Abstract
Malaria and typhoid fever are two febrile illnesses prevalent in the tropics that often present overlapping symptoms. In this work, we demonstrate an optical reader-based diagnostics platform for rapid codetection and quantification of two antigen targets: lipopolysaccharide (LPS) for typhoid fever and plasmodium lactate dehydrogenase (pLDH) for malaria infections. We report a limit of detection (LoD) of 5 ng/mL for LPS and 10 ng/mL for pLDH in a spiked serum test. We also validated the duplex test’s performance of differentiating malaria infection, typhoid fever infection, and coinfection by testing clinical samples in human serum. Our platform provides the potential for further multiplexing by encoding different color codes to various detection targets. The rapid result (~15 min), low cost (~$2), and minimal volume requirement for human serum clinical samples (4 μL) of our diagnostic platform offer great potential for deployment in resource-limited settings to help distinguish common causes for acute febrile illnesses at the point-of-need.
Graphical Abstract

1. INTRODUCTION
Acute febrile illnesses (AFIs) represent a significant global burden. It also remains a challenge to differentially diagnose different febrile illnesses, especially in low-income countries. Malaria and typhoid fever are two febrile illnesses with overlapping geographical distribution and clinical presentation.1,2 In 2019, 229 million new malaria infections caused 409,000 deaths worldwide according to the WHO malaria report.2 Typhoid fever, on the other hand, caused an estimated 11–21 million cases with around 128,000 to 161,000 deaths worldwide every year.3 Low-resource settings with poor public health infrastructure, such as many regions in sub-Saharan African, were endemic for both malaria and typhoid fever.1,2
In addition, malaria and typhoid fever present with similar symptoms, particularly during the early stage of typhoid fever.4,5 The coinfections of these two further complicate the diagnostic dilemma.6-8 The association of malaria and typhoid fever was first described in the 19th century and was named “typho-malarial” fever.9,10 The traditional malaria diagnosis is based on microscopy, and the diagnosis of typhoid fever usually involves the culture of blood, stool, or bone marrow samples.11-13 These methods are time-consuming and usually require sophisticated lab equipment and trained personnel.
Microfluidic paper-based analytical devices, including lateral flow assays (LFAs), have been used as a portable and affordable rapid diagnostic test (RDT) for many health and disease states.14-18 Traditional LFAs are usually based on single color conjugations such as gold nanoparticles. This presents a challenge to monitor cross-reactivity between species. Moreover, LFA results are typically qualitative, and while quantitative evaluation could be achieved with LFAs, they are usually imaged by the camera of a mobile device in low-resource settings. The variances of background intensity and lightening conditions affected affect the quantification accuracy.
In this work, we demonstrated a two-color optical reader-based LFA system for rapid codetection and quantification of two antigen targets: plasmodium lactate dehydrogenase (pLDH) and Salmonella typhimurium (S. typhi) lipopolysaccharide (LPS). Since antigen-based LFAs detect the presence and concentration of antigens for symptomatic people,19 they are chosen in the current study since the goal is to differentiate the causes for patients who have ongoing fever symptoms. Since pLDH is pan-specific to all four human malaria Plasmodium species,20 it was used as the target for malaria.21 For typhoid fever, we chose S. typhi LPS, a major component of S. typhi’s outer membrane, as our detection target.22 We first evaluated the duplex’s performance in a spiked serum test, followed by a validation study of human serum clinical samples qualitatively and quantitatively.
2. MATERIALS AND METHODS
2.1. Antibody Conjugation to Latex Nanoparticles.
Mouse pan pLDH monoclonal antibodies (MyBioSource) and mouse monoclonal Salmonella antibodies (Fitzgerald) were conjugated to 400 nm blue and red latex nanoparticles, respectively, using the latex conjugation kits (Expedeon). Stock antibodies were first diluted to 0.1 mg/mL before being moved to a mini vial to react with latex particles. Following the reaction, quenching, and centrifugation, the conjugated antibodies for each mini vial were resuspended to 0.5% (v/v) in a storage buffer containing 50 mM borate and 10% sucrose. The conjugated antibodies were then stored at 4 °C for future use.
