Abstract
Objectives
To investigate the association between leisure time physical activity (LTPA) and MRI-based diastolic function and the mediating role of metabolic health.
Methods
This cross-sectional analysis comprised 901 participants (46% women, mean age (SD): 56 (6) years (The Netherlands, 2008–2012)). LTPA was assessed via questionnaire, quantified in metabolic equivalent of tasks (METs)-minutes per week and participants underwent abdominal and cardiovascular MRI. Confirmatory factor analysis was used to construct the metabolic load factor. Piecewise structural equation model with adjustments for confounders was used to determine associations between LTPA and diastolic function and the mediating effect of metabolic load.
Results
Significant differences in mitral early/late peak filling rate (E/A) ratio per SD of LTPA (men=1999, women=1870 MET-min/week) of 0.18, (95% CI= 0.03 to 0.33, p=0.021) were observed in men, but not in women: −0.01 (−0.01 to 0.34, p=0.058). Difference in deceleration time of mitral early filling (E-DT) was 0.13 (0.01 to 0.24, p=0.030) in men and 0.17 (0.05 to 0.28, p=0.005) in women. Metabolic load, including MRI-based visceral and subcutaneous adipose tissue, fasting glucose, high-density lipoprotein cholesterol and triglycerides, mediated these associations as follows: E/A-ratio of 0.030 (0.000 to 0.067, 19% mediated, p=0.047) in men but not in women: 0.058 (0.027 to 0.089, p<0.001) and E-DT not in men 0.004 (−0.012 to 0.021, p=0.602) but did in women 0.044 (0.013 to 0.057, 27% mediated, p=0.006).
Conclusions
A larger amount of LTPA was associated with improved diastolic function where confirmatory factor analysis-based metabolic load partly mediated this effect. Future studies should assess whether improving indicators of metabolic load alongside LTPA will benefit healthy diastolic function even more.
Keywords: cardiovascular epidemiology, physical activity, MRI, cardiology, metabolism
WHATS IS ALREADY KNOWN ON THIS TOPIC
Increased leisure time physical activity (LTPA) is associated with improved overall cardiovascular and metabolic health.
Few have investigated the role of diastolic function, a precursor of heart failure with preserved ejection fraction, in this association and, if so, diastolic function was determined by echocardiography instead of MRI, like in this study.
WHATS THIS STUDY ADDS
More LTPA was associated with better MRI-based diastolic function and metabolic health in a large Dutch middle-aged cohort of men and women.
Metabolic load assessed by confirmatory factor analysis, consisting of continuously MRI-based subcutaneous and visceral fat, glucose, triglycerides and high-density lipoprotein cholesterol was also associated to diastolic function.
Metabolic load partly mediates the association between increased LTPA and improved diastolic function in both men and women.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
LTPA should be encouraged in middle-aged subjects to improve diastolic function and metabolic health.
Besides LTPA, other strategies that promote metabolic health could be considered to improve the interventional effectivity on diastolic function.
Introduction
Leisure time physical activity (LTPA) is associated with cardiovascular health benefits like reduced risk and progression of cardiovascular diseases (including heart disease and stroke) and mortality.1 2 The risk of heart failure and mortality is reduced, even by small doses of moderate to vigorous LTPA, particularly in initially inactive people who become active.3–7 However, the effect of LTPA on heart failure with preserved ejection fraction (HFpEF), currently a major cause of cardiac deaths worldwide, remains inconclusive.8 HFpEF is a disease characterised by diastolic dysfunction, which is largely an untreatable disease with high morbidity and mortality.9 Previous research on LTPA has focused mainly on exercise and athletes,10 11 but limited evidence exists on the effect of LTPA in populations where it is most relevant, namely in the general population that is enriched with obese and elderly people who are more prone to develop HFpEF. Therefore, associations between LTPA and diastolic function needs further research. Particularly MRI-quantified diastolic function measures are preferred as MRI provides superior readouts of diastolic function in comparison to echocardiography,12 13 which is often used in population-based studies.14–17
Besides LTPA, metabolic health is an important determinant associated with cardiovascular health benefits. Obesogenic behaviours, such as physical inactivity and an unhealthy diet, frequently lead to the metabolic syndrome (MetS), a cluster of cardiometabolic risk factors that often coincide. It is characterised by derangements of waist circumference, systolic and diastolic blood pressure, blood glucose, high-density lipoprotein cholesterol (HDL-c) and triglycerides.18 However, its definition is hampered by dichotomisation of indicators, as well as sex-specific differences in body fat distribution. Evidence whether visceral adipose tissue (VAT) or subcutaneous adipose tissue (SAT) is more important in the deterioration of diastolic function remains inconclusive and the role of MetS as a comorbidity needs further investigation.19–21 Thus, to fully understand the biological mechanisms on how LTPA affects cardiac health and in particular diastolic function, methods using full continuous information of the MetS are essential.
Confirmatory factor analysis (CFA) is an unsupervised dimensionality reduction technique often used in machine learning models, that does not require the categorisation of continuous information and can test the appropriateness of (group-)specific latent structures.22 23 Recently it has been shown that sex-specific metabolic impairment can be modelled with a one-factor approach using CFA with MetS indicators.24 Model fits improved when MRI-derived SAT and VAT were added obsoleting waist circumference.25 Importantly, the latent metabolic load factor was associated with reduced diastolic function, which was more pronounced in women. To investigate to what extent metabolic impairment mediates the association between LTPA and diastolic function, we aimed to use a one-factor CFA approach of metabolic load including MRI-derived SAT and VAT. A huge advantage using CFA in mediation analysis is that multiple observed variables are used as mediators of metabolic health and reduced to one latent factor, unlike standard mediation analysis where only one observed variable is used as mediator.
The aim of this study was therefore to investigate the association between LTPA and MRI-derived diastolic function in a large-scale population-based study. Importantly a comprehensive mediation analysis using a latent metabolic load factor as mediator in a structural equation model (SEM) was used. We defined the metabolic load factor using CFA which included continuous information of MetS indicators and MRI-derived SAT and VAT. We hypothesised that LTPA-related improvements in diastolic function are partially mediated by metabolic load.
Materials & methods
Study design and population
The Netherlands Epidemiology of Obesity (NEO) study is a population-based, prospective cohort study designed to investigate pathways that lead to obesity-related diseases. Between 2008 and 2012, 6671 individuals aged 45–65 years were included, with an oversampling of individuals with overweight or obesity. The study design and population characteristics have been described in detail elsewhere.26 Briefly, men and women living in the area of Leiden were invited to participate, aged between 45 and 65 years and had a self-reported body mass index (BMI) of 27 kg/m2 or higher. In addition, all inhabitants aged between 45 and 65 years from one municipality (Leiderdorp) were invited to participate, irrespective of their BMI, allowing for a reference distribution of BMI. Participants were predominantly white, therefore it is unclear whether our findings might generalise to different age and ethnic groups. The Medical Ethical Committee of the Leiden University Medical Center (LUMC) approved the design of the study (Protocol number: P08.109, date of approval: 4 August 2008). All participants provided written informed consent.
