Abstract

Well-performing organic–inorganic halide perovskites are susceptible to poor efficiency and instability due to their various defects at the interphases, grain boundaries (GBs), and surfaces. In this study, an in situ method is utilized for effectively passivating the under-coordinated Pb2+ defects of perovskite with new non-fullerene acceptors (NFAs) (INXBCDT; X = H, Cl, and Br) through their carbonyl and cyano functional groups during the antisolvent dripping process. It reveals that the bicyclopentadithiophene (BCDT) core with highly electron-withdrawing end-capping groups passivates GBs and boosts perovskite grain growth. This effective defect passivation decreases the trap density to increase the carrier recombination lifetime of the perovskite film. As a result, bromo-substituted dicyanomethylene indanone (INBr)-end-capped BCDT (INBrBCDT-b8; 3a)-passivated devices exhibit the highest power conversion efficiency (PCE) of 22.20% (vs those of 18.09% obtained for perovskite films without passivation) upon an optimized film preparation process. Note that devices treated with more soluble 2-ethylhexyl-substituted compounds (1a, 2a, and 3a) exhibit higher PCE than those treated with less soluble octyl-substituted compounds (1b, 2b, and 3b). It is also worth noting that BCDT is a cost-effective six-ring core that is easier to synthesize with a higher yield and therefore much cheaper than those with highly fused-ring cores. In addition, a long-term stability test in a glovebox for 1500 h reveals that the perovskite solar cells (PSCs) based on a perovskite absorber treated with compound 3a maintain ∼90% of their initial PCE. This is the first example of the simplest high-conjugation additive for perovskite film to achieve a PCE greater than 22% of the corresponding lead-based PSCs.
Keywords: perovskite solar cells, bicyclopentadithiophene, non-fullerene acceptors, grain boundary passivation, high performance
1. Introduction
Due to the growing demand for sustainable energy, researchers mainly focus on the development of photovoltaic (PV) technologies, which convert solar energy into electrical energy. Compared to other futuristic PV technologies, perovskite solar cells (PSCs) are a potential candidate for renewable clean energy technology.1−3 In the last 10 years, PSCs have shown impressive advancements in conversion efficiencies from 3.8% in 20094 to 26.1%5 today in a single-junction architecture. PSCs have therefore been regarded as the fastest-advancing solar technology in 2016.6 PSCs have also received widespread attention, primarily because of their exceptional optoelectronic characteristics,7−9 high efficiency, low weight, high flexibility, scalable low-temperature solution processability, color tunability, and simple fabrication processes, as well as low manufacturing cost.1,10−15 Despite their rapid advancement, the commercialization of PSC continues to confront obstacles, with inadequate stability being a prominent concern.16 In general, perovskite films fabricated at low-temperature result in inferior device efficiencies, mainly due to the defects at grain boundaries (GBs) and imperfect interfaces or surfaces.17−22 Besides, other instability problems are also caused by these defects, such as ion migration, current hysteresis, and device degradation.7,23 Passivation is a key strategy to obtain excellent surfaces and interfacial/GB properties while simultaneously promoting the efficiency and stability of the cells.24 Therefore, high-efficient and stable PSCs can be achieved through the efficient GB defect passivation of the perovskite films.11,25
Post passivation treatment is successfully applied to decrease the number of defects at the perovskite framework by coordinating with under-coordinated halide or metal ions.26−29 The smart passivation approach involves employing various materials such as metal cations,30,31 organic cations,32,33 anions,34,35 zwitterions,36,37 Lewis acids,38,39 and Lewis bases,26,40 which possess electron-donating/accepting capabilities.24 Among these materials, Lewis-base passivators are well-known passivators to reduce defect densities as well as enhance the device performance of the PSCs, which requires further investigation.29 Lewis bases, such as compounds containing sulfur/nitrogen/oxygen, graphene oxide, n-type π-conjugated molecules, and polymers, have been used as passivating agents.24 However, only a few reports have been found to study GB passivation using electron acceptors/n-type π-conjugated molecules as additives/passivators in PSCs.41−45 Non-fullerene acceptors (NFAs) are some of the π-conjugated Lewis base n-type molecules possessing a highly electron-donating planar molecular framework with suitable side chains and strong electron-withdrawing end-capped units. For example, recently reported IDIC,27IT-4F,7IT-M,46 and ITIC(45) NFAs are excellent performing n-type π-conjugated materials in organic PVs. They have also been demonstrated for effective passivation in PSCs to achieve power conversion efficiency (PCE) of 19.5, 18.3, 20.5, and 19.04%, respectively (Figure 1a). In addition, our group developed a chlorinated seven thiophene ring-fused (DCDTT)-based NFA, INCl-DCDTT, as a passivation agent, and enhanced PCE up to 21.39% was recently achieved.47 These compounds contain oxygen, nitrogen, and sulfur atoms of the carbonyl, cyano, and thiophene groups were proposed to coordinate with Pb2+ ions, passivating the defects through their nonbonded electrons.24 In addition, cyano/carbonyl units containing organic small molecules, SY2(48) and SGT-421,49 were developed and reported as donor-passivating agents in perovskite film and exhibited enhanced PCE of 18.96 and 17.27%, respectively (Figure 1b), for the corresponding PSCs. The results suggest that NFAs are potential efficient passivators for Pb-based perovskite. However, most of these highly efficient passivating NFAs feature a large fused-ring central core (5–7 fused-rings), which usually requires a tedious multiple-step synthesis. According to our own synthetic experience, the yields of these six- to seven-ring-fused NFAs are quite low, which will preclude their practical/commercial applications. Consequently, investigating NFAs with simple synthesis and easy modification in their optoelectronic properties is essential. This exploration is crucial for the production of affordable, high-efficient, and scalable Pb-based PSCs.13 In the current work, as shown in Figure 1c, six newly easy-accessible bicyclopentadithiophene (BCDT)-based NFAs, INBCDT-b8 (1a), INBCDT-8 (1b), INClBCDT-b8 (2a), INClBCDT-8 (2b), INBrBCDT-b8 (3a), and INBrBCDT-8 (3b), are developed to serve as passivating agents for Pb-based perovskites applied in solar cells.
Figure 1.
(a,b) Reported small molecules for the development of PSCs; (c) molecular structures and their performances with regard to BCDT-based compounds (1–3) of passivated lead-perovskite studied in this paper.
Figure S1 illustrates the molecular design and approach to efficient NFA passivators for perovskites based on the theoretical calculation and experimental results. This takes advantage of the simple synthesis of these six A–D–A-type NFAs, where acceptor (A) and donor (D) groups have been prepared individually and then coupled via Knoevenagel condensation. Even though fluorination on the acceptor group is a fruitful approach,50−52 the process of fluorination is generally complicated and expensive, which is adverse for commercial applications.53 Therefore, incorporation of cost-effective chlorinated and brominated indanones, derived from inexpensive starting materials, into the NFA enhances the intramolecular charge transfer (ICT) effect and thus helps to adjust their energy levels as well as perform comparative studies on the resulting cells. Moreover, strong electron-donating alkoxy phenyls were obtained from easier and more inexpensive synthetic procedures to replace the regular alkyl phenyl substitutions, which require catalyzed synthesis. Another important factor is solubility, which is a crucial issue for using NFAs as an additive. For better solubility, we incorporated branched alkyl chains into the structure. Among the six BCDT-based compounds, branched alkyl (2-ethylhexyl)-substituted compounds (1a, 2a, and 3a) possess higher solubility than the other three linear alkyl (octyl)-substituted compounds (1b, 2b, and 3b). Interestingly, the PCEs of PSCs based on perovskite passivated by 1a, 2a, and 3a are also higher than those of the cells based on perovskite passivated by their respective linear alkyl-substituted ones (1b, 2b, and 3b). The brominated-BCDT compound 3a exhibits suitable energy levels, and the perovskite films with the addition of 3a demonstrate larger grain sizes and enhanced crystallinity compared to the original pristine perovskite (CB) films. Thus, the highest PCE of 22.20% was achieved for the PSCs based on 3a passivated perovskite. Note that a dimer of cyclopentadithiophene (CDT), BCDT, is a cost-effective six-ring core that is easier to synthesize with a higher yield compared to those highly fused-ring cores. As far as our knowledge extends, this marks the prime instance of utilizing the simplest high conjugation system of a cost-effective brominated small molecular NFA to be utilized as a donor passivation agent during an antisolvent dripping process for Pb-based perovskites. This has led to the attainment of a PCE greater than 22% in the corresponding solar cells. It is noted that PCEs of all the newly synthesized compounds, 1 (a–b), 2 (a–b), and 3 (a–b)-passivated devices, are significantly exceeding the efficiency of the control device (18.09%). Overall, the results suggest that exceptionally simple NFAs are involved in efficient defect passivation and reveal their important aspects in the molecular design with great future application.
