Abstract
Candida albicans activates the classical and alternative complement pathways, leading to deposition of opsonic complement fragments on the cell surface. Our previous studies found that antimannan immunoglobulin G (IgG) in normal human serum (NHS) allows C. albicans to initiate the classical pathway. The purpose of this study was to determine whether antimannan IgG also plays a role in initiation of the alternative pathway. Pooled NHS was rendered free of classical pathway activity by chelation of serum Ca2+ with EGTA alone or in combination with immunoaffinity removal of antimannan antibodies. Kinetic analysis revealed a 6-min lag in detection of C3 binding to C. albicans incubated in EGTA-chelated NHS, compared to a 12-min lag in NHS that was both EGTA chelated and mannan absorbed. The 12-min lag was shortened to 6 min by addition of affinity-purified antimannan IgG. The accelerating effect of antimannan IgG on alternative pathway initiation was dose dependent and was reproduced in a complement binding reaction consisting of six purified proteins of the alternative pathway. Both Fab and F(ab′)2 fragments of antimannan IgG facilitated alternative pathway initiation in a manner similar to that observed with intact antibody. Immunofluorescence analysis showed that addition of antimannan IgG to EGTA-chelated and mannan-absorbed serum promoted an early deposition of C3 molecules on the yeast cells but had little or no effect on distribution of the cellular sites for C3 activation. Thus, antimannan IgG antibodies play an important regulatory role in interactions between the host complement system and C. albicans.
Candida albicans activates the human complement system via both the classical and alternative pathways, leading to deposition of opsonic complement fragments on the yeast cell surface (19, 21, 40). A naturally occurring antimannan immunoglobulin G (IgG) is required for activation of the classical pathway by C. albicans yeast cells incubated in normal human serum (NHS) (21, 40). C3 deposition via the antimannan IgG-dependent classical pathway occurs rapidly and can be detected within 1 min following incubation of the yeast cells with NHS (40). Moreover, initial C3 molecules bound through the classical pathway are uniformly distributed over the entire yeast cell surface (21, 40). C3 deposition through the alternative pathway exhibits distinctly different characteristics. If the classical pathway of NHS is blocked by either treatment with the Ca2+ chelator EGTA (21, 40) or mannan absorption to remove antimannan IgG (40), deposition of initial C3 molecules occurs at a few discrete sites on the yeast cell surface. Furthermore, deposition of initial C3 molecules via the alternative pathway requires a much longer incubation time than C3 binding through the antimannan IgG-dependent classical pathway. For example, if the classical pathway is blocked by treatment of serum with EGTA, there is a 6-min delay before readily detectable amounts of C3 accumulate on the yeast cells; if the classical pathway is blocked by absorption of NHS with mannan to remove initiating antibody, the delay is extended to 12 min (40).
Our interest has focused on the observed difference in the time required for C3 accumulation via the alternative pathway on C. albicans yeast cells incubated in EGTA-chelated serum versus mannan-absorbed serum. One explanation is that mannan absorption of serum reduced the activity of one or more of proteins that are involved in the alternative pathway. This possibility appears unlikely because addition of affinity-purified antimannan IgG to mannan-absorbed serum restores characteristic C3 binding kinetics to levels observed in intact serum (40). Alternatively, the naturally occurring antimannan IgG that is present in EGTA-chelated NHS may facilitate C3 deposition via the alternative pathway.
Antibody-dependent activation of the alternative pathway has been described for several particulate activators. Ratnoff et al. (31) identified three models that have been used to demonstrate antibody-dependent activation of the alternative pathway: (i) experiments done in the presence of EGTA which chelates Ca2+ and thereby prevents activation of C1, (ii) experiments done with purified proteins of the alternative pathway, and (iii) experiments done with sera that are genetically deficient in C4 or C2. Using one or more of these experimental approaches, studies with bacteria, protozoa, virus-infected cells, erythrocytes, cross-linked dextran, and zymosan have shown that antibodies can facilitate activation of the alternative pathway by some particles. The role of antibodies in alternative pathway initiation has not been studied with C. albicans or other pathogenic fungi.
The objectives of this report were to determine whether antimannan IgG in NHS influences alternative pathway-mediated deposition of C3 onto C. albicans yeast cells and to determine the molecular components of IgG molecules that are involved in this process. Our results show that (i) very little deposition of C3 occurs in the absence of antimannan IgG, (ii) antimannan IgG accelerates alternative pathway initiation in a dose-dependent manner, and (iii) alternative pathway initiation is facilitated by both Fab and F(ab′)2 fragments of antimannan IgG.
MATERIALS AND METHODS
Yeast and yeast mannan.
