Abstract
Background
Studies have shown that the absolute lymphocyte count (ALC) and the neutrophil-to-lymphocyte ratio (NLR) are related to the outcomes in patients with breast cancer receiving specific chemotherapies. However, the reports have focussed on the initial blood test and there is a lack of evidence or data to support that dynamic changes of ALC or NLR are associated with the patients’ survival outcomes.
Methods
We retrospectively reviewed electronic medical records from patients with breast cancer treated with eribulin from 2015 to 2019 at our institution. Blood test data were available prior to starting eribulin (baseline), and at 1, 3 and 6 months after initiating eribulin. We classified the patients into ALC and NLR high and low groups using the following cut-offs: 1000/µl for ALC and 3 for NLR. We defined ALC and NLR trends as increasing or decreasing compared with the initial data. We assessed the associations between the ALC and NLR with progression-free survival and overall survival.
Results
There were 136 patients with breast cancer treated with eribulin. Of these patients, 60 had complete blood tests and follow-up data. Neither a high ALC nor a low baseline NLR was associated with the survival outcome. One month after initiating eribulin treatment, a high ALC and a low NLR were significantly associated with longer progression-free survival (p = 0.044 for each). Three months after initiating eribulin, a high ALC was significantly associated with better overall survival (p = 0.006). A high NLR at 3 or 6 months after initiating eribulin was associated with worse overall survival (p = 0.017 and p = 0.001, respectively). The ALC and NLR trends across times were not associated with survivals.
Conclusion
We showed that 1, 3 and 6 months after initiating eribulin, a high ALC and a low NLR may be related to the patients’ survival outcomes. The ALC and NLR trends were not associated with survival. Accordingly, we believe patients who maintain a high ALC and a low NLR may have better clinical outcomes after initiating eribulin.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-11923-5.
Keywords: Breast cancer, Eribulin, Absolute lymphocyte count, Neutrophil-to-lymphocyte count
Background
Cytotoxic chemotherapy is still a mainstay of treatment for recurrent advanced or metastatic breast cancer (A/MBC) because most of the patients are usually treated with chemotherapy sooner or later. Although some hormone receptor-positive patients first receive endocrine therapy, most of them eventually would need chemotherapy as well [1]. Eribulin, a novel chemotherapeutic agent, was licensed for the treatment of A/MBC in patients who had previously received at least one chemotherapy regimen in the metastatic settings. It improved overall survival (OS) in women with A/MBC in the phase III EMBRACE clinical trial [2]. To date, there is no consensus regarding the use of biomarkers across times to predict whether a patient with A/MBC would benefit from eribulin or not.
Inflammation plays an important role in cancer development and responses to therapies, so researchers have evaluated the ability of inflammatory biomarkers in blood to predict the survival outcomes of patients with various types of cancers and receiving various treatments, including patients with A/MBC receiving eribulin [3–11]. They have examined the ability of the absolute lymphocyte count (ALC) and the neutrophil-to-lymphocyte ratio (NLR) - which can be measured easily and inexpensively - to predict the cancer prognosis [12–17]. Eribulin exerts an anticancer effect by inhibiting microtubule dynamics, and it is thought to have biological effects on the immune system by suppressing the epithelial-to-mesenchymal transition (EMT) and vascular remodelling and by improving the tumour microenvironment. These actions contribute to an improvement in OS in patients with A/MBC [18]. Several studies have shown that a high ALC or a low baseline NLR is associated with better survival outcomes in patients with A/MBC treated with eribulin [4, 5, 7, 8], but not for patients receiving traditional treatments [3, 19].
Almost all of available studies have focussed mainly on the ALC or NLR values at the baseline, i.e., before the initiation of a treatment, to identify patients who might benefit from monitoring these measures [3–5, 7, 8, 19]. Some researchers suggest that the associated factors could change dynamically during treatment with several therapies. For example, researchers have noted a significant increase in the ALC of patients with human epidermal growth factor receptor 2 (HER2)-positive A/MBC after one cycle of trastuzumab emtansine treatment, or dissimilar changes in the ALC and NLR during the treatment with eribulin and bevacizumab among patients with A/MBC or non-small cell lung cancer, respectively [6, 20, 21]. Such changes might reflect a patient’s real-time response to the treatments, or could be used to predict a patient’s prognosis more precisely [6, 20, 21]. Moreover, Nakamoto et al. [6] demonstrated that dynamic changes in ALC after one cycle of treatment with eribulin seemed to be an independent predictor for post-progress survival among patients with A/MBC, even when eribulin was discontinued. In this study, we examined the ability of the ALC and NLR at different times after eribulin had been initiated to predict progression-free survival (PFS) and OS of patients with A/MBC. We hypothesise that the changes in the ALC and NLR after eribulin initiation allow clinicians to predict a patient’s outcomes regardless of whether the patient is still receiving eribulin or not.
Methods
Patients
For this retrospective cohort study, data were retrieved from the electronic medical records (eMRs) of patients with A/MBC treated with eribulin at China Medical University Hospital (CMUH) between 1 January 2015 and 30 June 2019. Female patients with locally recurrent or metastatic breast cancer, age ≥ 20 years and who received at least one cycle of eribulin for A/MBC were included. Patients with a second cancer or who did not have complete blood counts at the initiation of eribulin treatment or at 1, 3 and 6 months following eribulin initiation were excluded for further analysis.
Treatment with eribulin
Eribulin was administered intravenously at a dose of 1.4 mg/m2 on days 1 and 8 of each 21-day cycle. The physician could adjust the eribulin dose based on the severity of adverse events. The treatment was continued until the disease being progressed or encounter intolerable of toxicity.
