Abstract
Vibrio vulnificus is an opportunistic human pathogen causing wound infections and septicemia, characterized by hemorrhagic and edematous damage to the skin. This human pathogen secretes a metalloprotease (V. vulnificus protease [VVP]) as an important virulence determinant. When several bacterial metalloproteases including VVP were injected intradermally into dorsal skin, VVP showed the greatest hemorrhagic activity. The level of the in vivo hemorrhagic activity of the bacterial metalloproteases was significantly correlated with that of the in vitro proteolytic activity for the reconstituted basement membrane gel. Of two major basement membrane components (laminin and type IV collagen), only type IV collagen was easily digested by VVP. Additionally, the immunoglobulin G antibody against type IV collagen, but not against laminin, showed sufficient protection against the hemorrhagic reaction caused by VVP. Capillary vessels are known to be stabilized by binding of the basal surface of vascular endothelial cells to the basement membrane. Therefore, specific degradation of type IV collagen may cause destruction of the basement membrane, breakdown of capillary vessels, and leakage of blood components including erythrocytes.
Metalloproteases, in which zinc is an essential metal ion for catalytic activity, are elaborated by various human pathogenic bacteria, as well as by nonpathogenic ones (8). On the basis of location of the zinc ligands, they can be classified into at least three families, thermolysin, serralysin, and neurotoxin (9). Collagenases produced by anaerobic bacteria including Clostridium histolyticum are also zinc-containing proteolytic enzymes (25); however, there is no evidence on location of the zinc ligands. Clostridial neurotoxins cleaving the synaptic vesicle membrane protein or the presynaptic plasma membrane protein have been reported to be decisive virulence determinants of Clostridium botulinum and Clostridium tetani (22). Proteolytic enzymes from other pathogenic organisms have also been demonstrated to play important roles in bacterial virulence (7, 20).
Vibrio vulnificus is an opportunistic human pathogen causing wound infections and septicemia (10, 23), characterized by formation of hemorrhagic and edematous lesions on the skin of limbs (10, 23). This human pathogen also produces a zinc metalloprotease (V. vulnificus protease [VVP]) of the thermolysin family (9, 18, 20). VVP has been documented to be the main virulence determinant for skin lesions, because this protease possesses the ability to enhance vascular permeability and to cause hemorrhagic damage (15, 17). The process of enhancement of hypodermic vascular permeability has been studied in detail, and it is currently believed that VVP directs in situ generation of inflammatory mediators such as bradykinin and histamine (15, 17). On the other hand, the mechanism by which VVP induces the hemorrhagic reaction has not been studied sufficiently, although proteolytic activity is essential to hemorrhagic activity (15, 17).
The venoms from crotalids and viperids contain hemorrhagic toxins, which are also zinc-containing metalloproteases (1). The precise mechanism causing hemorrhagic damage by snake toxins is not known yet. However, these toxins may degrade basement membrane (BM) lying along capillary vessels, because it has been reported that snake toxins have in vitro proteolytic activity for isolated BM components, such as laminin, type IV collagen, and nidogen-entactin (1). The hemorrhagic reaction caused by any of the snake hemorrhagic toxins is observed within a few minutes when the toxin is injected intradermally (1). Since this time course is very similar to that for VVP (15, 17), VVP may cause hypodermic hemorrhage through a process identical or analogous to that of snake hemorrhagic toxins.
In the present study, we report that VVP injected into dorsal skin possibly induces hemorrhagic reaction through disorganization of the BM layer due to specific degradation of type IV collagen, which is known to form the backbone structure of the BM layer.
MATERIALS AND METHODS
Bacterial metalloproteases.
The recombinant VVP (45 kDa) was isolated from the periplasmic fraction of transformant Escherichia coli DH5α(pVVP7-1), which carries the entire vvp gene subcloned into pBluescript II KS(+), as described recently (21). Serralysin (60 kDa) was purchased from Sigma Chemical (St. Louis, Mo.). Thermolysin (35 kDa) and C. histolyticum collagenase (105 kDa) were obtained from Seikagaku Corporation (Tokyo, Japan). Homogeneity of each of the bacterial metalloproteases was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (14).
IgG antibodies and control IgG.