C. albicans CA-1, provided by Jim E. Cutler (Montana State University), was used for this study. The culture was maintained and grown as described (15, 21). Briefly, yeast cells stored at −80°C in 50% glycerol were used to start a fresh culture in GYEP (2% glucose, 0.3% yeast extract, 1% peptone). The yeast culture was passaged three times at 24-h intervals at 37°C. Yeast cells were killed by treatment with 1% formaldehyde overnight at 4°C, harvested by centrifugation, washed, resuspended in phosphate-buffered saline (PBS) containing 0.02% azide, and stored at 4°C.
The mannan of yeast CA-1 was extracted and isolated as described elsewhere (18, 40). Water-soluble mannan was precipitated with Fehling solution; mannan was released from the precipitate into water containing Amberlite IR-120 (H+) resin (Aldrich, Milwaukee, Wis.), dialyzed against water, and lyophilized. C. albicans yeast mannan obtained by this method typically contains about 95% carbohydrate as mannan and about 2% protein (18). Candida mannan was coupled to CNBr-Sepharose 4B (Pharmacia Biotech, Uppsala, Sweden) as described elsewhere (40).
Serum and serum proteins.
C3 (20, 38), factor B (11, 16), factor D (24), and factor H (11, 35) were isolated from frozen human plasma (Reno Blood Services, Reno, Nev.). Purified proteins were stored at −80°C until use. Human factor I (Sigma, St. Louis, Mo.) and human properdin (Calbiochem, La Jolla, Calif.) were purchased. C3 was labeled with 125I as described previously (8) by use of Iodogen reagent (Pierce, Rockford, Ill.).
Pooled serum was prepared from peripheral blood collected from at least 10 normal donors after informed consent and was stored at −80°C. Serum was heated for 30 min at 56°C for studies requiring heat-inactivated serum. To prepare mannan-absorbed serum, NHS was incubated with mannan-Sepharose 4B beads in a ratio of 6:4 (vol/vol) for 60 min at 0°C with mixing every 10 to 15 min and then separated from the beads by centrifugation (40). The absorbed serum was filtered through a 0.45-μm-pore-size filter and used immediately. The concentration of absorbed serum was corrected for the dilution that occurred during the absorption procedure.
Naturally occurring human antimannan IgG was isolated from pooled human plasma by immunoaffinity chromatography using mannan-Sepharose 4B followed by affinity purification on protein A as described elsewhere (40). Fab or F(ab′)2 fragments were prepared by digestion of antimannan IgG with papain or pepsin, respectively, by use of commercial digestion kits (catalog no. 44885 and 44888; Pierce). Purity of the prepared IgG, Fab, or F(ab′)2 was confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis under reducing conditions and Coomassie blue staining. Concentrations of purified IgG, Fab, or F(ab′)2 were estimated by the bicinchoninic acid method (Pierce). The concentration of antimannan IgG was corrected to reflect the proportion of purified IgG that retained binding capacity after immunoaffinity purification (33% for the lot of antimannan IgG used for this study) as shown by absorption with Candida yeast cells (40). The affinity-purified antimannan IgG was used as a standard in enzyme-linked immunosorbent assays to estimate the amount of naturally occurring antimannan IgG antibodies in pooled serum preparations (40). The estimated quantity of naturally occurring antimannan IgG in the serum pool was used as the basis for addition of purified antimannan IgG to the pooled serum that was absorbed with immobilized mannan.
Quantitative analysis of C3 binding using 125I-C3.
Binding of C3 to candidal yeast cells was analyzed by the procedure of Kozel et al. (21). Briefly, each complement binding medium contained (i) 40% mannan-absorbed or nonabsorbed serum and (ii) 125I-labeled C3 sufficient to provide a specific activity of 50,000 cpm/μg of C3 for the mixture of labeled and unlabeled C3 in the serum (assuming that NHS contains 1,200 μg of C3/ml). To study classical pathway activation, the binding medium contained GVB2+ buffer (sodium Veronal [5 mM]-buffered saline [142 mM], pH 7.3, containing 0.1% gelatin, 1.5 mM CaCl2, and 1 mM MgCl2). To study alternative pathway activation, the medium contained GVB-Mg-EGTA (sodium Veronal [5 mM]-buffered saline [142 mM], pH 7.3, containing 0.1% gelatin, 5 mM EGTA, and 5 mM MgCl2). In some experiments, purified human antimannan IgG, or its Fab or F(ab′)2 fragments, was added to the binding medium prior to addition of the yeast cells. The reaction medium was warmed to and kept at 37°C, and 2.0 × 106 yeast cells per ml of reaction medium were added to initiate C3 binding. To study the kinetics of C3 binding, 50-μl samples were withdrawn in duplicate at various time intervals and added to 200 μl of a stop solution (PBS, 0.1% SDS, 20 mM EDTA) in Millipore MABX-N12 filter plates fitted with BV 1.2-μm-pore-size filter membranes (Millipore, Bedford, Mass.). The particles were washed five times with PBS containing 0.1% SDS. The membranes were removed, and the amount of radioactivity bound to the yeast cells collected on the membranes was determined with a Packard AUTO-GAMMA gamma counter. Specific binding was determined by subtracting the radioactivity of samples which used heat-inactivated serum from the total binding observed with NHS or other serum preparations.