ALC and NLR
This study focussed on the effect of eribulin after its initiation irrespective of whether it was continued or discontinued. Complete blood counts -including white blood cells, neutrophils and lymphocytes - were evaluated at four time points, which were determined based on the time since the eribulin initiation date (the index date). Practically, the lab data closest to the defined time points (within 2 weeks) were used for analysis. The first time point is baseline, just prior to the initial eribulin dose. At CMUH, a complete blood count is usually obtained at the beginning of each planed treatment cycle as part of the routine clinical practice. The subsequent three time points were at 1, 3 and 6 months after eribulin initiation. The NLR was calculated by dividing the number of neutrophils by the number of lymphocytes. The ALC and NLR were classified as high or low using a cut-off of 1000/µl for the ALC and 3 for the NLR; these values were chosen based on the available studies [5, 14, 22, 23]. The trends in the ALC and NLR 1, 3 and 6 months after eribulin initiation were categorised as decreasing if the ALC and NLR were lower compared with baseline and non-decreasing (including increasing) if they were equal or higher compared with baseline.
Outcomes
The primary outcome was PFS: the time from initial eribulin treatment until disease progression or death. If eribulin was discontinued due to treatment intolerance or any other reasons with no reported disease progression, then the patient was censored. The secondary outcome was OS, which was computed from eribulin initiation until death. Considering CMUH is a tertiary hospital with comprehensive cancer diagnosis and treatment facilities in middle of Taiwan, we assumed those patients who were lost to follow-up at CMUH might be due to either they decided to stop receiving further treatment in their end stages of cancer or they had encountered the competing risks for death with no records in CMUH. In practice, some patients might choose to either receive home hospice care or die at home with CMUH discharge medical records indicated “discharge against medical advice” for their end-stage care. Some might be transferred to the other medical institutes based on patients’ preferences or special needs of out-of-pocket treatments, which might be not available in CMUH. In this case, these patients were censored because we assumed they were not treated continuously at CMUH or until the study observation period had ended. Consequently, these patients were treated as death events at the time of loss to follow-up to obtain a stricter estimate of OS.
Statistical analysis
The patient characteristics are presented with descriptive statistics, including the median and interquartile range (IQR) and frequencies. Kaplan-Meier curves were generated to assess the trends in PFS and OS at each time point based on the ALC and NLR groups. The log-rank test was used to compare the survival outcomes between the groups. A univariate Cox proportional-hazards regression model was used to explore the factors associated with the ALC and NLR at different time points and the ALC and NLR trends and PFS and OS. Those factors associated with PFS and OS (with p ≤ 0.15) were used to adjust for the associations of the ALC, NLR and their trends with PFS and OS in the multivariate Cox proportional hazards regression model. When analysing the ALC and NLR trends, the baseline values were also used as a covariate for adjustment. When comparing the ALC and NLR 1, 3 and 6 months after eribulin initiation with the baseline ALC and NLR, whether the patient was still receiving eribulin treatment at the corresponding time points, except for some specific concerns, was also considered an important covariate for multivariate Cox regression. Statistical significance was set at p < 0.05. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
There were 136 patients with A/MBC who were prescribed with eribulin at CMUH from January 2015 to June 2019. We excluded patients without baseline or follow-up tests, who did not complete at least one cycle of eribulin or who did not have a sufficient follow-up time. Finally, we included 60 patients in the study. Their median age was 51 (IQR 41-59) years (Table 1). Of the patients, 57.6%, 50.0% and 37.3% were oestrogen receptor (ER) positive, progesterone receptor (PR) positive and HER2 positive, respectively, and 16.7% of them were diagnosed with triple-negative breast cancer. The majority of the patients had metastasis at two or more sites (66.7%). Moreover, 60% of the patients experienced visceral metastasis (15.0% visceral and 45.0% visceral and non-visceral metastasis). In terms of treatment features, 78.4% of all assessed patients had previously been treated with chemotherapy as adjuvant or neo-adjuvant therapy. One fourth of the patients received eribulin as first- or second-line chemotherapy, and three fourths received eribulin as third-line and higher chemotherapy for their advance disease. The median chemotherapy line of eribulin was 4 (IQR 3-5). The majority of patients (76.7%) received eribulin as monotherapy.
Table 1.
Characteristics among assessed advanced or metastatic breast cancer breast cancer (A/MBC) patients treated with Eribulin (N=60)
| Characteristics | N | % |
|---|---|---|
| Age (years): Median (IQR) | 51 (41 – 59) | |
| Estrogen receptora (N=59) | ||
| Positive | 34 | 57.6 |
| Negative | 25 | 42.4 |
| Progesterone receptora (N=58) | ||
| Positive | 29 | 50.0 |
| Negative | 29 | 50.0 |
| HER2 statusa (N=59) | ||
| Negative | 37 | 62.7 |
| Positive | 22 | 37.3 |
| Triple negative breast cancer | ||
| No | 50 | 83.3 |
| Yes | 10 | 16.7 |
| Number of metastatic sites: Median (IQR) | 2 (1-3) | |
| Number of metastatic sites: | ||
| <2 sites (0 or 1) | 20 | 33.3 |
| ≥2 sites | 40 | 66.7 |
| Types of metastasis: | ||
| Non-visceral | 24 | 40.0 |
| Visceral | 27 | 45.0 |
| Both visceral and non-visceral | 9 | 15.0 |
| (Neo)adjuvant chemotherapya (N=37) | ||
| No | 8 | 21.6 |
| Yes | 29 | 78.4 |
| Eribulin used line to treat recurrent or metastatic breast cancer: Median (IQR) | 4 (3 - 6) | |
| Early-line (First and second line) | 15 | 25.0 |
| Late-line (≥ 3rd line) | 45 | 75.0 |
| Eribulin duration (days): Median (IQR) | 123 (68 – 185) | |
| Eribulin treated | ||
| As monotherapy | 46 | 76.7 |
| With Trastuzumab (±pertuzumab) | 6 | 10.0 |
| With Hormone therapy | 5 | 8.3 |
| With Othersb | 3 | 5.0 |
IQR Interquartile range, HER2 Human epidermal growth factor receptor 2, TNBC Triple negative breast cancer
athe calculation was based on those patients with available data accordingly
bothers include those were prescribed with pembrolizumab, bevacizumab, capecitabin
In univariate Cox regression analysis, none of the patient demographic and clinical characteristics were significantly associated with PFS (Table 2). However, patients with an older age, more than one metastatic site and third-line or higher eribulin treatment were at increased risk of mortality (i.e. OS) compared with younger patients (hazard ratio [HR] 1.04, 95% confidence interval [CI] 1.01-1.07), those with one or no metastatic sites (HR 2.50, 95% CI 1.02-6.13) and those receiving first- or second-line eribulin (HR 4.30, 95% CI 1.30-14.22). The HRs for PFS considering patients who still used eribulin at 3 or 6 months after eribulin initiation were 4.43 (95% CI 2.11-9.30) and 4.04 (95% CI 1.97-8.30), respectively. These values are consistent with the expected PFS for the patients with A/MBC treated with eribulin and not included in multivariate analysis.
