Abstract
Musculoskeletal development and later post-natal homeostasis are highly dynamic processes, marked by rapid structural and functional changes across very short periods of time. Adult anatomy and physiology are derived from pre-existing cellular and biochemical states. Consequently, these early developmental states guide and predict the future of the system as a whole. Tools have been developed to mark, trace, and follow specific cells and their progeny either from one developmental state to the next or between circumstances of health and disease. There are now many such technologies alongside a library of molecular markers which may be utilized in conjunction to allow for precise development of unique cell ‘lineages’. In this review, we first describe the development of the musculoskeletal system beginning as an embryonic germ layer and at each of the key developmental stages that follow. We then discuss these structures in the context of adult tissues during homeostasis, injury, and repair. Special focus is given in each of these sections to the key genes involved which may serve as markers of lineage or later in post-natal tissues. We then finish with a technical assessment of lineage tracing and the techniques and technologies currently used to mark cells, tissues, and structures within the musculoskeletal system.
Keywords: Bone, Skeletomuscular System, Lineage Tracing, Fate Mapping, Model, Developmental Biology
1.0. Introduction:
Movement, structure, and support rely on a complex biomechanical network of static and dynamic tissues we define as the musculoskeletal system. Each component of the musculoskeletal system is highly specialized in form and function with unique approaches to homeostasis and repair. Each of these tissues in turn arose from a more primordial state, often developing via shared ancestral cells or tissues in a ‘lineage’ which can be traced across time. Adult form and function are derived from the interaction of these earlier states and, consequently, study of these lineages provides critical data to predict how adult tissues will respond to stress, injury, and disease. Because developmental states are typically transitory, the challenge of this study is accurate identification and tracking of each lineage across time. A range of tools have been developed to allow for precise ‘lineage tracing’ across animal models for study of musculoskeletal health and disease. In this review we will focus on those lineages which lead to the bones, muscles, cartilage, tendons, and ligaments necessary to facilitate structure and movement. We will explore the development of these structures, those markers which may be used to follow that development, and transitory patterns of expression which may be used to study key stages throughout.
2.0. Origins of the Musculoskeletal System:
Immediately after fertilization, the human ovum divides through several stages including the morula, blastocyst, and gastrula.1,2 The gastrula is a tri-laminar structure from which we derive the three commonly classified germ layers of ectoderm, endoderm, and mesoderm. The outer layer or ectoderm will become the nervous system and the epidermis and is the source for the neural crest cells. Neural crest cells are a migratory cell population which contributes to the musculoskeletal system of the head and neck. The inner layer or endoderm gives rise to the internal epithelium including both digestive and respiratory systems and their associated organs. The intermediate layer or mesoderm gives rise to a vast majority of the body’s structures including the substructures of the musculoskeletal system, the dermis, and the cardiovascular system.3 The mesoderm classically defines the musculoskeletal system and can be marked at this stage by the transcription factor brachyury also known as T-box transcription factor T (TBXT). Brachyury is necessary for mesodermal differentiation and, consequently, marks the entirety of the mesodermal lineage (Table 1&2).4–5
Table 1:
Sample Cre, CreERT, and Floxed Lines. Genes allowing for recognition and tracing of specific musculoskeletal lineage targets are represented as well as the class of modification (Cre, CreERT, Floxed). References provided are for manuscripts which first reference the generation of selected lines.
| Gene | Target Cells | Mouse Lines | References |
|---|---|---|---|
| TBXT (Brachyury) | Mesoderm | Cre | Perantoni et al., 2005 |
| CreERT | Anderson et al., 2013 | ||
| TBX6 | Paraxial Mesoderm (Pre-Somitic) | CreERT | Peter Lopez et al., 2012 |
| DLL1 | Paraxial Mesoderm (Pre-Somitic) | Cre | Ji et al., 2006 |
| PAX3 | Paraxial Mesoderm (Pre-Somitic); Muscle | Cre | Lang et al., 2005 |
| CreERT | Southard et al., 2014 | ||
| MEOX1 | Paraxial Mesoderm (Somitic) | Cre | Jukkola et al., 2005 |
| FOXF1 | Lateral Plate Mesoderm | Flox | Ren et al., 2014 |
| PRRX1 | Lateral Plate Mesoderm; Pre-Chondral Mesenchyme | Cre | Logan et al., 2002 |
| CreERT | Kawanami et al., 2009 | ||
| PAX7 | Muscle Satellite Cells | Cre | Lepper et al., 2009 |
| CreERT | Lepper et al., 2012 | ||
| Flox | Lepper et al., 2009 | ||
| MYF5 | Adult Muscle, Muscle Satellite Cells (Proliferating) | Cre | Tallquist et al., 2000 |
| CreERT | Biressi et al., 2013 | ||
| MYF6 (MRF4) | Adult Muscle, Muscle Satellite Cells (Differentiating) | Cre | Keller et al., 2004 |
| CreERT | Southard et al., 2014 | ||
| MYOD1 | Adult Muscle, Muscle Satellite Cells (Proliferating) | Cre | Haldar et al., 2014 |
| CreERT | Southard et al., 2014 | ||
| MYOG1 | Adult Muscle, Muscle Satellite Cells (Differentiating) | Cre | Li et al., 2005 |
| CreERT | Southard et al., 2014 | ||
| MCK/CKM | Adult Muscle | Cre | Bruning et al., 1998 |
| MYL 1/3 (MLC1/3) | Adult Muscle | Cre | Bothe et al., 2000 |
| CreERT | Southard et al., 2014 | ||
| NOTCH1 | Muscle Satellite Cells (Quiescent) | Flox | Yang et al., 2004 |
| STAT3 | Muscle Satellite Cells (Proliferating) | Flox | Moh et al., 2007 |
| SCX | Tenocyte/Ligamentocyte/Enthesis | Cre | Sugimoto et al., 2013 |
| CreERT | Howell et al., 2017 | ||
| MKX | Tenocyte/Ligamentocyte/Enthesis | Flox | Liu et al., 2010 |
| GLI1 | Tenocyte/Ligamentocyte Progenitors | CreERT | Ahn et al., 2004 |
| PDGFRα | Pre-Chondral Mesenchyme | Cre | Roesch et al., 2008 |
| CreERT | Kang et al., 2010 | ||
| Flox | Tallquist et al., 2003 | ||
| SOX9 | Pre-Chondrocytes, Chondrocytes, and Enthesis | Cre | Akiyama et al., 2005 |
| CreERT | Kopp et al., 2011 | ||
| Flox | Akiyama et al., 2002 | ||
| NKX3.2 (BAPX1) | Pre-Chondrocytes and Chondrocytes | Cre | Stanfel et al., 2006 |
| HES1 | Pre-Chondrocytes and Chondrocytes | Cre | Imayoshi et al., 2003 |
| CreERT | Kopinke et al., 2011 | ||
| COL2A1 | Chondrocytes, Stromal Cells | Cre | Ovchinnikov et al., 2000 |
| CreERT | Nakamura et al,. 2006 | ||
| ACAN | Pre-Chondrocytes and Chondrocytes | CreERT | Henry et al., 2009 |
| COL10A1 | Hypertrophic Chondrocytes | Cre | Chen et al., 2019 |
| RUNX2 | Hypertrophic Chondrocytes | Cre | Rauch et al., 2010 |
| Flox | Tu et al., 2012 | ||
| IHH | Hypertrophic Chondrocytes | Flox | Razzaque et al., 2005 |
| PTHrP | Resting Chondrocytes | CreERT | Mizuhashi et al., 2018 |
| Flox | He et al., 2001 | ||
| VEGF | Hypertrophic Chondrocytes | Flox | Gerber et al., 1999 |
| MMP13 | Hypertrophic Chondrocytes | Flox | Stickens et al., 2004 |
| GDF5 | Articular Cartilage (Interzone), Enthesis | Cre | Rountree et al., 2004 |
| CreERT | Shwartz et al., 2016 | ||
| WNT9A | Articular Cartilage (Interzone) | Flox | Spater et al., 2006 |
| TPPP3 | Synoviocytes | CreERT | Harvey et al., 2019 |
| PRG4 | Synoviocytes | CreERT | Kozhemyakina et al., 2015 |
| Flox | Hill et al., 2015 | ||
| OSX | Perichondrium, Periosteum, Stromal | Cre | Rodda et al., 2006 |
| CreERT | Maes et al., 2010 | ||
| HES1 | Perichondrium and Periosteum | Cre | Imayoshi et al., 2003 |
| CreERT | Kopinke et al., 2011 | ||
| DLX5 | Perichondrium and Periosteum | Cre | Ruest et al., 2003 |
| CreERT | Taniguchi et al., 2011 | ||
| OCN (BGLAP) | Osteoblasts | Cre | Zhang et al., 2002 |
| CreERT | Yoshikawa et al., 2011 | ||
| OPN (SPP1) | Osteoblasts | Cre | Español-Suñer et al., 2012 |
| DMP1 | Pre-Osteocytes and Osteocytes | Cre | Lu et al., 2007 |
| CreERT | Powell et al., 2011 | ||
| SOST | Pre-Osteocytes and Osteocytes | Cre | Xiong et al., 2015 |
| CreERT | Maurel et al., 2019 | ||
| TRAP (ACP5) | Osteoclasts | Cre | Chiu et al., 2004 |
| CTSK | Osteoclasts | Cre | Winkeler et al., 2012 |
| CreERT | Sanchez-Fernandez et al., 2012 | ||
| LYSM | Myloid (Pre-Osteoclast) | Cre | Clausen et al., 1999 |
| CreERT | Canli et al., 2017 | ||
| RANK (TNFRSF11a) | Myloid (Pre-Osteoclast) | Cre | Maeda et al., 2012 |
| LEPR | Marrow Stromal Cells | Cre | Leshan et al., 2006 |
| Flox | Coehn et al., 2001 | ||
| ADIPOQ | Marrow Adipogenic Progenitors | Cre | Eguchi et al., 2011 |
| CreERT | Jeffrey et al., 2015 | ||
| CLEC11A | Marrow Osteogenic Progenitors | Flox | Zhang et al., 2021 |
Table 2:
Mesodermal Developmental Structures, Stages, and Markers
| Mesodermal Subunit | Lineage Markers | Developmental Structures | Adult Structures |
|---|---|---|---|
| Mesoderm (Gastrula) | TBXT/Brachyury | ||
| Chordamesoderm | COL2A1 SHHA IHHB Cytokeratin 8/18/19 Galectin-3 |
Notochord | Vertebral Disks Intervertebral Bodies Nucleus Pulposus |
| Paraxial Mesoderm | MSGN1 (Posterior Paraxial) TBX6 (Anterior Paraxial) PAX3 (Anterior Paraxial) UNCX (Posterior Somite) TBX18 (Anterior Somite) |
Somites | Axial and Appendicular Dermis Axial and Appendicular Skeletal Muscle Axial Ligament/Tendon Axial Articulation (Cartilage/Synovium) Axial Bones |
| Intermediate Mesoderm | PAX2 LHX1 |
Kidney Gonads |
|
| Lateral Plate Mesoderm | FOXF1 PRRX1 |
Somatic Coelum | Thoracic and Abdominal Cavities |
| Somatic Mesoderm | Body Wall Limb Bud Appendicular Ligament/Tendon Appendicular Articulation (Cartilage/Synovium) Appendicular Bones Parietal Peritoneum Parietal Pleura Parietal Pericardium |
||
| Splanchnic Mesoderm | Visceral Peritoneum Visceral Pleura Viasceral Pericardium Visceral Linings |
2.1. The Mesodermal Lineage
The trilaminar gastrula develops axially-to-laterally around the neural tube into several distinct mesodermal structures.6 Centermost of these is the axial mesoderm, also known as the chorda-mesoderm, which underlies the neural tube and gives rise to the notochord.7 A majority of cells and structures derived from the axial mesoderm are transitory during development with only some components of the vertebral disks, intervertebral bodies, and nucleus pulposus present into adulthood.7
The next three mesodermal structures arranged from medial to lateral are the paraxial mesoderm, the intermediate mesoderm, and the lateral plate mesoderm (Table 2).8 Of these, the paraxial and lateral plate mesoderm are most relevant to musculoskeletal development lending to the generation of the somites and somatopleure respectively (Table 3).8 The intermediate mesoderm is not a primary contributor to musculoskeletal development instead developing into the urogenital system. From this is can be determined that lineage tracing of the musculoskeletal system must focus on expression of markers distinct to the paraxial (mesogenin 1 (MSGN1), T-Box transcription factor 6 (TBX6), delta 1 (DLL1), and paired box 3 (PAX3)) and lateral plate (forkhead box protein F1 (FOXF1), paired related homeobox 1 (PRRX1), and GATA binding protein 4 (GATA4)) mesoderm (Table 1&2).8
Table 3:
Somite Developmental and Adult Structures
| Somite Derivative | Developmental Structures | Adult Structures |
|---|---|---|
| Dermomyotome | Dermotome Myotome |
Dermis Skeletal Muscle |
| Sclerotome (Primordial) | Syndetome Arthrotome Endotome Sclerotome (Proper) |
Tendon Joints Endothelial Bones |
2.2. Paraxial Mesoderm and Somite Formation
The paraxial mesoderm is the source of the axial musculoskeletal system and has a unique pattern of forty-two to forty-four paired cranial to caudal segments called somites. After paraxial mesoderm first coalesces and elongates it develops from posterior to anterior with the posterior being more immature and the anterior more committed. Spatial restriction of MSGN1 and TBX6 marks the posterior somite while PAX 3 marks the more anterior component (Table 2).8 Along the craniocaudal axis elongation and differentiation are driven by wingless-type integration site family (WNT), neurogenic locus notch homolog protein (NOTCH), and fibroblast growth factor (FGF) signaling.9–11 Mediolateral development and identity is in turn driven by bone morphogenetic protein (BMP) signaling gradients. Consequently, the anterior zone of the paraxial mesoderm segments into the embryonic somites.9–11 Post-somite segmentation mesenchyme homeobox 1 (MEOX1) may be utilized to track the paraxial lineage (Table 1). As somites mature, WNT6 signaling in the dorsal ectoderm activates transcription factor 15 (TCF15/Paraxis) which organizes mesenchymal-to-epithelial transition of the anterior somite.66–67 This generates a trilaminar structure consisting of a mesenchymal core with both dorsal and ventral epithelial lamina. The epithelial lamina eventually forms a cleft which separates the developing somite.12–13 Once separated, somites compartmentalize along a dorsoventral and mediolateral axis in a gradient of the dermomyotome (further dividing into dermotome and myotome), syndetome, and sclerotome (Table 3). Each of these components are further subdivided into anterior and posterior zones marked by T-Box transcription factor 18 (TBX18) and UNC homeobox (UNCX) respectively.14–16 The dermatome will later develop into the dermis, connective tissues, and adipose of the neck and trunk. The myotome will develop into axial muscles with a further subdivision in the epimere (extensors) and the hypomere (anterior thoracic and abdominal walls).17–18 Continuing in this manner the sclerotome will give rise to the syndetome (tendon/ligaments), the arthrotome (articular cartilage), the endotome (some endothelial tissues), and the proper sclerotome which is origin for axial bones.