The kinetics of C3 deposition was also studied in an alternative pathway that was reconstituted in GVB containing 0.1% gelatin and 1 mM MgCl2 from six purified proteins of the alternative pathway as described elsewhere (22, 34). Factors B, D, H, and I and C3 were used at 40% of their physiological concentrations (22). As recommended by the supplier (Calbiochem), properdin was used at five times as much as 40% of its physiological concentration to compensate for loss of activity during purification and/or storage.
Immunofluorescence analysis of C3 binding.
The pattern of C3 binding to the yeast cell surface was determined by immunofluorescence (40). Yeast cells were opsonized as described above for the quantitative analysis except that the reaction mixtures did not contain 125I-C3. At various time intervals, 200-μl samples were transferred to 900 μl of ice-cold PBS containing 10 mM EDTA, and unbound C3 was removed by three washes with PBS. The yeast-bound C3 was detected with fluorescein isothiocyanate (FITC)-conjugated goat anti-human C3 antibodies (Kent Laboratories, Indianapolis, Ind.). The fluorescence images of C3 deposition on the yeast surface were acquired at 0.4-μm intervals through individual cells by use of an epifluorescence microscope equipped with a Colour CoolView image acquisition system (Photonic Science, East Sussex, United Kingdom) and were processed with the aid of image analysis software including Image-Pro Plus (Media Cybernetics, Silver Spring, Md.) and MicroTome (VayTeK, Fairfield, Iowa). Each stack of images acquired from a single cell was digitally deconvolved and projected onto a single plane.
RESULTS
Naturally occurring antimannan IgG facilitates C3 binding via the alternative pathway.
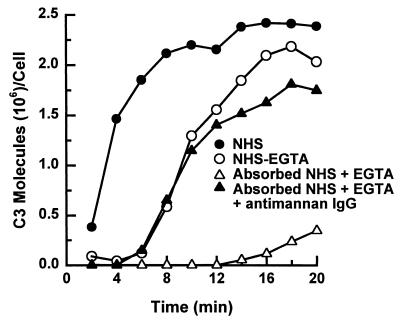
In our previous studies, we observed that alternative pathway activation by C. albicans yeast was faster in EGTA-treated NHS that contains naturally occurring antimannan IgG than in NHS that was rendered free of antimannan antibody by mannan absorption. This observation suggests that antimannan IgG may facilitate alternative pathway initiation. To test this hypothesis, we examined the effect of purified antimannan IgG on the kinetics of alternative pathway activation. Kinetics of C3 deposition to yeast cells was analyzed over 20 min in a complement binding medium containing (i) 40% NHS, (ii) 40% EGTA-treated NHS, (iii) 40% NHS that was both mannan absorbed and EGTA treated, or (iv) 40% NHS that was both mannan absorbed and EGTA treated and supplemented with antimannan IgG at 88 μg per ml of binding mixture, which was 40% of the antimannan IgG concentration found in the pooled serum used for this experiment. The results (Fig. 1) showed that addition of antimannan IgG to serum that was both mannan absorbed and EGTA treated reduced the lag in C3 deposition from 12 to 6 min and fully restored the kinetics of C3 deposition to that observed in EGTA-treated NHS.
FIG. 1.
Effect of antimannan IgG on the kinetics for C3 deposition on C. albicans yeast cells via the alternative pathway. Yeast cells were incubated in a C3 binding medium containing 40% NHS, 40% EGTA-chelated NHS, 40% mannan-absorbed and EGTA-chelated NHS, or 40% mannan-absorbed and EGTA-chelated NHS supplemented with antimannan IgG at 88 μg per ml of reaction mixture, which was 40% of antimannan IgG concentration found in the pooled serum used for this experiment.
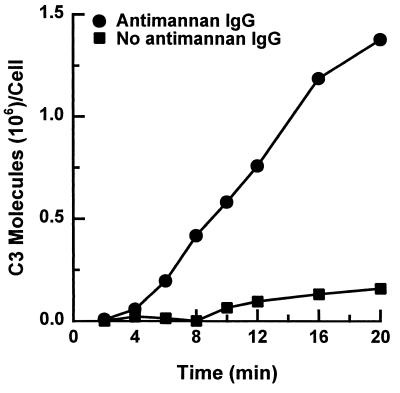
To confirm the role of antimannan IgG as an accelerator of alternative pathway activation, serum in the complement binding reactions was replaced with the six isolated proteins required for alternative pathway activity: factors B, D, H, I, and properdin and C3. Yeast cells were incubated for 2 to 20 min with these six proteins alone or with the six proteins supplemented with antimannan IgG at 30 μg per ml of reaction mixture. Use of 30 μg per ml of reaction mixture was based on dose-response experiments which established the optimal antibody concentrations required for antibody-accelerated activation of the alternative pathway (see below). In the absence of antimannan IgG, deposition of C3 on the yeast was very limited (Fig. 2); addition of antimannan IgG reduced the time required for binding of readily measurable amounts of C3 via the alternative pathway and promoted a sevenfold increase in the amount of accumulated C3 at the end of the 20-min incubation.