Table 2.
Factors associated with progression-free-survival (PFS) and overall survival (OS) among A/MBC patients treated with Eribulin (univariate cox proportional-hazards analysis)
| PFS | OS | |||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Demographic and clinical characteristics | ||||
| Age (years) | 1.00 (0.97 – 1.03) | 0.778 | 1.04 (1.00 – 1.07) | 0.032 |
| Estrogen receptor (Negative vs. Positive) | 1.12 (0.59 – 2.14) | 0.727 | 0.87 (0.41 – 1.83) | 0.714 |
| Progesterone receptor (Negative vs. Positive) | 1.30 (0.68 – 2.46) | 0.426 | 0.74 (0.36 – 1.55) | 0.430 |
| HER2 status (Positive vs. Negative) | 1.22 (0.63 – 2.37) | 0.557 | 0.88 (0.43 – 1.82) | 0.732 |
| TNBC (Yes vs. No) | 0.97 (0.40 – 2.34) | 0.947 | 1.12 (0.39 – 3.22) | 0.840 |
| Number of metastatic site (> 1 vs. ≤1) | 1.77 (0.82 – 3.82) | 0.150 | 2.50 (1.02 -6.13) | 0.045 |
| Metastatic sites | ||||
| Non visceral metastasis | 1 | 1 | ||
| Visceral and bone (2) | 1.24 (0.61 – 2.54) | 0.550 | 1.79 (0.81 – 4.00) | 0.152 |
| Visceral only (3) | 1.60 (0.57 – 4.49) | 0.389 | 1.28 (0.43 – 3.81) | 0.658 |
| Eribulin line of therapy for recurrent and metastatic breast cancer (≥3rd vs. <3rd) | 1.62 (0.76 – 3.46) | 0.212 | 4.30 (1.30 – 14.22) | 0.017 |
| (Neo)adjuvant (No vs. Yes) | 1.37 (0.53 – 3.49) | 0.516 | 0.95 (0.34 – 2.64) | 0.925 |
| Monotherapy of eribulin (No vs. Yes) | 0.97 (0.46 – 2.07) | 0.945 | 1.03 (0.46 -2.32) | 0.941 |
| Still use eribulin or not at the time point of test as compare with that at baseline | ||||
| Off/on eribulin treatment at 1-month-test | 0.99 (0.23 – 4.34) | 0.988 | 4.21 (0.89 – 19.82) | 0.069 |
| Off/on eribulin treatment at 3-month-test | 4.43 (2.11 – 9.30) | <0.0001 | 0.99 (0.45 – 2.15) | 0.973 |
| Off/on eribulin treatment at 6-month-test | 4.04 (1.97 – 8.30) | <0.0001 | 1.921 (0.88 – 4.21) | 0.103 |
A/MBC Advanced or metastatic breast cancer, PFS Progression-free-survival, OS Overall survival
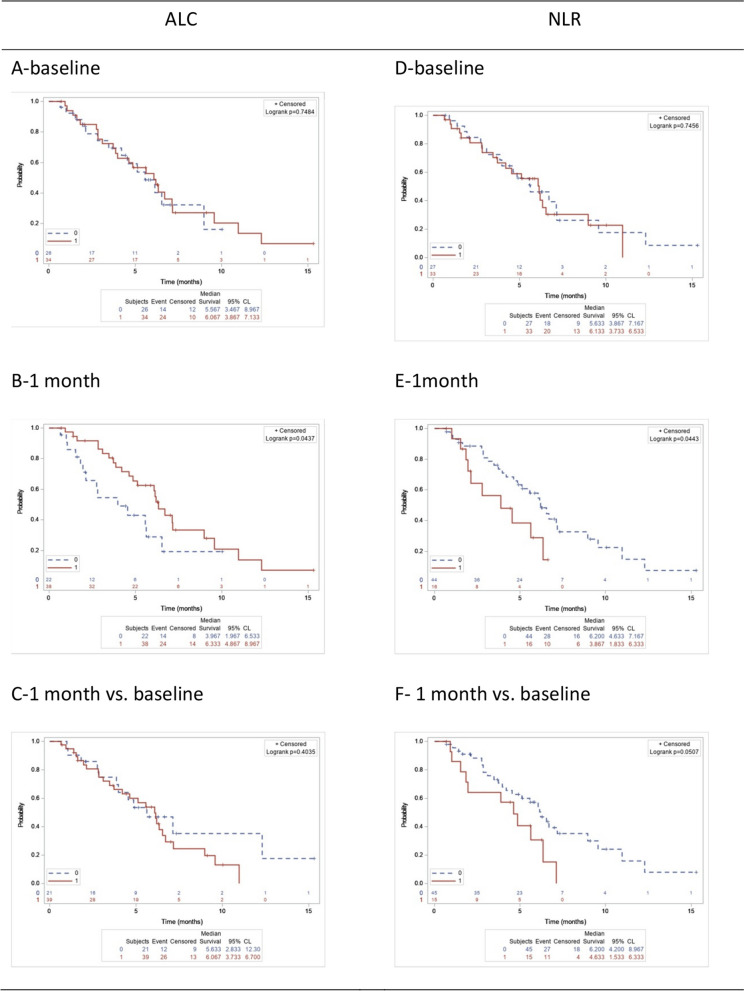
Kaplan-Meier analysis followed by the log-rank test revealed no association between a high ALC or a low NLR at baseline with PFS (all p > 0.05, Fig. 1A and D). In contrast, 1 month after eribulin initiation, a high ALC and a low NLR were significantly associated with longer PFS compared with a low ALC (p = 0.044, Fig. 1B) or a high NLR (p = 0.044, Fig. 1E). The ALC trend was not associated with PFS (p = 0.403, Fig. 1C). Moreover, a decreasing trend for NLR at 1 month after eribulin initiation showed no significant association with better PFS compared with a non-decreasing trend (p = 0.051, Fig. 1F).
