Abstract
The KEAP1-NRF2 axis is pivotal in the cellular mechanism against oxidative and electrophilic stress. NRF2, under standard conditions, undergoes proteasomal degradation mediated by the E3 ubiquitin ligase KEAP1. Stress conditions lead to KEAP1 inactivation, facilitating NRF2 stability and subsequent activation of defensive genes. NRF2 signaling anomalies are associated with cancer progression and neurodegenerative diseases. Continuous activation of the NRF2 pathway aids in the survival of cancer cells, while a deficiency in NRF2 functionality intensifies inflammation and oxidative injury in neurodegenerative disease models. Thus, the modulation of this pathway is being investigated for therapeutic applications in both cancer and neurodegenerative diseases.
Important Compound Classes
Titles
Oxadiazole Derivatives, Preparation Process Thereof and Their Use in Treating Inflammatory Diseases; and Tetrahydroisoquinoline Compounds that are KEAP1 Binders
Patent Publication Numbers
WO 2023247958 A1 (https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2023247958&_cid=P10-LQV3YC-90555-1)
WO 2023073364 A1 (https://patents.google.com/patent/WO2023073364A1/en?oq=WO+2023073364+A1)
Publication Dates
December 28, 2023, and May 4, 2023
Priority Applications
EP 23159047.2 and GB 2115334.1
Priority Dates
February 28, 2023, and October 25, 2021
Inventors
Ahmed, S.; Cooke, M. L.; Mazzacani, A.; Waugh, T. (WO 2023247958 A1); and Lucas, C. L.; Ray, N. C.; Esmieu, W. R. K. (WO 2023073364 A1).
Assignee Companies
Sitryx Therapeutics Limited [GB/GB], 101 Bellhouse Building, Magdalen Center, The Oxford Science Park, Oxford OX4 4GA, GB (WO 2023247958 A1); and C4X Discovery Limited [GB/GB], Manchester One, Suite 4B, 53 Portland Street, Manchester MI 3LD, GB (WO 2023073364 A1)
Disease Area
Cancer
Biological Target
KEAP1
Summary
Nuclear factor erythroid 2-related factor 2 (NRF2) regulates the transcription of genes essential for cellular defense against oxidative and electrophilic occurrences. Kelch-like ECH-associated protein 1 (KEAP1), an essential regulator, facilitates NRF2 ubiquitination and degradation under non-stressful conditions. The KEAP1-NRF2 pathway is crucial in defending against various stresses, leading to chemoresistance in cancers and aggravation of neurodegenerative disorders upon NRF2 inactivity. Deciphering the exact molecular regulation of this pathway is pivotal for therapeutic targeting.
Molecular regulation of NRF2 signaling by KEAP1: KEAP1 employs a cullin3 (Cul3)-based E3 ubiquitin ligase complex for NRF2 degradation, ensuring minimal basal NRF2 activity. Electrophilic or oxidative stress alters KEAP1, inhibiting NRF2 ubiquitination and allowing NRF2 stabilization. This leads to NRF2’s interaction with small Maf proteins and activation of genes through antioxidant response elements or electrophile response elements.
KEAP1-NRF2 pathway in cancer: Mutations in NRF2, KEAP1, or Cul3 lead to NRF2 overaccumulation in various cancers, enhancing cell proliferation, survival, and resistance to therapies. The coexistence of NRF2 mutations with alterations in KRAS/PI3K/Akt signaling pathways indicate their collective role in tumor progression. Cancerous alterations include NRF2 activation through alternative splicing, mutations at the KEAP1 interaction site, or overexpression of KEAP1 inhibitors.
Role of KEAP1-NRF2 pathway in neurodegeneration: NRF2 activation is neuroprotective in neurodegenerative contexts, reducing inflammation and oxidative damage. NRF2 knockout models exhibit aggravated neurodegeneration, whereas treatments activating NRF2, such as dimethyl fumarate, show improved outcomes. The KEAP1-NRF2 pathway thus presents as a promising target for neuroprotection.
Therapeutic opportunities targeting the KEAP1-NRF2 pathway: Addressing aberrant NRF2 signaling in cancer through NRF2 inhibitors or targeting synthetic lethal interactions offers potential therapeutic avenues. Conversely, pharmacological NRF2 activation might confer neuroprotection. Understanding the molecular intricacies of this pathway is essential for therapeutic manipulation.
Recent advancements include utilizing KEAP1 for targeted protein degradation in proteolysis targeting chimeras (PROTACs), especially concerning central nervous system (CNS) diseases. A notable development is the PROTAC named MS83, which links a KEAP1 ligand to a BRD4 binder, exemplifying the potential of KEAP1-based PROTACs in selective protein degradation and offering new treatment possibilities for various CNS diseases.
Additional therapeutic developments: Emerging studies on oxadiazole derivatives, particularly in inflammatory diseases, complement the ongoing research on the KEAP1-NRF2 pathway. These derivatives, including dimethyl fumarate and 4-octyl itaconate, have demonstrated efficacy in modulating various cellular responses integral to inflammation and immune regulation.
Their mechanisms of action, notably the activation of the NRF2 pathway and the suppression of pro-inflammatory cytokines, align with the therapeutic targets of the KEAP1-NRF2 system. Incorporating these findings enhances the understanding of potential therapeutic strategies, particularly in addressing inflammatory and neurodegenerative diseases through NRF2 modulation. This convergence of research underscores the expanding landscape of targeted therapies in combating chronic inflammatory conditions and their associated immune responses.
As such, the KEAP1-NRF2 signaling axis is crucial in cellular stress response, with distinct functional anomalies in cancer and neurodegenerative diseases. The modulation of NRF2-KEAP1 signaling offers a compelling strategy for therapeutic intervention.
