Abstract
This work describes the studies on the direct C3-glycosylation of the C19-hydroxylated cardiotonic steroids strophanthidol, anhydro-ouabagenin, and ouabagenin using a strategy based on in situ protection of the C5 and C19 hydroxyl groups with boronic acids. While this strategy resulted in a successful one-pot C3-selective glycosylation of strophanthidol and anhydro-ouabegenin, it failed to provide ouabain from ouabagenin. The neuroprotective activity of the synthetic and natural glycosides against LPS-induced neuroinflammation was explored in neonatal mouse primary glia cells. Co-administration of natural and synthetic C3-glycosides at 200 nM concentrations resulted in the significant reduction of the LPS-induced neuroinflammatory markers IL-6, IL-1, TNFα, and IKBKE, with the anhydro-ouabagenin-3-(α)-l-rhamnoside (anhydro-ouabain) showing the most significant effect. At the same time, unglycosylated anhydro-ouabagenin enhanced rather than suppressed LPS-induced neuroinflammation.
Keywords: cardiotonic steroids, site-selective glycosylation, anti-inflammatory activity, glia cells
Cardenolides represent a broad family of glycosylated steroids found in animals and plants such as oleander, foxglove, lily of the valley, red squill, dogbane, etc.1,2 For centuries, herbal remedies containing cardenolides as active ingredients have been used to treat various conditions such as edema or “dropsy”.3 Cardenolides are known to serve as inotropic agents, and they are known to reversibly inhibit the Na,K-ATPase pump, which increases intracellular sodium and decreases intracellular potassium levels, which in turn results in intracellular accumulation of calcium. Due to this effect, cardiotonic steroids digoxin and digitoxin have been essential therapeutics for the treatment of heart failure and arrhythmia.2,4−7 However, the biological activity of cardenolides extends well beyond their cardiotonic activity. Among numerous other applications, cardiotonic steroids have been explored as potential therapeutic agents for the treatment of cancer,8−10 viral infections,11,12 neurodegenerative diseases,13,14 and stroke15−17 and as anti-inflammatory12,18,19 and senolytic20 agents. Particularly interesting are the recent reports indicating that cardiotonic steroids, such as ouabain and digoxin, may influence the peripheral and central nervous systems. Ouabain is an endogenous cardiotonic steroid produced by mammalians in subnanomolar concentrations by the adrenal gland, hypothalamus, and pituitary.21−24 Several recent in vivo studies demonstrated ouabain has an anti-inflammatory effect in the rat hippocampus and that ouabain demonstrated neuroprotection against liposaccharide (LPS) induced inflammation.25−28 This neuroprotective effect was attributed to the ability of ouabain to promote membrane lipid remodeling and increase the expression of glutamate transporter EAAT4, which helps to mitigate the oxidative stress induced by LPS. This effect is not limited to ouabain as cardiotonic steroids oleandrin and digoxin demonstrated neuroprotection for oxygen and glucose deprivation in ischemia models.15−17
While these prior examples suggest that cardenolides may exhibit neuroprotective properties, the specific structural features that are essential for these effects are unknown. It has been observed that both the carbohydrate structure and skeletal oxidation (Figure 1A) may impact the biological activity of cardenolides; however, no comparative study evaluating neuroprotective properties of cardenolides across a group of cardiotonic steroids with different oxidation patterns and glycosylation sites has been carried to date. This work describes selective (α)-l-rhamnosylation and subsequent exploration of C19-hydroxylated cardenolides with different oxidation patterns (1–3). The potential of the synthetic and natural steroids to reduce LPS-caused neuroinflammation was investigated at 200 nM concentration using primary glia cells derived from neonatal 1–2 day old mice. The effect of steroids was monitored using the gene expression of inflammation markers IL-1, IL-6, TNFα, and IKBKE (inhibitor of nuclear kappa B kinase subunit epsilon), and several significantly differing responses were observed. The synthetic and natural 3-(α)-l-rhamnosides of 1–3 as well as digitoxigenin were found to significantly reduce the LPS-induced inflammation with the synthetic analog anhydro-ouabagenin 3-(α)-l-rhamnoside (20) exhibiting the most significant effect. At the same time, the aglycone of 20, anhydro-ouabagenin (2), significantly enhanced the inflammation levels.
Figure 1.
Polyhydroxylated cardiotonic steroid aglycones and challenges with site-selective glycosylation.
