Abstract

Breast cancer is a leading cause of death in women, and its management highly depends on early disease diagnosis and monitoring. This remains challenging due to breast cancer’s heterogeneity and a scarcity of specific biomarkers that could predict responses to therapy and enable personalized treatment. This Perspective describes the diagnostic landscape for breast cancer management, molecular strategies targeting receptors overexpressed in tumors, the theranostic potential of the oxytocin receptor (OTR) as an emerging breast cancer target, and the development of OTR-specific optical and nuclear tracers to study, visualize, and treat tumors. A special focus is on the chemistry and pharmacology underpinning OTR tracer development, preclinical in vitro and in vivo studies, challenges, and future directions. The use of peptide-based tracers targeting upregulated receptors in cancer is a highly promising strategy complementing current diagnostics and therapies and providing new opportunities to improve cancer management and patient survival.
Significance
Receptors overexpressed in tumors but not healthy cells are promising targets for theranostics. The peptide hormone oxytocin receptor (OTR) is one such emerging target in breast cancer. Peptide-based optical and nuclear tracers are being developed that target OTR in breast cancer to validate OTR’s role and theranostic potential in breast cancer as well as to develop more effective, safer, and more personalized treatment options.
1. Breast Cancer—A Heterogeneous Disease
Breast cancer is the second most common type of cancer diagnosed in women after non-melanoma skin cancers.1 The global cancer statistics reported 2.3 million newly diagnosed breast cancer cases and 684,996 deaths (responsible for 15.5% of total mortalities in females) in 2020.2,3 Despite having a favorable prognosis,4 breast cancer remains the leading cause of death in women worldwide.2 Efficient diagnosis and classification of breast cancer subtypes facilitate the management of the disease, but the heterogeneity of the malignant cells present in the mammary epithelial tissue often hinders accurate diagnosis.5 Current breast cancer diagnosis relies primarily on the assessment of molecular markers, such as the estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2),6 and Ki67, a cancer antigen used as a marker for cell proliferation and now an accepted prognostic factor to differentiate between ER-positive (ER+) tumor subtypes (Figure 1).7,8 Based on the expression of hormonal receptors, breast tumors can be classified into the following four subtypes: luminal A, luminal B, HER2-positive (HER2+), and triple-negative breast cancer (TNBC).9 Luminal A tumors have the highest incidence (∼50%) among women but also have the best therapeutic outcome; by contrast, luminal B tumors have a lower incidence (∼15%) but worse prognosis.9 ER-negative (ER–) breast tumors overexpressing HER2 (incidence of ∼20%) initially had poor therapy outcomes,9 which have improved in recent years due to the introduction of combination therapies.10 FDA-approved anti-HER2 agents include monoclonal antibodies Pertuzumab, Trastuzumab, and Ado-Trastuzumab emtansine (T-DM1);11 tyrosine kinase inhibitors Lapatinib (TYKERB),11,12 Neratinib (Nerlynx),12 and Tucatinib (Tukysa);12 and the antibody–drug conjugate fam-trastuzumab deruxtecan-nxki (DS-8201a, T-DXd, ENHERTU).13 Finally, tumors that are negative for the biomarkers mentioned above (ER–, PR–, HER2–) fall in the TNBC category. TNBC includes basal (neoplastic cells constitutively expressing markers, such as cytokeratins or EGFR)14 and non-basal tumors,15 with most TNBCs expressing basal markers.14 TNBC patients have poor overall survival due to the tumors’ aggressiveness and increased risks of relapse16,17 and have an incidence rate of ∼15%.9,18 Pembrolizumab, a humanized monoclonal anti-programmed cell death protein 1 (PD1) antibody, is used as a neoadjuvant and adjuvant for treating patients with high-risk early-stage TNBC.19,20
Figure 1.
Intrinsic subtypes of breast cancer. They are represented as an accumulation of tumor cells in orange (luminal A), purple (luminal B), green (HER2), and dark pink (TNBC). The average incidence is presented in the pie chart, and the relative treatment outcome prognosis for the different tumor subtypes is shown in the bottom triangle (data on molecular profiles adopted from refs (22−24)). Ki67 is a nuclear protein associated with cell proliferation. A high fraction of Ki67-positive tumor cells suggests a high proliferation rate and is often indicative of more aggressive tumors.
According to the “5-year relative survival percentage” reported by the U.S. National Cancer Institute, the highest survival pattern was observed in women with luminal A subtype (94.4%), followed by the luminal B subtype (90.7%), HER2 subtype (84.8%), and finally TNBC (77.1%).9 Although mortality has decreased due to early detection and increasing therapeutic options, almost 30% of patients diagnosed with early stages of breast cancer still develop recurrent or metastatic diseases,9 with 5-year survival rates in those patients of only 27%.21
2. Diagnostic Tools for Breast Cancer Management
In most countries, manual breast palpation is the first screening method to detect breast tumors,25 followed by visualizing abnormalities through different breast imaging techniques. An overview of current technologies for breast cancer detection is presented in Table 1, including their advantages and limitations.
Table 1. Current Breast Cancer Detection and Diagnostic Methods, Including Their Advantages and Limitationsa.
A visual overview of the three main limitations is represented with colored circles. “Sensitivity”, in a clinical context, refers to the amount of correctly diagnosed patients (i.e., true positives), as opposed to the term “specificity”, which alludes to the number of healthy people correctly diagnosed as not having the disease (i.e., true negatives).29 Stage I, tumor less than 2 cm; stage II, tumor 2–5 cm.
Mammography utilizes a low dose of X-rays to visualize the internal structure of the breast.26−29 It is widely used to identify the early onset of breast cancer before the manifestation of physical symptoms.26,27 It has, however, low sensitivity in young women due to higher breast tissue density and higher tumor growth rate than in older women.28 Even though mammography is the gold standard used for breast cancer screening,30 other techniques such as ultrasonography31 and magnetic resonance imaging (MRI)32 are used to identify tumors that are not detectable in mammograms, as well as to determine tumor size more accurately. Each of these modalities has advantages and limitations, mostly due to their low specificity (i.e., high rate of false positives).33,34 Other techniques, such as sentinel lymph node biopsy (SLNB)35 or molecular profiling,36 aid in categorizing the stage of breast tumors.
Nuclear imaging techniques such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET) are used as auxiliary imaging tools to better characterize breast cancer. SPECT and PET can detect tumors using radiotracers (small molecules, peptides, antibodies, affibodies, or nanobodies labeled with radioisotopes) directed to receptors, transporters, or enzymes overexpressed in cancer cells.37−41 The clinical use of SPECT and PET remains limited due to the high costs (especially in the case of PET tracers that have short half-lives and require in-house cyclotron production) and scarcity of radiotracers. However, considering the ongoing technological advances, we expect to see a broader utilization of these technologies, particularly due to their high sensitivity and accuracy.42
SPECT uses radiotracers that emit γ-rays captured with a γ camera to acquire multiple 2D projections from different angles to produce a full 3D body image.56 SPECT-CT permits accurate 3D localization of primary and/or metastatic tumors, yet with a limited spatial resolution (∼10 mm).49 Typical radiopharmaceuticals used in SPECT imaging of breast cancer include [201Tl]thalluimchloride, [99mTc]technetium methoxyisobutylisonitrile ([99mTc]Tc-MIBI, [99mTc]Tc-sestamibi), and [99mTc]technetium diphosphonates (Figure 2).57 As an example, SPECT-CT with [99mTc]Tc-MIBI is used for finding proliferative tumoral tissue around a breast implant, in the remaining breast parenchyma, or on the chest wall after surgery.58
Figure 2.
Radiopharmaceuticals used in SPECT imaging.
PET imaging is based on detecting annihilation photons produced by the disintegration of positron-emitting radiotracers.59 The most commonly used radiotracer for the visualization of tumor distribution is [18F]fluorodeoxyglucose ([18F]FDG), a radiolabeled glucose analogue that cancer cells absorb in greater amounts than normal cells due to their increased metabolic activity.47 The different pharmacokinetic (PK) and pharmacodynamic (PD) rates are measured throughout the body with high spatial resolution, allowing the visualization of cancer metastases far away from the breast. A limitation of PET is its deficient detection rate for non-invasive breast cancers and small breast carcinomas.60,61 Sensitivity of PET highly depends on tumor size,50 varying from 95% for tumors larger than 1 cm to 25% for tumors smaller than 1 cm (resolution limit in modern clinical PET scanners is 4 mm).62 This fact renders the detection of early-stage breast cancer (stage I, tumors 1–19 mm) challenging.60,63 Moreover, the variability of glucose uptake in primary breast tumors differs in terms of tissue integrity and vascular density, which can result in false negatives that are difficult to differentiate from real signals.64
Both SPECT and PET can assess the presence and extent of disease as well as provide unique information about tumor biological characteristics, such as the rate of proliferation and metabolic activity.57 These methods are important and powerful techniques to complement traditional imaging modalities in breast cancer diagnosis and monitoring. The therapeutic potential of these techniques is leveraged when targeted approaches are employed, directing a radiopharmaceutical to the tissue of interest and differentiating it from healthy tissue.
3. Receptor-Targeted Approaches
The discovery that several cell surface receptors are overexpressed in tumor tissues compared to healthy tissue enables tumor-targeting strategies.65 Targeting entities include small molecules, peptides, antibodies, or antibody fragments, each having their advantages and limitations. Small molecules, for example, offer good bioavailability, stability, and tumor tissue penetration but are often not target-specific due to their small size and limited chirality.66 By contrast, antibodies provide high target specificity and affinity but have to be injected and have poor tumor penetration due to their large size.67 Peptide ligands display a good balance in biochemical properties between small molecules and antibodies, having higher tissue penetration than antibodies and better target specificity and affinity than small molecules.67−70 While some peptides have short half-lives, it is relatively easy to tune their metabolism and clearance rate, e.g., using fatty acid modifications for serum albumin binding.71 Moreover, peptides can be equipped easily with reporter tags compatible with optical (fluorescent tracers) or nuclear (radiotracers) imaging.68,72 Such peptide tracers, therefore, hold promising potential as diagnostic and therapeutic tools in oncology.72,73
3.1. Tracers for Optical Imaging
Optical imaging tracers are typically equipped with fluorophores to visualize membrane receptors expressed in cancer cells. While fluorescent ligands can be used in vitro, tissue autofluorescence needs to be considered for in vivo imaging since mammalian tissues are opaque to light in the visible spectrum (400–700 nm).74 In these cases, near-infrared (NIR) fluorophores can be used that function in wavelength regions above 700 nm.75 NIR fluorophores reduce light-scattering effects, enabling better tissue penetration (>1 cm). NIR imaging is applied in superficial tumor detection, integrated as part of endoscopies and open-surgery procedures, which assists surgeons in removing cancerous tissue;72,76 indocyanine green (ICG) and methylene blue (MB) are so far the only two NIR fluorophores approved by the FDA.75,77 NIR peptide tracers have been developed targeting overexpressed EGFR in glioblastomas78 and the integrin αvβ3 receptor expressed on sprouting tumor vasculatures.79 Most NIR tracer research is still preclinical, focusing on improving optical properties and toxicity profiles,80,81 with the exception of BLZ-100 (Tozuleristide, Tumor Paint; chlorotoxin peptide conjugated to ICG), which is expected to receive FDA approval for its use in visualizing pediatric brain cancer cells during tumor-removing surgery and has also been applied to breast cancer.82,83
3.2. Tracers for Nuclear Imaging
Nuclear imaging tracers use radioisotopes to visualize cancer cells. Particularly SPECT and PET techniques are employed to provide 3D positional images of targeted tumors in the body;47 this relates to whole-body as well as focused breast imaging, although the latter offers better diagnostic accuracy.84,85 Radiotracers can be used in the clinic for disease scanning, tumor characterization (staging), and treatment response monitoring.37 The design and synthesis of radiotracers for breast cancer (Figure 3) were initially based on the labeling of identified biomarkers (ER, PR, and HER2). The [18F]FES PET radiotracer has been used in ER+ tumors for preclinical evaluation of a therapeutic response and clinical visualization of breast cancer with an average of 90% specificity and 85% sensitivity.86−92 In the case of PR, [18F]FFNP was used in a clinical study, identifying ∼94% of PR+ breast tumors using PET.93 To visualize HER2+ breast tumors, both PET and SPECT radiopharmaceuticals based on the monoclonal antibody trastuzumab were tested,14−18,94 and the efficacy of 64Cu-labeled trastuzumab to detect HER2-positive breast cancer was confirmed.95
Figure 3.
Tracers used for nuclear imaging of different breast cancer types. Ligands, linkers, and chelators are colored in black, radionuclides in purple, and antibodies in mauve. Abu, 4-aminobutyric acid; Sta, statine ((3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid).
Less studied targets include the gastrin-releasing peptide receptor (GRPR) and vasoactive intestinal polypeptide receptor 1 (VIP-R1), yet their expression in breast cancer is documented (GRPR96−99 and VIP-R1100,101), and they were investigated clinically for the detection of breast malignancies.102−104 Radiotracers used for the detection of GRPR were [68Ga]Ga-SB3, achieving 50% tumor detection (n = 8),102 and [68Ga]Ga-RM2, reaching 72% breast cancer visualization (n = 18),103 both GRPR peptide antagonists. In a clinical study to detect VIP-R1, radiotracer [64Cu]Cu-TP3805 was used, achieving 100% detection of breast tumors (n = 20).104
Expression of target receptors often depends on breast cancer subtype and might be influenced by factors such as treatment or disease progression.37 TNBCs currently lack validated target receptors or biomarkers,105 and targeting metastatic disease is challenging, with a poor 5-year survival (<30%).106 It remains therefore important to identify and validate new receptor targets for breast cancer management, especially for TNBC. The peptide hormone oxytocin receptor (OTR) is such a new target.107,108
4. The Oxytocin Receptor: An Emerging Target for Breast Cancer Management
OTR belongs to the class I family (rhodopsin-like) of G protein-coupled receptors (GPCRs) and is activated by its endogenous peptide hormone OT.109 OT is a nonapeptide comprising a six-residue macrocycle linked by a disulfide bond between positions 1 and 6 and a three-residue tail with a C-terminal amide (Figure 4a). OTR’s structure has recently been resolved via X-ray diffraction, with antagonist retosiban bound to an inactive conformation,110 and via cryo-EM, with OT bound to an active conformation (Figure 4b,c).111,112 OTR can be upregulated to play important roles in the reproductive, cardiovascular, endocrine, and central nervous systems.109 OTR becomes upregulated in the female breast during pregnancy when the mammary glands develop in preparation for breastfeeding.107,109 During breastfeeding, OT is synthesized in the magnocellular neurons of the hypothalamus, transported to the posterior pituitary (neurohypophysis),109,113 from which it is released into the bloodstream to bind to OTR in the mammary glands, inducing contractions of the myoepithelial cells resulting in milk ejection.114 OTR is also expressed in breast tumors,107,115 as well as leiomyoma,116 neuroblastoma and glioma,117 adenocarcinoma of the endometrium,118 ovarian carcinoma,119 prostate cancer,120−122 small-cell lung carcinoma,123 trophoblast,124 choriocarcinoma,124 and osteosarcoma.125,126 Peptide radiotracers have been developed successfully targeting OTR in breast malignancies (mouse models),127,128 supporting sufficient receptor density in tumors for diagnosis and treatment. OTR mRNA was detected in up to 95% of human breast cancer cell lines (n = 60)129−134 and tissues (n = 57).130,131 OTR was detected at the protein level in 91% of such tissue samples,130,133 although immunohistochemistry results need to be considered cautiously due to potential problems related to OTR antibody specificity and lack of appropriate controls. Proliferation assays using human epithelial triple-negative MDA-MB-231 and ER+ MCF7135 breast cancer cell lines demonstrated increasing antiproliferative effects when OT was administered in a dose-dependent manner (1–100 nM), consistent with previous data.136
Figure 4.