2.2. LFA Strip Manufacturing and Testing Protocol.
The LFA test strip based on a Hi-Flow Plus 180 membrane card (EMD Millipore) contains the following components: a sample pad, a conjugate pad, a nitrocellulose membrane, and a collection pad (see Figure 1A). Each pad had a 0.2 mm overlap to ensure a consistent flow at the conjugation. Mouse pan pLDH monoclonal antibodies (MyBioSource), mouse anti-Salmonella typhimurium LPS monoclonal antibodies (Fitzgerald), and goat antimouse IgG (whole molecule) antibodies (Sigma-Aldrich, Inc.) were all diluted to 0.5 mg/mL in 1× PBS and dispensed as the test line for malaria, the test line for typhoid fever, and the control line, respectively. The membrane card was dried for 2 h at 37 °C for antibody immobilization. These lines were dispensed 3 mm apart on a nitrocellulose membrane by an automated lateral flow reagent dispenser (ClaremontBio Solutions). The assembled pad cut into 4 mm × 6 cm was caged in a plastic cassette with a predried conjugation pad containing 1.2 μL of blue latex nanoparticle conjugated pLDH antibodies in 0.5% (v/v) and 0.8 μL red latex nanoparticle conjugated LPS antibodies in 0.5% (v/v) for each strip.
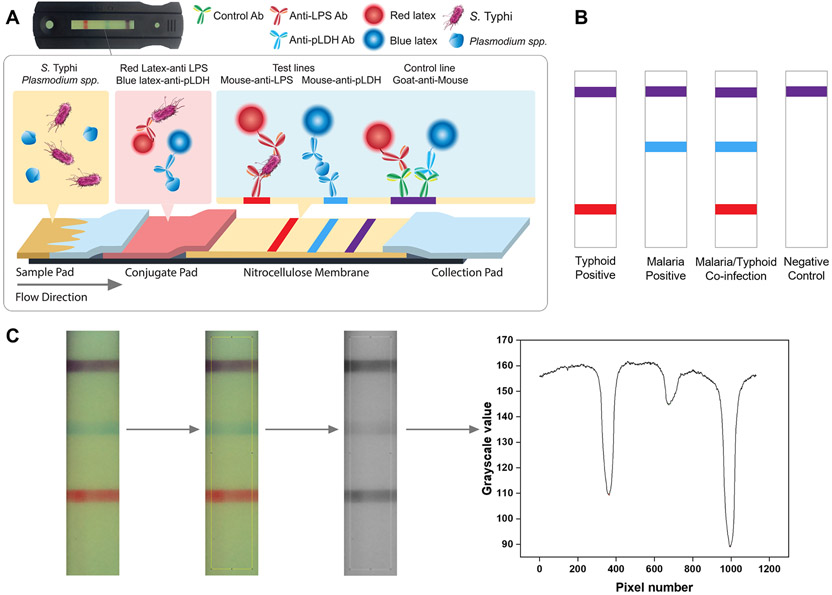
Figure 1.
Schematics, sample cases, and quantification protocol of a typhoid/malaria duplex lateral flow assay (LFA). (A) Schematics of a typhoid/malaria duplex LFA. A serum sample containing S. typhi and certain species of plasmodium (e.g., P. falciparum) is being added to the sample pad, followed by a buffer wash. S. typhi LPS and pLDH are chosen as the target for Typhoid fever and malaria, respectively. The conjugate pad contains both LPS and pLDH detection antibodies preconjugated with red and blue latex nanoparticles. S. typhi and P. falciparum bind to latex nanoparticle preconjugated LPS and pLDH detection antibodies, respectively. As the liquid sample flows through the nitrocellulose membrane, the nanoparticle-conjugated LPS detection antibody is captured by the first line (shown in red). The nanoparticle-conjugated pLDH detection antibody is captured by the second line (shown in blue). Unbound latex nanoparticle conjugates are captured by the third line to serve as the control (shown in purple). The excess liquid sample is absorbed on the collection pad. The cassette containing the latex nanoparticle-based LFA is inserted into a custom-built optical reader for visualization and subsequent quantification. (B) Sample cases for various diagnostic scenarios. Purple is used for the control line to denote the coinfection of typhoid fever and malaria. (C) Image processing algorithm to quantify intensity levels of test lines and the control line. The raw images are first cropped to suitable sizes, and the same region of interest (ROI) is applied to all the images to ensure consistency. Raw images are later converted to grayscale images. R, G, B, and grayscale values for the ROI are extracted from the images using the color profiler plug-in in ImageJ, and Simpson’s 3/8 rule is applied to calculate the areas under the peaks.
