gauge needle, and filtered through 3-μm-pore-size polycarbonate filters. The filter-purified tachyzoites were inoculated into a fresh HFF culture (25 cm2 T-flask), incubated for 1 h at 37°C to allow parasite penetration, and then washed with standard growth medium to remove extracellular tachyzoites, whereupon the incubation was continued. At various time intervals, the vacuole size (1, 2, 4, 8, 16, 32, 64 tachyzoites per vacuole) within a single T-25 flask was determined for a minimum of 50 vacuoles (5 to 10 fields at ×400) chosen at random without prior microscopic examination. The average parasite number per vacuole and standard deviation were calculated for each time point.
Experimental infections in mice.
Female CD-1 outbred mice and mice containing a targeted disruption of the gamma interferon gene (gko) (5) were used for experimental infections. Parasite inoculum (101 to 105) was purified from tachyzoite- or sporozoite-infected HFF cell monolayers as described above. At the appropriate parasite dose, five mice were infected by subcutaneous (s.q.) inoculation and monitored for 30 days, at which time the sera of surviving mice were analyzed by immunofluorescence assay (see next section). Serum exhibiting a >1:100 titer was considered confirmation of a Toxoplasma infection.
To assess the growth rate of parasites passed in mice, gko mice were inoculated s.q. with 103 MS-J tachyzoites or VEG tachyzoites from HFF cultures at 3 or 8 days post-sporozoite infection. The mice were euthanized 10 days postinoculation, the brain tissue was homogenized in HFF cell culture media, and 0.1 ml of the suspension was inoculated intraperitoneally into a second gko mouse. The peritoneal exudate of the second gko mouse was removed 7 days postinoculation and inoculated into HFF cultures. After a single passage, growth rate and percent BAG1 expression of the isolated parasite populations were evaluated (see next section).
Immunofluorescence assays.
HFF cells grown in 8-well chamber slides or on 6-well plates containing coverslips were inoculated with 105 sporozoites or tachyzoites. At various intervals, the slides were washed three times in PBS, fixed with 3% paraformaldehyde in PBS, treated in acetone for 10 min at 4°C, and air dried. The slides were incubated with anti-recombinant BAG1 mouse antiserum (3) or anti-SAG1 monoclonal antibody DG52 (4) for 1 h in a humid chamber and then washed three times in PBS followed by a single wash in PBS–1% bovine serum albumin (wt/vol). Matched irrelevant antibody controls were included for each antibody. The slides were treated with secondary antibody fluorescein-conjugated anti-mouse immunoglobulin G (IgG) (Sigma, St. Louis, Mo.) diluted 1:64 in DMEM containing 2.5% goat sera for 1 h in the dark, washed four times in PBS, mounted with Gel-mount solution containing 2.5% (wt/vol) diazabicyclo[2.2.2.7]octane, and evaluated with a Nikon epifluorescence microscope. BAG1-positive parasites were enumerated by examining at least 100 vacuoles in randomly selected fields.
RESULTS
Tachyzoites emerging from sporozoite-infected cells undergo a change in growth rate.
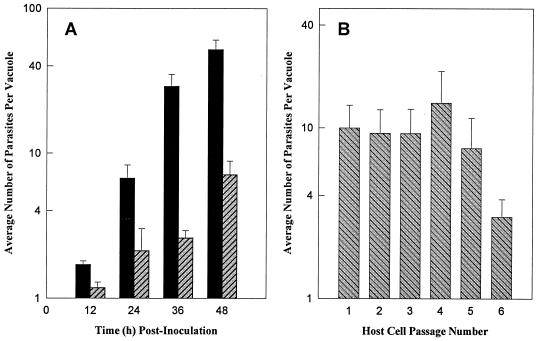
To measure the growth rate of VEG tachyzoites emerging from sporozoite-infected HFF cells (emergent tachyzoites), sporozoite cultures were disrupted by needle passage (48 h postinoculation), and tachyzoites were filter purified and reinoculated into a fresh HFF cell monolayer. Parasite growth was monitored by counting the number of tachyzoites per vacuole at 12-h intervals. Tachyzoites within a vacuole exhibit exponential growth, and by limiting the infection period (1 h), the range of vacuole sizes during growth was tightly distributed (11). Emergent tachyzoites doubled on average every 6 h and lysed their host cells (at 64 to 128 parasites per vacuole) beginning 48 h postinoculation (Fig. 1A, closed bars). The emergent replication rate and rapid expansion period were limited, however. Ten days after the establishment of a tachyzoite-infected culture from sporozoites (Day 10 VEG tachyzoites), the parasite growth rate had dramatically slowed (Fig. 1A) and host lysis (4 to 5 days) was significantly delayed. Whereas emerging tachyzoites divide approximately once every 6 h, the growth rate of day 10 VEG tachyzoites (Fig. 1A, hatched bars) was two- to threefold slower (∼15 h doubling). Once growth shifted, tachyzoites did not resume a fast growth rate through 3 months of continuous culture.
