Abstract
A prominent feature of Lyme disease is the perivascular accumulation of mononuclear leukocytes. Incubation of human umbilical vein endothelial cells (HUVEC) cultured on amniotic tissue with either interleukin-1 (IL-1) or Borrelia burgdorferi, the spirochetal agent of Lyme disease, increased the rate at which human monocytes migrated across the endothelial monolayers. Very late antigen 4 (VLA-4) and CD11/CD18 integrins mediated migration of monocytes across HUVEC exposed to either B. burgdorferi or IL-1 in similar manners. Neutralizing antibodies to the chemokine monocyte chemoattractant protein 1 (MCP-1) inhibited the migration of monocytes across unstimulated, IL-1-treated, or B. burgdorferi-stimulated HUVEC by 91% ± 3%, 65% ± 2%, or 25% ± 22%, respectively. Stimulation of HUVEC with B. burgdorferi also promoted a 6-fold ± 2-fold increase in the migration of human CD4+ T lymphocytes. Although MCP-1 played only a limited role in the migration of monocytes across B. burgdorferi-treated HUVEC, migration of CD4+ T lymphocytes across HUVEC exposed to spirochetes was highly dependent on this chemokine. The anti-inflammatory cytokine IL-10 reduced both migration of monocytes and endothelial production of MCP-1 in response to B. burgdorferi by approximately 50%, yet IL-10 inhibited neither migration nor secretion of MCP-1 when HUVEC were stimulated with IL-1. Our results suggest that activation of endothelium by B. burgdorferi may contribute to formation of the chronic inflammatory infiltrates associated with Lyme disease. The transendothelial migration of monocytes that is induced by B. burgdorferi is significantly less dependent on MCP-1 than is migration induced by IL-1. Selective inhibition by IL-10 further indicates that B. burgdorferi and IL-1 employ distinct mechanisms to activate endothelial cells.
Lyme disease is the most prevalent vector-borne illness in the United States (1). A prominent histopathologic feature of this disease is the presence of inflammatory infiltrates within infected tissues (15). During an inflammatory response, leukocytes leave the bloodstream and enter surrounding tissues by binding to and then traversing the endothelial cell monolayer that lines the blood vessel wall. This transendothelial migration is dependent on the interactions of adhesion molecules on endothelium and leukocytes and on the production of chemoattractants (43), which include the chemotactic cytokines known as chemokines. Chemokines are subdivided into groups based on the positions of their conserved cysteine residues. CXC chemokines, which include the GRO proteins and interleukin-8 (IL-8), tend to be chemotactic for neutrophils, whereas CC chemokines, such as monocyte chemoattractant protein 1 (MCP-1), tend to attract monocytes and lymphocytes (2, 3). Recently, an integral membrane protein with a chemokine-like domain at its amino terminus has also been identified. This protein, termed fractalkine or neurotactin, is classified as a CX3C chemokine and is expressed on the surfaces of human umbilical vein endothelial cells (HUVEC) stimulated by IL-1 or tumor necrosis factor alpha (TNF-α) (6, 35).
Endothelial cells actively control the trafficking of leukocytes and are therefore important regulators of the inflammatory response. Treatment of endothelial cells with the proinflammatory cytokines IL-1 and TNF-α results in upregulation of the expression of adhesion molecules for leukocytes, including vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and E-selectin (43), and in increased production of several chemokines, including IL-8 and MCP-1 (2). As a result of this stimulation, the transendothelial migration of both neutrophils (17, 19, 26) and monocytes (31, 36) is enhanced. Likewise, the causative organism of Lyme disease, Borrelia burgdorferi, stimulates cultured endothelial cells to increase their expression of VCAM-1, ICAM-1, and E-selectin (8, 41) and to secrete IL-8 (9). As a consequence of these changes, neutrophils migrate across endothelial monolayers that have been exposed to B. burgdorferi (9, 41). B. burgdorferi spirochetes do not contain lipopolysaccharide (LPS) (46), a potent activator of endothelial cells (2). Instead, endothelial activation is mediated, at least in part, by the outer surface lipoproteins of B. burgdorferi (16, 40, 50). Although the phenotype of HUVEC treated with B. burgdorferi is quite similar to that of HUVEC treated with IL-1 or TNF-α, these host cytokines do not mediate activation of HUVEC by the spirochetes (9).
The effects of B. burgdorferi on the transendothelial migration of mononuclear leukocytes in vitro have not been studied, even though these cells are typically found in the chronic inflammatory lesions associated with Lyme disease (15). Herein we show that B. burgdorferi is as strong a stimulus as IL-1 in terms of promoting the transendothelial migration of monocytes. However, IL-1 and B. burgdorferi induce this migration through different mechanisms.
MATERIALS AND METHODS
Antibodies and recombinant proteins.
Monoclonal antibody (MAb) HP1/2, immunoglobulin (Ig) type IgG1, directed against very late antigen 4 (VLA-4) (38), was provided by Roy R. Lobb (Biogen Inc., Cambridge, Mass.). MAb TS1/18 (IgG1) (39), directed against CD18, was provided by Richard T. Coughlin (Cambridge Biotech, Worcester, Mass.). Neutralizing MAbs (IgG1) to human MCP-1 were purchased from R&D Systems (Minneapolis, Minn.) and Anogen (Mississauga, Ontario, Canada). MAb MOPC-21 (IgG1), obtained from Sigma Chemical Co. (St. Louis, Mo.), was used as a control. Recombinant human IL-1β was supplied by Collaborative Biomedical Products (Bedford, Mass.). Recombinant human IL-4 and IL-10 were obtained from R&D Systems.
Culture of spirochetes.
B. burgdorferi HBD1, originally isolated from human blood (7), was cultured at 33°C in serum-free Barbour-Stoenner-Kelly medium modified to minimize the content of LPS (41). HBD1 spirochetes (passages 40 to 53) were used in all experiments unless noted otherwise. B. burgdorferi N40 (5), isolated from heart tissue of infected mice (12) and passaged one to three times in vitro, was used in some experiments. Spirochetes were harvested during late-log-phase growth, centrifuged, and resuspended in medium 199 (M199; Life Technologies Inc., Grand Island, N.Y.) containing 20% heat-inactivated (heated for 30 min at 56°C) fetal bovine serum (HIFBS; HyClone Laboratories, Logan, Utah) and, in conditioned media and enzyme-linked immunosorbent assay (ELISA) experiments, 25 mM HEPES (pH 7.2). To control for the introduction of exogenous LPS, a sham preparation was made by subjecting a volume of uninoculated growth medium equal to the largest volume of spirochete culture used in each experiment to the same manipulations as the spirochetes themselves.
Isolation and culture of cells.
Endothelial cells were isolated from human umbilical veins by collagenase perfusion and were maintained in M199–20% FBS supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 μg of amphotericin B per ml at 37°C (21, 41). After 3 to 5 days, cells from confluent cultures were trypsinized, pooled, and passaged onto tissue culture plates or acellular connective tissue substrates prepared from human amnion (18).
Leukocytes were isolated from the venous blood of healthy adults by dextran sedimentation followed by density gradient centrifugation. Monocytes, isolated by using a hyperosmotic medium (Accudenz; Accurate Chemical Co., Westbury, N.Y.) as previously described (31), were greater than 90% pure, as determined by their ability to phagocytose latex beads. CD4+ T cells, purified using Accu-Prep lymphocytes (Accurate) followed by positive selection with Dynabeads (product M-450 CD4; Dynal, Lake Success, N.Y.) were at least 98% pure, as determined by fluorescence-activated cell sorting (FACS) analysis using fluorescently labeled MAbs against both CD4 and CD3 (Simultest CD3/CD4; Becton Dickinson, San Jose, Calif.). Magnetic beads were removed from the lymphocytes prior to migration assays by using DETACHaBEAD (Dynal).