2. Results and Discussion
INXBCDT compounds were synthesized as represented in Scheme 1, and the detailed procedures are given in Schemes S1–S4. In brief, distannylated bithiophene 4 was first coupled with ethyl 2-bromothiophene-3-carboxylate (5) by using Pd(PPh3)4 to give diesters of tetrathiophene 6. Then, via an alkoxyphenyl anion (7) addition to the diesters of 6, benzylic alcohol 8 was produced. The latter was catalyzed by Amberlyst-15, and a BCDT core (BCDT; 9) was obtained. Further, treating 9 with POCl3/DMF via the Vilsmeier–Haack reaction yields dialdehyde 10. Finally, the Knoevenagel condensation of dialdehyde 10 with indanones (11–13) gives target compounds (1–3). The spectra of all characterized compounds are shown in Figures S12–S52. Note that the solubility factor of these compounds is explored by incorporating branched/linear octyl chain substituents onto the central BCDT core. Comparatively, all three branched C8H17-substituted compounds (1a, 2a, and 3a) possess better solubility than their linear C8H17-substituted analogues (1b, 2b, and 3b).
Scheme 1. Synthetic Route to INXBCDT Series (Compounds 1–3).
The physicochemical properties of the INXBCDT derivatives (1–3) are outlined in Table 1. Thermogravimetric analysis (TGA; Figure S2) showed that all six INXBCDT derivatives are thermally stable and exhibit Td in the range of 340–369 °C. Subsequently, the melting point for all BCDTs occurred at >320 °C, proving the exceptional thermal stability of these compounds, suitable for device fabrication and later operation. Figure 2 depicts the UV–vis of the INXBCDT compounds in o-dichlorobenzene (o-DCB). As expected, there is no large variation in the maximum absorption (λmax) by changing the alkyl chain (branched octyl vs linear octyl) of NFAs in the solution state.54,55 A distinct bathochromic shift (∼30 nm) has been observed for the maximum absorption peaks of brominated (3a and 3b; 784 and 779 nm) and chlorinated (2a and 2b; ∼780 nm) NFAs in contrast to the nonhalogenated derivatives (1a and 1b; 750 nm). The energy gaps [ΔEg (opt) values] obtained for compounds 2 and 3 (∼1.43 eV) are smaller than those of 1 (1.49 eV). The trend is consistent with the values obtained from the differential pulse voltammetry (DPV) measurement (Figure 3a), in which the energy gaps of 2 and 3 (∼1.50 eV) are confirmed to be smaller than those of 1 (1.56 eV; Table 1 and Figure 3b; vide infra).15,53,54,56,57
Table 1. Photophysical and Electrochemical Data of INXBCDTs.
| passivating agents | Tda [°C] | Tmb [°C] | λabs (soln) [nm]c | ΔEg (opt) [eV]d | Eox [V]e | HOMO [eV]f | Ered [V]e | LUMO [eV]f | ΔEg (DPV) [eV]g | ΔEg (DFT) [eV]h |
|---|---|---|---|---|---|---|---|---|---|---|
| INBCDT-b8 (1a) | 369 | 359 | 750 | 1.49 | 1.17 | –5.61 | –0.40 | –4.04 | 1.57 | 1.91 |
| INBCDT-8 (1b) | 369 | 358 | 750 | 1.49 | 1.17 | –5.61 | –0.40 | –4.04 | 1.56 | |
| INClBCDT-b8 (2a) | 340 | 335 | 780 | 1.44 | 1.23 | –5.67 | –0.29 | –4.15 | 1.52 | 1.86 |
| INClBCDT-8 (2b) | 355 | 328 | 779 | 1.44 | 1.23 | –5.67 | –0.28 | –4.16 | 1.51 | |
| INBrBCDT-b8 (3a) | 342 | 321 | 784 | 1.43 | 1.24 | –5.68 | –0.28 | –4.16 | 1.51 | 1.87 |
| INBrBCDT-8 (3b) | 347 | 341 | 779 | 1.44 | 1.23 | –5.67 | –0.28 | –4.16 | 1.50 |
By TGA.
From melting point apparatus.
Determined in o-DCB.
Calculated by using the optical absorption onset, ΔEg = 1240/λonset.
By DPV in o-DCB.
HOMO/LUMO = −(4.44 + 0.64 + Eox/Ered).
ΔEg = ELUMO – EHOMO from DPV.
The energy gap was derived from DFT calculations (alkyl chains are replaced with –CH3 groups).
Figure 2.
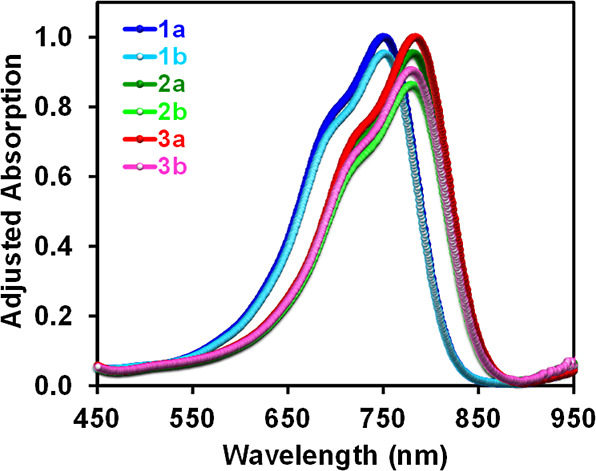
UV–vis absorption spectra of INXBCDTs.
Figure 3.
(a) DPV curves; (b) HOMO and LUMO values from DPV; and (c) DFT of INXBCDT compounds (alkyl chains are replaced with –CH3 groups for simplicity).
The electrochemical behavior of INXBCDTs (1–3) was examined with Bu4N+PF6– as an electrolyte (Figures 3a and S3).55 The electrochemically derived HOMO and LUMO energies (Figure 3b) and corresponding values (Table 1) are given. The LUMO and HOMO of compounds 1–3 are calculated according to the equation: HOMO/LUMO = −(4.44 + 0.64 + Eox/Ered). The DPV data clearly confirms that the variation in the alkyl chain (branched octyl vs linear octyl) has no significant effect on the electrochemical properties of NFAs, and each pair possesses similar oxidation and reduction potentials.54,58 In contrast, halogenated compounds 2 and 3 feature more positive oxidation (Eox = +1.23 V) and reduction (Ered = −0.28 V) potentials in comparison to their analogue 1 (Eox = +1.17 V, Ered = −0.40 V), which leads to lower HOMO (−5.67 eV) and LUMO (−4.16 eV) vs seen with nonhalogenated 1 [HOMO (−5.61 eV) and LUMO (−4.04 eV)]. Due to the electron-deficient nature of the halogenated indanones, compounds 2 and 3 display a shift toward higher values in both oxidation and reduction potentials in comparison to the potentials noticed for 1.56,59,60 In particular, the LUMO levels of halogenated NFAs (2 and 3) are much lower than those of nonhalogenated ones (1), comparing the difference in HOMO levels, resulting in the energy gap contractions of NFAs 2 and 3 compared to 1. Consequently, the narrower energy gaps were confirmed for the halogenated 2–3 (∼1.50 eV) vs 1 (1.56 eV), which is in line with their optical energy gap values: 2–3 (∼1.44 eV) < 1 (1.49 eV). Moreover, this trend is also consistent with the DFT-derived energy gap values as 2–3 (∼1.87 eV) < 1 (1.91 eV; vide infra).
To investigate the impact of the chemical geometry structure of the six INXBCDT molecules on passivation ability, DFT calculations were employed at the B3LYP/6-31G* level of the Gaussian 03W program. The computational results indicate that the HOMO is primarily distributed across the central BCDT, whereas the LUMO is situated on the end-group IN-derivatives or the whole backbone.61 Furthermore, it was found that INCl and INBr-substituted compounds possess near planar conformation with the smallest dihedral angles of 0.24 and 2.66°, respectively, between the two CDT units (whereas 8.74° for nonhalogenated derivatives). It was postulated that coplanarity in BCDTs was facilitated by noncovalent contacts (S···O), therefore extending the effective π-conjugation length (Figure S1). Indeed, these proposed interactions (S···O) were supported by DFT calculations, which showed that the dihedral angle between core and end-capping units was from −0.37 to 1.03°, which could conformationally lock the coplanar geometry for enhanced charge transport.62−64 The calculated HOMO/LUMO energy levels of the chloro- and bromo-substituted compounds 2 (−5.47 eV/–3.61 eV) and 3 (−5.45 eV/–3.58 eV) also exhibit lower HOMO and LUMO energy levels compared to 1 (−5.28 eV/–3.37 eV; Figure 3c). From Table 1, the optically, electrochemically, and theoretically derived energy gaps trends of these INXBCDTs are consistent with each other in the order of 2–3 < 1, as a result of the electron-deficient nature of the halogenated indanones.
The physicochemical properties, morphology, photophysics, efficiency, and stability of the respective PSCs were investigated both with and without passivation. The INXBCDT-treated perovskite films were prepared by dissolving INXBCDT in the chlorobenzene (CB) as an antisolvent during the spin coating process (see Supporting Information). The pristine perovskite (perovskite film prepared from the pure antisolvent CB) was used as a reference for the comparison. The valence band (VB) energy levels of perovskites were assessed using ultraviolet photoelectron spectra (UPS), as depicted in Figure S4, instead of relying on the electrochemical method to determine the VB of the INXBCDT passivators. The band gap of perovskite films was determined through a Tauc plot, as illustrated in Figure S5. The energy level diagrams for TiO2, pristine perovskite, INXBCDT-passivated perovskites, and HTL (Spiro-OMeTAD) are presented in Figure 4, with corresponding numerical data provided in Table S1 of the Supporting Information for the clarity. The HOMO energy level of the perovskite film treated with INBrBCDT-b8 (named 3a-PSK) is measured at −5.37 eV. This value closely aligns with the HOMO of the Spiro-OMeTAD, which is −5.22 eV, resulting in minimal voltage loss. The LUMO energy level of 3a-PSK is recorded at −3.75 eV, making it conducive for the efficient transport of electrons. Moreover, the UV–vis absorption spectra of both pristine perovskite and INXBCDT-treated perovskite films reveal that the passivated perovskite film exhibits stronger absorption in the 500–750 nm range (refer to Figure S6). This increased absorption implies an enhanced capacity to capture more light within this wavelength range, potentially attributed to the close packing, larger grain size, or greater crystallinity of the passivated perovskite film.13 It is worth noting that perovskite films passivated with INXBCDT featuring branched alkyl chains (1a-PSK, 2a-PSK, and 3a-PSK) have stronger absorption in the range of 400–500 nm (see Figure S6), suggesting a reduced hydration level in the perovskite. However, the energy gaps derived from the optical Tauc plots, as depicted in Figure S5, are comparable. This similarity indicates that the observed differences in film quality are not substantial enough to significantly impact the bandgap energy of the perovskite.