Data collection
Participants came to the NEO study centre of the LUMC for a study visit after an overnight fast of ≥10 hours. Prior to this study visit, participants completed a questionnaire at home to report demographic, lifestyle and clinical information. Participating individuals reported sex (women/men), ethnicity (white/other), tobacco smoking (never/former/current) and education (high/other). Anthropometry was acquired and questionnaires were used to report demographic, lifestyle and clinical information. Research nurses recorded names and dosages of current medication used in the month preceding the study visit. Exclusion criteria were use of systemic inflammation-lowering medication and a history of cardiovascular disease (myocardial infarction, angina, congestive heart failure, stroke or peripheral vascular disease). After excluding individuals not eligible for imaging determined by MRI contraindications (claustrophobia or metallic devices), a random subset underwent abdominal and cardiac MRI. The final study population was determined after exclusions, as shown in figure 1.
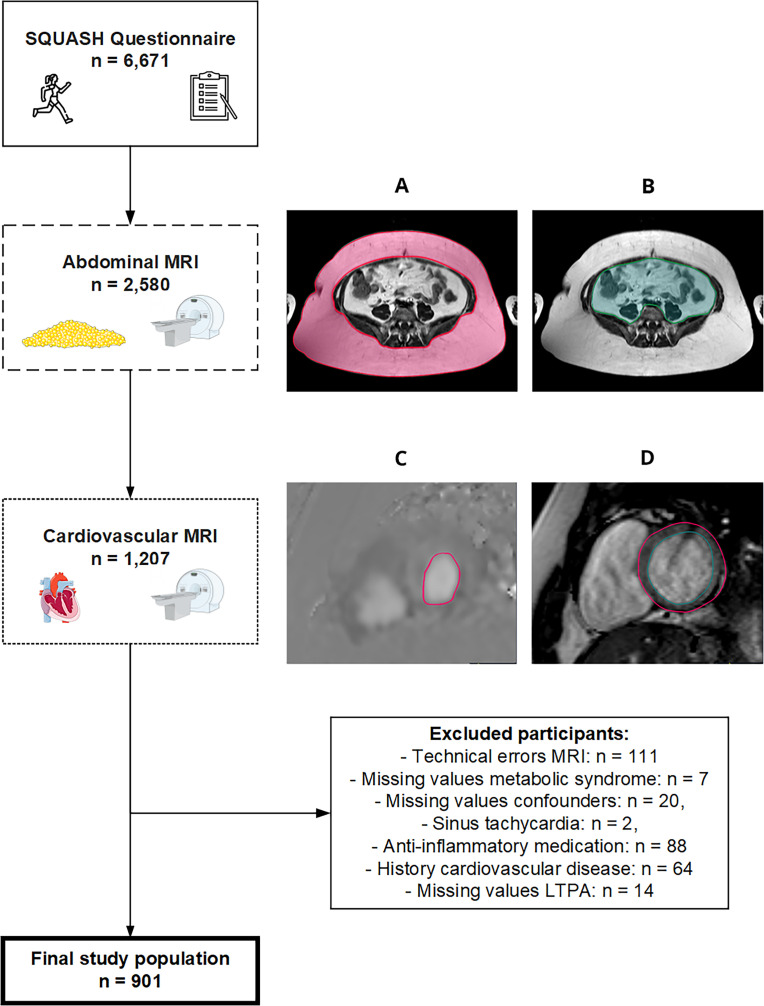
Figure 1.
Subject inclusion and exclusion flowchart. The total Netherlands Epidemiology of Obesity study population included 6671 participants of whom 1207 participants satisfied the inclusion criteria that they received both abdominal and cardiovascular MRI. Participants were additionally excluded for reasons of (1) missing data of n=111 due to technical errors in the E/A ratio, E-DT, LV mass, LV end-systolic and end-diastolic volume, VAT or SAT quantification, (2) n=7 due to missing data in one of the MetS variables, (3) n=20 due to missing data in confounders, (4) n=2 due to sinus tachycardia,46 (5) n=88 due to usage of systemic inflammation-lowering medication, (6) n=64 due to a history of cardiovascular disease and (7) n=14 due to missing data in LTPA. The remaining study population therefore comprised n=901 participants. E-DT, E-deceleration time; LV, left ventricle; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Metabolic syndrome indicators
Waist circumference was quantified in the middle of the lowest rib and the iliac crest. Blood samples to quantify glucose, triglycerides and HDL-c were acquired after a 10 hours overnight fast with standard methods at the LUMC central clinical chemistry laboratory.26 Blood pressure was measured during the morning of the baseline visit, after drawing blood samples and prior to the MRI examinations. Three consecutive seated systolic and diastolic blood pressure measurements with 5-min rest in between were averaged and calculated (OMRON, Model M10-IT, Omron Health Care, Illinois, USA).26
Leisure time physical activity
LTPA was assessed using the Short Questionnaire to Assess Health-enhancing physical activity (SQUASH), which includes questions on activities regarding a normal week in recent months.27 For each activity during leisure time, participants reported frequency, duration and intensity in minutes per week and all activities were converted to age-specific metabolic equivalent tasks (METs).28 1 MET equals the absolute energy expenditure while awake and sitting quietly. Specifically 1 MET equals 3.5 mL oxygen consumption per kilogram body weight per minute.29 Various activities are divided into light 1.5<3.0 METs; moderate 3.0–5.9 METs and vigorous ≥6.0 METs categories. It has been shown that primarily moderate-to-vigorous LTPA health benefits are most pronounced and therefore these have been included in the current analysis.2
Magnetic resonance imaging
MRI was performed on a 1.5 T MRI scanner (Philips Medical Systems, Best, The Netherlands) with a 16-channel phased-array coil. Details on the image acquisition settings are provided as online supplemental material. Analysis of the MR images was conducted in the time period of 2012–2014. To analyse diastolic function, we included the deceleration time of the early phase of transmitral flow (E-DT) and the ratio of the mitral early and late peak filling rates (E/A ratio) as primary endpoints. The E/A ratio and E-DT were determined using an electrocardiographically (ECG) prospectively gated gradient echo sequence with velocity encoding over the mitral valve in 40 cardiac phases. An ECG prospective gated breath-hold balanced steady-state free precession sequence was used in standard long-axis orientations, and for a stack of short-axis cines. From these, indices of cardiac morphology were acquired including LV mass, end-systolic/end-diastolic volume, stroke volume, ejection fraction and cardiac output (online supplemental material). To analyse adiposity, we included abdominal VAT and SAT, which were determined by transforming the number of pixels to square centimetre using a turbo spin echo sequence. Three slices were acquired and averaged at the level of the fifth lumbar vertebra.