Immunofluorescence detection of B. burgdorferi.
B. burgdorferi spirochetes were incubated with HUVEC-amnion cultures for 8 h. Cultures were then fixed in 10% buffered formalin for 1 h, washed in a large volume of phosphate-buffered saline (PBS) overnight, and incubated with 50 μg of fluorescein-labeled, affinity-purified, anti-B. burgdorferi polyclonal antibody (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) per ml in PBS for 2 h at 37°C in the dark. Tissues were washed in PBS and prepared for fluorescence microscopy by mounting in a mixture of 90% glycerol–10% PBS containing 1 mg of 1,4-phenylenediamine (Aldrich, Milwaukee, Wis.) per ml. The number of spirochetes associated with each HUVEC-amnion culture was determined in five randomly selected 400× fields. The number of spirochetes beneath the endothelial cells was assessed by counting all spirochetes in focal planes underneath that of the endothelial monolayer.
Quantitation of MCP-1.
HUVEC plated at 2 × 105 cells per well in 24-well tissue culture plates were grown to confluence and incubated at 37°C with 1.0 ml of M199–20% HIFBS–25 mM HEPES (pH 7.2) or test preparations for various times. Conditioned media were collected and centrifuged at 20,000 × g for 30 min. Amounts of MCP-1 in supernatants were measured by using a commercial ELISA kit (Anogen).
Chemotaxis assay.
Conditioned media were collected from HUVEC (2 × 105 cells per well in 24-well tissue culture plates) incubated for 24 h at 37°C with 1 ml of M199–20% HIFBS–25 mM HEPES (pH 7.2) containing 5 U of IL-1β per ml or B. burgdorferi at a ratio of 10 spirochetes per endothelial cell (Bb/EC). Conditioned media were diluted 10-fold in M199–25 mM HEPES (pH 7.2) and tested for the ability to induce chemotaxis of monocytes, in the presence or absence of MAbs, in leading-front Boyden chamber assays as previously described (36).
Leukocyte transendothelial migration assay.
HUVEC were plated at a density of 1.5 × 105 cells per cm2 on amniotic tissue and cultured for 7 to 10 days (21). Confluent monolayers were washed, incubated with M199–20% HIFBS or various test preparations for 8 h at 37°C, and again washed. Monocytes (2 × 105 cells per cm2) or CD4+ T cells (6.5 × 105 to 1 × 106 cells per cm2) were added to the HUVEC monolayers for 20 min, 1 h, or 2 h at 37°C. The cultures were then fixed in 10% buffered formalin, rinsed in saline, and stained with Wright’s stain. The total number of leukocytes associated with each tissue was determined by counting nine ×400 fields, using light microscopy to view whole mounts en face. The percentage of leukocytes that migrated beneath the endothelium compared to the percentage that were adherent to the apical side of the endothelium was assessed by analysis of sections cut perpendicularly to the plane of the HUVEC monolayer as previously described (36).
In some experiments, monocytes were preincubated for 30 min at 22°C with MAbs directed against adhesion molecules before addition to HUVEC cultures. These MAbs were used at concentrations previously determined to be saturating (31). In other experiments, antibody to MCP-1 was added both above and beneath HUVEC-amnion cultures, which were elevated on silicone rubber supports to allow better access of the antibody (36). To test the effect of IL-10 on activation of HUVEC by IL-1 or B. burgdorferi, HUVEC cultures were preincubated with 20 ng of IL-10 per ml for 1 h at 37°C. IL-10 was also present during the 8-h stimulation period.
After 2 h, monocytes migrated to the same extent across all endothelial monolayers regardless of treatment. Therefore, in experiments where monocytes were allowed to migrate for 2 h, activation of HUVEC was confirmed by including separate groups in which migration of monocytes was also assessed after only 20 min.
Monocyte exiting assay.
Exiting of monocytes from HUVEC-amnion cultures was determined essentially as previously described (37). In brief, HUVEC cultures were incubated for 8 h at 37°C with control media or various spirochete preparations. In some cases, spirochetes were then opsonized by incubating HUVEC cultures for 1 h at 37°C with high-titer anti-B. burgdorferi human serum diluted 1:2 in M199 (provided by Marc Golightly, State University of New York at Stony Brook). Cultures were then washed, and monocytes were added for 2 h to allow for maximal migration. Cultures were washed extensively to remove nonadherent monocytes and again incubated at 37°C for up to 96 h. The number of monocytes associated with each culture was then assessed as described above.
Statistics.
Data from all experimental groups were subjected to an unpaired analysis of variance with the Tukey-Kramer multiple-comparison test. For direct comparisons, the means ± standard deviations (SD) of experimental groups were subjected to either unpaired Student t tests or alternate Welch t tests to determine a two-tailed P value. P of <0.05 was used as the alpha value to determine statistical significance for all analyses. All stated inhibitions due to intervention with MAbs or IL-10 are statistically significant unless noted otherwise.
RESULTS
Monocytes migrate at an increased rate across endothelium stimulated with B. burgdorferi.
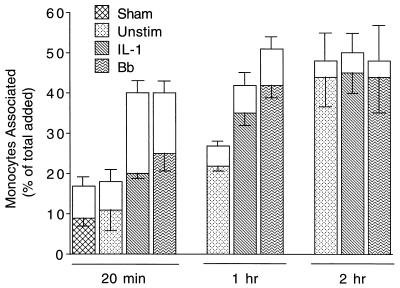
Approximately 40% of added human monocytes adhere to and migrate across unstimulated endothelial monolayers grown on amniotic connective tissue (31). Time course experiments were performed to determine if pretreatment of endothelial monolayers with B. burgdorferi spirochetes affected the rate or extent of association of monocytes. In these experiments, monocytes were added to HUVEC-amnion cultures that had been preincubated with control medium, IL-1, or B. burgdorferi for 8 h, a time that permits maximum stimulation of the transendothelial migration of neutrophils by spirochetes (41). At 20 min or 1 h following their addition, more monocytes adhered to and migrated across HUVEC cultures that had been stimulated with IL-1 or B. burgdorferi compared to unstimulated endothelial monolayers (Fig. 1). In contrast, pretreatment of HUVEC with a sham preparation lacking spirochetes increased neither adhesion nor migration. After 2 h, similar numbers of monocytes migrated across unstimulated HUVEC or HUVEC treated with IL-1 or B. burgdorferi (Fig. 1). Therefore, addition of B. burgdorferi to HUVEC resulted in an increase in the rate, but not the extent, of migration of monocytes.
FIG. 1.
Time course of the migration of monocytes across HUVEC stimulated with B. burgdorferi or IL-1. Monocytes were incubated for indicated times with HUVEC-amnion cultures that had been pretreated for 8 h with either control medium (Unstim), a sham preparation, 5 U of IL-1 per ml, or B. burgdorferi (Bb) at a ratio of 10 Bb/EC. Transendothelial migration was assessed as described in Materials and Methods. The total height of each bar represents the number of monocytes associated with each culture as a percentage of the total number added. The lower (patterned) portion of each bar represents the percentage that migrated beneath the endothelium; the upper (unfilled) portion represents the percentage adherent to the apical surface. Bars represent the means ± SD of three to four replicate samples. This experiment was repeated twice with similar results.