2.3. Lateral Plate Mesoderm and the Limb Bud
Expression within the early lateral plate is highly heterogeneous with individual markers either a) not marking all lateral plate lineages (FOXF1, PRRX1, GATA4) or b) marking non-lateral plate lineages (TBXT). Grossly, the lateral plate mesoderm divides into a dorsal somatic mesoderm (somatopleure) and a ventral splanchnic mesoderm (splanchnopleure).19–20 Between these sheets is the intraembryonic or somatic coelom which gives rise to the abdominal and thoracic cavities. Of these, the somatopleure contributes primarily to the musculoskeletal system. The somatopleure gives rise to body wall and is subdivided further into the anterior limb bud, the posterior limb bud, and the non-limb forming wall including the parietal peritoneum, pleura, and pericardium. The anterior and posterior limb buds may be marked by T-box transcription factor 5 and 4 (TBX5, TBX4) respectively (Table 4). As the nascent limb bud begins to develop, cells derived from the somitic paraxial mesoderm migrate including myogenic populations which will later form the appendicular musculature. Polarity and positional signaling is a complex and highly conserved system, however, a full accounting of the early limb development process is beyond the scope of this review. As the limb begins to form along the gradient axes established during the early limb bud musculoskeletal structures beginning as early mesenchymal condensates will form which will develop later into established structures. Higher level patterning of these structures appears dependent on homeobox (HOX) gene transcription factors. During development the proximodistal axis of the limb divides into three regions which from proximal to distal include the stylopod (HOX9/10), the zeugopod (HOX11), and the autopod (HOX 12/13) (Table 4).21–22 Bones which form from the stylopod include the humerus and femur. Those forming the zeugopod include the radius, ulna, tibia, and fibula. The carpus/tarsus, metacarpus/metatarsus, and phalanges form the autopod (Table 4).23
Table 4:
Limb Development, Markers, and Adult Structures
| Limb Development | Lineage Markers | Adult Structures |
|---|---|---|
| Forelimb Bud Mesenchyme | TBX5 | Forelimb |
| Hindlimb Bud Mesenchyme | TBX4 PITX1 |
Hindlimb |
| Stylopod | HOX9/10 | Humerus Femur |
| Zeugopod | HOX11 | Radius/Ulna Tibia/Fibula |
| Autopod | HOX12/13 | Carpus/Metacarpus Tarsus/Metatarsus Phalanges |
3.0. Musculoskeletal Development:
3.1. Cartilage and Bone:
Initial formation of appendicular bone occurs by the process of endochondral ossification. A condensation of lateral plate mesoderm-derived mesenchyme, typically marked by platelet-derived growth factor receptor alpha (PDGFRα), forms in the area which has been patterned by HOX genes to form bone primordia (Table 1&5).23–27 As the mesenchymal condensate expands cells which are more centrally located will transdifferentiate in chondrocytes. In a majority of pre-chondrocytes this event is marked expression of the master chondral regulatory SRY-box transcription factor 9 (SOX9) followed by the osteogenic inhibitor Nirenberg and Kim 3 homeobox 2 (NKX3.2).34–35 Peripheral to this, a separate SOX9-negative group of pre-chondrocytes expressing hairy and enhancer of split-1 (HES1) begins to emerge. At this time the surrounding mesenchyme will also begin to develop into the multilaminar perichondrium (Table 5).28–29 Closest to the chondral template, in the deepest perichondral layers multi-potent osterix (OSX) positive cells can be found with both potential as both chondrogenic and osteogenic precursors.30–31 A further HES1 and distal-less homeobox 5 (DLX5) positive population stratifies to the superficial perichondrium which will later contribute both osteoblasts and later marrow stem cells.32–33 The pre-chondrocytes then begin to mature and express chondroid matrix markers including collagen type 2 alpha 1 chain (COL2A1) and aggrecan (ACAN). In the deepest portion of the cartilage template chondrocytes will begin to mature then hypertrophy.36–37 These hypertrophic chondrocytes begin to express collagen type X alpha 1 chain (COL10A1) as well as vascular signals such as vascular endothelial growth factor (VEGF) to trigger surround vasculature to invade the chondral template (Table 5).38 Vascular invasion triggers both simultaneous resorption of cartilage and ossification as well as the generation of the marrow space.
Table 5:
Appendicular Musculoskeletal Structures and Derivatives
| Appendicular Musculoskeletal Component | Lineage Markers | Mesodermal Lineage |
|---|---|---|
| Pre-Osseous Mesenchymal Condensate |
PRRX1
PDGFRa |
Lateral Plate |
| Chondral Template/Immature Chondrocytes |
SOX9
NKX3.2 HES1 COL2A1 ACAN |
Lateral Plate |
| Hypertrophic Chondrocytes |
COL10A1
RUNX2 VEGF NOTCH 1/2 IHH MMP13 |
Lateral Plate |
| Perichrondrium |
OSX
HES1 DLX5 WNT5A WNT11 |
Lateral Plate |
| Trabecular Bone (Osteoblasts) |
OCN
OPN COL1A1 |
Lateral Plate |
| Osteoclasts |
TRAP
Cathepsin K |
Lateral Plate |
| Articular Cartilage |
GDF5
GDF6 WNT9A TPPP3 |
Lateral Plate |
| Synovium | PRG4 | Lateral Plate |
| Tendon/Ligament |
SCX
MKX |
Lateral Plate |
| Enthesis |
SCX
SOX9 GDF5 |
Lateral Plate |
| Myotendinous Junction | COLXXII | Mixed |
| Muscle |
PAX3
MYF5 |
Paraxial |
| Muscle Satellite Cells |
PAX7
NOTCH |
Paraxial |
As bone matures a highly diverse mesenchymal and hematopoietic niche develops in the medullary space such that common lineage markers may represent a myriad of distinct sub-populations. Hematopoietic stem cells (HSCs) are incredibly heterogenous such that a combination of lineage positive and negative markers are required to identify specific populations.39–40 One common combination includes signaling lymphocytic activation molecule 1 (SLAMF1/CD150) and stem cell antigen-1 (SCA1) positivity alongside SLAMF2/CD48 and lineage (LIN: CD3, CD45R (B220; PTPRC), CD11B, Ly6G, TER119) negativity.39–40 Some cre-driven lineages have also been developed which included FMS like tyrosine kinase 3 (FLT3), HOXB5, and alpha-catulin (CTNNAL1).39–40 In addition to hematopoietic stem cells marrow is enriched for leukocyte populations (monocytes/macrophages, dendritic cells), megakaryocytes, endothelial, and neuronal tissues as well as resident mesenchymal stromal cells (MSCs).39–40 During early post-natal development nestin (NES) marks both marrow stromal cells and osteoblasts. These marrow stromal cells include several mesenchymal progenitor populations (OSX, COL2A1, HOXA11) which modulate osseous homeostasis.39–40 Within the early metaphysis OSX and HOXA11 positive population generate a stable leptin receptor (LEPR) expressing stromal population which includes the presumptive skeletal stem cells.41–42 These LEPR positive populations may then subdivide into an adiponectin (ADIPOQ) positive and osteolectin (CLEC11A) positive lineage of adipocytes and osteogenic progenitors respectively (Table 1&5).43–44 Recent data suggests that the spatial relation of these LEPR positive populations (perisinusoidal vs. periarteriolar) may help mark those cells of adipogenic (perisinusoidal) vs. osteogenic (periarteriolar).44
3.2. Growth Plates:
Upon formation of the initial or primary ossification center the immature chondrocytes which remain proximally and distally become aligned and organize into zones of rest, proliferation, and hypertrophy. These parallel disks and the primary ossification center when taken as a unit are referred to as the epiphyseal or growth plate. This is an ultimately transient structure that is maintained by negative feedback of parathyroid hormone-related protein (PTHrP) secreted by resting chondrocytes which in turn binds reciprocal receptors on proliferating chrondrocytes in a paracrine manner.45–47 Once bound this suppresses terminal differentiation to the indian hedgehog (IHH) producing hypertrophic chondrocytes. As this is a paracrine effect, sustained proliferation of chrondrocytes eventually moves away from the resting zone, reducing the PTHrP signal and IHH signaling increases resulting in activation of runt-related transcription factor 2 (RUNX2) supporting terminal osteogenesis (Table 1&5).
3.3. Joints/Articulations:
Articular surfaces form at the surface between the bones of developing limb fields and the earliest histologic precursor to joint formation is the development of an interzone between distinct cartilage templates. These are avascular zones of densely packed mesenchymal cells which are flattened and oriented perpendicular the adjacent chondral templates. Several markers have been identified which may mark interzone cells joint formation. WNT9A and growth differentiation factor 5/6 (GDF5/6) are some of the earliest consistent markers of interzone cells consequently defining an articular cartilage lineage.48–51 Interzone markers appear to have complex and overlapping expression patterns driven by both positive (odd-skipped-related transcription factor 1/2 (OSR1/2) and NOG) and negative regulatory elements (BMP 2/4 and fibronectin receptor/alpha5beta1 integrin (FNR)).51–55 Synovial demarcation can further be identified by expression of tubulin polymerization-promoting protein family member 3 (TPPP3).56 TPPP3 expression aligns with cavitation and alongside proteoglycan 4 (PRG4/lubricin) mark synoviocytes (Table 1&5).56
3.4. Ligaments, Tendons, and their Interfaces:
Tendons and ligaments are force-transmitting structures which interface between muscle and bone or bone and bone respectively. The developmental differences between tendons and ligaments are poorly defined and for the sake of this review they will mostly be discussed as a single developmental entity. Tenocytes and ligamentocytes are the primary cell of the tendon and ligament respectively and are of mesodermal lineage. Tenocytes of the appendicular and axial body have different origins with those of the trunk arising the syndetome, a somite sub-element derived from the paraxial mesoderm.57 In the limb, tenocytes are derived from the mesenchyme of the lateral plate mesoderm and differentiate in response axial-to-appendicular migration of myoblasts from the myotomes. Currently it is believed that tenocytes of either origin are dependent on expression of scleraxis (SCX) and mohawk (MKX) during differentiation (Table 1&5).58–62 Consequently, a majority tenocytes are presumed to be of a SCX-lineage regardless of paraxial or lateral plate source. This is not universal, however, and some anchoring ligaments as well as short tendons are not entirely dependent on SCX expression. Once tenocytes have developed tendon development occurs in two stages. During the first stage, ribbons of tenocytes delineate a zone of collagen fibril deposition. This is followed by a second mohawk homeobox (MKX) dependent second stage in which the collagen template is utilized to grow and develop mature tendons. In force-transmitting tendons and ligaments this process is presumed to be related to mechanical stimulation.
Where tendons and ligaments connect to bones, we find a distinct transitional structure termed the enthesis. This is a zonal structure primarily composed of fibrocartilage which gradually transitions between the primarily fibrous elements of the tendon/ligament to the primarily mineralized elements which transition to bone. The fiber-cartilage-bone transition of the enthesis shares some histologic similarities with endochondral ossification at the cartilage-bone interface. Interestingly, a gradient of overlapping expression including SCX, SOX9, GDF5, and hedgehog are critical for both identity and maturation.63–65 The exact signaling process is as of yet unknown, however as with ligaments and tendons proper maturation of the system is presumed to be dependent on transmission of mechanical signals.