In clinical testing, a human serum sample containing target analytes was placed on the sample pad. It was washed by a buffer containing 1× TBS, 1% BSA, and 1% Tween-20 to avoid nonspecific binding. As the sample flowed through the membrane, red latex nanoparticle-conjugated LPS detection antibodies were captured by the first line. Blue latex nanoparticle-conjugated pLDH detection antibodies were captured by the second line. The third line captured unbound red and blue latex nanoparticle conjugates to serve as the control. Purple was used for the control line to denote typhoid fever and malaria coinfections. The excess liquid sample was absorbed on the collection pad. After 15 min, test strip images were taken by a custom-built optical reader. Sample cases for various diagnostic scenarios were shown in Figure 1B.
Qualitative diagnostic results can be obtained by visual assessment of the images. Quantification of analyte concentrations could be achieved by analyzing strip images taken by our custom-built optical reader system.23 Figure S1 contains the testing protocol and the interior view of the reader. Peak intensities were subsequently analyzed for the images taken by the reader system. Figure 1C shows the image analysis protocol to quantify the intensity levels of both test lines and the control line. Briefly, the raw images were cropped to suitable sizes before being converted to grayscale images. RGB and grayscale values were extracted with the “Color Profiler” plug-in command in ImageJ, and a program in LabView integrated areas under the peaks.20 The relationship between peak intensities and concentrations was established to get a calibration curve for later quantification. A consistent ROI selection for all images is essential in quantification,24 and the effect of ROI selections on quantification is shown in Figure S2.
2.3. Calibration Curve Preparation in Spiked Serum.
The calibration curve was prepared by spiking LPS and pLDH antigens of known concentrations ranging from 5 to 500 ng/mL to pooled human serum samples to simulate typhoid fever and malaria infections. All tests were conducted in triplicates. Peak area intensities for LPS and pLDH test lines were plotted against concentration levels to generate their respective calibration curves. These calibration curves were later used for predicting the analyte concentrations in the validation tests with the human clinical samples in serum.
2.4. Validation with Human Clinical Samples.
The clinical validation was conducted to evaluate the performance of the duplex platform with 25 human samples (Discovery Life Sciences). These included eight malaria-positive clinical samples containing P. falciparum species in human serum, two typhoid fever-positive samples containing S. typhi in whole blood, and 15 whole blood negative samples. Serum samples were separated from whole blood through centrifugation at 2000g for 10 min. The two S. typhi-positive samples were confirmed by blood culture. The remaining 23 samples were confirmed by microscopy results, and the pLDH concentrations of the P. falciparum samples were quantified by ELISA kits (Cellabs, #Quantimal pLDH CELISA). All 25 samples were run three times with the LFA duplex. A dilution factor of 5 was found to be optimal based on the concentration range of clinical samples and test line capture reagents.
