Abstract

Precision oncology is revolutionized by targeted therapies like talazoparib, a PARP inhibitor, leveraging advanced understanding of DNA repair mechanisms such as ribonucleotide excision repair and homologous recombination repair. CRISPR-Cas technology has been pivotal in unraveling these pathways, facilitating personalized treatment strategies. The identification of genomic loss of heterozygosity as a biomarker targets HRR-deficient cancers, enhancing talazoparib’s efficacy. These breakthroughs represent a significant advancement in precision medicine, offering more effective, individualized cancer therapies.
Important Compound Classes
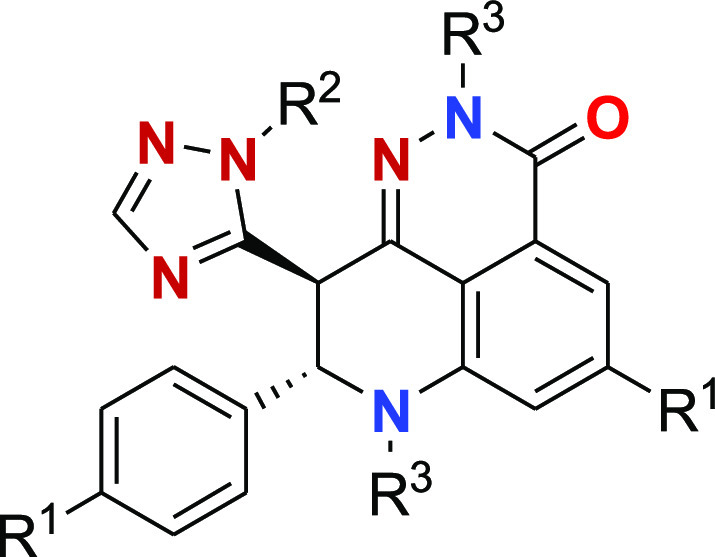
Titles
Genomic Loss of Heterozygosity as a Predictive Biomarker for Treatment with Talazoparib and Methods of Treatment Thereof; Methods of Identifying a Tumor that is Sensitive to Treatment with Talazoparib and Methods of Treatment Thereof; and Combination Therapies Including PARP1 Inhibitors
Patent Publication Numbers
WO 2023/131894 A1 (URL: https://patents.google.com/patent/WO2023131894A1/en?oq=WO+2023%2f131894+A1);
WO 2022/123427 A1 (https://patents.google.com/patent/WO2022123427A1/en?oq=WO+2022%2f123427+A1); and
WO 2023/133409 A2 (https://patents.google.com/patent/WO2023133409A2/en?oq=WO+2023%2f133409+A2)
Publication Dates
July 13, 2023, and June 16 2022
Priority Applications
US 63/321,170; US 63/122,453; and US 63/296,266
Priority Dates
March 18, 2022; December 7, 2020; and January 4, 2022
Inventors
Laird, A. D. (WO 2023/131894 A1); Gruber, J. J.; Telli, M. (WO 2022/123427 A1); Moscat-Guillen, J.; Diaz-Meco Conde, M. T.; Linares Rodriguez, J. F.; Diaz, T. C. (WO 2023/133409 A2)
Assignee Companies
Pfizer Inc. [US/US], 235 East 42nd Street, New York, New York 10017, United States (WO 2023/131894 A1 and WO 2022/123427 A1); Cornell University [US/US], 395 Pine Tree Road, Suite 310, Ithaca, New York 14850, United States (WO 2023/133409 A2)
Disease Area
Cancer
Biological Target
Poly(ADP-ribose) polymerase 1 (PARP 1)
Summary
The battle against cancer is witnessing a paradigm shift with the introduction of targeted therapies like talazoparib, a PARP inhibitor. Understanding the intricacies of DNA repair has become increasingly important in cancer research. Recent advancements have shed light on ribonucleotide excision repair (RER), a crucial process where enzymes scan DNA for misplaced ribonucleotides. This mechanism maintains genomic stability, a critical cancer development and progression factor.
Another pivotal process is homologous recombination repair (HRR), which is responsible for repairing DNA double-strand breaks. The dynamics of specific proteins involved in HRR, such as Rad51, are critical for genome integrity. The failure of this repair mechanism can lead to genomic instability, often observed in various cancers.
The emergence of CRISPR-Cas technology has revolutionized the understanding of DNA repair pathways. This ground-breaking tool enables precise genetic manipulations, shedding light on the molecular underpinnings of DNA repair processes. Studies leveraging this technology have significantly contributed to our understanding of how cells respond to DNA damage and the implications for cancer therapy.
These developments in DNA repair research are particularly relevant in the context of PARP inhibitors, a class of drugs that target specific DNA repair pathways in cancer cells. The insights gained from recent studies have enhanced our understanding of these mechanisms and opened new avenues for targeted cancer therapies, making personalized treatment more attainable.
Innovative biomarker strategies: Patent WO 2023/131894 A1 introduces genomic loss of heterozygosity (gLOH) as a biomarker to identify patients who would benefit from talazoparib, especially in cancers with HRR deficiency. This patent underscores the growing trend of personalized medicine in oncology, as it targets specific genetic profiles in breast and ovarian cancers.