bmjsem-2023-001778supp001.pdf (10KB, pdf)
bmjsem-2023-001778supp002.pdf (53.2KB, pdf)
Statistical analyses
To correctly represent baseline associations in the general population, adjustments were made for the oversampling of individuals with BMI ≥27 kg/m2.30 This was done by weighting all participants towards the BMI distribution of participants from the Leiderdorp municipality, whose BMI distribution was similar to the BMI distribution in the general Dutch population.31 32 Consequently, all results apply to a population-based study without oversampling of individuals with a high BMI. Baseline characteristics are presented as mean (SD), median (IQR) or as percentage, and stratified by sex. CFA was used as a measurement model to construct a metabolic load factor. CFA is a form of factor analysis that tests whether a postulated latent measurement model befits the observed data. First, all of the MetS variables and MRI-derived abdominal VAT and SAT were included to analyse the individual factor loadings. Consequently, a selection of included variables which loaded, that is, correlated significantly was made (p<0.05), defining indicators of metabolic load. Model fits were assessed using a comparative fit index (CFI), the root mean square error of approximation (RMSEA) and the standardised root mean square residual (SRMR). Indication of goodness of model fitting was CFI ≥0.95, RMSEA ≤0.05 and SRMR ≤0.05. Stepwise exclusion of indicators with lowest loading was carried out until the model fit did not improve any further. An evaluation of measurement invariance was carried out to examine presence of sex-specific difference on loadings of each single indicator of the metabolic load factor. We used a piecewise SEM approach, combining individual models with estimated paths merged to construct a mediation model.23 Hereby we examined associations between LTPA, diastolic function and cardiac morphology and haemodynamics. Subsequently, metabolic load was added to examine mediation. All analyses were performed separately for men and women. Associations are presented as regression coefficients (β) with 95% CIs per SD of the determinant. Variables were transformed into standardised scores with mean of 0 and SD of 1. The crude model was adjusted for the potential confounding factors age, ethnicity, education and tobacco smoking. Unadjusted values for multiple comparisons with p<0.05 were considered significant. Bonferroni-corrected significance levels depicted in italic are also given. Analyses were conducted in accordance to the CHecklist for statistical Assessment of Medical Papers (the CHAMP) guidelines.33 For all statistical analyses R V.4.2.1 was used.
Patient and public involvement
The research was conducted without the involvement of patients or the general public in the study design, data analysis, writing, or editing.
Results
Baseline characteristics
A total of 6671 participants were included in the NEO study. Of those, 1207 participants were randomly allocated to undergo cardiovascular and abdominal MRI. After excluding participants with invalid MRI images, missing covariates or medical conditions (see figure 1), data from n=901 participants were included in the present study.
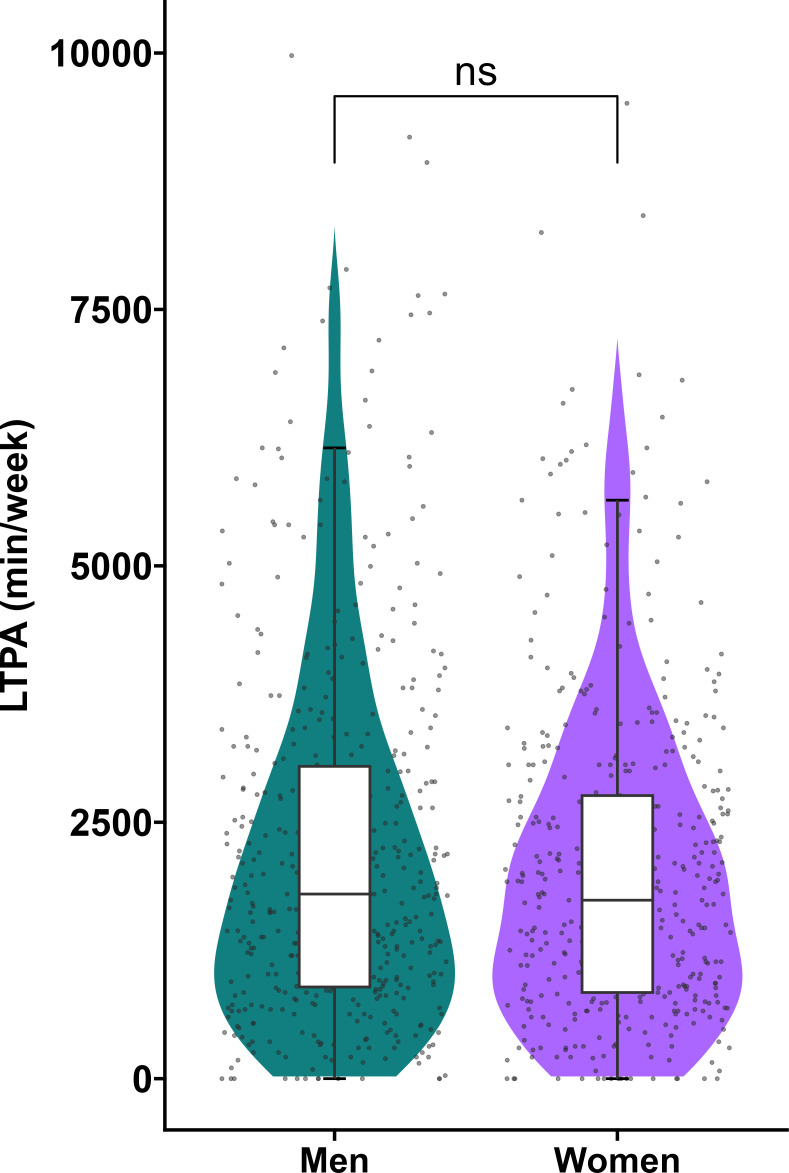
Table 1 shows the demographic and lifestyle characteristics of the study population stratified by sex. Mean (SD) age was 56 (6) years, 46% were women and mean BMI was 26 (3.9) kg/m2. Participants engaged a median (25th, 75th percentile) of 1928 (968, 3195) MET min/week in LTPA (figure 2). There was no difference in LTPA between men and women. When participants were categorised according to the US guidelines only 5% was considered inactive (0 MET-min/week), 8% low active (1–499 MET-min/week), 16% moderately active (500–1000 MET-min/week) and 71% highly active (>1000 MET-min/week).2 All of the MetS variables were significantly different between men and women. Table 2 shows the cardiac and abdominal MRI variables stratified by sex. With the exception of the E/A ratio 1.3 (0.5) and E-DT 182.7 (47.1) ms, all variables were different between men and women.
Table 1.