FIG. 1.
(A) Growth of VEG tachyzoite populations obtained following sporozoite inoculation. The day 10 population was purified after a single passage (at 5 days post-sporozoite inoculation) into a second HFF monolayer. The day 2 (closed bars) and day 10 (hatched bars) VEG parasites were allowed to invade confluent HFF monolayers for 1 h before extracellular parasites were removed. Vacuole sizes (number of parasites per vacuole) were determined every 12 h over 48 h. All growth data represent the average of at least 50 randomly selected vacuoles and are plotted on a log10 scale. (B) Rapid growth of emergent tachyzoites is stable for 5 days and is not limited by the number of host cell invasions. Beginning at 48 h post-sporozoite inoculation, purified tachyzoites were inoculated into fresh HFF monolayers (1 h infection), and the average vacuole size was determined 24 h later. This process was repeated every 24 h through five host cell passages (see Materials and Methods for complete details). The vacuole size in host cell passage no. 1 represents tachyzoite growth within the sporozoite-infected host cell (24 to 48 h post-sporozoite inoculation).
In order to follow the growth rate of emergent tachyzoites beyond a single infectious cycle, parasites were passed (prior to host cell lysis) into new HFF cell monolayers at 24-h intervals over 7 days. Beginning with sporozoite-infected cells (Fig. 1B, host cell 1, growth was monitored 24 to 48 h postsporozoite) and continuing through the fifth host cell, tachyzoites grew at the emergent rate of three to four divisions in a 24-h period (Fig. 1B, host cell 1 to 5), but by the sixth host cell (day 7 postinoculation), the growth of the population as a whole was dramatically slower (Fig. 1B, host cell 6). Whether tachyzoites were passed less frequently (every 36 or 48 h) or sporozoites were inoculated sparsely to avoid exhausting the initial HFF monolayer, a reduced rate of parasite growth appeared in a similar time frame in each case (data not shown).
Change in tachyzoite growth rate precedes a spontaneous switch to bradyzoite development.
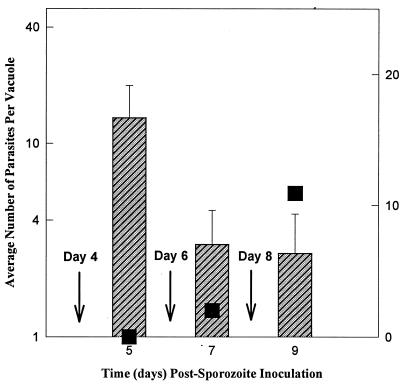
The induction of bradyzoite differentiation by drug treatments or physiological stress leads to a concomitant reduction in the growth rate of the affected tachyzoites (2, 30). We therefore examined whether the spontaneous shift of emergent tachyzoites to slower growth was associated with a switch to bradyzoite development. To assess bradyzoite differentiation, antiserum prepared against recombinant bradyzoite-specific antigen 1 (BAG1) (3) was used in an immunofluorescent assay to identify differentiating parasites. BAG1 expression was not observed in sporozoites (data not shown) (3) or during rapid growth of emergent VEG tachyzoites. BAG1 expression was detected, however, following the shift in growth rate as demonstrated by the results shown in Fig. 2. VEG tachyzoites from day 4 post-sporozoite cultures were reinoculated into a new monolayer, and the vacuole size and the frequency of BAG1-positive parasites were determined 24 h later (Fig. 2, day 5). As noted, tachyzoites at this stage grow at the fast rate (three to four divisions/24 h, see Fig. 1B), and examination under fluorescence did not reveal BAG1-positive parasites here or at earlier times post-sporozoite infection (percent BAG1 is denoted by the closed squares). In the next passage (day 6, see arrow), tachyzoite growth was slowed (Fig. 2, day 7), in agreement with our previously established time frame (Fig. 1B), and we observed a small fraction (2%) of parasites staining positive for BAG1. The frequency of BAG1 parasites increased in the next passage (10%, day 9) and continued to rise (14.6%, day 10), reaching a maximum of ∼50% by 15 days post-sporozoite inoculation.