Fig. 1.
Progression-free survival (PFS) among patients with high and low ALC and NLR
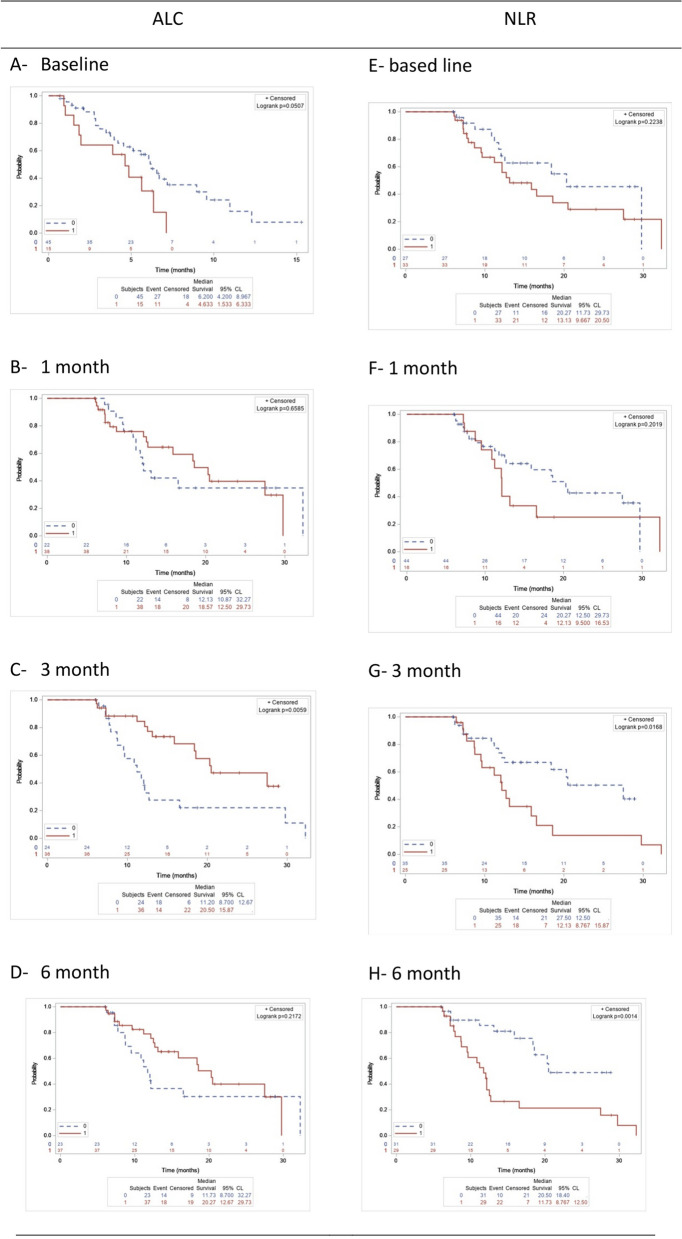
The trends of OS among patients with high and low ALC and NLR at 1-, 3-,6-month tests were listed in Fig. 2A-2H, accordingly. In particular, a high ALC at baseline or 3 months after eribulin initiation was significantly associated with better OS compared with a low ALC (p = 0.011 and p = 0.006 respectively, Fig. 2A and C). A high NLR at 3 or 6 months after eribulin initiation was significantly associated with worse OS compared with a low NLR (p = 0.017 and p = 0.001, respectively, Fig. 2G and H). The ALC and NLR trends at all the time points did not show an association with OS (Supplementary Fig. 1). However, there were significant differences among the groups of patients stratified by ALC trends according to the baseline ALC level (Supplementary Fig. 2), and among different groups of patients stratified by the NLR baseline level and the NLR trend at 6 months after eribulin initiation compared with baseline (Supplementary Fig. 3).
Fig. 2.
Overall survival (OS) among patients with high and low ALC and NLR. ALC= absolute lymphocyte count; NLR= neutrophil to lymphocyte ratio; OS= Overall survival; 0=low; 1=high in (A) (B) (C) (D) (E) (F) (G) (H). OS of patients with high and low ALC at the baseline (A), 1month (B), 3-month (C) and 6-month test (D); OS of patients with high and low NLR at the baseline (E), 1- month (F), 3-month (G) and 6-month test (H)
In the multivariate Cox regression analysis, only the ALC at 1 month after eribulin initiation was significantly associated with PFS. Specifically, patients with a low ALC had 2.26 times (95% CI 1.12-4.55) higher risk of disease progression after adjusting for the number of metastatic sites and off/on treatment with eribulin (Table 3). For OS, a low ALC at the baseline was significantly associated with a higher risk of mortality than a high baseline ALC (HR 2.89, 95% CI 1.33-6.26). Patients with a low ALC or a high NLR at 3 months after eribulin initiation had a higher risk of mortality compared with those with a high ALC (HR 2.30, 95% CI 1.03-5.11) or a low NLR (HR 2.37, 95% CI 1.07-5.24). After adjusting for the confounding factors, a non-decreasing trend for the NLR at 6 months after eribulin initiation was significantly associated with a higher risk of mortality compared with a decreasing trend (HR 6.12, 95% CI 1.83-20.45). We also considered the associations between OS and a high versus low NLR/ALC stratified by on/off treatment with eribulin at 3 or 6 months after eribulin initiation. There was a significant association between OS and the NLR at 3 and 6 months after eribulin initiation, and a significant association between OS and the ALC at 3 months after eribulin initiation (Supplementary Fig. 4).