OT chemical structure and the OT-OTR complex. (a) Molecular structure of OT (blue) and the recommended labeling position 8 indicated in purple. (b) Side view of OT bound to OTR. (c) Top view of OT bound to OTR, as determined via single-particle cryo-electron microscopy of OTR in complex with OT (adapted from ref (112)). For a detailed map of molecular interactions, please refer to Waltenspühl et al.112
Administration of OT and OT analogues in different rodent breast cancer models reduced breast tumors substantially (44–82%).107,115,137−139 For example, OT or atosiban (biased OTR ligand) was administered via osmotic pumps into xenograft models of BALB/c mice (n = 43) and Fisher rats (n = 22) bearing mammary carcinomas TS/A and D-R3230AC, respectively, for 14 days.115 This resulted in up to 72% tumor reduction in animals treated with OT or atosiban compared to controls. Tumor reduction by atosiban was unexpected since it was thought to be an OTR antagonist; further studies, however, demonstrated atosiban to be a biased ligand, selectively activating Gi signaling while blocking Gq signaling.140 In another study that used BALB/c mice bearing MC4-L2 mouse mammary adenocarcinomas, treatment with OT for 42 days reduced tumor growth rate and tumor size (∼82%).137 Treatment with atosiban was also studied; however, no significant changes in tumor growth or size were observed. Using the same xenograft system, BALB/c mice bearing MC4-L2 mouse mammary adenocarcinomas (n = 56), a 44% tumor reduction upon OT treatment and a 12% increase in tumor volume with atosiban were observed.138 In another study, OT was administered for 21 days in BALB/C mice bearing mammary carcinomas TS/A, which led to a tumor reduction of ∼50%.107,139 While tumor reduction upon OT treatment seems fairly consistent among studies, atosiban displayed varying effects (reduction/proliferation of tumors or no effect in tumor growth), warranting more systematic studies. OT also seems to be released upon physical activity, acting protectively against breast cancer:141 mice bearing breast tumors were assigned to a treadmill for 5 days, increasing OT concentration in plasma. Since an endogenous OT increase was also observed in parallel to tumor growth inhibition effects, a different group of animals was externally administered with OT (no exercise), confirming OT-dependent tumor reduction (tumor volume 42 days after tumor transplantation, tumor + exercise training, and tumor + OT was 1.10, 0.81, and 0.56 cm3, respectively).138 All of the used breast cancer cell lines express OTR. The MCF7 cell line originates from a patient with metastatic breast cancer and is the most studied human breast cancer cell line in the world,142 while TS/A originated from a spontaneous mammary tumor of an inbred BALB/c female mouse exhibiting features typical of human breast cancer.143 MC4-L2 xenograft models are experimental models of mammary carcinogenesis in which the administration of medroxyprogesterone acetate to female BALB/c mice induces progestin-dependent ductal metastatic mammary tumors with high levels of both ER and PR.144 Despite the overall beneficial effects of OT in these animal models, more studies are required to investigate the effects of OT and optimized/selective OTR drug candidates on human xenograft models across a variety of different subtypes. OT itself is considered a poor drug candidate due to its short circulation half-life and activity at closely related vasopressin receptors (VPRs).
In a clinical study, endogenous OT increased up to 9-fold in the peripheral blood from tumor tissue of breast cancer patients (n = 40) compared to contralateral (healthy) breast; this effect increased in the advanced stages of the disease (p ≤ 0.004).145 Interestingly, OTR gene and protein expression were up to 11-fold downregulated.145
The relationship between breastfeeding and breast cancer has been studied since the 1950s.146 The consensus is that breastfeeding reduces the risk of developing breast cancer.147−155 The most comprehensive study with the highest statistical significance to date was published in 2002, a worldwide collaborative analysis comprising 47 studies which included population-based, case-control, and follow-up analyses in parous women of different ages and ethnic origins in developed and developing countries, and with a wide range of reproductive and breastfeeding patterns.148 The relative risk of breast cancer development in this study was reduced by 4.3% per year in women who breastfed for at least 12 months. Another study with women in Sri Lanka (n = 19,755) demonstrated a striking reduction of 87–94% in the risk of developing breast cancer among women who breastfed for more extended periods (24–47 months).155 The exact mechanisms underpinning these protective effects require further investigation; however, evidence points to an involvement of the OT/OTR signaling system.
The exact expression profiles of OTR in breast tumors of different subtypes and stages remain an active research question; however, OTR-specific nuclear imaging tracers accumulated in breast malignancies in vivo, suggesting that OTR expression is sufficient for tumor-specific targeting, at least in the rodent models employed.127,128,156 More systematic studies investigating OTR expression across a large panel of breast cancer patient samples will be critical in revealing cancer subtypes that display robust OTR overexpression to support tumor imaging and targeted radiotherapy.
5. Oxytocin Receptor Tracer Development
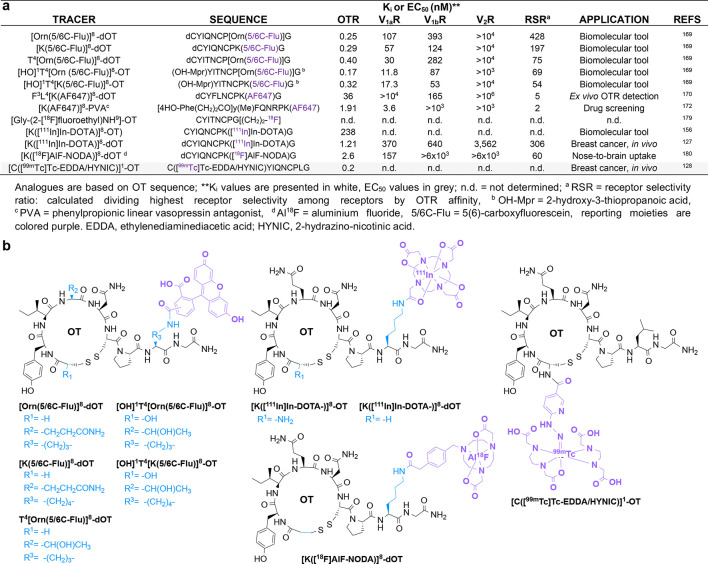
A major bottleneck in OTR ligand development is OTR’s similarity to the closely related VPRs, V1aR, V1bR, and V2R.157−159 Considerable efforts have been invested in structure–activity relationship (SAR) studies and medicinal chemistry approaches, with thousands of ligands synthesized and pharmacologically characterized.157,158,160−164 This resulted in several approved peptide drugs acting via OTR (e.g., OT, demoxytocin, atosiban, carbetocin); however, none of these ligands is OTR-selective,157 with selectivity defined as a 100-fold affinity preference for one receptor over the others.165Table 2 lists a range of agonists and antagonists with selectivity/preference for OTR. Models of ligand–receptor interactions originally suggested that the OT ring fits deeply into the transmembrane (TM) core, interacting with a cluster of residues located in TM3, TM5, and TM6, whereas the tail interacts with the upper part of the first TM helix and the second extracellular domain of the receptor (Figure 4b,c).166−168 Recently elucidated OT-bound OTR structure via single-particle cryo-EM revealed that all 9 amino acids of the OT participate in OTR binding with the cyclic part (residues 1–6) being deeply buried inside of the pocket while the C-terminal tripeptide (7–9) is situated toward extracellular loops (Figure 4b,c).112 Leu8 is oriented toward the extracellular space, explaining why position 8 is best suited for attaching fluorophores or other modifications in OT.112 Position 8 of OT (Figure 4a, purple) has been replaced or modified with several moieties without substantially affecting OTR binding or activation. Particularly replacement of Leu8 with lysine or ornithine provides a suitable handle for OT-like tracer production.127,169−172 This modification is often combined with removing the N-terminal amine, rendering the peptide less susceptible to aminopeptidases and more hydrophobic with a better binding pocket fit and enhanced potency.173 Several OTR tracers have been developed169 and are listed and depicted in Figure 5, along with their pharmacological profiles across OTR and VPRs and their applications.
Table 2. OT Analogues with OTR Preference or Selectivity over VPRs.
RSR, receptor selectivity ratio: calculated dividing highest receptor selectivity among receptors by OTR affinity.
Disulfide bridge formed with substituted dibromoxylene (m-xylene bridged between residues 1 and 6).
thio = S–S bridge was substituted for CH2–S, with CH2 in position 6.
BuG, N-(n-butyl)glycine.
thP = trans-4-hydroxyproline.
L(Me) = γ-methylleucine.
thio = S–S bridge was substituted for CH2–S, with CH2 in position 1.
X′ amino acids used to form a lactam bridge.
zG = azaglycine.
G(2-ThiMe) = N-alkylated glycines with 2-thiophenylmethyl.
MeBzlG = N-(3methylbenzyl)glycine.
FBzlG = N-(4-fluorobenzyl)glycine.
C8 = octanoic acid.
Biphasic fitting (Hill coefficient for both phases was fixed to −1; “EC50 hi” refers to the high-affinity site and “EC50 lo” to the low-affinity site.178 All assay values were obtained from pharmacological testing on human isoforms of OTR, V1aR, V1bR, and V2R. *Analogues are based on OT sequence (indicates C-terminal amide). **Ki values are presented in white, EC50 values in gray. n.d. = not determined; “d” = for desamino (no N-terminal amine); d(CH2)5 = 1-[β-mercapto-β,β-cyclopentamethylene]propionic acid.
Figure 5.
Overview of developed OTR tracers with information on OTR and VPR pharmacology, OTR selectivity, application, and chemical structure. (a) OTR-targeting fluorescent tracers and radiotracers with their sequence information, the OTR and VPR pharmacology, the OTR selectivity ratio (RSR), and the application information. (b) Chemical structures of all listed OTR tracers. Residues that differ from endogenous OT are highlighted in blue and labeling groups (fluorophores, radionuclides, and metal chelators) in purple.
Some of these OTR radiotracers have been used to target breast cancer.127,128 ([K([111In]In-DOTA)]8-OT) displayed high affinity for OTR (MCF7 breast cancer cells) and tumor uptake (BALB/c mice bearing OTR+ TS/A tumor) with a tumor-to-blood ratio of 2.67.156 The desamino version of this tracer, [K([111In]In-DOTA)]8-dOT, was evaluated in a preclinical study aiming to determine the amount and specificity of the receptor-mediated uptake using an experimental model of OTR+ TS/A tumors growing in Balb/c mice.127 This biodistribution study demonstrated higher tracer uptake in tumors than in blood or liver (tumor/blood and tumor/liver uptake ratios were 7.58 and 1.42, respectively) but lower than in kidneys (tumor/kidney uptake ratio was 0.06), highlighting a rapid clearing process.127 OTR-specificity was confirmed by administering 50 μg of OT 30 min before radiotracer administration, resulting in a 3-fold-reduced tumor uptake.127 [K([111In]In-DOTA)]8-dOT internalized with OTR within 5 min, supporting tracer tumor accumulation, which could be beneficial for tumor imaging or targeted radiotherapy.127
[C([99mTc]Tc-EDDA/HYNIC)]1-OT is another radiotracer developed to target OTR.128 Here, 2-hydrazinonicotinic acid (HYNIC) chelates technetium-99m, a γ-emitting radionuclide with a half-life of 6 h commonly used for SPECT.181 As such complexes may exist in numerous isomeric forms, adding ethylenediaminediacetic acid (EDDA) as co-ligand helps prepare complexes of higher stability and symmetry, resulting in fewer coordination isomers.182 Interestingly, the addition of the chelator HYNIC to the N-terminal amine of OT did not affect OTR binding,183 while introducing [99mTc]Tc-EDDA/HYNIC to position 8 reduced binding affinity, as determined by a radioimmunocompetition assay (IC50 values of 0.2 and 1 nM, respectively). Both Lys8- and Cys1-labeled tracers were internalized in OTR-expressing MCF7 cells. In vivo, breast tumors were induced in athymic male mice by subcutaneous injection of MCF7 cells. From the two studied labeling positions, only [C([99mTc]Tc-EDDA/HYNIC)]1-OT achieved tumor uptake, besides the typical non-specific uptake in kidneys and liver.128
6. OTR Tracers for Breast Cancer—Opportunities and Challenges
OTR radiotracers have shown promise in breast cancer mouse models with OTR and tumor-specific uptake.127,128,156 Rational design to advance OTR tracer development is feasible, particularly considering the many SAR studies with OT analogues along with the well-established OTR/VPR pharmacology. Such tracer development will provide novel imaging tools, if not theranostic leads, that should be beneficial not only for tumor imaging and cancer management but also for fundamental research investigating OTR’s role in health and disease.
While discoveries linking OTR to breast cancer are promising, only a single study evaluated OTR tracers in animals bearing human breast tumors, however, with poor pharmacological tracer characterization (no VPR data) and limited biodistribution info (no tumor/background tracer uptake ratios).127 Further in vivo studies using xenograft tumor models with different human breast cancer subtypes are needed to assess translational and clinical perspectives. OTR levels need to be systematically profiled across different subtypes to provide a clearer picture of OTR’s potential as a molecular target for imaging and therapy. OTR quantification at the protein level remains challenging due to the high extracellular homology of OTR with VPRs (V1aR, V1bR, and V2R) and a lack of specific antibodies.157,159,184,185 OTR tracers may be able to help in this task, given sufficient signal/brightness and selectivity and affinity to reliably quantify OTR in cells and tissue.