3. RESULTS
3.1. Calibration Curve for Samples in Spiked Serum.
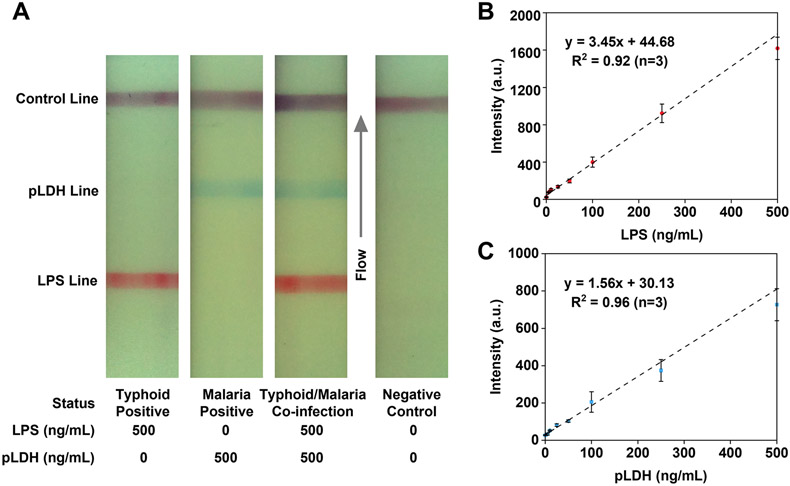
The LFA strips testing LPS and pLDH antigens with concentrations ranging from 5 to 500 ng/mL spiked into pooled human sera were imaged with the custom-built optical reader described above. Figure 3A shows test strips representing various diagnostic scenarios: Typhoid fever-positive, malaria-positive, coinfection, and a negative control case simulated with a pooled human serum sample. Linear fittings were established to fit the calibration curves to correlate the test line intensities of LPS and pLDH antigens to their respective concentrations. The calibration curve for LPS (Figure 3B) was fitted linearly to an equation so that LPS intensity = 3.45 × (LPS) + 44.68, where (LPS) represents LPS antigen concentration, with an R2 value of 0.92. The calibration curve for pLDH (Figure 3C) was fitted linearly to an equation so that pLDH intensity = 1.56 × (pLDH) + 30.13 where (pLDH) represents pLDH antigen concentration, with an R2 value of 0.96. To evaluate the efficacy for both assays, the limit of detection (LoD) was calculated on the basis of a standard method to compare the intensity value of the negative control sample (blank sample) plus three times the standard deviation (Sblank + 3SD) with the intensities of test lines at various concentrations. With this criterion, the LoD was estimated to be 5 ng/mL for LPS and 10 ng/mL for pLDH, respectively. Test strips from the spiked serum samples confirmed the assay platform’s capability of differentially detecting multiple diagnostic scenarios with low detection limits.
Figure 3.
Clinical sample test to validate the assay performance for the malaria/typhoid fever codetection diagnostic platform. (A) Images of test strips of eight malaria-positive human serum samples and two malaria-negative samples as examples for the negative controls. All malaria clinical samples are confirmed by microscopy results and quantified by ELISA for the antigen concentrations. All samples are diluted five times before testing. (B) Correlation plot of the predicted serum pLDH concentrations with those determined by ELISA. A good correlation is established between the two methods, as proven by the R2 value for the linear relationship. Error bars represent standard deviations from triplicate tests. (C) Images of test strips of two human clinical samples that are confirmed by blood culture to be positive for S. typhi. Samples are also diluted five times before testing. (D) Simulation of malaria/typhoid fever coinfection scenarios by spiking LPS antigens on clinical sample #VIII under different dilution ratios. With the decrease of antigen concentrations, both pLDH and LPS test line intensities decrease.
3.2. Clinical Sample Validation.
We tested eight P. falciparum-positive clinical samples in human serum, two S. typhi-positive clinical samples in whole blood, and 15 negative whole blood samples. All whole blood samples were centrifuged to separate serum samples before testing. The test strip images of all eight malaria-positive samples and two negative samples are presented in Figure 3A. The results of all eight malaria-positive and 15 negative samples were confirmed by microscopy characterization, and the results of two S. typhi-positive clinical samples were confirmed by blood culture. All these results were provided by the supplier. The pLDH antigen concentrations of the eight malaria-positive samples were quantified by ELISA kits. All malaria-positive samples were diluted five times before testing so that their concentrations were within the calibration curve range for further quantification. A rise in pLDH line intensities with rising concentrations can be observed qualitatively (Figure 3A). For quantitative analysis, the test line intensities of the pLDH lines were extracted, and their concentration values were predicted using the calibration curve (Figure 2C). A correlation plot of serum pLDH concentrations predicted from the LFA results against their corresponding ELISA results is shown in Figure 3B. Results showed a correlation efficient of 0.98 (P < 0.0001) with an R2 value of 0.97 for linear fitting. This quantitatively proved the efficacy of the LFA-based platform presented herein for the accurate determination of pLDH concentrations. The LFA results of two S. typhi-positive clinical samples were shown in Figure 3C, and their corresponding LPS concentrations were calculated accordingly (Figure 2B). However, since no quantitative results were available for these two samples, other than the blood culture test, we could only demonstrate the duplex LFA’s capability of detecting S. typhi qualitatively.