Baseline study population characteristics
| Characteristics | Total population | Men (54%) | Women (46%) |
| Age (years) | 56 (6) | 55 (7) | 56 (6) |
| Education (% High) | 46 | 50 | 43 |
| Ethnicity (% white) | 97 | 96 | 98 |
| Weight (kg) | 78.2 (14.8) | 86.8 (12.5) | 70.7 (12.4) |
| Body mass index, (kg/m2) | 26 (3.9) | 27 (3.3) | 25 (4.3) |
| Smoking | |||
| Current (%) | 13 | 14 | 12 |
| Previous (%) | 44 | 44 | 44 |
| Never (%) | 43 | 42 | 44 |
| LTPA (MET-min/week) | 2347 (1933) 1928 (968–3195) |
2460 (1999) 2100 (1050–3240) |
2249 (1870) 1830 (900–3125) |
| Inactive: 0 MET-min/w (%) | 5 | 5 | 7 |
| Low: 1–499 MET-min/w (%) | 8 | 9 | 7 |
| Moderate: 500–1000 MET-min/w (%) | 16 | 15 | 17 |
| High: > 1000 MET-min/w (%) | 71 | 71 | 70 |
| Waist circumference (cm) | 91.2 (12.4) | 97.4 (9.5) | 85.8 (12.2) |
| Systolic blood pressure (mmHg) | 130.6 (17.8) | 134.9 (15.9) | 126.9 (18.5) |
| Diastolic blood pressure (mmHg) | 83.5 (10.7) | 85.2 (10.4) | 82.0 (10.8) |
| Fasting glucose (mmol/l) | 5.5 (1.0) | 5.7 (1.1) | 5.3 (0.8) |
| HDL-c (mmol/l) | 1.5 (0.4) | 1.3 (0.3) | 1.7 (0.4) |
| Triglycerides (mmol/l) | 1.1 (0.8–1.5) | 1.3 (0.9–1.7) | 0.9 (0.7–1.4) |
| Central Obese (%) | 36 | 32 | 39 |
| Hypertension (%) | 61 | 69 | 55 |
| Disturbed glucose metabolism (%) | 30 | 37 | 23 |
| Low-HDL-c (%) | 16 | 17 | 14 |
| Hypertriglyceridemia (%) | 19 | 24 | 16 |
| Metabolic syndrome (%) | 23 | 28 | 19 |
| Diabetes mellitus (%) | 2.9 | 3.9 | 2.1 |
Results were weighted toward the BMI distribution of the general population. Data are presented as mean and standard deviation (SD) for continuous variables with a normal distribution, median (25th, 75th percentile) for continuous variables with a non-normal distribution or percentage (number).
HDL-c, high-density lipoproteins cholesterol; LTPA, leisure time physical activity.
Figure 2.
Leisure time physical activity. Distribution of LTPA (MET-min/week) in men (median: 2100 min, 1050 to 3240) and women (median 1830 min, 900 to 3126 min). ns=differences were non-significant.
Table 2.
Cardiovascular parameters
| Imaging measurements | Total population | Men | Women |
| Diastolic function | |||
| E/A ratio | 1.3 (0.5) | 1.3 (0.5) | 1.4 (0.5) |
| E-DT(ms) | 182.7 (47.1) | 181.5 (48.4) | 183.7 (46.0) |
| Cardiac morphology | |||
| LV mass (g) | 99.5 (25.8) | 118.6 (22.2) | 82.9 (15.1) |
| LV mass index (g/m2) | 51.1 (10.2) | 57.0 (9.4) | 46.1 (7.9) |
| Cardiac haemodynamic | |||
| LV end-systolic volume, (ml) | 54.2 (16.5) | 61.5 (17.7) | 48.0 (12.2) |
| LV end-diastolic volume (ml) | 148.1 (32.8) | 165.6 (32.9) | 133.0 (24.1) |
| LV stroke volume (ml) | 93.9 (20.9) | 104.1 (21.6) | 85.0 (15.7) |
| LV ejection fraction (%) | 63.6 (5.9) | 63.1 (6.4) | 64.1 (5.4) |
| Cardiac output (l/min) | 6.2 (1.4) | 6.8 (1.4) | 5.6 (1.1) |
| Adiposity | |||
| VAT (cm2) | 88.3 (53.9) | 113.0 (55.4) | 66.9 (42.3) |
| SAT (cm2) | 235.3 (94.0) | 207.6 (73.3) | 259.4 (103.0) |
Results were weighted toward the BMI distribution of the general population. Data are presented as mean (SD) for continuous variables with a normal distribution.
E-DT, E-wave deceleration time; LV, left ventricle; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Associations of LTPA with diastolic function
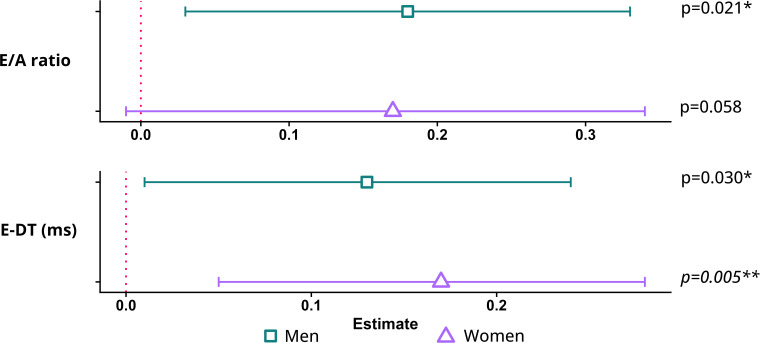
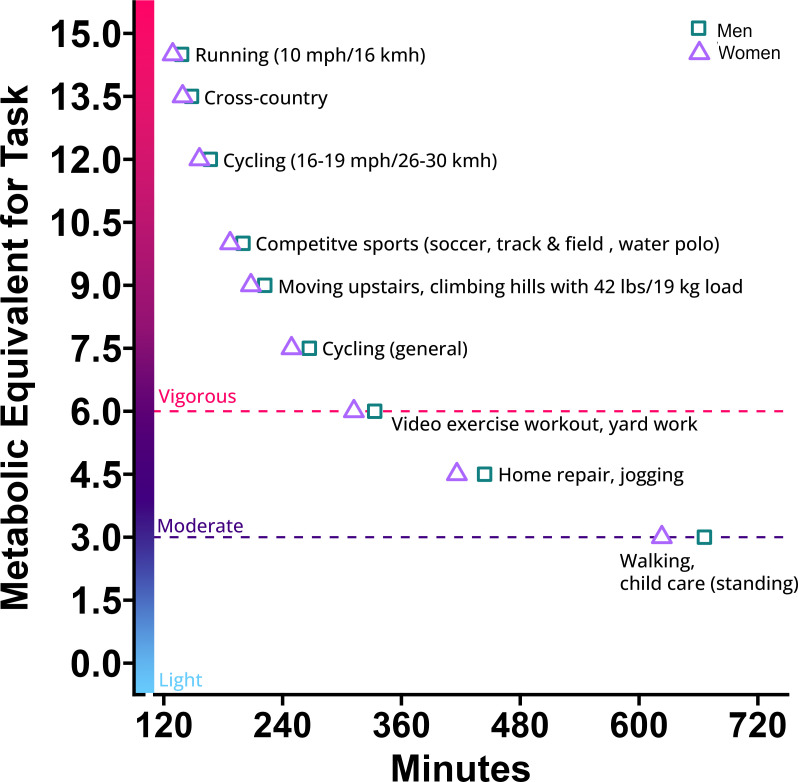
Table 3 and figure 3 present sex-specific crude and adjusted associations between LTPA and diastolic parameters. Figure 4 illustrates various activities that represent the proportion of 1 SD MET-minutes per week (men: 1999 min, women:1870 min) of moderate to vigorous LTPA. In the unadjusted model, LTPA was not associated with the E/A ratio for either men or women. After adjusting for confounders, each SD of LTPA was associated with a higher E/A ratio in men (β=0.18 SD; 95% CI = 0.03 to 0.33, per SD LTPA). LTPA was associated with E-DT for both men and women (men: β=0.11 SD; 95% CI = 0.00 to 0.24, women: β=0.11 SD; 95% CI=0.00 to 0.22) per SD LTPA. This association sustained after adjustment with E-DT of 0.13 SD (95% CI=0.01 to 0.24) higher in men and 0.17 SD (95% CI=0.05 to 0.28) higher in women. Results from sensitivity analyses, after exclusion of participants with diabetes mellitus, are presented in online supplemental table S2.