Table 3.
Associations between ALC, NLR, their trends and progression-free-survival (PFS) or overall survival (OS) among patients used Eribulin using multivariate cox proportional-hazards analysis
| PFSa | OSa | |||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| ALC level (Low vs.High [ref]) b | ||||
| At baseline | 1.05 (0.53 – 2.08) | 0.888 | 2.89 (1.33 – 6.26) | 0.007* |
| At 1-month-testc | 2.26 (1.12 – 4.55) | 0.023* | 1.33 (0.63 – 2.83) | 0.462 |
| At 3-month-testc | - | - | 2.30 (1.03 – 5.11) | 0.042* |
| At 6-month-testc | - | - | 1.14 (0.54 – 2.40) | 0.728 |
| ALC trends (Decreasing vs. Non-decreasing[ref]) | ||||
| 1-month-test compared with the baselined,c | 0.82 (0.38 – 1.75) | 0.608 | 0.88 (0.38 – 2.04) | 0.768 |
| 3-month-test compared with the baselined,c | - | - | 2.08 (0.88 – 4.94) | 0.095 |
| 6-month-test compared with the baselined,c | - | - | 0.76 (0.34 – 1.73) | 0.515 |
| NLR level (High vs. Low[ref])e | ||||
| At baseline | 0.98 (0.50 – 1.91) | 0.944 | 1.59 (0.74 – 3.43) | 0.240 |
| At 1-month-testc | 1.97 (0.96 – 4.27) | 0.087 | 1.45 (0.67 – 3.14) | 0.344 |
| At 3-month-testc | - | - | 2.37 (1.07 – 5.24) | 0.033* |
| At 6-month-testc | - | - | 2.06 (0.85 – 5.00) | 0.109 |
| NLR trends (Non-decreasing vs. non-decreasing [ref]) | ||||
| 1-month-test compared with the baselinef,c | 2.18 (0.92 – 5.18) | 0.078 | 2.15 (0.86 – 5.39) | 0.103 |
| 3-month-test compared with the baselinefc | - | - | 2.50 (0.92 – 6.85) | 0.074 |
| 6-month-test compared with the baselinef,c | - | - | 6.12 (1.83 – 20.45) | 0.003* |
HR Hazard ratio, CI Confidence interval, ALC Absolute lymphocyte count, PFS Progression-free-survival, OS Overall survival
aAdjusted for the factors have p≤0.15 in the univariate analysis above (number of metastatic sites for PFS; number of metastatic sites, eribulin line of treatment and age for OS).
bALC high: ≥1000/µl, low: <1000/µl
*p<0.05
cAdditionally, model was adjusted for off/on eribulin treatment at time point corresponding with the tests
dAdditionally, model was adjusted for ALC at baseline
eNLR high: ≥3, low: <3
fAdditionally, model was adjusted for NLR at baseline
Discussion
To our knowledge, this is the first study to explore the changes in the ALC and NLR across times and proved blood test results in different timing after initiating eribulin treatment. The changes in the ALC and NLR are important predictors for PFS and OS among patients with A/MBC. Specifically, a high ALC or a low NLR at 1 month after eribulin initiation was significantly associated with better PFS in patients with A/MBC. Moreover, a high ALC at 1 month after eribulin initiation was an independent predictor of PFS after controlling for the other factors. After controlling for the other factors, a high baseline ALC, a low NLR at 3 months after eribulin initiation or a non-decreasing NLR trend at 6 months after eribulin initiation were the independent factors associated with extended OS in patients with A/MBC.
After initiating eribulin treatment, the ALC status in patients with A/MBC could be a reliable predictor of the outcomes, with a high ALC being favourable. The ALC in the peripheral blood is considered as a possible indicator of the tumour immune microenvironment [3] and has well-known anti-tumour properties [24]. Moreover, eribulin can alter the tumour immune microenvironment by suppressing the EMT and vascular remodelling [18, 25, 26].
We found that an ALC > 1000/µl at 1 month after eribulin initiation was significantly associated with longer PFS than an ALC < 1000/µl. There have been a few studies in which researchers investigated the associations between ALC changes and clinical outcomes, and the available findings are inconsistent [6, 21]. Imamura et al. [21] reported that a significant increase in the ALC and a non-significant change in the neutrophil count following one cycle of trastuzumab emtansine treatment for patients with A/MBC. However, a significant decrease in the NLR (i.e. a significant increase in the ALC and a non-significant change in the neutrophil count) was significantly associated with improved PFS. Nakamoto et al. showed that a decrease in the ALC after one cycle with eribulin was linked to a longer time to treatment failure [6]. In contrast, we did not find an association between the ALC trend and PFS. However, the findings support the idea that a high ALC at 1 month after eribulin initiation was associated with improved PFS. Based on our study, we believe the ALC change at 1 month after eribulin initiation could be a potential predictor of better PFS among patients treated with eribulin. However, further cross-validation studies in different medical institutes are needed to confirm our findings.