FIG. 2.
Effect of antimannan IgG on alternative pathway activity reconstituted from six purified alternative pathway proteins. Yeast cells were incubated in a C3 binding medium containing the isolated alternative pathway proteins alone or with purified antimannan IgG at 30 μg per ml of reaction mixture.
Effect of antimannan IgG on alternative pathway initiation is dose dependent.
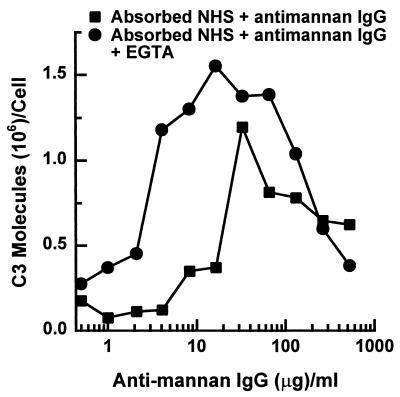
Because activation of the classical pathway by C. albicans yeast cells is dependent on antimannan IgG (40), the observation that antimannan IgG also accelerates alternative pathway initiation prompted us to compare dose requirements for antimannan IgG in C3 deposition via these two pathways. Yeast cells were incubated for 6 min in a complement binding medium containing mannan-absorbed NHS and various amounts of antimannan IgG, and the amount of C3 bound per cell was measured. Accumulation of C3 on yeast cells after a 6-min incubation was used as a measure of classical pathway activity because in the absence of the classical pathway activity, as in EGTA-chelated serum or mannan-absorbed serum, there is little or no detectable C3 on the yeast cells (Fig. 1 and reference 40). Consequently, C3 binding during the first 6 min of incubation reflects classical pathway activity. The dose effect of antimannan IgG on alternative pathway activity was assessed following an incubation of yeast cells for 12 min in NHS that was both mannan absorbed and EGTA chelated and supplemented with various amounts of antimannan IgG. Accumulation of C3 molecules on yeast cells after 12 min was taken as a measure of alternative pathway activity because in the absence of antimannan IgG, as in mannan-absorbed NHS, there is little or no detectable C3 on the yeast cells after 12 min (Fig. 1). EGTA chelation was needed to limit C3 binding to action of the alternative pathway.
Results in Fig. 3 show that antimannan IgG had both positive and negative effects on C3 binding via either the classical or the alternative pathway. When the IgG was supplemented up to approximately 30 μg per ml of binding reaction, it promoted C3 binding via either pathway. However, accumulation of C3 through the alternative pathway required less antimannan IgG than accumulation through the classical pathway. For example, it was estimated by linear regression that binding of 106 C3 molecules to yeast cells via the alternative pathway required 5 μg of antimannan IgG per ml of reaction mixture, compared to 30 μg of antimannan IgG by the classical pathway. When antimannan IgG reached a level higher than 30 μg/ml, it began to suppress accumulation of C3 activated by either pathway.
FIG. 3.
Dose effect of exogenous antimannan IgG on binding of C3 to C. albicans via the classical or alternative pathway. C3 binding reactions were supplemented with various amounts of antimannan IgG per milliliter of binding mixture that contained either 40% mannan-absorbed serum to assess the ability of yeast cells to activate the classical pathway following a 6-min incubation or 40% mannan-absorbed and EGTA-chelated serum to assess the ability of yeast cells to activate the alternative pathway following a 12 min incubation.
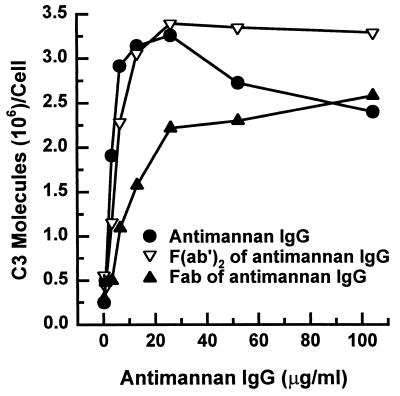
Fab or F(ab′)2 fragments of antimannan IgG facilitate C3 binding via the alternative pathway.