Peptide-based fluorescent tracers and radiotracers have certain opportunities and advantages over antibodies. Indeed, attempts to develop small molecules and biologics targeting OTR for clinical use have so far failed.158 First, peptides can be rapidly designed and chemically produced at reduced costs compared to antibodies.186 Peptides have good biocompatibility and low immunogenicity and can be modified to enhance the in vivo stability and pharmacokinetics. For instance, blood circulation of peptides can be increased through conjugation to polyethylene glycol (PEG) chains or serum albumin binders,187 which might enhance tumor accumulation. Since peptides are smaller than antibodies (∼3 kDa peptides vs ∼150 kDa antibodies), they have better tumor penetration; additionally, they can induce ligand–receptor-mediated internalization, which could lead to enhanced tumor uptake and visualization.188 This may be particularly important for targeted radiotherapy, where therapeutic radionuclides, e.g., 90Y or 177Lu, or chemotherapeutic agents would accumulate closer to the cancer cell nuclei than without internalization.73,189,190 Such targeted treatment is desirable for cancer subtypes that do not yet have a targeted therapy option (e.g., TNBC) and where systemic chemotherapy with its severe side effects remains the first-line treatment.191
This so-called peptide receptor radionucleotide therapy (PRRT) has already been successfully applied to neuroendocrine tumors (NETs) targeting the peptide hormone somatostatin receptor (SSTR).192−194 [177Lu]Lu-DOTA-TATE (Lutathera) was approved by the EMA in 2017 and the FDA in 2018 for treating SSTR-positive gastroenteropancreatic NETs.195 The success of somatostatin radiotracers paved the way for other peptide-based radiotracers, e.g., those based on the RGD peptide, vasoactive intestinal peptide (VIP), cholecystokinin (CCK)/gastrin peptide, α-melanocyte-stimulating hormone (α-MSH), neurotensin (NT), T140, exendin-4, neuropeptide Y (NPY), substance P, and tumor molecular targeted peptide 1 (TMTP1).65,196,197 For breast cancer management, chemerin-based [68Ga]Ga-DOTA peptide conjugates targeting the chemokine-like receptor 1 (CMKLR1) with high specificity and affinity could visualize CMKLR1-positive breast cancer xenografts via PET/MRI.198 [68Ga]Ga-NeoBOMB1, a DOTA-coupled GRPR antagonist, was tested in a study in four patients diagnosed with prostate cancer, where it rapidly localized in pathologic lesions, achieving high-contrast imaging during PET/CT.199 Breast cancer patients with GRPR-positive tumors are potential candidates for treatment with 177Lu-labeled NeoBOMB1.200 Taken together, these clinical trials support the safety and efficacy of peptide-based radiotracers and the theranostic opportunities of PRRT, providing a strong foundation for a thriving biotech industry that is already embracing these approaches and seeing the therapeutic potential of peptide-based theranostics.201,202
7. Conclusions and Perspectives
The landscape of breast cancer management has evolved significantly with the advent of targeted therapies, representing a marked departure from the era of indiscriminate chemotherapy or radiation treatments. This transformation translates into tangible benefits for patient survival, overall care, and the emergence of personalized treatment strategies. To further elevate these advancements, the focus must shift toward achieving earlier and more precise diagnoses, leveraging predictive biomarkers, and refining tumor-targeted therapies to mitigate side effects. Overcoming diagnostic challenges posed by the high heterogeneity of breast tumors and dense breast tissue requires an integrated approach, combining mammography with highly sensitive nuclear imaging techniques. Among these, PET stands out as a particularly promising modality, especially when it is coupled with cancer-specific radiotracers.
A notable stride in targeted therapies is the growing recognition of PRRT in the pharmaceutical realm, offering treatments that are both less toxic and more efficient, with recent approvals marking a pivotal milestone. PRRT underscores the advantages of employing peptide radiotracers in cancer treatment, characterized by their high target specificity, excellent tumor penetration, and swift clearance. Within this evolving landscape, OTR emerges as a novel target for breast cancer diagnosis and targeted therapy, particularly relevant in the context of TNBC. Despite its promise, more systematic studies are imperative to validate OTR in human breast cancer xenograft models and assess OTR tumor expression across diverse patient populations.
The development of OTR-specific ligands and tracers plays a critical role in advancing these studies, serving as molecular tools for investigating OTR in breast cancer and beyond. Recent advancements in OTR structural elucidation through X-ray and cryo-EM will enhance the rational designs of OTR-specific radiotracers, and previous studies highlight position 8 of OT as the optimal site for attaching tracer-related tags. It is important to underscore that, akin to successful PRRT approaches targeting overexpressed receptors in cancers (e.g., SSTR, GRPR), the efficacy and feasibility of OTR-targeted radiotherapy hinge on significant OTR overexpression in tumors and the OTR-selectivity of the radiotracers. Such OTR-selective radiotracers are poised to contribute substantially to the expanding frontier of enhancing cancer care—ranging from improved disease diagnosis and staging to precision therapy monitoring and tumor-targeted interventions with fewer side effects.
Acknowledgments
M.M. was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program (714366) and by the Australian Research Council (DP190101667, DP230102707, FT210100266). M.M., P.F.A., and A.M. were supported by grants from the Cancer Council Queensland and Cancer Australia (1146504). A.M. was supported by a grant from the National Breast Cancer Foundation Australia (IIRS18-159). We thank Dr. Katrina Woolcock and Monika Perisic for their help with this manuscript.
Glossary
Abbreviations Used
- α-MSH
α-melanocyte-stimulating hormone
- CCK
cholecystokinin
- CMKLR1
chemokine-like receptor 1
- EDDA
ethylenediaminediacetic acid
- ER
estrogen receptor
- GPCR
G protein-coupled receptor
- GRPR
gastrin-releasing peptide receptor
- HER2
human epidermal growth factor receptor 2
- ICG
indocyanine green
- MRI
magnetic resonance imaging
- NET
neuroendocrine tumor
- NIR
near-infrared
- NPY
neuropeptide Y
- NT
neurotensin
- OT
oxytocin
- OTR
oxytocin receptor
- PD
pharmacodynamic
- PD1
programmed cell death protein 1
- PEG
polyethylene glycol
- PET
positron emission tomography
- PK
pharmacokinetic
- PR
progesterone receptor
- PRRT
peptide receptor radionucleotide therapy
- SLNB
sentinel lymph node biopsy
- SPECT
single-photon emission computed tomography
- SSTR
somatostatin receptor
- T-DM1
trastuzumab and ado-trastuzumab emtansine
- T-Dxd
fam-trastuzumab deruxtecan-nxki
- TM
transmembrane
- TMTP1
tumor molecular targeted peptide 1
- TNBC
triple-negative breast cancer
- TYKERB
tyrosine kinase inhibitor lapatinib
- VIP
vasoactive intestinal peptide
- VIP-R1
vasoactive intestinal polypeptide receptor 1
- VPR
vasopressin receptor
Biographies
Predrag Kalaba is a medicinal chemist with a strong interest in multidisciplinary research, especially in the fields of GPCRs and neuroscience. His core interests include the synthesis and characterization of small molecules and neuropeptides, structure–activity relationship studies, and the development of molecular tools to study biological processes such as learning and memory and cognitive decline and enhancement. His background in biochemistry and medicinal chemistry allows him to tackle these problems from different angles and to develop innovative therapeutic strategies for diseases with unmet medical needs. He is also passionate about teaching and mentoring students. He obtained a Ph.D. degree in pharmaceutical sciences in 2018 from the University of Vienna and currently holds a senior post-doc position at the Muttenthaler lab, University of Vienna.
Cristina Sanchez de la Rosa obtained her Ph.D. degree from the University of Queensland in 2021. During her doctoral studies, she focused on peptide tracer development targeting the oxytocin and vasopressin receptor systems. Prior to her doctoral studies, she worked at the Gambus lab at the Medical School of the University of Birmingham, UK, where she focused on the regulation of DNA replication, and at the Biomedicine Department of the University of Cádiz, Spain, where she focused on Δ6 FAD and Elovl 5 activities through heterologous expression in yeast. She also holds a B.Sc. degree in biotechnology from the University of Cádiz, Spain.
Andreas Möller is a trained biochemist and cancer biologist with >20 years of experience in cancer research. He has a strong background in cancer cell biology, exosome biology, hypoxia research, cancer metastasis, and immunology. His research program focuses on novel approaches to understanding cancer metastasis and how the composition of the tumor microenvironment is coordinated, with the aim of translating the findings into clinical applications. He is an internationally recognized expert in cancer metastasis, extracellular vesicles, and cancer immune responses.
Paul F. Alewood is a pioneer in the fields of venom-based molecular discovery and solid-phase peptide synthesis, where his approaches have been adopted by many researchers in academia and industry. Prof. Alewood has helped develop the highly efficient venomics approach that spans high-throughput sequencing by integrating transcriptomics and proteomics, rapid solid-phase peptide synthesis, including high-throughput strategies to access cysteine-rich toxins, and peptide drug development of lead molecules with commercial potential. One exciting source of bioactive peptides is the marine predatory cone snail, on which Prof. Alewood and collaborators have published over 100 papers describing their identification, synthesis, structures, receptor target, and mode of action.
Markus Muttenthaler is a medicinal chemist working at the interface of chemistry and biology with a strong passion for translational research. His research focuses on neuropeptides and exploring nature’s biodiversity to develop molecular tools, diagnostics, and therapeutics. His background in drug discovery and development, as well as his interdisciplinary training in the fields of chemistry, molecular biology, and pharmacology, assist him in characterizing these often highly potent and selective compounds and studying their interactions with human physiology for medical innovations in pain, cancer, gut disorders, and neurodegenerative diseases.
Author Contributions
# P.K. and C.S.R. contributed equally to this work as co-first authors.
The authors declare no competing financial interest.
References