In the post hoc analysis of the EMBRACE phase III clinical trial, the baseline ALC was a specific predictor of OS in eribulin-treated patients [3]. Specifically, a high baseline ALC was a potential predictor of OS among patients with A/MBC in the eribulin group but not in the control group (other treatments based on physician’s choice). Other studies have reported that a high baseline ALC was associated with improved OS despite the use of different ALC cut-offs (e.g. 1000/µl, 1500/µl or the median ALC value) [4–6, 8]. In the current study, a baseline ALC > 1000/µl was an independent predictor of OS, a finding consistent with previous studies [9, 27–29]. Ideally, this finding could be used to facilitate clinical decision-making to identify patients who may benefit from treatment with eribulin. Although the baseline ALC has been robustly demonstrated to be a specific predictor of OS in patients with A/MBC treated with eribulin, the predictive value of the ALC regarding PFS remains unclear. Some studies did show the significant associations of high baseline ALC and improved PFS [9, 27], but some indicated a non-significant association [28, 29]. In this current study, we did not find an association between the baseline ALC and PFS.
In contrast to the ALC, the baseline NLR does not seem to be a good candidate biomarker to predict the response to eribulin therapy. A few systematic reviews and meta-analyses have indicated the role of baseline NLR as one of predictors for PFS and OS for patients with various types of cancer (including breast cancer) [30–34]. On additional study indicated that baseline NLR was one of predictors of partial response to eribulin but not for PFS among metastatic breast cancer patients [35]. Although the post hoc analysis of the EMBRACE clinical trial showed that the baseline NLR was predictor for OS [3], Corbeau et al’s systematic review did include this aforementioned study and indicted that the NLR was an independent predictor of survival in BC patients among those adjuvant treatment studies but not for but those early BC patients “receiving neo-adjuvant chemotherapy and advanced BC patients [14]. In our study, we did not find a significant correlation between the baseline NLR and PFS or OS. Therefore, we believe the baseline ALC should be considered a more specific predictor for patients treated with eribulin than the baseline NLR.
Nevertheless, changes in the NLR after eribulin initiation could be potential predictors of survival. Regarding PFS, NLR and its trend at 1 month after eribulin initiation was significantly associated with PFS in the Kaplan-Meier analysis. These findings are consistent with another study in which the authors found that a decrease in the NLR after one cycle was associated with improved PFS in patients with A/MBC treated with trastuzumab emtansine [21]. Other studies have revealed that the NLR after one or two chemotherapy cycles could be considered predictors for PFS among patients with advanced colorectal or urothelial cancer [36, 37]. In terms of OS, the dynamic changes in the NLR at 3 and 6 months after eribulin initiation were independent predictors of OS. This finding is consistent with a previous study that indicated an increasing trend in the NLR after six cycles of bevacizumab was linked to a higher mortality risk in patients with advanced non-small cell lung cancer [20]. In this case, we presume that the use of eribulin is associated with a long-term outcome like OS after sufficient follow-up (i.e. 3 months or 6 months after eribulin initiation), only if the effect of eribulin is stable. In contrast, Nakamoto et al. [6] revealed that the dynamic change in the NLR after one cycle or at the end of eribulin treatment among patients with A/MBC did not show a significant relationship with OS.
There are some limitations to this study. First, it was a retrospective single-centre study. As a result, the information used to determine the outcomes was heavily influenced by the local clinical practice. In particular, the definition of death is based on local practice; it includes those who were lost to follow-up and were deemed to be at high risk of dying at the CMUH. However, we applied this definition to all patient groups. Second, this study had a relatively small sample size and the findings might be not able to apply to those patients treated in the other hospitals. We were afraid to encounter type II error due to small sample size to make it unlikely to uncover significant relationships between prognostic factors and outcomes. In other words, the lack of a significant association from the multivariate analysis could be attributable to the small sample size. Further research to recruit more patients (conducting prospective cohort study) and to compare with the patients treated in different institutes or even in different countries might be helpful to confirm the robustness of the findings. Third, we do not know whether the changes in the ALC and NLR are particular predictors for eribulin because the associations obtained from the statistical analysis could not be interpreted as causality. Fourth, we cannot rule out that other factors might have interfered with the blood tests, such as acute infection and glucocorticoid usage, given we performed a retrospective study using electronic medical records. Future prospective research with a greater number of patients across times in different institutes might be able to address these aforementioned limitations.
In conclusion, we have shown that changes in the ALC and NLR following initial eribulin treatment could be potential predictors of PFS and OS in patients with A/MBC. Specifically, a high ALC at 1 month after eribulin initiation was significantly associated with better PFS, whereas a low ALC or a high NLR at 3 months after eribulin initiation or a non-decreasing NLR trend at 6 months were significantly associated with higher a mortality risk as compared with a high ALC, a low NLR or a decreasing NLR, respectively. Thus, the ALC and NLR, which can be measured relatively easily and inexpensively, at 1-, 3-, 6-month seem to be helpful to either identify individuals with A/MBC who could potentially benefit from eribulin, or to predict their responses and prognosis of cancer. Future studies should be conducted simultaneously at different medical institutes, use nationwide databases or perform a prospective study design to understand the impact of using the ALC and NLR across times as predictors of breast cancer survival outcomes.
PFS of patients with high and low ALC at the baseline (A), at the 1-month test (B), and its trends at 1-month test (as compared with baseline) (C); PFS of patients with high and low NLR at the baseline (D), at the 1-month test (E), and its trend at 1-month test (as compared with baseline) (F).
Supplementary Information
Additional file 1: Supplementary Figure 1. Overall survival (OS) among patients with increased or decreased trends of ALC, NLR (0: decreasing, 1: non-decreasing). Supplementary Figure 2. Comparing PFS and OS among different groups of patients stratified by ALC trends according to the baseline level. Supplementary Figure 3. Comparing PFS and OS among different groups of patients stratified by NLR baseline and trends. Supplementary Figure 4. Analysis on the association of high(1)/low(0) NLR/ALC with OS stratified by on(1)/off(0) treatment with eribulin.
Acknowledgements
We sincerely appreciate those who helped to complete this project, including Yu-Fen Chen and Lin-Chun Yang for assistance with the research project.