The association of monocytes with B. burgdorferi-stimulated HUVEC after 20 min of incubation was examined in 17 separate experiments. In all experiments, pretreatment with B. burgdorferi significantly increased the number of monocytes that migrated, whereas pretreatment with sham preparations was without effect. Migration across B. burgdorferi-stimulated HUVEC after 20 min was directly compared to migration across IL-1-stimulated HUVEC in 10 experiments. In six of these experiments, significantly more monocytes migrated across the B. burgdorferi-stimulated monolayers, whereas no difference was seen in the remaining four experiments. Monocytes did not migrate at an increased rate across HUVEC that had been pretreated with B. burgdorferi for only 4 h. When HUVEC were exposed to B. burgdorferi for 24 h, monocytes migrated at the same rate as they did across HUVEC that had been stimulated for 8 h (data not shown).
Immunofluorescence analysis indicated that even after extensive washing, up to 1% of the B. burgdorferi HBD1 spirochetes used in these experiments remained bound to HUVEC monolayers. Therefore, some spirochetes were still present during periods of monocyte migration. To establish whether spirochetes could directly activate monocytes and cause them to undergo transendothelial migration at an increased rate, monocytes were added together with B. burgdorferi (at a ratio of 10 Bb/EC) to previously unstimulated HUVEC cultures for 20 min. Monocytes coincubated with B. burgdorferi migrated across unstimulated HUVEC monolayers to the same extent as did control monocytes. By contrast, in the same experiment, monocytes migrated at an increased rate across HUVEC that had been preincubated for 8 h with B. burgdorferi (data not shown). B. burgdorferi thus exerts its effects on migration of monocytes through activation of HUVEC, rather than the leukocytes themselves.
B. burgdorferi does not affect the rate at which monocytes exit from endothelial-amnion cultures.
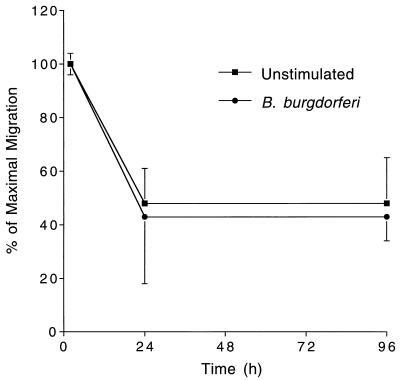
Many of the monocytes that cross HUVEC monolayers and enter the underlying amniotic tissue later exit the cultures by traversing the monolayers in the basal to apical direction. Pretreatment of endothelium with IL-1 increases the rate of this reverse transendothelial migration (37). To investigate whether B. burgdorferi affects the kinetics with which monocytes exit, experiments were performed in which HUVEC-amnion cultures were left untreated or were treated for 8 h with spirochetes. Monocytes were then added for 2 h to allow maximum numbers to migrate (31). Cultures were washed extensively to remove nonadherent monocytes and incubated with control medium for up to 96 h. Tissues were then analyzed to determine the extent of loss of monocytes. In four separate experiments, monocytes exited from cultures that had been stimulated with B. burgdorferi HBD1 at the same rate as they did from unstimulated cultures (data not shown). Immunofluorescence analysis showed that HBD1 spirochetes bound to HUVEC at low levels and did not migrate into the underlying amniotic tissue. In contrast, when cultures were incubated with B. burgdorferi N40 for 8 h at a ratio of 100 Bb/EC, at least 1 Bb/EC migrated beneath the HUVEC monolayers. However, the presence of N40 spirochetes within the amniotic tissue did not lead to increased retention of the monocytes (Fig. 2), even when the spirochetes were first opsonized with antibody (data not shown).
FIG. 2.
B. burgdorferi does not affect the rate at which monocytes exit from HUVEC-amnion cultures. HUVEC-amnion cultures were either left unstimulated or pretreated for 8 h with B. burgdorferi N40 at a ratio of 100 Bb/EC. Monocytes were then added to the cultures for 2 h. Cultures were washed and either fixed immediately or incubated for an additional 22 or 96 h. The number of monocytes remaining in the amniotic tissue was then determined as described in Materials and Methods. Data are presented as the percentage of monocytes that remained in the amniotic tissue relative to the number that had migrated after 2 h. Bars represent the means ± SD of four to five replicate samples. This experiment was repeated twice with similar results.
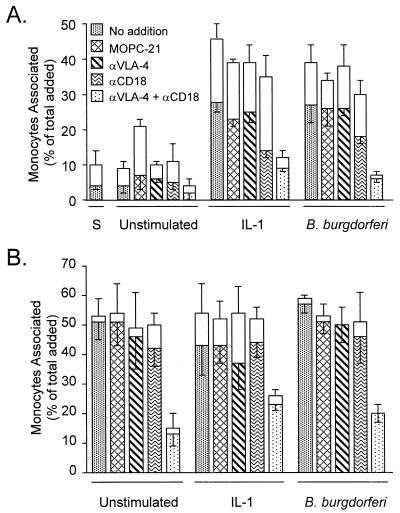
B. burgdorferi-stimulated transendothelial migration of monocytes is dependent on VLA-4 and CD11/CD18 integrins.
Monocytes use both CD11/CD18 and VLA-4 integrins to migrate across unstimulated or IL-1-stimulated HUVEC monolayers (11, 31). To ascertain whether monocytes employ these same adhesion molecules to cross B. burgdorferi-stimulated endothelial monolayers, HUVEC were left unstimulated or were pretreated with IL-1 or B. burgdorferi. Monocytes were then added to the cultures in the presence of no antibody, a control MAb, or anti-CD18 and anti-VLA-4 MAbs (used alone or in combination). As expected, when added for 20 min, more monocytes adhered to and migrated across both IL-1- and B. burgdorferi-stimulated HUVEC monolayers compared to unstimulated or sham-treated monolayers (Fig. 3A). MAb to VLA-4 alone had little effect on this migration, whereas MAb to CD18 partially inhibited migration across both IL-1-stimulated and B. burgdorferi-stimulated HUVEC (51 and 35%, respectively). When both VLA-4 and CD18 were blocked, more complete inhibition was observed: migration across IL-1- or B. burgdorferi-stimulated monolayers was decreased by 69 or 79%, respectively, in the experiment shown in Fig. 3A and by 88 or 89% in a replicate experiment. An isotype-matched control MAb had no effect.
FIG. 3.
VLA-4 and CD11/CD18 integrins mediate the migration of monocytes across endothelium stimulated with B. burgdorferi or IL-1. Monocytes were suspended in medium containing either no addition, 50 μg of isotype-matched control MAb (MOPC-21) per ml, 10 μg of HP1/2 (anti-VLA-4) per ml, 40 μg of TS1/18 (anti-CD18) per ml, or 10 μg of HP1/2 plus 40 μg of TS1/18 per ml. The monocytes were then incubated for 20 min (A) or 2 h (B) with HUVEC that were either unstimulated or pretreated with a sham preparation (S), 5 U of IL-1 per ml, or B. burgdorferi at a ratio of 10 Bb/EC. The legend to panel A also applies to panel B. Transendothelial migration was assessed as described in Materials and Methods. The total height of each bar represents the number of monocytes associated with each culture as a percentage of the total number added. The lower (patterned) portion of each bar represents the percentage that migrated beneath the endothelium; the upper (unfilled) portion represents the percentage adherent to the apical surface. Bars represent the means ± SD of three to four replicate samples.
