Abstract

Intermolecular (Coulombic) interactions are pivotal for aggregation, solvation, and crystallization. We demonstrate that the collective strong coupling of several molecules to a single optical mode results in notable changes in the molecular excitations around a single perturbed molecule, thus representing an impurity in an otherwise ordered system. A competition between short-range coulombic and long-range photonic correlations inverts the local transition density in a polaritonic state, suggesting notable changes in the polarizability of the solvation shell. Our results provide an alternative perspective on recent work in polaritonic chemistry and pave the way for the rigorous treatment of cooperative effects in aggregation, solvation, and crystallization.
The coherent interaction of molecules with confined optical modes leads to hybrid light–matter states called polaritons.1−3 In the strong coupling regime, usually achieved by coupling several molecules to the optical cavity, experiments show significant modifications of chemical properties.4−19 As a result of their delocalized nature, polaritons in quantum optics are studied from a collective perspective where the molecules are modeled as few-level systems that indirectly interact solely through the photon field.20−26 However, chemistry is governed by local interactions, and the molecular complexity requires refined chemical methods to analyze processes like reactions. The behavior of molecules is, hence, susceptible to their immediate surroundings. For example, their spectral absorption and emission can be altered by their interplay with the solvent (solvatochromic) or by the close proximity to identical molecules (concentration-dependent). Experiments under strong coupling often use organic molecules that are sensitive to their surroundings, show intense excitations, or even form aggregates.6−9,27−32 The chemical environment can then play an active role and exert significant influence, as emphasized by several vibrational strong coupling (VSC) experiments showing modification of assembly33−35 and reactivity.14,36,37 For a clear chemical understanding of polaritonic processes, we must then investigate the coexisting roles of the molecule, the solvent (chemical environment), and the cavity (optical environment). To this end, ab initio quantum electrodynamics (QED) merges the knowledge of quantum optics and quantum chemistry to explore single-molecule effects retaining the chemical complexity.38−50 Even if collective states can show larger contributions from selected molecules,45,51 local changes tend to reduce as a result of collective delocalization and an increasing number of quasi-dark states.52 While recent work indicates a larger relevance of dynamic electronic polarization,50,53,54 it remains puzzling how the collective nature of polaritons enters chemical processes. The role of changes in the chemical environment (solvents and aggregates) under strong coupling has been widely disregarded up to this point.
In this letter, we add another facet to the understanding of polaritonic chemistry by reintroducing the chemical environment. Using QED coupled cluster (QED-CC),46 which is at present the most accurate approach for medium-sized molecular polaritons, we study the yet unexplored competition between photon and intermolecular interactions. We extracted the single-molecule response in collective ensembles and investigated the microscopic changes of aggregates or solvation systems in polaritonic chemistry. We demonstrate local response modifications unambiguously distinguishable from collective delocalization arising from the interplay between Coulomb and transverse fields. Moreover, our results show a slower decrease than conventional local effects in polaritonics, i.e., not with the total number of coupled molecules but with the number of affected solvation shells. As a result, the immediate surroundings of the impurity, representing a solute, nucleation, or reaction center, undergo notable changes. Structural changes in the chemical environment can then influence the dynamics of the impurity. Our simple model, a stretched molecule embedded in a perfectly ordered environment, can represent a solute in a solvent, a nucleation center, or an impurity in an aggregate. Indeed, these physical realizations are associated with intermolecular (Coulombic) interactions with the surrounding chemical environment. Therefore, solvation, aggregation, and nucleation are used interchangeably in the following as a result of their conceptual similarity.
Modeling Solvation and Collective Coupling. Molecular
properties are significantly modified by chemical environments, such
as polymer matrices, solvents, or aggregates. The interaction of a
solute with its surrounding molecules leads to changes in the ground
and excited electronic densities, which affect energy levels, molecular
geometries, and even chemical reactivity. In addition, the solvent
also directly contributes to properties, such as spectroscopic signals.
To study the impact of polaritons on the complex chemical response,
we must simultaneously include photon coupling and Coulomb interactions
in the solute–solvent system. Therefore, we focus on a system
with non-negligible intermolecular interactions as a prototype for
Coulomb forces in supramolecular structures. Our model system is a
chain of N hydrogen molecules that form a H-aggregate
illustrated in Figure 1. An impurity (P), representing a solute or reactive molecule, is
introduced by stretching the bond of the central hydrogen, and we
investigate different intermolecular distances to alter the Coulomb
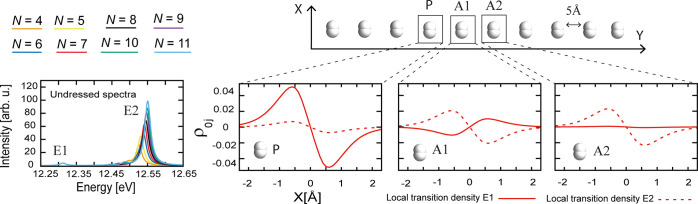
forces. Figure 1 shows
the absorption spectrum of the undressed (out-of-cavity) chain for
different N and 5 Å intermolecular separation.