An affinity-purified rabbit immunoglobulin G (IgG) preparation, which was used as the control IgG preparation, was obtained from Seikagaku Corporation. The rabbit IgG antibody against mouse laminin or type IV collagen was prepared as follows: a solution containing 1.0 mg of purified mouse laminin (Becton Dickinson, Bedford, Mass.) or type IV collagen (Becton Dickinson) was emulsified with an equal volume of Freund’s complete adjuvant and injected subcutaneously into the dorsal skin of a rabbit (ca. 3.0 kg in weight) at 2-week intervals. The antiserum was collected at 7 days after the third injection, and the IgG fraction was obtained by ammonium sulfate fractionation and column chromatography on a Hitrap protein A column (Amersham Pharmacia Biotech, Uppsala, Sweden).
Quantitative immunoprecipitation experiments demonstrated that 1.0 mg of each of the IgG antibodies could specifically react with 0.4 mg of laminin and 0.2 mg of type IV collagen.
Hemorrhagic and permeability-enhancing reactions.
The reactions of the bacterial metalloproteases were measured as described elsewhere (15). For the hemorrhagic reaction, a male Hartley guinea pig (ca. 400 g in weight) was anesthetized with pentobarbital (20 mg/kg of body weight), and then VVP, thermolysin, serralysin, or C. histolyticum collagenase (1.0 or 10 μg in 0.1 ml of saline) was injected intradermally into the dorsal skin. At 30 min postinjection, the animal was sacrificed, the dorsal skin was stripped off, and the area of the hemorrhagic spot was measured.
For the permeability-enhancing reaction, each of the proteases (1.0 or 10 μg in 0.1 ml of saline) was injected into the dorsal skin of an anesthetized guinea pig which had previously been administered 5% Evans blue (1 ml/kg) intravenously. At 30 min postinjection, the blue spot caused by extravasation of the Evans blue-serum albumin complex was measured.
In order to test the effects of the IgG antibodies and control IgG on the hemorrhagic reaction, an appropriate amount of each of the IgG preparations was mixed with 10 μg of VVP. Each admixture (0.1 ml) thus prepared was injected into the dorsal skin of a male ICR mouse (7 or 8 weeks old), and the area of the hemorrhagic spot was measured at 30 min postinjection.
Proteolytic activity for the reconstituted BM gel.
Matrigel solution (Becton Dickinson), BM materials extracted from Engelbreth-Holm-Swarm tumors in lathyritic mice (11), was dialyzed overnight against 20 mM Tris-HCl buffer (pH 8.0) containing 150 mM NaCl and 1 mM CaCl2 (Tris-buffered saline [TBS]). Then, in order to reconstitute the BM gel, 50 μl of the dialyzed Matrigel solution (0.4 mg of total protein) in a centrifuge tube was incubated at 37°C for 1 h. Thereafter, 25 μl of each of the bacterial metalloproteases (0 to 10 μg) was layered on the reconstituted BM gel. After incubation at 37°C for an appropriate period, 20 μl of the supernatant was withdrawn, and the protein released from the reconstituted gel was quantified by the ninhydrin method.
Measurement of residual amounts of laminin and type IV collagen in the protease-treated BM gel.
Each bacterial metalloprotease (0 to 5 μg in 5 μl of TBS) was allowed to react on 10 μl (80 μg of total protein) of the reconstituted BM gel at 37°C for 1 h. After incubation, the protease was inactivated by addition of 10 μl of 10 mM o-phenanthroline, and the protease-treated gel was cooled in an ice-cold water bath in order to separate the components. Then, this cooled sample was incubated with 0.3 mg of the IgG antibody against laminin or type IV collagen for 1 h in an ice-cold water bath. Thereafter, the insoluble immunocomplex aggregate was collected by centrifugation at 3,000 × g for 5 min, the precipitate obtained was dissolved in 0.5 ml of 1 M NaOH, and the protein was quantified by measuring absorbance at 280 nm.
Proteolytic activity of VVP and thermolysin for purified mouse type IV collagen.
Mouse type IV collagen (50 μg) and the protease (0 to 3 μg) were incubated at 37°C for an appropriate period in a total of 50 μl of TBS. After incubation, the reaction was terminated by addition of o-phenanthroline, and the degree of proteolysis of type IV collagen was analyzed by SDS-PAGE.
RESULTS
Hemorrhage and vascular permeability enhancement by bacterial metalloproteases.