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68 (6), 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Sung H.; Ferlay J.; Siegel R. L.; Laversanne M.; Soerjomataram I.; Jemal A.; Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71 (3), 209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Arnold M.; Morgan E.; Rumgay H.; Mafra A.; Singh D.; Laversanne M.; Vignat J.; Gralow J. R.; Cardoso F.; Siesling S.; Soerjomataram I. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast Edinb. Scotl. 2022, 66, 15–23. 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allemani C.; Matsuda T.; Di Carlo V.; Harewood R.; Matz M.; Nikšić M.; Bonaventure A.; Valkov M.; Johnson C. J.; Estève J.; Ogunbiyi O. J.; Azevedo e Silva G.; Chen W.-Q.; Eser S.; Engholm G.; Stiller C. A.; Monnereau A.; Woods R. R.; Visser O.; Lim G. H.; Aitken J.; Weir H. K.; Coleman M. P.; Bouzbid S.; Hamdi-Chérif M.; Zaidi Z.; Meguenni K.; Regagba D.; Bayo S.; Cheick Bougadari T.; Manraj S. S.; Bendahhou K.; Fabowale A.; Bradshaw D.; Somdyala N. I. M.; Kumcher I.; Moreno F.; Calabrano G. H.; Espinola S. B.; Carballo Quintero B.; Fita R.; Diumenjo M. C.; Laspada W. D.; Ibañez S. G.; Lima C. A.; De Souza P. C. F.; Del Pino K.; Laporte C.; Curado M. P.; de Oliveira J. C.; Veneziano C. L. A.; Veneziano D. B.; Latorre M. R. D. O.; Tanaka L. F.; Rebelo M. S.; Santos M. O.; Galaz J. C.; Aparicio Aravena M.; Sanhueza Monsalve J.; Herrmann D. A.; Vargas S.; Herrera V. M.; Uribe C. J.; Bravo L. E.; Garcia L. S.; Arias-Ortiz N. E.; Morantes D.; Jurado D. M.; Yépez Chamorro M. C.; Delgado S.; Ramirez M.; Galán Alvarez Y. H.; Torres P.; Martínez-Reyes F.; Jaramillo L.; Quinto R.; Castillo J.; Mendoza M.; Cueva P.; Yépez J. G.; Bhakkan B.; Deloumeaux J.; Joachim C.; Macni J.; Carrillo R.; Shalkow Klincovstein J.; Rivera Gomez R.; Poquioma E.; Tortolero-Luna G.; Zavala D.; Alonso R.; Barrios E.; Eckstrand A.; Nikiforuk C.; Noonan G.; Turner D.; Kumar E.; Zhang B.; McCrate F. R.; Ryan S.; MacIntyre M.; Saint-Jacques N.; Nishri D. E.; McClure C. A.; Vriends K. A.; Kozie S.; Stuart-Panko H.; Freeman T.; George J. T.; Brockhouse J. T.; O’Brien D. K.; Holt A.; Almon L.; Kwong S.; Morris C.; Rycroft R.; Mueller L.; Phillips C. E.; Brown H.; Cromartie B.; Schwartz A. G.; Vigneau F.; Levin G. M.; Wohler B.; Bayakly R.; Ward K. C.; Gomez S. L.; McKinley M.; Cress R.; Green M. D.; Miyagi K.; Ruppert L. P.; Lynch C. F.; Huang B.; Tucker T. C.; Deapen D.; Liu L.; Hsieh M. C.; Wu X. C.; Schwenn M.; Gershman S. T.; Knowlton R. C.; Alverson G.; Copeland G. E.; Bushhouse S.; Rogers D. B.; Jackson-Thompson J.; Lemons D.; Zimmerman H. J.; Hood M.; Roberts-Johnson J.; Rees J. R.; Riddle B.; Pawlish K. S.; Stroup A.; Key C.; Wiggins C.; Kahn A. R.; Schymura M. J.; Radhakrishnan S.; Rao C.; Giljahn L. K.; Slocumb R. M.; Espinoza R. E.; Khan F.; Aird K. G.; Beran T.; Rubertone J. J.; Slack S. J.; Garcia L.; Rousseau D. L.; Janes T. A.; Schwartz S. M.; Bolick S. W.; Hurley D. M.; Whiteside M. A.; Miller-Gianturco P.; Williams M. A.; Herget K.; Sweeney C.; Johnson A. T.; Keitheri Cheteri M. B.; Migliore Santiago P.; Blankenship S. E.; Farley S.; Borchers R.; Malicki R.; Espinoza J. R.; Grandpre J.; Wilson R.; Edwards B. K.; Mariotto A.; Lei Y.; Wang N.; Chen J. S.; Zhou Y.; He Y. T.; Song G. H.; Gu X. P.; Mei D.; Mu H. J.; Ge H. M.; Wu T. H.; Li Y. Y.; Zhao D. L.; Jin F.; Zhang J. H.; Zhu F. D.; Junhua Q.; Yang Y. L.; Jiang C. X.; Biao W.; Wang J.; Li Q. L.; Yi H.; Zhou X.; Dong J.; Li W.; Fu F. X.; Liu S. Z.; Chen J. G.; Zhu J.; Li Y. H.; Lu Y. Q.; Fan M.; Huang S. Q.; Guo G. P.; Zhaolai H.; Wei K.; Zeng H.; Demetriou A. V.; Mang W. K.; Ngan K. C.; Kataki A. C.; Krishnatreya M.; Jayalekshmi P. A.; Sebastian P.; Nandakumar A.; Malekzadeh R.; Roshandel G.; Keinan-Boker L.; Silverman B. G.; Ito H.; Nakagawa H.; Sato M.; Tobori F.; Nakata I.; Teramoto N.; Hattori M.; Kaizaki Y.; Moki F.; Sugiyama H.; Utada M.; Nishimura M.; Yoshida K.; Kurosawa K.; Nemoto Y.; Narimatsu H.; Sakaguchi M.; Kanemura S.; Naito M.; Narisawa R.; Miyashiro I.; Nakata K.; Sato S.; Yoshii M.; Oki I.; Fukushima N.; Shibata A.; Iwasa K.; Ono C.; Nimri O.; Jung K. W.; Won Y. J.; Alawadhi E.; Elbasmi A.; Ab Manan A.; Adam F.; Sanjaajmats E.; Tudev U.; Ochir C.; Al Khater A. M.; El Mistiri M. M.; Teo Y. Y.; Chiang C. J.; Lee W. C.; Buasom R.; Sangrajrang S.; Kamsa-ard S.; Wiangnon S.; Daoprasert K.; Pongnikorn D.; Leklob A.; Sangkitipaiboon S.; Geater S. L.; Sriplung H.; Ceylan O.; Kög I.; Dirican O.; Köse T.; Gurbuz T.; Karaşahin F. E.; Turhan D.; Aktaş U.; Halat Y.; Yakut C. I.; Altinisik M.; Cavusoglu Y.; Türkköylü A.; Üçüncü N.; Hackl M.; Zborovskaya A. A.; Aleinikova O. V.; Henau K.; Van Eycken L.; Valerianova Z.; Yordanova M. R.; Šekerija M.; Dušek L.; Zvolský M.; Storm H.; Innos K.; Mägi M.; Malila N.; Seppä K.; Jégu J.; Velten M.; Cornet E.; Troussard X.; Bouvier A. M.; Guizard A. V.; Bouvier V.; Launoy G.; Arveux P.; Maynadié M.; Mounier M.; Woronoff A. S.; Daoulas M.; Robaszkiewicz M.; Clavel J.; Goujon S.; Lacour B.; Baldi I.; Pouchieu C.; Amadeo B.; Coureau G.; Orazio S.; Preux P. M.; Rharbaoui F.; Marrer E.; Trétarre B.; Colonna M.; Delafosse P.; Ligier K.; Plouvier S.; Cowppli-Bony A.; Molinié F.; Bara S.; Ganry O.; Lapôtre-Ledoux B.; Grosclaude P.; Bossard N.; Uhry Z.; Bray F.; Piñeros M.; Stabenow R.; Wilsdorf-Köhler H.; Eberle A.; Luttmann S.; Löhden I.; Nennecke A. L.; Kieschke J.; Sirri E.; Emrich K.; Zeissig S. R.; Holleczek B.; Eisemann N.; Katalinic A.; Asquez R. A.; Kumar V.; Petridou E.; Ólafsdóttir E. J.; Tryggvadóttir L.; Clough-Gorr K.; Walsh P. M.; Sundseth H.; Mazzoleni G.; Vittadello F.; Coviello E.; Cuccaro F.; Galasso R.; Sampietro G.; Giacomin A.; Magoni M.; Ardizzone A.; D’Argenzio A.; Castaing M.; Grosso G.; Lavecchia A. M.; Sutera Sardo A.; Gola G.; Gatti L.; Ricci P.; Ferretti S.; Serraino D.; Zucchetto A.; Celesia M. V.; Filiberti R. A.; Pannozzo F.; Melcarne A.; Quarta F.; Russo A. G.; Carrozzi G.; Cirilli C.; Cavalieri d’Oro L.; Rognoni M.; Fusco M.; Vitale M. F.; Usala M.; Cusimano R.; Mazzucco W.; Michiara M.; Sgargi P.; Boschetti L.; Borciani E.; Seghini P.; Maule M. M.; Merletti F.; Tumino R.; Mancuso P.; Vicentini M.; Cassetti T.; Sassatelli R.; Falcini F.; Giorgetti S.; Caiazzo A. L.; Cavallo R.; Cesaraccio R.; Pirino D. R.; Contrino M. L.; Tisano F.; Fanetti A. C.; Maspero S.; Carone S.; Mincuzzi A.; Candela G.; Scuderi T.; Gentilini M. A.; Piffer S.; Rosso S.; Barchielli A.; Caldarella A.; Bianconi F.; Stracci F.; Contiero P.; Tagliabue G.; Rugge M.; Zorzi M.; Beggiato S.; Brustolin A.; Berrino F.; Gatta G.; Sant M.; Buzzoni C.; Mangone L.; Capocaccia R.; De Angelis R.; Zanetti R.; Maurina A.; Pildava S.; Lipunova N.; Vincerževskiené I.; Agius D.; Calleja N.; Siesling S.; Larønningen S.; Møller B.; Dyzmann-Sroka A.; Trojanowski M.; Góźdoź S.; Mężyk R.; Mierzwa T.; Molong L.; Rachtan J.; Szewczyk S.; Błaszczyk J.; Kępska K.; Kościańska B.; Tarocińska K.; Zwierko M.; Drosik K.; Maksimowicz K. M.; Purwin-Porowska E.; Reca E.; Wójcik-Tomaszewska J.; Tukiendorf A.; Grądalska-Lampart M.; Radziszewska A. U.; Gos A.; Talerczyk M.; Wyborska M.; Didkowska J. A.; Wojciechowska U.; Bielska-Lasota M.; Forjaz de Lacerda G.; Rego R. A.; Bastos J.; Silva M. A.; Antunes L.; Laranja Pontes J.; Mayer-da-Silva A.; Miranda A.; Blaga L. M.; Coza D.; Gusenkova L.; Lazarevich O.; Prudnikova O.; Vjushkov D. M.; Egorova A. G.; Orlov A. E.; Kudyakov L. A.; Pikalova L. V.; Adamcik J.; Safaei Diba C.; Primic-Žakelj M.; Zadnik V.; Larrañaga N.; Lopez de Munain A.; Herrera A. A.; Redondas R.; Marcos-Gragera R.; Vilardell Gil M. L.; Molina E.; Sánchez Perez M. J.; Franch Sureda P.; Ramos Montserrat M.; Chirlaque M. D.; Navarro C.; Ardanaz E. E.; Guevara M. M.; Fernández-Delgado R.; Peris-Bonet R.; Carulla M.; Galceran J.; Alberich C.; Vicente-Raneda M.; Khan S.; Pettersson D.; Dickman P.; Avelina I.; Staehelin K.; Camey B.; Bouchardy C.; Schaffar R.; Frick H.; Herrmann C.; Bulliard J. L.; Maspoli-Conconi M.; Kuehni C. E.; Redmond S. M.; Bordoni A.; Ortelli L.; Chiolero A.; Konzelmann I.; Matthes K. L.; Rohrmann S.; Broggio J.; Rashbass J.; Fitzpatrick D.; Gavin A.; Clark D. I.; Deas A. J.; Huws D. W.; White C.; Montel L.; Rachet B.; Turculet A. D.; Stephens R.; Chalker E.; Phung H.; Walton R.; You H.; Guthridge S.; Johnson F.; Gordon P.; D’Onise K.; Priest K.; Stokes B. C.; Venn A.; Farrugia H.; Thursfield V.; Dowling J.; Currow D.; Hendrix J.; Lewis C. Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 391 (10125), 1023–1075. 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turashvili G.; Brogi E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. 10.3389/fmed.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini G.; Balko J. M.; Mayer I. A.; Sanders M. E.; Gianni L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13 (11), 674–690. 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik S.; Tang G.; Shak S.; Kim C.; Baker J.; Kim W.; Cronin M.; Baehner F. L.; Watson D.; Bryant J.; Costantino J. P.; Geyer C. E.; Wickerham D. L.; Wolmark N. Gene Expression and Benefit of Chemotherapy in Women With Node-Negative, Estrogen Receptor-Positive Breast Cancer. J. Clin. Oncol. 2006, 24 (23), 3726–3734. 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- Nishimura R.; Osako T.; Okumura Y.; Hayashi M.; Toyozumi Y.; Arima N. Ki-67 as a Prognostic Marker According to Breast Cancer Subtype and a Predictor of Recurrence Time in Primary Breast Cancer. Exp. Ther. Med. 2010, 1 (5), 747–754. 10.3892/etm.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrantia-Borunda E.; Anchondo-Nuñez P.; Acuña-Aguilar L. E.; Gómez-Valles F. O.; Ramírez-Valdespino C. A.. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz H. N., Ed.; Exon Publications: Brisbane, 2022. [PubMed] [Google Scholar]
- Merino Bonilla J. A.; Torres Tabanera M.; Ros Mendoza L. H. Breast Cancer in the 21st Century: From Early Detection to New Therapies. Radiol. Engl. Ed. 2017, 59 (5), 368–379. 10.1016/j.rxeng.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Sauter E. R. Reliable Biomarkers to Identify New and Recurrent Cancer. Eur. J. Breast Health 2017, 13 (4), 162–167. 10.5152/ejbh.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam I.; Swain S. M. HER2-Positive Breast Cancer and Tyrosine Kinase Inhibitors: The Time Is Now. NPJ. Breast Cancer 2021, 7 (1), 56. 10.1038/s41523-021-00265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar M. K.; Manore S. G.; Regua A. T.; Lo H.-W. Antibody-Drug Conjugates for the Treatment of HER2-Positive Breast Cancer. Genes 2022, 13 (11), 2065. 10.3390/genes13112065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft D. J.; Cryns V. L. Minireview: Basal-Like Breast Cancer: From Molecular Profiles to Targeted Therapies. Mol. Endocrinol. 2011, 25 (2), 199–211. 10.1210/me.2010-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann B. D.; Pietenpol J. A.; Tan A. R. Triple-Negative Breast Cancer: Molecular Subtypes and New Targets for Therapy. Am. Soc. Clin. Oncol. Educ. Book 2015, (35), e31–e39. 10.14694/EdBook_AM.2015.35.e31. [DOI] [PubMed] [Google Scholar]
- Podo F.; Buydens L. M. C.; Degani H.; Hilhorst R.; Klipp E.; Gribbestad I. S.; Van Huffel S.; van Laarhoven H. W. M.; Luts J.; Monleon D.; Postma G. J.; Schneiderhan-Marra N.; Santoro F.; Wouters H.; Russnes H. G.; Sørlie T.; Tagliabue E.; Børresen-Dale A.-L. Triple-Negative Breast Cancer: Present Challenges and New Perspectives. Mol. Oncol. 2010, 4 (3), 209–229. 10.1016/j.molonc.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes W. D.; Smith I. E.; Reis-Filho J. S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363 (20), 1938–1948. 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- Nielsen T. O.; Hsu F. D.; Jensen K.; Cheang M.; Karaca G.; Hu Z.; Hernandez-Boussard T.; Livasy C.; Cowan D.; Dressler L.; Akslen L. A.; Ragaz J.; Gown A. M.; Gilks C. B.; van de Rijn M.; Perou C. M. Immunohistochemical and Clinical Characterization of the Basal-Like Subtype of Invasive Breast Carcinoma. Clin. Cancer Res. 2004, 10 (16), 5367–5374. 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- Shah M.; Osgood C. L.; Amatya A. K.; Fiero M. H.; Pierce W. F.; Nair A.; Herz J.; Robertson K. J.; Mixter B. D.; Tang S.; Pazdur R.; Beaver J. A.; Amiri-Kordestani L. FDA Approval Summary: Pembrolizumab for Neoadjuvant and Adjuvant Treatment of Patients with High-Risk Early-Stage Triple-Negative Breast Cancer. Clin. Cancer Res. 2022, 28 (24), 5249–5253. 10.1158/1078-0432.CCR-22-1110. [DOI] [PubMed] [Google Scholar]
- Kwok G.; Yau T. C. C.; Chiu J. W.; Tse E.; Kwong Y.-L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12 (11), 2777–2789. 10.1080/21645515.2016.1199310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Muttenthaler M. High Oxytocin Receptor Expression Linked to Increased Cell Migration and Reduced Survival in Patients with Triple-Negative Breast Cancer. Biomedicines 2022, 10 (7), 1595. 10.3390/biomedicines10071595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B.; Baehner F. L.; Reis-Filho J. S. The Contribution of Gene Expression Profiling to Breast Cancer Classification, Prognostication and Prediction: A Retrospective of the Last Decade: A Commentary on Microarrays in Breast Cancer Research. J. Pathol. 2010, 220 (2), 263–280. 10.1002/path.2648. [DOI] [PubMed] [Google Scholar]