The global response of the system is governed by the transition density
ϱj0(x, y, z), which is, for instance, connected
to the optical properties via the transition dipole moment 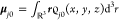 . To characterize
the local behavior of
each dimer, we integrate ϱj0(x, y, z) perpendicularly
to the excitation transition dipole moments (that is, in the y and z directions) around P, its nearest
neighbor A1 (first solvation shell), and its next nearest neighbor
A2. The integrated local transition densities ρIj0(x) = ∫+∞–∞ dz ∫Y(2)IY(1)I dy ϱj0(x, y, z) (I = P,
A1, and A2) for the excited states E1 and E2 are depicted in Figure 1. The excitation
E1 is mainly localized on P and A1 with anti-aligned local transition
dipoles, while E2 is delocalized and shows an all-parallel transition
moment alignment. In Figure 2, we show the polaritonic absorption spectrum with the photon
resonant to the (isolated) unperturbed H2 excitation with
light–matter coupling strength
. To characterize
the local behavior of
each dimer, we integrate ϱj0(x, y, z) perpendicularly
to the excitation transition dipole moments (that is, in the y and z directions) around P, its nearest
neighbor A1 (first solvation shell), and its next nearest neighbor
A2. The integrated local transition densities ρIj0(x) = ∫+∞–∞ dz ∫Y(2)IY(1)I dy ϱj0(x, y, z) (I = P,
A1, and A2) for the excited states E1 and E2 are depicted in Figure 1. The excitation
E1 is mainly localized on P and A1 with anti-aligned local transition
dipoles, while E2 is delocalized and shows an all-parallel transition
moment alignment. In Figure 2, we show the polaritonic absorption spectrum with the photon
resonant to the (isolated) unperturbed H2 excitation with
light–matter coupling strength 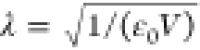 = 0.005 au, corresponding to an effective
mode volume V = 74.5 nm3, which is achievable
with, e.g., plasmonic resonators. We will demonstrate in the following
that the fundamental coupling strength is nonetheless of secondary
relevance. Three polaritonic branches, the lower polariton (LP), middle
polariton (MP), and upper polariton (UP) emerge. The electron and
electron–photon correlation are accurately described by the ab initio QED-CC ansatz wave function, thus capturing any
modification of inter- (dispersion) and intramolecular forces.37,46,47,55,56 Nevertheless, as a result of the low coupling
strength, the molecular ground state, here, is essentially unaffected
by the embedding in the optical environment and polaritonic effects
effectively arise from collective coupling. The dimers are indirectly
coupled through the cavity, establishing an interplay between longitudinal
(Coulomb) and transverse (photon) fields. The simplicity and engineerability
of our model allow us to separate intermolecular and cavity-induced
effects, providing a satisfactory proof-of-concept model for investigating
microscopic molecular responses in complex chemical and photonic environments.
= 0.005 au, corresponding to an effective
mode volume V = 74.5 nm3, which is achievable
with, e.g., plasmonic resonators. We will demonstrate in the following
that the fundamental coupling strength is nonetheless of secondary
relevance. Three polaritonic branches, the lower polariton (LP), middle
polariton (MP), and upper polariton (UP) emerge. The electron and
electron–photon correlation are accurately described by the ab initio QED-CC ansatz wave function, thus capturing any
modification of inter- (dispersion) and intramolecular forces.37,46,47,55,56 Nevertheless, as a result of the low coupling
strength, the molecular ground state, here, is essentially unaffected
by the embedding in the optical environment and polaritonic effects
effectively arise from collective coupling. The dimers are indirectly
coupled through the cavity, establishing an interplay between longitudinal
(Coulomb) and transverse (photon) fields. The simplicity and engineerability
of our model allow us to separate intermolecular and cavity-induced
effects, providing a satisfactory proof-of-concept model for investigating
microscopic molecular responses in complex chemical and photonic environments.
Figure 1.
Structure of the (H2)N aggregate and absorption spectra outside the cavity for 5 Å intermolecular separation. The local transition densities ρj0(x) [i.e., the transition density ϱj0(x, y, z) integrated perpendicularly to the dipole moment direction, that is, perpendicular to the H2 bond ρIj0(x) = ∫+∞–∞ d z ∫YI+2 ÅYI–2 Å dy ϱj0(x, y, z), where I = P, A1, and A2; see section 1 of the Supporting Information] of the undressed excitations E1 (solid lines) and E2 (dotted lines) of (H2)7 are shown, showing specific transition moment alignment patterns caused by intermolecular interactions. Only the left densities are shown because the right densities display the same behavior (see section 1 of the Supporting Information).
Figure 2.
Polaritonic spectra for 5 Å intermolecular separation with coupling strength 0.005 au, polarization along the H2 bonds, and photon frequency tuned to the undressed excitation of the isolated unperturbed dimers. The energies of the lower and middle polaritons as a function of the number of dimers N in the chain, depicted in the top left panel, highlight an avoided crossing. The integrated local transition density of the lower polariton (LP) for P, A1, and A2, are shown. The Coulomb forces compete with the transverse field (photon) to determine the alignment pattern of the transition moments, generating a sign flip in the transition density of A1.
Modification of Solvation. In Figure 2, we plot the LP local transition
densities for P, A1, and A2. As N increases, the
A1 local transition density changes sign; i.e., the transition dipole
of A1 aligns with the other molecules. This effect requires that both
of the (undressed) excitations E1 and E2 contribute to the LP state
and, hence, occur around the avoided crossing domain illustrated in Figure 2. While the alignment
clearly originates from collective effects, its impact is evidently
localized in the first solvation shell and requires explicit treatment
of the solute–solvent interface. The local change arises from
competition between the short-range intermolecular forces, which pattern
E1 and E2, and the collective interaction to the optical mode, which
tends to align the molecular transition moments (see also section 3 of the Supporting Information). Indeed,
for larger intermolecular separations, the LP local moments are aligned
(see section 5 of the Supporting Information),
confirming that the sign change stems from the interplay between Coulomb
and photon fields. Which effect prevails depends upon the setup, specifically
the excitation energies (solute–solvent system), the chain
length N (collective coupling), the light–matter
coupling strength  (optical device features), and the intermolecular
forces (solute–solvent system and intermolecular separation).
Notice also that there is a collective strength for which the excitation
of the nearest dimers is effectively quenched by collective strong
coupling (N = 9 in Figure 2). Analogous quenching effects play an important
role in limiting the refractive index of atomic media.57
(optical device features), and the intermolecular
forces (solute–solvent system and intermolecular separation).
Notice also that there is a collective strength for which the excitation
of the nearest dimers is effectively quenched by collective strong
coupling (N = 9 in Figure 2). Analogous quenching effects play an important
role in limiting the refractive index of atomic media.57
The number of solvent molecules heavily
outweighs the concentration
of solutes. It is therefore instructive to verify how our observation
scales in the thermodynamic limit Ne, V → ∞, Ne/V = constant, i.e., with fixed Ne/V but increasing particle number Ne and quantization volume V (controlling
the fundamental coupling strength). To this end, we resort to a simplified
Tavis–Cummings (TC)–Kasha58−61 model (parametrized according
to the electronic CC calculations), with a nearest neighbor transition
dipole–dipole coupling between the molecular excitations (see sections 3.3 and 5.1 of the Supporting Information).