FIG. 2.
BAG1 expression is detected following a reduction in tachyzoite growth rate. VEG tachyzoites purified from days 4, 6, and 8 (arrows) post-sporozoite inoculation cultures were passed into fresh HFF monolayers, and 24 h later the average vacuole size and number of BAG1-positive parasites were evaluated (see Materials and Methods). Hatched bars, average vacuole size; closed squares, percentage of BAG1-positive parasites.
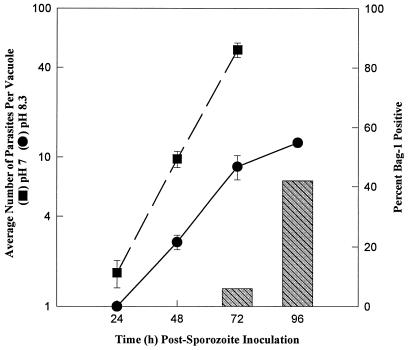
Figure 2 indicates that lengthening of the tachyzoite cell cycle is closely correlated with the bradyzoite development. We were interested, therefore, in whether emergent VEG tachyzoites could be induced to differentiate. Sporozoite-infected HFF cultures were changed to alkaline media (pH 8.3) 12 h postinoculation, and the growth rate and frequency of BAG1 expression were followed over 96 h (Fig. 3). Sporozoite development into tachyzoites did not appear to be affected by the alkaline culture conditions; they moved from the first into the second parasitophorous vacuole by 24 h postinoculation, and the induction of the tachyzoite-specific, SAG1 antigen followed established kinetics (data not shown) (33, 34). Emergent tachyzoites cultured in pH 7 medium do not express BAG1 and begin to lyse from sporozoite-infected host cells by 72 h (Fig. 3, closed squares). In contrast, parasites shifted to alkaline conditions (Fig. 3, closed circles) grew at a slow rate, equivalent to that of growth-shifted tachyzoites (Fig. 1A, hatched bars). BAG1-positive parasites were clearly present in the alkaline cultures by 72 h postinoculation, and nearly 50% of the parasites were expressing BAG1 at 96 h (Fig. 3, hatched bars). Thus, emergent tachyzoites have the capacity to differentiate into bradyzoites within 48 h of their forming in the sporozoite-infected cell (≤24 h postinoculation).
FIG. 3.
Tachyzoites formed within sporozoite-infected cells can be induced to express BAG1. Sporozoite-infected cultures were switched to alkaline medium 12 h postinoculation. The average vacuole size (pH 8.3, closed circles) and number of BAG1-positive parasites (hatched bars) were then determined over 96 h and compared to those obtained with control cultures (pH 7 growth, closed squares). BAG1 expression was not detected in control parasites, which had completely lysed out of their host cells by 96 h post-sporozoite infection.
The spontaneous switch to slow growth and bradyzoite differentiation occurs in vivo and influences virulence in mice.