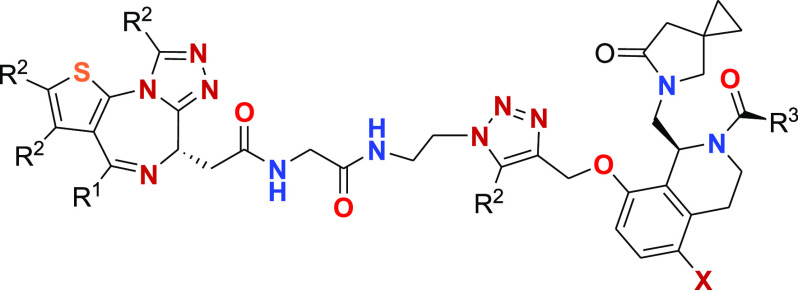
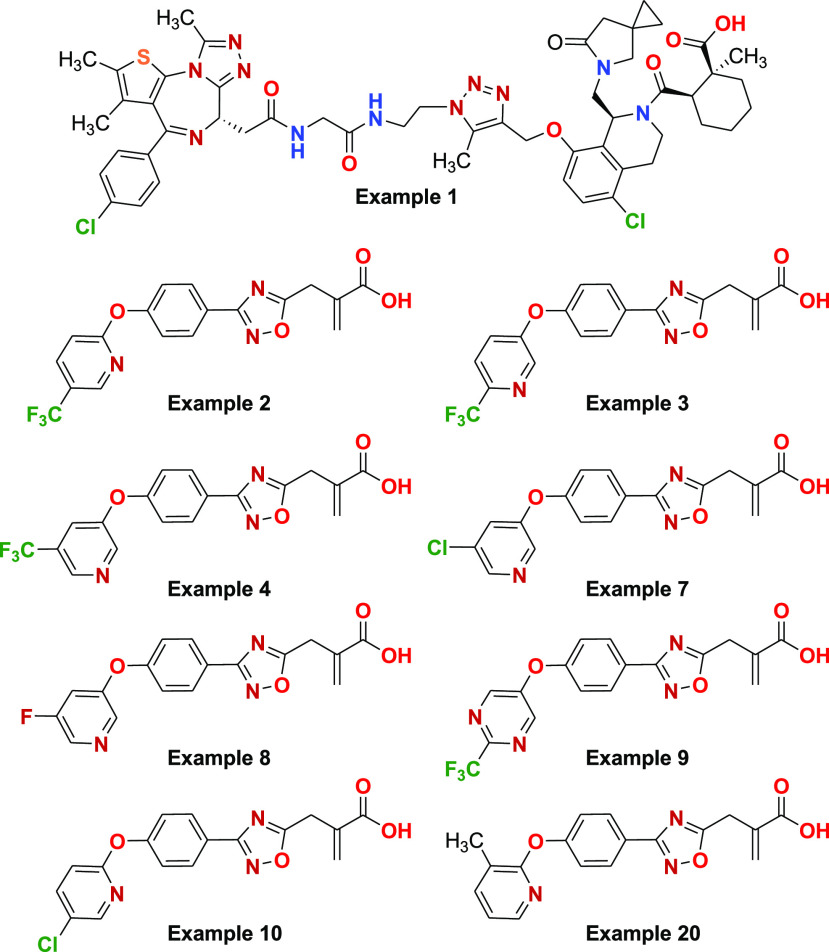
Key Structures
Biological Assay
BRD4 Degradation assay, mRNA cell-based assay, alphaLISA IL-1β, and IL-6 cytokine assay
Biological Data
The table below shows THP-1 cell IL-1β and IL-6 IC50 values (μM) for exemplary compounds.
For Example 1, the degradation of BRD4 was nearly
complete when treated with concentrations ≥0.1 μM. Furthermore,
this treatment resulted in up to 75% degradation of the target protein
at a lower concentration of 0.01 μM (WO 2023073364 A1).
Recent Review Articles
The author declares no competing financial interest.
References
- Wang J.; Cao Y.; Lu Y.; Zhu H.; Zhang J.; Che J.; Zhuang R.; Shao J. Recent progress and applications of small molecule inhibitors of Keap1-Nrf2 axis for neurodegenerative diseases. Eur. J. Med. Chem. 2024, 264, 115998. 10.1016/j.ejmech.2023.115998. [DOI] [PubMed] [Google Scholar]
- Yang F.; Smith M. J. Metal profiling in coronary ischemia-reperfusion injury: Implications for KEAP1/NRF2 regulated redox signaling. Free Radic. Biol. Med. 2024, 210, 158–171. 10.1016/j.freeradbiomed.2023.11.013. [DOI] [PubMed] [Google Scholar]
- Yang W.; Wang Y.; Tao K.; Li R. Metabolite itaconate in host immunoregulation and defense. Cell. Mol. Biol. Lett. 2023, 28, 100. 10.1186/s11658-023-00503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusetskaya N. Y.; Loginova N. Y.; Pokrovskaya E. P.; Chesovskikh Y. S.; Titova L. E. Redox regulation of the NLRP3-mediated inflammation and pyroptosis. Biomed. Khim. 2023, 69, 333–352. 10.18097/pbmc20236906333. [DOI] [PubMed] [Google Scholar]
- Ge M.; Papagiannakopoulos T.; Bar-Peled L. Reductive stress in cancer: coming out of the shadows. Trends Cancer. 2023, 10.1016/j.trecan.2023.10.002. [DOI] [PubMed] [Google Scholar]
- Qian H.; Zhu M.; Tan X.; Zhang Y.; Liu X.; Yang L. Superenhancers and the superenhancer reader BRD4: tumorigenic factors and therapeutic targets. Cell Death Discovery 2023, 9, 470. 10.1038/s41420-023-01775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]