When monocytes were added for 2 h to allow maximal migration, MAbs to either CD18 or VLA-4 alone did not significantly inhibit migration across unstimulated or stimulated HUVEC monolayers (Fig. 3B). In contrast, when both adhesion molecules were blocked, migration across unstimulated, IL-1-treated, or B. burgdorferi-stimulated monolayers was reduced by 75, 46, or 65%, respectively, in the experiment shown and by 80, 37, or 62%, respectively, in a replicate experiment.
Endothelial cells secrete increased amounts of MCP-1 in response to B. burgdorferi.
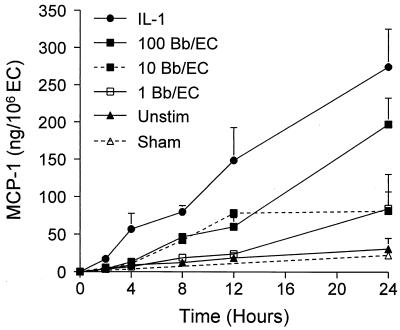
Unstimulated endothelium secreted only low levels of MCP-1, but production was markedly enhanced by either IL-1 or B. burgdorferi (Fig. 4). IL-1 was a stronger stimulus than were spirochetes. At all time points tested, HUVEC treated with IL-1 produced significantly more MCP-1 than did unstimulated cultures. In contrast, secretion in response to 10 or 100 Bb/EC was significantly above that of unstimulated HUVEC only after 8 h of coculture. The amounts of MCP-1 in conditioned media collected from HUVEC that had been incubated for 8 h with control medium, IL-1, or B. burgdorferi were measured in eight separate experiments, with variable results. Secretion of MCP-1 by unstimulated HUVEC ranged from 3 to 35 ng per 106 EC and averaged 11 ng per 106 EC. Stimulation with IL-1 increased production of MCP-1 11-fold ± 3.8-fold, averaging 90 ng per 106 EC and ranging from 42 to 178 ng per 106 EC. Coculture with 10 Bb/EC resulted in a 3.7-fold ± 1.8-fold increase, averaging 38 ng per 106 EC and ranging from 6 to 96 ng per 106 EC. HUVEC incubated with a sham preparation lacking spirochetes did not secrete any more MCP-1 than did unstimulated endothelium.
FIG. 4.
Time- and dose-dependent production of MCP-1 by HUVEC in response to B. burgdorferi or IL-1. Conditioned media were collected from HUVEC incubated with medium alone (Unstim), sham preparations, 5 U of IL-1 per ml, or B. burgdorferi at a ratio of 1, 10 or 100 Bb/EC for the indicated times. Amounts of MCP-1 were measured by ELISA. Datum points represent the means ± SD of three replicate samples. This experiment was repeated once with similar results.
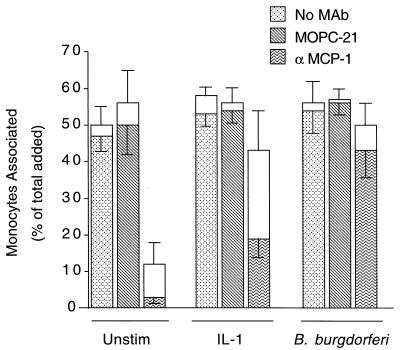
MCP-1 plays a limited role in the migration of monocytes across endothelium exposed to B. burgdorferi.
The migration of monocytes across unstimulated HUVEC is nearly completely dependent on MCP-1, whereas IL-1-stimulated migration only partially depends on this cytokine (36). The role of MCP-1 in B. burgdorferi-stimulated migration was investigated by incubating HUVEC with control medium, IL-1, or B. burgdorferi for 8 h in the presence of no antibody, an isotype-matched control MAb, or a neutralizing MAb against MCP-1. Monocytes were then added to the cultures for 2 h, either alone or with the appropriate MAb. With no antibody or control MAb, monocytes migrated to the same extent across all HUVEC cultures, regardless of the stimulus. When the anti-MCP-1 MAb was added, migration across unstimulated HUVEC was inhibited by 93% ± 3%, whereas migration across IL-1-treated HUVEC was decreased by 64% ± 9%. In contrast, migration across B. burgdorferi-stimulated monolayers was inhibited by only 21% ± 13%, an amount that was not statistically significant (Fig. 5). Similar results were also obtained when a different neutralizing anti-MCP-1 MAb was used. In three separate experiments, anti-MCP-1 MAbs inhibited migration across HUVEC cultures that were left unstimulated or were stimulated with IL-1 or B. burgdorferi by averages of 91% ± 3%, 65% ± 2%, and 25% ± 22%, respectively (Table 1). Migration across B. burgdorferi-stimulated monolayers was decreased significantly in only one experiment. In all experiments, migration across IL-1-stimulated HUVEC was inhibited by the MAbs to MCP-1 to a greater extent than was migration across HUVEC exposed to B. burgdorferi. Therefore, MCP-1 contributed less to migration of monocytes across B. burgdorferi-stimulated HUVEC compared to HUVEC that were unstimulated or treated with IL-1.
FIG. 5.
MCP-1 is not the major chemoattractant mediating the migration of monocytes across B. burgdorferi-stimulated HUVEC. HUVEC cultures, elevated on silicone supports, were incubated for 8 h with either control medium (Unstim), 5 U of IL-1 per ml, or B. burgdorferi at a ratio of 10 Bb/EC in the presence of no MAb, 20 μg of control MAb (MOPC-21) per ml, or 20 μg of neutralizing anti-MCP-1 MAb per ml. Subsequently added monocytes, resuspended in fresh media containing the same MAbs, were incubated with the cultures for 2 h, and transendothelial migration was assessed as described in Materials and Methods. The total height of each bar represents the number of monocytes associated with each culture as a percentage of the total number added. The lower portion of each bar represents the percentage that migrated beneath the endothelium; the upper portion represents the percentage adherent to the apical surface. Bars represent the means ± SD of three replicate samples.
TABLE 1.
Effect of MAb to MCP-1 on migration of monocytes across HUVEC stimulated with IL-1 or B. burgdorferi
| Expt | Stimulus | % Inhibition by anti-MCP-1a | P value |
|---|---|---|---|
| 1 | None | 88 ± 5 | 0.0095 |
| IL-1 | 63 ± 16 | 0.0073 | |
| B. burgdorferi | 5 ± 15 | NSb | |
| 2 | None | 93 ± 3 | <0.0001 |
| IL-1 | 64 ± 9 | 0.004 | |
| B. burgdorferi | 21 ± 13 | NS | |
| 3 | None | 92 ± 2 | <0.0001 |
| IL-1 | 67 ± 6 | 0.0004 | |
| B. burgdorferi | 49 ± 8 | 0.0043 |
Mean ± SD of three replicate samples.
NS, not significant.
We confirmed that we were using saturating amounts of MAb to MCP-1 by two methods. First, increasing the concentration of MAb resulted in no further inhibition of migration (data not shown). Second, when culture media were collected after experiments, no MCP-1 could be detected in samples that contained the anti-MCP-1 MAb by a commercial MCP-1 ELISA which used, as one of the detection antibodies, the same MAb included in the transmigration experiments. The ability of the neutralizing MAb to mask detection of MCP-1 by ELISA indicates that the MAb had indeed bound all available MCP-1.