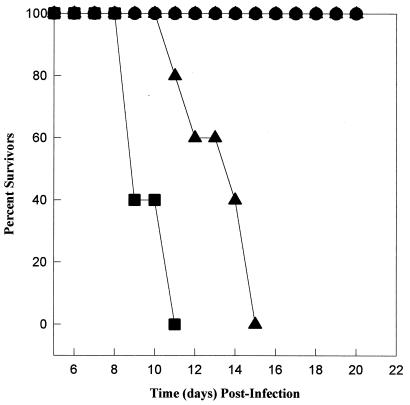
A fast-growing tachyzoite line (MS-J) was established by continuous passage of a sporozoite-infected culture. The MS-J tachyzoite growth rate (∼7 h, doubling time is estimated from growth curves not shown) is similar to that of day 2 (postsporozoite) VEG tachyzoites (Fig. 1A, ∼6 h) and somewhat slower than that of tachyzoites of the RH strain (∼4.5 h). Inoculated s.q. into CD-1 mice, MS-J and RH tachyzoites caused 100% mortality at parasite doses of ≥101 (only 103 dose is shown [Fig. 4]). Doses of fewer than 10 parasites were not attempted; however, we noted that the time to death of CD-1 mice infected with the faster growing RH strain (closed circles) was consistently shorter than that of MS-J-infected mice, which likely reflects a higher virulence (16).
FIG. 4.
Tachyzoites obtained from VEG sporozoite-infected cultures are avirulent in CD-1 mice. The virulence of day 3 and day 8 (post-sporozoite infection) VEG tachyzoite populations was compared to that for RH (closed squares) and MS-J (closed triangles) tachyzoite lines. CD-1 mice (groups of 5) were inoculated s.q., and mortality was monitored over 30 days (only 22 days are shown). The results obtained from a 103 parasite dose are displayed. CD-1 mice infected with emergent VEG tachyzoites were asymptomatic, although all showed positive serology to T. gondii, indicating that they were infected.
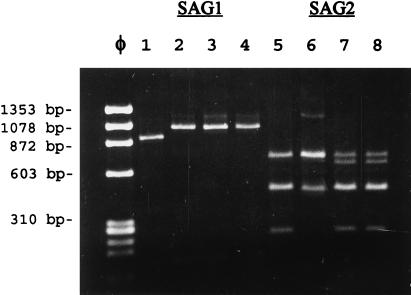
Acute virulence of T. gondii type 1 strains (28), and some natural recombinants (16), is correlated with allele 1 of the SAG1 locus, while avirulent strains, such as the type III VEG strain, usually have the SAG1-2 allele (15, 23). Accordingly, we eliminated the possibility that MS-J arose from a contamination event. Results of RFLP analyses performed on PCR-amplified DNA are shown in Fig. 5. Strains ME49-P(lk), VEG, and the mutant MS-J display identical alleles at the SAG1 locus, consistent with avirulence in mice (only the DdeI digest is shown, lanes 2 to 4, Fig. 5), while the allele for RH is distinct for virulent strains (Fig. 5, lane 1) (15, 16). At the SAG2 locus, RFLP patterns for VEG and MS-J are identical as expected (Fig. 5, lanes 7 and 8), while those for RH and ME49-P(lk) are distinct (Fig. 5, lanes 5 and 6). These data serve to illustrate the authenticity of the type I, II, and III clonal lineages (15) used in this comparison and demonstrate the avirulent genotype of the mutant MS-J at SAG1.
FIG. 5.
RFLP analysis of MS-J parasites. Restriction enzyme patterns of the SAG1 (DdeI digest shown, lanes 1 to 4) and SAG2 (Sau3A shown, lanes 4 to 8) loci from RH, ME49-P(lk), VEG, and the VEG mutant MS-J are shown. Genomic DNAs were PCR amplified, the products were restricted, and the fragments were resolved on 1.2% agarose gels. Lanes: 1 and 5, RH; 2 and 6, ME49-P(lk); 3 and 7, VEG; 4 and 8, MS-J. Molecular size standards are from HaeIII-digested Φx174 DNA.