Figure 4.
Schematic representation of the energy levels.
The scanning electron microscopes (SEMs) depicted in Figure 5a highlight the morphological differences between the pristine and passivated INXBCDT perovskite films. The pristine perovskite film exhibits an irregular texture characterized by roughness and an uneven grain size, resulting in distinctly visible GBs. Surprisingly, the perovskite films prepared using INXBCDT CB solution as the antisolvent are flat films with large grain size. SEM topographies demonstrate that antisolvent containing INXBCDT (1a–1b, 2a–2b, and 3a–3b) has the potential to influence the crystallization process of perovskite, leading to the formation of perovskite films of superior quality. In Figure 5b, cross-sectional SEM images reveal that the pristine perovskite film exhibits pinhole defects in the vertical direction. Additionally, the perovskite grain appears to be loosely attached to the TiO2 layer. In contrast, perovskite films passivated with INXBCDT, especially 3a-PSK, exhibit no obvious pinhole and attach closely to the TiO2 underlayer. The presence of pinhole defects at the interface adversely affects charge transport and collection. That is why PSCs based on INXBCDT-treated perovskite demonstrate superior PV performance compared to those utilizing a pristine perovskite absorber. X-ray diffraction (XRD) patterns showcased in Figure 5c reveal the crystallinity and phase characteristics of both pristine perovskite and INXBCDT-passivated perovskite films (1a–1b(CB), 2a–2b(CB), and 3a–3b(CB)). Notably, all perovskite films exhibit identical characteristic peaks in their XRD patterns. However, a PbI2 diffraction peak was observed in the pristine perovskite film. This occurrence is attributed to the film preparation process, which involves exposure to air for 2 h. Consequently, some perovskite in the pristine film decomposed to PbI2. Moreover, among all of the perovskite films investigated in this study, the (100) diffraction peak intensity of the 3a-PSK film stands out as the strongest. These results proved that treatment with INBrBCDT-b8 (3a) distinctly enhances both the quality and stability of the perovskite film. To illustrate the distribution of compound 3a in perovskite film, SEM X-ray energy dispersive spectroscopic (EDS) element mapping (Figure 5d,e) was conducted on both the top and cross-section of 3a-PSK film. The uniform distribution of INBrBCDT-b8 across the entire film indicates that this compound effectively passivates the entire perovskite film. Figure 5d,e also reveals the thorough penetration of INBrBCDT-b8 (3a) throughout the entire perovskite layer, displaying a randomized vertical distribution. Moreover, the branched alkyl chains of compound 3a that existed in the GBs can prevent water vapor from damaging the perovskite via the frail GB.
Figure 5.
(a) Surface topography and (b) cross-section SEM images; (c) XRD profiles; (d) top view; and (e) cross-section view of SEM-EDS elements mapping for 3a-PSK (sulfur, oxygen, and bromine atoms are in red, green, and blue colors, respectively).
Fourier-transform infrared (FTIR) analysis was performed for compound 3a and the mixture of 3a and PbI2, to further investigate the interactions between INBrBCDT-b8 (3a) and under-coordinated Pb2+ ions on the grain surface/interface (Figure S7). As a result, lower wavenumbers are observed for the mixture of 3a and PbI2 [C=O bond (1687 cm–1) and C≡N bond (2210 cm–1)], compared to those of compound 3a [C=O bond (1715 cm–1) and C≡N bond (2238 cm–1)]. This shift indicates that 3a possesses the ability to passivate Pb2+ defects in perovskite, a finding consistent with observations in various reports.42,65Figure 6 illustrates the schematic representation of the interaction between the carbonyl and cyano moieties of 3a and Pb2+, along with the hydrophobic nature of the branched alkyl chain of 3a within the perovskite layer. In the 3a-PSK system, compound 3a acts as a passivator, contributing to the development of high-density perovskite films with large grains. This functionality is expected to lead to better performance and stability of devices based on INXBCDT-treated perovskite absorbers.
Figure 6.
Schematic diagram of GB passivation by compound 3a, which can prevent H2O from entering to degrade the perovskite.
Steady-state (PL) and time-resolved (TRPL) were conducted for both pristine and INXBCDT-passivated perovskite films to investigate the impact of passivation on the hole transporting efficiency. These measurements were taken on glass substrates as well as on films coated with HTL, and the outcomes are presented in Figure 7a–d. The PL intensity and estimated exciton lifetimes for all of the perovskite films are listed in Tables S2–S4, respectively. With the application of HTL, the perovskite film incorporated with compound 3a exhibits significantly lower PL intensity compared to other INXBCDT-treated perovskite films (Figure 7b). This reduction in PL intensity is attributed to the enhanced charge transfer efficiency from the perovskite to the HTL. As depicted in Figure 7a, in the absence of the HTL top layer, the PL intensity of the 3a-PSK is the strongest, suggesting that the INBrBCDT-b8-treated perovskite film exhibits superior characteristics with fewer defect sites. In accordance with previous literature, the exciton lifetime can be extracted from the normalized curves of TRPL.66 All of the passivated perovskite films showed a significant increase in the PL decay time. Furthermore, 3a-PSK demonstrated the longest PL decay time of 7.52 ns, indicating the best quality (the lowest defect density) of the INBrBCDT-b8-treated perovskite film. Conversely, the PL decay time was dramatically reduced for all passivated perovskite films overlaid with HTL. Nevertheless, a transient decay time of 0.45 ns was observed for 3a-PSK with HTL among all of the perovskite films studied in this paper, suggesting that 3a-PSK exhibits the most effective hole transport ability. The TRPL data support the conclusion that INBrBCDT-b8 (3a) is the best passivator for perovskite film among the passivators studied in this paper.
Figure 7.
Steady-state PL (a,b) and TRPL (c,d) spectra of perovskite films (on glass/with Spiro-OMeTAD overlayer).
PSCs with a regular (n–i–p) configuration were fabricated by using the architecture FTO/c-TiO2/m-TiO2/PSK/HTL/MoO3/Ag. Detailed device fabrication is given in the Supporting Information. In these cells, pristine and INXBCDTs (1a–1b, 2a–2b, and 3a–3b) were employed as passivators. The schematic representation of the cell structure is provided in Figure S8. These cells were specifically designed to evaluate the passivation effects on the performance of the corresponding cells. Initially, the concentration of INXBCDTs in the antisolvent was optimized to 0.2 wt % (vs solvent) (Table S5). Figure 8a displays the J–V curves of the best-performing devices, utilizing various passivators, while Figure 8b provides the corresponding IPCE curves. Figure 8c,d illustrates the J–V curves for both forward and reverse voltage scans of the cells. Table 2 summarizes the PV parameters of all the devices studied in this paper. The highest PCE among all PSC devices was achieved by INBrBCDT-b8 (3a) passivated perovskite, attaining a champion PCE of 22.20%. This superior performance included a Voc of 1.15 V, a Jsc of 24.44 mA/cm2, and an FF of 79. In contrast, the pristine perovskite film showed a maximum PCE of 18.09%, featuring a Voc of 1.03 V, a Jsc of 24.06 mA/cm2, and an FF of 73. PSCs fabricated with films treated by compounds 1a–1b, 2a–2b, and 3b, which displayed maximum PCE values of 20.39, 19.55, 21.51, 20.61, and 21.49%, respectively. Note that all six passivators can increase the PCE of the corresponding cells, and among these, the INBrBCDT-b8 (3a)-treated cell has a PCE up to 22.20% (vs 18.09% for the reference cell). The successful GB passivation leads to the improved PCE of the PSCs.67 It is worth emphasizing that devices employing 3a-PSK show no hysteresis (0%), in contrast to the pristine PSK, which exhibits a hysteresis index of 37% (refer to Figure 8c,d and Tables S6 and S7). Therefore, a more symmetrical distribution of ions in both scan directions leads to a higher efficiency of 3a-PSK. Furthermore, Figure 8b (and Table S8) presents the Jsc values derived from integrated EQE measurements: 21.92 mA/cm2 for the reference device and 22.55, 22.44, 22.19, 22.01, 22.34, and 21.82 mA/cm2 for the six passivated PSCs using INXBCDTs. These Jsc values obtained from the IPCE curves are in agreement with the Jsc values determined from the J–V curves.
Figure 8.
(a) J–V curves; (b) EQE spectra; (c) J–V curves of the devices with 3a-PSK; and (d) with pristine PSK (in both forward and reverse scans).