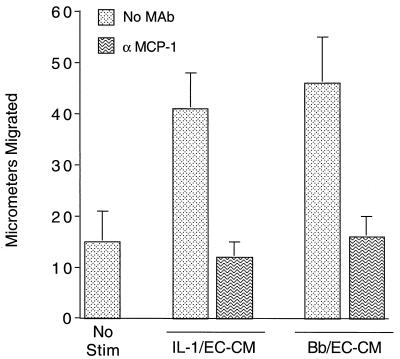
These transmigration experiments indicated that a factor(s) other than MCP-1 plays a role in the migration of monocytes across both IL-1- and B. burgdorferi-stimulated HUVEC. Compared to unconditioned medium, conditioned media collected from IL-1-treated or B. burgdorferi-stimulated HUVEC promoted the migration of monocytes into cellulose nitrate filters in Boyden chamber assays. When a neutralizing anti-MCP-1 MAb was added, this stimulation of migration was completely blocked (Fig. 6). In a replicate experiment, the anti-MCP-1 MAb blocked the chemotaxis of monocytes in response to the same B. burgdorferi-HUVEC conditioned medium by 72%, whereas an isotype-matched control MAb (MOPC-21) had no effect. Thus, despite its limited role in migration of monocytes across HUVEC exposed to B. burgdorferi, MCP-1 is the major soluble chemoattractant for monocytes produced by endothelium in response to spirochetes.
FIG. 6.
MCP-1 is the major soluble chemoattractant for monocytes produced by HUVEC in response to both IL-1 and B. burgdorferi. Conditioned media were collected from HUVEC incubated with either 5 U of IL-1 per ml (IL-1/EC-CM) or B. burgdorferi at a ratio of 10 Bb/EC (Bb/EC-CM) for 24 h. IL-1/EC-CM and Bb/EC-CM were diluted 1:10 in M199 and were either left untreated (No MAb) or incubated with 10 μg of neutralizing anti-MCP-1 MAb per ml. Conditioned media or control medium (No Stim) were tested for chemotactic activity toward monocytes in Boyden chambers. Bars represent the means ± SD of three replicate samples.
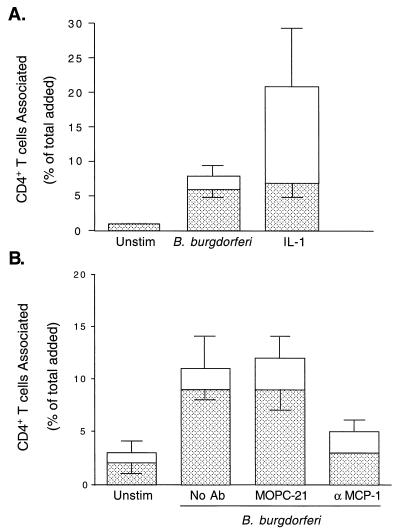
B. burgdorferi promotes the transendothelial migration of CD4+ T lymphocytes in an MCP-1-dependent manner.
To investigate whether B. burgdorferi promotes the transendothelial migration of CD4+ T cells, HUVEC were either left unstimulated or stimulated with IL-1 or B. burgdorferi for 8 h. Human peripheral blood CD4+ T cells were then added to the cultures for 2 h. Less than 1% of the CD4+ T cells migrated across unstimulated endothelium, whereas 10-fold more migrated across HUVEC treated with B. burgdorferi. Although more T cells adhered to IL-1-stimulated HUVEC, the numbers of cells that migrated across B. burgdorferi- and IL-1-stimulated endothelium did not differ significantly (Fig. 7A). In four experiments, treatment of HUVEC with B. burgdorferi resulted in a 6-fold ± 2-fold increase in the migration of CD4+ T cells. In contrast, coincubation of CD4+ T cells with B. burgdorferi during migration did not significantly increase their ability to penetrate unstimulated HUVEC monolayers (data not shown). MAb to MCP-1 inhibited migration of the T cells in response to B. burgdorferi by 68%, whereas a control MAb had no effect (Fig. 7B). A similar level of inhibition was obtained in a replicate experiment in which a different neutralizing MAb against MCP-1 was used. B. burgdorferi-induced migration of CD4+ T cells is thus largely dependent on MCP-1.
FIG. 7.
CD4+ T cells migrate across B. burgdorferi-stimulated HUVEC in an MCP-1-dependent manner. CD4+ T cells were incubated for 2 h with HUVEC-amnion cultures that had been pretreated with either control medium (Unstim), 5 U of IL-1 per ml, or B. burgdorferi at a ratio of 10 Bb/EC (A). CD4+ T cells were incubated for 2 h with HUVEC-amnion cultures that had been pretreated with either control medium (Unstim) or B. burgdorferi at a ratio of 10 Bb/EC in the presence of no MAb, an isotype-matched control MAb (MOPC-21), or 20 μg of neutralizing anti-MCP-1 MAb per ml (B). Transendothelial migration was assessed as described in Materials and Methods. The total height of each bar represents the number of T cells associated with each culture as a percentage of the total number added. The lower portion of each bar represents the percentage that migrated beneath the endothelium; the upper portion represents the percentage adherent to the apical surface. Bars represent the means ± SD of four to five replicate samples.
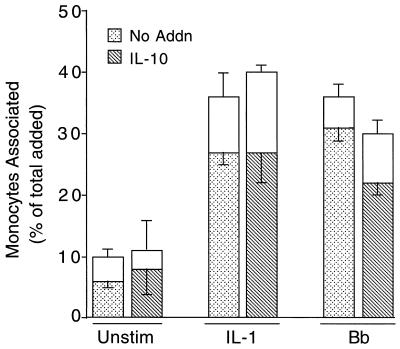
IL-10 inhibits the migration of monocytes across endothelium stimulated with B. burgdorferi but not with IL-1.
In mice, T helper (Th) type 2 (Th2) cells and their associated cytokines, which include IL-4 and IL-10, provide protection from the symptoms of Lyme disease (23, 24, 30). Human recombinant IL-4 was toxic to HUVEC at concentrations as low as 0.2 ng/ml and therefore was not tested further. Human recombinant IL-10 had no adverse effects on HUVEC or on the growth or motility of spirochetes (data not shown). To determine if IL-10 affected the transendothelial migration of monocytes, HUVEC were incubated with control medium, IL-1, or B. burgdorferi for 8 h in the presence or absence of 20 ng of IL-10 per ml. Monocytes were then added to the cultures for 20 min. IL-10 did not inhibit migration of monocytes across unstimulated HUVEC or HUVEC treated with IL-1. In contrast, IL-10 decreased the number of monocytes that migrated (above the basal, unstimulated level) across HUVEC stimulated with B. burgdorferi by 38% (Fig. 8; P = 0.0007). In three additional experiments, IL-10 reduced B. burgdorferi-induced migration of monocytes by 62% (P = 0.0008), 63% (P = 0.0004), and 51% (P < 0.0001), for an average of 54% ± 12%. In a dose-response experiment, IL-10 at concentrations of 20, 2, or 0.2 ng/ml suppressed B. burgdorferi-stimulated migration by 51, 49, or 28%, respectively. Pretreatment of monocytes with 20 ng of IL-10 per ml for 1 h had no effect on their migration across either unstimulated, IL-1-treated, or B. burgdorferi-stimulated endothelial monolayers (data not shown). IL-10 therefore modulates B. burgdorferi-stimulated migration by acting not on monocytes but on the endothelium.
FIG. 8.
IL-10 inhibits the migration of monocytes across HUVEC stimulated by B. burgdorferi but not IL-1. Monocytes were added for 20 min to HUVEC-amnion cultures that had been pretreated for 8 h with control medium (Unstim), 5 U of IL-1 per ml, or B. burgdorferi at a ratio of 10 Bb/EC in the absence (No Addn) or presence of 20 ng of IL-10 per ml. Transendothelial migration was assessed as described in Materials and Methods. The total height of each bar represents the number of monocytes associated with each culture as a percentage of the total added. The lower portion of each bar represents the percentage that migrated beneath the endothelium; the upper portion represents the percentage adherent to the apical surface. Bars represent the means ± SD of three to five replicate samples. This experiment yielded similar results when repeated once using both IL-1- and B. burgdorferi-stimulated samples and three additional times with B. burgdorferi-treated samples only.