The Nagorny group has long-standing interests in streamlining the medicinal chemistry exploration of cardiotonic steroids by developing new strategies for the aglycone synthesis and their selective glycosylation.29−32 Previously, we described strategies for the installation of native (α)-l-rhamnoside moiety at the C3-position of various cardiotonic steroids.33−35 It was observed that cardiotonic steroids such as 5 containing C3 and C19 oxidation undergo competitive C19 glycosylation that produces a complex mixture of products such as 7 and 8 (cf. Figure 1B).33 While this challenge could be overcome by selective introduction of a C19-protecting group such as methoxyacetate,33 this may require significant protecting group optimization and might be problematic for the substrates that have additional hydroxyl groups such as 1–3 (Figure 1A).36−38
In the context of their studies focused on regioselective glycosylation of 6-deoxyerythronolide B,39 we utilized boronic acid esters as the traceless protecting groups.40−43In situ protection of the 1,3-diol moiety of 2-deoxyerythronolide B (6-dEB) was followed by the addition of glycosyl trichloroacetimidate and TMSOTf as the promoter to accomplish selective glycosylation. The basic reaction work up was used to remove the boronic acid and produce the desired glycoside as the single regioisomer. This concept could be applied to highly oxidized cardiotonic steroids 1–3 containing 1,3,5-hydroxyl groups (Scheme 1).34 However, unlike the previous glycosylation developed for 6-dEB, the presence of multiple hydroxyl groups may lead to several regioisomeric protection products in addition to the desired boronic acid ester 9. This would lead to multiple regioisomeric glycosylation products in addition to 9. Assuming that boronic acid formation is a reversible process and the most thermodynamically stable boronate is formed under the protection conditions, the regioselectivity of boronic acid ester formation can be assessed computationally. The results of these computations (DFT, B3LYP, 6-31+G*) are summarized in Table 1. Ouabagenin (1) contains 5 hydroxyl groups that may participate in boronic acid ester formation and may form 8 potential products. The C1/C19 boronic acid ester 13 was found to have the lowest energy with 2.3 kcal/mol higher stability than the second most stable C5/C19 boronate 9. Only 3 cyclic boronates are possible in the case of anhydro-ouabagenin (2) and strophanthidol (3). In both cases, the most stable ester is formed by the complexation of the C5/C19 sites, and the formation of the second most stable C3/C5 boronate would be disfavored by 1.9 kcal/mol (2) or 1.7 kcal/mol (1). The results in Table 1 suggest that the reaction of methyl boronic acid with 2 and 3 should lead to clean protection of the C5 and C19 positions, and only the C3 alcohols would be available to react. At the same time, the reaction of ouabagenin will result in a selective protection of the C1 and C19 alcohols, and the C3 and C11 alcohols can be potentially glycosylated. Indeed, when strophanthidol (3) was treated with methyl boronic acid in the presence of 4 Å MS, exclusive formation of boronate 14 was observed by NMR (Scheme 2). The reaction of 14 with trichloroacetimidate 6 required optimization.34 Under the optimized conditions, that included using 2 equiv of 6, activation with triflic acid (30 mol %), 4 Å MS at 0 °C to r.t. resulted in the efficient formation of the glycosylated product. The subsequent removal of the boronic acid ester with methanol resulted in an 81% yield of the desired α-product 15. The benzoyl protecting groups present on the rhamnose moiety of 15 were selectively removed with a saturated solution of ammonia in methanol (18 h) to provide the desired deprotection product in quantitative yield. This procedure was subsequently optimized to be performed as a one-pot telescoped operation to provide α-16 directly from 3 in 73% overall yield.
Scheme 1. Traceless Boronic Acid Protecting Group for the Selective Protection of the C19 Position of Cardiotonic Steroids.
Table 1. Computed Relative Stabilities of Methyl Boronic Acid Esters Derived from Steroids 1–3 (DFT, B3LYP, 6-31+G*).
| realtive
energy for the regioisomeric boronates (DFT, B3LYP, 6-31+G*) (kcal/mol) |
||||||||
|---|---|---|---|---|---|---|---|---|
| scaffold | C1/C3 | C1/C5 | C1/C11 | C1/C19 | C3/C5 | C3/C19 | C5/C19 | C11/C19 |
| 1a | +8.3 | +8.9 | +7.6 | 0 | +7.8 | +18.3 | +2.3 | +7.6 |
| 2b | - | - | - | - | +1.9 | +22.2 | 0 | - |
| 3c | - | - | - | - | +1.7 | +17.2 | 0 | - |
Referenced to the energy of cyclic C3/C5 boronate derived from 1.