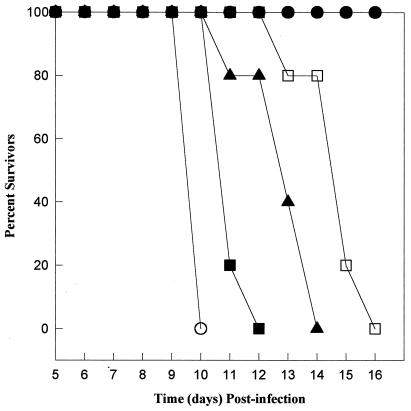
In contrast to the acute virulence of MS-J and RH, CD-1 mice survived infections with >104 day 3 or day 8 (postsporozoite) VEG tachyzoites (103 dose shown, Fig. 4). In all surviving mice, infections were confirmed by a positive immunofluorescent assay result. It may be inferred from these results that the fast growth of day 3 VEG tachyzoites is limited and therefore that these parasites pose no more of a threat to an immunocompetent animal than the growth-shifted day 8 VEG tachyzoites. We explored this question further in gko mice, whose defective immune system is unable to eliminate infections with avirulent strains (27). The virulence studies in gko mice were performed twice with identical results, and the results of one experiment are shown in Fig. 6 (103 doses shown). In gko mice, all strains tested were lethal, with the time to death correlating with the relative differences in their respective growth characteristics. As expected, gko mice infected with RH (open circles) and MS-J (closed squares) tachyzoites succumbed in the shortest time, whereas some mice inoculated with day 8 VEG parasites (open squares) survived nearly a week longer. Because day 3 VEG tachyzoites (closed triangles) grow fast initially and then shift to slower growth, the time to death of mice infected with these parasites fell between the MS-J and day 8 VEG parasites.
FIG. 6.
Tachyzoite growth differences are consistent with the time to death for infected gko mice. RH (open circles), MS-J (closed squares), and day 3 (closed triangles) and day 8 (open squares) VEG tachyzoites were inoculated (103 parasites) s.q. into gko mice (groups of five), and mortality was monitored. Mock-infected controls (closed circles).
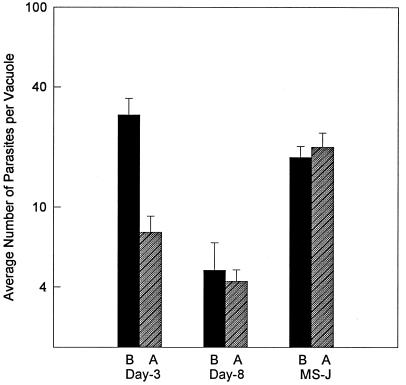
In order to confirm that day 3 VEG tachyzoites had growth shifted in the gko mice, parasites were obtained from the brain tissue of gko infected mice and reinoculated intraperitoneally into new gko mice to expand the parasite population. The peritoneal exudate was then removed, and the recovered parasites were passed once in HFF cells before evaluating their growth rate (Fig. 7). The time from sporozoite (or MS-J tachyzoite) inoculation until the parasites were reestablished in cell culture was 17 days. Tachyzoites obtained from animals infected with day 3 VEG tachyzoites (Fig. 7, day 3A) grew approximately threefold more slowly than the starting parasite population (day 3B), indicating that they had shifted their growth in the gko mice. Because day 8 VEG tachyzoites had already growth shifted in cell culture, the phenotype of parasites recovered from gko mice was unchanged (day 8B, A). The growth behavior of the mutant MS-J, which does not growth shift, was also unaltered by passage in gko mice (MS-JA, B). Spontaneous BAG1 expression was readily detected (>10%) in the parasite populations recovered from day 3 or day 8 mice, indicating that the switch to bradyzoite differentiation had also occurred in these animals (data not shown). MS-J tachyzoites did not express BAG1 before or after passage through gko mice, in agreement with our inability to detect tissue cysts in CD-1 mice infected with MS-J parasites.
FIG. 7.
Fast-growing, emergent tachyzoites display a slower growth rate after passage in gko mice. Day 3 and day 8 post-sporozoite infection and MS-J (VEG) tachyzoites (103 parasites) were inoculated s.q. into gko mice. Infected brain tissue (10 days postinfection) was inoculated intraperitoneally into new gko mice, and after a period of amplification (7 days), parasites were recovered from the peritoneal exudate. Upon first passage into fresh HFF cells, vacuole sizes were determined 36 h postinoculation (see Materials and Methods for complete details). Closed bars (B), 36 h vacuole size prior to passage through mice; hatched bars (A), vacuole size post-gko passage.