Abbreviations
- ALC
Absolute lymphocyte count
- NLR
Neutrophil-to-lymphocyte ratio
- A/MBC
Advanced or metastatic breast cancer
- OS
Overall survival
- EMT
Epithelial-to-mesenchymal transition
- HER2
Human epidermal growth factor receptor 2
- PFS
Progression-free survival
- eMRs
Electronic medical records
- CMUH
China Medical University Hospital
- IQR
Interquartile range
- ER
Oestrogen receptor
- PR
Progesterone receptor
- HR
Hazard ratio
- CI
Confidence interval
- IT
Information technology
- IRB
Institutional Review Board
Authors’ contributions
All authors were involved in the preparation of this manuscript. MXS, HWL, HTHN, LCL, CHH contributed to the study conception, design and comparisons. Material/literature preparation and data collection were performed by and MXS, HW L, TCL, CJC, HCW, CTW, YCW, GYH, CHH. Data analysis was performed by HWL, HTHN. The first draft of the manuscript was written by MXS, HWL, HTHN, LCL, CHH All listed authors reviewed the manuscript, contributed to, and approved the final manuscript.
Funding
This research was partially supported by the National Science and Technology Counsil, Taiwan (NSTC 112-2320-B-039-048; MOST 109-2320-B-039-023); China Medical University (CMU) (CMU110-Z-07; CMU109-MF-40; CMU109-Z-07); and China Medical University Hospital (DMR-110-079; DMR-110-080) in the course of project preparation and implementation. These funding agencies played no role in the study implementation, the analysis or interpretation of data, or preparation, and review and approval of the manuscript.
Availability of data and materials
The detailed patient databases generated and analyzed during this study are not publicly available due to appropriate protection of patient personal information but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This is a retrospective data analysis study. The written informed consent has been waived and the study protocol and implementation procedure were approved by the Research Ethics Committee (Institutional Review Board [IRB] of China Medical University Hospital (CMUH): CMUH109-REC2-122). Accordingly, all procedures involving human participants were performed in accordance with the ethical standards of the institutional research committee, the 1964 Declaration of Helsinki, Good Clinical Practices guideline, governmental laws and regulations, and its later amendments or comparable ethical standards. Importantly, this study meets the criteria of waving the inform consent because we used “de-identified” data, which were retrospectively collected and processed by the information technology (IT) center through formal application process in this specific hospital upon the request of the available reginal IRB. Specifically, the used data cannot be identified for the personal information after being processed by the IT center. In Taiwan, it is emphasized that “ as for the personal data regarding medical records, medical treatment, genetics, sexual life, health examinations and criminal records, which were prepared or collected by public agencies or medical/academic research institutes for medical, health or crime prevention purposes, only the data meet the following criteria can be used to fulfill the necessity of analysis purpose or academic research: data cannot be identified for the personal information after being processed by the providers or which were collected through deidentify the personal information based on the method of disclosure.” Thus, the aforementioned data can be used without having inform consents from patients or individuals, upon the “Personal Data Protection Law” announced by the Committee of Personal Data Protection, Executive Yuan, Taiwan, and approved by CMUH IRB accordingly.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meng-Xia Su, Hsiang-Wen Lin and Hanh T.H. Nguyen contributed equally to this work and share first authorship.
Liang-Chih Liu and Chih-Hao Huang contributed equally to this work and share corresponding authorship.
Contributor Information
Liang-Chih Liu, Email: dr0363@yahoo.com.tw.
Chih-Hao Huang, Email: 022651@tool.caaumed.org.tw.
References
- 1.Mayer EL, Burstein HJ. Chemotherapy for metastatic breast cancer. Hematol Oncol Clin North Am. 2007;21:257–72. doi: 10.1016/j.hoc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Cortes J, O'Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet (London, England) 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 3.Miyoshi Y, Yoshimura Y, Saito K, et al. High absolute lymphocyte counts are associated with longer overall survival in patients with metastatic breast cancer treated with eribulin-but not with treatment of physician's choice-in the EMBRACE study. Br. Cancer. 2020;27:706–715. doi: 10.1007/s12282-020-01067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sata A, Fukui R, Miyagawa Y, et al. C-Reactive Protein and Absolute Lymphocyte Count Can Predict Overall Survival of Patients Treated With Eribulin. Anticancer Res. 2020;40:4147–4156. doi: 10.21873/anticanres.14414. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe J, Saito M, Horimoto Y, et al. A maintained absolute lymphocyte count predicts the overall survival benefit from eribulin therapy, including eribulin re-administration, in HER2-negative advanced breast cancer patients: a single-institutional experience. Breast Cancer Res Treat. 2020;181:211–220. doi: 10.1007/s10549-020-05626-1. [DOI] [PubMed] [Google Scholar]
- 6.Nakamoto S, Ikeda M, Kubo S, et al. Dynamic changes in absolute lymphocyte counts during eribulin therapy are associated with survival benefit. Anticancer Res. 2021;41:3109–3119. doi: 10.21873/anticanres.15095. [DOI] [PubMed] [Google Scholar]