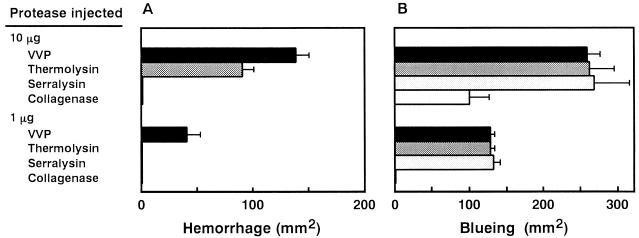
VVP and three prototype enzymes of bacterial metalloprotease families were injected intradermally into the dorsal skin of guinea pigs. At 30 min postinjection, the areas of hemorrhagic spots were measured (Fig. 1A). Both VVP and thermolysin, belonging to the thermolysin family, formed the visual hemorrhagic area, while VVP was found to have the greater hemorrhagic activity. On the other hand, neither serralysin nor C. histolyticum collagenase formed the hemorrhagic area. The hemorrhagic reaction caused by thermolysin, as well as that caused by VVP (15), was demonstrated to be transitory. Thus, the hemorrhagic area was not expanded at 6 h postinjection (data not shown).
FIG. 1.
(A) Hemorrhagic activity of bacterial metalloproteases. VVP, thermolysin, serralysin, or C. histolyticum collagenase (1.0 or 10 μg) was injected intradermally into the dorsal skin of a guinea pig. At 30 min postinjection, the animal was sacrificed, the dorsal skin was stripped off, and the area of the hemorrhagic spot was measured (n = 2). (B) Permeability-enhancing activity of bacterial metalloproteases. Each of the proteases (1.0 or 10 μg) was injected into the dorsal skin of a guinea pig which had previously been administered 5% Evans blue (1 ml/kg) intravenously. At 30 min postinjection, the blue spot caused by extravasation of the Evans blue-serum albumin complex was measured (n = 2).
As shown in Fig. 1B, all bacterial metalloproteases tested enhanced hypodermic vascular permeability. It should be noted that serralysin, having no hemorrhagic activity, showed permeability-enhancing activity comparable to those of VVP and thermolysin. Miyoshi et al. (15, 17) previously reported that the substances modulating the permeability-enhancing reaction did not affect the hemorrhagic reaction caused by VVP. Thus, hemorrhagic damage caused by the bacterial metalloproteases is most probably independent of enhancement of hypodermic vascular permeability.
Degradation of the reconstituted BM gel by bacterial metalloproteases.
The BM associates with the basal surface of vascular endothelial cells and stabilizes capillary vessels (5, 6, 24), and its components can induce morphological differentiation of endothelial cells into the capillary structure (4, 13). Therefore, it was thought that destruction of the vascular BM might be a crucial event for inducing hemorrhagic damage.
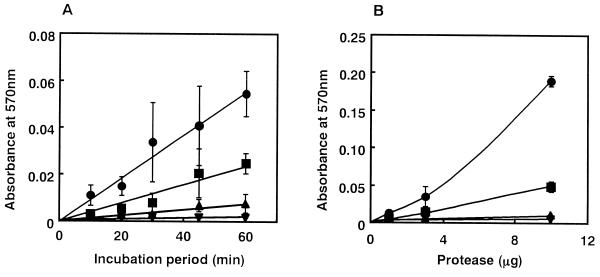
In order to test this idea, we first tested the proteolytic action of each bacterial metalloprotease for the reconstituted BM gel. Each of the proteases (3 μg) was added to the top of the reconstituted gel (0.4 mg of total protein) and allowed to incubate at 37°C. The supernatant was withdrawn periodically, and the protein liberated from the reconstituted gel was measured. As shown in Fig. 2A, VVP could degrade the reconstituted BM gel most effectively in a time-dependent manner. Thermolysin was also found to have significant proteolytic activity for the gel, while serralysin and C. histolyticum collagenase showed either no or negligible proteolytic action for the gel. Some degradation of the reconstituted BM gel was observed when 1 μg of VVP was allowed to react for 1 h; however, C. histolyticum collagenase could not degrade the gel even when 10 μg was allowed to react for 1 h (Fig. 2B). These findings demonstrate that only hemorrhagic bacterial metalloproteases, namely, VVP and thermolysin, show sufficient proteolytic action for the reconstituted BM gel. In other words, the level of the in vivo hemorrhagic activity was similar to that of the in vitro proteolytic activity for the reconstituted gel.