The total number of molecules Ne = NrepN is given by the number
of molecules N per aggregate (i.e., the same number
used in Figures 1 and 2) and how many times the system appears in the cavity Nrep. In Figure 3a, we report the eigenvector coefficients for single
P and A1 to quantify how the individual molecules contribute to the
collective excitations of the TC–Kasha model. The ratio Ne/V is fixed; i.e., the Rabi
splitting is kept constant while increasing N. The
simulations for Nrep = 1 and 4 show analogous
results and, thus, prove that the described behavior is qualitatively
resistant to the thermodynamic limit. Still, the single-molecule contribution
(i.e., the TC–Kasha eigenvector coefficients of a single dimer)
decreases by approximately 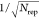 . Similar conclusions are drawn from panels
b and c of Figure 3, where, for each panel, λ and Nrep are fixed while increasing N. This allows us to
investigate the effect of changing the number of coupled molecules
and, thus, the collective effects. Increasing the number N – 1 of coupled solvent molecules at fixed Nrep and λ shortens the critical intermolecular distance
at which the A1 coefficient shows a change. Panels b1 and b2 of Figure 3, computed at different
couplings, show similar behavior and order of magnitude, but the N-value for which the transition moment of A1 changes sign
is shifted to longer chains for smaller couplings. Comparing panels
b1 and c of Figure 3 shows the effect of increasing Nrep/N while rescaling λ by
. Similar conclusions are drawn from panels
b and c of Figure 3, where, for each panel, λ and Nrep are fixed while increasing N. This allows us to
investigate the effect of changing the number of coupled molecules
and, thus, the collective effects. Increasing the number N – 1 of coupled solvent molecules at fixed Nrep and λ shortens the critical intermolecular distance
at which the A1 coefficient shows a change. Panels b1 and b2 of Figure 3, computed at different
couplings, show similar behavior and order of magnitude, but the N-value for which the transition moment of A1 changes sign
is shifted to longer chains for smaller couplings. Comparing panels
b1 and c of Figure 3 shows the effect of increasing Nrep/N while rescaling λ by 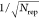 , which thus retains the overall
collective
coupling. The panels show the same qualitative behavior, with coefficients
rescaled by
, which thus retains the overall
collective
coupling. The panels show the same qualitative behavior, with coefficients
rescaled by 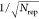 , as in Figure 3a. We also confirm this trend by simulating
two H2 chains in our QED-CC calculations (see section 5.1 of the Supporting Information). Therefore, Figure 3 demonstrates that
the magnitude of the change in the environmental response for fixed Ne/V decreases with the number
of activated species P, which is significantly smaller than the number
of coupled molecules Ne, and P does not
need to be strongly coupled to the device. In other words, if the
solvent exists in vast excess as in experiments showing modifications
of crystallization33−35 and ionic conductivity,62 each solvation shell will experience a considerably larger effect
from the cavity than the bulk of the solvent molecules. Moreover,
keeping the solute–solvent ratio fixed (increasing Nrep) shortens the critical length N as seen from panels b and c of Figure 3. Therefore, the intermolecular distance
and chain length N for which the transition moment
of A1 changes sign depend solely upon the solvent strong coupling
defined by the ratio Nrep(N – 1)/V.
, as in Figure 3a. We also confirm this trend by simulating
two H2 chains in our QED-CC calculations (see section 5.1 of the Supporting Information). Therefore, Figure 3 demonstrates that
the magnitude of the change in the environmental response for fixed Ne/V decreases with the number
of activated species P, which is significantly smaller than the number
of coupled molecules Ne, and P does not
need to be strongly coupled to the device. In other words, if the
solvent exists in vast excess as in experiments showing modifications
of crystallization33−35 and ionic conductivity,62 each solvation shell will experience a considerably larger effect
from the cavity than the bulk of the solvent molecules. Moreover,
keeping the solute–solvent ratio fixed (increasing Nrep) shortens the critical length N as seen from panels b and c of Figure 3. Therefore, the intermolecular distance
and chain length N for which the transition moment
of A1 changes sign depend solely upon the solvent strong coupling
defined by the ratio Nrep(N – 1)/V.
Figure 3.

Wave function coefficients cI, where I = P and A1
from the TC–Kasha
model as a function of the environment molecules N – 1 and the intermolecular separation. (a) The coupling strength
is set to  . We qualitatively recover the same sign
flip for A1 as Figure 2 for Nrep = 1 (upper panel) and 4 (lower
panel), but the coefficient values approximately scale as
. We qualitatively recover the same sign
flip for A1 as Figure 2 for Nrep = 1 (upper panel) and 4 (lower
panel), but the coefficient values approximately scale as 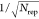 . Notably, such a decay is much smaller
than
. Notably, such a decay is much smaller
than  . (b) Wave function coefficients obtained
by fixing λ and Nrep = 1 as N varies. This is consistent with experimental setups where
the same cavity (i.e., λ fixed) is employed at different molecular
concentrations (here, the solvent molecules N –
1). (c) Same as panel b but for Nrep =
4. In comparison to panel b1, the overall collective coupling is maintained,
but as a result of the larger number of solute molecules, the coefficients
are rescaled by
. (b) Wave function coefficients obtained
by fixing λ and Nrep = 1 as N varies. This is consistent with experimental setups where
the same cavity (i.e., λ fixed) is employed at different molecular
concentrations (here, the solvent molecules N –
1). (c) Same as panel b but for Nrep =
4. In comparison to panel b1, the overall collective coupling is maintained,
but as a result of the larger number of solute molecules, the coefficients
are rescaled by 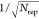 . The impurity concentration P then defines
the decay of the wave function coefficients. Notice that P does not
need to be in a strong coupling. See section 5.1 of the Supporting Information for more results.
. The impurity concentration P then defines
the decay of the wave function coefficients. Notice that P does not
need to be in a strong coupling. See section 5.1 of the Supporting Information for more results.
Thus far, we considered all of the molecules at the same distance. However, analogous results are found for a small H2 cluster, with the other hydrogens at larger distances. This is illustrated in Figure 4 for (H2)3 with intermolecular distance 3.7 Å and slightly increased λ = 0.01 au to compensate for the larger blue shift of E1. The remaining N – 3 hydrogens are placed at a 20 Å distance. The results show the same behavior as in Figure 2 and emphasize the locality induced by the short-range Coulomb interactions, clearly highlighting the interplay between the chemical surroundings and the collective optical dressing.
Figure 4.