Previous studies found that the Fc fragment of IgG was not needed for antibody-dependent activation of the alternative pathway; however, a requirement for bivalency in activation of the alternative pathway depended on the system under study (6, 7, 13, 17, 26, 29, 32, 36, 39). To determine whether Fab or F(ab′)2 fragments were able to accelerate alternative pathway activation, yeast cells were incubated for 12 min in complement binding mixtures containing NHS that was both mannan absorbed and EGTA chelated and was supplemented with various amounts of either intact antimannan IgG or its Fab or F(ab′)2 fragments. The results (Fig. 4) showed that both Fab or F(ab′)2 fragments accelerated C3 deposition via the alternative pathway in a dose-dependent manner. F(ab′)2 fragments appeared to be more effective than F(ab′) fragments and acted in a manner indistinguishable from that of intact antimannan IgG. The pattern of the results shown in Fig. 4 was not appreciably affected if the amount of input antimannan IgG or its fragments was expressed as either molarity or normality. Taken together, these results indicate that neither the Fc region nor bivalency is essential for facilitation of the alternative pathway by antimannan IgG.
FIG. 4.
Effect of Fab or F(ab′)2 fragments of antimannan IgG on alternative pathway mediated-C3 deposition to yeast cells. Yeast cells were incubated in a C3 binding medium containing 40% mannan-absorbed and EGTA-chelated NHS that was supplemented with various amounts of intact antimannan IgG or its Fab or F(ab′)2 fragments.
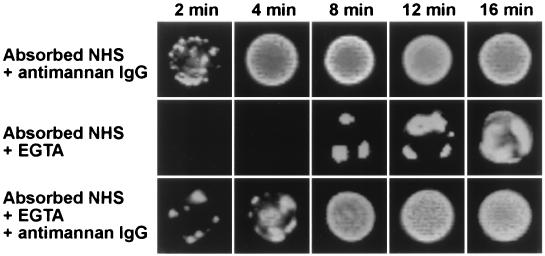
Antimannan IgG promotes early formation of alternative pathway initiation sites.
The above observations describe quantitative effects of antimannan IgG on C3 binding via the alternative pathway. Previous studies from our laboratory used immunofluorescence analysis to show that initial binding of C3 via the classical pathway is rapid and occurs over the entire C. albicans surface (21, 40). The binding of initial C3 molecules activated through the alternative pathway, as in mannan-absorbed serum, is slow and begins from a few discrete sites on the cell surface (21, 40). Consequently, we sought to determine the effect of antimannan IgG on the pattern of alternative-pathway-dependent C3 deposition on the yeast cell surface. Yeast cells were incubated for 2 to 16 min in serum that had been (i) mannan absorbed and supplemented with antimannan IgG (antibody-mediated activation of the classical pathway), (ii) both mannan absorbed and EGTA chelated (antibody-independent activation of the alternative pathway), or (iii) both mannan absorbed and EGTA chelated and supplemented with antimannan IgG (antibody-mediated activation of the alternative pathway). The binding patterns of C3 molecules were detected with FITC-labeled anti-C3 antibodies (Fig. 5). Yeast cells incubated in mannan-absorbed serum that was supplemented with antimannan IgG showed early binding (2 min) of C3 at numerous sites on the yeast surface, and the yeast cell was uniformly covered with C3 after 4 min of incubation. If C3 binding was restricted to antibody-independent activation of the alternative pathway due to mannan absorption and EGTA treatment of the serum, C3 binding occurred as focal initiation sites that were delayed in their occurrence (8 min) and appeared to expand with time to cover the cell (Fig. 5). However, addition of antimannan IgG to the mannan-absorbed and EGTA-treated serum promoted early deposition of initial C3 molecules to the yeast (2 min) but had little effect on the pattern of distribution of initiation sites (Fig. 5). Thus, the critical difference between antibody-dependent and antibody-independent activation of the alternative pathway was the time required for formation of the initiation foci; foci formed more rapidly in the presence of antimannan antibody.
FIG. 5.
Effect of exogenous antimannan IgG on formation of alternative pathway initiation sites. Yeast cells were incubated in a complement binding medium that contained 40% mannan-absorbed NHS and 26 μg of antimannan IgG per ml of reaction mixture (classical pathway intact), 40% mannan-absorbed and EGTA-chelated NHS (antibody-independent activation of the alternative pathway), or 40% mannan-absorbed and EGTA-chelated NHS and 26 μg antimannan IgG per ml reaction mixture (antibody-dependent activation of the alternative pathway). Yeast cells were stained with FITC-labeled goat anti-human C3 antibodies.