FIG. 2.
(A) Time dependence of degradation of reconstituted BM gel by bacterial metalloproteases. Each of the proteases (3 μg) was layered on the reconstituted BM gel (0.4 mg of total protein) and was allowed to incubate at 37°C. After an appropriate incubation period, the supernatant was withdrawn, and the protein released from the reconstituted gel was quantified by the ninhydrin method (n = 3). (B) Dose dependence of degradation of the reconstituted gel by bacterial metalloproteases. An appropriate amount (0 to 10 μg) of each protease was allowed to react on the reconstituted gel at 37°C for 1 h. Thereafter, the protein released from the reconstituted gel was quantified (n = 3). Symbols: •, VVP; ■, thermolysin; ▴, serralysin; ▾, C. histolyticum collagenase.
Like the in vivo hemorrhagic action (15, 17), the in vitro degrading action of VVP on the reconstituted BM gel was significantly blocked by addition of phosphoramidone, a competitive inhibitor of VVP (reference 16 and data not shown).
Degradation of type IV collagen in the reconstituted BM gel by VVP and thermolysin.
Although the BM gel has several protein components, laminin and type IV collagen are dominant (12). Specifically, laminin is almost 60% of the materials in the gel and type IV collagen is about 30%. We secondly studied which of the protein components is digested primarily by VVP and thermolysin.
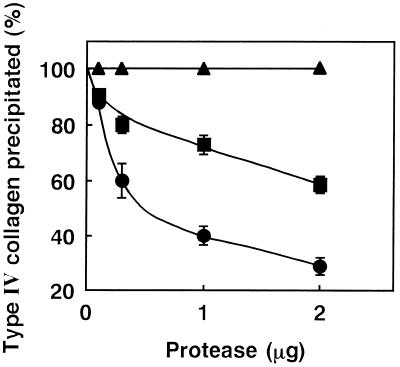
Various amounts of each of the proteases (0 to 5 μg) were allowed to react on the reconstituted BM gel (80 μg of total protein) at 37°C for 1 h. After incubation, the gel was separated into its components, intact laminin or type IV collagen was precipitated individually with the monospecific antibody, and the protein of each immunocomplex precipitated was then quantified. Laminin was found not to decrease in reactivity to the antibody even when a dose as high as 5 μg of VVP was used for the experiment (data not shown), indicating the inability of VVP to digest laminin. On the other hand, type IV collagen was demonstrated to be dose-dependently degraded by VVP (Fig. 3). When 0.1, 0.3, 1.0, or 2.0 μg of VVP was allowed to react on the reconstituted BM gel, the amount of type IV collagen reacting to the antibody was reduced to 88, 60, 40, and 29% of original level, respectively. Thermolysin was also able to digest only type IV collagen, while its digestive ability was half that of VVP (Fig. 3). However, neither serralysin nor C. histolyticum collagenase showed proteolytic activity for laminin (data not shown) and type IV collagen (Fig. 3).
FIG. 3.
Amount of intact type IV collagen in the reconstituted BM gel which was incubated with bacterial metalloproteases. An appropriate amount (0 to 2 μg) of each protease was allowed to react on the reconstituted BM (80 μg of total protein) at 37°C for 1 h. After incubation, each of the protease-treated gels was separated into its components, and only type IV collagen was precipitated with the specific IgG antibody. Thereafter, the relative amount of intact type IV collagen precipitated was determined (n = 3). Symbols: •, VVP; ■, thermolysin; ▴, serralysin or C. histolyticum collagenase.
