Abstract
Cancer cell migration is related to malignancy and patient prognosis. We previously reported that intracellular reactive oxygen species (ROS) promoted cancer cellular migration and invasion and that an antioxidant enzyme could help to attenuate the malignancy. Catechin is known as an antioxidant, and we have developed a catechin analog, planar catechin, which showed an antioxidant activity significantly stronger than that of the parent (+)-catechin. In this study, we examined the effects of the planar catechin on the migration of gastric normal and cancer cells. A scratched assay showed that the planar catechin suppressed the cellular migration rates in both normal and cancer cells, while the prevention levels in cancer cells were remarkable compared to the normal cells. These results suggest that the planar catechin with the enhanced antioxidant activity effectively scavenged the ROS overexpressed in the cancer cells and inhibited cancer cellular activities, including migration.
Keywords: Planar catechin, Gastric cancer cells, Cell migration
Cancer cellular migration and invasion are related to the metastasis and subsequent tumor formation.1,2 Therefore, the migration of cancer cells is associated with patient prognosis. To activate the ability of migration and invasion in cancer cells, reactive oxygen species (ROS), which are mainly produced in mitochondria, are reported to play an important role.3,4 ROS can activate nuclear factor-kappa B (NF-κB), which is a transcription factor, plays a pivotal role in inflammation, and is related to cancer cellular migration through the induction of matrix metalloproteinase-9 expression.5−8 On the other hand, overexpression of manganese superoxide dismutase (MnSOD), a superoxide scavenging enzyme localized in mitochondria, was reported to suppress cancer cellular migration and invasion.9 Thus, intracellular ROS scavenging by a powerful antioxidant may contribute to the inhibition of cell migration in cancer cells.
Catechin is known as an antioxidant and has some physiological effects, such as antiatherogenic effects, abdominal fat reduction, and so on.10,11 We have developed a novel catechin analog, planar catechin, which shows a 10-fold stronger scavenging capacity than the parent (+)-catechin against 2,2-diphenyl-1-picrylhydrazyl radicals solubilized in water by β-cyclodextrin in phosphate buffer (0.1 M, pH 7.4) at 298 K (Figure 1).12,13 Furthermore, we recently reported that the planar catechin showed cancer cell dominant cytotoxicity through the decrease of mitochondrial membrane potential.14 Hence, the planar catechin may also inhibit cancer cellular migration and could be a promising agent for improvement of cancer patient prognosis. We report herein the inhibitory effects of the planar catechin on the cell migration in comparison with those of (+)-catechin using a rat normal gastric cell line, RGM1, and its chemically mutated cell line, RGK1.
Figure 1.
Chemical structures of (+)-catechin and planar catechin.
ROS produced in cells are related to the cell migration, and thus, a treatment with antioxidants, such as N-acetylcysteine and vitamin C, could inhibit cancer cellular migration.15−17 In this study, we examined the effects of the planar catechin, which showed a higher ROS-scavenging ability than the parent (+)-catechin, on cell migration of gastric normal and cancer cells. As shown in Figure 2a,b, the scratched areas in both RGM1 and RGK1 control samples were healed in 12 h after the assay started and those with (+)-catechin treatment showed almost the same results. However, a treatment with the planar catechin suppressed the healing, and the scratched areas were left after 12 h. Interestingly, the remarkable inhibition of the healing was observed in RGK1 cancer cells compared to RGM1 normal cells after treatment with the planar catechin (Figure 2c,d), indicating that the planar catechin predominantly suppressed the cancer cell migration. Cell migration may be related to cell proliferation. In cancer cells, ROS production is elevated and the high levels of ROS induce cell proliferation.18,19 Therefore, administration of antioxidants would enable scavenging of ROS overgenerated in cancer cells and subsequent prevention of proliferation. In fact, we have reported that a red dye derived from Monascus purpureus had high antioxidant ability and inhibited cancer cell proliferation by decreasing mitochondrial ROS.20 In addition, water-containing nanobubbled hydrogen shows superior ROS scavenging ability, and we have clarified that the nanobubbled hydrogen can inhibit cancer cell growth in vitro and tumor growth in vivo.21,22 Thus, it is suggested that the suppression of cancer cell migration in this study was induced by the strong antioxidant capacity of the planar catechin. On the other hand, several antioxidants are reported to promote migration and proliferation of skin cells and fibroblasts in vitro and in vivo.23 Moreover, some antioxidant enzymes are related to wound healing in genetically modified mice, and the induction of a cyclin-dependent kinase inhibitor is lower in SOD1-deficient mice than in wild-type mice.24 The types of antioxidants, the parts of the tissue, and genetic backgrounds may also be associated with the wound-healing process.
