Abstract
The activation of a predominant T-helper-cell subset plays a critical role in disease resolution. In the case of Toxoplasma gondii, the available evidence indicates that CD4+ protective cells belong to the Th1 subset. The aim of this study was to determine whether T. gondii antigens (in T. gondii sonicate [TSo]) presented by splenic dendritic cells (DC) were able to induce a specific immune response in vivo and to protect CBA/J mice orally challenged with T. gondii cysts. CBA/J mice immunized with TSo-pulsed DC exhibited significantly fewer cysts in their brains after oral infection with T. gondii 76K than control mice did. Protected mice developed a strong humoral response in vivo, with especially high levels of anti-TSo immunoglobulin G2a antibodies in serum. T. gondii antigens such as SAG1 (surface protein), SAG2 (surface protein), MIC1 (microneme protein), ROP2 through ROP4 (rhoptry proteins), and MIC2 (microneme protein) were recognized predominantly. Furthermore, DC loaded with TSo, which synthesized high levels of interleukin-12 (IL-12), triggered a strong cellular response in vivo, as assessed by the proliferation of lymph node cells in response to TSo restimulation in vitro. Cellular proliferation was associated with gamma interferon and IL-2 production. Taken together, these results indicate that immunization of CBA/J mice with TSo-pulsed DC can induce both humoral and Th1-like cellular immune responses and affords partial resistance against the establishment of chronic toxoplasmosis.
Toxoplasma gondii is an obligate intracellular protozoan parasite that is responsible for toxoplasmosis in different species of birds and mammals, including humans. Usually asymptomatic in hosts with intact immunity, toxoplasmosis may lead to severe or lethal damage when associated with immunosuppressive states such as AIDS, because of the reactivation of encysted parasites, or when transmitted to the fetus during pregnancy (19, 52). Although an effective live vaccine is available for animals (6), such a vaccine is inappropriate for use in humans.
There is increasing evidence that protection against parasites or foreign antigens not only depends on the initiation of a specific immune response but also strongly relies on the character of the response, i.e., the Th1-Th2 balance. Indeed, murine CD4+ Th lymphocytes consist of several subsets, including two subpopulations named Th1 and Th2 which differ by their lymphokine secretion pattern, and the development of an appropriate CD4+ Th subset has been shown to be important for disease resolution. The major mechanism by which immunocompetent hosts control T. gondii infection is considered to be cell-mediated immunity (21), and the available evidence indicates that CD4+ protective cells belong to the Th1 subset (22, 25). CD4+ cells are protective mainly through gamma interferon (IFN-γ) production and can also activate CD8+ cells. CD8+ cytotoxicity (34, 35) aided by the helper activities of CD4+ cells (1) and the microbicidal or microbiostatic activity of IFN-γ-activated macrophages (61) and nonphagocytic cells (14, 50, 63) are two major mechanisms of resistance to Toxoplasma infection. Indeed, a synergistic role of CD4+ and CD8+ T lymphocytes has been demonstrated in protective immunity against T. gondii (22). The physiologic regulation of Th phenotype development is still poorly understood, but because of major histocompatibility complex (MHC) restriction, attention has been focused on the major role of antigen-presenting cells (APC) in the initiation of the immune response. In vitro experiments have shown that activation of Th1 clones requires the presence of particular APC, i.e., dendritic cells (DC); in contrast, Th2 cells respond optimally to antigen presented by B cells (20). DC have recently been reported to promote the development of CD4+ Th1 cells through their production of interleukin-12 (IL-12) (28, 39).
In agreement with this hypothesis, it was demonstrated that in vitro antigen-pulsed DC initiate a strong humoral response in vivo, especially high levels of immunoglobulin G2a (IgG2a) antibodies, indicating that the helper population induced by DC belongs to the Th1 subset (13, 58). Moreover, recent studies have demonstrated in different models that DC loaded with tumor protein or live bacteria were able to induce a specific immune response and a strong protection of mice against subsequent challenge (16, 42, 64).
The aim of this study was to determine whether T. gondii antigens presented by splenic DC were able to induce a specific immune response in vivo and to protect CBA/J mice subsequently orally challenged with T. gondii cysts. After adoptive transfer of in vitro T. gondii antigen-pulsed DC, the specific antibody response in the serum was investigated. The proliferative ability and cytokine patterns of immune lymph node cells after specific restimulation in vitro were also studied. Protection was evaluated by the decrease in brain cyst load 1 month after the oral challenge.
MATERIALS AND METHODS
Mice.
Female CBA/J mice (H-2k) aged 6 to 8 weeks were purchased from Charles River Wiga (Sulzfeld, Germany) and maintained in a pathogen-free environment.
Parasites.
Tachyzoites of the RH strain of T. gondii were harvested from the peritoneal fluids of Swiss OF1 mice (Institut Pasteur, Brussels, Belgium) which had been infected intraperitoneally 3 to 4 days earlier. Cysts of T. gondii 76K were obtained from the brains of orally infected Swiss OF1 mice. The virulence of strain 76K was maintained by repeated monthly passage in mice.
Preparation of Toxoplasma sonicate.
Tachyzoites of T. gondii RH were washed, sonicated, and centrifuged as previously described (55). The supernatant from the last centrifugation, which was used as the source of antigen, was concentrated through dialysis tubing to achieve aliquots of 1 ml containing 1 mg of protein each, as determined by a protein assay reagent kit (Bio-Rad) with bovine serum albumin (BSA) as the standard. The aliquots of T. gondii sonicate (TSo) were stored at −20°C until use.
Purification and antigen pulsing of DC.