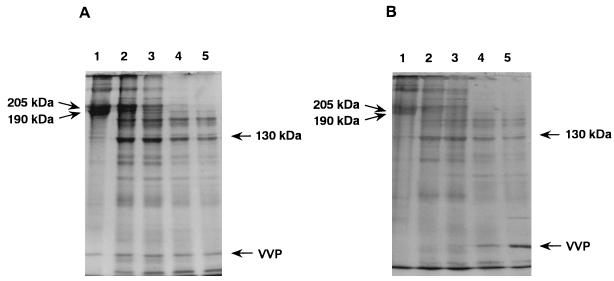
In order to confirm type IV collagen-degrading ability, VVP and purified mouse type IV collagen were incubated at 37°C, and the degree of proteolysis of the collagen was periodically analyzed by SDS-PAGE. It was shown that when 1 μg of VVP was allowed to react on 50 μg of the substrate, the protein bands corresponding to type IV collagen subunits (205 and 190 kDa) were gradually cleaved into several fragments including the 130-kDa major one, and no intact type IV collagen molecule was detected at after 10 min of incubation (Fig. 4A). Additionally, this proteolytic action for type IV collagen was dose dependent (Fig. 4B), and significant proteolysis of the collagen molecule was observed even when an amount as small as 0.1 μg of VVP was allowed to react on the substrate at 37°C for 10 min. Thermolysin was also found to cause proteolysis of the type IV collagen in a time- and dose-dependent manner (data not shown). The SDS-PAGE profile of thermolysin-treated type IV collagen was very similar to that of VVP-treated collagen. However, the collagenolytic activity of thermolysin might be slightly weaker than that of VVP, because some intact type IV collagen molecules were detected when 1 μg of thermolysin was allowed to react at 37°C for 10 min.
FIG. 4.
(A) Time dependence of proteolytic action of VVP on purified mouse type IV collagen. VVP (1 μg) was allowed to react on mouse type IV collagen (50 μg) at 37°C. After an appropriate incubation period, an aliquot of the sample was withdrawn, and the degree of proteolysis of type IV collagen was analyzed by SDS-PAGE. Lanes: 1, admixture of type IV collagen and VVP; 2, type IV collagen incubated with VVP for 3 min; 3, type IV collagen incubated with VVP for 5 min; 4, type IV collagen incubated with VVP for 10 min; 5, type IV collagen incubated with VVP for 20 min. (B) Dose dependence of proteolytic action of VVP on purified mouse type IV collagen. Various amounts of VVP (0 to 3 μg) were allowed to react on mouse type IV collagen (50 μg) at 37°C for 10 min. After incubation, an aliquot of the sample was withdrawn, and the degree of proteolysis of type IV collagen was analyzed by SDS-PAGE. Lanes: 1, type IV collagen alone; 2, type IV collagen incubated with 0.1 μg of VVP; 3, type IV collagen incubated with 0.3 μg of VVP; 4, type IV collagen incubated with 1 μg of VVP; 5, type IV collagen incubated with 3 μg of VVP.
Taken together, it may be concluded that VVP and thermolysin have proteolytic activity for type IV collagen but not for laminin.
Effects of IgG antibodies against BM components on the hemorrhagic reaction caused by VVP.
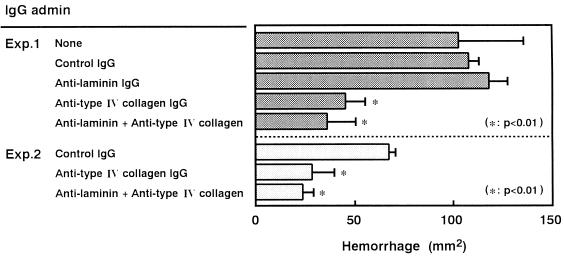
As shown in Fig. 5, when 0.6 mg of the IgG antibody against mouse type IV collagen was injected simultaneously into the dorsal skin of mice, the hemorrhagic damage caused by 10 μg of VVP was sufficiently blocked. However, the antilaminin IgG antibody (0.6 mg) did not show any effect. This hemorrhage-preventing ability of the anti-type IV collagen antibody was shown to be dose dependent (data not shown). For example, 0.2 mg of the antibody could significantly block VVP-induced hemorrhagic damage; however, 60 μg or less of the antibody showed insufficient protection. These findings suggest that VVP may act in vivo only on type IV collagen in the BM thin layer.
FIG. 5.
Effects of IgG antibodies against BM components on the hemorrhagic reaction caused by VVP. VVP (10 μg) was mixed with control IgG or IgG antibody (0.6 mg), and each admixture was injected intradermally into the dorsal skin of a mouse. At 30 min postinjection, the area of the hemorrhagic spot was measured (n = 4). Eight-week-old mice were used in experiment 1, while 7-week-old mice were employed for experiment 2. admin, administered.
On balance, it is possible to conclude that VVP injected intradermally into the dorsal skin directly degrades only type IV collagen, which results in disorganization of the BM layer, in breakdown of capillary vessels, and finally in hypodermic hemorrhage.