Figure 2.
Test strips showing different diagnostic scenarios and calibration curves for LPS and pLDH antigens spiked in pooled human serum. (A) Images of test strips representing various diagnostic scenarios: Typhoid fever-positive, malaria-positive, the coinfection of typhoid fever and malaria, and the negative control. (B) Calibration curve for LPS antigens with concentrations from 5 to 500 ng/mL. In this range, the LPS test line intensity increases as the concentration rises. Error bars represent standard deviations of the mean of the triplicate measurements. (C) Calibration curve for pLDH antigens with concentrations from 5 to 500 ng/mL. The pLDH signal intensity increases with a rising concentration in this range. Error bars represent standard deviations of the mean of triplicate measurements..
Due to the lack of access to malaria/typhoid fever coinfection human clinical samples, we spiked LPS antigens of known concentrations in a malaria-positive clinical sample to simulate coinfection. We tested the samples under various dilution conditions (Figure 3D). The results further proved no cross-reactivity between malaria and typhoid fever test lines. Both pLDH and LPS test line intensities decreased with further dilutions. They exhibited similar intensity values compared to the results in the spiked serum test.
4. DISCUSSION
Our optical reader-based LFA platform provided a differential quantification of two antigens responsive to malaria and typhoid fever. We first evaluated the LFA performance by spiking antigens with known concentrations into pooled human serum samples and acquired calibration curves for later quantification. Our duplex LFA combined two targets on the same strip and achieved detection limits (5 ng/mL for LPS and 10 ng/mL for pLDH) comparable to the best LFA singleplex.21 We also evaluated the duplex assay’s quantification performance by testing malaria clinical samples in human serum. These clinical samples were precharacterized by microscopy and quantified by ELISA to serve as the gold standard pLDH values. We applied the calibration curves generated during the spiked serum test to predict the concentrations of these clinical samples. A good correlation was established between ELISA results and values predicted by LFA with a correlation coefficient close to one. We also spiked LPS antigens of known concentrations to a selected malaria-positive clinical sample to mimic coinfection and further validated no cross-reactivity.
The primary innovation for the present study is to differentially detect and quantify two antigen targets inferring malaria and typhoid fever at the same time. Our differential diagnostic platform provides a rapid, accurate, and simultaneous quantification of two targets with a single test strip based on a low-cost optical reader, which is optimized for colorimetric detection, has minimal sample volume requirement, and can pick up faint signals to enable sensitive detection. The differential diagnosis of these two febrile illnesses with overlapping symptoms will lead to better clinical management and reduce the health burden for low-resource settings. We first showed our system’s ability to differentiate malaria and typhoid fever in spiked serum tests, followed by a clinical validation using malaria-positive and typhoid fever-positive human samples as well as simulated coinfection samples. Meanwhile, we validated the quantification potential of our LFA platform for determining pLDH concentrations by testing eight malaria-positive human samples with known pLDH concentrations. Since the current study only involved two typhoid fever-positive human samples characterized by blood culture tests for qualitative confirmation, we anticipate having a better evaluation of our LFA platform’s quantification potential for LPS antigens with access to a larger quantity of clinical samples.