DISCUSSION
Mannan is a prominent structural component displayed on the cell surface of C. albicans yeast cells (4), and antimannan IgG is present ubiquitously in the general human population (10, 14, 23). Previously, we showed that this naturally occurring antimannan IgG is required for activation of the classical complement pathway when C. albicans is incubated in NHS (40). We now present evidence that this naturally occurring antimannan IgG also regulates activation of the alternative pathway by C. albicans yeast cells. In NHS that is deficient in antimannan antibodies and lacks classical pathway activity as a result of EGTA chelation of serum Ca2+, addition of exogenous antimannan IgG accelerated C3 binding via the alternative pathway in a dose-dependent manner (Fig. 1 and 3). This accelerating effect of antimannan IgG was confirmed in a serum-free complement binding medium that consisted of only purified proteins of the alternative pathway (Fig. 2) as well as in immunofluorescence analysis (Fig. 5). A similar regulatory role for antibodies in alternative pathway activation has been reported in experimental systems using bacteria (3, 7, 37), protozoa (2, 12, 17), virus-infected cells (6, 36), erythrocytes (26, 29, 30), and zymosan (32, 33). In most cases, the antibody was a ubiquitous natural antibody found in NHS. To our knowledge, our study provides the first documented observation of antibody-facilitated activation of the alternative pathway by pathogenic fungi. Moreover, this study identifies mannan as the antigen recognized by the facilitating antibody. This current observation together with our previous report that antimannan IgG is an initiator of the classical pathway in NHS establish an essential function of naturally occurring antimannan IgG in modulation of interactions between the host complement defense system and C. albicans.
Acceleration of alternative pathway activation by antimannan IgG was also demonstrated in experiments involving only Fab or F(ab′)2 fragments (Fig. 4). Fab or F(ab′)2 fragments of IgG as functional components in antibody-mediated alternative pathway activation have been reported for several other experimental systems, including bacteria (7, 13, 39), protozoa (17), virus-infected cells (6, 36), erythrocytes (1, 26, 29), and zymosan (32). In each of these studies, F(ab′)2 fragments were effective activators of the alternative pathway. Fab fragments were found to activate the alternative pathway in some studies (1, 6, 7, 17, 26, 39) but not in others (13, 29, 32, 36). The failure of Fab fragments to facilitate alternative pathway activation may be due to reduced avidity of the Fab fragments. This may account for our observation that Fab fragments were somewhat less effective on a dose basis than F(ab′)2 fragments (Fig. 4).
The mechanism by which antibody facilitates activation of the alternative pathway by C. albicans remains to be determined. Mechanisms that have been proposed to explain antibody-dependent activation of the alternative pathway in other systems include (i) increased efficiency in binding of metastable C3b to the particles (27, 32, 33), (ii) stabilization of the alternative pathway C3 convertase (29), and (iii) reduced interaction of particle-bound C3b with factor H (5, 29). Perhaps the mechanism varies with the particle under study. It is clear from our examination of the sites of early C3 binding via antibody-dependent and antibody-independent alternative pathway activation (Fig. 5) that the antimannan antibody does not change the fundamental pattern of C3 deposition; rather, the antibody accelerates the rate at which this pattern emerges. This increase in the rate of formation of focal initiation sites is compatible with any of the mechanisms proposed for antibody-dependent activation of the alternative pathway.
Perhaps the most striking result of our study is the almost complete dependence of complement activation by C. albicans on antimannan IgG. Immunoaffinity absorption of NHS to remove antimannan IgG markedly delayed the rate of accumulation of C3 on cells incubated in the antibody-depleted serum. Incubation of C. albicans in a mixture of highly purified proteins of the alternative pathway produced very little C3 deposition during a 20-min incubation. These results indicate that C. albicans has little intrinsic ability to mediate C3 deposition at the cell surface via the alternative pathway. Consequently, antimannan IgG is critical to rapid and maximal complement opsonization of C. albicans yeast cells.
Animal models have shown that complement activation is a critical innate host defense mechanism in experimental candidiasis (9, 25, 28); consequently, efficient activation of the host complement system may well determine the fate of invading C. albicans. Given the importance of antimannan antibody to opsonization of C. albicans, does the absence of antimannan IgG represent a risk factor for disseminated candidiasis? Conversely, the importance of antibodies in complement activation lends support for immunization as a means to increase resistance to systemic candidiasis. We (40) and others (10, 14, 23) have shown that levels of antimannan antibody are highly variable among normal donors. Moreover, there is a direct correlation between the level of antibody in individual sera and the rate of accumulation of C3 on C. albicans via the classical pathway (40). Our observation that lower amounts of antimannan IgG are required to facilitate activation of the alternative pathway than are required for activation of the classical pathway suggests that antibody-dependent activation of the alternative pathway may be a first-line defense in disseminated candidiasis. On the other hand, our dose-response experiment suggests that maximal activation of the alternative complement pathway by C. albicans may require an optimal amount of antimannan IgG (Fig. 3). How the amount and specificity of antimannan IgG influences the interactions between human complement defense system and C. albicans is largely unknown. Studies in progress are designed to assess the contribution of quantitative and qualitative variability in antimannan antibodies in individual sera to C3 deposition via the alternative pathway.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants AI 14209 and AI 37194.