Table 2. PV Parameters of High-Performing PSCs Using Pristine and INXBCDT Passivators.
| passivating agents | Jsc (mA/cm2) | Voc (V) | FF | max. PCE (%) | average PCE (%) |
|---|---|---|---|---|---|
| pristine | 24.06 | 1.03 | 73 | 18.09 | 17.13 ± 0.88 |
| INBCDT-b8 (1a) | 24.71 | 1.10 | 75 | 20.39 | 19.36 ± 0.70 |
| INBCDT-8 (1b) | 24.57 | 1.09 | 73 | 19.55 | 18.58 ± 0.78 |
| INClBCDT-b8 (2a) | 24.41 | 1.13 | 78 | 21.51 | 20.41 ± 0.52 |
| INClBCDT-8 (2b) | 24.21 | 1.12 | 76 | 20.61 | 19.63 ± 0.82 |
| INBrBCDT-b8 (3a) | 24.44 | 1.15 | 79 | 22.20 | 21.37 ± 0.45 |
| INBrBCDT-8 (3b) | 24.17 | 1.14 | 78 | 21.49 | 20.01 ± 0.78 |
Moreover, the photocurrent of the best-performing 3a-PSK devices was measured to evaluate the actual power output at the maximum power point (1.00 V), revealing stabilized efficiencies of 22.00% (Figure 9). Further, the PCE distribution of the PSC device metrics based on INBrBCDT-b8 was assessed, as depicted in Figure S9. Notably, the PCE of the INBrBCDT-b8-treated cells demonstrates an average value of 21.37 ± 0.45% for 40 cells, indicating the reproducibility of the high efficiency.
Figure 9.
Steady-state output of PCE and current density at maximum power point (at 1.00 V) as a function of time for the champion cell (INBrBCDT-b8-PSK/3a-PSK) under simulated 1 sun illumination.
The hole mobility was measured for all the PSCs with the device structure of ITO/PEDOT:PSS/perovskite/MoO3/Ag, and their corresponding I–V characteristics are provided in Figure S10, SI. The measured hole mobilities are 1.70 × 10–3 and 5.26 × 10–3 cm2 V–1 s–1 for pristine and 3a-PSK films, respectively. Concurrently, the calculated trap densities are 5.19 × 1015 and 3.00 × 1015 cm–3 for pristine and 3a-PSK films, respectively. In general, the trap density is inversely proportional to hole mobility. Therefore, the higher mobility and lower trap density observed in 3a-PSK films are advantageous for charge collection, contributing to enhanced PV performance compared to the pristine films.
The long-term stability plays a vital role in the development of PSCs.68 The stability test was carried out for the nonencapsulated devices within a N2-filled glovebox. The pristine film exhibited rapid PCE decay, losing 45% of its original PCE after 1500 h. In contrast, perovskite films passivated with 1a–1b, 2a–2b, and 3a–3b maintained good stability, retaining 87, 67, 91, 77, 93, and 80% of their initial efficiency, respectively, as depicted in Figure 10. It is interesting to note that perovskite passivation with branched alkyl chains of INXBCDT (1a-PSK, 2a-PSK, and 3a-PSK) has better long-term stability than those cells based on linear-chain passivated absorbers (1b-PSK, 2b-PSK, and 3b-PSK), due to the branched alkyl chain having better coverage on the perovskite surface. Furthermore, as a representative of the best-performing NFA, the devices using compound 3a were tested for thermal stability at high temperatures (85 °C) and moisture resistivity at 50% relative humidity (Figure S11). According to Figure S11a, after 216 h of heating at 85 °C, the PCE of the reference devices loses 62% of its initial PCE, whereas the devices based on 3a-PSK lose just 38% of their initial PCE. The reference devices decayed completely (PCE ∼ 0) after 216 h of storage in an ambient atmosphere at 50% relative humidity, whereas the devices based on 3a-PSK retained 40% of their initial PCE (Figure S11b). These results demonstrate that the new NFA-treated PSK (3a-PSK) has increased long-term stability, thermal stability, and moisture resistance, which could be beneficial for high-performance and stable PSCs.
Figure 10.
PCE decay of the PSCs based on various perovskite absorbers (stored at RT; N2).
It is known69 that defects at the GBs of perovskite materials initiate degradation when exposed to moisture and oxygen. Compounds 1a–1b, 2a–2b, and 3a–3b-treated perovskite films are much more hydrophobic than the pristine perovskite film, as revealed by the water contact angles illustrated in Figure 11. The water contact angle of the pristine perovskite film is 44.9°, while the contact angles of the compounds 1a–1b, 2a–2b, and 3a–3b-treated perovskite films are 84.8, 83.7, 85.2, 82.8, 86.5, and 80.3°, respectively. Among all the devices, 3a-PSK films showed the largest water contact angle, leading to the improved stability of the devices. This improvement is ascribed to the superior quality of the perovskite film, which is characterized by a larger grain size and greater crystallinity.70
Figure 11.

Water contact angles of pristine, 1a–1b, 2a–2b, and 3a–3b-treated perovskite films.
3. Conclusions
In conclusion, the study effectively showcased a straightforward and effective method to enhance both the efficiency and the stability of PSCs. This was achieved by incorporating six new organic small molecules bearing three kinds of end groups, serving as effective GB passivators in perovskite films. Specifically, A–D–A-type small molecule INXBCDTs (compounds 1–3) were designed and synthesized, featuring various end-capping units (IN, INCl, and INBr). These compounds were strategically introduced into perovskite films during antisolvent dripping. Results from SEM and XRD measurements indicated that compound 3a-incorporated perovskite films exhibited the largest grain size and the highest crystallinity relative to those of pristine perovskite films and those passivated with other compounds. EDS and FTIR data suggest that the presence of C=O and C≡N units in compound 3a facilitates coordination with the under-coordinated Pb2+ ions, effectively passivating the defect sites within the perovskite films. TRPL results showed that compound 3a promotes the transport of the holes to HTL. Overall, PSCs based on the optimized 3a-treated perovskite absorber demonstrated outstanding performance, achieving a remarkable PCE of 22.20% along with an FF of 79%, a Jsc of 24.44 mA cm–2, and a Voc of 1.15 V. This represents a substantial improvement compared to 18.09% obtained with the control device utilizing a pristine perovskite absorber. The solubility factor involves an increase in the PCE of compounds 1a, 2a, and 3a (2-ethylhexyl-substituted) compared to 1b, 2b, and 3b (octyl-substituted). Perovskite devices treated with INBrBCDT-b8 (3a) maintain approximately 90% of their original PCE after 1500 h of storage inside a glovebox without packing. In contrast, the control device experiences a 45% reduction in its original PCE under identical test conditions. This work shows that GB and interface passivation by small molecular NFA are excellent approaches to achieving efficient and stable PSCs.
4. Experimental Section
4.1. Synthesis of Compound INBCDT-b8 (1a)
Under anhydrous conditions, diCHO-BCDT-b8 (10a) (100 mg, 0.081 mmol) and 2-(3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (11) (63 mg, 0.326 mmol) were dissolved in 30 mL CHCl3, 1 mL pyridine was added, and then heated to reflux for 24 h. The reaction mixture was cooled to room temperature, and 2 M HCl(aq) was added slowly at 0 °C. The reaction mixture was extracted with dichloromethane and washed with water. The organic layer was dried over MgSO4, concentrated, and purified by column chromatography (DCM/hexane) to give a deep blue-red solid 1a (yield = 47%). 1H NMR (500 MHz, CDCl3): δ (ppm) 8.64 (m, 2H), 8.45 (s, 2H), 8.03 (m, 2H), 7.83–7.81 (m, 4H), 7.63 (s, 2H), 7.09–6.82 (m, 18H), 3.81–3.76 (m, 8H), 1.71–1.66 (m, 4H), 1.49–1.35 (m, 16H), 1.29–1.27 (m, 16H), 0.90–0.87 (m, 24H); 13C NMR (125 MHz, CDCl3): δ (ppm) 187.96, 164.14, 159.31, 158.63, 155.53, 145.34, 140.63, 139.71, 139.45, 138.04, 136.50, 135.75, 134.42, 133.84, 128.59, 124.98, 123.66, 119.91, 119.07, 114.87, 114.67, 114.61, 70.55, 67.14, 61.40, 39.40, 30.53, 30.48, 29.09, 23.87, 23.00, 14.03, 11.12, 11.09; HRMS (MALDI, [M]+) calcd. for C100H98N4O6S4, 1578.6369; found, 1578.6364.
4.2. Synthesis of Compound INBCDT-8 (1b)
Followed the procedure for preparing 1a using 10b and 11. The crude product was purified by column chromatography (DCM/hexane) to give a deep blue-red solid 1b (yield = 50%). 1H NMR (500 MHz, CDCl3): δ (ppm) 8.64 (m, 2H), 8.41 (s, 2H), 8.02 (m, 2H), 7.85–7.79 (m, 4H), 7.63 (s, 2H), 7.14–6.81 (m, 18H), 3.93–3.86 (m, 8H), 1.77–1.71 (m, 8H), 1.45–1.39 (m, 8H), 1.30–1.25 (m, 32H), 0.87–0.84 (m, 12H); 13C NMR (125 MHz, CDCl3): δ (ppm) 187.96, 164.14, 159.04, 158.46, 155.54, 145.26, 140.70, 139.69, 139.44, 138.11, 136.50, 135.70, 134.44, 133.96, 128.75, 124.98, 123.62, 120.02, 119.10, 114.91, 114.68, 114.61, 68.18, 67.16, 61.41, 31.82, 29.36, 29.26, 29.23, 26.05, 22.62, 14.05; HRMS (MALDI, [M]+) calcd. for C100H98N4O6S4, 1578.6369; found, 1578.6364.
4.3. Synthesis of Compound INClBCDT-b8 (2a)
Under anhydrous conditions, diCHO-BCDT-b8 (10a) (100 mg, 0.081 mmol) and 2-(5,6-dichloro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (12) (86 mg, 0.326 mmol) were dissolved in 30 mL of CHCl3, 1 mL of pyridine was added, and then heated to reflux for 24 h. The reaction mixture was cooled to room temperature, and 2 M HCl(aq) was added slowly at 0 °C. The reaction mixture was extracted with dichloromethane and washed with water. The organic layer was dried over MgSO4, concentrated, and purified by column chromatography (DCM/hexane) to give a deep blue solid 2a (yield = 55%). 1H NMR (500 MHz, CDCl3): δ (ppm) 8.78 (s, 2H), 8.66 (s, 2H), 7.95 (s, 2H), 7.65 (s, 2H), 7.18 (m, 8H), 6.85 (m, 10H), 3.83–3.77 (m, 8H), 1.71–1.66 (m, 4H), 1.47–1.36 (m, 16H), 1.30–1.28 (m, 16H), 0.91–0.87 (m, 24H); 13C NMR (125 MHz, CDCl3): δ (ppm) 185.77, 165.48, 159.52, 159.28, 156.79, 145.96, 140.73, 139.97, 139.52, 138.49, 138.20, 136.43, 135.66, 133.37, 128.59, 126.67, 124.98, 119.80, 119.41, 114.92, 114.27, 113.98, 70.58, 67.96, 61.49, 39.38, 30.52, 30.49, 29.08, 23.86, 23.0, 14.03, 11.11, 11.09; HRMS (MALDI, [M]+) calcd. for C100H94Cl4N4O6S4, 1714.4810; found, 1714.4805.
4.4. Synthesis of Compound INClBCDT-8 (2b)
Followed the procedure for preparing 2a using 10b and 12. The crude product was purified by column chromatography (DCM/hexane) to give a deep blue solid 2b (yield = 56%). 1H NMR (500 MHz, CDCl3): δ (ppm) 8.73 (s, 2H), 8.58 (s, 2H), 7.96 (s, 2H), 7.66 (s, 2H), 7.23 (m, 8H), 6.87 (m, 10H), 3.93–3.87 (m, 8H), 1.77–1.72 (m, 8H), 1.43–1.39 (m, 8H), 1.30–1.26 (m, 32H), 0.87–0.85 (m, 12H); 13C NMR (125 MHz, CDCl3): δ (ppm) 185.80, 165.50, 159.51, 159.02, 156.75, 145.87, 140.70, 139.95, 139.52, 138.54, 138.24, 136.39, 135.69, 133.44, 128.64, 126.70, 124.95, 119.89, 119.47, 114.92, 114.31, 114.01, 68.18, 67.97, 61.49, 31.81, 30.90, 29.36, 29.23, 26.05, 25.61, 22.63, 14.07; HRMS (MALDI, [M]+) calcd. for C100H94Cl4N4O6S4, 1714.4810; found, 1714.4805.
4.5. Synthesis of Compound INBrBCDT-b8 (3a)
Under anhydrous conditions, diCHO-BCDT-b8 (10a) (100 mg, 0.081 mmol) and 2-(5,6-dibromo-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (13) (115 mg, 0.326 mmol) were dissolved in 30 mL of CHCl3, 1 mL of pyridine was added, and then heated to reflux for 24 h. The reaction mixture was cooled to room temperature, and 2 M HCl(aq) was added slowly at 0 °C. The reaction mixture was extracted with dichloromethane and washed with water. The organic layer was dried over MgSO4, concentrated, and purified by column chromatography (DCM/hexane) to give a deep blue-green solid 3a (yield = 52%). 1H NMR (500 MHz, CDCl3): δ (ppm) 8.78 (m, 4H), 8.10 (s, 2H), 7.65 (s, 2H), 7.17 (m, 8H), 6.85 (m, 10H), 3.81–3.78 (m, 8H), 1.71–1.66 (m, 4H), 1.48–1.36 (m, 16H), 1.29–1.27 (m, 16H), 0.90–0.87 (m, 24H); 13C NMR (125 MHz, CDCl3): δ (ppm) 185.73, 165.40, 159.32, 156.89, 156.65, 146.01, 140.85, 140.19, 138.73, 138.56, 136.50, 135.98, 133.43, 132.87, 132.29, 129.67, 128.61, 128.02, 119.73, 119.21, 114.95, 114.34, 114.02, 70.60, 67.97, 61.50, 39.40, 30.54, 30.51, 29.10, 23.88, 23.01, 14.05, 11.14, 11.11; HRMS (MALDI, [M]+) calcd. for C100H94Br4N4O6S4, 1890.2790; found, 1890.2784.
4.6. Synthesis of Compound INBrBCDT-8 (3b)
Followed the procedure for preparing 3a using 10b and 13. The crude product was purified by column chromatography (DCM/hexane) to give a deep blue-green solid 3b (yield = 58%). 1H NMR (500 MHz, CDCl3): δ (ppm) 8.73 (m, 4H), 8.10 (s, 2H), 7.66 (s, 2H), 7.21 (m, 8H), 6.87 (m, 10H), 3.93–3.85 (m, 8H), 1.77–1.72 (m, 8H), 1.43–1.39 (m, 8H), 1.30–1.26 (m, 32H), 0.87–0.85 (m, 12H); 13C NMR (125 MHz, CDCl3): δ (ppm) 185.53, 165.19, 159.10, 157.07, 156.26, 146.04, 140.90, 140.32, 138.53, 136.57, 135.80, 133.53, 132.93, 132.30, 129.55, 128.65, 127.85, 119.48, 118.90, 114.94, 114.27, 113.90, 68.16, 67.76, 61.42, 31.81, 29.67, 29.36, 29.23, 26.05, 22.62, 14.07; HRMS (MALDI, [M]+) calcd. for C100H94Br4N4O6S4, 1890.2790; found, 1890.2784.
Acknowledgments
The authors gratefully acknowledge the funding provided by the Ministry of Science and Technology of Taiwan (MOST 111-2113-M-008-004-MY3), the National Science and Technology Council in Taiwan (NSTC 111-2622-8-008-006), and the NCU-Covestro Research Center. The device fabrication and PV parameter measurements were carried out in the Advanced Laboratory of Accommodation and Research for Organic Photovoltaics, MOST, Taiwan, ROC.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.3c15774.
Experimental details, TGA, DPV curves, UPS, optical Tauc plots, and other characterization data (PDF)
Author Contributions
∥ A.V. and S.N.A. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Fu L.; Li H.; Wang L.; Yin R.; Li B.; Yin L. Defect Passivation Strategies in Perovskites for an Enhanced Photovoltaic Performance. Energy Environ. Sci. 2020, 13, 4017–4056. 10.1039/D0EE01767A. [DOI] [Google Scholar]
- Zhang M.; Zhan X. Nonfullerene N-Type Organic Semiconductors for Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1900860. 10.1002/aenm.201900860. [DOI] [Google Scholar]
- Velusamy A.; Yau S.; Liu C.-L.; Ezhumalai Y.; Kumaresan P.; Chen M.-C. Recent Studies on Small Molecular and Polymeric Hole-Transporting Materials for High-Performance Perovskite Solar Cells. J. Chin. Chem. Soc. 2023, 70, 2046–2063. 10.1002/jccs.202300326. [DOI] [Google Scholar]
- Kojima A.; Teshima K.; Shirai Y.; Miyasaka T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- Szabó G.; Park N.-G.; De Angelis F.; Kamat P. V. Are Perovskite Solar Cells Reaching the Efficiency and Voltage Limits?. ACS Energy Lett. 2023, 8, 3829–3831. 10.1021/acsenergylett.3c01649. [DOI] [Google Scholar]
- Manser J. S.; Christians J. A.; Kamat P. V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. 10.1021/acs.chemrev.6b00136. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Ma J.; Lei H.; Yao F.; Li B.; Xiong L.; Fang G. Enhanced Performance of Perovskite Solar Cells Via Anti-Solvent Nonfullerene Lewis Base IT-4F Induced Trap-Passivation. J. Mater. Chem. A 2018, 6, 5919–5925. 10.1039/C8TA00583D. [DOI] [Google Scholar]
- Yu B.; Zhang L.; Wu J.; Liu K.; Wu H.; Shi J.; Luo Y.; Li D.; Bo Z.; Meng Q. Application of a New π-Conjugated Ladder-Like Polymer in Enhancing the Stability and Efficiency of Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 1417–1424. 10.1039/C9TA10475E. [DOI] [Google Scholar]
- Miyazawa Y.; Ikegami M.; Chen H.-W.; Ohshima T.; Imaizumi M.; Hirose K.; Miyasaka T. Tolerance of Perovskite Solar Cell to High-Energy Particle Irradiations in Space Environment. iScience 2018, 2, 148–155. 10.1016/j.isci.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke W.; Priyanka P.; Vegiraju S.; Stoumpos C. C.; Spanopoulos I.; Soe C. M. M.; Marks T. J.; Chen M.-C.; Kanatzidis M. G. Dopant-Free Tetrakis-Triphenylamine Hole Transporting Material for Efficient Tin-Based Perovskite Solar Cells. J. Am. Chem. Soc. 2018, 140, 388–393. 10.1021/jacs.7b10898. [DOI] [PubMed] [Google Scholar]
- Vegiraju S.; Ke W.; Priyanka P.; Ni J.-S.; Wu Y.-C.; Spanopoulos I.; Yau S. L.; Marks T. J.; Chen M.-C.; Kanatzidis M. G. Benzodithiophene Hole-Transporting Materials for Efficient Tin-Based Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1905393. 10.1002/adfm.201905393. [DOI] [Google Scholar]
- Sutanto A. A.; Joseph V.; Igci C.; Syzgantseva O. A.; Syzgantseva M. A.; Jankauskas V.; Rakstys K.; Queloz V. I. E.; Huang P.-Y.; Ni J.-S.; Kinge S.; Asiri A. M.; Chen M.-C.; Nazeeruddin M. K. Isomeric Carbazole-Based Hole-Transporting Materials: Role of the Linkage Position on the Photovoltaic Performance of Perovskite Solar Cells. Chem. Mater. 2021, 33, 3286–3296. 10.1021/acs.chemmater.1c00335. [DOI] [Google Scholar]
- Joseph V.; Sutanto A. A.; Igci C.; Syzgantseva O. A.; Jankauskas V.; Rakstys K.; Queloz V. I. E.; Kanda H.; Huang P.-Y.; Ni J.-S.; Kinge S.; Chen M.-C.; Nazeeruddin M. K. Stable Perovskite Solar Cells Using Molecularly Engineered Functionalized Oligothiophenes as Low-Cost Hole-Transporting Materials. Small 2021, 17, 2100783. 10.1002/smll.202100783. [DOI] [PubMed] [Google Scholar]
- Govindan V.; Yang K.-C.; Fu Y.-S.; Wu C.-G. Low-Cost Synthesis of Heterocyclic Spiro-Type Hole Transporting Materials for Perovskite Solar Cell Applications. New J. Chem. 2018, 42, 7332–7339. 10.1039/C8NJ01082J. [DOI] [Google Scholar]
- Velusamy A.; Afraj S. N.; Yau S.; Liu C.-L.; Ezhumalai Y.; Kumaresan P.; Chen M.-C. Fused Thiophene Based Materials for Organic Thin-Film Transistors. J. Chin. Chem. Soc. 2022, 69, 1253–1275. 10.1002/jccs.202200214. [DOI] [Google Scholar]
- Yang Y.; You J. Make Perovskite Solar Cells Stable. Nature 2017, 544, 155–156. 10.1038/544155a. [DOI] [PubMed] [Google Scholar]
- Wei H.; Fang Y.; Mulligan P.; Chuirazzi W.; Fang H.-H.; Wang C.; Ecker B. R.; Gao Y.; Loi M. A.; Cao L.; Huang J. Sensitive X-Ray Detectors Made of Methylammonium Lead Tribromide Perovskite Single Crystals. Nat. Photonics 2016, 10, 333–339. 10.1038/nphoton.2016.41. [DOI] [Google Scholar]
- Huang J.; Shao Y.; Dong Q. Organometal Trihalide Perovskite Single Crystals: A Next Wave of Materials for 25% Efficiency Photovoltaics and Applications Beyond?. J. Phys. Chem. Lett. 2015, 6, 3218–3227. 10.1021/acs.jpclett.5b01419. [DOI] [Google Scholar]
- Saidaminov M. I.; Abdelhady A. L.; Murali B.; Alarousu E.; Burlakov V. M.; Peng W.; Dursun I.; Wang L.; He Y.; Maculan G.; Goriely A.; Wu T.; Mohammed O. F.; Bakr O. M. High-Quality Bulk Hybrid Perovskite Single Crystals within Minutes by Inverse Temperature Crystallization. Nat. Commun. 2015, 6, 7586. 10.1038/ncomms8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suding K.; Higgs E.; Palmer M.; Callicott J. B.; Anderson C. B.; Baker M.; Gutrich J. J.; Hondula K. L.; LaFevor M. C.; Larson B. M. H.; Randall A.; Ruhl J. B.; Schwartz K. Z. S. Committing to Ecological Restoration. Science 2015, 348, 638–640. 10.1126/science.aaa4216. [DOI] [PubMed] [Google Scholar]
- Yun J. S.; Ho-Baillie A.; Huang S.; Woo S. H.; Heo Y.; Seidel J.; Huang F.; Cheng Y.-B.; Green M. A. Benefit of Grain Boundaries in Organic-Inorganic Halide Planar Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 875–880. 10.1021/acs.jpclett.5b00182. [DOI] [PubMed] [Google Scholar]
- Shao Y.; Xiao Z.; Bi C.; Yuan Y.; Huang J. Origin and Elimination of Photocurrent Hysteresis by Fullerene Passivation in CH3NH3PbI3 Planar Heterojunction Solar Cells. Nat. Commun. 2014, 5, 5784. 10.1038/ncomms6784. [DOI] [PubMed] [Google Scholar]
- Shao Y.; Fang Y.; Li T.; Wang Q.; Dong Q.; Deng Y.; Yuan Y.; Wei H.; Wang M.; Gruverman A.; Shield J.; Huang J. Grain Boundary Dominated Ion Migration in Polycrystalline Organic-Inorganic Halide Perovskite Films. Energy Environ. Sci. 2016, 9, 1752–1759. 10.1039/C6EE00413J. [DOI] [Google Scholar]
- Li Y.; Wu H.; Qi W.; Zhou X.; Li J.; Cheng J.; Zhao Y.; Li Y.; Zhang X. Passivation of Defects in Perovskite Solar Cell: From a Chemistry Point of View. Nano Energy 2020, 77, 105237. 10.1016/j.nanoen.2020.105237. [DOI] [Google Scholar]
- Afraj S. N.; Zheng D.; Velusamy A.; Ke W.; Cuthriell S.; Zhang X.; Chen Y.; Lin C.; Ni J.-S.; Wasielewski M. R.; Huang W.; Yu J.; Pan C.-H.; Schaller R. D.; Chen M.-C.; Kanatzidis M. G.; Facchetti A.; Marks T. J. 2,3-Diphenylthieno[3,4-b]Pyrazines as Hole-Transporting Materials for Stable, High-Performance Perovskite Solar Cells. ACS Energy Lett. 2022, 7, 2118–2127. 10.1021/acsenergylett.2c00684. [DOI] [Google Scholar]
- Noel N. K.; Abate A.; Stranks S. D.; Parrott E. S.; Burlakov V. M.; Goriely A.; Snaith H. J. Enhanced Photoluminescence and Solar Cell Performance Via Lewis Base Passivation of Organic-Inorganic Lead Halide Perovskites. ACS Nano 2014, 8, 9815–9821. 10.1021/nn5036476. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Shen L.; Dai J.; Deng Y.; Wu Y.; Bai Y.; Zheng X.; Wang J.; Fang Y.; Wei H.; Ma W.; Zeng X. C.; Zhan X.; Huang J. π-Conjugated Lewis Base: Efficient Trap-Passivation and Charge-Extraction for Hybrid Perovskite Solar Cells. Adv. Mater. 2017, 29, 1604545. 10.1002/adma.201604545. [DOI] [PubMed] [Google Scholar]
- Lee S.; Park J. H.; Lee B. R.; Jung E. D.; Yu J. C.; Di Nuzzo D.; Friend R. H.; Song M. H. Amine-Based Passivating Materials for Enhanced Optical Properties and Performance of Organic-Inorganic Perovskites in Light-Emitting Diodes. J. Phys. Chem. Lett. 2017, 8, 1784–1792. 10.1021/acs.jpclett.7b00372. [DOI] [PubMed] [Google Scholar]
- Zheng X.; Chen B.; Dai J.; Fang Y.; Bai Y.; Lin Y.; Wei H.; Zeng X. C.; Huang J. Defect Passivation in Hybrid Perovskite Solar Cells Using Quaternary Ammonium Halide Anions And cations. Nat. Energy 2017, 2, 17102. 10.1038/nenergy.2017.102. [DOI] [Google Scholar]
- Bi C.; Zheng X.; Chen B.; Wei H.; Huang J. Spontaneous Passivation of Hybrid Perovskite by Sodium Ions from Glass Substrates: Mysterious Enhancement of Device Efficiency Revealed. ACS Energy Lett. 2017, 2, 1400–1406. 10.1021/acsenergylett.7b00356. [DOI] [Google Scholar]
- Wang L.; Zhou H.; Hu J.; Huang B.; Sun M.; Dong B.; Zheng G.; Huang Y.; Chen Y.; Li L.; Xu Z.; Li N.; Liu Z.; Chen Q.; Sun L.-D.; Yan C.-H. A Eu3+-Eu2+ Ion Redox Shuttle Imparts Operational Durability to Pb-I Perovskite Solar Cells. Science 2019, 363, 265–270. 10.1126/science.aau5701. [DOI] [PubMed] [Google Scholar]
- Alharbi E. A.; Alyamani A. Y.; Kubicki D. J.; Uhl A. R.; Walder B. J.; Alanazi A. Q.; Luo J.; Burgos-Caminal A.; Albadri A.; Albrithen H.; Alotaibi M. H.; Moser J.-E.; Zakeeruddin S. M.; Giordano F.; Emsley L.; Grätzel M. Atomic-Level Passivation Mechanism of Ammonium Salts Enabling Highly Efficient Perovskite Solar Cells. Nat. Commun. 2019, 10, 3008. 10.1038/s41467-019-10985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E. H.; Jeon N. J.; Park E. Y.; Moon C. S.; Shin T. J.; Yang T.-Y.; Noh J. H.; Seo J. Efficient, Stable and Scalable Perovskite Solar Cells Using Poly(3-Hexylthiophene). Nature 2019, 567, 511–515. 10.1038/s41586-019-1036-3. [DOI] [PubMed] [Google Scholar]
- Li N.; Tao S.; Chen Y.; Niu X.; Onwudinanti C. K.; Hu C.; Qiu Z.; Xu Z.; Zheng G.; Wang L.; Zhang Y.; Li L.; Liu H.; Lun Y.; Hong J.; Wang X.; Liu Y.; Xie H.; Gao Y.; Bai Y.; Yang S.; Brocks G.; Chen Q.; Zhou H. Cation and Anion Immobilization through Chemical Bonding Enhancement with Fluorides for Stable Halide Perovskite Solar Cells. Nat. Energy 2019, 4, 408–415. 10.1038/s41560-019-0382-6. [DOI] [Google Scholar]
- Zhao H.; Han Y.; Xu Z.; Duan C.; Yang S.; Yuan S.; Yang Z.; Liu Z.; Liu S. A Novel Anion Doping for Stable CsPbI2Br Perovskite Solar Cells with an Efficiency of 15.56% and an Open Circuit Voltage of 1.30 V. Adv. Energy Mater. 2019, 9, 1902279. 10.1002/aenm.201902279. [DOI] [Google Scholar]
- Ye S.; Rao H.; Zhao Z.; Zhang L.; Bao H.; Sun W.; Li Y.; Gu F.; Wang J.; Liu Z.; Bian Z.; Huang C. A Breakthrough Efficiency of 19.9% Obtained in Inverted Perovskite Solar Cells by Using an Efficient Trap State Passivator Cu(Thiourea)I. J. Am. Chem. Soc. 2017, 139, 7504–7512. 10.1021/jacs.7b01439. [DOI] [PubMed] [Google Scholar]
- Wang B.; Wu F.; Bi S.; Zhou J.; Wang J.; Leng X.; Zhang D.; Meng R.; Xue B.; Zong C.; Zhu L.; Zhang Y.; Zhou H. A Polyaspartic Acid Sodium Interfacial Layer Enhances Surface Trap Passivation in Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 23895–23903. 10.1039/C9TA01947B. [DOI] [Google Scholar]
- Liang P.-W.; Chueh C.-C.; Williams S. T.; Jen A. K.-Y. Roles of Fullerene-Based Interlayers in Enhancing the Performance of Organometal Perovskite Thin-Film Solar Cells. Adv. Energy Mater. 2015, 5, 1402321. 10.1002/aenm.201402321. [DOI] [Google Scholar]
- Yang Z.; Dou J.; Kou S.; Dang J.; Ji Y.; Yang G.; Wu W.-Q.; Kuang D.-B.; Wang M. Multifunctional Phosphorus-Containing Lewis Acid and Base Passivation Enabling Efficient and Moisture-Stable Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1910710. 10.1002/adfm.201910710. [DOI] [Google Scholar]
- Ahmed G. H.; Yin J.; Bose R.; Sinatra L.; Alarousu E.; Yengel E.; AlYami N. M.; Saidaminov M. I.; Zhang Y.; Hedhili M. N.; Bakr O. M.; Brédas J. L.; Mohammed O. F. Pyridine-Induced Dimensionality Change in Hybrid Perovskite Nanocrystals. Chem. Mater. 2017, 29, 4393–4400. 10.1021/acs.chemmater.7b00872. [DOI] [Google Scholar]
- Zhang M.; Dai S.; Chandrabose S.; Chen K.; Liu K.; Qin M.; Lu X.; Hodgkiss J. M.; Zhou H.; Zhan X. High-Performance Fused Ring Electron Acceptor-Perovskite Hybrid. J. Am. Chem. Soc. 2018, 140, 14938–14944. 10.1021/jacs.8b09300. [DOI] [PubMed] [Google Scholar]
- Song C.; Li X.; Wang Y.; Fu S.; Wan L.; Liu S.; Zhang W.; Song W.; Fang J. Sulfonyl-Based Non-Fullerene Electron Acceptor-Assisted Grain Boundary Passivation for Efficient and Stable Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 19881–19888. 10.1039/C9TA06439G. [DOI] [Google Scholar]
- Gao Y.; Wu Y.; Liu Y.; Lu M.; Yang L.; Wang Y.; Yu W. W.; Bai X.; Zhang Y.; Dai Q. Interface and Grain Boundary Passivation for Efficient and Stable Perovskite Solar Cells: The Effect of Terminal Groups in Hydrophobic Fused Benzothiadiazole-Based Organic Semiconductors. Nanoscale Horiz. 2020, 5, 1574–1585. 10.1039/D0NH00374C. [DOI] [PubMed] [Google Scholar]
- Qin M.; Cao J.; Zhang T.; Mai J.; Lau T.-K.; Zhou S.; Zhou Y.; Wang J.; Hsu Y.-J.; Zhao N.; Xu J.; Zhan X.; Lu X. Fused-Ring Electron Acceptor ITIC-Th: A Novel Stabilizer for Halide Perovskite Precursor Solution. Adv. Energy Mater. 2018, 8, 1703399. 10.1002/aenm.201703399. [DOI] [Google Scholar]
- Niu T.; Lu J.; Munir R.; Li J.; Barrit D.; Zhang X.; Hu H.; Yang Z.; Amassian A.; Zhao K.; Liu S. Stable High-Performance Perovskite Solar Cells Via Grain Boundary Passivation. Adv. Mater. 2018, 30, 1706576. 10.1002/adma.201706576. [DOI] [PubMed] [Google Scholar]
- Yang G.; Qin P.; Fang G.; Li G. A Lewis Base-Assisted Passivation Strategy Towards Highly Efficient and Stable Perovskite Solar Cells. Sol. RRL 2018, 2, 1800055. 10.1002/solr.201800055. [DOI] [Google Scholar]
- Afraj S. N.; Velusamy A.; Chen C.-Y.; Ni J.-S.; Ezhumalai Y.; Pan C.-H.; Chen K.-Y.; Yau S.-L.; Liu C.-L.; Chiang C.-H.; Wu C.-G.; Chen M.-C. Dicyclopentadithienothiophene (DCDTT)-Based Organic Semiconductor Assisted Grain Boundary Passivation for Highly Efficient and Stable Perovskite Solar Cells. J. Mater. Chem. A 2022, 10, 11254–11267. 10.1039/D2TA00617K. [DOI] [Google Scholar]
- Wang S.-Y.; Chen C.-P.; Chung C.-L.; Hsu C.-W.; Hsu H.-L.; Wu T.-H.; Zhuang J.-Y.; Chang C.-J.; Chen H. M.; Chang Y. J. Defect Passivation by Amide-Based Hole-Transporting Interfacial Layer Enhanced Perovskite Grain Growth for Efficient p-i-n Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 40050–40061. 10.1021/acsami.9b13952. [DOI] [PubMed] [Google Scholar]
- Lu C.; Paramasivam M.; Park K.; Kim C. H.; Kim H. K. Phenothiazine Functionalized Multifunctional A-π-D-π-D-π-A-Type Hole-Transporting Materials Via Sequential C-H Arylation Approach for Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 14011–14022. 10.1021/acsami.8b20646. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Dai S.; Wu Y.; Zhang Q.; Wang J.; Jiang L.; Ling Q.; Wei Z.; Ma W.; You W.; Wang C.; Zhan X. Single-Junction Binary-Blend Nonfullerene Polymer Solar Cells with 12.1% Efficiency. Adv. Mater. 2017, 29, 1700144. 10.1002/adma.201700144. [DOI] [PubMed] [Google Scholar]
- Nguyen T. L.; Choi H.; Ko S. J.; Uddin M. A.; Walker B.; Yum S.; Jeong J. E.; Yun M. H.; Shin T. J.; Hwang S.; Kim J. Y.; Woo H. Y. Semi-Crystalline Photovoltaic Polymers with Efficiency Exceeding 9% in a ∼ 300 nm Thick Conventional Single-Cell Device. Energy Environ. Sci. 2014, 7, 3040–3051. 10.1039/C4EE01529K. [DOI] [Google Scholar]
- Deng D.; Zhang Y.; Zhang J.; Wang Z.; Zhu L.; Fang J.; Xia B.; Wang Z.; Lu K.; Ma W.; Wei Z. Fluorination-Enabled Optimal Morphology Leads to over 11% Efficiency for Inverted Small-Molecule Organic Solar Cells. Nat. Commun. 2016, 7, 13740. 10.1038/ncomms13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y.; Yang C.; Yao H.; Zhu J.; Wang Y.; Jia G.; Gao F.; Hou J. Efficient Semitransparent Organic Solar Cells with Tunable Color Enabled by an Ultralow-Bandgap Nonfullerene Acceptor. Adv. Mater. 2017, 29, 1703080. 10.1002/adma.201703080. [DOI] [PubMed] [Google Scholar]
- Velusamy A.; Yu C.-H.; Afraj S. N.; Lin C.-C.; Lo W.-Y.; Yeh C.-J.; Wu Y.-W.; Hsieh H.-C.; Chen J.; Lee G.-H.; Tung S.-H.; Liu C.-L.; Chen M.-C.; Facchetti A. Thienoisoindigo (TII)-Based Quinoidal Small Molecules for High-Performance N-Type Organic Field Effect Transistors. Adv. Sci. 2021, 8, 2002930. 10.1002/advs.202002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegiraju S.; He G.-Y.; Kim C.; Priyanka P.; Chiu Y.-J.; Liu C.-W.; Huang C.-Y.; Ni J.-S.; Wu Y.-W.; Chen Z.; Lee G.-H.; Tung S.-H.; Liu C.-L.; Chen M.-C.; Facchetti A. Solution-Processable Dithienothiophenoquinoid (DTTQ) Structures for Ambient-Stable N-Channel Organic Field Effect Transistors. Adv. Funct. Mater. 2017, 27, 1606761. 10.1002/adfm.201606761. [DOI] [Google Scholar]
- Chen M.-C.; Chiang Y.-J.; Kim C.; Guo Y.-J.; Chen S.-Y.; Liang Y.-J.; Huang Y.-W.; Hu T.-S.; Lee G.-H.; Facchetti A.; Marks T. J. One-Pot [1 + 1+1] Synthesis of Dithieno[2,3-b:3′,2′-d]Thiophene (DTT) and Their Functionalized Derivatives for Organic Thin-Film Transistors. Chem. Commun. 2009, 1846–1848. 10.1039/b820621j. [DOI] [PubMed] [Google Scholar]
- Vegiraju S.; Amelenan Torimtubun A. A.; Lin P.-S.; Tsai H.-C.; Lien W.-C.; Chen C.-S.; He G.-Y.; Lin C.-Y.; Zheng D.; Huang Y.-F.; Wu Y.-C.; Yau S.-L.; Lee G.-H.; Tung S.-H.; Wang C.-L.; Liu C.-L.; Chen M.-C.; Facchetti A. Solution-Processable Quinoidal Dithioalkylterthiophene-Based Small Molecules Pseudo-Pentathienoacenes Via an Intramolecular S···S Lock for High-Performance N-Type Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2020, 12, 25081–25091. 10.1021/acsami.0c03477. [DOI] [PubMed] [Google Scholar]
- Velusamy A.; Chen Y.-Y.; Lin M.-H.; Afraj S. N.; Liu J.-H.; Chen M.-C.; Liu C.-L. Diselenophene-Dithioalkylthiophene Based Quinoidal Small Molecules for Ambipolar Organic Field Effect Transistors. Adv. Sci. 2023, 2305361. 10.1002/advs.202305361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velusamy A.; Yang Y.-C.; Lin C.-C.; Afraj S. N.; Jiang K.; Chen P.-S.; Yau S.-L.; Osaka I.; Tung S.-H.; Chen M.-C.; Liu C.-L. Solution Processable Pentafluorophenyl End-Capped Dithienothiophene Organic Semiconductors for Hole-Transporting Organic Field Effect Transistors. Adv. Electron. Mater. 2022, 8, 2100648. 10.1002/aelm.202100648. [DOI] [Google Scholar]
- Youn J.; Huang P.-Y.; Huang Y.-W.; Chen M.-C.; Lin Y.-J.; Huang H.; Ortiz R. P.; Stern C.; Chung M.-C.; Feng C.-Y.; Chen L.-H.; Facchetti A.; Marks T. J. Versatile α,ω-Disubstituted Tetrathienoacene Semiconductors for High Performance Organic Thin-Film Transistors. Adv. Funct. Mater. 2012, 22, 48–60. 10.1002/adfm.201101053. [DOI] [Google Scholar]
- Dey S. Recent Progress in Molecular Design of Fused Ring Electron Acceptors for Organic Solar Cells. Small 2019, 15, 1900134. 10.1002/smll.201900134. [DOI] [PubMed] [Google Scholar]
- Afraj S. N.; Lin C.-C.; Velusamy A.; Cho C.-H.; Liu H.-Y.; Chen J.; Lee G.-H.; Fu J.-C.; Ni J.-S.; Tung S.-H.; Yau S.; Liu C.-L.; Chen M.-C.; Facchetti A. Heteroalkyl-Substitution in Molecular Organic Semiconductors: Chalcogen Effect on Crystallography, Conformational Lock, and Charge Transport. Adv. Funct. Mater. 2022, 32, 2200880. 10.1002/adfm.202200880. [DOI] [Google Scholar]
- Lin C.-C.; Afraj S. N.; Velusamy A.; Yu P.-C.; Cho C.-H.; Chen J.; Li Y.-H.; Lee G.-H.; Tung S.-H.; Liu C.-L.; Chen M.-C.; Facchetti A. A Solution Processable Dithioalkyl Dithienothiophene (DSDTT) Based Small Molecule and Its Blends for High Performance Organic Field Effect Transistors. ACS Nano 2021, 15, 727–738. 10.1021/acsnano.0c07003. [DOI] [PubMed] [Google Scholar]
- Vegiraju S.; Chang B.-C.; Priyanka P.; Huang D.-Y.; Wu K.-Y.; Li L.-H.; Chang W.-C.; Lai Y.-Y.; Hong S.-H.; Yu B.-C.; Wang C.-L.; Chang W.-J.; Liu C.-L.; Chen M.-C.; Facchetti A. Intramolecular Locked Dithioalkylbithiophene-Based Semiconductors for High-Performance Organic Field-Effect Transistors. Adv. Mater. 2017, 29, 1702414. 10.1002/adma.201702414. [DOI] [PubMed] [Google Scholar]
- Wang K.; Liu J.; Yin J.; Aydin E.; Harrison G. T.; Liu W.; Chen S.; Mohammed O. F.; De Wolf S. Defect Passivation in Perovskite Solar Cells by Cyano-Based π-Conjugated Molecules for Improved Performance and Stability. Adv. Funct. Mater. 2020, 30, 2002861. 10.1002/adfm.202002861. [DOI] [Google Scholar]
- Chiang C.-H.; Wu C.-G. Bulk Heterojunction Perovskite-PCBM Solar Cells with High Fill Factor. Nat. Photonics 2016, 10, 196–200. 10.1038/nphoton.2016.3. [DOI] [Google Scholar]
- Nie W.; Tsai H.; Asadpour R.; Blancon J.-C.; Neukirch A. J.; Gupta G.; Crochet J. J.; Chhowalla M.; Tretiak S.; Alam M. A.; Wang H.-L.; Mohite A. D. High-Efficiency Solution-Processed Perovskite Solar Cells with Millimeter-Scale Grains. Science 2015, 347, 522–525. 10.1126/science.aaa0472. [DOI] [PubMed] [Google Scholar]
- Khenkin M. V.; Katz E. A.; Abate A.; Bardizza G.; Berry J. J.; Brabec C.; Brunetti F.; Bulović V.; Burlingame Q.; Di Carlo A.; Cheacharoen R.; Cheng Y.-B.; Colsmann A.; Cros S.; Domanski K.; Dusza M.; Fell C. J.; Forrest S. R.; Galagan Y.; Di Girolamo D.; Grätzel M.; Hagfeldt A.; von Hauff E.; Hoppe H.; Kettle J.; Köbler H.; Leite M. S.; Liu S.; Loo Y.-L.; Luther J. M.; Ma C.-Q.; Madsen M.; Manceau M.; Matheron M.; McGehee M.; Meitzner R.; Nazeeruddin M. K.; Nogueira A. F.; Odabaşı C. ¸.; Osherov A.; Park N.-G.; Reese M. O.; De Rossi F.; Saliba M.; Schubert U. S.; Snaith H. J.; Stranks S. D.; Tress W.; Troshin P. A.; Turkovic V.; Veenstra S.; Visoly-Fisher I.; Walsh A.; Watson T.; Xie H.; Yıldırım R.; Zakeeruddin S. M.; Zhu K.; Lira-Cantu M. Consensus Statement for Stability Assessment and Reporting for Perovskite Photovoltaics Based on ISOS Procedures. Nat. Energy 2020, 5, 35–49. 10.1038/s41560-019-0529-5. [DOI] [Google Scholar]
- Wang F.; Geng W.; Zhou Y.; Fang H.-H.; Tong C.-J.; Loi M. A.; Liu L.-M.; Zhao N. Phenylalkylamine Passivation of Organolead Halide Perovskites Enabling High-Efficiency and Air-Stable Photovoltaic Cells. Adv. Mater. 2016, 28, 9986–9992. 10.1002/adma.201603062. [DOI] [PubMed] [Google Scholar]
- Xue J.; Lee J.-W.; Dai Z.; Wang R.; Nuryyeva S.; Liao M. E.; Chang S.-Y.; Meng L.; Meng D.; Sun P.; Lin O.; Goorsky M. S.; Yang Y. Surface Ligand Management for Stable FAPbI3 Perovskite Quantum Dot Solar Cells. Joule 2018, 2, 1866–1878. 10.1016/j.joule.2018.07.018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.