Figure 2.
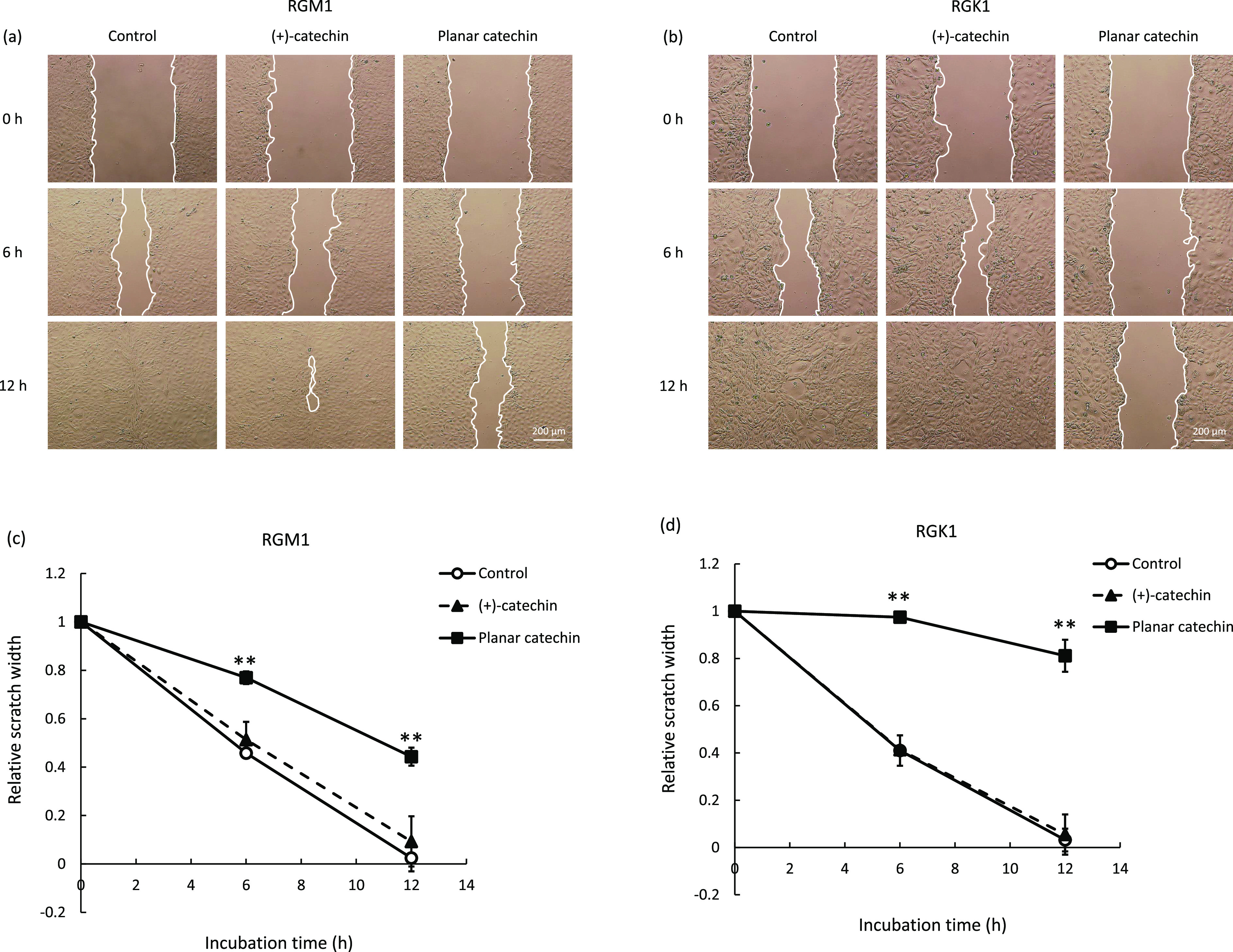
Observation and measurement of cell migration distances after treatment of (+)-catechin or the planar catechin for 0, 6, and 12 h. Representative images of migration assay of (a) RGM1 and (b) RGK1. Scale bar: 200 μm. Results of the distances between the scratched areas in (c) RGM1 and (d) RGK1. Statistical significance was tested by Tukey HSD. n = 5, mean ± SD, **p < 0.01 vs other samples at the same incubation time.