DISCUSSION
The vascular BM, a continuous thin layer around the capillary vessels, associates with the basal surface of vascular endothelial cells (5, 6, 24), and thus, the capillary vessels are stabilized by this thin layer. The BM has several protein components. Among them, type IV collagen is known to form the framework of the membrane, and laminin is known to link the endothelial cell to the framework made by cross-linked type IV collagen (5, 6, 24). The present study demonstrated that VVP and thermolysin, which cause hypodermic hemorrhage, possess proteolytic activity specific for type IV collagen and that the antibody against type IV collagen prevented the hemorrhagic reaction caused by VVP. In contrast, serralysin and C. histolyticum collagenase, which have no hemorrhagic activity, showed an inability to digest type IV collagen. Therefore, destruction of the type IV collagen framework may be a crucial event resulting in disorganization of the BM thin layer and in hemorrhage.
When VVP was allowed to react on the reconstituted BM gel, no laminin digestion was observed. However, this protease showed significant proteolytic action for purified laminin (data not shown). It is not clear why laminin molecules incorporated into the BM gel resist proteolytic action. However, it is speculated that VVP may not be able to make contact with laminin, which binds to the extended framework of cross-linked type IV collagen, due to the steric hindrance. Otherwise, in the laminin molecule, the domain containing the VVP cleavage site may function in attachment to the type IV collagen molecule.
Although disorganization of the vascular BM is likely to be the most important mechanism, VVP may induce hemorrhagic damage through several cooperative mechanisms. For instance, Miyoshi et al. (19) previously reported strong fibrinolytic and fibrinogenolytic activities of VVP. These activities possibly cause delay in plasma coagulation and increase the escape of erythrocytes from damaged capillary vessels.
Both in vitro (16) and in vivo (3), VVP shows collagenolytic activity for type I collagen, a main material in the connective tissue. In vivo degradation of collagen fibrils may facilitate bacterial dissemination. However, this collagenolytic activity is unlikely to contribute to hemorrhagic activity, because simultaneous injection of C. histolyticum collagenase, whose activity is highly specific for type I collagen, did not augment the hemorrhagic reaction caused by VVP (data not shown). Additionally, this bacterial collagenase alone formed no visual hemorrhagic area even when a dose as high as 10 μg was injected into dorsal skin.
Many hemorrhagic toxins having the in vitro proteolytic activity for BM materials including type IV collagen have been isolated from venoms of crotalids and viperids (1). Electron microscopic examinations have shown that hypodermic hemorrhage caused by these snake toxins is generally accompanied by disappearance of the BM thin layer and by attenuation and/or disruption of vascular endothelial cells without alteration of the intercellular tight junction, which indicate that hemorrhage is by rhexis, not by diapedesis (1). Since the reagents modulating the vascular permeability-enhancing reaction are known to have no effect on the hemorrhagic reaction (15, 17), hemorrhage caused by VVP may be also by rhexis. Recent in vitro studies using cultured endothelial cells revealed that none of the snake hemorrhagic toxins examined had direct cytotoxic activity (1, 2). Therefore, it is believed that in vivo disruption of vascular endothelial cells is due to the indirect action of the hemorrhagic toxins. Experiments to determine whether VVP possesses direct cytotoxic activity against vascular endothelial cells are in progress.
In conclusion, the data presented herein indicate that specific proteolysis of type IV collagen in the vascular BM by VVP may be a crucial event causing hypodermic hemorrhagic damage and suggest that hemorrhagic skin damage, a characteristic symptom of V. vulnificus infection, may be caused by VVP produced by the bacterial cells invading the interstitial tissue space.