The spleens of CBA/J mice were digested with collagenase (CLSIII; Worthington Biochemical Corp., Freehold, N.J.) and separated into low- and high-density fractions on a BSA gradient (Bovuminar Cohn fraction V powder; Armour Pharmaceutical Co., Tarrytown, N.Y.). The splenic DC were purified by a procedure described by Crowley et al. (10). Briefly, low-density cells were cultured for 2 h at 37°C under 5% CO2 in complete medium containing RPMI 1640 (Seromed; Biochem KG, Berlin, Germany) supplemented with 10% fetal calf serum (Gibco BRL), penicillin, streptomycin, nonessential amino acids, sodium pyruvate, 2-mercaptoethanol, and l-glutamine (Flow ICN Biomedicals), and the nonadherent cells were removed by vigorous pipetting. The adherent cells were cultured for 1 h in serum-free medium, and the nonadherent cells were removed by gentle pipetting. The remaining cells (DC and macrophages [Mφ]) were incubated overnight in complete medium containing 50 μg of TSo per ml, and the nonadherent cells containing the DC-enriched fraction (as assessed by morphology and specific staining) were thoroughly washed, counted, and used for immunization. At that point, since passive adsorption of the antigen onto DC could not be excluded, a control including mice injected intravenously with 5 μg of TSo was added to the experimental design.
The overnight culture supernatants (15 ml) were concentrated to 1 ml through dialysis tubing and were tested for the presence of IL-12. As a control, culture medium was added to the remaining adherent cells (Mφ), which were incubated for an additional night. Simultaneously, thioglycolate-elicited Mφ were harvested from mouse peritoneal cavities and cultured overnight in the presence (TSo-pulsed Mφ) or absence (unpulsed Mφ) of TSo. The overnight culture supernatants from these Mφ cultures were collected, concentrated, and tested for the presence of IL-12.
FACScan analysis.
DC were incubated with 2.4G2 (a rat anti-mouse Fc receptor [FcR] monoclonal antibody [MAb]) for 10 min before being stained, to prevent antibody binding to FcR, and incubated with fluorescein isothiocyanate-coupled MAbs: 14.4.4 (murine IgG2a anti-I-Ek,d), N418 (hamster anti-murine CD11c [45]), 16-10A1 (rat IgG2a anti-B7.1 [51]), and GL1 (hamster MAb anti-B7.2 [27]). Tagged DC were analyzed on a FACScan apparatus (Becton Dickinson & Co.).
Immunization schedules.
In the humoral response and protection studies, CBA/J mice received an intravenous injection of 3 × 105 TSo-pulsed or unpulsed DC. Control mice were untreated or injected with 5 μg of TSo alone. All the mice were bled on day 24 and were orally infected with 100 cysts of T. gondii 76K on day 28.
In the cellular response study, CBA/J mice were injected in the fore and hind footpads with 3 × 105 TSo-pulsed DC or unpulsed DC or with 5 μg of TSo alone per footpad or were untreated, as specified in a protocol described by Inaba et al. (33). In the study of antigen-specific proliferative response, draining lymph nodes (popliteal and brachial) were harvested 5 days later. In the cytotoxicity assay, draining lymph nodes were harvested 7 days later.
Determination of antigen-specific antibody levels.
Levels of antigen-specific antibodies in serum were determined by enzyme-linked immunoabsorbent assays (ELISA) by standard procedures. Briefly, flat-bottom wells of microdilution flexible plates (Falcon) were coated with TSo at 12 μg/ml in 10 mM phosphate-buffered saline (pH 7.4) (PBS). After overnight incubation at 4°C, nonspecific binding sites were blocked with PBS–1% bovine serum albumin for 3 h at room temperature. After three washes in PBS, threefold serial dilutions of either immune or preimmune sera in PBS–0.1% BSA were added to the wells and incubated overnight at 4°C. The plates were washed with PBS to remove unbound antibodies, and an anti-mouse Ig-peroxidase conjugate was applied to each well for 90 min at 37°C. The conjugate was a polyclonal goat anti-mouse Ig reagent (Boehringer GmbH, Mannheim, Germany) or isotype-specific (IgG1 and IgG2a) rat MAb (38) and was used in both cases at 1/2,000 in PBS–0.1% BSA. After being washed in PBS, the plates were developed with o-phenylenediamine (4 mg/10 ml) and urea hydrogen peroxide (4 mg/10 ml) in citrate buffer (24 mM citrate, 58 mM Na2HPO4 · 2H2O [pH 5]). The reaction was stopped by the addition of 2 N H2SO4, and the optical densities were read at 492 nm in a Titertek Multiskan ELISA reader (Flow Laboratories, Inc.). The values are given as the mean optical densities from duplicates for a serum dilution of 1:150.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with purified T. gondii tachyzoites as the source of antigen were performed as described previously (7). T. gondii antigens recognized by serum IgG antibodies and MAbs were detected with a goat anti-mouse IgG-alkaline phosphatase conjugate (Sigma).
MAbs.
The following anti-T. gondii MAbs were used: 3G11 (anti-p22 [SAG2]), 1E5 (anti-p30 [SAG1]), 1F7 (anti-gp60 [MIC1]), 4A7 (anti-55–60-kDa [ROP2 to ROP4]), and 4A11 (anti-p100 [MIC2]). These MAbs were generously donated by J.-F. Dubremetz (Institut National de la Santé et de la Recherche Médicale, Villeneuve d’Ascq, France).
Measurement of resistance to challenge infection.
At 28 days after passive DC transfer, CBA/J mice were infected orally with 100 cysts of strain 76K obtained from the brains of chronically infected mice. One month after challenge, the mice were sacrificed and their brains were recovered. Each brain was homogenized in 4 ml of PBS with a pestle and mortar. The cysts were enumerated microscopically by counting four samples (25 μl each) of each brain homogenate. The results are expressed as means ± standard deviations for each group.
Measurement of antigen-specific proliferative response.
Popliteal and brachial lymph nodes were harvested and pressed. Single-cell suspensions were obtained by filtration through nylon mesh. After being washed, the cells were resuspended in Click’s medium (Irvine Scientific, Santa Ana, Calif.) supplemented with 0.5% heat-inactivated mouse serum, penicillin, streptomycin, nonessential amino acids, sodium pyruvate, 2-mercaptoethanol, and l-glutamine and seeded in triplicate in flat-bottom 96-well microtiter plates (Nunc) at 6 × 105 cells per well in 200 μl of culture medium alone or with various concentrations of TSo. The plates were incubated for 4 days at 37°C under 5% CO2 and pulsed with 0.5 μCi of [3H]thymidine per well for an additional 18 h. Incorporation into cellular DNA was measured by liquid scintillation counting. The results are expressed as Δcpm, calculated by subtracting the mean counts per minute (cpm) of unstimulated cells from the mean cpm of stimulated cells. Culture supernatants were harvested at 24 h for the IL-2 assay, at 72 h for the IFN-γ assay, and at 96 h for the IL-5 assay.