DISCUSSION
Soon after host cell invasion, VEG sporozoites begin to differentiate, as evidenced by the concurrent induction of tachyzoite-specific (33, 34) and the disappearance of sporozoite-specific proteins (17). On the basis of these examinations, the parasites that release from sporozoite-infected cells do not appear to express sporozoite proteins and are therefore undifferentiated tachyzoites. Emergent tachyzoites grow uniformly and at a relatively fast rate in different host cell types (HFF cells, Fig. 1A and B). Remarkably, however, this rate of growth was proscribed to ∼20 parasite divisions in three (6 to 7 divisions/cell) to five host cells (24-h passage; Fig. 1B), indicating that emergent tachyzoite growth is somehow restricted by a mechanism that is unrelated to host cell invasion. It is unlikely that this growth limit is due to a cell culture deficiency, because rapidly dividing VEG tachyzoites develop directly from sporozoites in vitro (Fig. 1A, day 2 tachyzoites), and fast growth was not sustainable and slow growth was not reversible when various tachyzoites were introduced into mice (compare days 3 and 8, Fig. 7). It is conceivable that the spontaneous slowing of emergent VEG tachyzoite growth reflects a cell cycle regulatory mechanism that may limit the biotic potential of sporozoite infections.
The appearance of parasites expressing bradyzoite antigen, BAG1, occurred only subsequent to a reduction in VEG tachyzoite growth rate (Fig. 2 and 3). Thus, lengthening of the tachyzoite cell cycle in vitro is correlated with bradyzoite development. A similar connection between tachyzoite growth rate and stage conversion was noted in earlier attempts to induce differentiation (2). However, in contrast to the conclusions offered by these studies, our results demonstrate that following the fast growth period, VEG tachyzoite differentiation was spontaneous (Fig. 2), indicating that these events are most likely genetically controlled rather than host induced. A delay in VEG tachyzoite in vitro differentiation is consistent with the reported time lag (7 to 10 days) between T. gondii oocyst infection and the appearance of functional bradyzoites in various mouse tissues (M-7741 and VEG strains) (8, 10). Thus, the VEG strain used here is developmentally competent, although we have not tested in vitro-produced VEG bradyzoites in the feline bioassay (10). Cumulatively, the duration of sporozoite differentiation (≤24 h) (10, 32, 33) combined with the estimated interval for tachyzoite to bradyzoite conversion (∼2 days) (8, 22) is insufficient to account for this delay. Indeed, our results appear to confirm this minimal time frame, since BAG1 was detectable within 48 h (72 h postsporozoite; Fig. 3) of tachyzoite formation (24 h postsporozoite) in alkaline-treated sporozoite cultures. Despite these results, BAG1-positive parasites were not apparent in mouse intestine (BAG1=BAG5; see reference 10) and were absent from uninduced VEG tachyzoite cultures (Fig. 2) until >5 days post-sporozoite infection. Thus, even though newly formed tachyzoites have the capacity to differentiate in vitro, the extent of the bradyzoite delay in oocyst-infected mice seems to support the hypothesis that tachyzoites inherently expand before they differentiate. It is not known if bradyzoite infections of mice are governed by similar mechanisms. On the basis of the cat bioassay, bradyzoites reappear in tissue cyst-infected mice in a delayed fashion that is remarkably similar (7 to 9 days) (8) to sporozoite infections. There are also data indicating that, like sporozoites (33, 34), bradyzoites transform quickly into tachyzoites without parasite replication being required (1, 29). It is intriguing to speculate, based on this evidence, that the intermediate cycle of T. gondii in mice may be similarly presented, regardless of whether infection is initiated by sporozoites or bradyzoites.
The growth constraint of emergent VEG tachyzoites appears to be a brake that can be lost, as exemplified by the mutant strain MS-J. MS-J tachyzoites grow nearly as fast as emergent VEG tachyzoites, but their growth was unaffected by long-term cell culture or passage in mice (Fig. 7). Accordingly, MS-J tachyzoites caused 100% mortality in CD1 mice at a dose more than 3 orders of magnitude lower than that of either emergent or growth-shifted VEG tachyzoites (Fig. 4). The specific mutation in MS-J parasites is unknown; however, MS-J is identical to its avirulent, type III parent at the SAG1 locus (Fig. 5) (23), thereby ruling out an obvious virulence marker (16, 28). Although it is possible that a mutation other than one affecting growth is responsible for MS-J virulence, the correlation between the time of mortality and the relative growth differences of the various tachyzoite strains tested (compare RH to MS-J in CD-1 mice, Fig. 4; see gko mice, Fig. 6) suggests that growth rate and the capacity to growth shift are virulent determinants. The connection between T. gondii virulence and elevated multiplication rate has long been recognized (18, 19), as has the role of continuous parasite culture or passage in mice in selecting tachyzoites with higher virulence (13, 16). The importance of the studies shown here is the discovery that emergent VEG tachyzoites have a naturally rapid growth rate, albeit of limited duration. The loss of this regulatory mechanism, which may readily occur when tachyzoites are removed from their natural developmental context, could explain some of these virulent strains.