Referenced to the energy of cyclic C5/C19 boronate of 2.
Referenced to the energy of cyclic C5/C19 boronate derived from 3.
Scheme 2. C3-Selective (α)-l-Rhamnosylation of Strophanthidol (3).
Our subsequent studies focused on investigating the C3-selective (α)-l-rhamnosylation of anhydro-ouabagenin (2) (Scheme 3). Anhydro-ouabagenin (2) is a known degradation product of ouabain.44−46 While this compound carries all of the major structural features present in ouabagenin (1) and strophanthidol (3), the glycosylated variants of 2 have not been previously synthesized and evaluated. As in the case of 3, computational studies (Table 1) predicted selective formation of the C5/C19 boronate. Indeed, upon exposure to methylboronic acid, single boronate 17 was observed by 1H NMR. However, subjecting 17 to reaction with 6 under previously developed conditions resulted in a 1.8:1 mixture of the C3- and C19-glycosylated products 18 and 19 in 65% yield. This eroded selectivity could be attributed to in situ boronate isomerization under acidic conditions, which is more favored for the conformationally restricted 2.
 |
1 |
Both 18 and 19 were separated and subsequently deprotected to provide regioisomeric glycosides 20 and 21. To avoid the isomerization of 17, a base promoted glycosylation was investigated as a one-pot transformation (eq 1). Thus, cyclic boronate 17 was formed in situ and the resultant crude reaction mixture was subjected to glycosylation with glycosyl bromide 22 in the presence of silver(I) carbonate. The resultant mixture was filtered through Celite to remove excess Ag(I) salts and treated with ammonia in methanol to provide a 6:1 mixture of 20 and 21 in 56% yield.
Scheme 3. Initial Studies on C3-Selective (α)-l-Rhamnosylation of Anhydro-ouabagenin (2).
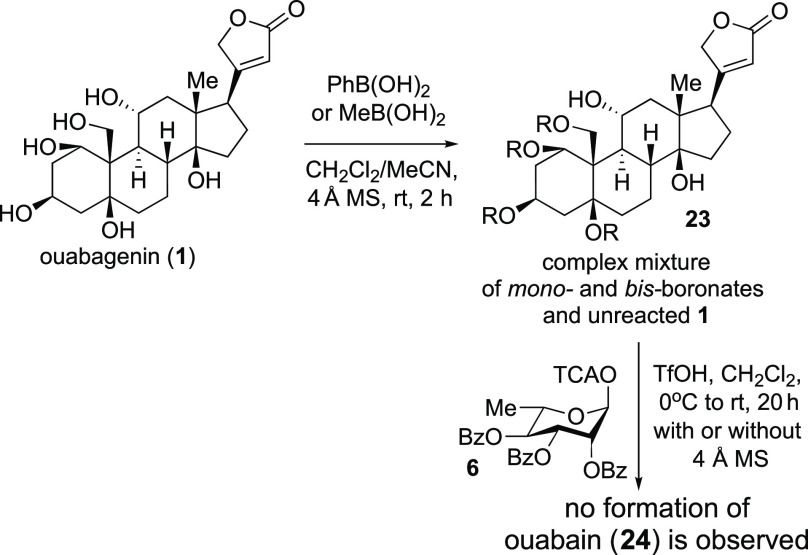
Unlike 2 and 3, ouabagenin (1) is expected to form a C1/C19 boronate (cf. Table 1). However, the low solubility of 1 in dichloromethane prevented this reaction (Scheme 4). The addition of acetonitrile as a cosolvent seemed to aid the formation of boronic acid esters; however, complexation with either methyl or phenyl boronic acids resulted in the formation of multiple mono- and bis-protected esters. Subjecting these mixtures to acid-catalyzed glycosylation with 6 did not lead to the formation of ouabain (24), and multiple decomposition products were isolated along with unreacted 1.
Scheme 4. Attempts to Accomplish the C3-Selective Glycosylation of Ouabagenin (1).
With access to synthetic aglycones 2 and 3, and their (α)-l-rhamnosides 16, 20, and 21, our subsequent studies focused on exploring neuroprotective properties of these derivatives along with natural cardenolides digoxin and ouabain (Figure 2).
Figure 2.