Cytokine analysis.
IL-12 in culture supernatants from unpulsed DC and TSo-pulsed DC or from unpulsed Mφ and TSo-pulsed Mφ was assayed by two-site ELISA with a polyclonal sheep anti-mouse IL-12 (p40) and a biotinylated polyclonal sheep anti-mouse IL-12 (p40) (generously supplied by the Immunology Department, Genetics Institute, Inc., Andover, Mass.).
IFN-γ in culture supernatants from lymph node cells was quantified by two-site ELISA with MAbs F1 and Db-1, kindly provided by A. Billiau (KUL, Leuven, Belgium) and P. H. Van Der Meide (TNO Health Research, Rijswijk, The Netherlands), respectively. IL-2 was assayed by a standard bioassay with an IL-2-dependent, IL-4-insensitive subclone of the CTL.L cell line. IL-5 was quantified by two-site ELISA with MAbs K4-TRF and K5-TRF, generously donated by R. L. Coffman (DNAX, Palo Alto, Calif.).
Cytotoxicity assay.
Cytotoxicity assay was performed as described previously (8). Target thioglycolate-elicited macrophages were dispensed in culture medium at a concentration of 3 × 104 cells/well in 96-well round-bottom tissue culture plates (Falcon, Lincoln Park, N.J.). They were incubated for at least 4 h at 37°C under 5% CO2 and then radiolabeled with 51Cr (1 μCi/well; specific activity, 469 mCi/mg [Dupont NEN, Boston, Mass.]) for 3 h. After being washed, the radiolabeled target cells were infected with tachyzoites of T. gondii RH at a 1:2 cell/parasite ratio and incubated overnight. Target Mφ were washed and incubated with lymph node cells at various effector-to-target-cell ratios in a final volume of 200 μl of culture medium. After centrifugation at 200 × g for 2 min, the culture plates were incubated for 3.5 h. They were then centrifuged at 200 × g, and 100 μl of supernatant was harvested from each well and assayed for release of cpm by scintillation counting (Packard, Canberra, Australia). The percentage of specific 51Cr release was calculated as [(mean cpm of tested sample − mean cpm of spontaneous release)/(mean cpm of maximal release − mean cpm of spontaneous release)] × 100.
Statistical analysis.
Levels of significance of the differences between groups were determined by the Student t test.
RESULTS
Characterization of DC.
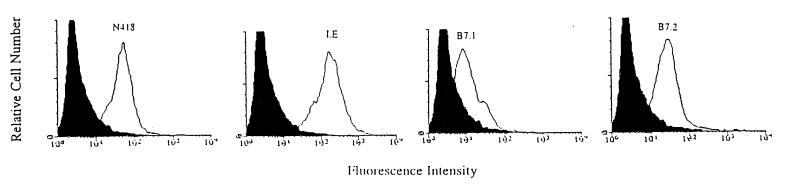
The phenotype profile of representative populations of splenic DC after overnight culture in medium alone or in medium containing TSo was determined (Fig. 1). Cell populations were stained for the dendritic cell marker (N418), for MHC class II expression (I-E), and for the expression of the costimulatory molecules B7-1 and B7-2. No difference was observed between the staining of unpulsed and TSo-pulsed DC, whatever the marker. Unpulsed or TSo-pulsed DC were at least 90% N418+ and strongly MHC class II+ (>90%). The costimulatory molecules B7-1 and B7-2 were differentially expressed on the DC. A high level of B7-2 (87%) was expressed on unpulsed and TSo-pulsed DC, whereas a lower level of B7-1 (56%) was expressed.
FIG. 1.
Phenotypic profile of DC. Splenic DC were purified and stained with MAbs as described in Materials and Methods.
These results indicated that unpulsed or TSo-pulsed DC expressed MHC class II molecule and costimulary molecules and thus could be able to present an antigen and sensitize T cells in vivo.
IL-12 synthesis by DC.
The presence of IL-12 (p40) was assayed in the overnight culture supernatants from unpulsed and TSo-pulsed DC or from Mφ and TSo-pulsed Mφ. DC responded to TSo pulsing by undergoing strong synthesis of IL-12 (2.10 ng of IL-12 p40 per ml). In contrast, no release of IL-12 was observed in overnight culture supernatant from unpulsed DC (<0.19 ng of IL-12 p40 per ml). Furthermore, whatever the origin of Mφ (remaining adherent cells after DC purification or thioglycolate-elicited peritoneal cavity Mφ), IL-12 was not detected in the overnight culture supernatants from unpulsed or TSo-pulsed Mφ (data not shown), indicating that IL-12 is produced exclusively by TSo-pulsed DC.
Protection against oral challenge.
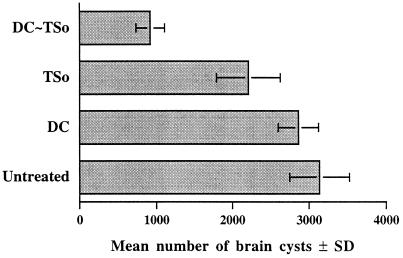
Mice were injected with 3 × 105 TSo-pulsed or unpulsed DC. Control mice received TSo alone or were untreated. The mice were then orally challenged with Toxoplasma cysts. One month after the oral challenge, the mouse brains were checked for the number of cysts (Fig. 2). Protection against cyst formation was highly significant in mice receiving TSo-pulsed DC in comparison with untreated mice (P < 0.01), with the former showing 70% fewer brain cysts. Moreover, the parasite burden in the brains of mice injected with TSo-pulsed DC was significantly lower than that in the brains of mice injected with unpulsed DC or TSo alone (P < 0.05). The difference between the unpulsed and TSo groups was not significant. A significantly smaller number of cysts (P < 0.05) was also observed in the brains of mice immunized with TSo alone compared with the numbers observed in the brains of untreated mice.
FIG. 2.