Opposite to the enthesis is the myotendinous junction. This is the interface between muscle and tendon or more accurately between the investing fascia of muscle and the tendon-proper. As opposed to the gradual transition of the enthesis the myotendinous junction resembles long extended processes each sheet of extracellular matrix between sarcolemma units of muscle directly conjoins together and into tendon collagen fibers.66 While maturation of this structure is well described in adults the exact developmental origin of the myotendinous junction is unclear. Collagen XXII is one of the few proteins primarily restricted to the myotendinous junction and thus may be helpful in marking fate of resident cells.67
3.5. Muscles:
Skeletal muscle, whether axial or appendicular, is derived from the paraxial mesoderm. Skeletal muscle first arises with the determination of pre-myogenic progenitor cells and eventually myoblasts in the somite. Earliest myogenesis initiates with activation of first PAX3 then myogenic factor 5 (MYF5) in the dorsomedial somite.68–69 Embryonic or primary myogenesis involves the production myofibers from PAX3 positive dermatomyotome progenitors.70–71 Myf5 then marks the primary myotome present immediately ventral to the dermomyotome proper. Mononuclear myocytes arise in this zone gradually elongating along a craniocaudal axis to span the somite. Simultaneously cells from the dermomyotome continue to translocate to the myotome acting as myogenic progenitors while more laterally myogenic cells migrate to the lateral plate and into limb bud to form appendicular skeletal muscle. These appendicular myocytes will then coalesce and form myofibers of their own. This process of primary myogenesis forms the primordial template for each of the skeletal muscles of the trunk and limbs.72–73 Secondary myogenesis occurs as further PAX3 positive progenitors express PAX7 then translocate to fuse to the primary fibers.74 Growth throughout this stage involves cell fusion rather than hypertrophy as is described in adult tissues. A portion of the PAX7 positive progenitors from the central dermatomyotome will maintain their developmental characteristics under Notch signaling and will contribute to the satellite cell population of adult muscle stem cells.
The transition from mononuclear myoblast to myotube to myofiber begins with recognition and adhesion of adjacent myoblasts. Elongation is formed by the successive fusion of chains of myoblasts and or multiple myotubes. Myotubes then undergo maturation in a process call myofibrillogenesis in which the myotube begins to segment into contractile modules called sarcomeres which are surrounded by and anchored to specialized connective tissue called the endomysium. In addition to mechanically supporting the sarcomere the endomysium transmits neuronal signals. This segmentation gives rise to the myofibrils present in adult muscle which extend the length of the muscle and are anchored by the myotendinous junctions.
4.0. The Adult Musculoskeletal System
Here we will discuss the process of musculoskeletal healing with a focus on those tissue- and site-specific markers which may be utilized as reporters during or after the healing process.
4.1. Fracture Healing, Bone Homeostasis, and Pathologic Bone Formation:
Adult bone is inhabited by several cells directly involved in osseous homeostasis including osteocytes/osteoblasts (osteocalcin (OCN), dentin-matrix protein (DMP1), sclerostin (SOST)) and osteoclasts (tartrate resistance acid phosphatase (TRAP)) as well as those of the periosteal and endosteal linings and the medullary/stromal space (Leptin Receptor (LEPR), neuron-glial antigen 2 (NG2)/chondroitin sulfate proteoglycan 4 (CSPG4), NES, Gremlin 1 (GREM1)).39–44 Extent of injury helps define which cellular populations are involved in repair. While microfractures can be managed during normal bone homeostasis significant fracture sets off an organized cascade of steps which utilizes the cellular and biochemical machinery of endochondral ossification to restore osseous integrity. As endochondral ossification utilizes developmental machinery, mesenchymal (PDGFRα), early chondral (SOX9, COL2A1), and hypertrophic cartilage (COL10A1, RUNX2) markers are valuable to track new versus pre-existing bone. Bone healing typically begins at time of fracture with immediate hematoma formation secondary to rupture of periosteal and endosteal vessels as well as disruption of the marrow cavity (Fig. 1A). The subsequent clotted hematoma serves as both scaffold and signaling center for leukocytes, endothelial cells, and ultimately mesodermal lineage cells. Disruption of the medullary canal and consequent sinusoidal space allows for activation of megakaryocytes, leukocyte populations, endothelial cells (platelet endothelial cell adhesion molecule 1 (PECAM1/CD31) and apelin (APLN)), and mesenchymal stromal cells (LEPR (Fig. 1B&C), stem cell factor (SCF), and C-X-C motif chemokine ligand 12 (CXCL12)) responsible of skeletal and hematopoietic maintenance.39–44 Ossification then occurs by two simultaneous processes. First, endochondral ossification will proceed from within the callus as described above. Second, at the level of the periosteum woven bone will be formed directly from resident osteoprogenitors. OSX-lineage positive cells in the periosteum are noted to proliferate and co-localize to/co-invade alongside vascular populations contributing to woven bone (Fig. 1B&C).30–31 Over time immature bone will remodel until the initial injury is functionally resolved.
Figure 1:
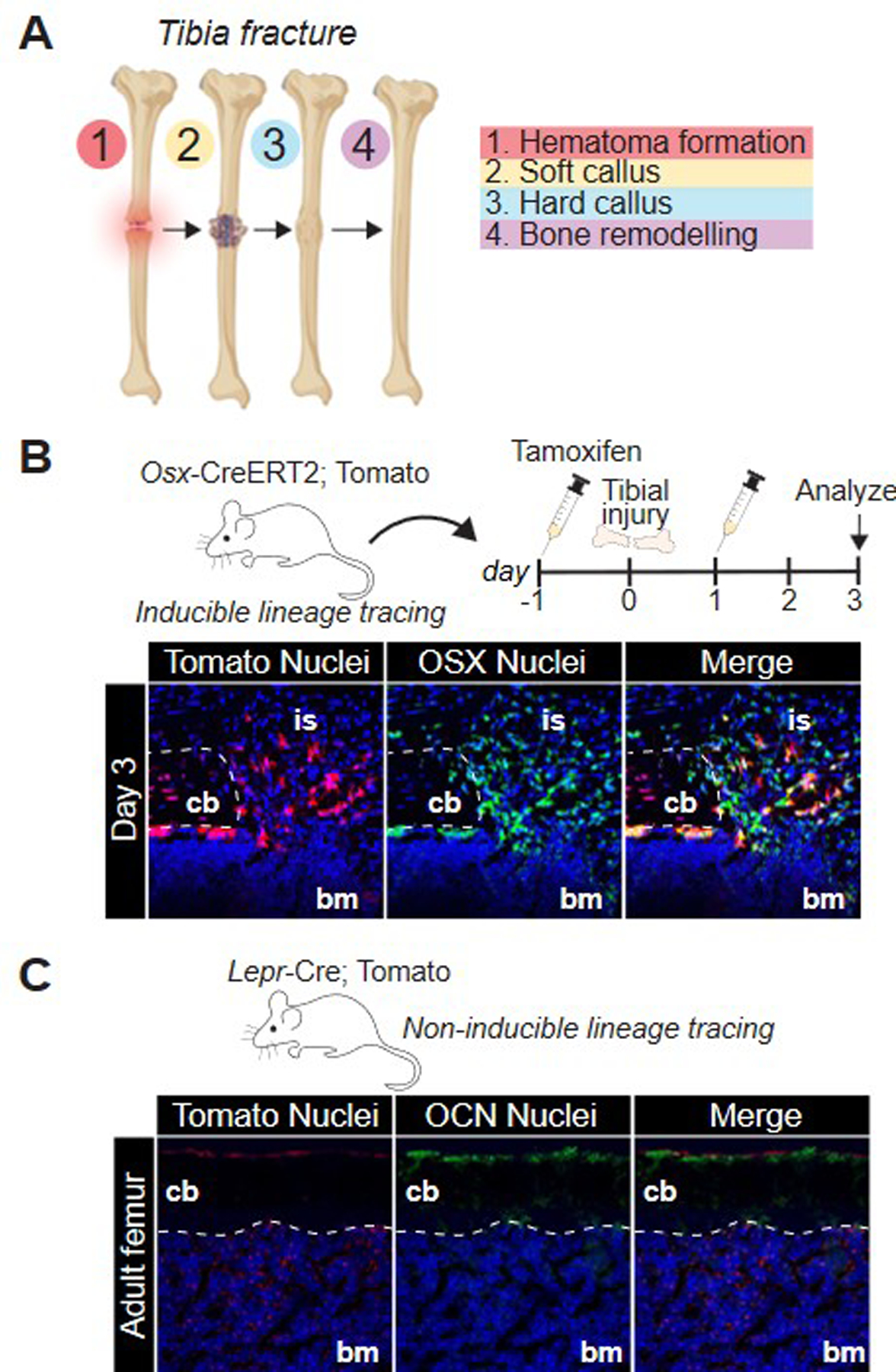
(A) Stages of fracture repair. (B) inducible cre strategy using an osx-cre-ERT2 transgenic mouse crossed with a Rosa-tdTomato mouse. After tamoxifen injection, osterix-expressing cells will express tdTomato, as shown here in post-operative day 3 section of a tibial injury. Immunofluorescence against osterix revealed co-labeling of osx and tomato-positive cells. (C) Non-inducible labeling strategy utilizing a LepR-cre and tdTomato mouse in which all LepR-expressing cells and their progeny will express tdTomato.
The body retains capacity for de novo bone and cartilage formation well into adulthood. While this can be physiologic there are several conditions of pathologic bone formation. One common variant is the formation of osteophytes. Inflammatory injury to periosteum or synovial lining stimulates proliferation of mesenchymal stem-like cells present in the periosteum stimulating rapid proliferation.75–76 These cell masses may then undergo endochondral ossification and ankylosis to the original bone.75–76 During this process intramembranous ossification may occur and contribute to the final osteophyte. A less common but potentially debilitating process is heterotopic ossification or ectopic bone formation.77 Similar to fracture healing, this condition can be traced by the same genes which mark each stage of endochondral ossification in which a mesenchymal anlage undergoes chondrification followed by ossification and eventually maturation as described above.77–78
4.2. Articular Cartilage Injury and Repair:
Articular cartilage is a well-organized, stratified/zonal structure. Articular cartilage is primarily avascular and receives nutrient exchange superficially by synovial fluid and at the deep surface by subchondral bone. Developmental lineage markers including SOX9 may be retained into adulthood, however, joints may also be effectively marked by ubiquitous chondrocyte extracellular matrix markers (COL2A1, ACAN, Cartilage Matrix Protein (Matrilin)).79 In mature articular cartilage expression patterns of many extracellular matrix components are zonally restricted. Aggrecan is primarily limited to those less-mineralized chondrocytes towards the articular surface away from the ‘tidemark’.80 Matrilin-3 localizes to joints and is further restricted to tangential and upper middle cartilage zone chondrocytes.81 Proteoglycan 4 (PRG4)/Lubricin is expressed more abundantly in chondrocytes of the superficial zone as well as synoviocytes.82–83 Other cartilage matrix proteins are less specific with Matrilin-1 present primarily during development and more in adult joints whereas Matrilin-2, while common in adult joints is also present in several extraskeletal tissues.84–85
Cartilage healing is limited under physiologic conditions. Given the relative avascularity of the superficial zones, partial-thickness injuries often demonstrate poor healing. Full-thickness defects, however, with disruption of subchondral bone are more able to demonstrate a limited form of regeneration. Under these conditions a fibrocartilaginous anlage similar to that seen in endochondral ossification forms. Healing at this level involves recruitment of GDF5 (articular) and NES (skeletal) lineage progenitors to form an osseocartilaginous callus, however, in absence of additional trauma or inflammation this typically arrests without further ossification.85–87 Repetitive injury/healing cycles such as seen in osteoarthritis (OA) further degrade the articular surface and can tip the balance towards osteogenesis as described below.
4.3. Tendinous and Ligamentous Healing:
Tendons and ligaments share similar pathways to healing, each undergoing overlapping stages of hematoma, inflammation, proliferation, and remodeling after trauma. Endothelial injury, relative hypoxia, and breakdown of coagulation byproducts signals for both endothelial and immunologic infiltration. Stages of inflammation include rapid response neutrophils followed by macrophages and then fibroblasts. Macrophages as well as resident cells of the epitenon and endotenon are key mediators of fibroproliferative signaling, expressing high levels of connective tissue growth factor (CTGF), transforming growth factor beta (TGFβ), and insulin-like growth factor 1 (IGF1) throughout the transition from inflammation to proliferation.88–89 The epitenon is a critical source for tendon progenitor cells (PDGFRA, Tubulin polymerization-promoting protein family member 3 (TPPP3), NES, SCX) with both perivascular and resident fibroproliferative cells contributing to tenogenic healing.90 At the enthesis additional contributions from tendon, bone, and the fibrocartilaginous anlage contribute to repair of the bone-cartilage-fibrous transition. In neonates, glioma-associated oncogene/zinc-finger protein (GLI1) marks a resident progenitor population capable of replenishing mineralize fibrocartilage (Table 1).91 This population loses Gli1 expression in adulthood and terminally differentiates with reduced regenerative capacity after injury.
4.4. Muscle Homeostasis, Healing, and Degeneration:
Muscle healing is highly dependent on the degree of injury and similar to bone is a balance of ordered regenerative processes and more fibrotic terminal processes in the cases of extensive or critically sized injuries. Repair after injury is typically managed in stages of degeneration followed by regeneration. Degeneration occurs after any injury sufficient to disrupt myofiber and consequently sarcolemma integrity. This results in rapid necrosis of affected myofibers and both signals for and is followed by inflammatory infiltration by phagocytic populations – first neutrophils then predominantly macrophages. Muscle regeneration follows and in healthy tissues this is typically marked by myogenic proliferation in which new myofibers are formed from the division, differentiation, and fusion of fibers to injured tissue. Injury additionally induces M-Cadherin expression in both injured myotubes and in satellite cells and M-Calpain in fusing myoblasts.92 New myofibers will then undergo hypertrophy prior to transition back to a morphologically consistent state with surrounding muscle.