One distinct advantage of the optical reader platform is the reduced background disparity to enable accurate quantification of analytes. We have demonstrated the reader’s ability to keep a consistent background for different cases23 and identified a method to directly save the raw data taken by the CMOS camera.25 The reader’s ability to recognize faint signals led to a minimal sample requirement at ~4 μL as demonstrated in the clinical validation. The cost for the optical reader was estimated to be ~ $70,23 and we envision placing the reader in clinics or primary health care centers to aid physicians or community health workers in making management decisions in resource-limited settings. The duplex cost was estimated to be ~$2 with over 60% of the cost from latex nanoparticles (Table S1). The current study demonstrates the optical reader’s efficacy in visualizing and quantifying latex nanoparticle-based LFAs, and we have shown the system can also be directly applied in gold nanoparticle-based LFAs and easily adapted for fluorophore-based applications.25-27 We envision that alternative conjugation materials such as europium nanoparticles can help increase the detection sensitivity. Upconversion nanoparticles have also shown promise in enabling a low detection limit while reducing the cost of the test strip.28
Our two-color multiplex LFA encoding each disease with a different color has the distinct advantage of facilitating rapid visual assessment for qualitative evaluation. By incorporating image analysis, we could predict the antigen concentrations quantitatively by analyzing the intensity levels of the respective test lines using predetermined calibration curves. The color separation algorithm also enables a simple method for evaluating the cross-reactivity between different infections. We envision our system could be easily expanded for other febrile illnesses by incorporating other detection targets such as viral proteins from dengue, chikungunya, and COVID-19. While the physical limitation of test strips could limit the total number of test lines, this problem can be solved by combining different test lines. We have shown the feasibility of detecting and quantifying pLDH and PfHRP2 with one single test line for the differential diagnosis of different human malaria species via our unique color-mixing encoding and color separation algorithm.20 With more robust color separation and optimization on color-mixing ratios, we envision that our LFA system can be further multiplexed while mitigating the physical limitations of traditional LFA test strips.
5. CONCLUSIONS
In summary, we developed an optical reader-based diagnostic platform for differential quantification of antigen targets for malaria and typhoid fever. Our colorimetric detection duplex LFA, shown to have good agreement with gold standard methods, presents a rapid and low-cost approach for differential detection and quantification of multiple targets with a minimal sample volume requirement.
Supplementary Material
ACKNOWLEDGMENTS
S.M. and D.E. acknowledge funding from the National Institutes of Health through 1R01EB021331. We also thank Vicky Simon in the Division of Nutritional Sciences at Cornell University for conducting the validation ELISA assays to determine the gold standard concentrations for pLDH antigens in human serum samples.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.1c03298.
Additional experimental protocol, details on the optical reader system and signal quantification, and cost breakdown of the duplex LFA strip (PDF)
The authors declare the following competing financial interest(s): D.E. and S.M. are founders and board members for a diagnostic start-up focused on measuring nutritional biomarkers at the point-of-care.
Contributor Information
Xiangkun Elvis Cao, Sibley School of Mechanical and Aerospace Engineering, Cornell University, Ithaca, New York 14853, United States of America.
Jinsu Kim, Division of Nutritional Sciences, Cornell University, Ithaca, New York 14853, United States of America.
Saurabh Mehta, Division of Nutritional Sciences and Institute for Nutritional Sciences, Global Health, and Technology, Cornell University, Ithaca, New York 14853, United States of America.
David Erickson, Sibley School of Mechanical and Aerospace Engineering, Cornell University, Ithaca, New York 14853, United States of America; Division of Nutritional Sciences and Institute for Nutritional Sciences, Global Health, and Technology, Cornell University, Ithaca, New York 14853, United States of America.
REFERENCES
- (1).Stanaway JD; Reiner RC; Blacker BF; Goldberg EM; Khalil IA; Troeger CE; Andrews JR; Bhutta ZA; Crump JA; Im J; Marks F; Mintz E; Park SE; Zaidi AKM; Abebe Z; Abejie AN; Adedeji IA; Ali BA; Amare AT; Atalay HT; et al. Lancet Infect. Dis 2019, 19, 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).World Health Organization. World Malaria Report 2020:20 Years of Global Progress And Challenges World Health Organization: Geneva, 2020. [Google Scholar]
- (3).World Health Organization. Malaria Rapid Diagnostic Test Performance: Results of Who Product Testing of Malaria RDTs: Round 8 (2016–2018); https://www.who.int/publications/i/item/9789241514965 (accessed 2021–07-29).