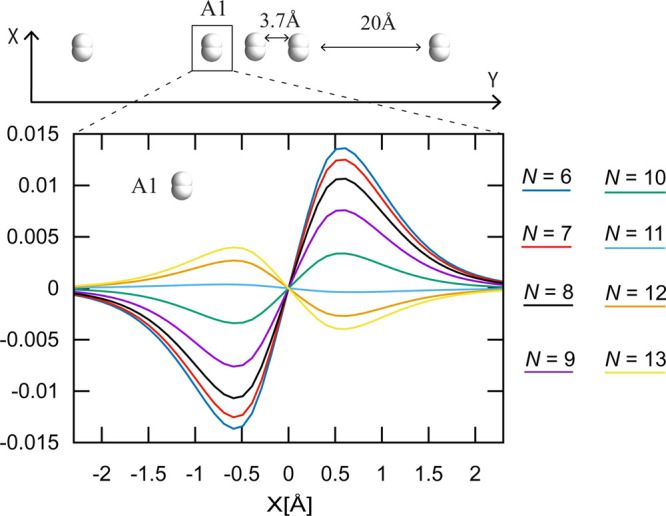
LP-integrated local transition densities for A1 for a cluster (H2)3 with intermolecular separation of 3.7 Å, together with N – 3 intermolecular dimers at a distance of 20 Å. As a result of the larger intermolecular forces between A1 and P, the excitation E1 is strongly blue-shifted. Therefore, we use a slightly larger coupling and a larger number N compared to Figure 2 to reach the avoided crossing region between the LP and MP. The results show the same qualitative behavior as Figure 2.
The TC–Kasha model successfully reproduces the modifications in the dynamic response of the first solvation shell. Because the model is parametrized with bare (electronic) transitions, it stresses the importance of collective behavior, which alters the LP microscopic response near P once a critical cooperative coupling strength is reached. Nevertheless, the TC–Kasha model has apparent limitations in that it will perform poorly in modeling the electronic dynamic of more complex systems and cannot account for nonlinear effects included in the QED-CC calculations; i.e., a self-consistent and more realistic treatment is likely to give similar or more favorable scaling. It should also be noted that the strength and structure of solvation vary widely with the type of intermolecular interaction such that the onset of this effect can change dramatically with the specific choice of solvent and solute. Quantum chemistry then provides a flexible tool set to explore such solvent effects beyond the heavily simplified dipole–dipole picture.63−65
Disorder and Resonance Dependence. While aggregation, solvation, and crystallization imply a somewhat structured environment, disorder (in the form of orientation or inhomogeneous broadening) is inevitable in chemistry under standard ambient conditions. Inhomogeneous broadening and the molecular orientation relative to the cavity polarization, for all solvent molecules besides A1 (long-range disorder), are included in our TC–Kasha model by sampling the excitation energies from a Gaussian distribution and assigning random H2 orientations (see section 5.1 of the Supporting Information for details). As demonstrated in Figure 5 and section 5.1 of the Supporting Information, the long-range disorder merely increases the required number of molecules N, but the overall solute–solvent effect remains unaffected. At the same time, local disorder severely impacts the intermolecular interactions because the Coulomb forces are strongly directional; i.e., other forms of aggregation will impact the solute differently. In the Supporting Information, we report the local transition densities for a J-aggregate (head–tail) (H2)N configuration and show a sign flip for the local transition dipole of A1 in the MP instead of the LP. The modification of the solvent–solute response is conceptually identical and merely moves to a different polaritonic state. This stems from the different transition moment patterns in the undressed excitations of the J-aggregate. Hence, we can expect that a realistic solvation system will partially exhibit the discussed effect in the LP and MP.
Figure 5.

Wave function coefficients obtained from a disordered TC–Kasha model, where the first and second solvation shells around P are fixed, while orientational disorder and inhomogeneous broadening are applied to the other dimers. The coupling strength is set to 0.005 au. We still recover the same qualitative behavior and coefficient magnitude of Figures 2 and 3b although for larger N because of the smaller collective coupling to the device as a result of disorder. Analogous results are obtained for different coupling strengths; see section 5.1 of the Supporting Information for more results.
Lastly, the solute–solvent polarization features a clear resonant behavior. When the cavity is detuned from the E2 excitation (entirely off-resonant or tuned to E1), the local changes are negligible or indistinguishable from collective delocalization effects. If the field is tuned to E2 (instead of the excitation of isolated unperturbed H2), the results are in line with the foregoing discussion, although this requires a slightly higher N to achieve the sign flip in A1. These results are consistent with the position of the avoided crossing between the LP and MP, which depends upon the photon frequency and determines the mixing of E1 and E2 (and, thus, the local impact of the strong coupling regime).
Conclusion. Using state-of-the-art ab
initio QED-CC and quantum optics models, we illustrate how
the intermolecular forces interplay with collective coupling in cooperative
systems and induce considerable changes in the dynamic polarizability
of the first solvation shell. Notice that the employed coupling strength
( = 0.005 au) is, in principle, achievable
in few-molecule experimental plasmonic cavities and ensures that the
QED-CC computed molecular ground state is fundamentally electronic.
Nevertheless, the thermodynamic limit study in Figure 3 and 5 shows that
the microscopic response changes of the LP are a collective effect
and, therefore, are also achieved for lower light–matter couplings
and a larger number of molecules. Such changes arise from a competition
between the collective interaction with the optical mode and the local
Coulomb interactions, which favors aligned (J-aggregate) or anti-aligned
(H-aggregate) patterns in the transition moments. Which effect prevails
depends upon the local chemical environment around the solute and
the collective coupling strength. The described effects
are also expected for systems carrying other types of interactions,
such as dipole–dipole and hydrogen bonds. Dipole–dipole
forces are more long-ranged in comparison to the dispersion interactions
of H2, and hence, such effects could also be observed at
larger distances. On the other hand, hydrogen bonds are shorter ranged,
favor a somewhat ordered local structure involving specific functional
groups, and can substantially alter both the electronic and vibrational
states. Although we do not expect a modification of the hydrogen bond
itself (as a result of the low coupling strength), its competition
with the transverse fields might alter the solvent response (e.g.,
by quenching the excitation of the surrounding solvent molecules as
in Figure 2) and, thus,
promote novel interesting effects in the framework of both electronic
strong coupling (ESC) and VSC. Indeed, our work suggests that cooperative
coupling to a solvent induces strong modifications in the dynamic
polarization of the first solvation shell of the solute. A change
in the dynamic polarization of the first solvation shell is then likely
to affect the solute dynamics, possibly leading to changes in solvent
rearrangement, chemical reactivity, nucleation, aggregation, and ionic
conductivity. Such a mechanism could be experimentally investigated
using ultrafast ultraviolet (UV) electronic spectroscopy.31,32,66,67 Moreover, our results suggest that these effects feature a clear
resonance dependence and will be relevant even in the thermodynamic
limit and for disordered systems. Hence, our work provides an alternative
perspective on cooperative strong coupling and the underlying mechanism
that modifies chemistry via changes in the interactions within solvents
and aggregates. Several VSC experiments have shown that the resonant
coupling can induce modifications in solvent effects14,36,37 and assembly,33−35 a feature proposed
to emerge from collective coupling and structural reorganization rather
than significant individual molecular changes.68 Longitudinal (coulombic) interactions are ubiquitous and
fundamental in understanding chemical effects, and the concepts developed
in this letter can help and inspire further discussions on both ESC
and VSC. While we fixed the nuclear positions in this work, motivated
by a separation of time scales, it is apparent that vibrational strong
coupling will require the inclusion of nuclear motion. We can therefore
expect that changes in the first solvation shell might result in reorganization
of the solvation structure. These results also encourage the development
and refinement of multiscale approaches45,69 to extend
our study to experimentally investigated systems.