Table 3.
Associations of LTPA (MET-min/week) with diastolic function
| Men | Women | |||||
| Outcome | β | 95% CI | P value | β | 95% CI | P value |
| Diastolic function | ||||||
| E/A ratio | ||||||
| Crude | 0.09 | −0.04 to 0.23 | 0.167 | 0.05 | −0.18 to 0.28 | 0.682 |
| Adjusted | 0.18 | 0.03 to 0.33 | 0.021 | 0.17 | −0.01 to 0.34 | 0.058 |
| E-DT (ms) | ||||||
| Crude | 0.11 | 0.00 to 0.21 | 0.041 | 0.11 | 0.00 to 2.02 | 0.045 |
| Adjusted | 0.13 | 0.01 to 0.24 | 0.030 | 0.17 | 0.05 to 0.28 | 0.005 |
Results represents linear regression coefficients and 95% CI, weighted toward the BMI distribution of the general population. β, regression coefficients reflect the difference in outcome per 1 SD of leisure time physical activity (LTPA) in MET-minutes/week: 1999 min in men/1870 min in women. Crude models were adjusted for age, sex, smoking, ethnicity and education.
Unadjusted values for multiple comparisons with p<0.05 were considered significant.
Italic depicted values meet the Bonferroni corrected significance level of p<0.004.
E-DT, E-wave deceleration time; LV, left ventricle; ms, milliseconds.
Figure 3.
Associations of LTPA with diastolic function. Plots representing linear regression coefficients and 95% CIs adjusted for age, sex, smoking, ethnicity and education. Regression coefficients reflect the difference in outcome per 1 SD of LTPA in MET-minutes/week: 1999 min in men/1870 min in women. Green=men, purple=women. Unadjusted values for multiple comparisons with p<0.05 were considered significant where *p<0.05, **p<0.01. Italic depicted values meet the Bonferroni-corrected significance level of p<0.0167. E-DT, E-deceleration time; LTPA, leisure time physical activity.
Figure 4.
Various activities that represent 1 SD of MET minutes (men=1999 min, women=1870 min) per week of moderate (3–6 METs, deep purple) to vigorous (>6 METs, red) leisure time physical activity. Running (10 mph/16 kmh=14.5 METs, cross-country=13.5 METs, cycling (16–19 mph/26–30 kmh)=12 METs, competitive sports=10 METs, moving upstairs, etc=9 METs, cycling (general)=7.5 METs, video exercise workout, etc=6 METs, home repair, etc=4.5 METs, walking, etc=3 METs. kmh, kilometres per hour; MET, metabolic equivalent of task; mph, miles per hour.
Online supplemental table S1 presents sex-specific crude and adjusted associations between LTPA (per SD) and cardiac morphology and haemodynamics parameters. In the unadjusted model, LTPA was associated with all variables except LV ejection fraction in both men and women and LV end-systolic volume, LV end-diastolic volume, LV stroke volume and LV cardiac output in women. These associations persisted after adjustment where other variables were associated to as follows: (men/women) LV mass—β=0.11/0.02 SD; 95% CI=0.03 to 0.19/0.02 to 0.18, LV mass index—β=0.16/0.17 SD; 95% CI=0.07 to 0.42/0.27 to 3.18, LV end-systolic volume—β=0.15/0.10 SD; 95% CI=0.03 to 0.26/0.02 to 0.18, LV end-diastolic volume: β=0.21/0.11 SD; 95% CI=0.15 to 0.38/0.02 to 0.22 and the LV stroke volume: β=0.21 SD; 95% CI=0.10; 0.31 and LV cardiac output in men: β=0.11 SD; 95% CI=0.03 to 0.20.
CFA of metabolic load
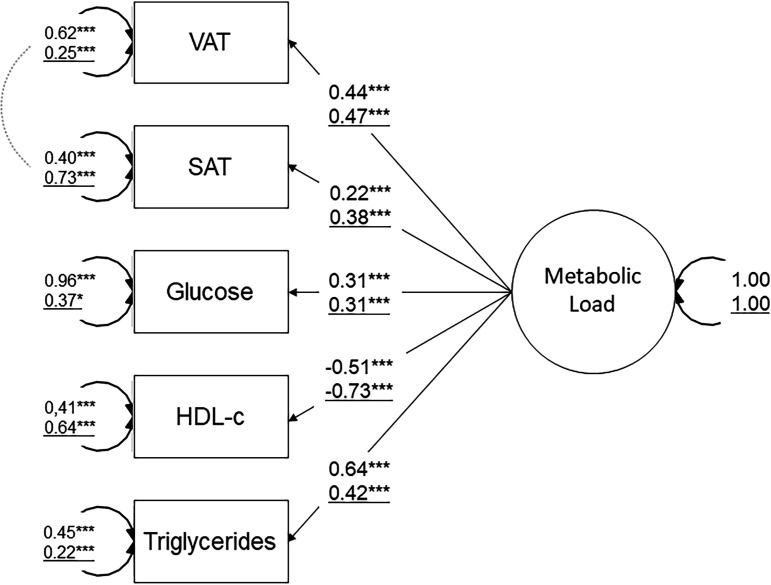
According to previous results,25 fitting of CFA with glucose, HDL-c, triglycerides and MRI-derived VAT and SAT was considered good (CFI=0.948; RMSEA=0.073; SRMR=0.039) and all indicators loaded significantly on the metabolic load factor (p<0.05): (standardised estimates: VAT=0.69, SAT=0.19, glucose=0.42, HDL-c=−0.71 and triglycerides=0.65). By assessing measurement of invariance, we found that the chosen indicators to define metabolic load displayed sex-specific differences. The model fit was not preserved after constraining the factor loadings to be equal across sex, thereby failing metric invariance (difference in robust CFI=−0.024, p<0.001). Figure 5 illustrates the standardised estimates stratified on sex.
Figure 5.