Evaluation of anti-inflammatory activity of 200 nM solutions of (A) digoxin; (B) ouabain (1); (C) anhydro-ouabagenin-3-(α)-l-rhamnoside (20); (D) anhydro-ouabagenin-19-(α)-l-rhamnoside (21); (E) strophanthidol-3-(α)-l-rhamnoside (16); and (F) anhydro-ouabagenin (2) during 24 h in the primary glial cell culture. The gene expression of IL-1, IL-6, TNFα, and IKBKE was stimulated using LPS (250 ng/mL), and DMSO was used as a negative control. Data were expressed as the mean ± SEM and analyzed by two-way ANOVA followed by Newman-Keuls post hoc analysis. (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
A primary glial cell culture extracted from the whole brain was used to assess the potential neuroprotective properties of cardiotonic steroids. The whole brain from 1 to 2 day-old C57BL/6J mice neonatal mice was collected, without the meninges, placed in HBSS modified medium without calcium or magnesium, and processed following the standard protocol to separate the oligodendrocytes and neurons from the glial cell culture.47 The primary glial cells were treated with 200 nM solution of cardiotonic steroids and stimulated with LPS (250 ng/mL), to induce inflammatory cytokine gene expression. DMSO was used as a negative control. The IL-1, IL-6, TNF-α, and IKBKE gene expressions were determined using qPCR.
The findings from these experiments are presented in Figure 2. In all cases, LPS stimulation triggered substantial increase in the expression of the inflammatory IL-1, IL-6, TNF-α, and IKBKE genes relative to the control treatment with DMSO. In contrast, applying 200 nM solutions of digoxin and compounds 2, 16, 20, 21, and 24 did not trigger significant inflammatory gene expression. When cells were sequentially treated with LPS and then with 200 nM solutions of 16, 24, 20 and digoxin, a significant reduction in the expression of all four inflammation genes was observed. The unnatural ouabain analogue 20 demonstrated the strongest anti-inflammatory activity. In contrast, the use of its isomer, C19-glycoside 21, enhanced the expression of IL-6, but reduced the production of TNFα as well as IKBKE markers. Finally, aglycone 2 enhanced inflammation relative to the LPS treatment alone, and all four genes were expressed at significantly higher levels.
The results above suggest that both glycosylation and skeletal substitution are important to the anti-inflammatory activity of cardenolides. The C3-glycosylation was found to have the most significant impact as the unglycosylated anhydro-ouabagenin (2) enhanced rather than reduced the levels of neuroinflammation. Similarly, altering the glycosylation site from the C3- to C19-position resulted in a reduced activity of 21 in comparison to 20. The side-by-side comparison of cardenolides 16, 20, and 24 containing 3-l-(α)-rhamnosylation indicates that changes in skeletal substitution may lead to the enhancement of the anti-inflammatory activity. The unnatural analogue anhydro-ouabain (20) represents a hybrid of 16 and 24 featuring the C1/C11 ether bridge that locks the conformation of otherwise flexible cis-AB rings. The direct comparison of 16, anhydro-ouabain (20), and ouabain (24) indicates that 20 has the highest levels of anti-inflammatory activity across all four inflammatory genes.
In summary, this article describes a direct strategy for achieving C3-(α)-l-rhamnosylation of common polyhydroxylated cardenolide aglycones 1–3 containing a primary C19-hydroxyl group. Based on the computational predictions suggesting that the reaction of 1–3 with boronic acids would favor the formation of the cyclic C5/C19 or C1/C19 esters, we have developed one pot glycosylation protocols that directly lead to strophanthidol-3-(α)-l-rhamnoside (16), and unnatural steroid anhydro-ouabagenin-3-(α)-l-rhamnoside (20). Despite the promising computational predictions, attempts to achieve selective glycosylation of ouabagenin (1) failed due to its low solubility in organic solvents coupled with the propensity to form bis-boronates.
The potential of 200 nM solutions of synthetic C3-(α)-l-rhamnosides 16 and 20, C19-(α)-l-rhamnoside 21, anhydro-ouabagenin (2), ouabain (24) and digoxin to reduce LPS-induced neuroinflammation was subsequently investigated using primary murine glial cells. While the C3-glycosylated steroids reduced the expression of inflammatory IL-1, IL-6, TNFα, and IKBKE, the coadministration of unnatural C19-glycoside 21 or aglycone 2 resulted in enhanced inflammation. In addition to glycosylation, the oxidation of the cardenolide skeleton was found to play a significant role in determining the extent of the anti-inflammatory activity exhibited by the steroids. Surprisingly, the previously unexplored unnatural anhydro-ouabagenin-3-(α)-l-rhamnoside (20) was found to exhibit the highest levels of anti-inflammatory activity. Further exploration of anhydro-ouabagenin glycosides and related synthetic derivatives of ouabain may lead to the analogs with enhanced anti-inflammatory properties and is currently the subject of ongoing studies in our laboratories.
Glossary
Abbreviations
- IL-1
interleukin-1 cytokine
- IL-6
interleukin-6 cytokine
- TNF-α
tumor necrosis factor alpha cytokine
- IKBKE
inhibitor of nuclear kappa B kinase subunit epsilon
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00517.
Experimental procedures and 1H and 13C NMR spectra for 2, 16, 20, and 21 as well as detailed description of the biological studies (PDF)
P.N. was supported by NIH R35GM136341 grant. M.S. was supported by NIH P30ES020957, R01ES033171, RF1AG078170, and P42ES030991. L.S. was supported by 5T32HL120822-09.
The authors declare no competing financial interest.
Notes
A preprint of this manuscript has been available through ChemRxiv since January 3, 2024: Rutkoski, R.; Debarba, L. K.; Stilgenbauer, L.; Rosenthal, T.; Sadagurski, M.; Nagorny, P. Selective (α)-l-Rhamnosylation and Neuroprotective Activity Exploration of Cardiotonic Steroids. DOI: 10.26434/chemrxiv-2024-v49vb.
Supplementary Material
References
- Wasserstrom J. A.; Aistrup G. L. Digitalis: New Actions for an Old Drug. American Journal of Physiology-Heart and Circulatory Physiology 2005, 289 (5), H1781–H1793. 10.1152/ajpheart.00707.2004. [DOI] [PubMed] [Google Scholar]
- Nesher M.; Shpolansky U.; Rosen H.; Lichtstein D. The Digitalis-like Steroid Hormones: New Mechanisms of Action and Biological Significance. Life Sciences 2007, 80 (23), 2093–2107. 10.1016/j.lfs.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Senthilkumaran S.; Meenakshisundaram R.; Thirumalaikolundusubramanian P.. Chapter 5 - Plant Toxins and the Heart. In Heart and Toxins; Ramachandran M., Ed.; Academic Press: Boston, 2015; pp 151–174. [Google Scholar]
- Schoner W.; Scheiner-Bobis G. Endogenous and Exogenous Cardiac Glycosides: Their Roles in Hypertension, Salt Metabolism, and Cell Growth. J. Physiol. Cell Physiol. 2007, 293 (2), C509–C536. 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- Prassas I.; Diamandis E. P. Novel Therapeutic Applications of Cardiac Glycosides. Nat. Rev. Drug. Discovery 2008, 7, 926–935. 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- Bagrov A. Y.; Shapiro J. I.; Fedorova O. V. Endogenous Cardiotonic Steroids: Physiology, Pharmacology, and Novel Therapeutic Targets. Pharmacol Rev. 2009, 61 (1), 9–38. 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.; Gao X.; Guo X.; Wang N.; Wang X. Research Progress in Pharmacological Activities and Applications of Cardiotonic Steroids. Frontiers in Pharmacology 2022, 13 (1), 902459. 10.3389/fphar.2022.902459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic T.; Van Quaquebeke E.; Delest B.; Debeir O.; Darro F.; Kiss R. Cardiotonic Steroids on the Road to Anti-Cancer Therapy. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2007, 1776 (1), 32–57. 10.1016/j.bbcan.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Babula P.; Masarik M.; Adam V.; Provaznik I.; Kizek R. From Na+/K+-ATPase and Cardiac Glycosides to Cytotoxicity and Cancer Treatment. Anti-Cancer Agents in Medicinal Chemistry- Anti-Cancer Agents) 2013, 13 (7), 1069–1087. 10.2174/18715206113139990304. [DOI] [PubMed] [Google Scholar]