- Ades F.; Zardavas D.; Bozovic-Spasojevic I.; Pugliano L.; Fumagalli D.; de Azambuja E.; Viale G.; Sotiriou C.; Piccart M. Luminal B Breast Cancer: Molecular Characterization, Clinical Management, and Future Perspectives. J. Clin. Oncol. 2014, 32 (25), 2794–2803. 10.1200/JCO.2013.54.1870. [DOI] [PubMed] [Google Scholar]
- Masuda H.; Baggerly K. A.; Wang Y.; Zhang Y.; Gonzalez-Angulo A. M.; Meric-Bernstam F.; Valero V.; Lehmann B. D.; Pietenpol J. A.; Hortobagyi G. N.; Symmans W. F.; Ueno N. T. Differential Response to Neoadjuvant Chemotherapy Among 7 Triple-Negative Breast Cancer Molecular Subtypes. Clin. Cancer Res. 2013, 19 (19), 5533–5540. 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher L.; Hogue J. C.; Desbiens C.; Poirier B.; Poirier E.; Boudreau D.; Joyal M.; Diorio C.; Duchesne N.; Chiquette J. Is Clinical Breast Examination Important for Breast Cancer Detection?. Curr. Oncol. 2016, 23 (4), 332–339. 10.3747/co.23.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gøtzsche P. C.; Jørgensen K. J. Screening for Breast Cancer with Mammography. Cochrane Database Syst. Rev. 2013, 2013 (6), CD001877. 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlikowske K. Efficacy of Screening Mammography Among Women Aged 40 to 49 Years and 50 to 69 Years: Comparison of Relative and Absolute Benefit. JNCI Monogr. 1997, 1997 (22), 79–86. 10.1093/jncimono/1997.22.79. [DOI] [PubMed] [Google Scholar]
- Buist D. S. M.; Porter P. L.; Lehman C.; Taplin S. H.; White E. Factors Contributing to Mammography Failure in Women Aged 40–49 Years. JNCI J. Natl. Cancer Inst. 2004, 96 (19), 1432–1440. 10.1093/jnci/djh269. [DOI] [PubMed] [Google Scholar]
- Yerushalmy J. Statistical Problems in Assessing Methods of Medical Diagnosis, with Special Reference to X-Ray Techniques. Public Health Rep. 1896–1970 1947, 62 (40), 1432. 10.2307/4586294. [DOI] [PubMed] [Google Scholar]
- Pinsky R. W.; Helvie M. A. Mammographic Breast Density: Effect on Imaging and Breast Cancer Risk. J. Natl. Compr. Canc. Netw. 2010, 8 (10), 1157–1165. 10.6004/jnccn.2010.0085. [DOI] [PubMed] [Google Scholar]
- Kolb T. M.; Lichy J.; Newhouse J. H. Comparison of the Performance of Screening Mammography, Physical Examination, and Breast US and Evaluation of Factors That Influence Them: An Analysis of 27,825 Patient Evaluations. Radiology 2002, 225 (1), 165–175. 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- Morrow M.; Waters J.; Morris E. MRI for Breast Cancer Screening, Diagnosis, and Treatment. Lancet 2011, 378 (9805), 1804–1811. 10.1016/S0140-6736(11)61350-0. [DOI] [PubMed] [Google Scholar]
- Hooley R. J.; Scoutt L. M.; Philpotts L. E. Breast Ultrasonography: State of the Art. Radiology 2013, 268 (3), 642–659. 10.1148/radiol.13121606. [DOI] [PubMed] [Google Scholar]
- Taskin F.; Polat Y.; Erdogdu I. H.; Turkdogan F. T.; Ozturk V. S.; Ozbas S. Problem-Solving Breast MRI: Useful or a Source of New Problems?. Diagn. Interv. Radiol. 2018, 24 (5), 255–261. 10.5152/dir.2018.17504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krag D. N.; Julian T. B.; Harlow S. P.; Weaver D. L.; Ashikaga T.; Bryant J.; Single R. M.; Wolmark N. NSABP-32: Phase III, Randomized Trial Comparing Axillary Resection with Sentinal Lymph Node Dissection: A Description of the Trial. Ann. Surg. Oncol. 2004, 11 (S3), 208S–210S. 10.1007/BF02523630. [DOI] [PubMed] [Google Scholar]
- Tang G.; Shak S.; Paik S.; Anderson S. J.; Costantino J. P.; Geyer C. E.; Mamounas E. P.; Wickerham D. L.; Wolmark N. Comparison of the Prognostic and Predictive Utilities of the 21-Gene Recurrence Score Assay and Adjuvant! For Women with Node-Negative, ER-Positive Breast Cancer: Results from NSABP B-14 and NSABP B-20. Breast Cancer Res. Treat. 2011, 127 (1), 133–142. 10.1007/s10549-010-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalm S.; Verzijlbergen J.; De Jong M. Review: Receptor Targeted Nuclear Imaging of Breast Cancer. Int. J. Mol. Sci. 2017, 18 (2), 260. 10.3390/ijms18020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa A. M.; Martel F. Targeting Glucose Transporters for Breast Cancer Therapy: The Effect of Natural and Synthetic Compounds. Cancers 2020, 12 (1), 154. 10.3390/cancers12010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E.; Koo J. S. Glucose Metabolism and Glucose Transporters in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 728759. 10.3389/fcell.2021.728759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y.; Kim E.-S.; Koo J. Amino Acid Transporters and Glutamine Metabolism in Breast Cancer. Int. J. Mol. Sci. 2018, 19 (3), 907. 10.3390/ijms19030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Wang J. Targeting Uptake Transporters for Cancer Imaging and Treatment. Acta Pharm. Sin. B 2020, 10 (1), 79–90. 10.1016/j.apsb.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crişan G.; Moldovean-Cioroianu N. S.; Timaru D.-G.; Andrieş G.; Căinap C.; Chiş V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23 (9), 5023. 10.3390/ijms23095023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gøtzsche P. C.; Olsen O. Is Screening for Breast Cancer with Mammography Justifiable?. Lancet 2000, 355 (9198), 129–134. 10.1016/S0140-6736(99)06065-1. [DOI] [PubMed] [Google Scholar]
- Gøtzsche P. C.; Nielsen M. Screening for Breast Cancer with Mammography. Cochrane Database Syst. Rev. 2011, 19, CD001877. 10.1002/14651858.CD001877.pub4. [DOI] [PubMed] [Google Scholar]
- Guo R.; Lu G.; Qin B.; Fei B. Ultrasound Imaging Technologies for Breast Cancer Detection and Management: A Review. Ultrasound Med. Biol. 2018, 44 (1), 37–70. 10.1016/j.ultrasmedbio.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokuhi S.; Parker S. J. Management of Breast Cancer: Basic Principles. Surg. Oxf. 2007, 25 (6), 261–263. 10.1016/j.mpsur.2007.05.008. [DOI] [Google Scholar]
- Akram M.; Iqbal M.; Daniyal M.; Khan A. U. Awareness and Current Knowledge of Breast Cancer. Biol. Res. 2017, 50 (1), 33. 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhajosula S.Molecular Imaging: Radiopharmaceuticals for PET and SPECT; Springer-Verlag: Berlin, New York, 2009. [Google Scholar]
- Adak S.; Bhalla R.; Vijaya Raj K. K.; Mandal S.; Pickett R.; Luthra S. K. Radiotracers for SPECT Imaging: Current Scenario and Future Prospects. ract 2012, 100 (2), 95–107. 10.1524/ract.2011.1891. [DOI] [Google Scholar]
- Almubarak M.; Osman S.; Marano G.; Abraham J. Role of Positron-Emission Tomography Scan in the Diagnosis and Management of Breast Cancer. Oncology (Williston Park) 2009, 23 (3), 255–261. [PubMed] [Google Scholar]
- Paik S.; Shak S.; Tang G.; Kim C.; Baker J.; Cronin M.; Baehner F. L.; Walker M. G.; Watson D.; Park T.; Hiller W.; Fisher E. R.; Wickerham D. L.; Bryant J.; Wolmark N. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N. Engl. J. Med. 2004, 351 (27), 2817–2826. 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- Cardoso F.; van’t Veer L. J.; Bogaerts J.; Slaets L.; Viale G.; Delaloge S.; Pierga J.-Y.; Brain E.; Causeret S.; DeLorenzi M.; Glas A. M.; Golfinopoulos V.; Goulioti T.; Knox S.; Matos E.; Meulemans B.; Neijenhuis P. A.; Nitz U.; Passalacqua R.; Ravdin P.; Rubio I. T.; Saghatchian M.; Smilde T. J.; Sotiriou C.; Stork L.; Straehle C.; Thomas G.; Thompson A. M.; van der Hoeven J. M.; Vuylsteke P.; Bernards R.; Tryfonidis K.; Rutgers E.; Piccart M. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375 (8), 717–729. 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- Wallden B.; Storhoff J.; Nielsen T.; Dowidar N.; Schaper C.; Ferree S.; Liu S.; Leung S.; Geiss G.; Snider J.; Vickery T.; Davies S. R.; Mardis E. R.; Gnant M.; Sestak I.; Ellis M. J.; Perou C. M.; Bernard P. S.; Parker J. S. Development and Verification of the PAM50-Based Prosigna Breast Cancer Gene Signature Assay. BMC Med. Genomics 2015, 8 (1), 54. 10.1186/s12920-015-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok E. J.; Bastiaannet E.; van den Hout W. B.; Liefers G. J.; Smit V. T. H. B. M.; Kroep J. R.; van de Velde C. J. H. Systematic Review of the Clinical and Economic Value of Gene Expression Profiles for Invasive Early Breast Cancer Available in Europe. Cancer Treat. Rev. 2018, 62, 74–90. 10.1016/j.ctrv.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Hannouf M. B.; Zaric G. S.; Blanchette P.; Brezden-Masley C.; Paulden M.; McCabe C.; Raphael J.; Brackstone M. Cost-Effectiveness Analysis of Multigene Expression Profiling Assays to Guide Adjuvant Therapy Decisions in Women with Invasive Early-Stage Breast Cancer. Pharmacogenomics J. 2020, 20 (1), 27–46. 10.1038/s41397-019-0089-x. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) and Institute of Medicine (US) Committee on the Mathematics and Physics of Emerging Dynamic Biomedical Imaging Mathematics and Physics of Emerging Biomedical Imaging; National Academies Press: Washington, DC, 1996. [PubMed] [Google Scholar]
- Bénard F.; Turcotte É. Imaging in Breast Cancer: Single-Photon Computed Tomography and Positron-Emission Tomography. Breast Cancer Res. 2005, 7 (4), 153. 10.1186/bcr1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergieva S.; Alexandrova E.; Baitchev G.; Parvanova V. SPECT-CT in Breast Cancer. Arch. Oncol. 2012, 20 (3–4), 127–131. 10.2298/AOO1204127S. [DOI] [Google Scholar]
- Ziegler S. I. Positron Emission Tomography: Principles, Technology, and Recent Developments. Nucl. Phys. A 2005, 752, 679–687. 10.1016/j.nuclphysa.2005.02.067. [DOI] [Google Scholar]
- Avril N.; Rosé C. A.; Schelling M.; Dose J.; Kuhn W.; Bense S.; Weber W.; Ziegler S.; Graeff H.; Schwaiger M. Breast Imaging With Positron Emission Tomography and Fluorine-18 Fluorodeoxyglucose: Use and Limitations. J. Clin. Oncol. 2000, 18 (20), 3495–3502. 10.1200/JCO.2000.18.20.3495. [DOI] [PubMed] [Google Scholar]
- Yang S. K.; Cho N.; Moon W. K. The Role of PET/CT for Evaluating Breast Cancer. Korean J. Radiol. 2007, 8 (5), 429. 10.3348/kjr.2007.8.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdi Y. E. Limits of Tumor Detectability in Nuclear Medicine and PET. Mol. Imaging Radionucl. Ther. 2012, 21 (2), 23–28. 10.4274/Mirt.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narod S. A.; Iqbal J.; Jakubowska A.; Huzarski T.; Sun P.; Cybulski C.; Gronwald J.; Byrski T.; Lubinski J. Are Two-Centimeter Breast Cancers Large or Small?. Curr. Oncol. 2013, 20 (4), 205–211. 10.3747/co.20.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafra L. Positron Emission Tomography (PET) and Mammography (PEM) for Breast Cancer: Importance to Surgeons. Ann. Surg. Oncol. 2006, 14 (1), 3–13. 10.1245/s10434-006-9019-7. [DOI] [PubMed] [Google Scholar]
- Sun X.; Li Y.; Liu T.; Li Z.; Zhang X.; Chen X. Peptide-Based Imaging Agents for Cancer Detection. Adv. Drug Delivery Rev. 2017, 110–111, 38–51. 10.1016/j.addr.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Van Valkenburgh J.; Hong X.; Conti P. S.; Zhang X.; Chen K. Small Molecules as Theranostic Agents in Cancer Immunology. Theranostics 2019, 9 (25), 7849–7871. 10.7150/thno.37218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17 (1), 134–143. 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottelius M.; Wester H.-J. Molecular Imaging Targeting Peptide Receptors. Methods 2009, 48 (2), 161–177. 10.1016/j.ymeth.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Sachdeva S.; Joo H.; Tsai J.; Jasti B.; Li X. A Rational Approach for Creating Peptides Mimicking Antibody Binding. Sci. Rep. 2019, 9 (1), 997. 10.1038/s41598-018-37201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttenthaler M.; King G. F.; Adams D. J.; Alewood P. F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discovery 2021, 20 (4), 309–325. 10.1038/s41573-020-00135-8. [DOI] [PubMed] [Google Scholar]
- Lee P.; Wu X. Review: Modifications of Human Serum Albumin and Their Binding Effect. Curr. Pharm. Des. 2015, 21 (14), 1862–1865. 10.2174/1381612821666150302115025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen F. W. B.; Hardwick J. C. H.; van Erkel A. R. Luminescence-Based Imaging Approaches in the Field of Interventional Molecular Imaging. Radiology 2015, 276 (1), 12–29. 10.1148/radiol.2015132698. [DOI] [PubMed] [Google Scholar]