Assay for protection against oral challenge. CBA/J mice injected with 3 × 105 unpulsed DC (DC) or TSo-pulsed DC (DC∼TSo) or with TSo alone (TSo) and untreated mice were orally infected with 100 cysts of T. gondii 76K 28 days later. The brain cyst load was evaluated 1 month after challenge. The results are the means of the numbers of brain cysts in each mouse ± standard deviations (SD). Results from one of three similar experiments are shown.
These results demonstrated that DC loaded with T. gondii antigens were effective APC inducing strong resistance to cyst formation.
Antibody responses.
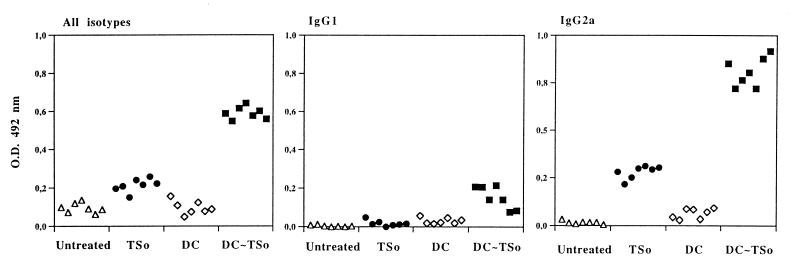
The total specific response (all isotypes), as well as specific antibodies of IgG1 and IgG2a isotypes, was tested in serum from mice immunized with unpulsed or TSo-pulsed DC or TSo alone (Fig. 3). A strong antibody response was elicited in mice injected with TSo-pulsed DC, compared to the low antibody response in mice injected with TSo and to the nondetectable antibody response in mice injected with unpulsed DC or not treated. The humoral response in mice injected with TSo-pulsed DC was characterized by a high level of TSo-specific IgG2a antibodies compared to the weak IgG1 response. Moreover, mice injected with TSo-pulsed DC produced much higher levels of specific IgG2a antibodies than did mice injected with TSo alone. Levels of specific IgG1 antibodies were barely detectable in mice immunized with TSo.
FIG. 3.
Isotype analysis of TSo-specific responses. Mice were injected with 3 × 105 TSo-pulsed DC (DC∼TSo) or with unpulsed DC (DC). Control mice were injected with TSo alone (TSo) or untreated. The total specific response (all isotypes) and specific antibodies of IgG1 and IgG2a isotypes were tested on day 24 after immunization by ELISA for each mouse serum (dilution of 1:150). O.D., optical density. The results are representative of three separate experiments.
These results demonstrated that TSo-pulsed DC stimulated a strong humoral response in vivo, and especially a high level of IgG2a antibodies, indicating that the helper population induced by DC belongs to the Th1 subset.
Toxoplasma antigens recognized by IgG antibodies in serum.
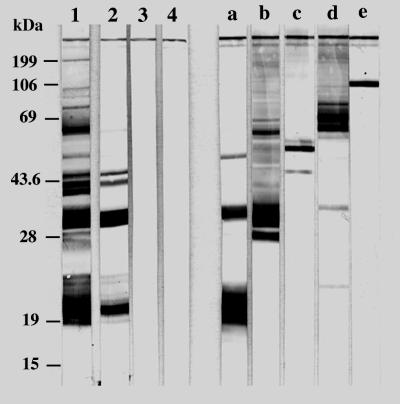
To investigate the T. gondii antigens processed and presented by splenic DC, serum IgG antibodies from mice injected with unpulsed or TSo-pulsed DC or with TSo alone were assayed with T. gondii in immunoblots (Fig. 4). Immune sera detected antigens with apparent molecular masses of 21, 30, and 40 to 45 kDa in mice immunized with TSo-pulsed DC or with TSo alone. The intensity of the 21-, 30-, and 40- to 45-kDa bands was enhanced when the sera were derived from mice injected with TSo-pulsed DC, in comparison to those from mice injected with TSo alone. Furthermore, in the group injected with TSo-pulsed DC, anti-T. gondii IgG antibodies recognized two extra antigens with apparent molecular masses of 50 and 100 kDa (faint bands) and two antigens with apparent molecular masses between 55 and 60 kDa. Antigens recognized by anti-T. gondii IgG antibodies (21-, 30-, 50-, 55- to 60-, and 100-kDa bands) showed a migration pattern similar to that of major Toxoplasma antigens (SAG2, SAG1, MIC1, ROP2 to ROP4, and MIC2, respectively) as detected by specific MAbs.
FIG. 4.
Western blot analysis of T. gondii antigens recognized by serum IgG antibodies and MAbs. Mice were injected with TSo-pulsed DC (lane 1), TSo alone (lane 2), or unpulsed DC (lane 3) or were untreated (lane 4). The following MAbs were used: 3G11 (anti-p22 [SAG2]) (lane a), 1E5 (anti-p30 [SAG1]) (lane b), 1F7 (anti-gp60 [MIC1]) (lane c), 4A7 (anti-55- and 60-kDa [ROP2 to ROP4]) (lane d), and 4A11 (anti-p100 [MIC2]) (lane e). The molecular masses (in kilodaltons) of protein standards are given on the left.
Cellular responses.
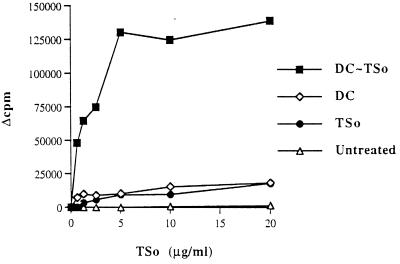
TSo-induced proliferation was assessed with lymph node cells from mice immunized with unpulsed or TSo-pulsed DC or with TSo alone (Fig. 5). Lymph node cells from TSo-pulsed DC-injected mice showed a strong dose-dependent proliferative response to TSo restimulation. Only weak proliferation of cells from mice immunized with TSo alone or with unpulsed DC was observed in response to TSo stimulation, whatever the concentration. Finally, no TSo-specific cell proliferation was observed with cells from untreated mice. The phenotypic analysis revealed that lymph nodes from TSo-pulsed DC-immunized mice are composed of 23% CD22 cells and 92% Thy1.2 cells and that T cells are composed of 60% CD4+ cells and 31% CD8α+ cells.
FIG. 5.