In two separate experiments, IL-10 did not lessen the amount of MCP-1 produced by unstimulated or IL-1-stimulated HUVEC. In contrast, in three experiments, IL-10 at 20 ng/ml decreased B. burgdorferi-stimulated production of MCP-1 by 55% ± 11%. A similar level of inhibition occurred when 10-fold-less IL-10 was used. A representative experiment is shown in Table 2. The effects of IL-10 on both migration of monocytes and secretion of MCP-1 indicate that this cytokine inhibits activation of HUVEC by B. burgdorferi but not by IL-1.
TABLE 2.
Effect of IL-10 on production of MCP-1 by HUVEC
| Stimulusa | MCP-1 produced (ng/106 EC)
|
||
|---|---|---|---|
| No IL-10 | +IL-10 (2 ng/ml) | +IL-10 (20 ng/ml) | |
| None | 9.5 ± 1.2 | NDb | 12.6 ± 0.7c |
| IL-1 (0.1 U/ml) | 52.9 ± 6.3 | ND | 59.3 ± 1.8 |
| IL-1 (5 U/ml) | 100.9 ± 6.2 | ND | 101.8 ± 1.4 |
| B. burgdorferi | 32.7 ± 1.2 | 23.8 ± 3.1d | 22.7 ± 0.7e |
HUVEC were incubated with IL-1 or B. burgdorferi at a ratio of 10 Bb/EC for 8 h with or without addition of IL-10. MCP-1 was measured by ELISA.
ND, not determined.
Significantly greater than without IL-10; P = 0.02.
38% inhibition of B. burgdorferi-stimulated increase; P < 0.01.
43% inhibition of B. burgdorferi-stimulated increase; P < 0.0003.
DISCUSSION
Previously, it has been shown that peripheral blood monocytes adhere to and migrate across unstimulated HUVEC monolayers grown on amniotic tissue (31). In this study, we found that treatment of HUVEC monolayers with B. burgdorferi increased the rate of transmigration of monocytes, whereas the number of monocytes that eventually migrated was not affected. Stimulation of HUVEC with B. burgdorferi also increased the number of CD4+ T lymphocytes that migrated. B. burgdorferi was as strong a stimulus as IL-1 in terms of promoting the transendothelial migration of both monocytes and CD4+ T cells. Since exposure of leukocytes to spirochetes during migration was without effect, increases in migration were due to the actions of B. burgdorferi on endothelium. Significant extravascular accumulation of leukocytes, including monocytes/macrophages and lymphocytes, occurs in the early erythema migrans rash, neurologic lesions, and late skin lesions that can be associated with Lyme disease (15). Monocytes/macrophages and T lymphocytes, the majority of which are CD4+, are also found in the synovial lesions of patients with Lyme arthritis (44). Our data suggest that in vivo, activation of vascular endothelial cells by B. burgdorferi may contribute to the formation of the chronic inflammatory infiltrates associated with Lyme disease.
Accumulation of monocytes/macrophages at sites of inflammation may result from both increased recruitment from the circulation and decreased clearance from the extravascular tissues. In vitro, preincubation of HUVEC monolayers with B. burgdorferi for either 8 h or 24 h similarly enhanced the rate at which monocytes transmigrated. In contrast, maximal numbers of neutrophils migrate when HUVEC are pretreated with spirochetes for 8 h; very little migration occurs when cultures are exposed for 24 h (41). The ability of HUVEC pretreated with B. burgdorferi for 24 h to support maximal migration of monocytes is consistent with the idea that spirochetes may cause sustained recruitment of these leukocytes in vivo. We also used our HUVEC-amnion culture system to examine whether B. burgdorferi might decrease the rate at which extravasated monocytes/macrophages are cleared from sites of infection. In the HUVEC-amnion system, many monocytes that initially traverse the endothelium in the apical to basal direction later exit the cultures by crossing the monolayer again via the opposite route (37). This observation led to the hypothesis that such reverse migration might contribute to clearance of macrophages from chronic inflammatory lesions in vivo (37), an idea that has yet to be tested experimentally. Nonetheless, we reasoned that spirochetes might provide a proinflammatory signal that would increase retention of monocytes in HUVEC-amnion cultures. However, monocytes exited from unstimulated or spirochete-treated cultures with similar kinetics. Although the physiologic relevance of this result is not known, it is clear that neither spirochetes themselves nor endothelial factors induced by B. burgdorferi influence the rate at which monocytes leave the extravascular compartment of our vessel wall constructs.
Monocytes use two adhesion molecule pathways during migration across unstimulated or IL-1-treated cultured endothelial monolayers. CD11/CD18 integrins on monocytes interacting with ICAM-1 and other ligands on endothelial cells constitute one path, whereas VLA-4 on monocytes interacting with VCAM-1 and fibronectin on endothelial cells constitute the other. These are alternative pathways, since migration is not substantially inhibited unless both are blocked (11, 31, 32). Using MAbs to CD11/CD18 and VLA-4, we determined that the migration of monocytes across B. burgdorferi-stimulated HUVEC monolayers was also mediated by these integrins. When both CD11/CD18 and VLA-4 were blocked, the amounts of residual migration of monocytes across IL-1- and B. burgdorferi-stimulated monolayers were similar (Fig. 3). This result suggests that monocytes do not employ unique adhesion molecule pathways to cross endothelial monolayers activated by B. burgdorferi.
Although our results suggest that monocytes use the same adhesion molecules to cross both B. burgdorferi- and IL-1-stimulated endothelial monolayers, the utilization of chemoattractants is different. Whereas the movement of monocytes across unstimulated and IL-1-stimulated endothelial cell monolayers is mediated, in large part, by the chemokine MCP-1 (36), we found that their movement across HUVEC stimulated with B. burgdorferi was not. Neutralizing MAbs directed against MCP-1 inhibited migration across unstimulated and IL-1-treated HUVEC by averages of 91 and 65%, respectively, verifying the efficacy of the antibodies. In contrast, these MAbs decreased migration across B. burgdorferi-stimulated HUVEC by an average of only 25%. The limited role of MCP-1 in the migration of monocytes across HUVEC treated with spirochetes indicates that compared to IL-1, B. burgdorferi more strongly induces an additional chemoattractant for monocytes. However, MCP-1 was the major soluble chemoattractant for monocytes made by endothelium exposed to spirochetes. Therefore, the additional chemotactic factor (or factors) for monocytes produced by endothelial cells in response to B. burgdorferi and, to a lesser extent, IL-1 is likely immobilized on the HUVEC-amnion cultures. In contrast to monocytes, CD4+ T lymphocytes largely employed MCP-1 to cross B. burgdorferi-stimulated HUVEC. Thus, the bound factor produced by endothelium in response to spirochetes is probably not a strong attractant for CD4+ T cells. The identity of this factor is unknown. It may be a chemokine bound to the surface of endothelial cells through interactions with glycosaminoglycans (49), or perhaps a transmembrane protein accounts for the activity. One possible candidate is the chemokine fractalkine, which is chemotactic for monocytes but not CD4+ T lymphocytes (22). In a murine model of rheumatoid arthritis, treatment with an antagonist of MCP-1 suppresses both swelling of joints and infiltration of mononuclear cells (20), suggesting that inhibitors of MCP-1 could be used as therapeutic agents to control inflammation in humans. Despite the similarity of synovial lesions associated with Lyme arthritis and rheumatoid arthritis (44), the fact that MCP-1 played only a minor role in the migration of monocytes across B. burgdorferi-stimulated endothelium raises the question of whether such an approach would be effective in the treatment of inflammation associated with Lyme disease.