There are some chemical compounds that induce the expression of antioxidant enzyme to exert ROS scavenging ability. Nakao et al. reported that oral intake of hydrogen-rich water for 8 weeks increased SOD levels.25 Sulforaphane, which is rich in broccoli sprouts, shows an antioxidant activity through the activation of the Nrf2-Keap1 system and subsequent induction of antioxidant enzyme, such as hemeoxygenase-1.26,27 Epigallocatechin gallate, which is one of the catechin species and abundant in green tea, is also reported to activate Nrf2 via inactivating Keap1.28 Furthermore, reactive antioxidants could be pro-oxidants and new reactants with other biomolecules, because antioxidants are easily oxidized, and may be a possible inducer of Nrf2 activation.29,30 Thus, planar catechin might cause the prevention of cellular migration through the activation of antioxidant signaling pathways such as Nrf2-Keap1.
In conclusion, the catechin analog, planar catechin, which has much higher antioxidant ability than the parent (+)-catechin, showed outstanding inhibition effects on cell migration, especially in RGK1 cancer cells. We suggest that the powerful antioxidant ability of the planar catechin scavenged the ROS overexpressed in the cancer cells and resulted in the subsequent attenuation of cancer cellular activities, leading to migration prevention. These results indicate that the planar catechin could be a promising agent for attenuation of cancer cellular malignancy and could lead to improved prognosis for patients with cancer. However, the behavior of the planar catechin, including the process of uptake and metabolism in cells, is still unclear, and clarification of the detailed mechanism on the suppression of cell migration by the planar catechin is currently being pursued.
Glossary
Abbreviations
- ROS
reactive oxygen species
- NF-κB
nuclear factor-kappa B
- SOD
superoxide dismutase
- Nrf2
nuclear factor erythroid 2-related factor 2
- Keap1
Kelch like ECH associated protein 1
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00499.
Detailed description of experimental methods (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lambert A. W.; Pattabiraman D. R.; Weinberg R. A. Emerging Biological Principles of Metastasis. Cell 2017, 168 (4), 670–691. 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikov N. M.; Zolotaryova S. Y.; Gautreau A. M.; Denisov E. V. Mutational Drivers of Cancer Cell Migration and Invasion. Br. J. Cancer 2021, 124 (1), 102–114. 10.1038/s41416-020-01149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal V.; Tuli H.; Varol A.; Thakral F.; Yerer M.; Sak K.; Varol M.; Jain A.; Khan M.; Sethi G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9 (11), 735. 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indo H. P.; Davidson M.; Yen H. C.; Suenaga S.; Tomita K.; Nishii T.; Higuchi M.; Koga Y.; Ozawa T.; Majima H. J. Evidence of ROS Generation by Mitochondria in Cells with Impaired Electron Transport Chain and Mitochondrial DNA Damage. Mitochondrion 2007, 7 (1–2), 106–118. 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A.; Ghosh S. The NF-KappaB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1 (4), a000034. 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Yi S.; Zhou J.; Zhang Y.; Guo F. The NF-ΚB Subunit RelB Regulates the Migration and Invasion Abilities and the Radio-Sensitivity of Prostate Cancer Cells. Int. J. Oncol. 2016, 49 (1), 381–392. 10.3892/ijo.2016.3500. [DOI] [PubMed] [Google Scholar]