Satellite cells are undifferentiation myogenic cells located peripheral to mature myofibers. These are mononuclear cells typically arrested under NOTCH signaling in a progenitor state and are most commonly derived from PAX7 positive populations from the central dermatomyotome.93–94 When muscle is uninjured and at rest these cells are typically quiescent and are only activated in response to trauma or high demand. Satellite cells are marked by myocyte nuclear factor (MNF) signaling.95 MNF is distinct in that it has two distinct MNFα and MNFβ with the beta isoform more abundant when quiescent and the alpha isoform more abundant during satellite cell activation. Satellite cells are capable of stem-like behavior and when activated the pool will asymmetrically proliferate with a portion their progeny differentiating to generate new myoblasts with the capacity to fuse during regeneration. Asymmetry of this process is thought to be necessary for maintenance of the stem cell population and is related to the variability in ratios between NUMB and NOTCH1 signaling in daughter cells.93–96 Upon activation satellite cells will express MYF5, myogenic differentiation 1 (MYOD), and signal transducer and activator of transcription (STAT3) during proliferation followed by myogenin 1 (MYOG1), myogenic factor 6/myogenic regulatory factor 4 (MYF6/MRF4), and Snail family transcriptional repressor 2 (SNAI2) during differentiation and fusion.97–100 Given the range of factors associated with muscle regeneration several animal models now exist with which to aid in the study and tracking myocyte and satellite cell populations (Table 1). In addition to satellite cell activity, current literature supports contribution from circulating NES-positive MSC and HSC progenitors.101–102
When myogenic regeneration fails, volumetric muscle loss and/or replacement occur. Muscle fibrosis, fibrofatty change, and sarcopenia make up a cluster of diseases related in part to the failure of normal muscle homeostasis. In both muscle fibrosis and fibrofatty change invasion and proliferation of non-myogenic cells are key to pathology with mesenchymal tissues (PDGFRA, SCA1).103–105 Because of the similarities between the two processes a significant body of work has gone into the identification of a common fibro/adipogenic progenitor. Extent and type of injury guides fibrotic vs. adipogenic (peroxisome proliferator- activated receptor gamma (PPARγ) and glycogen synthase kinase 3 (GSK3)) differentiation.106–108 Intra-muscular ectopic ossification, as described in heterotopic ossification above, may incorporate this fibro/adipogenic progenitor population, however, tracing experiments suggest alternate cell sources for these lesions.109–110
5.0. Technical Aspects of Lineage Tracing and Fate Mapping:
The musculoskeletal system is marked by its diversity of adult and developmental structures. Because of the transient nature of processes such as somite differentiation, endochondral ossification, or satellite cell activation, lineage tracing of population across time is necessary to study the system as a whole.111–112 Consequently, tracking a cell or cellular lineage across time requires the ability to mark the target in such a way that we are able to accurately identify either the original cell or its progeny at a later date. Though this may be achieved by direct observation in physically small, developmentally immature, or translucent specimens, markers become critical in the study of health and disease in larger, adult, or mammalian models.114–116 Accuracy of any such technique is dependent on several key factors. First, the marker must persist to the desired generation without loss of sufficient signal. Second, the marker may not be spread or in some manner diffuse to cells not of the desired lineage. Thirdly, the marker must not affect the normal proliferation, differentiation, migration or any other part of the cell lines development in such a way as to alter the normal function of the cell or its progeny. This can be achieved in a variety of ways which we will briefly summarize below.
5.1. Model Organisms
Historically invertebrates and lower vertebrates have been utilized for fate mapping and lineage tracing as their small size, availability, and rapid generational turnover has facilitated such endeavors. In the modern era, however, more and more clinically translatable studies draw data from mammalian models. Rodents, as example, are logistically amenable to both germline mutations and study of both developmental and pathologic processes. A wide range of existing reporter animals now exist for commercial purchase or generation with high degrees of accuracy (Table 1).117–118 In this review we will not focus on individual strains of animals, but rather those genes which, during development or disease, are relevant to lineage tracing of musculoskeletal cells. Briefly, it is important to note that despite the highest degree of clinical relevance, direct genetic labelling in humans is not supported in the setting of current legislative and bioethical concerns relating to germline modification. More available are those nuclear and mitochondrial somatic mutations which occur naturally and allow for post-differentiation characterization of shared lineages between cells and tissues, or in the labelling of tissues in vitro for autotransplantation. Given the relative infancy of this field, however, no comprehensive lineage analysis is present for the musculoskeletal system by these techniques.
5.2. Direct and Indirect Labeling
The addition of a dye to a cell population is one of the earliest techniques utilized for temporo-spatial cell tracking.114,119–120 Non-toxic or vital dyes were critical to the earliest embryonic fate mapping, and successive improvements in dye techniques have led to a broad range of lipid- and water-soluble and insoluble dyes as well as radiolabeled or fluorophore-conjugated compounds capable of sustaining signal across several generations of cell division. Dyes can be taken up and trapped in cells, incorporated into DNA, or can irreversibly bind to lipids and/or proteins of the cell membrane and several commercially available products exist to make use of these various mechanisms. Depending on the technique, dyes can additionally be used for pulse-chase experiments to track cell division and or to identify cell populations in which no genetic marker is currently known.121–122 Even modern dyes have some limitations. Because dyes are limited to their original exposure signal strength can be lost with each cellular division. Consequently, cells which divide rapidly are more likely to deplete their signal. Furthermore, particularly with those dyes that bind to proteins or lipids there is the risk that either the dyed cell or other phagocytic cells will recognize the foreign material which may lead to signal degradation, changes to cell function, or even cell death.
5.3. Gene Constructs
Modern lineage tracing primarily utilizes one or more genetic constructs to generate a stable and selective reporter. The actual insertion of these constructs into organisms whether germline or in adult cells and whether by mechanical123–125, electrical126, chemical127, viral (adenoviral, lentiviral)128, or proteomic (Zinc Finger Nucleases, TALENs, CRISPR/Cas9)129–131 is beyond the scope of this review in which we will focus primarily on specific constructs and their application to lineage tracing in the setting of musculoskeletal development.
Historically lineage tracing utilizing gene constructs is dependent on a system of inducible insertion/deletion centered around Cre-Lox recombination described by Sternberg et al. Successive iterations have let to the identification of a range of site-specific recombinases and more refined control of recombination events.132–133 In brief, a promoter, typically that of a specific gene of interest which expression limited to an ancestral cell or cells within the lineage of interest, is linked to the production of a site-specific recombinase. In cells which produce this recombinase a second, typically inert, transgene flanked by some identifying sequence undergoes a recombination event to activate said transgene and thus marks the cell (Fig. 2A, B).134 These transgenes are often colorimetric, genetic, functional, or fluorescent reporters, described later in this review, which will remain present and active into the progeny of a given cell consequently marking all descendent in a specific lineage. As these reporter cassettes and their activating promoters become more complex, systems of alternating color can be used to mark extensive and cellular lineage with ever increasing accuracy (Table 1).
Figure 2:
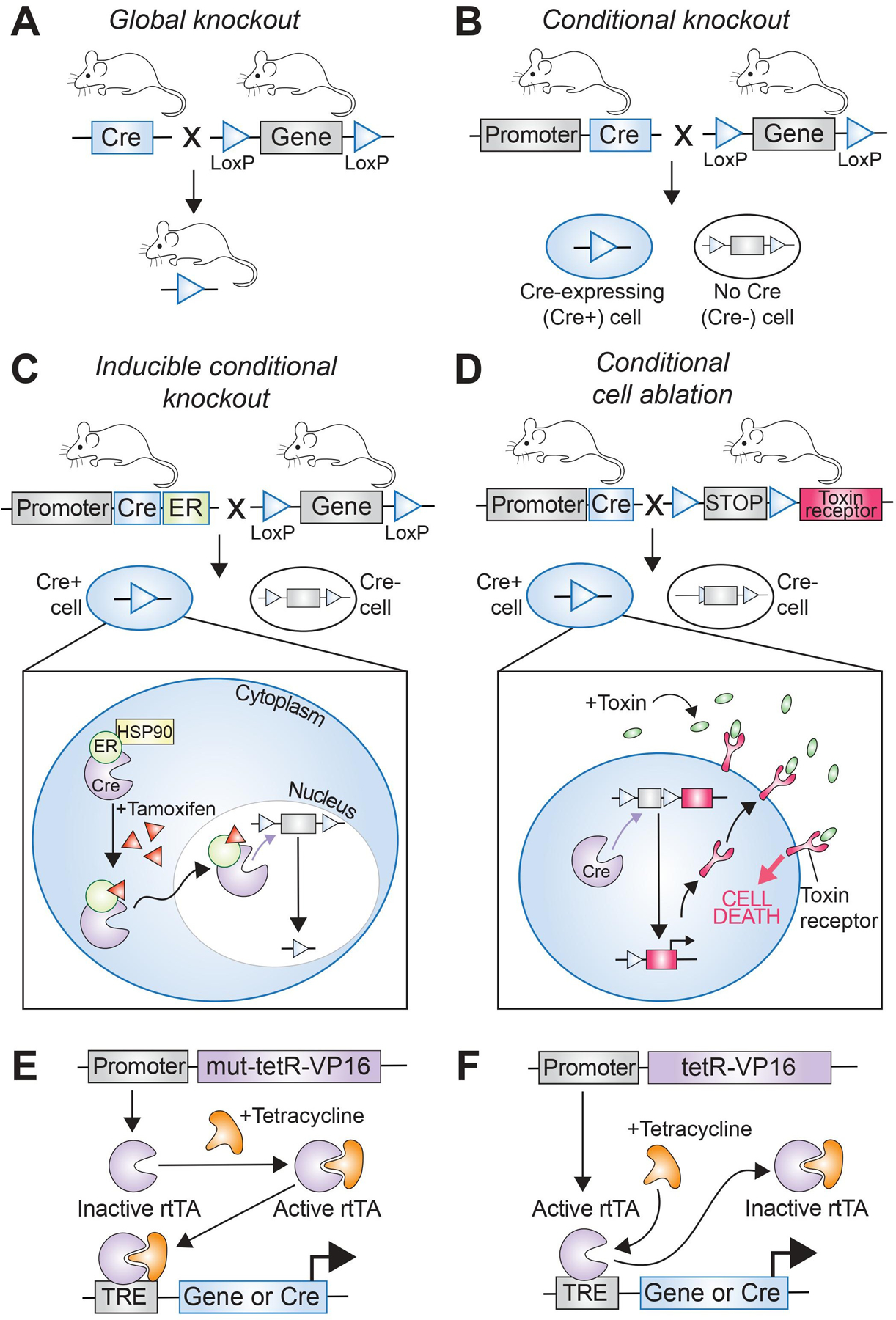
Schematic depicting the difference between a (A) global knockout and a (B) conditional knockout strategy. (C) Inducible conditional knockout using the cre-ERT2 system, in which the cre recombinase is translocated into the nucleus only after administration of tamoxifen. (D) Cell ablation using an inducible conditional strategy in which the toxin receptor is expressed in cells after cre recombination excised the stop codon located adjacent to the coding sequence of the receptor. Once the toxin is delivered systemically, only cells that express the toxin receptor will be ablated. (E, F) Tetracycline-responsive elements either activate or inactivate the cre recombinase, resulting in inhibition or activation of downstream gene expression.
The Cre (P1 bacteriophage cyclization recombinase) of the Cre-LoxP system is one of a family of tyrosine recombinases which includes other commonly utilized Flp (flippase),135 Dre (D6 site-specific DNA recombinase),136–137 and Vika recombinases (Fig. 3A, B).138–139 A tyrosine recombinase functions such that the enzyme targets a specific sequence of base pairs such as LoxP (Cre), Frt (Flp), rox (Dre), or vox (Vika). Base pair sequences targeted by tyrosine recombinases have a directionality determined by palindromic repeats centered around an asymmetric core. Specific recombinases then utilize these targeting sequences to guide recombination events. Cre-LoxP systems target paired LoxP sites with the intervening genomic locus being described as floxed. Location and orientation of LoxP sites can lead to excision (unidirectional, cis-placement of LoxP flanking sites), inversion (bidirectional, cis-placement of LoxP flanking sites), or translocation (trans placement of LoxP sites) with other tyrosine recombinases functioning similarly.140 Because the enzymes in these systems target specific and typically non-overlapping target sequences, complex arrays of specific recombination events can be utilized to alter transcription of one or more genes of interest with increasing specificity. Variations of this technology have been described such as in the setting of DeaLT (dual-recombinase-activated lineage tracing) described by He et al., 2017 in which the Dre-Rox system is utilized to improve specificity of Cre-LoxP system for more precise fate mapping.141 Tyrosine recombinases are not the only option for site-specific recombination, though benefit significantly from not needing accessory factors for activation. Integrases, both tyrosine and serine based have been described with the PhiC31 serine integrase having been described for use in mammals.142 As opposed to the multidirectional tyrosine recombinases, serine integrases are unidirectional and function only at a limited range of genomic acceptor sites. This is beneficial during initial insertion of transgenes as there are more limited risks of off-target insertion.