In vitro proliferation of lymph node cells from mice injected in the fore and hind footpads with 3 × 105 TSo-pulsed DC (DC∼TSo) or unpulsed DC (DC) per footpad. Control mice were injected with TSo alone (TSo) or untreated. Results are expressed as Δcpm. The results are representative of at least three experiments.
To demonstrate T. gondii-specific cytotoxic T lymphocytes in the popliteal and brachial lymph nodes after injection of TSo-pulsed DC, lymph node cells from mice immunized with unpulsed or TSo-pulsed DC were assayed, 7 days after immunization, for cytotoxicity toward radiolabeled infected Mφ. Whatever the groups, no significant level of cytotoxicity activity was detected.
Cytokine production.
The presence of IFN-γ, IL-2, and IL-5 was assayed in the supernatants of lymph node cell cultures stimulated with TSo and obtained from mice receiving unpulsed or TSo-pulsed DC or TSo alone (Table 1). Lymph node cells from mice immunized with TSo-pulsed DC responded to TSo stimulation by increased production of IFN-γ and IL-2 compared with that obtained from lymph node cells from untreated mice or mice immunized with unpulsed DC or with TSo alone. No specific release of IL-5 from any culture supernatant was demonstrable.
TABLE 1.
Cytokine production by lymph node cells from mice immunized in the footpads with TSo-pulsed or unpulsed DC or TSo alonea
| Immunization regimen | IFN-γ concn (U/ml) | IL-2 concn (U/ml) | IL-5 concn (U/ml) |
|---|---|---|---|
| DC∼TSo | 1,050 | 0.70 | <0.45 |
| DC | <0.9 | <0.08 | <0.45 |
| TSo | <0.9 | <0.08 | <0.45 |
Mice were injected in the footpads with TSo-pulsed DC (DC∼TSo) or unpulsed DC (DC) or with TSo alone (TSo) or were untreated. Lymph node cells recovered 5 days after immunization were cultured with TSo. The culture supernatants were examined for cytokine production. The peak levels of cytokine in the supernatant are given. Values for IL-2, IFN-γ, and IL-5 were measured at 24, 72, and 96 h of culture, respectively. The results are representative of at least three experiments.
Taken together, these results demonstrated that DC loaded with T. gondii antigens triggered a strong cellular response in vivo that is associated with the CD4+ Th1 subset. However, under our experimental conditions, this cellular response did not involve potent cytotoxic activity.
DISCUSSION
Th1 and Th2 T-cell subsets mediate separate effector functions, and the development of one population has important biologic consequences. Protective immunity in T. gondii infection is generally considered to be cell mediated (21). Many studies have emphasized the importance of helper CD4+ Th1 lymphocytes in checking acute infection (22, 25) and also in preventing chronic infection (1). CD4+ cells might also stimulate CD8+-cell differentiation, which has been described as protective in a mouse model (35). For the development of an effective vaccine, it is of major importance to favor the differentiation of Th1 protective cells in vivo. Several factors such as antigen density, MHC genotype, and lymphokines present during the initiation of the immune response have been shown to influence the predominance of the Th1 or Th2 subset in vivo (15). The discovery of MHC restriction has focused attention on the major role of APC in the activation of Th cells, which in turn leads to the differentiation of the effector cells of the immune response. DC appear to play a major role in initiating T-cell immune responses (33) and are able to stimulate a Th1-associated antibody response in vivo (13, 58). We show in this study that DC loaded in vitro with T. gondii antigens were effective for use as APC to induce in vivo not only potent humoral and cellular immune responses but also a strong resistance to cyst formation upon oral infection with T. gondii.
Murine susceptibility to toxoplasmosis depends on the mouse H-2 haplotype. It has been clearly demonstrated that the presence of the Ld gene in mice is correlated with resistance to brain cyst formation (4). The putative mechanism of this resistance might be better control of the parasitemia stage through the early stimulation of CD8+ T cells by protective peptides of T. gondii presented in the context of the H-2Ld molecule. Both CD4+ T cells and CD8+ T cells actively participate in the development of resistance to T. gondii and in the mechanisms controlling brain cyst formation in mice (1). Injection of DC pulsed in vitro with TSo into CBA/J mice (H-2k), a cyst-susceptible strain, effectively prevented brain cyst formation following oral challenge with T. gondii 76K. A smaller reduction in the number of brain cysts was also observed in mice injected with TSo alone. This mild protection could be due to the in vivo presentation of TSo antigens by DC. Specific immune responses were then investigated in the protected mice.
We first studied the specific antibody response induced in vivo after a single injection of in vitro TSo-pulsed DC. The humoral response in mice injected with TSo-pulsed DC was characterized by a high level of TSo-specific IgG2a antibodies. This increase in the level of IgG2a specific for TSo is in agreement with the increase already observed in other models involving myoglobin or human gamma globulin (13, 58). However, in contrast to these findings, mice injected with TSo-pulsed DC produce only a low level of TSo-specific IgG1 antibodies. The regulation of Ig isotype production is under the control of a number of pluripotent cytokines acting as either a B-cell switch or differentiation factors. Although the role of IL-12 in the humoral immune response is less clear, recent studies found that treatment with IL-12 at the time of immunization with MHC class I alloantigen in rats (26) and with TNP-KLH in mice (43) strongly inhibited the IgG1-specific response. We showed that TSo-pulsed DC produced a high level of IL-12. This may explain the low level of TSo-specific IgG1 antibodies induced in vivo. Furthermore, it has been shown that Th1- and Th2-derived lymphokines reciprocally regulate the determination of Ig isotype response (57). The production of IgG2a is linked to the activation of IFN-γ-producing T cells (Th1 function), whereas IL-4 is important in the regulation of IgE production (Th2 function) (60). The high production of specific IgG2a in sera from mice immunized with TSo-pulsed DC suggests that they mainly activate specific Th1 cells in vivo.