Studies using mice have provided insight into cytokines that may regulate host responses to B. burgdorferi. When murine Th cells become activated, they can be subtyped as Th2 cells, which produce IL-4, IL-5, and IL-10, or Th1 cells, which secrete gamma interferon (IFN-γ) and IL-2. Cytokines produced by each type of Th cell inhibit the development and functions of the other type (34). When infected with B. burgdorferi, mice that produce primarily Th2 cytokines are relatively resistant to the symptoms of Lyme disease, whereas mice that display a predominately Th1 response are susceptible (24, 30). When the development of a Th2-type profile is favored by treating mice with either anti-IFN-γ antibodies (24, 30) or recombinant IL-4 (23), the symptoms of Lyme disease are reduced. Even when mice lack B cells, recombinant IL-4 reduces the severity of their symptoms (23), indicating that Th2 cytokines confer resistance to the symptoms of Lyme disease, at least in part, through a mechanism that does not depend on production of antibodies.
It may be, then, that IL-4, IL-5, and IL-10 suppress inflammatory activation of various host cells by B. burgdorferi. There is ample evidence that IL-10 acts in such an anti-inflammatory manner (14). In mice, for example, IL-10 prevents lethal shock induced by LPS or staphylococcal enterotoxin B (14). In vitro, IL-10 inhibits the production of many proinflammatory cytokines and chemokines, including MCP-1, by activated monocytes (14, 51). IL-10 also reduces binding of a human monocytic cell line and a lymphoblastic T-cell line to HUVEC stimulated by IL-1 (25), adhesion of monocytes to explanted human saphenous veins (45), and migration of peripheral blood mononuclear cells across HUVEC activated with LPS (28). The effect of IL-10 on production of the CXC chemokine IL-8 by endothelial cells is controversial. In one report, secretion of IL-8 by HUVEC stimulated with LPS was increased threefold by IL-10 (13), whereas a 33% decrease was observed by others (10). In this study, we observed that IL-10 reduced migration of monocytes across B. burgdorferi-stimulated HUVEC by an average of 54%. Similarly, IL-10 inhibited endothelial production of MCP-1 in response to spirochetes by 55%. Since migration of monocytes across B. burgdorferi-stimulated HUVEC occurs independently of MCP-1, the inhibitory effect of IL-10 is likely due to repression of a proinflammatory factor other than MCP-1. However, reduced production of MCP-1 may hamper B. burgdorferi-stimulated migration of other types of leukocytes. Yin et al. have shown that IL-10 also decreases production of IFN-γ and TNF-α by B. burgdorferi-stimulated mononuclear cells isolated from synovial fluids of patients with Lyme arthritis (52). In combination with our results, this finding suggests that in vivo, IL-10 may provide protection from the symptoms of Lyme disease by inhibiting B. burgdorferi-induced proinflammatory activation of both endothelium and other cell types, as well.
Although IL-10 suppressed both migration of monocytes and production of MCP-1 in response to B. burgdorferi, neither parameter was affected when IL-1 was used as a stimulus. This selective inhibition may be due to induction of receptors for IL-10 on HUVEC by B. burgdorferi but not IL-1. Only B. burgdorferi-stimulated endothelium would then be susceptible to the anti-inflammatory actions of IL-10. Such an induced expression of IL-10 receptors has been observed in murine fibroblasts treated with LPS (48). It is also possible that IL-1 and B. burgdorferi use distinct intracellular signaling cascades to activate transcription of proinflammatory genes. For example, expression of genes in response to B. burgdorferi, but not IL-1, may be dependent on a transcription factor whose activation is blocked by IL-10. Another possibility is that B. burgdorferi and IL-1 employ different mechanisms to activate the same transcription factors. In endothelial cells, one transcription factor known to be activated by both B. burgdorferi (16, 50) and IL-1 (29) is nuclear factor κB (NF-κB), which is involved in inducible transcription of many proinflammatory genes, including that encoding MCP-1 (4, 29). In monocytes, IL-10 reduces activation of NF-κB in response to LPS (47). During IgG immune complex-induced lung injury in mice, inhibition of activation of NF-κB by IL-10 correlates with lack of degradation of the inhibitor protein IκB (27). In endothelial cells, then, B. burgdorferi and IL-1 perhaps use different pathways to inactivate IκB, one that can be inhibited by IL-10 and one that cannot.
B. burgdorferi induces production of both CXC and CC chemokines, including MCP-1, in human peripheral blood monocytes (42). This finding suggests that in vivo, chemokines produced by stimulated macrophages may attract additional leukocytes to sites that are infected with B. burgdorferi. However, our results indicate that endothelial cells exposed to B. burgdorferi also produce chemotactic factors for monocytes, including a surface-bound attractant. Due to their close proximity to the bloodstream, chemoattractants bound to endothelial cells or their underlying matrix are likely to play a particularly important role in recruitment of circulating leukocytes in vivo (33). Stimulation of HUVEC with either IL-1 or B. burgdorferi caused monocytes to migrate across endothelial monolayers at an increased rate. However, the movement of monocytes across HUVEC treated with spirochetes, but not IL-1, occurred independently of MCP-1. This differential utilization of MCP-1 suggests that compared to IL-1, B. burgdorferi more strongly induces the expression of an additional chemotactic factor(s). Furthermore, the migration of monocytes across endothelial monolayers stimulated with spirochetes, but not IL-1, is inhibited by IL-10. Therefore, although both B. burgdorferi and IL-1 activate endothelial cells in a proinflammatory manner, it is clear from our results that they do so through distinct mechanisms.
ACKNOWLEDGMENTS
This work was supported by a research award from the Arthritis Foundation.
We thank Roy Lobb, Richard Coughlin, Marc Golightly, and Jorge Benach for generous gifts of reagents, Chris Pullis for performing FACS analysis, and LoriDawn Horb and Jennifer Raffanello for expert technical assistance. M.J.B. thanks Jorge Benach, Howard Fleit, Richard Kew, and Todd Miller for helpful discussions and guidance.