- Mező G.; Manea M. Receptor-Mediated Tumor Targeting Based on Peptide Hormones. Expert Opin. Drug Delivery 2010, 7 (1), 79–96. 10.1517/17425240903418410. [DOI] [PubMed] [Google Scholar]
- Johnsen S. Hidden in Plain Sight: The Ecology and Physiology of Organismal Transparency. Biol. Bull. 2001, 201 (3), 301–318. 10.2307/1543609. [DOI] [PubMed] [Google Scholar]
- Hong G.; Antaris A. L.; Dai H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1 (1), 0010. 10.1038/s41551-016-0010. [DOI] [Google Scholar]
- Hussain T.; Nguyen Q. T. Molecular Imaging for Cancer Diagnosis and Surgery. Adv. Drug Delivery Rev. 2014, 66, 90–100. 10.1016/j.addr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; Pu K. Near-Infrared Fluorescent Molecular Probes for Imaging and Diagnosis of Nephro-Urological Diseases. Chem. Sci. 2021, 12 (10), 3379–3392. 10.1039/D0SC02925D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnes R. S.; Broome A.-M.; Wang J.; Verma A.; Lavik K.; Basilion J. P. An Optical Probe for Noninvasive Molecular Imaging of Orthotopic Brain Tumors Overexpressing Epidermal Growth Factor Receptor. Mol. Cancer Ther. 2012, 11 (10), 2202–2211. 10.1158/1535-7163.MCT-12-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.; Wan S.; Tian J.; Li S.; Deng D.; Qian Z.; Gu Y. Fast Clearing RGD-Based near-Infrared Fluorescent Probes for in Vivo Tumor Diagnosis: Fast Clearing RGD-Based near-Infrared Probes. Contrast Media Mol. Imaging 2012, 7 (4), 390–402. 10.1002/cmmi.1464. [DOI] [PubMed] [Google Scholar]
- Ding F.; Zhan Y.; Lu X.; Sun Y. Recent Advances in Near-Infrared II Fluorophores for Multifunctional Biomedical Imaging. Chem. Sci. 2018, 9 (19), 4370–4380. 10.1039/C8SC01153B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.; Hu Z.; Tian R.; Yung B. C.; Yang Q.; Zhao S.; Kiesewetter D. O.; Niu G.; Sun H.; Antaris A. L.; Chen X. Repurposing Cyanine NIR-I Dyes Accelerates Clinical Translation of Near-Infrared-II (NIR-II) Bioimaging. Adv. Mater. 2018, 30 (34), 1802546. 10.1002/adma.201802546. [DOI] [PubMed] [Google Scholar]
- Patil C. G.; Walker D. G.; Miller D. M.; Butte P.; Morrison B.; Kittle D. S.; Hansen S. J.; Nufer K. L.; Byrnes-Blake K. A.; Yamada M.; Lin L. L.; Pham K.; Perry J.; Parrish-Novak J.; Ishak L.; Prow T.; Black K.; Mamelak A. N. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults With Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85 (4), E641–E649. 10.1093/neuros/nyz125. [DOI] [PubMed] [Google Scholar]
- Dintzis S. M.; Hansen S.; Harrington K. M.; Tan L. C.; Miller D. M.; Ishak L.; Parrish-Novak J.; Kittle D.; Perry J.; Gombotz C.; Fortney T.; Porenta S.; Hales L.; Calhoun K. E.; Anderson B. O.; Javid S. H.; Byrd D. R. Real-Time Visualization of Breast Carcinoma in Pathology Specimens From Patients Receiving Fluorescent Tumor-Marking Agent Tozuleristide. Arch. Pathol. Lab. Med. 2019, 143 (9), 1076–1083. 10.5858/arpa.2018-0197-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinyak J. E.; Berg W. A.; Schilling K.; Madsen K. S.; Narayanan D.; Tartar M. Breast Cancer Detection Using High-Resolution Breast PET Compared to Whole-Body PET or PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41 (2), 260–275. 10.1007/s00259-013-2553-1. [DOI] [PubMed] [Google Scholar]
- Hsu D. F. C.; Freese D. L.; Levin C. S. Breast-Dedicated Radionuclide Imaging Systems. J. Nucl. Med. 2016, 57 (Supplement 1), 40S–45S. 10.2967/jnumed.115.157883. [DOI] [PubMed] [Google Scholar]
- Mintun M. A.; Welch M. J.; Siegel B. A.; Mathias C. J.; Brodack J. W.; McGuire A. H.; Katzenellenbogen J. A. Breast Cancer: PET Imaging of Estrogen Receptors. Radiology 1988, 169 (1), 45–48. 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- Peterson L. M.; Mankoff D. A.; Lawton T.; Yagle K.; Schubert E. K.; Stekhova S.; Gown A.; Link J. M.; Tewson T.; Krohn K. A. Quantitative Imaging of Estrogen Receptor Expression in Breast Cancer with PET and 18 F-Fluoroestradiol. J. Nucl. Med. 2008, 49 (3), 367–374. 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- Mortimer J. E.; Dehdashti F.; Siegel B. A.; Katzenellenbogen J. A.; Fracasso P.; Welch M. J. Positron Emission Tomography with 2-[18F]Fluoro-2-Deoxy-D-Glucose and 16alpha-[18F]Fluoro-17beta-Estradiol in Breast Cancer: Correlation with Estrogen Receptor Status and Response to Systemic Therapy. Clin. Cancer Res. 1996, 2 (6), 933–939. [PubMed] [Google Scholar]
- Dehdashti F.; Mortimer J. E.; Siegel B. A.; Griffeth L. K.; Bonasera T. J.; Fusselman M. J.; Detert D. D.; Cutler P. D.; Katzenellenbogen J. A.; Welch M. J. Positron Tomographic Assessment of Estrogen Receptors in Breast Cancer: Comparison with FDG-PET and in Vitro Receptor Assays. J. Nucl. Med. 1995, 36 (10), 1766–1774. [PubMed] [Google Scholar]
- Gemignani M. L.; Patil S.; Seshan V. E.; Sampson M.; Humm J. L.; Lewis J. S.; Brogi E.; Larson S. M.; Morrow M.; Pandit-Taskar N. Feasibility and Predictability of Perioperative PET and Estrogen Receptor Ligand in Patients with Invasive Breast Cancer. J. Nucl. Med. 2013, 54 (10), 1697–1702. 10.2967/jnumed.112.113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesewetter D. O.; Kilbourn M. R.; Landvatter S. W.; Heiman D. F.; Katzenellenbogen J. A.; Welch M. J. Preparation of Four Fluorine-18-Labeled Estrogens and Their Selective Uptakes in Target Tissues of Immature Rats. J. Nucl. Med. 1984, 25 (11), 1212–1221. [PubMed] [Google Scholar]
- He S.; Wang M.; Yang Z.; Zhang J.; Zhang Y.; Luo J.; Zhang Y. Comparison of 18F-FES, 18F-FDG, and 18F-FMISO PET Imaging Probes for Early Prediction and Monitoring of Response to Endocrine Therapy in a Mouse Xenograft Model of ER-Positive Breast Cancer. PLoS One 2016, 11 (7), e0159916. 10.1371/journal.pone.0159916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehdashti F.; Laforest R.; Gao F.; Aft R. L.; Dence C. S.; Zhou D.; Shoghi K. I.; Siegel B. A.; Katzenellenbogen J. A.; Welch M. J. Assessment of Progesterone Receptors in Breast Carcinoma by PET with 21- 18 F-Fluoro-16α,17α-[(R)-(1′-α-Furylmethylidene)Dioxy]-19-Norpregn-4-Ene-3,20-Dione. J. Nucl. Med. 2012, 53 (3), 363–370. 10.2967/jnumed.111.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey L. A.; Perou C. M.; Livasy C. A.; Dressler L. G.; Cowan D.; Conway K.; Karaca G.; Troester M. A.; Tse C. K.; Edmiston S.; Deming S. L.; Geradts J.; Cheang M. C. U.; Nielsen T. O.; Moorman P. G.; Earp H. S.; Millikan R. C. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA 2006, 295 (21), 2492. 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Kurihara H.; Hamada A.; Yoshida M.; Shimma S.; Hashimoto J.; Yonemori K.; Tani H.; Miyakita Y.; Kanayama Y.; Wada Y.; Kodaira M.; Yunokawa M.; Yamamoto H.; Shimizu C.; Takahashi K.; Watanabe Y.; Fujiwara Y.; Tamura K. 64Cu-DOTA-Trastuzumab PET Imaging and HER2 Specificity of Brain Metastases in HER2-Positive Breast Cancer Patients. EJNMMI Res. 2015, 5 (1), 8. 10.1186/s13550-015-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C.; Wenger S.; Schmuckli-Maurer J.; Schaer J.-C.; Gugger M. Bombesin Receptor Subtypes in Human Cancers: Detection with the Universal Radioligand (125)I-[D-TYR(6), Beta-ALA(11), PHE(13), NLE(14)] Bombesin(6–14). Clin. Cancer Res. 2002, 8 (4), 1139–1146. [PubMed] [Google Scholar]
- Reubi J.; Gugger M.; Waser B. Co-Expressed Peptide Receptors in Breast Cancer as a Molecular Basis for in Vivo Multireceptor Tumour Targeting. Eur. J. Nucl. Med. Mol. Imaging 2002, 29 (7), 855–862. 10.1007/s00259-002-0794-5. [DOI] [PubMed] [Google Scholar]
- Gugger M.; Reubi J. C. Gastrin-Releasing Peptide Receptors in Non-Neoplastic and Neoplastic Human Breast. Am. J. Pathol. 1999, 155 (6), 2067–2076. 10.1016/S0002-9440(10)65525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalm S. U.; Martens J. W. M.; Sieuwerts A. M.; van Deurzen C. H. M.; Koelewijn S. J.; de Blois E.; Maina T.; Nock B. A.; Brunel L.; Fehrentz J.-A.; Martinez J.; de Jong M.; Melis M. In Vitro and In Vivo Application of Radiolabeled Gastrin-Releasing Peptide Receptor Ligands in Breast Cancer. J. Nucl. Med. 2015, 56 (5), 752–757. 10.2967/jnumed.114.153023. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Aruva M. R.; Shanthly N.; Cardi C. A.; Patel C. A.; Rattan S.; Cesarone G.; Wickstrom E.; Thakur M. L. Vasoactive Intestinal Peptide (VIP) and Pituitary Adenylate Cyclase Activating Peptide (PACAP) Receptor Specific Peptide Analogues for PET Imaging of Breast Cancer: In Vitro/in Vivo Evaluation. Regul. Pept. 2007, 144 (1–3), 91–100. 10.1016/j.regpep.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C. In Vitro Identification of Vasoactive Intestinal Peptide Receptors in Human Tumors: Implications for Tumor Imaging. J. Nucl. Med. 1995, 36 (10), 1846–1853. [PubMed] [Google Scholar]
- Maina T.; Bergsma H.; Kulkarni H. R.; Mueller D.; Charalambidis D.; Krenning E. P.; Nock B. A.; de Jong M.; Baum R. P. Preclinical and First Clinical Experience with the Gastrin-Releasing Peptide Receptor-Antagonist [68Ga]SB3 and PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43 (5), 964–973. 10.1007/s00259-015-3232-1. [DOI] [PubMed] [Google Scholar]
- Stoykow C.; Erbes T.; Maecke H. R.; Bulla S.; Bartholomä M.; Mayer S.; Drendel V.; Bronsert P.; Werner M.; Gitsch G.; Weber W. A.; Stickeler E.; Meyer P. T. Gastrin-Releasing Peptide Receptor Imaging in Breast Cancer Using the Receptor Antagonist 68 Ga-RM2 And PET. Theranostics 2016, 6 (10), 1641–1650. 10.7150/thno.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M. L.; Zhang K.; Berger A.; Cavanaugh B.; Kim S.; Channappa C.; Frangos A. J.; Wickstrom E.; Intenzo C. M. VPAC1 Receptors for Imaging Breast Cancer: A Feasibility Study. J. Nucl. Med. 2013, 54 (7), 1019–1025. 10.2967/jnumed.112.114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson B.; Eaves C. J. Basal-like Breast Cancers: From Pathology to Biology and Back Again. Stem Cell Rep. 2018, 10 (6), 1676–1686. 10.1016/j.stemcr.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Steeg P. S.; Price J. E.; Krishnamurthy S.; Mani S. A.; Reuben J.; Cristofanilli M.; Dontu G.; Bidaut L.; Valero V.; Hortobagyi G. N.; Yu D. Breast Cancer Metastasis: Challenges and Opportunities. Cancer Res. 2009, 69 (12), 4951–4953. 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- Liu H.; Gruber C. W.; Alewood P. F.; Möller A.; Muttenthaler M. The Oxytocin Receptor Signalling System and Breast Cancer: A Critical Review. Oncogene 2020, 39 (37), 5917–5932. 10.1038/s41388-020-01415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman B.; Harricharran T.; Ogunwobi O. O. Oxytocin and Cancer: An Emerging Link. World J. Clin. Oncol. 2018, 9 (5), 74–82. 10.5306/wjco.v9.i5.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B.; Neumann I. D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98 (3), 1805–1908. 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- Waltenspühl Y.; Schöppe J.; Ehrenmann J.; Kummer L.; Plückthun A. Crystal Structure of the Human Oxytocin Receptor. Sci. Adv. 2020, 6 (29), eabb5419. 10.1126/sciadv.abb5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz J. G.; Robertson M. J.; Barros-Álvarez X.; Panova O.; Nwokonko R. M.; Gao Y.; Skiniotis G. The Oxytocin Signaling Complex Reveals a Molecular Switch for Cation Dependence. Nat. Struct. Mol. Biol. 2022, 29 (3), 274–281. 10.1038/s41594-022-00728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltenspühl Y.; Ehrenmann J.; Vacca S.; Thom C.; Medalia O.; Plückthun A. Structural Basis for the Activation and Ligand Recognition of the Human Oxytocin Receptor. Nat. Commun. 2022, 13 (1), 4153. 10.1038/s41467-022-31325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveque T. F.; Scharrer E. Pituicytes and the Origin of the Antidiuretic Hormone 1. Endocrinology 1953, 52 (4), 436–447. 10.1210/endo-52-4-436. [DOI] [PubMed] [Google Scholar]
- Neville M. C.; McFadden T. B.; Forsyth I. Hormonal Regulation of Mammary Differentiation and Milk Secretion. J. Mammary Gland Biol. Neoplasia 2002, 7 (1), 49–66. 10.1023/A:1015770423167. [DOI] [PubMed] [Google Scholar]
- Cassoni P.; Sapino A.; Papotti M.; Bussolati G. Oxytocin and Oxytocin-Analogue F314 Inhibit Cell Proliferation and Tumor Growth of Rat and Mouse Mammary Carcinomas. Int. J. Cancer 1996, 66 (6), 817–820. . [DOI] [PubMed] [Google Scholar]