Figure 3:
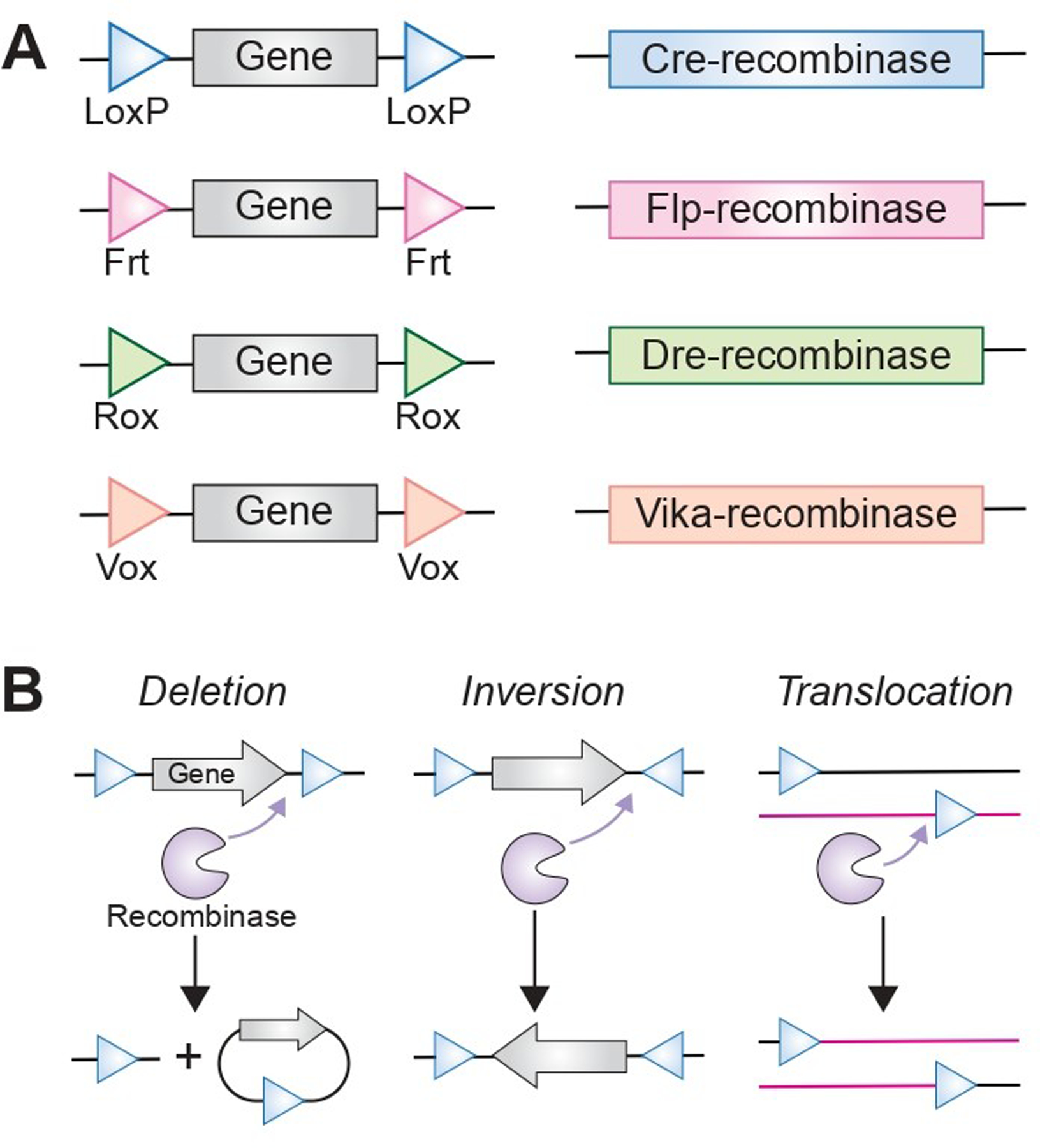
(A) Schematic of different recombinase strategies that result in either (B) deletion, (C) inversion or (D) translocation.
5.4. Inducibility, Ablation, and Transplantation
A benefit and limitation of traditional promoter-linked recombinases is that the initial stimulation of the promoter generates a cascade leading the transcription and activation of the recombinase and then essentially permanent change post-recombination. This is effective when the goal is to mark a lineage from first presence of a specific protein, however, limits specificity across the axis of time and in cases where mutation may induce embryonic lethality can lead to failure of the model as a whole. Numerous techniques have been developed to account for this by introducing a requirement for a second-agent at various stages of the recombination process (i.e., generation of a conditional transgene). One powerful example is incorporation of tetracycline or doxycycline-sensitive elements of the bacterial tet-operon which undergo conformational changes to either turn on or off (Tet-On, Tet-Off) in the presence of tetracycline/doxycycline (Fig. 2E, F).143–144 Mutant recombinases provide further control as is the case of the inducible estrogen receptor (ER) transgene (Cre-ERT2) which allows for activation by the synthetic estrogen receptor ligand tamoxifen (4-hydroxytamoxifen) but not by physiologic 17β-estradiol (Fig. 2C; Table 1).145–146 In each of these cases the absence of the conditional agent at baseline allows for exogenous control of the system – albeit at the cost of any unwanted side-effects associated with drug administration.
Depending on the promoter and/or recombinase/integrase, a single cell, an anatomic region, or a whole organism may be marked. This allows for a wide range of techniques for the modelling of development, health, and disease. Traditional techniques track the proliferation and differentiation of tissues within a single organism which, through elegant use of modifiable systems such as Cre-Lox and Flp-Frt activation and deactivation of markers in time may be utilized to track specific developmental or pathologic events. More complex constructs may be incorporated such as toxin receptors for cell ablation (diphtheria toxin, herpes simplex virus 1 thymidine kinase (HSV-1-tk)) or other functional proteins and or receptors which alter the actions and biology of the targeted cells (Fig. 2D).147–150 In organisms where germline modification is not easily amenable alternate techniques such as cell or tissue transplantation or parabiosis may provide additional information.151 Parabiosis, as example, allows for the direct connection between the bloodstream of two separate organisms such that one with a reporter may supply circulating cells to another without the recipient carrying any mutation. This allows for further discrimination between local, regional, and circulating cell population.152–153
5.5. Protein Reporters
Currently most forms of lineage tracing or fate mapping are dependent on the use of some form of label or reporter. A prerequisite for successful reporter selection is a predictable pattern of expression and/or retention and the absence of off-target effects that may unduly influence the surrounding system. The introduction of new peptide or protein can most simply be assessed with traditional histologic or immunoblot techniques, however, it is common with protein reporters to provide some visual cue for ease of assay without potentially destructive second-stage procedures. Historically 2-staged enzymatic reactions were used to generate a colorimetric effect, as seen in LacZ (β-Galactosidase) or GusA (β-Glucuronidase) with a variety of substrates providing different final colors.154 Luminescent effects can be achieved with Firefly or Renilla luciferase in presence of luciferin or coelenterazine respectively which can then be utilized in a stoichiometric manner to determine activity in conditions of in vivo imaging.155 Generation of mouse lines with pre-existing gene-trap loci such as the Gt(ROSA)26Sor strain developed in the 1990s further simplified the insertion of reporters allowing for the expansion of targeted animal models.156–157 These advances set the stage for the use of endogenous fluorescent proteins. Fluorescent reporter constructs improve of the spectrophotometric specificity of luciferase-based constructs and allow for simultaneous live assay across a range of fluorophores. Early fluorescent proteins were modified from xenologous sources such as green fluorescent protein (GFP: Aequorea jellyfish) and red fluorescent protein (RFP/dsRed: Discosoma coral).158–159 These have since been further modified directly or in the setting of fluorescence resonance energy transfer (FRET) between paired fluorophore constructs to generate a rainbow of fluorescent options which may be used to better discriminate tissues.160–162 Further improvements to these techniques include dual and multi-color transgenic labeling systems.163–165 One of the earliest of these was the mTmG developed by Muzumdar et al., in 2007 which expresses membrane-targeted Tomato (mT) in absence of Cre and transitions to membrane-targeted green fluorescent protein (mG) post-Cre recombination.166 Multi-copy single-cassette multi-color fluorescent reporters were introduced with the Brainbow technology developed by the Lichtman group in 2007.167–168 Initially based on the use of serially-fluorescent proteins arranged in tandem after a single promoter with expression controlled by cre-mediated deletion, successive iterations of this technology have accounted for cre-mediated inversion and a greater array of colors. Multiple copies of an injected transgene can activate expression of a wide range of fluorophores. Later iterations of this include the now well described Confetti Mouse - which utilized nuclear GFP, cytoplasmic yellow fluorescent protein (YFP) or RFP, and membrane bound cyan fluorescent protein (CFP) to provide a range of expression options within a single cell.169 This Confetti mouse provides a similar applicability to the earlier mTmG animals in that a traditional Cre-reporter system can be crossed without requiring extensive back-crossing or application of further transgenic modification.
5.6. DNA Reporters
Lineage tracing may additionally be performed in absence of the production of protein markers. Variant form of lineage tracing utilizing the tracking of cells by artificial or inherited DNA sequences.170 Artificial gene-constructs called ‘barcodes; may be inserted via the same techniques used to generate promoter/reporter constructs described above. One example of this is Polylox described in Pei et al., 2017 which utilizes the Cre-lox system for insertion of ten loxP sites spaced with alternating orientations.171 This technique allows for extensive excision/inversion events and consequently barcode diversity sufficient to allow for single cell fate mapping.
6.0. Conclusions
Lineage tracing in health and disease is a highly complex process which combines developmental biology, a clinical understanding of anatomy, physiology, and pathology with novel tools for cell tracking. Understanding the developmental processes that give rise to adult structures provides a guide for examining both regenerative and pathologic changes to adult tissue. The musculoskeletal system is composed of a wide range of highly specialized and functionally divergent tissues stemming from common mesodermal ancestors. Decades of research have generated a library of hundreds of genetic markers, some specific and some overlapping which mark the fate of cells belonging to each specific developmental lineage. Currently, lineage tracing provides a powerful tool with which to track these cells, however, the process is slow and limited by the availability of constructs and the heterogeneity of cell populations at each stage of development. Currently, the gold standard in lineage tracing remains models such as CreERT, Tet, and flippase systems, however, expansion of available animal models, development of novel methods for construct insertion such as CRISPR, and new systems for lineage tracing such as Barcode and Polylox continue to expand our ability to ask and answer questions with scientific and clinical relevance to better improve our understanding of musculoskeletal health and disease.
Supplementary Material
Acknowledgements
The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund which contributes to funding for SL. B.L. funded by R01 AR079863 and R01 AR078324.
Footnotes
Financial Disclosure Statement: No Financial Disclosures Associated with This Work.