Confirmatory factor analysis of metabolic load. The schematic shows the proposed sex-specific one-factor structure approach of metabolic load and outcome values. Adjusting for potential confounders was made by including age, ethnicity, education and smoking. Standardised parameter estimates representing factor loadings (ie, correlation coefficients) are shown on the arrow pathways. Numbers on the paths refer to the men (top) and women (bottom/underline) group. Double headed arrows indicate covariance and error terms of indicators. Coefficients were significant at *p<0.05, **p<0.01, ***p<0.001. HDL-c, high-density lipoprotein cholesterol; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
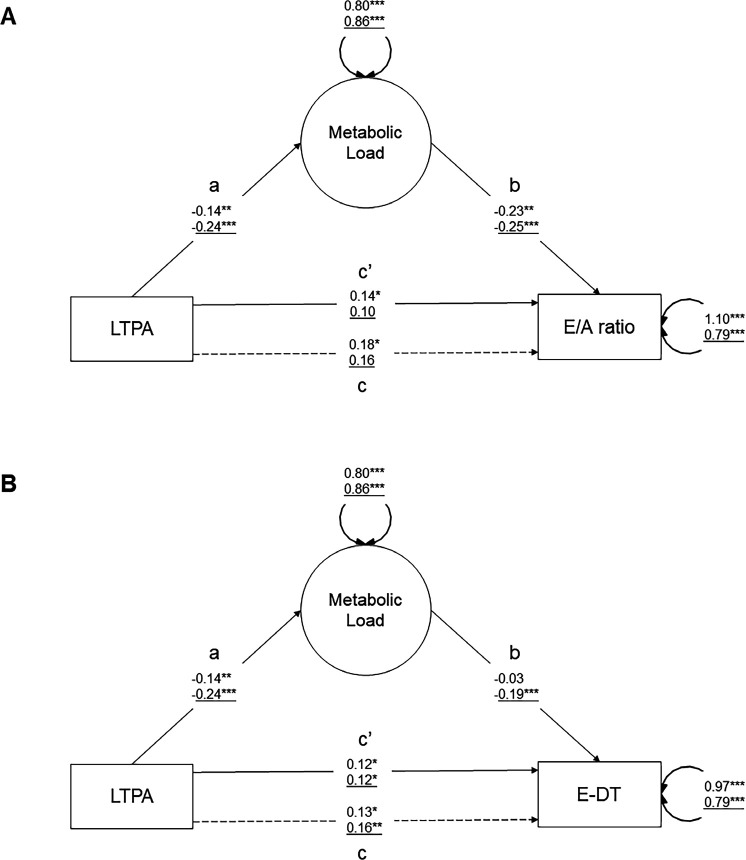
Mediating role of the metabolic load factor
The association between LTPA and diastolic function was partially mediated via metabolic load in men and women (figure 6a/b and table 4). There was an indirect effect on the E/A ratio of 0.034 SD (95% CI=0.000 to 0.067, 19% mediated, p=0.047) for men, but not for women (no association LTPA–E/A ratio, p=0.058). There was an indirect effect on E-DT of 0.044 SD (95% CI=0.013 to 0.075, 27% mediated, p=0.006) for women, but not in men. Online supplemental table S3 summarises the sex-specific mediation effect of metabolic load on cardiac morphology and haemodynamics. For cardiac morphology, metabolic load mediated partially the effect of LTPA on LV mass (men/women: –0.020/–0.018 SD, 95% CI=−0.037 to −0.004/−0.035 to −0.001, 18%/18% mediated, p=0.017/0.033). For cardiac haemodynamics, no mediation of metabolic load appeared except for LV cardiac output in men (−0.021 SD, 95% CI=−0.039 to −0.002, 18% mediated, p=0.027).
Figure 6.
Mediation analysis of metabolic load between LTPA and diastolic function. The schematics show the structure and outcomes of the mediation analysis between LTPA and (A) E/A ratio and (B) ED-T. Adjusting for potential confounding was made by including age, ethnicity, education and smoking. Standardised parameter estimates representing factor loadings (ie, correlation coefficients) are shown on the arrow paths. Numbers on paths refer to the men (top) and women (bottom/underline) group. Double headed arrows report on covariance and error terms of the indicators. The mediation models decomposes the total effect c, into the direct effect a*b and the direct effect c′. The total effect can be described as c=c′+ab, and the indirect effect as a*b=c–c′. Coefficients were significant at *p<0.05, **p<0.01, ***p<0.001. E-DT, E-deceleration time; EDV, left ventricular end diastolic volume; LTPA, leisure time physical activity (MET-min/week); LV, left ventricle.
Table 4.
Mediation of the association between LTPA and diastolic function by metabolic load
| Men | Women | |||||||
| Outcome | Indirect effect | 95% CI | Proportion mediated (%) | P value | Indirect effect | 95% CI | Proportion mediated (%) | P value |
| Diastolic function | ||||||||
| E/A ratio | 0.030 | 0.000 to 0.067 | 19 | 0.047 | 0.058 | 0.027 to 0.089 | – | <0.001 |
| E-DT (ms) | 0.004 | −0.012 to 0.021 | 0.602 | 0.044 | 0.013 to 0.075 | 27 | 0.006 | |
Results represents percentage mediation of total effect, weighted toward the BMI distribution of the general population in the adjusted models. Besides presented mediator, the model was also adjusted for age, sex, smoking, ethnicity and education.
Unadjusted values for multiple comparisons with p<0.05 were considered significant.
Italic depicted values meet the Bonferroni corrected significance level of p<0.0167.
E-DT, E-wave deceleration time; LV, left ventricle.
Discussion
In this study, we have shown in a general middle-aged population that increased LTPA is associated with several parameters of diastolic function as well as cardiac morphology and haemodynamics. More importantly we showed that these positive associations between LTPA and diastolic function were mediated by beneficial changes in metabolic health.
Our results show that the positive association between LTPA and diastolic function is a complex process consisting of various direct cardiac and indirect metabolic pathways. Exercise-induced cardiac remodelling is caused by integrative metabolic changes combined with augmented cardiac workload.34 Our study therefore corroborates with previous research which showed that increased doses of LTPA were associated with favourable LV diastolic function. Measures of diastolic function, such as the E/A ratio, increased with higher doses of LTPA.14 Also, ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (e'), an indicator for LV filling pressures, was higher in low-dose LTPA participants.14–17 Though less pronounced, we have shown a prolonged E-DT in high-dose LTPA participants which could be due to lower LV filling pressures. Improved diastolic function via increased LTPA might be explained by altered haemodynamic stress inducing favourable cardiac remodelling.35
Our study showed that an unsupervised one-factor approach of metabolic load partly mediates the association between LTPA and diastolic function. It has been shown that metabolic health improves by LTPA: according to a comprehensive meta-analysis, a larger amount of LTPA decreased the risk of developing the MetS.36 This is in concordance with our results that LTPA was associated with lower metabolic load in both men and women (pathway ‘a’ in figure 6). However, studies that have investigated the mediating role of metabolic health in the relation between LTPA and diastolic function are lacking to our knowledge. Though inference has been made on unadjusted values, which might be perceived as p-hacking, our present study is of exploratory nature with a clear theoretical model and hypothesis making p values less stringent.37 We observed a mediation effect of metabolic load that accounted for 19%–27% of the total association between LTPA and diastolic function. We can speculate that other lifestyle interventions such as certain nutritional strategies including caloric restrictions, which can positively influence indicators of the metabolic load factor and increase metabolic health, could be beneficial for diastolic function.38 39
Potentially, future work could focus on wearable quantified LTPA by accelerometry and cardiac devices like the Actiheart to improve the accuracy of the physical activity readouts.40 Self-reported LTPA via the SQUASH questionnaire can vary considerably within subjects compared with wearables.41 Also, self-reported intensity is challenging to quantify, where low-intensity PA seems to be underestimated and moderate/high-intensity PA overestimated. LTPA determined by accelerometry allows a more precise determination of intensity and duration of physical activity. This may be important as specifically high-intensity activity appears to be associated with greatest improvements in metabolic and cardiac health.42 To highlight physiological mechanisms responsible for associations between LTPA and diastolic function, future research should also include overall fitness parameters. Currently, it can be hypothesised that LTPA in combination with higher muscle mass leads to a different (diastolic) cardiac workload response than in lower muscle mass participants. Nonetheless, this can only be speculated from the NEO dataset. Thus future work should also quantify overall fitness using, for example, maximal oxygen consumption cardiopulmonary exercise testing43 or even wearable lactate sensors.44
Clinical implications
This study yields important insights into the relationship among LTPA, MRI-based diastolic function and metabolic health within a substantial Dutch middle-aged cohort in both men and women. The findings demonstrate an association between more LTPA and better MRI-derived diastolic function, as well as a reduced metabolic load encompassing MRI-based subcutaneous and visceral fat, glucose, triglycerides and HDL-c. Importantly, this study unveils the pivotal role of metabolic load, as determined through CFA, in mediating the association between LTPA and diastolic function across both men and women. Thus, encouraging LTPA among middle-aged individuals is an effective strategy for improving diastolic function and metabolic health. Interventions, alongside LTPA, that alleviate metabolic load might address a considerable proportion of diastolic function.