- Lee K. H.; Khan-Dawood F. S.; Dawood M. Y. Oxytocin Receptor and Its Messenger Ribonucleic Acid in Human Leiomyoma and Myometrium. Am. J. Obstet. Gynecol. 1998, 179 (3), 620–627. 10.1016/S0002-9378(98)70054-7. [DOI] [PubMed] [Google Scholar]
- Cassoni P.; Sapino A.; Stella A.; Fortunati N.; Bussolati G. Presence and Significance of Oxytocin Receptors in Human Neuroblastomas and Glial Tumors. Int. J. Cancer 1998, 77 (5), 695–700. . [DOI] [PubMed] [Google Scholar]
- Cassoni P.; Fulcheri E.; Carcangiu M. L.; Stella A.; Deaglio S.; Bussolati G. Oxytocin Receptors in Human Adenocarcinomas of the Endometrium: Presence and Biological Significance. J. Pathol. 2000, 190 (4), 470–477. . [DOI] [PubMed] [Google Scholar]
- Morita T.; Shibata K.; Kikkawa F.; Kajiyama H.; Ino K.; Mizutani S. Oxytocin Inhibits the Progression of Human Ovarian Carcinoma Cellsin Vitro Andin Vivo. Int. J. Cancer 2004, 109 (4), 525–532. 10.1002/ijc.20017. [DOI] [PubMed] [Google Scholar]
- Xu H.; Fu S.; Chen Q.; Gu M.; Zhou J.; Liu C.; Chen Y.; Wang Z. The Function of Oxytocin: A Potential Biomarker for Prostate Cancer Diagnosis and Promoter of Prostate Cancer. Oncotarget 2017, 8 (19), 31215–31226. 10.18632/oncotarget.16107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington K.; Assinder S.; Gould M.; Nicholson H. Oxytocin, Oxytocin-Associated Neurophysin and the Oxytocin Receptor in the Human Prostate. Cell Tissue Res. 2004, 318 (2), 375–382. 10.1007/s00441-004-0968-5. [DOI] [PubMed] [Google Scholar]
- Whittington K.; Connors B.; King K.; Assinder S.; Hogarth K.; Nicholson H. The Effect of Oxytocin on Cell Proliferation in the Human Prostate Is Modulated by Gonadal Steroids: Implications for Benign Prostatic Hyperplasia and Carcinoma of the Prostate. Prostate 2007, 67 (10), 1132–1142. 10.1002/pros.20612. [DOI] [PubMed] [Google Scholar]
- Péqueux C.; Keegan B. P.; Hagelstein M.-T.; Geenen V.; Legros J.-J.; North W. G. Oxytocin- and Vasopressin-Induced Growth of Human Small-Cell Lung Cancer Is Mediated by the Mitogen-Activated Protein Kinase Pathway. Endocr. Relat. Cancer 2004, 11 (4), 871–885. 10.1677/erc.1.00803. [DOI] [PubMed] [Google Scholar]
- Cassoni P.; Sapino A.; Munaron L.; Deaglio S.; Chini B.; Graziani A.; Ahmed A.; Bussolati G. Activation of Functional Oxytocin Receptors Stimulates Cell Proliferation in Human Trophoblast and Choriocarcinoma Cell Lines*. Endocrinology 2001, 142 (3), 1130–1136. 10.1210/endo.142.3.8047. [DOI] [PubMed] [Google Scholar]
- Petersson M. Opposite Effects of Oxytocin on Proliferation of Osteosarcoma Cell Lines. Regul. Pept. 2008, 150 (1–3), 50–54. 10.1016/j.regpep.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Petersson M.; Lagumdzija A.; Stark A.; Bucht E. Oxytocin Stimulates Proliferation of Human Osteoblast-like Cells. Peptides 2002, 23 (6), 1121–1126. 10.1016/S0196-9781(02)00041-4. [DOI] [PubMed] [Google Scholar]
- Chini B.; Chinol M.; Cassoni P.; Papi S.; Reversi A.; Areces L.; Marrocco T.; Paganelli G.; Manning M.; Bussolati G. Improved Radiotracing of Oxytocin Receptor-Expressing Tumours Using the New [111In]-DOTA-Lys8-Deamino-Vasotocin Analogue. Br. J. Cancer 2003, 89 (5), 930–936. 10.1038/sj.bjc.6601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Olvera A. D.; Ferro-Flores G.; Pedraza-López M.; de Murphy C. A.; De León-Rodríguez L. M. Synthesis of Oxytocin HYNIC Derivatives as Potential Diagnostic Agents for Breast Cancer. Bioconjugate Chem. 2007, 18 (5), 1560–1567. 10.1021/bc070047a. [DOI] [PubMed] [Google Scholar]
- Cassoni P.; Marrocco T.; Sapino A.; Allia E.; Bussolati G. Oxytocin Synthesis within the Normal and Neoplastic Breast: First Evidence of a Local Peptide Source. Int. J. Oncol. 2006, 28 (5), 1263–1268. 10.3892/ijo.28.5.1263. [DOI] [PubMed] [Google Scholar]
- Ito Y.; Kobayashi T.; Kimura T.; Matsuura N.; Wakasugi E.; Takeda T.; Shimano T.; Kubota Y.; Nobunaga T.; Makino Y.; Azuma C.; Saji F.; Monden M. Investigation of the Oxytocin Receptor Expression in Human Breast Cancer Tissue Using Newly Established Monoclonal Antibodies. Endocrinology 1996, 137 (2), 773–779. 10.1210/endo.137.2.8593829. [DOI] [PubMed] [Google Scholar]
- Bussolati G.; Cassoni P.; Ghisolfi G.; Negro F.; Sapino A. Immunolocalization and Gene Expression of Oxytocin Receptors in Carcinomas and Non-Neoplastic Tissues of the Breast. Am. J. Pathol. 1996, 148 (6), 1895–1903. [PMC free article] [PubMed] [Google Scholar]
- Amico J. A.; Rauk P. N.; Cai H. Estradiol and Progesterone Regulate Oxytocin Receptor Binding and Expression in Human Breast Cancer Cell Lines. Endocrine 2002, 18 (1), 79–84. 10.1385/ENDO:18:1:79. [DOI] [PubMed] [Google Scholar]
- Copland J. A.; Jeng Y.-J.; Strakova Z.; Ives K. L.; Hellmich M. R.; Soloff M. S. Demonstration of Functional Oxytocin Receptors in Human Breast Hs578T Cells and Their Up-Regulation through a Protein Kinase C-Dependent Pathway*. Endocrinology 1999, 140 (5), 2258–2267. 10.1210/endo.140.5.6723. [DOI] [PubMed] [Google Scholar]
- Cassoni P.; Marrocco T.; Bussolati B.; Allia E.; Munaron L.; Sapino A.; Bussolati G. Oxytocin Induces Proliferation and Migration in Immortalized Human Dermal Microvascular Endothelial Cells and Human Breast Tumor-Derived Endothelial Cells. Mol. Cancer Res. 2006, 4 (6), 351–359. 10.1158/1541-7786.MCR-06-0024. [DOI] [PubMed] [Google Scholar]
- Cassoni P.; Sapino A.; Fortunati N.; Munaron L.; Chini B.; Bussolati G. Oxytocin Inhibits the Proliferation of MDA-MB231 Human Breast-Cancer Cells via Cyclic Adenosine Monophosphate and Protein Kinase A. Int. J. Cancer 1997, 72 (2), 340–344. . [DOI] [PubMed] [Google Scholar]
- Cassoni P.; Sapino A.; Negro F.; Bussolati G. Oxytocin Inhibits Proliferation of Human Breast Cancer Cell Lines. Virchows Arch 1994, 425 (5), 467–472. 10.1007/BF00197549. [DOI] [PubMed] [Google Scholar]
- Khori V.; Alizadeh A. M.; Khalighfard S.; Heidarian Y.; Khodayari H. Oxytocin Effects on the Inhibition of the NF-κB/miR195 Pathway in Mice Breast Cancer. Peptides 2018, 107, 54–60. 10.1016/j.peptides.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Alizadeh A. M.; Heydari Z.; Rahimi M.; Bazgir B.; Shirvani H.; Alipour S.; Heidarian Y.; Khalighfard S.; Isanejad A. Oxytocin Mediates the Beneficial Effects of the Exercise Training on Breast Cancer. Exp. Physiol. 2018, 103 (2), 222–235. 10.1113/EP086463. [DOI] [PubMed] [Google Scholar]
- Sapino A.; Cassoni P.; Stella A.; Bussolati G. Oxytocin Receptor within the Breast: Biological Function and Distribution. Anticancer Res. 1998, 18 (3C), 2181–2186. [PubMed] [Google Scholar]
- Reversi A.; Rimoldi V.; Marrocco T.; Cassoni P.; Bussolati G.; Parenti M.; Chini B. The Oxytocin Receptor Antagonist Atosiban Inhibits Cell Growth via a “Biased Agonist” Mechanism. J. Biol. Chem. 2005, 280 (16), 16311–16318. 10.1074/jbc.M409945200. [DOI] [PubMed] [Google Scholar]
- Imanieh M. H.; Bagheri F.; Alizadeh A. M.; Ashkani-Esfahani S. Oxytocin Has Therapeutic Effects on Cancer, a Hypothesis. Eur. J. Pharmacol. 2014, 741, 112–123. 10.1016/j.ejphar.2014.07.053. [DOI] [PubMed] [Google Scholar]
- Lee A. V.; Oesterreich S.; Davidson N. E. MCF-7 Cells-Changing the Course of Breast Cancer Research and Care for 45 Years. JNCI J. Natl. Cancer Inst. 2015, 107 (7), djv073–djv073. 10.1093/jnci/djv073. [DOI] [PubMed] [Google Scholar]
- De Giovanni; Nicoletti; Landuzzi; Palladini; Lollini; Nanni Bioprofiling TS/A Murine Mammary Cancer for a Functional Precision Experimental Model. Cancers 2019, 11 (12), 1889. 10.3390/cancers11121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanari C.; Lüthy I.; Lamb C. A.; Fabris V.; Pagano E.; Helguero L. A.; Sanjuan N.; Merani S.; Molinolo A. A. Five Novel Hormone-Responsive Cell Lines Derived from Murine Mammary Ductal Carcinomas: In Vivo and in Vitro Effects of Estrogens and Progestins. Cancer Res. 2001, 61 (1), 293–302. [PubMed] [Google Scholar]
- Ariana M.; Pornour M.; Mehr S. S.; Vaseghi H.; Ganji S. M.; Alivand M. R.; Salari M.; Akbari M. E. Preventive Effects of Oxytocin and Oxytocin Receptor in Breast Cancer Pathogenesis. Pers. Med. 2019, 16 (1), 25–34. 10.2217/pme-2018-0009. [DOI] [PubMed] [Google Scholar]
- Lewison E. F. Breast Cancer and Pregnancy or Lactation. Int. Abstr. Surg. 1954, 99 (5), 417–424. [PubMed] [Google Scholar]
- Lyons T. R.; Schedin P. J.; Borges V. F. Pregnancy and Breast Cancer: When They Collide. J. Mammary Gland Biol. Neoplasia 2009, 14 (2), 87–98. 10.1007/s10911-009-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breast Cancer and Breastfeeding: Collaborative Reanalysis of Individual Data from 47 Epidemiological Studies in 30 Countries, Including 50 302 Women with Breast Cancer and 96 973 Women without the Disease. Lancet 2002, 360 (9328), 187–195. 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- Lipworth L. History of Breast-Feeding in Relation to Breast Cancer Risk: A Review of the Epidemiologic Literature. J. Natl. Cancer Inst. 2000, 92 (4), 302–312. 10.1093/jnci/92.4.302. [DOI] [PubMed] [Google Scholar]
- Yang L.; Jacobsen K. H. A Systematic Review of the Association between Breastfeeding and Breast Cancer. J. Womens Health 2008, 17 (10), 1635–1645. 10.1089/jwh.2008.0917. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos J.; Lubinski J.; Salmena L.; Lynch H. T.; Kim-Sing C.; Foulkes W. D.; Ghadirian P.; Neuhausen S. L.; Demsky R.; Tung N.; Ainsworth P.; Senter L.; Eisen A.; Eng C.; Singer C.; Ginsburg O.; Blum J.; Huzarski T.; Poll A.; Sun P.; Narod S. A. Hereditary Breast Cancer Clinical Study Group. Breastfeeding and the Risk of Breast Cancer in BRCA1 and BRCA2Mutation Carriers. Breast Cancer Res. BCR 2012, 14 (2), R42. 10.1186/bcr3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkula M.; Pukkala E.; Kyyrönen P.; Kauppila A. Grand Multiparity and the Risk of Breast Cancer: Population-Based Study in Finland. Cancer Causes Control. 2001, 12 (6), 491–500. 10.1023/A:1011253527605. [DOI] [PubMed] [Google Scholar]
- Lee S. Y.; Kim M. T.; Kim S. W.; Song M. S.; Yoon S. J. Effect of Lifetime Lactation on Breast Cancer Risk: A Korean Women’s Cohort Study. Int. J. Cancer 2003, 105 (3), 390–393. 10.1002/ijc.11078. [DOI] [PubMed] [Google Scholar]
- Shema L.; Ore L.; Ben-Shachar M.; Haj M.; Linn S. The Association between Breastfeeding and Breast Cancer Occurrence among Israeli Jewish Women: A Case Control Study. J. Cancer Res. Clin. Oncol. 2007, 133 (8), 539. 10.1007/s00432-007-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva M.; Senarath U.; Gunatilake M.; Lokuhetty D. Prolonged Breastfeeding Reduces Risk of Breast Cancer in Sri Lankan Women: A Case-Control Study. Cancer Epidemiol. 2010, 34 (3), 267–273. 10.1016/j.canep.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Bussolati G.; Chinol M.; Chini B.; Nacca A.; Cassoni P.; Paganelli G. 111In-Labeled 1,4,7,10-Tetraazacyclododecane-N,N’,N″,N″’-Tetraacetic Acid-Lys(8)-Vasotocin: A New Powerful Radioligand for Oxytocin Receptor-Expressing Tumors. Cancer Res. 2001, 61 (11), 4393–4397. [PubMed] [Google Scholar]
- Gruber C. W.; Muttenthaler M.; Freissmuth M. Ligand-Based Peptide Design and Combinatorial Peptide Libraries to Target G Protein-Coupled Receptors. Curr. Pharm. Des. 2010, 16 (28), 3071–3088. 10.2174/138161210793292474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M.; Misicka A.; Olma A.; Bankowski K.; Stoev S.; Chini B.; Durroux T.; Mouillac B.; Corbani M.; Guillon G. Oxytocin and Vasopressin Agonists and Antagonists as Research Tools and Potential Therapeutics. J. Neuroendocrinol. 2012, 24 (4), 609–628. 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G.; Fahrenholz F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001, 81 (2), 629–683. 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Wiśniewski K.; Alagarsamy S.; Galyean R.; Tariga H.; Thompson D.; Ly B.; Wiśniewska H.; Qi S.; Croston G.; Laporte R.; Rivière P. J.-M.; Schteingart C. D. New, Potent, and Selective Peptidic Oxytocin Receptor Agonists. J. Med. Chem. 2014, 57 (12), 5306–5317. 10.1021/jm500365s. [DOI] [PubMed] [Google Scholar]