Competing Interest Statement: No Competing Interests to Declare
Supplemental Table 1: List of Abbreviations and Acronyms
References
- 1.Solnica-Krezel L Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol 2005. Mar 29;15(6):R213–28. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Muhr J, Ackerman KM. Embryology, Gastrulation. [Updated 2022 Apr 13]. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2022. Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554394/ [PubMed] [Google Scholar]
- 3.Ferretti E, Hadjantonakis AK. Mesoderm specification and diversification: from single cells to emergent tissues. Curr Opin Cell Biol 2019. Dec;61:110–116. doi: 10.1016/j.ceb.2019.07.012. Epub 2019 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey ES, O’Reilly MA, Conlon FL, Smith JC. The T-box transcription factor Brachyury regulates expression of eFGF through binding to a non-palindromic response element. Development 1998. Oct;125(19):3887–94. doi: 10.1242/dev.125.19.3887. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Kwan KM, Mackem S. Putative oncogene Brachyury (T) is essential to specify cell fate but dispensable for notochord progenitor proliferation and EMT. Proc Natl Acad Sci U S A 2016. Apr 5;113(14):3820–5. doi: 10.1073/pnas.1601252113. Epub 2016 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tani S, Chung UI, Ohba S, Hojo H. Understanding paraxial mesoderm development and sclerotome specification for skeletal repair. Exp Mol Med 2020. Aug;52(8):1166–1177. doi: 10.1038/s12276-020-0482-1. Epub 2020 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corallo D, Trapani V, Bonaldo P. The notochord: structure and functions. Cell Mol Life Sci 2015. Aug;72(16):2989–3008. doi: 10.1007/s00018-015-1897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chal J, Pourquié O. Making muscle: skeletal myogenesis in vivo and in vitro. Development 2017. Jun 15;144(12):2104–2122. doi: 10.1242/dev.151035. [DOI] [PubMed] [Google Scholar]
- 9.Wahl MB, Deng C, Lewandoski M, Pourquié O. FGF signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development 2007. Nov;134(22):4033–41. doi: 10.1242/dev.009167. [DOI] [PubMed] [Google Scholar]
- 10.Wang HY, Huang YX, Zheng LH, Bao YL, Sun LG, Wu Y, Yu CL, Song ZB, Sun Y, Wang GN, Ma ZQ, Li YX. Modelling coupled oscillations in the Notch, Wnt, and FGF signaling pathways during somitogenesis: a comprehensive mathematical model. Comput Intell Neurosci 2015;2015:387409. doi: 10.1155/2015/387409. Epub 2015 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aulehla A, Pourquié O. Signaling gradients during paraxial mesoderm development. Cold Spring Harb Perspect Biol 2010. Feb;2(2):a000869. doi: 10.1101/cshperspect.a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Šošić D, Brand-Saberi B, Schmidt C, Christ B, Olson EN. Regulation of paraxis expression and somite formation by ectoderm- and neural tube-derived signals. Dev Biol 1997. May 15;185(2):229–43. doi: 10.1006/dbio.1997.8561. [DOI] [PubMed] [Google Scholar]
- 13.Burgess R, Cserjesi P, Ligon KL, Olson EN. Paraxis: a basic helix-loop-helix protein expressed in paraxial mesoderm and developing somites. Dev Biol 1995. Apr;168(2):296–306. doi: 10.1006/dbio.1995.1081. [DOI] [PubMed] [Google Scholar]
- 14.Bussen M, Petry M, Schuster-Gossler K, Leitges M, Gossler A, Kispert A. The T-box transcription factor Tbx18 maintains the separation of anterior and posterior somite compartments. Genes Dev 2004. May 15;18(10):1209–21. doi: 10.1101/gad.300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stafford DA, Brunet LJ, Khokha MK, Economides AN, Harland RM. Cooperative activity of noggin and gremlin 1 in axial skeleton development. Development 2011. Mar;138(5):1005–14. doi: 10.1242/dev.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanaki-Matsumiya M, Matsuda M, Gritti N, Nakaki F, Sharpe J, Trivedi V, Ebisuya M. Periodic formation of epithelial somites from human pluripotent stem cells. Nat Commun 2022. Apr 28;13(1):2325. doi: 10.1038/s41467-022-29967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollway G, Currie P. Vertebrate myotome development. Birth Defects Res C Embryo Today 2005. Sep;75(3):172–9. doi: 10.1002/bdrc.20046. [DOI] [PubMed] [Google Scholar]
- 18.Vieira L Embryology of the Fascial System. Cureus 2020. Aug 30;12(8):e10134. doi: 10.7759/cureus.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahlapuu M, Ormestad M, Enerbäck S, Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 2001. Jan;128(2):155–66. doi: 10.1242/dev.128.2.155. [DOI] [PubMed] [Google Scholar]
- 20.Zeller R, López-Ríos J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet 2009. Dec;10(12):845–58. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- 21.Raines AM, Magella B, Adam M, Potter SS. Key pathways regulated by HoxA9,10,11/HoxD9,10,11 during limb development. BMC Dev Biol 2015. Jul 19;15:28. doi: 10.1186/s12861-015-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pineault KM, Wellik DM. Hox genes and limb musculoskeletal development. Curr Osteoporos Rep 2014. Dec;12(4):420–7. doi: 10.1007/s11914-014-0241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bénazet JD, Zeller R. Vertebrate limb development: moving from classical morphogen gradients to an integrated 4-dimensional patterning system. Cold Spring Harb Perspect Biol 2009. Oct;1(4):a001339. doi: 10.1101/cshperspect.a001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsushita Y, Ono W, Ono N. Skeletal Stem Cells for Bone Development and Repair: Diversity Matters. Curr Osteoporos Rep 2020. Jun;18(3):189–198. doi: 10.1007/s11914-020-00572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Andre P, Ye L, Yang YZ. The Hedgehog signalling pathway in bone formation. Int J Oral Sci 2015. Jun 26;7(2):73–9. doi: 10.1038/ijos.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol 2008;40(1):46–62. doi: 10.1016/j.biocel.2007.06.009. Epub 2007 Jun 29. [DOI] [PubMed] [Google Scholar]
- 27.Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol 2013. Jan 1;5(1):a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronenberg HM. The role of the perichondrium in fetal bone development. Ann N Y Acad Sci 2007. Nov;1116:59–64. doi: 10.1196/annals.1402.059. [DOI] [PubMed] [Google Scholar]
- 29.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 2010. Aug 17;19(2):329–44. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha KM, Zhou X. Genetic and molecular control of osterix in skeletal formation. J Cell Biochem 2013. May;114(5):975–84. doi: 10.1002/jcb.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Zhang Z, Feng JQ, Dusevich VM, Sinha K, Zhang H, Darnay BG, de Crombrugghe B. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci U S A 2010. Jul 20;107(29):12919–24. doi: 10.1073/pnas.0912855107. Epub 2010 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suh JH, Lee HW, Lee JW, Kim JB. Hes1 stimulates transcriptional activity of Runx2 by increasing protein stabilization during osteoblast differentiation. Biochem Biophys Res Commun 2008. Feb 29;367(1):97–102. doi: 10.1016/j.bbrc.2007.12.100. [DOI] [PubMed] [Google Scholar]
- 33.Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun 2003. Sep 26;309(3):689–94. doi: 10.1016/j.bbrc.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 34.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet 1999. May;22(1):85–9. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 35.Hardingham TE, Oldershaw RA, Tew SR. Cartilage, SOX9 and Notch signals in chondrogenesis. J Anat 2006. Oct;209(4):469–80. doi: 10.1111/j.1469-7580.2006.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Rigueur D, Lyons KM. TGFβ signaling in cartilage development and maintenance. Birth Defects Res C Embryo Today 2014. Mar;102(1):37–51. doi: 10.1002/bdrc.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res 2002. Mar;12(1):19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 38.Gu J, Lu Y, Li F, Qiao L, Wang Q, Li N, Borgia JA, Deng Y, Lei G, Zheng Q. Identification and characterization of the novel Col10a1 regulatory mechanism during chondrocyte hypertrophic differentiation. Cell Death Dis 2014. Oct 16;5(10):e1469. doi: 10.1038/cddis.2014.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014. Jan 16;505(7483):327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Comazzetto S, Shen B, Morrison SJ. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell 2021. Jul 12;56(13):1848–1860. doi: 10.1016/j.devcel.2021.05.018. Epub 2021 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trinh T, Ropa J, Aljoufi A, Cooper S, Sinn A, Srour EF, Broxmeyer HE. Leptin receptor, a surface marker for a subset of highly engrafting long-term functional hematopoietic stem cells. Leukemia 2021. Jul;35(7):2064–2075. doi: 10.1038/s41375-020-01079-z. Epub 2020 Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comazzetto S, Murphy MM, Berto S, Jeffery E, Zhao Z, Morrison SJ. Restricted Hematopoietic Progenitors and Erythropoiesis Require SCF from Leptin Receptor+ Niche Cells in the Bone Marrow. Cell Stem Cell 2019. Mar 7;24(3):477–486.e6. doi: 10.1016/j.stem.2018.11.022. Epub 2019 Jan 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu L, Tu Q, Han Q, Zhang L, Sui L, Zheng L, Meng S, Tang Y, Xuan D, Zhang J, Murray D, Shen Q, Cheng J, Kim SH, Dong LQ, Valverde P, Cao X, Chen J. Adiponectin regulates bone marrow mesenchymal stem cell niche through a unique signal transduction pathway: an approach for treating bone disease in diabetes. Stem Cells 2015. Jan;33(1):240–52. doi: 10.1002/stem.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen B, Tasdogan A, Ubellacker JM, Zhang J, Nosyreva ED, Du L, Murphy MM, Hu S, Yi Y, Kara N, Liu X, Guela S, Jia Y, Ramesh V, Embree C, Mitchell EC, Zhao YC, Ju LA, Hu Z, Crane GM, Zhao Z, Syeda R, Morrison SJ. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 2021. Mar;591(7850):438–444. doi: 10.1038/s41586-021-03298-5. Epub 2021 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burdan F, Szumiło J, Korobowicz A, Farooquee R, Patel S, Patel A, Dave A, Szumiło M, Solecki M, Klepacz R, Dudka J. Morphology and physiology of the epiphyseal growth plate. Folia Histochem Cytobiol 2009;47(1):5–16. doi: 10.2478/v10042-009-0007-1. [DOI] [PubMed] [Google Scholar]
- 46.Kindblom JM, Nilsson O, Hurme T, Ohlsson C, Sävendahl L. Expression and localization of Indian hedgehog (Ihh) and parathyroid hormone related protein (PTHrP) in the human growth plate during pubertal development. J Endocrinol 2002. Aug;174(2):R1–6. doi: 10.1677/joe.0.174r001. [DOI] [PubMed] [Google Scholar]
- 47.Kronenberg HM. Developmental regulation of the growth plate. Nature 2003. May 15;423(6937):332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 48.Takahata Y, Hagino H, Kimura A, Urushizaki M, Yamamoto S, Wakamori K, Murakami T, Hata K, Nishimura R. Regulatory Mechanisms of Prg4 and Gdf5 Expression in Articular Cartilage and Functions in Osteoarthritis. Int J Mol Sci 2022. Apr 23;23(9):4672. doi: 10.3390/ijms23094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kania K, Colella F, Riemen AHK, Wang H, Howard KA, Aigner T, Dell’Accio F, Capellini TD, Roelofs AJ, De Bari C. Regulation of Gdf5 expression in joint remodelling, repair and osteoarthritis. Sci Rep 2020. Jan 13;10(1):157. doi: 10.1038/s41598-019-57011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Später D, Hill TP, O’sullivan RJ, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 2006. Aug;133(15):3039–49. doi: 10.1242/dev.02471. Epub 2006 Jul 3. [DOI] [PubMed] [Google Scholar]
- 51.Pacifici M, Koyama E, Shibukawa Y, Wu C, Tamamura Y, Enomoto-Iwamoto M, Iwamoto M. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann N Y Acad Sci 2006. Apr;1068:74–86. doi: 10.1196/annals.1346.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xuan F, Yano F, Mori D, Chijimatsu R, Maenohara Y, Nakamoto H, Mori Y, Makii Y, Oichi T, Taketo MM, Hojo H, Ohba S, Chung UI, Tanaka S, Saito T. Wnt/β-catenin signaling contributes to articular cartilage homeostasis through lubricin induction in the superficial zone. Arthritis Res Ther 2019. Nov 27;21(1):247. doi: 10.1186/s13075-019-2041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garciadiego-Cázares D, Rosales C, Katoh M, Chimal-Monroy J. Coordination of chondrocyte differentiation and joint formation by alpha5beta1 integrin in the developing appendicular skeleton. Development 2004. Oct;131(19):4735–42. doi: 10.1242/dev.01345. Epub 2004 Aug 25. [DOI] [PubMed] [Google Scholar]
- 54.Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, Baban K, Affolter M, Vale WW, Izpisua Belmonte JC, Choe S. Structural basis of BMP signaling inhibition by Noggin, a novel twelve-membered cystine knot protein. J Bone Joint Surg Am 2003;85-A Suppl 3:52–8. doi: 10.2106/00004623-200300003-00010. [DOI] [PubMed] [Google Scholar]
- 55.Ray A, Singh PN, Sohaskey ML, Harland RM, Bandyopadhyay A. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development 2015. Mar 15;142(6):1169–79. doi: 10.1242/dev.110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staverosky JA, Pryce BA, Watson SS, Schweitzer R. Tubulin polymerization-promoting protein family member 3, Tppp3, is a specific marker of the differentiating tendon sheath and synovial joints. Dev Dyn 2009. Mar;238(3):685–92. doi: 10.1002/dvdy.21865. [DOI] [PubMed] [Google Scholar]