Our ability to resolve questions about the T. gondii cell cycle and its relationship to development and the acquisition of virulence is hampered by a lack of experimental models. As a first step, we have recently introduced thymidine kinase into T. gondii and through this modification have synchronized tachyzoite populations (24). Experiments are now under way to define the basic features of the tachyzoite cell cycle.
ACKNOWLEDGMENTS
We thank John Boothroyd for kindly donating antibodies to SAG1 and David Pascual for supplying the gamma interferon knockout mice used in these studies.
This work was supported in part by USDA CSREES NRI competitive grants 95-02064 and 97-02461 (M.W.W.) and NIH grants AI-28724 and AI-31808 (D.S.R.). D.S.R. is a Burroughs Wellcome New Investigator in Molecular Parasitology.
Footnotes
Contribution J-5194 from the Montana State University Agriculture Experiment Station.
REFERENCES
- 1.Bohne W, Heesemann J, Gross U. Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect Immun. 1993;61:1141–1145. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohne W, Gross U, Ferguson D J P, Heesemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 4.Burg J L, Perelman D, Kaspar L H, Ware P L, Boothroyd J C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- 5.Dalton D K, Pitts M S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 6.Dubey J P. Toxoplasma, Hammondia, Besnotia, Sarcocystis, and other tissue cyst-forming coccidia of man and animals. In: Krier J P, editor. Parasitic protozoa. Vol. 3. New York, N.Y: Academic Press; 1977. pp. 101–237. [Google Scholar]
- 7.Dubey J P, Frenkel J K. Cyst-induced toxoplasmosis in cats. J Protozool. 1972;19:155–177. doi: 10.1111/j.1550-7408.1972.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 8.Dubey J P, Frenkel J K. Feline toxoplasmosis from acutely infected mice and the development of Toxoplasma cysts. J Protozool. 1976;23:537–546. doi: 10.1111/j.1550-7408.1976.tb03836.x. [DOI] [PubMed] [Google Scholar]
- 9.Dubey J P, Beattie C P. Toxoplasmosis of animals and man. Boca Raton, Fla: CRC Press, Inc.; 1988. [Google Scholar]
- 10.Dubey J P, Speer C A, Shen S K, Kwok O C H, Blixt J A. Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii cysts. J Parasitol. 1997;83:870–882. [PubMed] [Google Scholar]
- 11.Fichera M E, Bhopale M K, Roos D S. In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii. Antimicrob Agents Chemother. 1995;39:1530–1537. doi: 10.1128/aac.39.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frenkel J K, Dubey J P, Hoff R L. Loss of stages after continuous passage of Toxoplasma gondii and Besnoitia jellisoni. J Protozool. 1976;23:421–424. doi: 10.1111/j.1550-7408.1976.tb03799.x. [DOI] [PubMed] [Google Scholar]
- 13.Frenkel J K, Ambroise-Thomas P. Genomic drift of Toxoplasma gondii. Parasitol Res. 1997;83:1–5. doi: 10.1007/s004360050197. [DOI] [PubMed] [Google Scholar]
- 14.Freyre A, Dubey J P, Smith D D, Frenkel J K. Oocyst-induced Toxoplasma gondii infections in cats. J Parasitol. 1989;75:750–755. [PubMed] [Google Scholar]
- 15.Howe D K, Sibley L D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 16.Howe D K, Summers B C, Sibley L D. Acute virulence in mice is associated with markers on chromosome VIII in Toxoplasma gondii. Infect Immun. 1996;64:5193–5198. doi: 10.1128/iai.64.12.5193-5198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerome, M. E., and M. W. White. Unpublished results.