To measure directly the T-cell activation elicited by DC in vivo, we studied the specific proliferative response and the specific cytotoxic T-lymphocyte response of lymph node cells from mice injected in the footpad with unpulsed or TSo-pulsed DC. To study the cellular immune response triggered by the transfer of TSo-pulsed DC, a route of DC injection that could efficiently target the T-cell areas of lymphoid tissue was chosen. Since DC injected subcutaneously have been reported to spontaneously and rapidly migrate to draining lymph nodes (2), we decided to use subcutaneous injection into the footpad, drained by popliteal and brachial lymph nodes, which are easily removed, to investigate T-cell activation. Protective immunity against T. gondii infection involves IFN-γ activity and also CD8+ cytotoxicity (21). Moreover, DC are potent APC capable of priming cytotoxic T-lymphocyte responses in vivo after being loaded in vitro with soluble protein (49). However, under our experimental conditions, lymph node cells from mice immunized with TSo-pulsed DC did not exhibit potent cytotoxic activity but, in contrast, showed a strong dose-dependent proliferative response to TSo restimulation. This lack of cytotoxic activity could be explained by the short interval between immunization and the assay (7 days). At that time, T cells in the lymph nodes are mainly CD4+. Activation and recruitment of CD8+ T cells could need more time. At the time of the challenge (28 days after the transfer), specific CD8+ cytotoxic activity could not be ruled out. The proliferative response is associated with increased production of IFN-γ and IL-2, whereas no specific release of IL-5 was observed. These results confirm that the cellular immune response elicited in vivo by TSo-pulsed DC belongs to the CD4+ Th1 subset. De Becker et al. (12) recently showed that Mφ stimulate Th2 differentiation in vivo whereas DC drive the development of cells producing Th1- and Th2-derived cytokines. In our study, TSo-pulsed DC transfer led only to Th1 differentiation. The nature (crude parasite antigenic extract TSo versus well-defined antigen human gamma globulin) and the amount of antigens seem to play a role in the development of the Th subset (9). The factors which influence the differentiation of Th0 cells into cells with Th1 or Th2 phenotypes include the type of APC, the nature and amounts of antigens, and the expression of costimulatory molecules (18, 37) and cytokine microenvironment (29, 30, 40, 41, 54, 62).
Soluble and membrane-bound signals delivered between T cells and APC have been reported to influence Th1 or Th2 commitment. During overnight culture corresponding to in vitro maturation of DC, the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) were expressed similarly on unpulsed and on TSo-pulsed DC. However, a higher level of B7-2 was expressed on these cells than the level of B7-1, whose expression was weak. Indeed, B7-2 appeared to be the dominant costimulatory ligand during the primary immune response, whereas B7-1, which is up-regulated later in the immune response and binds T-cell surface molecules CD28 and CTLA-4 with low affinity, may be critical in prolonging the primary T-cell response or costimulating the secondary T-cell response (3, 44). It has been suggested that the presence of B7-1 favors the activation of Th1 lymphocytes whereas B7-2 favors the priming of Th2 cells (18, 37). Despite a high level of B7-2 on TSo-pulsed DC, we found that these cells elicited a strong and specific Th1 response in vivo. These results support the hypothesis that the B7-2 molecule could also costimulate IL-2 production, a Th1 function (17).
Cytokines can also play a critical role in Th-cell phenotype differentiation. Many studies have reported that IL-12 and IFN-γ promote the differentiation of antigen-specific T cells into Th1 cells (30, 40, 41, 54) and that IL-4 and, to a lesser extent, IL-10 stimulate the development of Th2 cells (29, 40, 62). Recent studies have indicated that IL-12 is produced by DC and favors the development of Th1 cells from naive CD4+ T cells (28, 39). In our model, the high level of IL-12 synthesis by TSo-pulsed DC may lead to the differentiation of only Th1.
Moreover, IL-12 synthesis by TSo-pulsed DC is very important since IL-12 appears to play a major role in natural immunity to T. gondii (31, 36). Indeed, IL-12 and other cytokines (IL-1 and tumor necrosis factor alpha) are produced by Mφ when exposed to live parasites or parasite extracts and stimulate NK cells to produce IFN-γ (23, 24, 32, 56). Moreover, a recent study has demonstrated that DC are the initial cells that synthesize IL-12 in the spleens of mice exposed in vivo to an extract of T. gondii (59). The production of IFN-γ by NK cells early in the course of T. gondii infection results in the activation of Mφ (61) and nonphagocytic cells such as fibroblasts (50), endothelial cells (63), and enterocytes (14) to kill parasites or inhibit parasite replication. This innate response affords protection to the host before the development of an adaptive immune response. DC could therefore play an important role not only in the initiation of adaptive immunity to T. gondii but also in the early induction of innate immunity to the parasite. After primary T. gondii infection, IL-12 produced by DC would be able to (i) activate NK cells to produce IFN-γ (53), (ii) promote T-cell differentiation toward the Th1 phenotype which produces IFN-γ (28, 39), and (iii) synergize with the CD28-B7 interaction for efficient proliferation and production of IFN-γ by T cells (48).
Finally, to investigate the T. gondii antigens processed and presented by DC, we determined the T. gondii antigens recognized by serum IgG antibodies from mice injected with unpulsed or TSo-pulsed DC. The presentation of T. gondii antigens by DC triggered the IgG response to the 21-, 30-, 40- to 45-, and 55- to 60-kDa bands and the faint bands at 50 and 100 kDa which were detected following injection of TSo-pulsed DC. These major T. gondii antigens showed identical migration patterns to those described in our previous paper (7) and to those of major well-known T. gondii proteins identified with MAbs (SAG2, SAG1, MIC1, ROP2 to ROP4, and MIC2). The SAG1 protein is the major surface antigen of the proliferative form of T. gondii. Recent data indicate that SAG1 is involved in the process of invasion (46, 47). In addition, the value of the 30-kDa protein (SAG1) of T. gondii as a protective antigen following parenteral immunization (5, 34) and mucosal immunization (11) is well known. Other major T. gondii antigens such as SAG2 (surface protein), MIC1 (microneme protein), ROP2 to ROP4 (rhoptry proteins), and MIC2 (microneme protein) seem to stimulate protective immunity. Although there is only a correlation between bands recognized by antibodies from TSo-pulsed DC-immunized mice and the major antigens so far recognized as important in T. gondii protection, the study indicates that antigen presentation by TSo-pulsed DC seems to be well adapted for a large sample of major T. gondii antigens.