Limitations
Besides strengths which are a large sample size, MRI-based cardiac and adiposity measurements and a comprehensive state-of-the-art statistical method to model metabolic load, we are aware that our research also has limitations. Our analysis is based on cross-sectional observational data which hinders inferring causality. Further, LTPA quantification via self-reported questionnaires may have considerable variation in the test–retest reliability and validity.41 Moreover, although we assessed diastolic function using MRI, which is more precise than echocardiography techniques, ideally, a multiparametric approach should be used which includes more specific cardiac variables, such as the E/e' ratio, to assess diastolic function.45 However this was not a standard procedure at the time of segmenting and analysing the MR images in this study (2012–2014). Lastly, various other studies have proposed that metabolic dysregulation consists of multiple latent factors.22 23 Nevertheless, these studies included substantially more variables. Our one-factor approach provides support by its fit indices and was stable across sexes.
Conclusion
A larger amount of LTPA was associated with improved diastolic function where CFA-based metabolic load partly mediates this effect. Interventions, alongside LTPA, that alleviate metabolic load might address a considerable proportion of diastolic function. Whereas these analyses were cross-sectional in nature, future studies should investigate whether improving indicators of metabolic load will benefit healthy diastolic function even more.
Acknowledgments
We express our gratitude to all individuals who participated in the Netherlands Epidemiology of Obesity (NEO) study. We are grateful to all participating general practitioners for inviting eligible participants and R L Widya, J Amersfoort, E Ghariq, M F Rodrigues and R P B Tonino, all Leiden University Medical Center, for their help in MR data collection.
Footnotes
Contributors: HK performed data cleaning and analysis, interpreted the data, drafted and finalised the manuscript and is responsible for the overall content as guarantor. JHPMvdV and CFWP were involved in analysing and interpreting the data. IAD, HJL, SMB and GJS participated in study conception and interpretation of the data. JWJ, FRR, RdM and HJL contributed to the conception and design of the NEO Study and the research described in this manuscript, contributed to the acquisition and interpretation of the data and revised the manuscript critically for important intellectual content. TL and MF helped to draft the manuscript. All authors provided final approval of the version to be published.
Funding: The NEO study is supported by the participating departments, the Division and the Board of Directors of the Leiden University Medical Centre, and by the Leiden University, Research Profile Area ‘Vascular and Regenerative Medicine’. This work was supported by a grant (CHANCE, #14741) from the Dutch Heart Foundation and the Netherlands Organization for Scientific Research (NWO), domain Applied and Engineering Sciences (TTW).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Access to the raw data supporting the conclusions of this article can be granted by the authors upon a reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The Medical Ethical Committee of the Leiden University Medical Center (LUMC) approved the design of the study (Protocol number: P08.109, date of approval: 4 August 2008). Participants gave informed consent to participate in the study before taking part.
References
- 1. Kohl HW, Craig CL, Lambert EV, et al. The pandemic of physical inactivity: global action for public health. Lancet 2012;380:294–305. 10.1016/S0140-6736(12)60898-8 [DOI] [PubMed] [Google Scholar]
- 2. Piercy KL, Troiano RP. Physical Activity Guidelines for Americans From the US Department of Health and Human Services. Circ Cardiovasc Qual Outcomes 2018;11:e005263. 10.1161/CIRCOUTCOMES.118.005263 [DOI] [PubMed] [Google Scholar]
- 3. Eijsvogels TMH, George KP, Thompson PD. Cardiovascular benefits and risks across the physical activity continuum. Curr Opin Cardiol 2016;31:566–71. 10.1097/HCO.0000000000000321 [DOI] [PubMed] [Google Scholar]
- 4. Pedisic Z, Shrestha N, Kovalchik S, et al. Is running associated with A lower risk of all-cause, cardiovascular and cancer mortality, and is the more the better? A systematic review and meta-analysis. Br J Sports Med 2020;54:898–905. 10.1136/bjsports-2018-100493 [DOI] [PubMed] [Google Scholar]
- 5. Hegde SM, Claggett B, Shah AM, et al. Physical Activity and Prognosis in the TOPCAT Trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist). Circulation 2017;136:982–92. 10.1161/CIRCULATIONAHA.117.028002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kraigher-Krainer E, Lyass A, Massaro JM, et al. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study. Eur J Heart Fail 2013;15:742–6. 10.1093/eurjhf/hft025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pandey A, LaMonte M, Klein L, et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol 2017;69:1129–42. 10.1016/j.jacc.2016.11.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pandey A, Parashar A, Kumbhani D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 2015;8:33–40. 10.1161/CIRCHEARTFAILURE.114.001615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 10. Galanti G, Stefani L, Mascherini G, et al. Left ventricular remodeling and the athlete’s heart, irrespective of quality load training. Cardiovasc Ultrasound 2016;14:46. 10.1186/s12947-016-0088-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pluim BM, Zwinderman AH, van der Laarse A, et al. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 2000;101:336–44. 10.1161/01.cir.101.3.336 [DOI] [PubMed] [Google Scholar]
- 12. de Haan S, de Boer K, Commandeur J, et al. Assessment of left ventricular ejection fraction in patients eligible for ICD therapy: Discrepancy between cardiac magnetic resonance imaging and 2D echocardiography. Neth Heart J 2014;22:449–55. 10.1007/s12471-014-0594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gruszczyńska K, Krzych LJ, Gołba KS, et al. Statistical agreement of left ventricle measurements using cardiac magnetic resonance and 2D echocardiography in ischemic heart failure. Med Sci Monit 2012;18:MT19–25. 10.12659/msm.882507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hegde SM, Gonçalves A, Claggett B, et al. Cardiac structure and function and leisure-time physical activity in the elderly: The Atherosclerosis Risk in Communities Study. Eur Heart J 2016;37:2544–51. 10.1093/eurheartj/ehw121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heitmann KA, Løchen M-L, Hopstock LA, et al. Cross-sectional associations between accelerometry-measured physical activity, left atrial size, and indices of left ventricular diastolic dysfunction: The Tromsø Study. Prev Med Rep 2021;21:101290. 10.1016/j.pmedr.2020.101290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joseph G, Mogelvang R, Biering-Sørensen T, et al. The association between physical activity and cardiac performance is dependent on age: the Copenhagen City Heart Study. Int J Cardiovasc Imaging 2019;35:1249–58. 10.1007/s10554-019-01566-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brinker SK, Pandey A, Ayers CR, et al. Association of cardiorespiratory fitness with left ventricular remodeling and diastolic function: the Cooper Center Longitudinal Study. JACC Heart Fail 2014;2:238–46. 10.1016/j.jchf.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 2004;24:e13–8. 10.1161/01.ATV.0000111245.75752.C6 [DOI] [PubMed] [Google Scholar]