- Muttenthaler M.; Andersson Å.; Vetter I.; Menon R.; Busnelli M.; Ragnarsson L.; Bergmayr C.; Arrowsmith S.; Deuis J. R.; Chiu H. S.; Palpant N. J.; O’Brien M.; Smith T. J.; Wray S.; Neumann I. D.; Gruber C. W.; Lewis R. J.; Alewood P. F. Subtle Modifications to Oxytocin Produce Ligands That Retain Potency and Improved Selectivity across Species. Sci. Signal. 2017, 10 (508), eaan3398. 10.1126/scisignal.aan3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehbach J.; O’Brien M.; Muttenthaler M.; Miazzo M.; Akcan M.; Elliott A. G.; Daly N. L.; Harvey P. J.; Arrowsmith S.; Gunasekera S.; Smith T. J.; Wray S.; Göransson U.; Dawson P. E.; Craik D. J.; Freissmuth M.; Gruber C. W. Oxytocic Plant Cyclotides as Templates for Peptide G Protein-Coupled Receptor Ligand Design. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (52), 21183–21188. 10.1073/pnas.1311183110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard R.; Stucki A.; Schmitt M.; Py G.; Grundschober C.; Gee A. D.; Tate E. W. Building Bridges for Highly Selective, Potent and Stable Oxytocin and Vasopressin Analogs. Bioorg. Med. Chem. 2018, 26 (11), 3039–3045. 10.1016/j.bmc.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Adachi Y.; Sakimura K.; Shimizu Y.; Nakayama M.; Terao Y.; Yano T.; Asami T. Potent and Selective Oxytocin Receptor Agonists without Disulfide Bridges. Bioorg. Med. Chem. Lett. 2017, 27 (11), 2331–2335. 10.1016/j.bmcl.2017.04.030. [DOI] [PubMed] [Google Scholar]
- Alnouri M. W.; Jepards S.; Casari A.; Schiedel A. C.; Hinz S.; Müller C. E. Selectivity Is Species-Dependent: Characterization of Standard Agonists and Antagonists at Human, Rat, and Mouse Adenosine Receptors. Purinergic Signal. 2015, 11 (3), 389–407. 10.1007/s11302-015-9460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R.; Kojro E.; Fahrenholz F.. Identification of Neurohypophysial Hormone Receptor Domains Involved in Ligand Binding and G Protein Coupling. In Vasopressin and Oxytocin; Zingg H. H., Bourque C. W., Bichet D. G., Eds.; Advances in Experimental Medicine and Biology449; Springer US: Boston, MA, 1998; pp 371–385. 10.1007/978-1-4615-4871-3_48. [DOI] [PubMed] [Google Scholar]
- Fanelli F.; Barbier P.; Zanchetta D.; de Benedetti P. G.; Chini B. Activation Mechanism of Human Oxytocin Receptor: A Combined Study of Experimental and Computer-Simulated Mutagenesis. Mol. Pharmacol. 1999, 56 (1), 214–225. 10.1124/mol.56.1.214. [DOI] [PubMed] [Google Scholar]
- Favre N.; Fanelli F.; Missotten M.; Nichols A.; Wilson J.; di Tiani M.; Rommel C.; Scheer A. The DRY Motif as a Molecular Switch of the Human Oxytocin Receptor. Biochemistry 2005, 44 (30), 9990–10008. 10.1021/bi0509853. [DOI] [PubMed] [Google Scholar]
- Terrillon S.; Cheng L. L.; Stoev S.; Mouillac B.; Barberis C.; Manning M.; Durroux T. Synthesis and Characterization of Fluorescent Antagonists and Agonists for Human Oxytocin and Vasopressin V 1 a Receptors. J. Med. Chem. 2002, 45 (12), 2579–2588. 10.1021/jm010526+. [DOI] [PubMed] [Google Scholar]
- Corbani M.; Trueba M.; Stoev S.; Murat B.; Mion J.; Boulay V.; Guillon G.; Manning M. Design, Synthesis, and Pharmacological Characterization of Fluorescent Peptides for Imaging Human V1b Vasopressin or Oxytocin Receptors. J. Med. Chem. 2011, 54 (8), 2864–2877. 10.1021/jm1016208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillac B.; Manning M.; Durroux T. Fluorescent Agonists and Antagonists for Vasopressin/Oxytocin G Protein-Coupled Receptors: Usefulness in Ligand Screening Assays and Receptor Studies. Mini-Rev. Med. Chem. 2008, 8 (10), 996–1005. 10.2174/138955708785740607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albizu L.; Teppaz G.; Seyer R.; Bazin H.; Ansanay H.; Manning M.; Mouillac B.; Durroux T. Toward Efficient Drug Screening by Homogeneous Assays Based on the Development of New Fluorescent Vasopressin and Oxytocin Receptor Ligands. J. Med. Chem. 2007, 50 (20), 4976–4985. 10.1021/jm061404q. [DOI] [PubMed] [Google Scholar]
- Hope D. B.; Murti V. V.; Du Vigneaud V. A Highly Potent Analogue of Oxytocin, Desamino-Oxytocin. J. Biol. Chem. 1962, 237, 1563–1566. 10.1016/S0021-9258(19)83740-7. [DOI] [PubMed] [Google Scholar]
- Manning M.; Miteva K.; Pancheva S.; Stoev S.; Wo N. C.; Chan W. Y. Design and Synthesis of Highly Selective in Vitro and in Vivo Uterine Receptor Antagonists of Oxytocin: Comparisons with Atosiban. Int. J. Pept. Protein Res. 1995, 46 (3–4), 244–252. 10.1111/j.1399-3011.1995.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Chini B.; Mouillac B.; Ala Y.; Balestre M. N.; Trumpp-Kallmeyer S.; Hoflack J.; Elands J.; Hibert M.; Manning M.; Jard S. Tyr115 Is the Key Residue for Determining Agonist Selectivity in the V1a Vasopressin Receptor. EMBO J. 1995, 14 (10), 2176–2182. 10.1002/j.1460-2075.1995.tb07211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M.; Saulière A.; Manning M.; Bouvier M.; Galés C.; Chini B. Functional Selective Oxytocin-Derived Agonists Discriminate between Individual G Protein Family Subtypes. J. Biol. Chem. 2012, 287 (6), 3617–3629. 10.1074/jbc.M111.277178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowbridge J.; Manning M.; Haldar J.; Sawyer W. H. Synthesis and Some Pharmacological Properties of [4-Threonine,7-Glycine]Oxytocin, [1-(L-2-Hydroxy-3-Mercaptopropanoic Acid),4-Threonine,7-Glycine]Oxytocin (Hydroxy[Thr4, Gly7]Oxytocin), and [7-Glycine]Oxytocin, Peptides with High Oxytocic-Antidiuretic Selectivity. J. Med. Chem. 1977, 20 (1), 120–123. 10.1021/jm00211a025. [DOI] [PubMed] [Google Scholar]
- Busnelli M.; Kleinau G.; Muttenthaler M.; Stoev S.; Manning M.; Bibic L.; Howell L. A.; McCormick P. J.; Di Lascio S.; Braida D.; Sala M.; Rovati G. E.; Bellini T.; Chini B. Design and Characterization of Superpotent Bivalent Ligands Targeting Oxytocin Receptor Dimers via a Channel-Like Structure. J. Med. Chem. 2016, 59 (15), 7152–7166. 10.1021/acs.jmedchem.6b00564. [DOI] [PubMed] [Google Scholar]
- Jelinski M.; Hamacher K.; Coenen H. H. C-Terminal18F-Fluoroethylamidation Exemplified on [Gly-OH9] Oxytocin. J. Label. Compd. Radiopharm. 2002, 45 (3), 217–229. 10.1002/jlcr.547. [DOI] [Google Scholar]
- Beard R.; Singh N.; Grundschober C.; Gee A. D.; Tate E. W. High-Yielding 18 F Radiosynthesis of a Novel Oxytocin Receptor Tracer, a Probe for Nose-to-Brain Oxytocin Uptake in Vivo. Chem. Commun. 2018, 54 (58), 8120–8123. 10.1039/C8CC01400K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monoclonal Antibody and Peptide-Targeted Radiotherapy of Cancer; Reilly R., Ed.; Wiley: Hoboken, NJ, 2010. [Google Scholar]
- Liu S.; Edwards D. S.; Looby R. J.; Harris A. R.; Poirier M. J.; Barrett J. A.; Heminway S. J.; Carroll T. R. Labeling a Hydrazino Nicotinamide-Modified Cyclic IIb/IIIa Receptor Antagonist with 99mTc Using Aminocarboxylates as Coligands. Bioconjugate Chem. 1996, 7 (1), 63–71. 10.1021/bc950069+. [DOI] [PubMed] [Google Scholar]
- Abrams M. J.; Juweid M.; tenKate C. I.; Schwartz D. A.; Hauser M. M.; Gaul F. E.; Fuccello A. J.; Rubin R. H.; Strauss H. W.; Fischman A. J. Technetium-99m-Human Polyclonal IgG Radiolabeled via the Hydrazino Nicotinamide Derivative for Imaging Focal Sites of Infection in Rats. J. Nucl. Med. 1990, 31 (12), 2022–2028. [PubMed] [Google Scholar]
- Zingg H. H.; Laporte S. A. The Oxytocin Receptor. Trends Endocrinol. Metab. 2003, 14 (5), 222–227. 10.1016/S1043-2760(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Chini B.; Mouillac B.; Balestre M.-N.; Trumpp-Kallmeyer S.; Hoflack J.; Hibert M.; Andriolo M.; Pupier S.; Jard S.; Barberis C. Two Aromatic Residues Regulate the Response of the Human Oxytocin Receptor to the Partial Agonist Arginine Vasopressin. FEBS Lett. 1996, 397 (2–3), 201–206. 10.1016/S0014-5793(96)01135-0. [DOI] [PubMed] [Google Scholar]
- Harding F. A.; Stickler M. M.; Razo J.; DuBridge R. The Immunogenicity of Humanized and Fully Human Antibodies: Residual Immunogenicity Resides in the CDR Regions. mAbs 2010, 2 (3), 256–265. 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbasi Gharibkandi N.; Conlon J. M.; Hosseinimehr S. J. Strategies for Improving Stability and Pharmacokinetic Characteristics of Radiolabeled Peptides for Imaging and Therapy. Peptides 2020, 133, 170385. 10.1016/j.peptides.2020.170385. [DOI] [PubMed] [Google Scholar]
- AlDeghaither D.; Smaglo B. G.; Weiner L. M. Beyond Peptides and mAbs-Current Status and Future Perspectives for Biotherapeutics with Novel Constructs. J. Clin. Pharmacol. 2015, 55 (S3), S4–S20. 10.1002/jcph.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodei L.; Cremonesi M.; Zoboli S.; Grana C.; Bartolomei M.; Rocca P.; Caracciolo M.; Mäcke H. R.; Chinol M.; Paganelli G. Receptor-Mediated Radionuclide Therapy with 90Y-DOTATOC in Association with Amino Acid Infusion: A Phase I Study. Eur. J. Nucl. Med. Mol. Imaging 2003, 30 (2), 207–216. 10.1007/s00259-002-1023-y. [DOI] [PubMed] [Google Scholar]
- Sofou S. Radionuclide Carriers for Targeting of Cancer. Int. J. Nanomedicine 2008, 181. 10.2147/IJN.S2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urruticoechea A.; Alemany R.; Balart J.; Villanueva A.; Vinals F.; Capella G. Recent Advances in Cancer Therapy: An Overview. Curr. Pharm. Des. 2010, 16 (1), 3–10. 10.2174/138161210789941847. [DOI] [PubMed] [Google Scholar]
- Feijtel D.; de Jong M.; Nonnekens J. Peptide Receptor Radionuclide Therapy: Looking Back, Looking Forward. Curr. Top. Med. Chem. 2020, 20 (32), 2959–2969. 10.2174/1568026620666200226104652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opalińska M.; Sowa-Staszczak A.; Grochowska A.; Olearska H.; Hubalewska-Dydejczyk A. Value of Peptide Receptor Radionuclide Therapy as Neoadjuvant Treatment in the Management of Primary Inoperable Neuroendocrine Tumors. Front. Oncol. 2021, 11, 687925. 10.3389/fonc.2021.687925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus B.; Cottereau A.-S.; Palmieri L.-J.; Dermine S.; Tenenbaum F.; Brezault C.; Coriat R. Indications of Peptide Receptor Radionuclide Therapy (PRRT) in Gastroenteropancreatic and Pulmonary Neuroendocrine Tumors: An Updated Review. J. Clin. Med. 2021, 10 (6), 1267. 10.3390/jcm10061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennrich U.; Kopka K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12 (3), 114. 10.3390/ph12030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgouros G.; Bodei L.; McDevitt M. R.; Nedrow J. R. Radiopharmaceutical Therapy in Cancer: Clinical Advances and Challenges. Nat. Rev. Drug Discovery 2020, 19 (9), 589–608. 10.1038/s41573-020-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kręcisz P.; Czarnecka K.; Królicki L.; Mikiciuk-Olasik E.; Szymański P. Radiolabeled Peptides and Antibodies in Medicine. Bioconjugate Chem. 2021, 32 (1), 25–42. 10.1021/acs.bioconjchem.0c00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann S.; Niederstadt L.; Koziolek E. J.; Gómez J. D. C.; Prasad S.; Wagener A.; von Hacht J. L.; Reinicke S.; Exner S.; Bandholtz S.; Beindorff N.; Brenner W.; Grötzinger C. CMKLR1-Targeting Peptide Tracers for PET/MR Imaging of Breast Cancer. Theranostics 2019, 9 (22), 6719–6733. 10.7150/thno.34857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock B. A.; Kaloudi A.; Lymperis E.; Giarika A.; Kulkarni H. R.; Klette I.; Singh A.; Krenning E. P.; de Jong M.; Maina T.; Baum R. P. Theranostic Perspectives in Prostate Cancer with the Gastrin-Releasing Peptide Receptor Antagonist NeoBOMB1: Preclinical and First Clinical Results. J. Nucl. Med. 2017, 58 (1), 75–80. 10.2967/jnumed.116.178889. [DOI] [PubMed] [Google Scholar]
- Djaileb L.; Morgat C.; van der Veldt A.; Virgolini I.; Cortes F.; Demange A.; Orlandi F.; Wegener A. Preliminary Diagnostic Performance of [68Ga]-NeoBOMB1 in Patients with Gastrin-Releasing Peptide Receptor-Positive Breast, Prostate, Colorectal or Lung Tumors (NeoFIND). J. Nucl. Med. 2020, 61, 346. [Google Scholar]
- Nhàn N. T. T.; Yamada T.; Yamada K. H. Peptide-Based Agents for Cancer Treatment: Current Applications and Future Directions. Int. J. Mol. Sci. 2023, 24 (16), 12931. 10.3390/ijms241612931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman P.; Joshi S.; Sharma S.; Preet S.; Sharma S.; Saini A. Strategic Approaches to Improvise Peptide Drugs as Next Generation Therapeutics. Int. J. Pept. Res. Ther. 2023, 29 (4), 61. 10.1007/s10989-023-10524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]