We thank Randall MacGill and Kevin Wall for technical assistance.
REFERENCES
- 1.Albar J P, Juarez C, Vivanco-Martinez F, Bragado R, Ortiz F. Structural requirements of rabbit IgG F(ab′)2 fragment for activation of the complement system through the alternative pathway. I. disulfide bonds. Mol Immunol. 1981;18:925–934. doi: 10.1016/0161-5890(81)90015-8. [DOI] [PubMed] [Google Scholar]
- 2.Aydintug M K, Leid R W, Widders P R. Antibody enhances killing of Tritrichomonas foetus by the alternative bovine complement pathway. Infect Immun. 1990;58:944–948. doi: 10.1128/iai.58.4.944-948.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornson A B, Lobel J S. Lack of a requirement for the Fc region of IgG in restoring pneumococcal opsonization via the alternative complement pathway in sickle cell disease. J Infect Dis. 1986;154:760–769. doi: 10.1093/infdis/154.5.760. [DOI] [PubMed] [Google Scholar]
- 4.Calderone R A, Braun P C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991;55:1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards M S, Nicholson-Weller A, Baker C J, Kasper D L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980;151:1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrnst A. Separate pathways of C activation by measles virus cytotoxic antibodies: subclass analysis and capacity of F(ab) molecules to activate C via the alternative pathway. J Immunol. 1978;121:1206–1212. [PubMed] [Google Scholar]
- 7.Eisenberg R A, Schwab J H. Arthropathic group A streptococcal cell walls require specific antibody for activation of human complement by both the classical and alternative pathways. Infect Immun. 1986;53:324–330. doi: 10.1128/iai.53.2.324-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraker P J, Speck J C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a, 6a-diphenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 9.Gelfand J A, Hurley D L, Fauci A S, Frank M M. Role of complement in host defense against experimental disseminated candidiasis. J Infect Dis. 1978;138:9–16. doi: 10.1093/infdis/138.1.9. [DOI] [PubMed] [Google Scholar]
- 10.Greenfield R A, Stephens J L, Bussey M J, Jones J M. Quantitation of antibody to Candida mannan by enzyme-linked immunosorbent assay. J Lab Clin Med. 1983;101:758–771. [PubMed] [Google Scholar]
- 11.Hammer C H, Wirtz G H, Renfer L, Gresham H D, Tack B F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981;256:3995–4003. [PubMed] [Google Scholar]
- 12.Hoover D L, Berger M, Oppenheim M H, Hockmeyer W T, Meltzer M S. Cytotoxicity of human serum for Leishmania donovani amastigotes: antibody facilitation of alternate complement pathway-mediated killing. Infect Immun. 1985;47:247–252. doi: 10.1128/iai.47.1.247-252.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joiner K A, Goldman R C, Hammer C H, Leive L, Frank M M. Studies of the mechanism of bacterial resistance to complement-mediated killing. V. IgG and F(ab′)2 mediate killing of E. coli 0111B4 by the alternative complement pathway without increasing C5b-9 deposition. J Immunol. 1983;131:2563–2569. [PubMed] [Google Scholar]
- 14.Jones J M. Quantitation of antibody against cell wall mannan and a major cytoplasmic antigen of Candida in rabbits, mice, and humans. Infect Immun. 1980;30:78–89. doi: 10.1128/iai.30.1.78-89.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanbe T, Jutila M A, Cutler J E. Evidence that Candida albicans binds via a unique adhesion system on phagocytic cells in the marginal zone of the mouse spleen. Infect Immun. 1992;60:1972–1978. doi: 10.1128/iai.60.5.1972-1978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr M A. Human factor B. J Pathol Bacteriol. 1981;80:102–111. [Google Scholar]
- 17.Kipnis T L, Krettli A U, Dias Da Silva W. Transformation of trypomastigote forms of Trypanosoma cruzi into activators of alternative complement pathway by immune IgG fragments. Scand J Immunol. 1985;22:217–226. doi: 10.1111/j.1365-3083.1985.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi H, Shibata N, Mitobe H, Ohkubo Y, Suzuki S. Structural study of phosphomannan of yeast-form cells of Candida albicans J-1012 strain with special reference to application of mild acetolysis. Arch Biochem Biophys. 1989;272:364–375. doi: 10.1016/0003-9861(89)90230-0. [DOI] [PubMed] [Google Scholar]