The findings presented in this work emphasize the central role played by DC in T. gondii resistance. Indeed, our findings show that DC pulsed in vitro with T. gondii antigens elicit a strong resistance to cyst formation after oral challenge in vivo. This protection is associated with a high level of specific IgG2a antibodies, suggesting that CD4+ protective cells belong to the Th1 subset. We have defined some major T. gondii antigens, such as SAG1, SAG2, MIC1, ROP2 to ROP4, and MIC2, which were recognized by the sera of TSo-pulsed DC-immunized mice. Some of these antigens (SAG1 and MIC2) were shown to play a major role in both parasite attachment and penetration. Therefore, specific immunity against this antigen could be involved in the induction of protective immunity. Furthermore, DC loaded with T. gondii antigens, which synthesize high levels of IL-12, are also able to induce a strong cellular response in vivo, characterized by the production of IFN-γ and IL-2.
ACKNOWLEDGMENTS
We thank H. Bazin for providing isotype-specific rat MAbs, J.-F. Dubremetz for donating anti-T. gondii MAbs, and G. De Becker for helpful comments. We especially thank M. Vercammen, Institut Pasteur, Brussels, Belgium, for the generous use of laboratory facilities and her staff for technical help. We are indebted to G. Dewasme, P. Vaerman, and M. Swaenepoel for excellent technical assistance. Correction of the manuscript by D. Raine is gratefully acknowledged.
This work was supported by grants from the European Commission (Joint Research contract CI1-CT94-0057). I.B. has a fellowship from the Région Centre (France).
REFERENCES
- 1.Araujo F G. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect Immun. 1991;59:1614–1619. doi: 10.1128/iai.59.5.1614-1619.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barratt-Boyes S M, Watkins S C, Finn O J. In vivo migration of dendritic cells differentiated in vitro. A chimpanzee model. J Immunol. 1997;158:4543–4547. [PubMed] [Google Scholar]
- 3.Bluestone J A. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 4.Brown C R, Hunter A, Estes R G, Beckmann E, Formann J, David C, Remington J S, McLeod R. Definitive identification of a gene that confers resistance against Toxoplasma gondii cyst burden and encephalitis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 5.Bülow R, Boothroyd J C. Protection of mice from fatal Toxoplasma gondii infection by immunization with P30 antigen in liposomes. J Immunol. 1991;147:3496–3500. [PubMed] [Google Scholar]
- 6.Buxton D. Toxoplasmosis: the first commercial vaccine. Parasitol Today. 1993;9:335–337. doi: 10.1016/0169-4758(93)90236-9. [DOI] [PubMed] [Google Scholar]
- 7.Chardès T, Bourguin I, Mévélec M-N, Dubremetz J-F, Bout D. Serum and secretory IgA antibodies specific for Toxoplasma gondii. Kinetics of the murine humoral response to infection and characterization of target antigens. Infect Immun. 1990;58:1240–1246. doi: 10.1128/iai.58.5.1240-1246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chardès T, Buzoni-Gatel D, Lepage A, Bernard F, Bout D. Toxoplasma gondii oral infection induces specific cytotoxic CD8α/β+ Thy-1+ gut intraepithelial lymphocytes, lytic for parasite-infected enterocytes. J Immunol. 1994;153:4596–4603. [PubMed] [Google Scholar]
- 9.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley M, Inaba K, Witmer-Pack M, Steinman R M. The cell surface of mouse dendritic cells: FACS analyses of dendritic cells from different tissues including thymus. Cell Immunol. 1989;118:108–125. doi: 10.1016/0008-8749(89)90361-4. [DOI] [PubMed] [Google Scholar]
- 11.Debard N, Buzoni-Gatel D, Bout D. Intranasal immunization with SAG1 protein of Toxoplasma gondii in association with cholera toxin dramatically reduces development of cerebral cysts after oral infection. Infect Immun. 1996;64:2158–2166. doi: 10.1128/iai.64.6.2158-2166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Becker G, Moulin V, Van Mechelen M, Tielemans F, Urbain J, Leo O, Moser M. Dendritic cells and macrophages induce the development of distinct T helper cell populations in vivo. Adv Exp Med Biol. 1997;417:369–373. doi: 10.1007/978-1-4757-9966-8_60. [DOI] [PubMed] [Google Scholar]
- 13.De Becker G, Sornasse T, Nabavi N, Bazin H, Tielemans F, Urbain J, Leo O, Moser M. Immunoglobulin isotype regulation by antigen-presenting cells in vivo. Eur J Immunol. 1994;24:1523–1528. doi: 10.1002/eji.1830240710. [DOI] [PubMed] [Google Scholar]
- 14.Dimier I, Bout D. Rat intestinal epithelial cell-line IEC-6 are activated by IFN-gamma to inhibit replication of the coccidian Toxoplasma gondii. Eur J Immunol. 1993;23:981–983. doi: 10.1002/eji.1830230435. [DOI] [PubMed] [Google Scholar]
- 15.Fitch F W, McKisic M D, Lancki D W, Gajewski T J. Differential regulation of murine T lymphocyte subsets. Annu Rev Immunol. 1993;11:29–48. doi: 10.1146/annurev.iy.11.040193.000333. [DOI] [PubMed] [Google Scholar]
- 16.Flamand V, Sornasse T, Thielemans K, Demanet C, Bakkus M, Bazin H, Tielemans F, Leo O, Urbain J, Moser M. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Eur J Immunol. 1994;24:605–610. doi: 10.1002/eji.1830240317. [DOI] [PubMed] [Google Scholar]
- 17.Freeman G J, Borriello F, Hodes R J, Reiser H, Gribben J G, Ng J W, Kim J, Goldberg J M, Hathcock K, Laszlo G, Lombard L A, Wang S, Gray G S, Nadler L M, Sharpe A H. Murine B7-2, an alternative CTLA-4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993;178:2185–2192. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman G J, Boussiotis V A, Anumanthan A, Bernstein G M, Ke X-Y, Rennert P D, Gray G S, Gribben J G, Nadler L M. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 19.Frenkel J K. Pathophysiology of toxoplasmosis. Parasitol Today. 1988;4:273–278. doi: 10.1016/0169-4758(88)90018-x. [DOI] [PubMed] [Google Scholar]
- 20.Gajewski T F, Pinnas M, Wong T, Fitch F. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991;146:1750–1758. [PubMed] [Google Scholar]
- 21.Gazzinelli R T, Denkers E Y, Sher A. Host resistance to Toxoplasma gondii: model for studying the selective induction of cell-mediated immunity by intracellular parasites. Infect Agents Dis. 1993;2:139–149. [PubMed] [Google Scholar]
