Abstract
Most nontyphoidal Salmonella (NTS) illnesses in the United States are thought to be foodborne. However, transmission routes likely vary among the different serotypes. We developed a relative ranking of NTS serotypes according to the strength of their association with foodborne transmission. We used Laboratory-based Enteric Disease Surveillance data to estimate the proportion of infections for each Salmonella serotype reported from 1998 to 2015 and Foodborne Disease Outbreak Surveillance System data to calculate the proportion of foodborne outbreak-associated Salmonella illnesses caused by each serotype. We calculated the ratios of these proportions to create a foodborne relatedness (FBR) measure for each serotype. Of the top 20 serotypes, Saintpaul (2.14), Heidelberg (1.61), and Berta (1.48) had the highest FBR measures; Mississippi (0.01), Bareilly (0.13), and Paratyphi B variant L(+) tartrate(+) (0.20) had the lowest. The FBRs for the three most prevalent serotypes were 1.22 for Enteritidis, 0.77 for Typhimurium, and 1.16 for Newport. This method provides a quantitative approach to estimating the relative differences in the likelihood that an illness caused by a particular serotype was transmitted by food, which may aid in tailoring strategies to prevent Salmonella illnesses and guide future research into serotype-specific source attribution.
Keywords: Salmonella, serotypes, foodborne transmission
Introduction
Nontyphoidal Salmonella (NTS) is estimated to cause more than 1.2 million illnesses each year in the United States, with more than 23,000 hospitalizations and 450 deaths (Scallan et al., 2011). Salmonella can be transmitted to humans through food, water, direct contact with animals, the environment, and person-to-person contact (Voetsch et al., 2009; Hale et al., 2012; Barton Behravesh and Griffin, 2015). A 2011 study based on available outbreak data and case–control studies used 94% as the estimate for NTS illnesses transmitted by food in the United States (Mermin et al., 2004; Scallan et al., 2011). A later study completed after additional data became available estimated that 11% of NTS illnesses were due to contact with animals and their environments, suggesting the proportion from foodborne transmission was lower than 89% (Hale et al., 2012). Transmission pathways vary among serotypes, because they have different reservoirs and exposure sources, making control challenging (Crim et al., 2014). For example, some serotypes, such as Enteritidis, are strongly associated with foodborne transmission, whereas others, such as Poona and Javiana, have been more commonly associated with other exposures, such as water and contact with animals (Woodward et al., 1997; Guard-Petter, 2001; