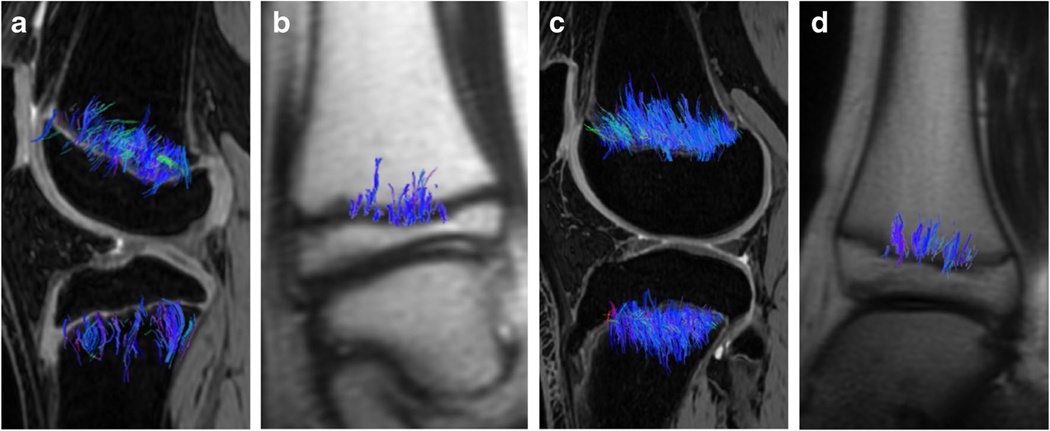
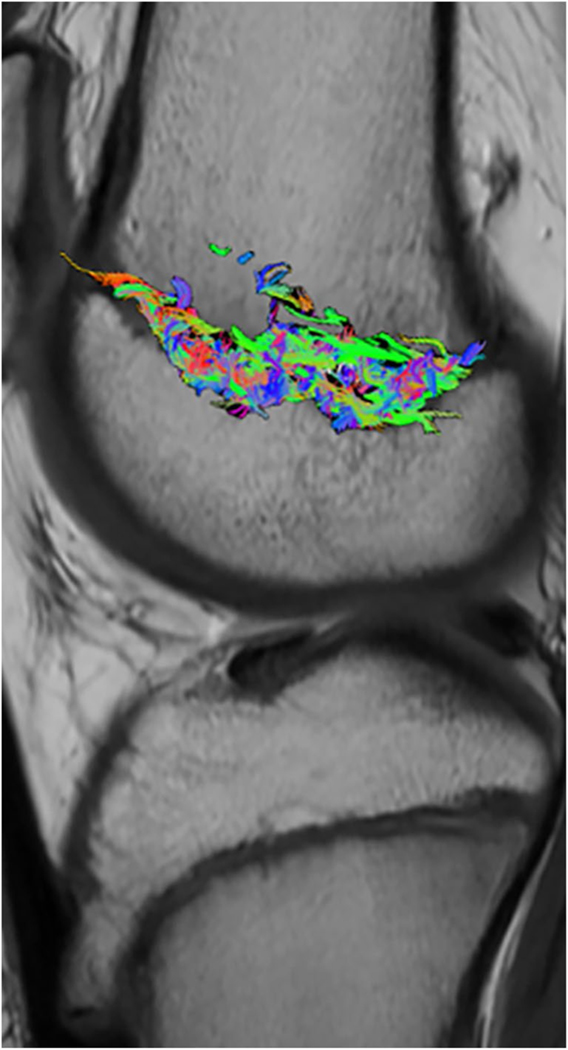
Abstract
The physis, or growth plate, is the primary structure responsible for longitudinal growth of the long bones. Diffusion tensor imaging (DTI) is a technique that depicts the anisotropic motion of water molecules, or diffusion. When diffusion is limited by cellular membranes, information on tissue microstructure can be acquired. Tractography, the visual display of the direction and magnitude of water diffusion, provides qualitative visualization of complex cellular architecture as well as quantitative diffusion metrics that appear to indirectly reflect physeal activity. In the growing bones, DTI depicts the columns of cartilage and new bone in the physeal-metaphyseal complex. In this “How I do It”, we will highlight the value of DTI as a clinical tool by presenting DTI tractography of the physeal-metaphyseal complex of children and adolescents during normal growth, illustrating variation in qualitative and quantitative tractography metrics with age and skeletal location. In addition, we will present tractography from patients with physeal dysfunction caused by growth hormone deficiency and physeal injury due to trauma, chemotherapy, and radiation therapy. Furthermore, we will delineate our process, or “DTI pipeline,” from image acquisition to data interpretation.
Keywords: Anisotropy, Child, Diffusion tensor imaging, Growth plate, Magnetic resonance imaging, Physis, Tractography
Graphical Abstract

Introduction
Vertical growth occurs at the physis and adjacent metaphysis, which together constitute the primary growth structure which will be referred here as the physeal-metaphyseal complex (PMC) [1].
The physis or growth plate in the growing child is a cartilaginous structure at the end of a bone, made up of parallel chondrocyte columns in a “palisade” or columnar arrangement [2]. The cartilage is converted into bone in the metaphysis. The newly formed bone in the metaphysis adjacent to the cartilage retains the columnar organization. Chondrocyte proliferation and hypertrophy result in increased extremity length, or longitudinal bone growth which peaks during puberty. Growth is subsequently halted by physeal closure after late adolescence [3].
Current growth prediction tools in clinical use do not provide information on the physes, although they are primarily responsible for height gain. Diffusion tensor imaging (DTI) of the physis is an MRI technique that provides a snapshot of physeal microarchitecture and growth physiology, indirectly representing physeal health and development. DTI depicts the motion and directionality of water molecules, or diffusion. The presence of membranes in the columns of cartilage and newly formed bone within the physis and metaphysis restricts the diffusion of water, producing a longitudinal vector on DTI depicted as tracts on diffusion tractography. When performed in subjects during the pubertal growth spurt, tractography provides individualized information about height change based on the number, volume, length, and morphology of the child’s tracts, which are indirectly based on the growth of the physis and proximal metaphysis [2, 4]. As the healthy child grows, the qualitative appearance and quantitative diffusion metrics of their physeal-metaphyseal tractography change, with diffusion metrics (tract count, volume, and length) reflecting the height change during the growth spurt [5]. This technique has been utilized to determine physeal activity and accurately predict height gain and final height [6, 7]. In addition, it may determine response to exogenous growth hormone treatment [7–9].
The physeal-metaphyseal complex is the growth organ comprised of cartilage columns within the physis and metaphysis. In this “How I do It,” we delineate the connection between the physeal-metaphyseal complex and zonal anatomy and tractography, the changes that occur during adolescence, and the possibility of predicting post-imaging growth and physeal closure and response to growth hormone therapy. We will also showcase our DTI workflow for analysis of the physeal-metaphyseal complex.
Physeal anatomy
The primary physis is a structure between the epiphysis and metaphysis at either end of a long bone [1]. The physis is made up of highly cellular cartilage which undergoes ossification into newly formed bone [3]. In the growing child, the cartilage of the primary physis is organized into columns of chondrocytes. Chondrocyte replication and hypertrophy result in added length at each column, which in turn increases the length of the bone (Fig. 1) [1]. This columnar architecture is preserved in the newly formed bone of the metaphyseal spongiosa, in which a parallel array of vessels meets the columns of cartilage. Increased velocity of growth as children transition into puberty is associated with an increased number and length of the columns. In later adolescence, the physis becomes progressively thinner and is bridged by small columns of bone, leading to physeal closure and skeletal maturity [1, 2].
Fig. 1.
Normal anatomy of the physis. The area of anatomy depicted by diffusion tensor imaging (DTI) is the physeal-metaphyseal complex. The left side of the physeal-metaphyseal complex is a line drawing representation and the right side is a superimposed safranin O stain highlighting the columnar architecture of cartilage and bone at the physeal-metaphyseal complex. The primary physis contains chondrocytes arranged in zones: reserve cells (Resting Zone) and proliferative cells arranged in columns (Proliferative Zone) which increase in size to become hypertrophic (Hypertrophic Zone) resulting in height gain. Histologically, the “palisading” arrangement of chondrocyte columns in the proliferative and hypertrophic zones allows for organized diffusion of water. At the end of the hypertrophic zone, decreasing oxygen levels triggers chondrocyte apoptosis and subsequent mineralization to form the primary spongiosa, which maintains the parallel arrangement of the physis
Diffusion tensor imaging: technique
In an obstacle-free environment, water will diffuse freely, a property known as isotropy. Brownian motion results in molecules diffusing a similar distance in all directions. However, if there are obstacles in the environment, such as cell membranes, molecular diffusion will be restricted in some directions but not in others. This restriction can range in organization from completely unrestricted (isotropy), to unidirectionally restricted (anisotropy) (Fig. 2) [10]. Diffusion-weighted imaging (DWI) measures the displacement of water molecules in a voxel over milliseconds. If the molecules diffuse freely, the MRI signal decreases in all directions, but if diffusion is restricted, it does not. If the boundaries (chondrocyte columns in the case of the physis) are organized to form a structured shape or directionality, the MRI signal will also be influenced by the differences in diffusion gradients relative to the orientation of the tissue [11].
Fig. 2.
Isotropic versus anisotropic diffusion. In this diagram, the beaker filled with water (top) represents an isotropic system; when a drop of blue dye is added to the beaker, the dye diffuses freely in all directions. In the bottom row of images, the beaker contains water and acrylic rods. When a drop of dye is added, it rapidly diffuses in a vertical direction along the columns of water between the acrylic rods, but is much slower to diffuse horizontally. The acrylic rods are analogous to cells arranged in a parallel and vertical configuration, with the cellular membranes limiting horizontal diffusion. This is an anisotropic system
DTI of the physis uses the directionality of the diffusion of water molecules to calculate a dominant tensor from the diffusing vectors of the water that runs through the physeal-metaphyseal complex [2]. This results in tracts in the direction of unrestricted diffusion. In other words, it is the vertical columnar organization of the chondrocytes in the physis and adjacent metaphysis that produces organized water diffusion which is in turn depicted by DTI tractography (Fig. 3) [2]. On tractography, the growth spurt is associated with more numerous, longer columns, and ongoing physeal closure is associated with fewer, shorter, and less organized tracts.
Fig. 3.
Standard convention for color coding of tractographic reconstruction. Blue tracts represent diffusion in the superior-inferior direction, red tracts in the left-to-right direction, and green tracts in the antero-posterior direction [10]. In this image, blue fiber tracts run perpendicular to the physis, corresponding to the direction of least restricted diffusion which is parallel to the cartilage columns within the physis and adjacent metaphysis. The right side is a superimposed safranin O stain highlighting the columnar architecture explain on Fig. 1
DTI of the physeal‑metaphyseal complex: how we do it
An efficient DTI workflow must be established to obtain reproducible and accurate tractography from which DTI metrics are derived. A step-wise approach is taken for collection and processing of data to enable data analysis and interpretation for growth prediction. We apply this technique in clinical practice at our tertiary pediatric hospital, to aid physicians seeking additional information on physeal-metaphyseal activity in cases of growth plate abnormality or after a growth plate injury.
Our data represents research and clinical work performed by us and a growing number of institutions that have started adopting the technique.
Acquisition
MRIs of the knee are acquired at 3T (GE Medical Systems, Waukesha, WI), using a fat-suppressed single shot spin echo echo-planar sequence with 20 noncollinear directions; b values 0 s/mm2 and 600 s/mm2. Imaging parameters include repetition time (TR)/echo time (TE); 3,000/51.7 ms; bandwidth 1953.12 Hz/pixel; parallel imaging factor, 2; signal averages, 5; matrix 255×255; and field of view, 256×256 mm. Voxel dimensions were 2.0×2.0 mm2 in-plane; section thickness, 3.0 mm; and with no gap between sections. The acquisition protocol and workflow can be adapted to other scanners; please refer to Kvist et al. and Jaimes et al. for diffusion tensor acquisition parameters using Phillips and Siemens magnets, respectively, at other centers [2, 4].
Post‑processing
Post-processing is done primarily by a research scientist at our institution. Other personnel (radiologists, residents, medical students) can be trained easily with supervised segmentation on practice cases. A previous study has shown the results to be highly reproducible [5]. Post-processing is done using tractography software Diffusion Toolkit v. 0.6.4 and TrackVis (FACT algorithm) (Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA), to manually draw regions of interest (ROIs) along the physes of the tibia and femur [12]. Diffusion Toolkit is a set of command-line tools for preprocessing of DTI data. It performs data reconstruction for conversion of the raw DICOM images to analyze format and an image orientation information file [12]. Afterwards, “tracking” is done using the tensor data and generating knee fiber tracks which are saved to a track data file. The segmentation of femur and tibia is done by using the generated tracks, locating the physis using the brightest voxel as a starting reference point and subsequently hand drawing the ROI transecting the physis.
A step-by-step tutorial on how to post-process images acquired with different MR vendors on Diffusion Toolkit and TrackVis can be found on Supplementary Material 1. Learners using this tutorial have been successful at performing DTI processing with high reproducibility.
Data interpretation and analysis
DTI metrics (tract count, volume, length, apparent diffusion coefficient (ADC), and FA) are obtained from both femur and tibia on every subject. Tract count refers to the number of tracts in a given ROI. The tract volume is the product of the number and length of the tracts [8]. Tract length denotes the millimeter distance separating two endpoints, where FACT tracking becomes unable to trace the diffusivity direction to adjacent voxels within the angle threshold specified during image reconstruction [13].
The diffusion metrics are subsequently compared with variables known to affect growth: sex, weight, parental height, and both chronological age and bone age.
Image superimposition
Physeal segmentation is done by identifying the brightest pixels to draw ROIs on post-processing software, as a result, the final image can look pixelated and appears noisy to the clinical radiologist. Superimposition of tractography on conventional MR images facilitates the understanding of tractographic information.
In our workflow, once we obtain the femur and tibia fiber tracts and collect diffusion metrics, we take a screenshot of the fiber tracts (TrackVis interface allows for visualization of the fiber tracts in the sagittal, coronal, and axial planes denominated “x,” “y,” and “z,” when these planes are deselected fiber tracks throughout the physes can be visualized in a 2D image). This step allows for a complete visualization of the fibers on the femur and tibia physes. Next, we use available free tools for background removal (e.g., Photoshop or Adobe) to eliminate the background from the screenshot.
After acquiring the DTI sequences, we can also obtain and view conventional MR images using any Digital Imaging and Communications in Medicine (DICOM) image viewing software. We then take a screenshot of a single slice in these conventional MR images. Subsequently, we superimpose the 2D tractography onto the screenshot of 3D-acquired conventional MR images (see Fig. 4).
Fig. 4.
Image superimposition. a A screenshot of the femur and tibia tractography is obtained. b The tractography screenshot is processed using any software for background removal; subsequently, a screenshot of any conventional 3D MR image in the coronal plane is obtained. Please note, we take a screenshot from one single coronal slice of the 3D image. c With the available background-less tractography, superimpose the tractography over the screenshot of the conventional MR images
A more detailed step-by-step tutorial on fiber tract super imposition on clinical images can be found on Supplementary Material 2.
Characteristic qualitative tractographic patterns during puberty in healthy children according to sex (girls ages 8‑15, boys ages 10‑16)
Physeal tractography patterns change qualitatively with age as the height of the columns increases, corresponding to increased velocity of growth. Femoral and tibial tracts will typically begin to lengthen around 8 years of age in females and 10 years of age in males [5]. In addition to changes with age, there are normal differences in tract appearance between different physes of the long bones. We have characterized mostly distal femoral and proximal tibial physeal tracts.
The femoral physeal tracts are predominantly located in the periphery, while tibial tracts are overall shorter and more central. The tibia has two distinct tract patterns: centrally located fibers with scant or absent fibers on the periphery, or uniform fiber tract distribution along the physis (Fig. 5). The sequence of normal physeal closure may explain why fiber tracts were more predominant in the periphery in the femoral physis and more centrally predominant in the tibia; MRI studies have shown that during normal development femoral physeal closure begins centrally––whereas in the tibia, although closure proceeds centrifugally, the anterior tuberosity is the last to unite with the metaphysis [14].
Fig. 5.
Femur versus tibia fiber tract patterns. Femur and tibial fiber tract patterns in three 11-year-old boys. There are qualitative differences in the tract patterns of the distal femoral physis versus the proximal tibial physis. (a) The femoral fiber tracts are longer and thicker than the tibial tracts (b, c) and femoral tracts are predominantly located peripherally (a) along the physis. (b, c) Tibial fiber tracts are either homogeneously distributed along the physis or predominantly central
In the prepubescent and early pubescent years, physeal tractography shows scant fiber tracts in children who have not yet experienced a significant growth spurt. On conventional MR of the physis at this stage, the physis appears as a distinct layer of cartilage between epiphyseal and metaphyseal bone. Due to slower growth velocity, fiber tracts will not be as dense or well-developed as in older children undergoing their growth spurt (Fig. 6).
Fig. 6.
Tractography on early pubescent children. Fiber tractography overlaid on conventional MR images, in three coronal T1 scans (a-c, e-g) and a sagittal proton-density (PD) image (d, h) in children transitioning into puberty, 8-year-old girls (a-d) and 10-year-old boys (e-h). The fiber tractography depicts the physeal-metaphyseal complex in both femur and tibia. Fiber tractography in both physes is scant, short, and parallel to the long axis of the bone in males and females. Females experience earlier appearance of fibers due to earlier growth spurt and during the growth spurt fiber tracts are overall finer and visibly less dense in females than in males
In children experiencing peak growth, the physeal tractography pattern increases in density and thickness associated with their rapid bone growth (Fig. 7). Rapid cell proliferation and hypertrophy result in both increased chondrocyte column height and increased stature. In this phase, the columns are longer, more densely packed, and organized in parallel, characteristics that are reflected in the tractography pattern.
Fig. 7.
Tractography of young adolescents during the growth spurt. Fiber tractography superimposed on conventional MR images, including the first three coronal T1 scans and a sagittal proton-density (PD) image, in children at peak growth: (a-d) 10-year-old females and (e-h) 12-year-old males. Femoral and tibial fiber tracts are elongated, thick, dense, and organized in parallel to the long axis of the bone. The males tend to have more numerous and longer tracts
In adolescents who have completed or are near completion of their growth spurt, physeal tractography will show smaller tracts due to physeal closure as bone growth slows down and eventually stops, a process that occurs gradually and at an individual rate depending on various factors [15]. As the physes close, the fibers that make up the physeal tracts become smaller, less well-defined, and less organized, resulting in a more fragmented and multicolored tractographic pattern. The tracts appear shorter and a clear directional pattern is absent (Fig. 8), with few blue tracts and more red and green tracts running perpendicular to the long axis of the bone, reflecting disorganized physeal and metaphyseal columns during partial or cotract patterns can help determine whether further growth remains or if skeletal maturity has been achieved (Fig. 9).
Fig. 8.
Tractography of older adolescents at the end of the growth spurt. Fiber tractography superimposed on conventional MR images, three coronal T1 images and a sagittal proton-density (PD), in adolescents at the end of growth: (a-d) 14-year-old females and (e-h) 15-year-old males. Femoral and tibial fiber tract patterns are not well-defined; tracts are short and multicolored indicating incoherent organization. Different degrees of physeal closure will be apparent at this phase, with some subjects demonstrating persistent remnant cartilage while others demonstrate complete closure. Figures 6a–h and 7a–h and (a-h) display similar qualitative tractographic patterns in both male and female subjects. The purpose of displaying these is to highlight the structural and organizational consistency of physeal tracts regardless of sex. The images show how the fiber tracts develop and change over time during the growth spurt according to sex under-scoring the earlier female growth spurt and the later appearance of the bulkier male fiber tractography. Both sexes initially have few fibers that grow, peak, and then decrease after the growth spurt
Fig. 9.
Tractography changes over time. Sequential results of DTI scans of the knee of a 12-year-old boy acquired over a 12-month span demonstrate the physeal changes that occur as the child progresses through puberty. a-b With the initial scan, tractography resulted in scant thin blue fiber tracts angled perpendicular to the femoral and tibial physes in coronal and sagittal planes. c-d At the 4-month follow-up, there was a visible increase in the number and volume of tracts, which corresponded to a 3.8-cm increase in the child’s height. e-f At the 12-month visit, the tracts had become progressively more widespread and volumetrically increased, corresponding to 5 cm of growth––or an overall height gain of 8.8 cm over 1 year
Lower extremity physes: tractography and diffusion metrics
Beyond the qualitative depiction of the tracts, the number, length, and volume of the tracts can be quantified (Fig. 10). Fractional anisotropy (FA), reflective of tissue organization, has values ranging from 0 when completely unrestricted to 1 if unidirectionally restricted.
Fig. 10.
Lower extremity PMC fiber tracts. a Tractography superimposed on sagittal MR images of the knee and coronal MR images of the ankle (b) from a 10-year-old girl during the growth spurt, versus tractography superimposed on sagittal MR images of the knee (c) and coronal MR images of the ankle (d) from a 12-year-old boy during peak growth. Comparing the images side by side showcases the generally more robust tractography patterns in males versus females, especially during peak growth. Note that the distal femoral tract count and volume are significantly larger than in the proximal tibia, which in turn has slightly larger tract count and volume than the distal tibia in both the female and male subjects; these differences are also qualitatively evident in the images
DTI tractography and diffusion metrics can predict post-imaging height gain and provide information on final height [5–7]. Tract length and volume have been validated as predictors of height velocity and total height gain (Fig. 11) with better accuracy than traditional bone age methods [6, 7]. Of all the DTI parameters, femoral tract volume best predicts the greatest change in height, after removing associations with age [6, 7]. In addition, fiber tract patterns can be qualitatively compared and provide information on the amount of future growth, as seen in Fig. 11. Traditional bone age methods showed bias and larger prediction errors associated with larger height gains compared to DTI [7].
Fig. 11.
Fiber tracts correlate to height gain. Diffusion tensor imaging tractography overlaid on coronal MR-images in two 11-year-old males shows a male with larger tract volume and a dense tractography pattern who achieved a subsequent 4.4 cm of growth over 4-months (a) and a second male with a scant tract pattern who achieved only 1.2 cm of subsequent growth (b)
Diffusion tensor imaging correlation with other imaging modalities
Although we lack human histologic validation of physeal tractography, studies of adolescent rabbits aged 16, 20, and 24-weeks old evaluated histologically, with DTI and with high-resolution micro-CT correlation (Fig. 12), confirmed that diffusion is in the direction of the physeal-metaphyseal complex [16–20]. Micro-CT depicted a brush-like appearance in that same area, and histology identified the area as the hypertrophic zone where enlarged columnar chondrocytes are responsible for organized diffusion. Femorotibial physeal height and volume was highest in the 16-week-old group and subsequently decreased with age in all imaging modalities [20], mimicking what has been found in healthy children in previous studies using DTI [5]. FA increased with age in rabbits and also in children approaching the end of the growth spurt [5, 20].
Fig. 12.
DTI correlation with other imaging modalities. “Coronal cross-section of the growth plate in multi-gradient echo 3D sequence on MRI, μCT, and histology (Masson’s trichrome staining) in each age group. The multi-gradient echo 3D sequence on MRI shows a high signal in the femoral (A) as well as the tibial (D) growth plate in the 16-wk-old rabbit; however, in the 24-wk-old, the growth plate is non-detectable (white arrows). μCT shows a low-attenuation growth plate in both the femur (B) and tibia (E). In the 20-wk-old rabbit, small bone bridging can be observed, and in the 24-wk-old rabbit, a completely fused growth plate is seen (red boxes). Histological representation shows the growth plate as a light blue line in 16- and 20-week-old rabbits in both the femur (C) and tibia (F). The light blue growth plate line cannot be seen in the 24-wk-old rabbit; instead, a dark blue sclerotic remnant of the growth plate is seen, which is sometimes referred to as a physeal scar (blue arrows).” From reference 19, open-access article permission not required [19]
Diffusion tensor imaging: growth hormone response evaluation
Growth hormone replacement is the preferred treatment for growth hormone deficiency in children to achieve normal adult height [21, 22]. However, response to therapy can vary and costs may be high [23–25]. DTI may offer an earlier assessment of response to therapy compared to current methods, which require detection of changes in height over a period of 1 to 2 years [26]. Individual tractography patterns can provide insight into physeal-metaphyseal complexes and zonal architecture conditions before treatment (Fig. 13), with more robust fiber tract patterns leading to greater growth (Fig. 14) [9].
Fig. 13.
DTI of the knee to evaluate GH response. Coronal MR images with diffusion tensor tractography superimposed in a 12-year-old male (a) and a 9-year-old female (b) before growth hormone was administered. Physes are intact in both patients on MR images; however, the fiber tractography was significantly different: tracts are abundant for the male (a) who grew 8 cm after 4 months of therapy, in contrast, to the scarce and thin fibers in the female (b) who achieved only 4 cm in the same time period
Fig. 14.
Longitudinal physeal-metaphyseal complex tractography changes in a growth hormone responder and representation of normalized tract volume and height. Growth hormone responder (Fig. 15a) displayed abundant and healthy-looking fiber tracts similar to those of healthy children after starting growth hormone treatment (Figs. 7, 8 and 9). Sagittal MR images with diffusion tensor tractography on a 12-year-old male before GH administration (left), and 4 months (center) and 8 months after (right). Tractography superimposed on sagittal 3D gradient echo images at the three times reveals scanty tracts before therapy (left). The number and density of the tracts increases progressively with growth hormone. Graphic representation of height and DTI changes. Values of stature and tract volume before therapy are normalized to 1. There is a 9% increase in height and a 220% increase in tract volume over an 8-month period
DTI tract patterns differ between GH responders and non-responders [9]. In neuroblastoma survivors receiving GH therapy post-cis-retinoic acid chemotherapy, physeal DTI showed distinct differences in tract patterns with scant and disorganized fibers in non-responders and copious predominantly blue fiber tracts in responders.
DTI of the physeal‑metaphyseal complex: clinical cases
In the context of growth, DTI of the knee physes can aid growth prediction and identify abnormal growth through tractography and the depiction of microstructural changes. The following clinical cases provide a glimpse of what DTI looks like in children with local insults to the physis that are known to potentially impair growth. Through clinical DTI images, we aim to better understand growth impairment and potential clinical implications on growth due to a number of illnesses.
Chronic repetitive physeal injury
In children with chronic repetitive physeal injury, areas of greatest pressure develop physeal widening and irregularity (Fig. 15) [27]. Histologic studies show that physeal widening in these children is caused by loss of the cartilaginous architecture and replacement by fibrous tissue that can be potentially due to repeated micromotion at the zone of cartilage transformation [27]. Chronic stress may widen the proximal uncalcified physis and make it susceptible to fracture and displacement [27]. The significance of DTI images in these patients is still unclear; however, the scarce fiber tracts in widened areas may be the result of disturbed diffusion due to physeal widening and associated loss of cartilage microarchitectural integrity (Fig. 15).
Fig. 15.
DTI in chronic repetitive physeal injury. DTI of the physis in an 11-year-old male with a medial femoral chronic repetitive physeal injury, genu varus deformity, and widening of the medial distal femoral physis shows femoral fiber tracts are abnormally scant and less dense than in the tibia
Salter‑Harris fracture
A Salter-Harris fracture is a fracture through the physis [28]. Fractures are classified into different types (I-V) according to physeal involvement, which influence prognosis and treatment [29]. Potential complications of physeal fractures are growth arrest, deformity, and limb-length discrepancy and can be evaluated by DTI tractography (Fig. 16) [29].
Fig. 16.
Salter-Harris fracture. a Sagittal proton-density (PD) image in a 12-year-old male with a Salter-Harris type 2 fracture affecting the medial distal femoral physis of the left knee. b Fiber tracts are absent where the fracture occurred and fiber tract length is decreased medially, adjacent to the fracture site. Longer tracts are seen laterally, farther from the fracture
Future goals
For MR-DTI of the physis to be used for clinical decision-making, the technique needs to be reliable and diffusion metrics need to be easily obtained. Physeal manual segmentation is time-consuming and operator-dependent. Using deep learning (DL) convolutional neural networks (CNNs) for automatization of physeal segmentation will provide more reliable results (Fig. 17). Furthermore, the use of deep learning (DL) algorithms for artifact elimination and increasing signal-to-noise ratios will provide better image quality and improve identification of physeal structures inside voxels. The automatization of superimposed DTI fiber tractography over clinical MR images allows clinicians to have a better understanding of the additional information provided using DTI which cannot be obtained with conventional MR images.
Fig. 17.
Physeal segmentation using artificial intelligence. a An AI-generated femoral ROI (pink) versus (d) manually drawn ROI (turquoise) in a 14-year-old male. a-c Both AI-generated ROI and the resultant tractography are identical to the manually drawn ROI (d-f). The AI-generated ROI was obtained after training a U-net transformer model for automated segmentation of the femur physis
Future areas for possible application of the technique and integration into routine clinical care include the prediction of the length of an individual bone in the context of leg length discrepancies or physeal injuries that may subsequently affect limb growth. For children with limb discrepancies, it may be possible to predict the differential growth of the femurs or tibias.
In children with growth arrest due to distal tibial physeal fractures, we are evaluating whether DTI can predict a slow-down of growth or growth arrest on part of the physis. Clinically, DTI metrics may be used to predict a discrepancy in growth months before it becomes apparent by conventional imaging. DTI may also be used to monitor the response to new therapies for systemic growth disorders in cases of achondroplasia, mucopolysaccharidosis, or hypophosphatasia (Fig. 18).
Fig. 18.
Hypophosphatasia. DTI in a 14-year-old male with hypophosphatasia with mild genotypic expression, short stature, and a delayed bone age of 12.5 years-old. Radiographs and MR images were unremarkable. Fiber tract pattern consists of predominantly green fibers interspersed with blue and red fiber tracts, with no clear directional pattern, which reflects physeal-metaphyseal complex structural disorganization in the context of a child with defective bone mineralization
Conclusion
In pediatric patients, DTI can be used as a valuable clinical tool, reflecting physeal and metaphyseal microarchitecture, and providing diffusion metrics for quantitative evaluation and prediction of skeletal growth. Tractography macroscopically reflects areas of increased growth in both the femoral and tibial physis. Physeal tractography patterns change with age: long parallel blue fibers in the physeal-metaphyseal complex likely reflect chondrocyte and bone columns in the child going through the growth spurt, which subsequently shorten into disorganized scant multicolored-fibers in the adolescent due to physeal closure. Tract volume shows growth velocity, with larger tract volumes representing increased growth velocity and adequate response to growth hormone.
Supplementary Material
Acknowledgements
We thank Dr. Ola Kvist, Dr. Sandra Diaz, and all the research team at the Pediatric Radiology Department Karolinska University Hospital in Stockholm, Sweden, as we borrowed an illustration from their open-access paper: “Magnetic resonance and diffusion tensor imaging of the adolescent rabbit growth plate of the knee” published by Wiley on Magnetic Resonance in Medicine Journal.
Funding
This study was funded by both SPR Research and Education Foundation and R01 HD104720.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00247-023-05753-z.
Declarations
Ethical approval and consent to participate The study was approved by the institutional review board of our institution and we certify that the study was performed in accordance with the ethical standards in the Declaration of Helsinki.
Conflicts of interest None
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available as it contains protected health information. However, de-identified data is available from the corresponding author on reasonable request.
References
- 1.Nguyen JC, Markhardt BK, Merrow AC, Dwek JR (2017) Imaging of pediatric growth plate disturbances. RadioGraphics 37(6):1791–1812. 10.1148/rg.2017170029 [DOI] [PubMed] [Google Scholar]
- 2.Jaimes C, Berman JI, Delgado J et al. (2014) Diffusion-tensor imaging of the growing ends of long bones: pilot demonstration of columnar structure in the physis and metaphysis of the knee. Radiology 273(2):491–501. 10.1148/radiol.14132136 [DOI] [PubMed] [Google Scholar]
- 3.Kvist O (2022) Magnetic resonance imaging and advanced imaging assessment of the growth plate in the adolescent and young adult. Karolinska Institute Press, Stockholm [Google Scholar]
- 4.Kvist O, Dorniok T, Sanmartin Berglund J et al. (2023) DTI assessment of the maturing growth plate of the knee in adolescents and young adults. Eur J Radiol. 162:110759. 10.1016/j.ejrad.2023.110759 [DOI] [PubMed] [Google Scholar]
- 5.Bedoya MA, Delgado J, Berman JI et al. (2017) Diffusion-tensor imaging of the physis: a possible biomarker for skeletal growth-experience with 151 children. Radiology 284(1):210–218. 10.1148/radiol.2017160681 [DOI] [PubMed] [Google Scholar]
- 6.Barrera CA, Bedoya MA, Delgado J et al. (2019) Correlation between diffusion tensor imaging parameters of the distal femoral physis and adjacent metaphysis, and subsequent adolescent growth. Pediatr Radiol 49(9):1192–1200. 10.1007/s00247-019-04443-z [DOI] [PubMed] [Google Scholar]
- 7.Jaramillo D, Duong P, Nguyen JC et al. (2022) Diffusion tensor imaging of the knee to predict childhood growth. Radiology 303(3):655–663. 10.1148/radiol.210484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong P, Mostoufi-Moab S, Raya JG et al. (2020) Imaging biomarkers of the physis: cartilage volume on MRI vs. tract volume and length on diffusion tensor imaging. J Magn Reson Imaging 52(2):544–551. 10.1002/jmri.27076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado J, Jaramillo D, Chauvin NA et al. (2019) Evaluating growth failure with diffusion tensor imaging in pediatric survivors of high-risk neuroblastoma treated with high-dose cis-retinoic acid. Pediatr Radiol 49(8):1056–1065. 10.1007/s00247-019-04409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soares JM, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. 7:31. 10.3389/fnins.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Hecke W, Emsell L, Sunaert S (2016) Diffusion tensor imaging: a practical handbook. 1st ed.Springer, New York [Google Scholar]
- 12.Wang R, Benner T, Sorensen AG, Wedeen VJ (2007) Diffusion Toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med 15:3720 [Google Scholar]
- 13.Torgerson CM, Irimia A, Leow AD et al. (2013) DTI tractography and white matter fiber tract characteristics in euthymic bipolar I patients and healthy control subjects. Brain Imaging Behav. 7(2):129–39. 10.1007/s11682-012-9202-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung T, Jaramillo D (1995) Normal maturing distal tibia and fibula: changes with age at MR imaging. Radiology 194(1):227–32. 10.1148/radiology.194.1.7997558 [DOI] [PubMed] [Google Scholar]
- 15.Kvist O, LuizaDallora A, Nilsson O et al. (2021) A cross-sectional magnetic resonance imaging study of factors influencing growth plate closure in adolescents and young adults. Acta Paediatr 110(4):1249–1256. 10.1111/apa.15617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seinsheimer F, Sledge CB (1981) Parameters of longitudinal growth rate in rabbit epiphyseal growth plates. J Bone Joint Surg Am. 63(4):627–30 [PubMed] [Google Scholar]
- 17.Buckwalter JA, Mower D, Ungar R et al. (1986) Morphometric analysis of chondrocyte hypertrophy. J Bone Joint Surg Am. 68(2):243–55 [PubMed] [Google Scholar]
- 18.Farnum CE, Lee R, O’Hara K, Urban JP (2002) Volume increase in growth plate chondrocytes during hypertrophy: the contribution of organic osmolytes. Bone. 30(4):574–81. 10.1016/s8756-3282(01)00710-4 [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Mirando AJ, Cofer G et al. (2019) Diffusion tractography of the rat knee at microscopic resolution. Magn Reson Med. 81(6):3775–3786. 10.1002/mrm.27652 [DOI] [PubMed] [Google Scholar]
- 20.*Kvist O, Damberg P, Dou Z et al. (2023) Magnetic resonance and diffusion tensor imaging of the adolescent rabbit growth plate of the knee. Magn Reson Med. 89(1):331–342. 10.1002/mrm.29432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanley T (2012) Diagnosis of growth hormone deficiency in childhood. Curr Opin Endocrinol Diabetes Obes. 19(1):47–52. 10.1097/MED.0b013e32834ec952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sizonenko PC, Clayton PE, Cohen P et al. (2001) Diagnosis and management of growth hormone deficiency in childhood and adolescence. Part 1: diagnosis of growth hormone deficiency. Growth Horm IGF Res. 11(3):137–65. 10.1054/ghir.2001.0203 [DOI] [PubMed] [Google Scholar]
- 23.Savage MO, Bang P (2012) The variability of responses to growth hormone therapy in children with short stature. Indian J Endocrinol Metab. 16(Suppl 2):S178–84. 10.4103/2230-8210.104034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplowitz P, Manjelievskaia J, Lopez-Gonzalez L et al. (2021) Economic burden of growth hormone deficiency in a US pediatric population. J Manag Care Spec Pharm. 27(8):1118–1128. 10.18553/jmcp.2021.21030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fideleff HL, Boquete HR, Suárez MG, Azaretzky M (2016) Burden of growth hormone deficiency and excess in children. Prog Mol Biol Transl Sci. 138:143–66. 10.1016/bs.pmbts.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 26.Grimberg A, DiVall SA, Polychronakos C et al. (2016) Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr 86(6):361–397. 10.1159/000452150 [DOI] [PubMed] [Google Scholar]
- 27.Rodgers WB, Schwend RM, Jaramillo D et al. (1997) Chronic physeal fractures in myelodysplasia: magnetic resonance analysis, histologic description, treatment, and outcome. J Pediatr Orthop. 17(5):615–21. 10.1097/00004694-199709000-00008 [DOI] [PubMed] [Google Scholar]
- 28.Cepela DJ, Tartaglione JP, Dooley TP, Patel PN (2016) Classifications in brief: Salter-Harris classification of pediatric physeal fractures. Clin Orthop Relat Res. 474(11):2531–2537. 10.1007/s11999-016-4891-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine RH, Thomas A, Nezwek TA, Waseem M (2023) Salter Harris fractures. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK430688/ Accessed 25 April 2023 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available as it contains protected health information. However, de-identified data is available from the corresponding author on reasonable request.