- Schneider N.; Cerella C.; Simoes C. M. O.; Diederich M. Anticancer and Immunogenic Properties of Cardiac Glycosides. Molecules 2017, 22 (11), 1932. 10.3390/molecules22111932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.-H.; Jeon S.; Lee J.; Kim S.; Jang M. S.; Park C. M.; Song J. H.; Kim H. R.; Kwon S. Broad Spectrum Antiviral Properties of Cardiotonic Steroids Used as Potential Therapeutics for Emerging Coronavirus Infections. Pharmaceutics 2021, 13 (11), 1839. 10.3390/pharmaceutics13111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Seedi H. R.; Yosri N.; El-Aarag B.; Mahmoud S. H.; Zayed A.; Du M.; Saeed A.; Musharraf S. G.; El-Garawani I. M.; Habib M. R.; Tahir H. E.; Hegab M. M.; Zou X.; Guo Z.; Efferth T.; Khalifa S. A. M. Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads. Molecules 2022, 27 (19), 6586. 10.3390/molecules27196586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid S.; Zerbes T.; Williams D.; Wang X.; Sackmann C.; Meier S.; Dulin N. O.; Nagorny P.; Schmitt-Ulms G. Identification of a Cardiac Glycoside Exhibiting Favorable Brain Bioavailability and Potency for Reducing Levels of the Cellular Prion Protein. International Journal of Molecular Sciences 2022, 23 (23), 14823. 10.3390/ijms232314823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markina A. A.; Kazanskaya R. B.; Timoshina J. A.; Zavialov V. A.; Abaimov D. A.; Volnova A. B.; Fedorova T. N.; Gainetdinov R. R.; Lopachev A. V. Na+,K+-ATPase and Cardiotonic Steroids in Models of Dopaminergic System Pathologies. Biomedicines 2023, 11 (7), 1820. 10.3390/biomedicines11071820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S.; Rehni A. K.; Singh N.; Jaggi A. S. Studies on Cerebral Protection of Digoxin against Ischemia/Reperfusion Injury in Mice. Yakugaku Zasshi 2009, 129 (4), 435–443. 10.1248/yakushi.129.435. [DOI] [PubMed] [Google Scholar]
- Dunn D. E.; He D. N.; Yang P.; Johansen M.; Newman R. A.; Lo D. C. In Vitro and in Vivo Neuroprotective Activity of the Cardiac Glycoside Oleandrin from Nerium Oleander in Brain Slice-Based Stroke Models. J. Neurochem 2011, 119 (4), 805–814. 10.1111/j.1471-4159.2011.07439.x. [DOI] [PubMed] [Google Scholar]
- Van Kanegan M. J.; He D. N.; Dunn D. E.; Yang P.; Newman R. A.; West A. E.; Lo D. C. BDNF Mediates Neuroprotection against Oxygen-Glucose Deprivation by the Cardiac Glycoside Oleandrin. J. Neurosci. 2014, 34 (3), 963–968. 10.1523/JNEUROSCI.2700-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana A. M.; Kinoshita P. F.; Leite J. A.; Kawamoto E. M.; Scavone C.. Cardiotonic Steroids as Modulators of Neuroinflammation. Frontiers in Endocrinology 2016, 7, 10.3389/fendo.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J. A.; Cavalcante-Silva L. H. A.; Ribeiro M. R.; de Morais Lima G.; Scavone C.; Rodrigues-Mascarenhas S.. Neuroinflammation and Neutrophils: Modulation by Ouabain. Frontiers in Pharmacology 2022, 13, 10.3389/fphar.2022.824907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smer-Barreto V.; Quintanilla A.; Elliott R. J. R.; Dawson J. C.; Sun J.; Campa V. M.; Lorente-Macías A.; Unciti-Broceta A.; Carragher N. O.; Acosta J. C.; Oyarzún D. A. Discovery of Senolytics Using Machine Learning. Nat. Commun. 2023, 14 (1), 3445. 10.1038/s41467-023-39120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn J. M.; Blaustein M. P.; Bova S.; DuCharme D. W.; Harris D. W.; Mandel F.; Mathews W. R.; Ludens J. H. Identification and Characterization of a Ouabain-like Compound from Human Plasma. Proc. Natl. Acad. Sci. U.S.A. 1991, 88 (14), 6259–6263. 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymiak A. A.; Norman J. A.; Bolgar M.; DiDonato G. C.; Lee H.; Parker W. L.; Lo L. C.; Berova N.; Nakanishi K.; Haber E. Physicochemical Characterization of a Ouabain Isomer Isolated from Bovine Hypothalamus. Proc. Natl. Acad. Sci. U. S. A. 1993, 90 (17), 8189–8193. 