Patent WO 2023/133409 A2 explores the combination of PARP1 inhibitors with agents affecting cancer-associated fibroblasts (CAFs). This approach reflects understanding the tumor microenvironment’s role in cancer progression, aiming to enhance treatment efficacy through strategic drug combinations.
Patent WO 2022/123427 A1 broadens talazoparib’s application scope by targeting tumors with non-BRCA1/2 homologous recombination pathway gene mutations. This development is crucial in offering therapy options to a broader range of cancer patients, especially those resistant to conventional treatments.
The clinical implications of these patents are profound. They promise enhanced treatment outcomes for patients with specific genetic mutations, making cancer therapy more effective and less generalized.
Talazoparib’s chemical structure enables it to inhibit PARP enzymes efficiently, which is crucial in DNA repair processes. This mechanism is particularly effective for HRR-deficient cancer cells, leading to increased DNA damage and cell death. Talazoparib’s effectiveness in targeting HRR-deficient tumors was recognized in previous approvals for treating certain breast cancers, especially in patients with inherited BRCA1/2 mutations.
These patents represent a significant stride in precision medicine, potentially extending talazoparib’s applicability to a broader range of cancers. They may set a precedent for future research and development in targeted cancer therapies. Talazoparib stands at the forefront of a new era in cancer treatment, demonstrating the power of precision medicine.
Key Structures
Biological Assay
A phase II clinical trial of talazoparib, PARP inhibitor in BRCA1 and BRCA2 wild-type patients with HER2-negative advanced breast cancer or other solid tumors.
Biological Data
The table below shows response rates
for patients treated with talazoparib monotherapy at 1 mg orally daily.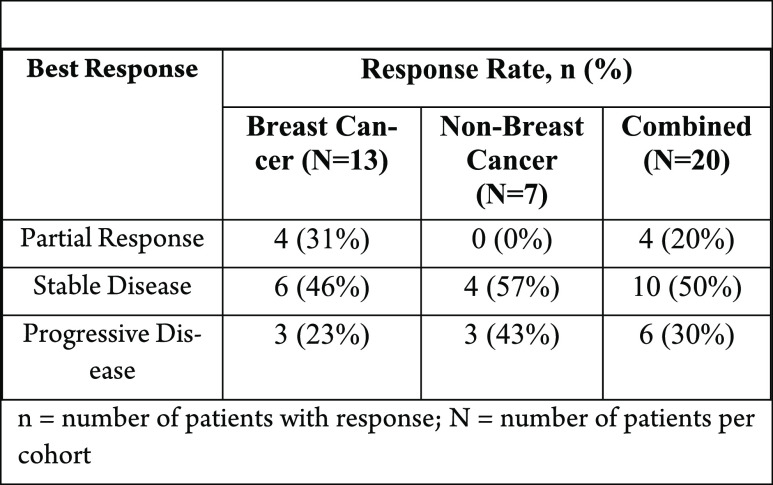
Recent Review Articles
The author declares no competing financial interest.
References
- Fu X.; Li P.; Zhou Q.; He R.; Wang G.; Zhu S.; Bagheri A.; Kupfer G.; Pei H.; Li J. Mechanism of PARP inhibitor resistance and potential overcoming strategies. Genes Dis. 2024, 11, 306–320. 10.1016/j.gendis.2023.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z.; Gowder M.; Proshkin S.; Bharati B. K.; Epshtein V.; Svetlov V.; Shamovsky I.; Nudler E. RNA polymerase drives ribonucleotide excision DNA repair in E. coli. Cell 2023, 186, 2425–2437.e21. 10.1016/j.cell.2023.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Miné-Hattab J.; Villemeur M.; Guerois R.; Pinholt H. D.; Mirny L. A.; Taddei A. In vivo tracking of functionally tagged Rad51 unveils a robust strategy of homology search. Nat. Struct. Mol. Biol. 2023, 30, 1582–1591. 10.1038/s41594-023-01065-w. [DOI] [PubMed] [Google Scholar]
- Thakur A.; Rana M.; Ritika; Mathew J.; Nepali S.; Pan C. H.; Liou J. P.; Nepali K. Small molecule tractable PARP inhibitors: Scaffold construction approaches, mechanistic insights and structure activity relationship. Bioorg. Chem. 2023, 141, 106893. 10.1016/j.bioorg.2023.106893. [DOI] [PubMed] [Google Scholar]
- Mai N.; Abuhadra N.; Jhaveri K. Molecularly Targeted Therapies for Triple Negative Breast Cancer: History, Advances, and Future Directions. Clin. Breast Cancer 2023, 23, 784–799. 10.1016/j.clbc.2023.05.012. [DOI] [PubMed] [Google Scholar]
- Maiorano B. A.; Conteduca V.; Catalano M.; Antonuzzo L.; Maiello E.; De Giorgi U.; Roviello G. Personalized medicine for metastatic prostate cancer: The paradigm of PARP inhibitors. Crit Rev. Oncol. Hematol. 2023, 192, 104157. 10.1016/j.critrevonc.2023.104157. [DOI] [PubMed] [Google Scholar]