= 0.005 au) is, in principle, achievable
in few-molecule experimental plasmonic cavities and ensures that the
QED-CC computed molecular ground state is fundamentally electronic.
Nevertheless, the thermodynamic limit study in Figure 3 and 5 shows that
the microscopic response changes of the LP are a collective effect
and, therefore, are also achieved for lower light–matter couplings
and a larger number of molecules. Such changes arise from a competition
between the collective interaction with the optical mode and the local
Coulomb interactions, which favors aligned (J-aggregate) or anti-aligned
(H-aggregate) patterns in the transition moments. Which effect prevails
depends upon the local chemical environment around the solute and
the collective coupling strength. The described effects
are also expected for systems carrying other types of interactions,
such as dipole–dipole and hydrogen bonds. Dipole–dipole
forces are more long-ranged in comparison to the dispersion interactions
of H2, and hence, such effects could also be observed at
larger distances. On the other hand, hydrogen bonds are shorter ranged,
favor a somewhat ordered local structure involving specific functional
groups, and can substantially alter both the electronic and vibrational
states. Although we do not expect a modification of the hydrogen bond
itself (as a result of the low coupling strength), its competition
with the transverse fields might alter the solvent response (e.g.,
by quenching the excitation of the surrounding solvent molecules as
in Figure 2) and, thus,
promote novel interesting effects in the framework of both electronic
strong coupling (ESC) and VSC. Indeed, our work suggests that cooperative
coupling to a solvent induces strong modifications in the dynamic
polarization of the first solvation shell of the solute. A change
in the dynamic polarization of the first solvation shell is then likely
to affect the solute dynamics, possibly leading to changes in solvent
rearrangement, chemical reactivity, nucleation, aggregation, and ionic
conductivity. Such a mechanism could be experimentally investigated
using ultrafast ultraviolet (UV) electronic spectroscopy.31,32,66,67 Moreover, our results suggest that these effects feature a clear
resonance dependence and will be relevant even in the thermodynamic
limit and for disordered systems. Hence, our work provides an alternative
perspective on cooperative strong coupling and the underlying mechanism
that modifies chemistry via changes in the interactions within solvents
and aggregates. Several VSC experiments have shown that the resonant
coupling can induce modifications in solvent effects14,36,37 and assembly,33−35 a feature proposed
to emerge from collective coupling and structural reorganization rather
than significant individual molecular changes.68 Longitudinal (coulombic) interactions are ubiquitous and
fundamental in understanding chemical effects, and the concepts developed
in this letter can help and inspire further discussions on both ESC
and VSC. While we fixed the nuclear positions in this work, motivated
by a separation of time scales, it is apparent that vibrational strong
coupling will require the inclusion of nuclear motion. We can therefore
expect that changes in the first solvation shell might result in reorganization
of the solvation structure. These results also encourage the development
and refinement of multiscale approaches45,69 to extend
our study to experimentally investigated systems.
Acknowledgments
The authors thank Göran Johansson for insightful discussions. Christian Schäfer acknowledges funding from the Horizon Europe Research and Innovation Programme of the European Union under the Marie Skłodowska-Curie Grant Agreement 101065117 and the Swedish Research Council (VR) through Grant 2016-06059. Matteo Castagnola and Henrik Koch acknowledge funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (Grant Agreement 101020016). Enrico Ronca acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon Europe Research and Innovation Programme (Grant ERC-StG-2021-101040197-QED-SPIN). This work was partially funded by the European Union. Views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or Research Executive Agency (REA). Neither the European Union nor the granting authority can be held responsible.
Data Availability Statement
The data of this study is freely available under https://zenodo.org/doi/10.5281/zenodo.10572145.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpclett.3c03506.