- (4).Odikamnoro O; Ikeh I; Okoh F; Ebiriekwe S; Nnadozie I; Nkwuda J; Asobie G Afr. J. Infect. Dis 2017, 12, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ohanu M; Mbah A; Okonkwo P; Nwagbo F West Afr. J. Med 2004, 22, 250–252. [DOI] [PubMed] [Google Scholar]
- (6).Samatha P; Rao CK; Sowmya SB J. Evol. Med. Dent. Sci 2015, 4, 11322–11327. [Google Scholar]
- (7).Pradhan PJ Med. Lab. Diagn 2011, 2, 22–26. [Google Scholar]
- (8).Sharma B; Matlani M; Gaind R; Pandey K IOSR-JDMS 2016, 15, 101–104. [Google Scholar]
- (9).Laffan JG Lancet 1887, 130, 215. [Google Scholar]
- (10).Exile. Lancet 1913, 181, 131. [Google Scholar]
- (11).Mweu E; English M Trop. Med. Int. Health 2008, 13, 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wain J; Diep TS; Bay PVB; Walsh AL; Vinh H; Duong NM; Ho VA; Hien TT; Farrar J; White NJ; Parry CM; Day NP J. J. Infect. Dev. Countries 2008, 2, 469. [DOI] [PubMed] [Google Scholar]
- (13).Payne D Bull. World Health Organ 1988, 66, 621. [PMC free article] [PubMed] [Google Scholar]
- (14).Li X; Zhao C; Liu X Microsyst. Nanoeng 2015, 1, 15014. [Google Scholar]
- (15).Kasetsirikul S; Shiddiky MJA; Nguyen N-T Microfluid. Nanofluid 2020, 24, 17. [Google Scholar]
- (16).Li Z; You M; Bai Y; Gong Y; Xu F Small Methods 2020, 4, 1900459. [Google Scholar]
- (17).He W; You M; Li Z; Cao L; Xu F; Li F; Li A Sens. Actuators, B 2021, 334, 129673. [Google Scholar]
- (18).Li F; You M; Li S; Hu J; Liu C; Gong Y; Yang H; Xu F Biotechnol. Adv 2020, 39, 107442. [DOI] [PubMed] [Google Scholar]
- (19).Centers for Disease Control and Prevention. Interim Guidance for Antigen Testing for SARS-CoV-2; https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html (accessed 2021-07-29).
- (20).Kim J; Cao XE; Finkelstein JL; Cárdenas WB; Erickson D; Mehta S Malar. J 2019, 18, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).World Health Organization. Immunization, Vaccines and Biologicals; https://www.who.int/teams/immunization-vaccines-andbiologicals/diseases/typhoid (accessed 2021-07-29).
- (22).Felgner J; Jain A; Nakajima R; Liang L; Jasinskas A; Gotuzzo E; Vinetz JM; Miyajima F; Pirmohamed M; Hassan-Hanga F; Umoru D; Jibir BW; Gambo S; Olateju K; Felgner PL; Obaro S; Davies DH PLoS Neglected Trop. Dis 2017, 11, No. e0005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wang R; Ongagna-Yhombi SY; Lu Z; Centeno-Tablante E; Colt S; Cao XE; Ren Y; Cárdenas WB; Mehta S; Erickson D Anal. Chem 2019, 91, 5415–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Scherr TF; Gupta S; Wright DW; Haselton FR Sci. Rep 2016, 6, 28645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lu Z; O’Dell D; Srinivasan B; Rey E; Wang R; Vemulapati S; Mehta S; Erickson D Proc. Natl. Acad. Sci. U. S. A 2017, 114, 13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Srinivasan B; Finkelstein JL; O’Dell D; Erickson D; Mehta S EBioMedicine 2019, 42, 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Srinivasan B; O’Dell D; Finkelstein JL; Lee S; Erickson D; Mehta S Biosens. Bioelectron 2018, 99, 115–121. [DOI] [PubMed] [Google Scholar]
- (28).You M; Lin M; Gong Y; Wang S; Li A; Ji L; Zhao H; Ling K; Wen T; Huang Y; Gao D; Ma Q; Wang T; Ma A; Li X; Xu F ACS Nano 2017, 11, 6261–6270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.