- 18.Kaufman H E, Remington J S, Jacob L. Toxoplasmosis: the nature of virulence. Am J Ophthalmol. 1958;46:255–261. doi: 10.1016/0002-9394(58)90805-5. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman H E, Melton M L, Remington J S, Jacob L. Strain differences of Toxoplasma gondii. J Parasitol. 1959;45:189–190. [PubMed] [Google Scholar]
- 20.Lindsay, D. S., M. A. Toivio-Kinnucan, and B. L. Blagburn. Ultrastructural determination of cystogenesis by various Toxoplasma gondii isolates in cell culture. J. Parasitol. 79:289–292. [PubMed]
- 21.Luft B J, Remington J S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 22.Odaert H, Soete M, Fortier B, Camus D, Dubremetz J F. Stage conversion of Toxoplasma gondii in mouse brain during infection and immunodepression. Parasitol Res. 1996;82:28–31. doi: 10.1007/BF03035408. [DOI] [PubMed] [Google Scholar]
- 23.Parmley S F, Gross U, Sucharczuk A, Windeck T, Sgarlato G D, Remington J S. Two alleles of the gene encoding surface antigen P22 in 25 strains of Toxoplasma gondii. J Parasitol. 1994;80:293–301. [PubMed] [Google Scholar]
- 24.Radke J, White M W. A cell cycle model for the tachyzoite of Toxoplasma gondii using the Herpes simplex virus thymidine kinase. Mol Biochem Parasitol. 1998;94:237–247. doi: 10.1016/s0166-6851(98)00074-7. [DOI] [PubMed] [Google Scholar]
- 25.Ricard J, Pelloux H, Pathak S, Pipy B, Ambroise-Thomas P. TNF-α enchances Toxoplasma gondii cyst formation in human fibroblasts through the sphingomyelinase pathway. Cell Signalling. 1996;8:439–442. doi: 10.1016/s0898-6568(96)00079-4. [DOI] [PubMed] [Google Scholar]
- 26.Roos D S, Donald R G K, Morrissette N S, Moulton A L C. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1995;45:25–61. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 27.Scharton-Kersten T M, Wynn T A, Denkers E Y, Bala S, Grunvald E, Heiny S, Gazzinelli R T, Sher A. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 28.Sibley L D, Boothroyd J C. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature (London) 1992;359:82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 29.Soete M, Fortier B, Camus D, Dubremetz J F. Toxoplasma gondii: kinetics of bradyzoite-tachyzoite interconversion in vitro. Exp Parasitol. 1993;76:259–264. doi: 10.1006/expr.1993.1031. [DOI] [PubMed] [Google Scholar]
- 30.Soete M, Camus D, Dubremetz J F. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–370. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 31.Speer C A, Hammond D M, Marhrt J L, Roberts W L. Structure of the oocyst and sporocyst walls and the excystation of sporozoites of Isospora canis. J Parasitol. 1973;59:35–40. [PubMed] [Google Scholar]
- 32.Speer C A, Dubey J P, Blixt J A, Prokop K. Time lapse video microscopy and ultrastructure of penetrating sporozoites, types 1 and 2 parasitophorous vacuoles, and the transformation of sporozoites to tachyzoites of the VEG strain of Toxoplasma gondii. J Parasitol. 1997;83:564–574. [PubMed] [Google Scholar]
- 33.Speer C A, Tilley M, Temple M E, Blixt J A, Dubey J P, White M W. Sporozoites of Toxoplasma gondii lack dense-granule protein GRA3 and form a unique parasitophorous vacuole. Mol Biochem Parasitol. 1995;75:75–86. doi: 10.1016/0166-6851(95)02515-4. [DOI] [PubMed] [Google Scholar]
- 34.Tilley M, Fichera M E, Jerome M E, Roos D S, White M W. Toxoplasma gondii sporozoites form a transient parasitophorous vacuole that is impermeable and contains only a subset of dense-granule proteins. Infect Immun. 1997;65:4598–4605. doi: 10.1128/iai.65.11.4598-4605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]