Computational details, left and right (QED-)CC local transition densities, ground and excited state densities, absorption spectra, additional model simulations for the J- and H-aggregates, and details on ab initio CC and QED-CC as well as the Jaynes–Cummings (JC), TC, Kasha, and TC–Kasha models (PDF)
Transparent Peer Review report available (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bhuyan R.; Mony J.; Kotov O.; Castellanos G. W.; Gómez Rivas J.; Shegai T. O.; Börjesson K. The Rise and Current Status of Polaritonic Photochemistry and Photophysics. Chem. Rev. 2023, 123, 10877–10919. 10.1021/acs.chemrev.2c00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K.; Hutchison J. A.; Uji-i H. Recent progress in vibropolaritonic chemistry. ChemPlusChem 2020, 85, 1981–1988. 10.1002/cplu.202000411. [DOI] [PubMed] [Google Scholar]
- Hirai K.; Hutchison J. A.; Uji-i H. Molecular Chemistry in Cavity Strong Coupling. Chem. Rev. 2023, 123, 8099–8126. 10.1021/acs.chemrev.2c00748. [DOI] [PubMed] [Google Scholar]
- Pang Y.; Thomas A.; Nagarajan K.; Vergauwe R. M.; Joseph K.; Patrahau B.; Wang K.; Genet C.; Ebbesen T. W. On the role of symmetry in vibrational strong coupling: The case of charge-transfer complexation. Angew. Chem., Int. Ed. 2020, 59, 10436–10440. 10.1002/anie.202002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sau A.; Nagarajan K.; Patrahau B.; Lethuillier-Karl L.; Vergauwe R. M.; Thomas A.; Moran J.; Genet C.; Ebbesen T. W. Modifying Woodward–Hoffmann stereoselectivity under vibrational strong coupling. Angew. Chem., Int. Ed. 2021, 60, 5712–5717. 10.1002/anie.202013465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T.; Hutchison J. A.; Léonard J.; Genet C.; Haacke S.; Ebbesen T. W. Polariton dynamics under strong light–molecule coupling. ChemPhysChem 2013, 14, 125–131. 10.1002/cphc.201200734. [DOI] [PubMed] [Google Scholar]
- George J.; Wang S.; Chervy T.; Canaguier-Durand A.; Schaeffer G.; Lehn J.-M.; Hutchison J. A.; Genet C.; Ebbesen T. W. Ultra-strong coupling of molecular materials: Spectroscopy and dynamics. Faraday Discuss. 2015, 178, 281–294. 10.1039/C4FD00197D. [DOI] [PubMed] [Google Scholar]
- Wang S.; Chervy T.; George J.; Hutchison J. A.; Genet C.; Ebbesen T. W. Quantum yield of polariton emission from hybrid light–matter states. J. Phys. Chem. Lett. 2014, 5, 1433–1439. 10.1021/jz5004439. [DOI] [PubMed] [Google Scholar]
- Zhong X.; Chervy T.; Zhang L.; Thomas A.; George J.; Genet C.; Hutchison J. A.; Ebbesen T. W. Energy transfer between spatially separated entangled molecules. Angew. Chem. 2017, 129, 9162–9166. 10.1002/ange.201703539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison J. A.; Schwartz T.; Genet C.; Devaux E.; Ebbesen T. W. Modifying chemical landscapes by coupling to vacuum fields. Angew. Chem., Int. Ed. 2012, 51, 1592–1596. 10.1002/anie.201107033. [DOI] [PubMed] [Google Scholar]
- Mony J.; Climent C.; Petersen A. U.; Moth-Poulsen K.; Feist J.; Börjesson K. Photoisomerization efficiency of a solar thermal fuel in the strong coupling regime. Adv. Funct. Mater. 2021, 31, 2010737. 10.1002/adfm.202010737. [DOI] [Google Scholar]
- Schwartz T.; Hutchison J. A.; Genet C.; Ebbesen T. W. Reversible switching of ultrastrong light-molecule coupling. Phys. Rev. Lett. 2011, 106, 196405. 10.1103/PhysRevLett.106.196405. [DOI] [PubMed] [Google Scholar]
- Thomas A.; George J.; Shalabney A.; Dryzhakov M.; Varma S. J.; Moran J.; Chervy T.; Zhong X.; Devaux E.; Genet C.; Hutchison J. A.; Ebbesen T. W. Ground-State Chemical Reactivity under Vibrational Coupling to the Vacuum Electromagnetic Field. Angew. Chem., Int. Ed. 2016, 55, 11462–11466. 10.1002/anie.201605504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lather J.; Bhatt P.; Thomas A.; Ebbesen T. W.; George J. Cavity catalysis by cooperative vibrational strong coupling of reactant and solvent molecules. Angew. Chem., Int. Ed. 2019, 58, 10635–10638. 10.1002/anie.201905407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.; Lethuillier-Karl L.; Nagarajan K.; Vergauwe R. M. A.; George J.; Chervy T.; Shalabney A.; Devaux E.; Genet C.; Moran J.; Ebbesen T. W. Tilting a ground-state reactivity landscape by vibrational strong coupling. Science 2019, 363, 615–619. 10.1126/science.aau7742. [DOI] [PubMed] [Google Scholar]
- Munkhbat B.; Wersäll M.; Baranov D. G.; Antosiewicz T. J.; Shegai T. Suppression of photo-oxidation of organic chromophores by strong coupling to plasmonic nanoantennas. Sci. Adv. 2018, 4, eaas9552. 10.1126/sciadv.aas9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaguier-Durand A.; Devaux E.; George J.; Pang Y.; Hutchison J. A.; Schwartz T.; Genet C.; Wilhelms N.; Lehn J.-M.; Ebbesen T. W. Thermodynamics of molecules strongly coupled to the vacuum field. Angew. Chem., Int. Ed. 2013, 52, 10533–10536. 10.1002/anie.201301861. [DOI] [PubMed] [Google Scholar]
- Hirai K.; Takeda R.; Hutchison J. A.; Uji-i H. Modulation of Prins cyclization by vibrational strong coupling. Angew. Chem. 2020, 132, 5370–5373. 10.1002/ange.201915632. [DOI] [PubMed] [Google Scholar]
- Polak D.; Jayaprakash R.; Lyons T. P.; Martínez-Martínez L. Á.; Leventis A.; Fallon K. J.; Coulthard H.; Bossanyi D. G.; Georgiou K.; Petty A. J. II; Anthony J.; Bronstein H.; Yuen-Zhou J.; Tartakovskii A. I.; Clark J.; Musser A. J. Manipulating molecules with strong coupling: Harvesting triplet excitons in organic exciton microcavities. Chem. Sci. 2020, 11, 343–354. 10.1039/C9SC04950A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michetti P.; La Rocca G. C. Simulation of J-aggregate microcavity photoluminescence. Phys. Rev. B 2008, 77, 195301. 10.1103/PhysRevB.77.195301. [DOI] [Google Scholar]