10.1073/pnas.90.17.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandi M.; Manunta P.; Balzan S.; Hamlyn J. M.; Bianchi G.; Ferrari P. Ouabain-like Factor Quantification in Mammalian Tissues and Plasma. Hypertension 1997, 30 (4), 886–896. 10.1161/01.HYP.30.4.886. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P.; Hamlyn J. M. Ouabain, Endogenous Ouabain and Ouabain-like Factors: The Na+ Pump/Ouabain Receptor, Its Linkage to NCX, and Its Myriad Functions. Cell Calcium 2020, 86, 102159. 10.1016/j.ceca.2020.102159. [DOI] [PubMed] [Google Scholar]
- Kinoshita P. F.; Yshii L. M.; Vasconcelos A. R.; Orellana A. M. M.; Lima L. de S.; Davel A. P. C.; Rossoni L. V.; Kawamoto E. M.; Scavone C. Signaling Function of Na,K-ATPase Induced by Ouabain against LPS as an Inflammation Model in Hippocampus. Journal of Neuroinflammation 2014, 11 (1), 218. 10.1186/s12974-014-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I. J. P.; Kinoshita P. F.; de Oliveira Braga I.; Parreira G. M.; Mignaco J. A.; Scavone C.; Barbosa L. A.; de Lima Santos H. Ouabain Attenuates the Oxidative Stress Induced by Lipopolysaccharides in the Cerebellum of Rats. Journal of Cellular Biochemistry 2018, 119 (2), 2156–2167. 10.1002/jcb.26377. [DOI] [PubMed] [Google Scholar]
- Garcia I. J. P.; Kinoshita P. F.; Silva L. N. D. e; De Souza Busch M.; Atella G. C.; Scavone C.; Cortes V. F.; Barbosa L. A.; De Lima Santos H. Ouabain Attenuates Oxidative Stress and Modulates Lipid Composition in Hippocampus of Rats in Lipopolysaccharide-Induced Hypocampal Neuroinflammation in Rats. Journal of Cellular Biochemistry 2019, 120 (3), 4081–4091. 10.1002/jcb.27693. [DOI] [PubMed] [Google Scholar]
- Garcia I. J. P.; Kinoshita P. F.; de Moura Valadares J. M.; de Carvalho L. E. D.; Cortes V. F.; Barbosa L. A.; Scavone C.; Santos H. d. L. Effect of Ouabain on Glutamate Transport in the Hippocampus of Rats with LPS-Induced Neuroinflammation. Biomedicines 2023, 11 (3), 920. 10.3390/biomedicines11030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichowicz N. R.; Kaplan W.; Khomutnyk Y.; Bhattarai B.; Sun Z.; Nagorny P. Concise Enantioselective Synthesis of Oxygenated Steroids via Sequential Copper(II)-Catalyzed Michael Addition/Intramolecular Aldol Cyclization Reactions. J. Am. Chem. Soc. 2015, 137 (45), 14341–14348. 10.1021/jacs.5b08528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan W.; Khatri H. R.; Nagorny P. Concise Enantioselective Total Synthesis of Cardiotonic Steroids 19-Hydroxysarmentogenin and Trewianin Aglycone. J. Am. Chem. Soc. 2016, 138 (22), 7194–7198. 10.1021/jacs.6b04029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri H. R.; Bhattarai B.; Kaplan W.; Li Z.; Curtis Long M. J.; Aye Y.; Nagorny P. Modular Total Synthesis and Cell-Based Anticancer Activity Evaluation of Ouabagenin and Other Cardiotonic Steroids with Varying Degrees of Oxygenation. J. Am. Chem. Soc. 2019, 141 (12), 4849–4860. 10.1021/jacs.8b12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejedelem Z.; Carney N.; Nagorny P. Synthesis of Cardiotonic Steroids Oleandrigenin and Rhodexin B. J. Org. Chem. 2021, 86 (15), 10249–10262. 10.1021/acs.joc.1c00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai B.; Nagorny P. Enantioselective Total Synthesis of Cannogenol-3-O-α-l-Rhamnoside via Sequential Cu(II)-Catalyzed Michael Addition/Intramolecular Aldol Cyclization Reactions. Org. Lett. 2018, 20 (1), 154–157. 10.1021/acs.orglett.7b03513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay J.