- 22.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 23.Gazzinelli R T, Hieny S, Wynn T, Wolf S, Sher A. IL-12 is required for the T-cell-independent induction of IFN-γ by an intracellular parasite and induces resistance in T-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 25.Gazzinelli R T, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ lymphocytes is required to re-activate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 26.Gracie J A, Bradley J A. Interleukin-12 induces interferon-γ-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26:1217–1221. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- 27.Hathcock K S, Laszlo G, Dickler H B, Bradshaw J, Linsley P, Hodes R J. Identification of an alternative CTLA-4 ligand costimulatory for T-cell activation. Science. 1993;262:905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 28.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman R M, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh C-S, Heimberger A B, Gold J S, O’Garra A, Murphy K M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ-T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh C-S, Macatonia S E, Tripp C S, O’Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 31.Hunter C A, Candolfi E, Subauste C, Van Cleave V, Remington J S. Studies on the role of interleukin-12 in acute murine toxoplasmosis. Immunology. 1995;84:16–20. [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter C A, Chizzonite R, Remington J S. Interleukin 1β is required for the ability of IL-12 to induce production of IFN-γ by NK cells: a role for IL-1β in the T cell independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 33.Inaba K, Metlay J P, Crowley M T, Steinman R M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan I A, Ely K H, Kasper L H. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- 35.Khan I A, Ely K H, Kasper L H. Antigen specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J Immunol. 1994;152:1856–1860. [PubMed] [Google Scholar]
- 36.Khan I A, Matsuura T, Kasper L H. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun. 1994;62:1639–1642. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 38.Lefebvre M, Vincenzotto C, Digneffe C, Cormont F, Genart C, Bazin H. Rat monoclonal antibodies against murine immunoglobulins. In: Bazin H, editor. Rat hybridomas and rat monoclonal antibodies. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 231–234. [Google Scholar]
- 39.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C-S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 40.Maggi E, Parronchi P, Manetti R, Simonelli C, Piccinni M-P, Rugiu F S, De Carli M, Ricci M, Romagnani S. Reciprocal regulatory effects of IFN-γ and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148:2142–2147. [PubMed] [Google Scholar]
- 41.Manetti R, Parronchi P, Giudizi M G, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mbow M L, Zeidner N, Panella N, Titus R G, Piesman J. Borrelia burgdorferi-pulsed dendritic cells induce a protective immune response against tick-transmitted spirochetes. Infect Immun. 1997;65:3386–3390. doi: 10.1128/iai.65.8.3386-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKnight A J, Zimmer G J, Fogelman I, Wolf S F, Abbas A K. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol. 1994;152:2172–2179. [PubMed] [Google Scholar]
- 44.McLellan A D, Starling G C, Williams L A, Hock B D, Hart D N J. Activation of human peripheral blood dendritic cells induces the CD86 co-stimulatory molecule. Eur J Immunol. 1995;25:2064–2068. doi: 10.1002/eji.1830250739. [DOI] [PubMed] [Google Scholar]
- 45.Metlay J P, Witmer-Pack M D, Agger R, Crowley M T, Lawless D, Steinman R M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mineo J R, Kasper L H. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30) Exp Parasitol. 1994;79:11–20. doi: 10.1006/expr.1994.1054. [DOI] [PubMed] [Google Scholar]
- 47.Mineo J R, McLeod R, Mack D, Smith J, Khan I A, Ely K H, Kasper L H. Antibodies to Toxoplasma gondii surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J Immunol. 1993;150:3951–3964. [PubMed] [Google Scholar]
- 48.Murphy E, Terres G, Macatonia S, Hsie C-S, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O’Garra A. B7 and interleukin 12 cooperate for proliferation and interferon-γ production by mouse T helper cell clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paglia P, Chiodoni C, Rodolfo M, Colombo M P. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfefferkorn E R, Guyre P M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984;44:211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razi-Wolf Z, Galvin F, Gray G, Reiser H. Evidence for a novel ligand, distinct from B7, for the CTLA-4 receptor. Proc Natl Acad Sci USA. 1993;90:11182–11186. doi: 10.1073/pnas.90.23.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Remington J S, McLeod R, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1995. p. 140. [Google Scholar]
- 53.Scharton-Kersten T M, Wynn T A, Denkers E Y, Bala S, Grunvald E, Hieny S, Gazzinelli R T, Sher A. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 54.Seder R A, Gazzinelli R, Sher A, Paul W E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma S D, Mullenak J, Araujo F G, Erlich H A, Remington J S. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983;131:977–983. [PubMed] [Google Scholar]
- 56.Sher A, Oswald I P, Hieny S, Gazzinelli R T. Toxoplasma gondii induces a T-independent IFN-γ response in natural killer cells that requires both adherent accessory cells and TNF-α. J Immunol. 1993;150:3982–3989. [PubMed] [Google Scholar]
- 57.Snapper C M, Mond J J. Towards a comprehensive view of immunoglobulin switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 58.Sornasse T, Flamand V, De Becker G, Bazin H, Tielemans F, Thielemans K, Urbain J, Leo O, Moser M. Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J Exp Med. 1992;175:15–21. doi: 10.1084/jem.175.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sousa C R E, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain R N, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens T L, Bossie A, Sanders V M, Fernandez-Botran R, Coffman R L, Mosmann T R, Vitetta E S. Regulation of Ab isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 62.Swain S L, Weinberg A D, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 63.Woodman J P, Dimier I H, Bout D T. Human endothelial cells are activated by IFN-γ to inhibit Toxoplasma gondii replication. Inhibition is due to a different mechanism from that existing in mouse macrophages and human fibroblasts. J Immunol. 1991;147:2019–2023. [PubMed] [Google Scholar]
- 64.Zitvogel L, Mayordomo J I, Tjandrawan T, DeLeo A B, Clarke M R, Lotze M T, Storkus W J. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]