- Wellnitz D.; Pupillo G.; Schachenmayer J. Disorder enhanced vibrational entanglement and dynamics in polaritonic chemistry. Commun. Phys. 2022, 5, 120. 10.1038/s42005-022-00892-5. [DOI] [Google Scholar]
- Du M.; Martínez-Martínez L. A.; Ribeiro R. F.; Hu Z.; Menon V. M.; Yuen-Zhou J. Theory for polariton-assisted remote energy transfer. Chem. Sci. 2018, 9, 6659–6669. 10.1039/C8SC00171E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway B. M. The Dicke model in quantum optics: Dicke model revisited. Philos. Trans. R. Soc., A 2011, 369, 1137–1155. 10.1098/rsta.2010.0333. [DOI] [PubMed] [Google Scholar]
- Hagenmüller D.; Schachenmayer J.; Schütz S.; Genes C.; Pupillo G. Cavity-enhanced transport of charge. Phys. Rev. Lett. 2017, 119, 223601. 10.1103/PhysRevLett.119.223601. [DOI] [PubMed] [Google Scholar]
- Feist J.; Galego J.; Garcia-Vidal F. J. Polaritonic chemistry with organic molecules. ACS Photonics 2018, 5, 205–216. 10.1021/acsphotonics.7b00680. [DOI] [Google Scholar]
- Ribeiro R. F.; Martínez-Martínez L. A.; Du M.; Campos-Gonzalez-Angulo J.; Yuen-Zhou J. Polariton chemistry: Controlling molecular dynamics with optical cavities. Chem. Sci. 2018, 9, 6325–6339. 10.1039/C8SC01043A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles D. M.; Grant R. T.; Lidzey D. G.; Clark C.; Lagoudakis P. G. Imaging the polariton relaxation bottleneck in strongly coupled organic semiconductor microcavities. Phys. Rev. B 2013, 88, 121303. 10.1103/PhysRevB.88.121303. [DOI] [Google Scholar]
- Coles D. M.; Somaschi N.; Michetti P.; Clark C.; Lagoudakis P. G.; Savvidis P. G.; Lidzey D. G. Polariton-mediated energy transfer between organic dyes in a strongly coupled optical microcavity. Nat. Mater. 2014, 13, 712–719. 10.1038/nmat3950. [DOI] [PubMed] [Google Scholar]
- Georgiou K.; Jayaprakash R.; Othonos A.; Lidzey D. G. Ultralong-Range Polariton-Assisted Energy Transfer in Organic Microcavities. Angew. Chem. 2021, 133, 16797–16803. 10.1002/ange.202105442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigeon J.; Le Liepvre S.; Vassant S.; Belabas N.; Bardou N.; Minot C.; Yacomotti A.; Levenson A.; Charra F.; Barbay S. Strong coupling between self-assembled molecules and surface plasmon polaritons. J. Phys. Chem. Lett. 2017, 8, 5626–5632. 10.1021/acs.jpclett.7b02586. [DOI] [PubMed] [Google Scholar]
- Timmer D.; Gittinger M.; Quenzel T.; Stephan S.; Zhang Y.; Schumacher M. F.; Lützen A.; Silies M.; Tretiak S.; Zhong J.-H.; De Sio A.; Lienau C. Plasmon mediated coherent population oscillations in molecular aggregates. arXiv.org, e-Print Arch., Condens. Matter 2023, arXiv:2307.14708. 10.48550/arXiv.2307.14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa P.; Wang W.; Pomraenke R.; Lammers M.; Maiuri M.; Manzoni C.; Cerullo G.; Lienau C. Real-time observation of ultrafast Rabi oscillations between excitons and plasmons in metal nanostructures with J-aggregates. Nat. Photonics 2013, 7, 128–132. 10.1038/nphoton.2012.340. [DOI] [Google Scholar]
- Joseph K.; Kushida S.; Smarsly E.; Ihiawakrim D.; Thomas A.; Paravicini-Bagliani G. L.; Nagarajan K.; Vergauwe R.; Devaux E.; Ersen O.; Bunz U. H. F.; Ebbesen T. W. Supramolecular assembly of conjugated polymers under vibrational strong coupling. Angew. Chem., Int. Ed. 2021, 60, 19665–19670. 10.1002/anie.202105840. [DOI] [PubMed] [Google Scholar]
- Hirai K.; Ishikawa H.; Chervy T.; Hutchison J. A.; Uji-i H. Selective crystallization via vibrational strong coupling. Chem. Sci. 2021, 12, 11986–11994. 10.1039/D1SC03706D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeep K.; Joseph K.; Gautier J.; Nagarajan K.; Sujith M.; Thomas K. G.; Ebbesen T. W. Manipulating the Self-Assembly of Phenyleneethynylenes under Vibrational Strong Coupling. J. Phys. Chem. Lett. 2022, 13, 1209–1214. 10.1021/acs.jpclett.1c03893. [DOI] [PubMed] [Google Scholar]
- Singh J.; Lather J.; George J. Solvent Dependence on Cooperative Vibrational Strong Coupling and Cavity Catalysis. ChemPhysChem 2023, 24, e202300016. 10.1002/cphc.202300016. [DOI] [PubMed] [Google Scholar]
- Piejko M.; Patrahau B.; Joseph K.; Muller C.; Devaux E.; Ebbesen T. W.; Moran J. Solvent Polarity under Vibrational Strong Coupling. J. Am. Chem. Soc. 2023, 145, 13215–13222. 10.1021/jacs.3c02260. [DOI] [PubMed] [Google Scholar]
- Fregoni J.; Granucci G.; Coccia E.; Persico M.; Corni S. Manipulating azobenzene photoisomerization through strong light–molecule coupling. Nat. Commun. 2018, 9, 4688. 10.1038/s41467-018-06971-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregoni J.; Granucci G.; Persico M.; Corni S. Strong Coupling with Light Enhances the Photoisomerization Quantum Yield of Azobenzene. Chem 2020, 6, 250–265. 10.1016/j.chempr.2019.11.001. [DOI] [Google Scholar]
- Sun J.; Vendrell O. Modification of Thermal Chemical Rates in a Cavity via Resonant Effects in the Collective Regime. J. Phys. Chem. Lett. 2023, 14, 8397–8404. 10.1021/acs.jpclett.3c02199. [DOI] [PubMed] [Google Scholar]
- Li T. E.; Subotnik J. E.; Nitzan A. Cavity molecular dynamics simulations of liquid water under vibrational ultrastrong coupling. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 18324–18331. 10.1073/pnas.2009272117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavošević F.; Rubio A. Wavefunction embedding for molecular polaritons. J. Chem. Phys. 2022, 157, 094101. 10.1063/5.0095552. [DOI] [PubMed] [Google Scholar]
- Pavošević F.; Hammes-Schiffer S.; Rubio A.; Flick J. Cavity-Modulated Proton Transfer Reactions. J. Am. Chem. Soc. 2022, 144, 4995–5002. 10.1021/jacs.1c13201. [DOI] [PubMed] [Google Scholar]