-H.; Dorokhov V.; Wang S.; Nagorny P. Regioselective Single Pot C3-Glycosylation of Strophanthidol Using Methylboronic Acid as a Transient Protecting Group. J. Antibiot. 2019, 72 (6), 437–448. 10.1038/s41429-019-0172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney N.; Perry N.; Garabedian J.; Nagorny P. Development of α-Selective Glycosylation with l-Oleandral and Its Application to the Total Synthesis of Oleandrin. Org. Lett. 2023, 25 (6), 966–971. 10.1021/acs.orglett.2c04358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong B.-C.; Kim S.; Kim T.-S.; Corey E. J. Synthesis and Properties of Several Isomers of the Cardioactive Steroid Ouabain. Tetrahedron Lett. 2006, 47 (16), 2711–2715. 10.1016/j.tetlet.2006.02.089. [DOI] [Google Scholar]
- Zhang H.; Sridhar Reddy M.; Phoenix S.; Deslongchamps P. Total Synthesis of Ouabagenin and Ouabain. Angew. Chem., Int. Ed. 2008, 47 (7), 1272–1275. 10.1002/anie.200704959. [DOI] [PubMed] [Google Scholar]
- Urabe D.; Nakagawa Y.; Mukai K.; Fukushima K.; Aoki N.; Itoh H.; Nagatomo M.; Inoue M. Total Synthesis and Biological Evaluation of 19-Hydroxysarmentogenin-3-O-α-l-Rhamnoside, Trewianin, and Their Aglycons. J. Org. Chem. 2018, 83 (22), 13888–13910. 10.1021/acs.joc.8b02219. [DOI] [PubMed] [Google Scholar]
- Tay J.-H.; Argüelles A. J.; DeMars M. D. I.; Zimmerman P. M.; Sherman D. H.; Nagorny P. Regiodivergent Glycosylations of 6-Deoxy-Erythronolide B and Oleandomycin-Derived Macrolactones Enabled by Chiral Acid Catalysis. J. Am. Chem. Soc. 2017, 139 (25), 8570–8578. 10.1021/jacs.7b03198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Píš J.; Hykl J.; Buděšinský M.; Harmatha J. Regioselective Synthesis of 20-Hydroxyecdysone Glycosides. Tetrahedron 1994, 50 (32), 9679–9690. 10.1016/S0040-4020(01)85536-8. [DOI] [Google Scholar]
- Fenger T. H.; Madsen R. Regioselective Glycosylation of Unprotected Phenyl 1-Thioglycopyranosides with Phenylboronic Acid as a Transient Masking Group. Eur. J. Org. Chem. 2013, 2013 (26), 5923–5933. 10.1002/ejoc.201300723. [DOI] [Google Scholar]
- Kaji E.; Nishino T.; Ishige K.; Ohya Y.; Shirai Y. Regioselective Glycosylation of Fully Unprotected Methyl Hexopyranosides by Means of Transient Masking of Hydroxy Groups with Arylboronic Acids. Tetrahedron Lett. 2010, 51 (12), 1570–1573. 10.1016/j.tetlet.2010.01.048. [DOI] [Google Scholar]
- Mancini R. S.; Lee J. B.; Taylor M. S. Boronic Esters as Protective Groups in Carbohydrate Chemistry: Processes for Acylation, Silylation and Alkylation of Glycoside-Derived Boronates. Org. Biomol. Chem. 2017, 15 (1), 132–143. 10.1039/C6OB02278B. [DOI] [PubMed] [Google Scholar]
- Sneeden R. P. A.; Turner R. B.; Ouabagenin I. The Relationship between Ouabagenin Monoacetonide and “Anhydroöuabagenin”1. J. Am. Chem. Soc. 1953, 75 (14), 3510–3513. 10.1021/ja01110a059. [DOI] [Google Scholar]
- Volpp G.; Tamm Ch. 1α, 11α-Oxido-Strophanthidol (Monoanhydro-Ouabagenin) Sowie Weitere 1α, 11α-Oxido-Steroide Aus Ouabagenin. Helv. Chim. Acta 1963, 46 (1), 219–237. 10.1002/hlca.19630460122. [DOI] [Google Scholar]
- Volpp G.; Tamm Ch. 1α-11α-Epoxy-Steroids from Ouabagenin by Transannular Substitution. Tetrahedron Lett. 1960, 1 (48), 31–36. 10.1016/S0040-4039(01)99410-9. [DOI] [Google Scholar]
- Deierborg T. Preparation of Primary Microglia Cultures from Postnatal Mouse and Rat Brains. Methods Mol. Biol. 2013, 1041, 25–31. 10.1007/978-1-62703-520-0_4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.