- Helbig G.; Christopherson K. W.; Bhat-Nakshatri P.; Kumar S.; Kishimoto H.; Miller K. D.; Broxmeyer H. E.; Nakshatri H. NF-ΚB Promotes Breast Cancer Cell Migration and Metastasis by Inducing the Expression of the Chemokine Receptor CXCR4. J. Biol. Chem. 2003, 278 (24), 21631–21638. 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- Hsieh H.-L.; Wang H.-H.; Wu W.-B.; Chu P.-J.; Yang C.-M. Transforming Growth Factor-Β1 Induces Matrix Metalloproteinase-9 and Cell Migration in Astrocytes: Roles of ROS-Dependent ERK- and JNK-NF-ΚB Pathways. J. Neuroinflammation 2010, 7 (1), 88. 10.1186/1742-2094-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M.; Matsui H.; Tomita T.; Sadakata H.; Indo H. P.; Majima H. J.; Kaneko T.; Hyodo I. Mitochondrial Reactive Oxygen Species Accelerate Gastric Cancer Cell Invasion. J. Clin. Biochem. Nutr. 2014, 54 (1), 12–17. 10.3164/jcbn.13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y.; Chiba T.; Tomita I.; Koizumi H.; Miura S.; Umegaki K.; Hara Y.; Ikeda M.; Tomita T. Tea Catechins Prevent the Development of Atherosclerosis in Apoprotein E-Deficient Mice. J. Nutr. 2001, 131 (1), 27–32. 10.1093/jn/131.1.27. [DOI] [PubMed] [Google Scholar]
- Wang H.; Wen Y.; Du Y.; Yan X.; Guo H.; Rycroft J. A.; Boon N.; Kovacs E. M. R.; Mela D. J. Effects of Catechin Enriched Green Tea on Body Composition. Obesity 2010, 18 (4), 773–779. 10.1038/oby.2009.256. [DOI] [PubMed] [Google Scholar]
- Fukuhara K.; Nakanishi I.; Kansui H.; Sugiyama E.; Kimura M.; Shimada T.; Urano S.; Yamaguchi K.; Miyata N. Enhanced Radical-Scavenging Activity of a Planar Catechin Analog. J. Am. Chem. Soc. 2002, 124 (21), 5952–5953. 10.1021/ja0178259. [DOI] [PubMed] [Google Scholar]
- Sekine-Suzuki E.; Nakanishi I.; Imai K.; Ueno M.; Shimokawa T.; Matsumoto K. I.; Fukuhara K. Efficient Protective Activity of a Planar Catechin Analogue against Radiation-Induced Apoptosis in Rat Thymocytes. RSC Adv. 2018, 8 (19), 10158–10162. 10.1039/C7RA13111A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H.; Shoji Y.; Matsumoto K.; Fukuhara K.; Nakanishi I. Anti-Cancer Effect of a Planar Catechin Analog through the Decrease in Mitochondrial Membrane Potential. ACS Med. Chem. Lett. 2023, 14 (10), 1478–1481. 10.1021/acsmedchemlett.3c00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd T. R.; DeGennaro M.; Lehmann R. Redox Regulation of Cell Migration and Adhesion. Trends Cell Biol. 2012, 22 (2), 107–115. 10.1016/j.tcb.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J.; Liu A.-D.; Hou G.-Q.; Zhang X.; Ren K.; Chen X.-Z.; Li S. S. C.; Wu Y.-S.; Cao X. N-Acetylcysteine Decreases Malignant Characteristics of Glioblastoma Cells by Inhibiting Notch2 Signaling. J. Exp. Clin. Cancer Res. 2019, 38 (1), 2. 10.1186/s13046-018-1016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L. H.; Wang Q. M.; Feng L. Y.; Ke Y. D.; Xu Q. Z.; Wei A. Y.; Zhang C.; Ying R. B. High-Dose Vitamin C Suppresses the Invasion and Metastasis of Breast Cancer Cells via Inhibiting Epithelial-Mesenchymal Transition. Onco. Targets. Ther. 2019, 12, 7405–7413. 10.2147/OTT.S222702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatrowski T. P.; Nathan C. F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51 (3), 794–798. [PubMed] [Google Scholar]