REFERENCES
- 1.Anonymous. Lyme disease—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:531–535. [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 5.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 6.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 7.Benach J L, Bosler E M, Hanrahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 8.Böggemeyer E, Stehle T, Schaible U E, Hahne M, Vestweber D, Simon M M. Borrelia burgdorferi upregulates the adhesion molecules E-selectin, P-selectin, ICAM-1 and VCAM-1 on mouse endothelioma cells in vitro. Cell Adhes Commun. 1994;2:145–157. doi: 10.3109/15419069409004433. [DOI] [PubMed] [Google Scholar]
- 9.Burns M J, Sellati T J, Teng E I, Furie M B. Production of interleukin-8 (IL-8) by cultured endothelial cells in response to Borrelia burgdorferi occurs independently of secreted IL-1 and tumor necrosis factor alpha and is required for subsequent transendothelial migration of neutrophils. Infect Immun. 1997;65:1217–1222. doi: 10.1128/iai.65.4.1217-1222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C C, Manning A M. TGF-β1, IL-10 and IL-4 differentially modulate the cytokine-induced expression of IL-6 and IL-8 in human endothelial cells. Cytokine. 1996;8:58–65. doi: 10.1006/cyto.1995.0008. [DOI] [PubMed] [Google Scholar]
- 11.Chuluyan H E, Issekutz A C. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Investig. 1993;92:2768–2777. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman J L, Gebbia J A, Piesman J, Degen J L, Bugge T H, Benach J L. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 13.De Beaux A C, Maingay J P, Ross J A, Fearon K C H, Carter D C. Interleukin-4 and interleukin-10 increase endotoxin-stimulated human umbilical vein endothelial cell interleukin-8 release. J Interferon Cytokine Res. 1995;15:441–445. doi: 10.1089/jir.1995.15.441. [DOI] [PubMed] [Google Scholar]
- 14.de Vries J E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27:537–541. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]
- 15.Duray P H. Histopathology of clinical phases of human Lyme disease. Rheum Dis Clin North Am. 1989;15:691–710. [PubMed] [Google Scholar]
- 16.Ebnet K, Brown K D, Siebenlist U K, Simon M M, Shaw S. Borrelia burgdorferi activates nuclear factor-κB and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158:3285–3292. [PubMed] [Google Scholar]
- 17.Furie M B, Burns M J, Tancinco M C A, Benjamin C D, Lobb R R. E-selectin (endothelial-leukocyte adhesion molecule-1) is not required for the migration of neutrophils across IL-1-stimulated endothelium in vitro. J Immunol. 1992;148:2395–2404. [PubMed] [Google Scholar]
- 18.Furie M B, Cramer E B, Naprstek B L, Silverstein S C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984;98:1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furie M B, McHugh D D. Migration of neutrophils across endothelial monolayers is stimulated by treatment of the monolayers with interleukin-1 or tumor necrosis factor-α. J Immunol. 1989;143:3309–3317. [PubMed] [Google Scholar]
- 20.Gong J H, Ratkay L G, Waterfield J D, Clark-Lewis I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med. 1997;186:131–137. doi: 10.1084/jem.186.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang A J, Furie M B, Nicholson S C, Fischbarg J, Liebovitch L S, Silverstein S C. Effects of human neutrophil chemotaxis across human endothelial cell monolayers on the permeability of these monolayers to ions and macromolecules. J Cell Physiol. 1988;135:355–366. doi: 10.1002/jcp.1041350302. [DOI] [PubMed] [Google Scholar]
- 22.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall T J, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 23.Keane-Myers A, Maliszewski C R, Finkelman F D, Nickell S P. Recombinant IL-4 treatment augments resistance to Borrelia burgdorferi infections in both normal susceptible and antibody-deficient susceptible mice. J Immunol. 1996;156:2488–2494. [PubMed] [Google Scholar]
- 24.Keane-Myers A, Nickell S P. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–2028. [PubMed] [Google Scholar]
- 25.Krakauer T. IL-10 inhibits the adhesion of leukocytic cells to IL-1-activated human endothelial cells. Immunol Lett. 1995;45:61–65. doi: 10.1016/0165-2478(94)00226-h. [DOI] [PubMed] [Google Scholar]
- 26.Kuijpers T W, Hakkert B C, Hart M H L, Roos D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet-activating factor and IL-8. J Cell Biol. 1992;117:565–572. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lentsch A B, Shanley T P, Sarma V, Ward P A. In vivo suppression of NF-κB and preservation of IκBα by interleukin-10 and interleukin-13. J Clin Investig. 1997;100:2443–2448. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindner H, Holler E, Gerbitz A, Johnson J P, Bornkamm G W, Eissner G. Influence of bacterial endotoxin on radiation-induced activation of human endothelial cells in vitro and in vivo: interleukin-10 protects against transendothelial migration. Transplantation. 1997;64:1370–1373. doi: 10.1097/00007890-199711150-00023. [DOI] [PubMed] [Google Scholar]
- 29.Martin T, Cardarelli P M, Parry G C N, Felts K A, Cobb R R. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-κB and AP-1. Eur J Immunol. 1997;27:1091–1097. doi: 10.1002/eji.1830270508. [DOI] [PubMed] [Google Scholar]
- 30.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meerschaert J, Furie M B. Monocytes use either CD11/CD18 or VLA-4 to migrate across human endothelium in vitro. J Immunol. 1994;152:1915–1926. [PubMed] [Google Scholar]
- 32.Meerschaert J, Furie M B. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18 and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol. 1995;154:4099–4112. [PubMed] [Google Scholar]
- 33.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 34.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 35.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo J A, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos J C, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 36.Randolph G J, Furie M B. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol. 1995;155:3610–3618. [PubMed] [Google Scholar]
- 37.Randolph G J, Furie M B. Mononuclear phagocytes egress from an in vitro model of the vascular wall by migrating across endothelium in the basal to apical direction: role of intercellular adhesion molecule 1 and the CD11/CD18 integrins. J Exp Med. 1996;183:451–462. doi: 10.1084/jem.183.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Madrid F, De Landazuri M O, Morago G, Cebrian M, Acevedo A, Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986;16:1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Madrid F, Krensky A M, Ware C F, Robbins E, Strominger J L, Burakoff S J, Springer T A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci USA. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sellati T J, Abrescia L D, Radolf J D, Furie M B. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect Immun. 1996;64:3180–3187. doi: 10.1128/iai.64.8.3180-3187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sellati T J, Burns M J, Ficazzola M A, Furie M B. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect Immun. 1995;63:4439–4447. doi: 10.1128/iai.63.11.4439-4447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sprenger H, Krause A, Kaufmann A, Priem S, Fabian D, Burmester G R, Gemsa D, Rittig M G. Borrelia burgdorferi induces chemokines in human monocytes. Infect Immun. 1997;65:4384–4388. doi: 10.1128/iai.65.11.4384-4388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 44.Steere A C, Duray P H, Butcher E C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Arthritis Rheum. 1988;31:487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- 45.Stefano G B, Christensen V B, Tonnesen E, Liu Y, Hughes T K, Jr, Bilfinger T V. Interleukin-10 stimulation of endogenous nitric oxide release from human saphenous veins diminishes immunocyte adherence. J Cardiovasc Pharmacol. 1997;30:90–95. doi: 10.1097/00005344-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Takayama K, Rothenberg R J, Barbour A G. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987;55:2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P, Wu P, Siegel M I, Egan R W, Billah M M. Interleukin (IL)-10 inhibits nuclear factor κB (NF κB) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 48.Weber-Nordt R M, Meraz M A, Schreiber R D. Lipopolysaccharide-dependent induction of IL-10 receptor expression on murine fibroblasts. J Immunol. 1994;153:3734–3744. [PubMed] [Google Scholar]
- 49.Witt D P, Lander A D. Differential binding of chemokines to glycosaminoglycan subpopulations. Curr Biol. 1994;4:394–400. doi: 10.1016/s0960-9822(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 50.Wooten R M, Modur V R, McIntyre T M, Weis J J. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-κB and inflammatory activation in human endothelial cells. J Immunol. 1996;157:4584–4590. [PubMed] [Google Scholar]
- 51.Yano S, Yanagawa H, Nishioka Y, Mukaida N, Matsushima K, Sone S. T helper 2 cytokines differently regulate monocyte chemoattractant protein-1 production by human peripheral blood monocytes and alveolar macrophages. J Immunol. 1996;157:2660–2665. [PubMed] [Google Scholar]
- 52.Yin Z, Braun J, Neure L, Wu P, Eggens U, Krause A, Kamradt T, Sieper J. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997;40:69–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]