- Schäfer C.; Flick J.; Ronca E.; Narang P.; Rubio A. Shining light on the microscopic resonant mechanism responsible for cavity-mediated chemical reactivity. Nat. Commun. 2022, 13, 7817. 10.1038/s41467-022-35363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer C. Polaritonic Chemistry from First Principles via Embedding Radiation Reaction. J. Phys. Chem. Lett. 2022, 13, 6905–6911. 10.1021/acs.jpclett.2c01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland T. S.; Ronca E.; Kjønstad E. F.; Rubio A.; Koch H. Coupled cluster theory for molecular polaritons: Changing ground and excited states. Phys. Rev. X 2020, 10, 041043. 10.1103/PhysRevX.10.041043. [DOI] [Google Scholar]
- Haugland T. S.; Schäfer C.; Ronca E.; Rubio A.; Koch H. Intermolecular interactions in optical cavities: An ab initio QED study. J. Chem. Phys. 2021, 154, 094113. 10.1063/5.0039256. [DOI] [PubMed] [Google Scholar]
- Mordovina U.; Bungey C.; Appel H.; Knowles P. J.; Rubio A.; Manby F. R. Polaritonic coupled-cluster theory. Phys. Rev. Res. 2020, 2, 023262. 10.1103/PhysRevResearch.2.023262. [DOI] [Google Scholar]
- Vidal M. L.; Manby F. R.; Knowles P. J. Polaritonic effects in the vibronic spectrum of molecules in an optical cavity. J. Chem. Phys. 2022, 156, 204119. 10.1063/5.0089412. [DOI] [PubMed] [Google Scholar]
- Schäfer C.; Fojt J.; Lindgren E.; Erhart P. Machine Learning for Polaritonic Chemistry: Accessing Chemical Kinetics. arXiv.org, e-Print Arch., Phys. 2023, arXiv:2311.09739. 10.48550/arXiv.2311.09739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidler D.; Schäfer C.; Ruggenthaler M.; Rubio A. Polaritonic chemistry: Collective strong coupling implies strong local modification of chemical properties. J. Phys. Chem. Lett. 2021, 12, 508–516. 10.1021/acs.jpclett.0c03436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen-Zhou J.; Menon V. M. Polariton chemistry: Thinking inside the (photon) box. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 5214–5216. 10.1073/pnas.1900795116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidler D.; Schnappinger T.; Obzhirov A.; Ruggenthaler M.; Kowalewski M.; Rubio A. Unraveling a cavity induced molecular polarization mechanism from collective vibrational strong coupling. arXiv.org, e-Print Arch., Quantum Phys. 2023, arXiv:2306.06004. 10.48550/arXiv.2306.06004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnappinger T.; Sidler D.; Ruggenthaler M.; Rubio A.; Kowalewski M. Cavity Born–Oppenheimer Hartree–Fock Ansatz: Light–Matter Properties of Strongly Coupled Molecular Ensembles. J. Phys. Chem. Lett. 2023, 14, 8024–8033. 10.1021/acs.jpclett.3c01842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland T. S.; Philbin J. P.; Ghosh T. K.; Chen M.; Koch H.; Narang P. Understanding the polaritonic ground state in cavity quantum electrodynamics. arXiv.org, e-Print Arch., Phys. 2023, arXiv:2307.14822. 10.48550/arXiv.2307.14822. [DOI] [Google Scholar]
- Cao J.; Pollak E. Cavity-Induced Quantum Interference and Collective Interactions in van der Waals Systems. arXiv.org, e-Print Arch., Quantum Phys. 2023, arXiv:2310.12881. 10.48550/arXiv.2310.12881. [DOI] [Google Scholar]
- Andreoli F.; Gullans M. J.; High A. A.; Browaeys A.; Chang D. E. Maximum refractive index of an atomic medium. Phys. Rev. X 2021, 11, 011026. 10.1103/PhysRevX.11.011026. [DOI] [Google Scholar]
- Dicke R. H. Coherence in spontaneous radiation processes. Phys. Rev. 1954, 93, 99. 10.1103/PhysRev.93.99. [DOI] [Google Scholar]
- Tavis M.; Cummings F. W. Exact solution for an N-molecule—radiation-field Hamiltonian. Phys. Rev. 1968, 170, 379. 10.1103/PhysRev.170.379. [DOI] [Google Scholar]
- Kasha M. Energy transfer mechanisms and the molecular exciton model for molecular aggregates. Radiat. Res. 1963, 20, 55–70. 10.2307/3571331. [DOI] [PubMed] [Google Scholar]
- Hestand N. J.; Spano F. C. Expanded theory of H-and J-molecular aggregates: The effects of vibronic coupling and intermolecular charge transfer. Chem. Rev. 2018, 118, 7069–7163. 10.1021/acs.chemrev.7b00581. [DOI] [PubMed] [Google Scholar]
- Fukushima T.; Yoshimitsu S.; Murakoshi K. Unlimiting ionic conduction: Manipulating hydration dynamics through vibrational strong coupling of water. Chem. Sci. 2023, 14, 11441–11446. 10.1039/D3SC03364C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L.; Van Duijnen P. T.; Snijders J. G. A discrete solvent reaction field model for calculating molecular linear response properties in solution. J. Chem. Phys. 2003, 119, 3800–3809. 10.1063/1.1590643. [DOI] [Google Scholar]
- Tomasi J.; Mennucci B.; Cammi R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
- Giovannini T.; Cappelli C. Continuum vs. atomistic approaches to computational spectroscopy of solvated systems. Chem. Commun. 2023, 59, 5644–5660. 10.1039/D2CC07079K. [DOI] [PubMed] [Google Scholar]
- Wang W.; Vasa P.; Pomraenke R.; Vogelgesang R.; De Sio A.; Sommer E.; Maiuri M.; Manzoni C.; Cerullo G.; Lienau C. Interplay between strong coupling and radiative damping of excitons and surface plasmon polaritons in hybrid nanostructures. ACS Nano 2014, 8, 1056–1064. 10.1021/nn405981k. [DOI] [PubMed] [Google Scholar]
- Vasa P.; Pomraenke R.; Cirmi G.; De Re E.; Wang W.; Schwieger S.; Leipold D.; Runge E.; Cerullo G.; Lienau C. Ultrafast manipulation of strong coupling in metal–molecular aggregate hybrid nanostructures. ACS Nano 2010, 4, 7559–7565. 10.1021/nn101973p. [DOI] [PubMed] [Google Scholar]
- Ebbesen T.; Patrahau B.; Piejko M.; Mayer R.; Antheaume C.; Sangchai T.; Ragazzon G.; Jayachandran A.; Devaux E.; Genet C.; Moran J. Direct Observation of Polaritonic Chemistry by Nuclear Magnetic Resonance Spectroscopy. ChemRxiv 2023, 10.26434/chemrxiv-2023-349f5. [DOI] [PubMed] [Google Scholar]
- Luk H. L.; Feist J.; Toppari J. J.; Groenhof G. Multiscale Molecular Dynamics Simulations of Polaritonic Chemistry. J. Chem. Theory Comput. 2017, 13, 4324–4335. 10.1021/acs.jctc.7b00388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study is freely available under https://zenodo.org/doi/10.5281/zenodo.10572145.