- Zeng J.; Li M.; Xu J. Y.; Xiao H.; Yang X.; Fan J. X.; Wu K.; Chen S. Aberrant ROS Mediate Cell Cycle and Motility in Colorectal Cancer Cells Through an Oncogenic CXCL14 Signaling Pathway. Front. Pharmacol. 2021, 12, 764015. 10.3389/fphar.2021.764015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa H.; Ito H.; Matsui H. Monascus Purpureus Induced Apoptosis on Gastric Cancer Cell by Scavenging Mitochondrial Reactive Oxygen Species. J. Clin. Biochem. Nutr. 2017, 61 (3), 189–195. 10.3164/jcbn.17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S.; Matsuoka D.; Miwa N. Antioxidant Activities of Nano-Bubble Hydrogen-Dissolved Water Assessed by ESR and 2,2′-Bipyridyl Methods. Mater. Sci. Eng., C 2015, 53, 7–10. 10.1016/j.msec.2015.03.064. [DOI] [PubMed] [Google Scholar]
- Kurokawa H.; Matsui H.; Ito H.; Taninaka A.; Shigekawa H.; Dodbiba G.; Wei Y.; Fujita T. Antioxidant Effect of Hydrogen Nanobubble Contributes to Suppression of Tumor Cell Growth. Biomed. J. Sci. Technol. Res. 2019, 19 (5), 14592–14594. 10.26717/BJSTR.2019.19.003361. [DOI] [Google Scholar]
- Comino-Sanz I. M.; López-Franco M. D.; Castro B.; Pancorbo-Hidalgo P. L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10 (16), 3558. 10.3390/jcm10163558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T.; Fujii J. Roles of Antioxidative Enzymes in Wound Healing. J. Dev. Biol. 2015, 3 (2), 57–70. 10.3390/jdb3020057. [DOI] [Google Scholar]
- Nakao A.; Toyoda Y.; Sharma P.; Evans M.; Guthrie N. Effectiveness of Hydrogen Rich Water on Antioxidant Status of Subjects with Potential Metabolic Syndrome - An Open Label Pilot Study. J. Clin. Biochem. Nutr. 2010, 46 (2), 140–149. 10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Talalay P.; Cho C. G.; Posner G. H. A Major Inducer of Anticarcinogenic Protective Enzymes from Broccoli: Isolation and Elucidation of Structure. Proc. Natl. Acad. Sci. U. S. A. 1992, 89 (6), 2399–2403. 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum Y.; Yu S.; Chang P. P.; Yuan X.; Kim J.; Xu C.; Han J.; Agarwal A.; Kong A. T. Mechanism of Action of Sulforaphane: Inhibition of P38 Mitogen-Activated Protein Kinase Isoforms Contributing to the Induction of Antioxidant Response Element-Mediated Heme Oxygenase-1 in Human Hepatoma HepG2 Cells. Cancer Res. 2006, 66 (17), 8804–8813. 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- Sun W.; Liu X.; Zhang H.; Song Y.; Li T.; Liu X.; Liu Y.; Guo L.; Wang F.; Yang T.; Guo W.; Wu J.; Jin H.; Wu H. Epigallocatechin Gallate Upregulates NRF2 to Prevent Diabetic Nephropathy via Disabling KEAP1. Free Radic. Biol. Med. 2017, 108, 840–857. 10.1016/j.freeradbiomed.2017.04.365. [DOI] [PubMed] [Google Scholar]
- Childs A.; Jacobs C.; Kaminski T.; Halliwell B.; Leeuwenburgh C. Supplementation with Vitamin C and N-Acetyl-Cysteine Increases Oxidative Stress in Humans after an Acute Muscle Injury Induced by Eccentric Exercise. Free Radic. Biol. Med. 2001, 31 (6), 745–753. 10.1016/S0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- Shin J.; Song M. H.; Oh J. W.; Keum Y. S.; Saini R. K. Pro-oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants 2020, 9 (6), 532. 10.3390/antiox9060532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.