- 19.Kozel T R, Brown R R, Pfrommer G S T. Activation and binding of C3 by Candida albicans. Infect Immun. 1987;55:1890–1894. doi: 10.1128/iai.55.8.1890-1894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozel T R, Pfrommer G S T. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun. 1986;52:1–5. doi: 10.1128/iai.52.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozel T R, Weinhold L C, Lupan D M. Distinct characteristics of initiation of the classical and alternative complement pathways by Candida albicans. Infect Immun. 1996;64:3360–3368. doi: 10.1128/iai.64.8.3360-3368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozel T R, Wilson M A, Pfrommer G S T, Schlageter A M. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989;57:1922–1927. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann P F, Reiss E. Comparison by ELISA of serum anti-Candida albicans mannan IgG levels of a normal population and in diseased patients. Mycopathologia. 1980;70:89–93. doi: 10.1007/BF00443073. [DOI] [PubMed] [Google Scholar]
- 24.Lesavre P H, Hugli T E, Esser A F, Muller-Eberhard H J. The alternative pathway C3/C5 convertase: chemical basis of factor B activation. J Immunol. 1979;123:529–534. [PubMed] [Google Scholar]
- 25.Lyon F L, Hector R F, Domer J E. Innate and acquired immune responses against Candida albicans in congenic B10.D2 mice with deficiency of the C5 complement component. J Med Vet Mycol. 1986;24:359–367. doi: 10.1080/02681218680000551. [DOI] [PubMed] [Google Scholar]
- 26.Moore F D, Jr, Austen K F, Fearon D T. Antibody restores human alternative complement pathway activation by mouse erythrocytes rendered functionally deficient by pretreatment with pronase. J Immunol. 1982;128:1302–1306. [PubMed] [Google Scholar]
- 27.Moore F D, Jr, Fearon D T, Austen K F. IgG on mouse erythrocytes augments activation of the human alternative complement pathway by enhancing deposition of C3b. J Immunol. 1981;126:1805–1809. [PubMed] [Google Scholar]
- 28.Morelli R, Rosenberg L T. Role of complement during experimental Candida infection in mice. Infect Immun. 1971;3:521–523. doi: 10.1128/iai.3.4.521-523.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson B, Ruddy S. Enhancing role of IgG in lysis of rabbit erythrocytes by the alternative pathway of human complement. J Immunol. 1979;122:1994–1999. [PubMed] [Google Scholar]
- 30.Polhill R B, Jr, Newman S L, Pruitt K M, Johnston R B., Jr Kinetic assessment of alternative complement pathway activity in a hemolytic system. II. Influence of antibody on alternative pathway activation. J Immunol. 1978;121:371–376. [PubMed] [Google Scholar]
- 31.Ratnoff W D, Fearon D T, Austen K F. The role of antibody in the activation of the alternative complement pathway. Springer Semin Immunopathol. 1983;6:361–371. doi: 10.1007/BF02116280. [DOI] [PubMed] [Google Scholar]
- 32.Schenkein H A, Ruddy S. The role of immunoglobulins in alternative complement pathway activation by zymosan. I. Human IgG with specificity for zymosan enhances alternative pathway activation by zymosan. J Immunol. 1981;126:7–10. [PubMed] [Google Scholar]
- 33.Schenkein H A, Ruddy S. The role of immunoglobulins in alternative pathway activation by zymosan. II. The effect of IgG on the kinetics of the alternative pathway. J Immunol. 1981;126:11–15. [PubMed] [Google Scholar]
- 34.Schreiber R D, Pangburn M K, Lesavre P H, Müller-Eberhard H J. Initiation of the alternative pathway of complement: recognition of activators by bound C3b and assembly of the entire pathway from six isolated proteins. Proc Natl Acad Sci USA. 1978;75:3948–3952. doi: 10.1073/pnas.75.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sim R B, DiScipio R G. Purification and structural studies on the complement-system control protein β1H (factor H) Biochem J. 1982;205:285–293. doi: 10.1042/bj2050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sissons J G P, Cooper N R, Oldstone M B A. Alternative complement pathway-mediated lysis of measles virus infected cells: induction by IgG antibody bound to individual viral glycoproteins and comparative efficacy of F(ab′)2 and Fab′ fragments. J Immunol. 1979;123:2144–2149. [PubMed] [Google Scholar]
- 37.Steele N P, Munson R S, Granoff D M, Cummins J E, Levine R P. Antibody-dependent alternative pathway killing of Haemophilus influenzae type b. Infect Immun. 1984;44:452–458. doi: 10.1128/iai.44.2.452-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tack B F, Janatova J, Thomas M L, Harrison R A, Hammer C H. The third, fourth, and fifth components of human complement: isolation and biochemical properties. Methods Enzymol. 1981;80:64–101. [Google Scholar]
- 39.Wachter E, Brade V. Influence of surface modulations by enzymes and monoclonal antibodies on alternative complement pathway activation by Yersinia enterocolitica. Infect Immun. 1989;57:1984–1989. doi: 10.1128/iai.57.7.1984-1989.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M X, Lupan D M, Kozel T R. Mannan-specific IgG antibodies in normal human serum mediate classical pathway initiation of C3 binding to Candida albicans. Infect Immun. 1997;65:3822–3827. doi: 10.1128/iai.65.9.3822-3827.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]