- 7.Ueno A, Maeda R, Kin T, et al. Utility of the absolute lymphocyte count and neutrophil/lymphocyte ratio for predicting survival in patients with metastatic breast cancer on eribulin: a real-world observational study. Chemotherapy. 2019;64:259–269. doi: 10.1159/000507043. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi M, Inoue K, Mukai H, et al. Indices of peripheral leukocytes predict longer overall survival in breast cancer patients on eribulin in Japan. Br Cancer (Tokyo, Japan) 2021;28:945–955. doi: 10.1007/s12282-021-01232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araki K, Ito Y, Fukada I, et al. Predictive impact of absolute lymphocyte counts for progression-free survival in human epidermal growth factor receptor 2-positive advanced breast cancer treated with pertuzumab and trastuzumab plus eribulin or nab-paclitaxel. BMC Cancer. 2018;18:982. doi: 10.1186/s12885-018-4888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou P, Yang E, Li Z. Neutrophil-to-lymphocyte ratio is an independent predictor for survival outcomes in cervical cancer: a systematic review and meta-analysis. Sci Rep. 2020;10:21917–21917. doi: 10.1038/s41598-020-79071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: a systematic review and meta-analysis. Cancer Med. 2021;10:5983–5997. doi: 10.1002/cam4.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbeau I, Jacot W, Guiu S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in breast cancer patients: a systematic review. Cancers. 2020;12:958. doi: 10.3390/cancers12040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Huang W, Wu Y, et al. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: a systematic review and meta-analysis. Cancer Cell Int. 2020;20:15. doi: 10.1186/s12935-020-1094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang M, Gao X, Sun H, et al. Neutrophil-Lymphocyte ratio as a prognostic parameter in NSCLC patients receiving EGFR-TKIs: a systematic review and meta-analysis. J Oncol. 2021;2021:6688346–6688346. doi: 10.1155/2021/6688346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamoto S, Ikeda M, Kubo S, et al. Systemic immunity markers associated with lymphocytes predict the survival benefit from paclitaxel plus bevacizumab in HER2 negative advanced breast cancer. Sci Rep. 2021;11:6328. doi: 10.1038/s41598-021-85948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto W, Kashiwagi S, Asano Y, et al. Eribulin promotes antitumor immune responses in patients with locally advanced or metastatic breast cancer. Anticancer Res. 2018;38:2929–2938. doi: 10.21873/anticanres.12541. [DOI] [PubMed] [Google Scholar]
- 19.Miyagawa Y, Araki K, Bun A, et al. Significant association between low baseline neutrophil-to-lymphocyte ratio and improved progression-free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab-paclitaxel. Clin Breast Cancer. 2018;18:400–409. doi: 10.1016/j.clbc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Wang S, Li C, et al. The kinetic changes of systemic inflammatory factors during bevacizumab treatment and its prognostic role in advanced non-small cell lung cancer patients. J Cancer. 2019;10:5082–5089. doi: 10.7150/jca.30478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imamura M, Morimoto T, Egawa C, et al. Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with HER2-positive breast cancer treated with trastuzumab emtansine. Sci Rep. 2019;9:1811. doi: 10.1038/s41598-018-37633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afghahi A, Purington N, Han SS, et al. Higher absolute lymphocyte counts predict lower mortality from early-stage triple-negative breast cancer. Clin Cancer Res. 2018;24:2851–2858. doi: 10.1158/1078-0432.CCR-17-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Che YQ, Zhang Y, Wang D, et al. Baseline lymphopenia: a predictor of poor outcomes in HER2 positive metastatic breast cancer treated with trastuzumab. Drug Des Devel Ther. 2019;13:3727–3734. doi: 10.2147/DDDT.S212610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T, Ozawa Y, Kimura T, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer. 2014;110:1497–505. doi: 10.1038/bjc.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Funahashi Y, Okamoto K, Adachi Y, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014;105:1334–42. doi: 10.1111/cas.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno A, Maeda R, Kim K, et al. Impact of absolute lymphocyte count on the efficacy of eribulin and capecitabine in patients with metastatic breast cancer. JCO. 2020;38:e13082. doi: 10.1200/JCO.2020.38.15_suppl.e13082. [DOI] [Google Scholar]
- 28.Myojin M, Horimoto Y, Ito M, et al. Neutrophil-to-lymphocyte ratio and histological type might predict clinical responses to eriburin-based treatment in patients with metastatic breast cancer. Br Cancer. 2020;27:732–738. doi: 10.1007/s12282-020-01069-0. [DOI] [PubMed] [Google Scholar]
- 29.Yoichi Koyama SK, Natsuki Uenaka, Miki Okazaki, et al. Absolute Lymphocyte Count, Platelet-to-Lymphocyte Ratio, and Overall Survival in Eribulin-treated HER2-negative Metastatic Breast Cancer Patients. CDP. 2021;1(5): 435-441. [DOI] [PMC free article] [PubMed]
- 30.Pirozzolo G, Gisbertz SS, Castoro C, et al. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac Dis. 2019;11:3136–3145. doi: 10.21037/jtd.2019.07.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao Y, Wu B, Jia W, et al. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in renal cell carcinoma: a systematic review and meta-analysis. BMC Urol. 2020;20:90. doi: 10.1186/s12894-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tham T, Bardash Y, Herman SW, et al. Neutrophil-to-lymphocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Head Neck. 2018;40:2546–2557. doi: 10.1002/hed.25324. [DOI] [PubMed] [Google Scholar]
- 33.Suh J, Jung JH, Jeong CW, et al. Clinical significance of pre-treated neutrophil-lymphocyte ratio in the management of urothelial carcinoma: a systemic review and meta-analysis. Front Oncol. 2019;9:1365. doi: 10.3389/fonc.2019.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ethier J-L, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. BCR. 2017;19:2–2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Sanctis R, Agostinetto E, Masci G, et al. Predictive factors of Eribulin activity in metastatic breast cancer patients. Oncol. 2018;94(Suppl 1):19–28. doi: 10.1159/000489065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chua W, Charles KA, Baracos VE, et al. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288–1295. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi L, Santoni M, Crabb SJ, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol. 2015;22:1377–84. doi: 10.1245/s10434-014-4097-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure 1. Overall survival (OS) among patients with increased or decreased trends of ALC, NLR (0: decreasing, 1: non-decreasing). Supplementary Figure 2. Comparing PFS and OS among different groups of patients stratified by ALC trends according to the baseline level. Supplementary Figure 3. Comparing PFS and OS among different groups of patients stratified by NLR baseline and trends. Supplementary Figure 4. Analysis on the association of high(1)/low(0) NLR/ALC with OS stratified by on(1)/off(0) treatment with eribulin.
Data Availability Statement
The detailed patient databases generated and analyzed during this study are not publicly available due to appropriate protection of patient personal information but are available from the corresponding author on reasonable request.