ACKNOWLEDGMENT
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Bjarnason J B, Fox J W. Hemorrhagic metalloproteinases from snake venoms. Pharmacol Ther. 1994;62:325–372. doi: 10.1016/0163-7258(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 2.Borkow G, Gutierrez J M, Ovadia M. In vitro activity of BaH1, the main hemorrhagic toxin of Bothrops asper snake venom on bovine endothelial cells. Toxicon. 1997;33:1387–1391. doi: 10.1016/0041-0101(95)00078-z. [DOI] [PubMed] [Google Scholar]
- 3.Chuang Y C, Sheu H M, Ko W C, Chang T M, Chang M C, Huang K Y. Mouse skin damage caused by a recombinant extracellular metalloprotease from Vibrio vulnificus and by V. vulnificus infection. J Formos Med Assoc. 1997;96:677–684. [PubMed] [Google Scholar]
- 4.Grant D S, Kleinman H K, Martin G R. The role of basement membranes in vascular development. Ann N Y Acad Sci. 1990;588:61–72. doi: 10.1111/j.1749-6632.1990.tb13197.x. [DOI] [PubMed] [Google Scholar]
- 5.Grant D S, Kleinman H K. Regulation of capillary formation by laminin and other components of the extracellular matrix. Exper Suppl (Basel) 1997;79:317–333. doi: 10.1007/978-3-0348-9006-9_13. [DOI] [PubMed] [Google Scholar]
- 6.Grant M E, Heathcote J G, Orkin R W. Current concepts of basement membrane structure and function. Biosci Rep. 1981;1:819–842. doi: 10.1007/BF01114816. [DOI] [PubMed] [Google Scholar]
- 7.Harrington D J. Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infect Immun. 1996;64:1885–1891. doi: 10.1128/iai.64.6.1885-1891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hase C C, Finkelstein R A. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993;57:823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper N M. Families of zinc metalloproteases. FEBS Lett. 1994;354:1–6. doi: 10.1016/0014-5793(94)01079-x. [DOI] [PubMed] [Google Scholar]
- 10.Janda J M, Powers C, Bryant R G, Abbott S L. Current perspectives on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin Microbiol Rev. 1988;1:245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinman H K, McGarvey M L, Liotta L A, Robey P G, Tryggvason K, Martin G. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 12.Kleinman H K, McGarvey M L, Hassell J R, Star V L, Cannon F B, Laurie G W, Martin G R. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 13.Kubota Y, Kleinman H K, Martin G R, Lawley T J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Miyoshi N, Miyoshi S, Sugiyama K, Suzuki Y, Furuta H, Shinoda S. Activation of the plasma kallikrein-kinin system by Vibrio vulnificus protease. Infect Immun. 1987;55:1936–1939. doi: 10.1128/iai.55.8.1936-1939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyoshi N, Shimizu C, Miyoshi S, Shinoda S. Purification and characterization of Vibrio vulnificus protease. Microbiol Immunol. 1987;31:13–25. doi: 10.1111/j.1348-0421.1987.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 17.Miyoshi S, Sugiyama K, Suzuki Y, Furuta H, Miyoshi N, Shinoda S. Enhancement of vascular permeability due to histamine-releasing effect of Vibrio vulnificus protease in rat skin. FEMS Microbiol Lett. 1985;40:95–98. [Google Scholar]
- 18.Miyoshi S, Oh E G, Hirata K, Shinoda S. Exocellular toxic factors produced by Vibrio vulnificus. J Toxicol Toxin Rev. 1993;12:253–288. [Google Scholar]
- 19.Miyoshi S, Narukawa H, Tomochika K, Shinoda S. Actions of Vibrio vulnificus metalloprotease on human plasma proteinase-proteinase inhibitor systems: a comparative study of native protease with its derivative modified by polyethylene glycol. Microbiol Immunol. 1995;39:959–966. doi: 10.1111/j.1348-0421.1995.tb03299.x. [DOI] [PubMed] [Google Scholar]
- 20.Miyoshi S, Shinoda S. Bacterial metalloprotease as the toxic factor in infection. J Toxicol Toxin Rev. 1997;16:177–194. [Google Scholar]
- 21.Miyoshi S, Wakae H, Tomochika K, Shinoda S. Functional domains of a zinc metalloprotease from Vibrio vulnificus. J Bacteriol. 1997;179:7606–7609. doi: 10.1128/jb.179.23.7606-7609.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oguma K, Fujinaga Y, Inoue K. Structure and function of Clostridium botulinum toxins. Microbiol Immunol. 1995;39:161–168. doi: 10.1111/j.1348-0421.1995.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 23.Tacket C O, Brenner F, Blake P A. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558–561. doi: 10.1093/infdis/149.4.558. [DOI] [PubMed] [Google Scholar]
- 24.Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989;180:487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoshihara K, Matsushita O, Minami J, Okabe A. Cloning and nucleotide sequence analysis of the colH gene from Clostridium histolyticum encoding a collagenase and a gelatinase. J Bacteriol. 1994;176:6489–6496. doi: 10.1128/jb.176.21.6489-6496.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]