- 19. Sawada N, Daimon M, Kawata T, et al. The Significance of the Effect of Visceral Adiposity on Left Ventricular Diastolic Function in the General Population. Sci Rep;9:4435. 10.1038/s41598-018-37137-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borlaug BA, Reddy YNV. Getting at the Heart of Central Obesity and the Metabolic Syndrome. Circ Cardiovasc Imaging 2016;9:10.1161/CIRCIMAGING.116.005110 e005110. 10.1161/CIRCIMAGING.116.005110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Canepa M, Strait JB, Milaneschi Y, et al. The relationship between visceral adiposity and left ventricular diastolic function: results from the Baltimore Longitudinal Study of Aging. Nutr Metab Cardiovasc Dis 2013;23:1263–70. 10.1016/j.numecd.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen B-J, Todaro JF, Niaura R, et al. Are metabolic risk factors one unified syndrome? Modeling the structure of the metabolic syndrome X. Am J Epidemiol 2003;157:701–11. 10.1093/aje/kwg045 [DOI] [PubMed] [Google Scholar]
- 23. Peeters CFW, Dziura J, van Wesel F. Pathophysiological domains underlying the metabolic syndrome: an alternative factor analytic strategy. Ann Epidemiol 2014;24:762–70. 10.1016/j.annepidem.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 24. Düzel S, Buchmann N, Drewelies J, et al. Validation of a single factor representing the indicators of metabolic syndrome as a continuous measure of metabolic load and its association with health and cognitive function. PLoS One 2018;13:e0208231. 10.1371/journal.pone.0208231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klarenberg H, Dekkers IA, Peeters CFW, et al. Confirmatory factor analysis including MRI-derived adipose tissues quantification improves associations of metabolic dysregulation to diastolic dysfunction. J Diabetes Complications 2022;36:S1056-8727(22)00104-0. 10.1016/j.jdiacomp.2022.108202 [DOI] [PubMed] [Google Scholar]
- 26. de Mutsert R, den Heijer M, Rabelink TJ, et al. The Netherlands Epidemiology of Obesity (NEO) study: study design and data collection. Eur J Epidemiol 2013;28:513–23. 10.1007/s10654-013-9801-3 [DOI] [PubMed] [Google Scholar]
- 27. Wendel-Vos GCW, Schuit AJ, Saris WHM, et al. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 2003;56:1163–9. 10.1016/s0895-4356(03)00220-8 [DOI] [PubMed] [Google Scholar]
- 28. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–504. 10.1097/00005768-200009001-00009 [DOI] [PubMed] [Google Scholar]
- 29. Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol 1990;13:555–65. 10.1002/clc.4960130809 [DOI] [PubMed] [Google Scholar]
- 30. Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health 1991;81:1166–73. 10.2105/ajph.81.9.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lumley T. Analysis of Complex Survey Samples. J Stat Soft;9:19. 10.18637/jss.v009.i08 [DOI] [Google Scholar]
- 32. Ministerie van Volksgezondheid . Welzijn en Sport Nederland de Maat Genomen, Available: www.rivmnl/Onderwerpen/N/Nederland_de_Maat_Genomen
- 33. Mansournia MA, Collins GS, Nielsen RO, et al. A CHecklist for statistical Assessment of Medical Papers (the CHAMP statement): explanation and elaboration. Br J Sports Med 2021;55:1009–17. 10.1136/bjsports-2020-103652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fulghum K, Hill BG. Metabolic Mechanisms of Exercise-Induced Cardiac Remodeling. Front Cardiovasc Med 2018;5:127. 10.3389/fcvm.2018.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. Mar-Apr 2012;54:380–6. 10.1016/j.pcad.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 36. He D, Xi B, Xue J, et al. Association between leisure time physical activity and metabolic syndrome: a meta-analysis of prospective cohort studies. Endocrine 2014;46:231–40. 10.1007/s12020-013-0110-0 [DOI] [PubMed] [Google Scholar]
- 37. Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol 2001;54:343–9. 10.1016/s0895-4356(00)00314-0 [DOI] [PubMed] [Google Scholar]
- 38. Fontana L, Meyer TE, Klein S, et al. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A 2004;101:6659–63. 10.1073/pnas.0308291101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van de Rest O, Schutte BAM, Deelen J, et al. Metabolic effects of a 13-weeks lifestyle intervention in older adults: The Growing Old Together Study. Aging 2016;8:111–24. 10.18632/aging.100877 Available: https://www.aging-us.com/lookup/doi/10.18632/aging.v8i1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Velde JHPM, Boone SC, Winters-van Eekelen E, et al. Timing of physical activity in relation to liver fat content and insulin resistance. Diabetologia 2023;66:461–71. 10.1007/s00125-022-05813-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nicolaou M, Gademan MGJ, Snijder MB, et al. Validation of the SQUASH Physical Activity Questionnaire in a Multi-Ethnic Population: The HELIUS Study. PLoS One 2016;11:e0161066. 10.1371/journal.pone.0161066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laursen AH, Kristiansen OP, Marott JL, et al. Intensity versus duration of physical activity: implications for the metabolic syndrome. A prospective cohort study. BMJ Open 2012;2:e001711. 10.1136/bmjopen-2012-001711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. American Thoracic S. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am J Respir Crit Care Med 2003;167:211–77. 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 44. Song Z, Zhou S, Qin Y, et al. Flexible and Wearable Biosensors for Monitoring Health Conditions. Biosensors;13:630. 10.3390/bios13060630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. 10.1093/ehjci/jew082 [DOI] [PubMed] [Google Scholar]
- 46. Chung CS, Afonso L. Heart Rate Is an Important Consideration for Cardiac Imaging of Diastolic Function. JACC Cardiovasc Imaging 2016;9:756–8. 10.1016/j.jcmg.2015.10.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2023-001778supp001.pdf (10KB, pdf)
bmjsem-2023-001778supp002.pdf (53.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Access to the raw data supporting the conclusions of this article can be granted by the authors upon a reasonable request.