- 57.Dubrulle J, Pourquie O. Welcome to syndetome: a new somitic compartment. Dev Cell 2003. May;4(5):611–2. doi: 10.1016/s1534-5807(03)00133-3. [DOI] [PubMed] [Google Scholar]
- 58.Anderson DM, Arredondo J, Hahn K, Valente G, Martin JF, Wilson-Rawls J, Rawls A. Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev Dyn 2006. Mar;235(3):792–801. doi: 10.1002/dvdy.20671. [DOI] [PubMed] [Google Scholar]
- 59.Berthet E, Chen C, Butcher K, Schneider RA, Alliston T, Amirtharajah M. Smad3 binds Scleraxis and Mohawk and regulates tendon matrix organization. J Orthop Res 2013. Sep;31(9):1475–83. doi: 10.1002/jor.22382. Epub 2013 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otabe K, Nakahara H, Hasegawa A, Matsukawa T, Ayabe F, Onizuka N, Inui M, Takada S, Ito Y, Sekiya I, Muneta T, Lotz M, Asahara H. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J Orthop Res 2015. Jan;33(1):1–8. doi: 10.1002/jor.22750. Epub 2014 Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gumucio JP, Schonk MM, Kharaz YA, Comerford E, Mendias CL. Scleraxis is required for the growth of adult tendons in response to mechanical loading. JCI Insight 2020. Jul 9;5(13):e138295. doi: 10.1172/jci.insight.138295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshimoto Y, Takimoto A, Watanabe H, Hiraki Y, Kondoh G, Shukunami C. Scleraxis is required for maturation of tissue domains for proper integration of the musculoskeletal system. Sci Rep 2017. Mar 22;7:45010. doi: 10.1038/srep45010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dyment NA, Breidenbach AP, Schwartz AG, Russell RP, Aschbacher-Smith L, Liu H, Hagiwara Y, Jiang R, Thomopoulos S, Butler DL, Rowe DW. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev Biol 2015. Sep 1;405(1):96–107. doi: 10.1016/j.ydbio.2015.06.020. Epub 2015 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Killian ML, Thomopoulos S. Scleraxis is required for the development of a functional tendon enthesis. FASEB J 2016. Jan;30(1):301–11. doi: 10.1096/fj.14-258236. Epub 2015 Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ideo K, Tokunaga T, Shukunami C, Takimoto A, Yoshimoto Y, Yonemitsu R, Karasugi T, Mizuta H, Hiraki Y, Miyamoto T. Role of Scx+/Sox9+ cells as potential progenitor cells for postnatal supraspinatus enthesis formation and healing after injury in mice. PLoS One 2020. Dec 1;15(12):e0242286. doi: 10.1371/journal.pone.0242286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charvet B, Ruggiero F, Le Guellec D. The development of the myotendinous junction. A review. Muscles Ligaments Tendons J 2012. Sep 10;2(2):53–63. [PMC free article] [PubMed] [Google Scholar]
- 67.Jakobsen JR, Mackey AL, Knudsen AB, Koch M, Kjaer M, Krogsgaard MR. Composition and adaptation of human myotendinous junction and neighboring muscle fibers to heavy resistance training. Scand J Med Sci Sports 2017. Dec;27(12):1547–1559. doi: 10.1111/sms.12794. Epub 2016 Oct 26. [DOI] [PubMed] [Google Scholar]
- 68.Bajard L, Relaix F, Lagha M, Rocancourt D, Daubas P, Buckingham ME. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev 2006. Sep 1;20(17):2450–64. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato T, Rocancourt D, Marques L, Thorsteinsdóttir S, Buckingham M. A Pax3/Dmrt2/Myf5 regulatory cascade functions at the onset of myogenesis. PLoS Genet 2010. Apr 1;6(4):e1000897. doi: 10.1371/journal.pgen.1000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magli A, Schnettler E, Rinaldi F, Bremer P, Perlingeiro RC. Functional dissection of Pax3 in paraxial mesoderm development and myogenesis. Stem Cells 2013. Jan;31(1):59–70. doi: 10.1002/stem.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchberger A, Nomokonova N, Arnold HH. Myf5 expression in somites and limb buds of mouse embryos is controlled by two distinct distal enhancer activities. Development 2003. Jul;130(14):3297–307. doi: 10.1242/dev.00557. [DOI] [PubMed] [Google Scholar]
- 72.Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F. The formation of skeletal muscle: from somite to limb. J Anat 2003. Jan;202(1):59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 2012. Feb 1;4(2):a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buckingham M, Relaix F. PAX3 and PAX7 as upstream regulators of myogenesis. Semin Cell Dev Biol 2015. Aug;44:115–25. doi: 10.1016/j.semcdb.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 75.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage 2007. Mar;15(3):237–44. doi: 10.1016/j.joca.2006.11.006. Epub 2007 Jan 3. [DOI] [PubMed] [Google Scholar]
- 76.Roelofs AJ, Kania K, Rafipay AJ, Sambale M, Kuwahara ST, Collins FL, Smeeton J, Serowoky MA, Rowley L, Wang H, Gronewold R, Kapeni C, Méndez-Ferrer S, Little CB, Bateman JF, Pap T, Mariani FV, Sherwood J, Crump JG, De Bari C. Identification of the skeletal progenitor cells forming osteophytes in osteoarthritis. Ann Rheum Dis 2020. Dec;79(12):1625–1634. doi: 10.1136/annrheumdis-2020-218350. Epub 2020 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, Sono T, McCarthy E, Levi B, James AW. Heterotopic Ossification: A Comprehensive Review. JBMR Plus 2019. Feb 27;3(4):e10172. doi: 10.1002/jbm4.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan L, Gao R, Liu Y, He B, Lv S, Hao D. The Pathogenesis of Ossification of the Posterior Longitudinal Ligament. Aging Dis 2017. Oct 1;8(5):570–582. doi: 10.14336/AD.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pullig O, Weseloh G, Klatt AR, Wagener R, Swoboda B. Matrilin-3 in human articular cartilage: increased expression in osteoarthritis. Osteoarthritis Cartilage 2002. Apr;10(4):253–63. doi: 10.1053/joca.2001.0508. [DOI] [PubMed] [Google Scholar]
- 80.Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis 2009. Dec;47(12):805–14. doi: 10.1002/dvg.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muttigi MS, Han I, Park HK, Park H, Lee SH. Matrilin-3 Role in Cartilage Development and Osteoarthritis. Int J Mol Sci 2016. Apr 20;17(4):590. doi: 10.3390/ijms17040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qadri M, Jay GD, Zhang LX, Richendrfer H, Schmidt TA, Elsaid KA. Proteoglycan-4 regulates fibroblast to myofibroblast transition and expression of fibrotic genes in the synovium. Arthritis Res Ther 2020. May 13;22(1):113. doi: 10.1186/s13075-020-02207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qadri M, Jay GD, Zhang LX, Schmidt TA, Totonchy J, Elsaid KA. Proteoglycan-4 is an essential regulator of synovial macrophage polarization and inflammatory macrophage joint infiltration. Arthritis Res Ther 2021. Sep 14;23(1):241. doi: 10.1186/s13075-021-02621-9. Erratum in: Arthritis Res Ther. 2021 Oct 29;23(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fresquet M, Jowitt TA, Stephen LA, Ylöstalo J, Briggs MD. Structural and functional investigations of Matrilin-1 A-domains reveal insights into their role in cartilage ECM assembly. J Biol Chem 2010. Oct 29;285(44):34048–61. doi: 10.1074/jbc.M110.154443. Epub 2010 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piecha D, Wiberg C, Mörgelin M, Reinhardt DP, Deák F, Maurer P, Paulsson M. Matrilin-2 interacts with itself and with other extracellular matrix proteins. Biochem J 2002. Nov 1;367(Pt 3):715–21. doi: 10.1042/BJ20021069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roelofs AJ, Zupan J, Riemen AHK, Kania K, Ansboro S, White N, Clark SM, De Bari C. Joint morphogenetic cells in the adult mammalian synovium. Nat Commun 2017. May 16;8:15040. doi: 10.1038/ncomms15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takahata Y, Hagino H, Kimura A, Urushizaki M, Yamamoto S, Wakamori K, Murakami T, Hata K, Nishimura R. Regulatory Mechanisms of Prg4 and Gdf5 Expression in Articular Cartilage and Functions in Osteoarthritis. Int J Mol Sci 2022. Apr 23;23(9):4672. doi: 10.3390/ijms23094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res 2015. Jun;33(6):832–9. doi: 10.1002/jor.22806. Epub 2015 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Müller SA, Todorov A, Heisterbach PE, Martin I, Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc 2015. Jul;23(7):2097–105. doi: 10.1007/s00167-013-2680-z. [DOI] [PubMed] [Google Scholar]
- 90.Harvey T, Flamenco S, Fan CM. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat Cell Biol 2019. Dec;21(12):1490–1503. doi: 10.1038/s41556-019-0417-z. Epub 2019 Nov 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwartz AG, Galatz LM, Thomopoulos S. Enthesis regeneration: a role for Gli1+ progenitor cells. Development 2017. Apr 1;144(7):1159–1164. doi: 10.1242/dev.139303. Epub 2017 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hyatt HW, Powers SK. The Role of Calpains in Skeletal Muscle Remodeling with Exercise and Inactivity-induced Atrophy. Int J Sports Med 2020. Dec;41(14):994–1008. doi: 10.1055/a-1199-7662. Epub 2020 Jul 17. [DOI] [PubMed] [Google Scholar]
- 93.Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol 2005. Aug-Oct;16(4–5):612–22. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Jiang C, Wen Y, Kuroda K, Hannon K, Rudnicki MA, Kuang S. Notch signaling deficiency underlies age-dependent depletion of satellite cells in muscular dystrophy. Dis Model Mech 2014. Aug;7(8):997–1004. doi: 10.1242/dmm.015917. Epub 2014 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garry DJ, Meeson A, Elterman J, Zhao Y, Yang P, Bassel-Duby R, Williams RS. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc Natl Acad Sci U S A 2000. May 9;97(10):5416–21. doi: 10.1073/pnas.100501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.George RM, Biressi S, Beres BJ, Rogers E, Mulia AK, Allen RE, Rawls A, Rando TA, Wilson-Rawls J. Numb-deficient satellite cells have regeneration and proliferation defects. Proc Natl Acad Sci U S A 2013. Nov 12;110(46):18549–54. doi: 10.1073/pnas.1311628110. Epub 2013 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soleimani VD, Yin H, Jahani-Asl A, Ming H, Kockx CE, van Ijcken WF, Grosveld F, Rudnicki MA. Snail regulates MyoD binding-site occupancy to direct enhancer switching and differentiation-specific transcription in myogenesis. Mol Cell 2012. Aug 10;47(3):457–68. doi: 10.1016/j.molcel.2012.05.046. Epub 2012 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moretti I, Ciciliot S, Dyar KA, Abraham R, Murgia M, Agatea L, Akimoto T, Bicciato S, Forcato M, Pierre P, Uhlenhaut NH, Rigby PW, Carvajal JJ, Blaauw B, Calabria E, Schiaffino S. MRF4 negatively regulates adult skeletal muscle growth by repressing MEF2 activity. Nat Commun 2016. Aug 3;7:12397. doi: 10.1038/ncomms12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guadagnin E, Mázala D, Chen YW. STAT3 in Skeletal Muscle Function and Disorders. Int J Mol Sci 2018. Aug 2;19(8):2265. doi: 10.3390/ijms19082265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zammit PS. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol 2017. Dec;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 101.Lindqvist J, Torvaldson E, Gullmets J, Karvonen H, Nagy A, Taimen P, Eriksson JE. Nestin contributes to skeletal muscle homeostasis and regeneration. J Cell Sci 2017. Sep 1;130(17):2833–2842. doi: 10.1242/jcs.202226. Epub 2017 Jul 21. [DOI] [PubMed] [Google Scholar]
- 102.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 2013. Jan;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front Endocrinol (Lausanne) 2016. Jun 20;7:69. doi: 10.3389/fendo.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao Y, Haginoya K, Sun G, Dai H, Onuma A, Iinuma K. Platelet-derived growth factor and its receptors are related to the progression of human muscular dystrophy: an immunohistochemical study. J Pathol 2003. Sep;201(1):149–59. doi: 10.1002/path.1414. [DOI] [PubMed] [Google Scholar]
- 105.Paylor B, Fernandes J, McManus B, Rossi F. Tissue-resident Sca1+ PDGFRα+ mesenchymal progenitors are the cellular source of fibrofatty infiltration in arrhythmogenic cardiomyopathy. F1000Res 2013. Jun 19;2:141. doi: 10.12688/f1000research.2-141.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Molina T, Fabre P, Dumont NA. Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases. Open Biol 2021. Dec;11(12):210110. doi: 10.1098/rsob.210110. Epub 2021 Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manickam R, Duszka K, Wahli W. PPARs and Microbiota in Skeletal Muscle Health and Wasting. Int J Mol Sci 2020. Oct 29;21(21):8056. doi: 10.3390/ijms21218056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dammone G, Karaz S, Lukjanenko L, Winkler C, Sizzano F, Jacot G, Migliavacca E, Palini A, Desvergne B, Gilardi F, Feige JN. PPARγ Controls Ectopic Adipogenesis and Cross-Talks with Myogenesis During Skeletal Muscle Regeneration. Int J Mol Sci 2018. Jul 13;19(7):2044. doi: 10.3390/ijms19072044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, Sono T, McCarthy E, Levi B, James AW. Heterotopic Ossification: A Comprehensive Review. JBMR Plus 2019. Feb 27;3(4):e10172. doi: 10.1002/jbm4.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hwang CD, Pagani CA, Nunez JH, Cherief M, Qin Q, Gomez-Salazar M, Kadaikal B, Kang H, Chowdary AR, Patel N, James AW, Levi B. Contemporary perspectives on heterotopic ossification. JCI Insight 2022. Jul 22;7(14):e158996. doi: 10.1172/jci.insight.158996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gabbutt C, Wright NA, Baker AM, Shibata D, Graham TA. Lineage tracing in human tissues. J Pathol 2022. Jul;257(4):501–512. doi: 10.1002/path.5911. Epub 2022 May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spencer Chapman M, Ranzoni AM, Myers B, Williams N, Coorens THH, Mitchell E, Butler T, Dawson KJ, Hooks Y, Moore L, Nangalia J, Robinson PS, Yoshida K, Hook E, Campbell PJ, Cvejic A. Lineage tracing of human development through somatic mutations. Nature 2021. Jul;595(7865):85–90. doi: 10.1038/s41586-021-03548-6. Epub 2021 May 12. [DOI] [PubMed] [Google Scholar]
- 113.Ludwig LS, Lareau CA, Ulirsch JC, Christian E, Muus C, Li LH, Pelka K, Ge W, Oren Y, Brack A, Law T, Rodman C, Chen JH, Boland GM, Hacohen N, Rozenblatt-Rosen O, Aryee MJ, Buenrostro JD, Regev A, Sankaran VG. Lineage Tracing in Humans Enabled by Mitochondrial Mutations and Single-Cell Genomics. Cell 2019. Mar 7;176(6):1325–1339.e22. doi: 10.1016/j.cell.2019.01.022. Epub 2019 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hsu YC. Theory and Practice of Lineage Tracing. Stem Cells 2015. Nov;33(11):3197–204. doi: 10.1002/stem.2123. Epub 2015 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moody SA. Lineage Tracing and Fate Mapping in Xenopus Embryos. Cold Spring Harb Protoc 2018. Dec 3;2018(12). doi: 10.1101/pdb.prot097253. Erratum in: Cold Spring Harb Protoc. 2021 Oct 1;2021(10):pdb.corr107781. [DOI] [PubMed] [Google Scholar]
- 116.Kretzschmar K, Watt FM. Lineage tracing. Cell 2012. Jan 20;148(1–2):33–45. doi: 10.1016/j.cell.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 117.Chandras C, Zouberakis M, Salimova E, Smedley D, Rosenthal N, Aidinis V. CreZOO--the European virtual repository of Cre and other targeted conditional driver strains. Database (Oxford) 2012. Jun 21;2012:bas029. doi: 10.1093/database/bas029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jungke P, Hans S, Brand M. The zebrafish CreZoo: an easy-to-handle database for novel CreER(T2)-driver lines. Zebrafish 2013. Sep;10(3):259–63. doi: 10.1089/zeb.2012.0834. Epub 2013 May 13. [DOI] [PubMed] [Google Scholar]
- 119.Horton MB, Prevedello G, Marchingo JM, Zhou JHS, Duffy KR, Heinzel S, Hodgkin PD. Multiplexed Division Tracking Dyes for Proliferation-Based Clonal Lineage Tracing. J Immunol 2018. Aug 1;201(3):1097–1103. doi: 10.4049/jimmunol.1800481. Epub 2018 Jun 18. [DOI] [PubMed] [Google Scholar]
- 120.Gimlich RL, Braun J. Improved fluorescent compounds for tracing cell lineage. Dev Biol 1985. Jun;109(2):509–14. doi: 10.1016/0012-1606(85)90476-2. [DOI] [PubMed] [Google Scholar]
- 121.Humphreys BD. Cutting to the chase: taking the pulse of label-retaining cells in kidney. Am J Physiol Renal Physiol 2015. Jan 1;308(1):F29–30. doi: 10.1152/ajprenal.00538.2014. Epub 2014 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells 2009. Feb;27(2):442–50. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fechheimer M, Boylan JF, Parker S, Sisken JE, Patel GL, Zimmer SG. Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proc Natl Acad Sci U S A 1987. Dec;84(23):8463–7. doi: 10.1073/pnas.84.23.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wyber JA, Andrews J, D’Emanuele A. The use of sonication for the efficient delivery of plasmid DNA into cells. Pharm Res 1997. Jun;14(6):750–6. doi: 10.1023/a:1012198321879. [DOI] [PubMed] [Google Scholar]
- 125.Delalande A, Leduc C, Midoux P, Postema M, Pichon C. Efficient Gene Delivery by Sonoporation Is Associated with Microbubble Entry into Cells and the Clathrin-Dependent Endocytosis Pathway. Ultrasound Med Biol 2015. Jul;41(7):1913–26. doi: 10.1016/j.ultrasmedbio.2015.03.010. Epub 2015 Apr 27. [DOI] [PubMed] [Google Scholar]
- 126.Potter H Transfection by electroporation. Curr Protoc Mol Biol 2003. May;Chapter 9:Unit 9.3. doi: 10.1002/0471142727.mb0903s62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chong ZX, Yeap SK, Ho WY. Transfection types, methods and strategies: a technical review. PeerJ 2021. Apr 21;9:e11165. doi: 10.7717/peerj.11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future. Anal Bioanal Chem 2010. Aug;397(8):3173–8. doi: 10.1007/s00216-010-3821-6. Epub 2010 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 2013. Jul;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. Epub 2013 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shamshirgaran Y, Liu J, Sumer H, Verma PJ, Taheri-Ghahfarokhi A. Tools for Efficient Genome Editing; ZFN, TALEN, and CRISPR. Methods Mol Biol 2022;2495:29–46. doi: 10.1007/978-1-0716-2301-5_2. [DOI] [PubMed] [Google Scholar]
- 131.Nemudryi AA, Valetdinova KR, Medvedev SP, Zakian SM. TALEN and CRISPR/Cas Genome Editing Systems: Tools of Discovery. Acta Naturae 2014. Jul;6(3):19–40. [PMC free article] [PubMed] [Google Scholar]
- 132.Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol 1981. Aug 25;150(4):467–86. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- 133.Yarmolinsky M, Hoess R. The Legacy of Nat Sternberg: The Genesis of Cre-lox Technology. Annu Rev Virol 2015. Nov;2(1):25–40. doi: 10.1146/annurev-virology-100114-054930. [DOI] [PubMed] [Google Scholar]
- 134.Van Duyne GD. Cre Recombinase. Microbiol Spectr 2015. Feb;3(1):MDNA3-0014-2014. doi: 10.1128/microbiolspec.MDNA3-0014-2014. [DOI] [PubMed] [Google Scholar]
- 135.Meinke G, Bohm A, Hauber J, Pisabarro MT, Buchholz F. Cre Recombinase and Other Tyrosine Recombinases. Chem Rev 2016. Oct 26;116(20):12785–12820. doi: 10.1021/acs.chemrev.6b00077. Epub 2016 May 10. [DOI] [PubMed] [Google Scholar]
- 136.Anastassiadis K, Fu J, Patsch C, Hu S, Weidlich S, Duerschke K, Buchholz F, Edenhofer F, Stewart AF. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis Model Mech 2009. Sep-Oct;2(9–10):508–15. doi: 10.1242/dmm.003087. Epub 2009 Aug 19. [DOI] [PubMed] [Google Scholar]
- 137.Sajgo S, Ghinia MG, Shi M, Liu P, Dong L, Parmhans N, Popescu O, Badea TC. Dre - Cre sequential recombination provides new tools for retinal ganglion cell labeling and manipulation in mice. PLoS One 2014. Mar 7;9(3):e91435. doi: 10.1371/journal.pone.0091435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karimova M, Baker O, Camgoz A, Naumann R, Buchholz F, Anastassiadis K. A single reporter mouse line for Vika, Flp, Dre, and Cre-recombination. Sci Rep 2018. Sep 27;8(1):14453. doi: 10.1038/s41598-018-32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Karimova M, Abi-Ghanem J, Berger N, Surendranath V, Pisabarro MT, Buchholz F. Vika/vox, a novel efficient and specific Cre/loxP-like site-specific recombination system. Nucleic Acids Res 2013. Jan;41(2):e37. doi: 10.1093/nar/gks1037. Epub 2012 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim H, Kim M, Im SK, Fang S. Mouse Cre-LoxP system: general principles to determine tissue-specific roles of target genes. Lab Anim Res 2018. Dec;34(4):147–159. doi: 10.5625/lar.2018.34.4.147. Epub 2018 Dec 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.He L, Li Y, Li Y, Pu W, Huang X, Tian X, Wang Y, Zhang H, Liu Q, Zhang L, Zhao H, Tang J, Ji H, Cai D, Han Z, Han Z, Nie Y, Hu S, Wang QD, Sun R, Fei J, Wang F, Chen T, Yan Y, Huang H, Pu WT, Zhou B. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med 2017. Dec;23(12):1488–1498. doi: 10.1038/nm.4437. Epub 2017 Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thyagarajan B, Olivares EC, Hollis RP, Ginsburg DS, Calos MP. Site-specific genomic integration in mammalian cells mediated by phage phiC31 integrase. Mol Cell Biol 2001. Jun;21(12):3926–34. doi: 10.1128/MCB.21.12.3926-3934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Das AT, Tenenbaum L, Berkhout B. Tet-On Systems For Doxycycline-inducible Gene Expression. Curr Gene Ther 2016;16(3):156–67. doi: 10.2174/1566523216666160524144041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jouvet N, Bouyakdan K, Campbell SA, Baldwin C, Townsend SE, Gannon MA, Poitout V, Alquier T, Estall JL. The Tetracycline-Controlled Transactivator (Tet-On/Off) System in β-Cells Reduces Insulin Expression and Secretion in Mice. Diabetes 2021. Dec;70(12):2850–2859. doi: 10.2337/db21-0147. Epub 2021 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 2002. Apr 15;244(2):305–18. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 146.Donocoff RS, Teteloshvili N, Chung H, Shoulson R, Creusot RJ. Optimization of tamoxifen-induced Cre activity and its effect on immune cell populations. Sci Rep 2020. Sep 17;10(1):15244. doi: 10.1038/s41598-020-72179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol 2001. Aug;19(8):746–50. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 148.Cha JH, Chang MY, Richardson JA, Eidels L. Transgenic mice expressing the diphtheria toxin receptor are sensitive to the toxin. Mol Microbiol 2003. Jul;49(1):235–40. doi: 10.1046/j.1365-2958.2003.03550.x. [DOI] [PubMed] [Google Scholar]
- 149.Zhang Y, Huang SZ, Wang S, Zeng YT. Development of an HSV-tk transgenic mouse model for study of liver damage. FEBS J 2005. May;272(9):2207–15. doi: 10.1111/j.1742-4658.2005.04644.x. [DOI] [PubMed] [Google Scholar]
- 150.Heyman RA, Borrelli E, Lesley J, Anderson D, Richman DD, Baird SM, Hyman R, Evans RM. Thymidine kinase obliteration: creation of transgenic mice with controlled immune deficiency. Proc Natl Acad Sci U S A 1989. Apr;86(8):2698–702. doi: 10.1073/pnas.86.8.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chung JY, Mackay F, Alderuccio F. Efficient conditional gene expression following transplantation of retrovirally transduced bone marrow stem cells. J Immunol Methods 2015. Jan;416:183–8. doi: 10.1016/j.jim.2014.11.002. Epub 2014 Nov 8. [DOI] [PubMed] [Google Scholar]
- 152.Kamran P, Sereti KI, Zhao P, Ali SR, Weissman IL, Ardehali R. Parabiosis in mice: a detailed protocol. J Vis Exp 2013. Oct 6;(80):50556. doi: 10.3791/50556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alman B, Baht G. Parabiosis: Assessing the Effects of Circulating Cells and Factors on the Skeleton. Methods Mol Biol 2021;2230:105–113. doi: 10.1007/978-1-0716-1028-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiong AS, Peng RH, Zhuang J, Davies J, Zhang J, Yao QH. Advances in directed molecular evolution of reporter genes. Crit Rev Biotechnol 2012. Jun;32(2):133–42. doi: 10.3109/07388551.2011.593503. Epub 2011 Jul 21. [DOI] [PubMed] [Google Scholar]
- 155.Kenda M, Vegelj J, Herlah B, Perdih A, Mladěnka P, Sollner Dolenc M. Evaluation of Firefly and Renilla Luciferase Inhibition in Reporter-Gene Assays: A Case of Isoflavonoids. Int J Mol Sci 2021. Jun 28;22(13):6927. doi: 10.3390/ijms22136927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev 1991. Sep;5(9):1513–23. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 157.Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A 1997. Apr 15;94(8):3789–94. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-fluorescent protein. Gene 1992. Feb 15;111(2):229–33. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 159.Fradkov AF, Chen Y, Ding L, Barsova EV, Matz MV, Lukyanov SA. Novel fluorescent protein from Discosoma coral and its mutants possesses a unique far-red fluorescence. FEBS Lett 2000. Aug 18;479(3):127–30. doi: 10.1016/s0014-5793(00)01895-0. [DOI] [PubMed] [Google Scholar]
- 160.Piatkevich KD, Verkhusha VV. Guide to red fluorescent proteins and biosensors for flow cytometry. Methods Cell Biol 2011;102:431–61. doi: 10.1016/B978-0-12-374912-3.00017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bajar BT, Wang ES, Zhang S, Lin MZ, Chu J. A Guide to Fluorescent Protein FRET Pairs. Sensors (Basel) 2016. Sep 14;16(9):1488. doi: 10.3390/s16091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 2004. Dec;22(12):1567–72. doi: 10.1038/nbt1037. Epub 2004 Nov 21. [DOI] [PubMed] [Google Scholar]
- 163.Liu K, Tang M, Jin H, Liu Q, He L, Zhu H, Liu X, Han X, Li Y, Zhang L, Tang J, Pu W, Lv Z, Wang H, Ji H, Zhou B. Triple-cell lineage tracing by a dual reporter on a single allele. J Biol Chem 2020. Jan 17;295(3):690–700. doi: 10.1074/jbc.RA119.011349. Epub 2019 Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ellenberg J, Lippincott-Schwartz J, Presley JF. Dual-colour imaging with GFP variants. Trends Cell Biol 1999. Feb;9(2):52–6. doi: 10.1016/s0962-8924(98)01420-2. [DOI] [PubMed] [Google Scholar]
- 165.Stewart MD, Jang CW, Hong NW, Austin AP, Behringer RR. Dual fluorescent protein reporters for studying cell behaviors in vivo. Genesis 2009. Oct;47(10):708–17. doi: 10.1002/dvg.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 2007. Sep;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 167.Weissman TA, Pan YA. Brainbow: new resources and emerging biological applications for multicolor genetic labeling and analysis. Genetics 2015. Feb;199(2):293–306. doi: 10.1534/genetics.114.172510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 2007. Nov 1;450(7166):56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 169.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010. Oct 1;143(1):134–44. doi: [DOI] [PubMed] [Google Scholar]
- 170.Kebschull JM, Zador AM. Cellular barcoding: lineage tracing, screening and beyond. Nat Methods 2018. Nov;15(11):871–879. doi: 10.1038/s41592-018-0185-x. Epub 2018 Oct 30. [DOI] [PubMed] [Google Scholar]
- 171.Pei W, Feyerabend TB, Rössler J, Wang X, Postrach D, Busch K, Rode I, Klapproth K, Dietlein N, Quedenau C, Chen W, Sauer S, Wolf S, Höfer T, Rodewald HR. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 2017. Aug 24;548(7668):456–460. doi: 10.1038/nature23653. Epub 2017 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


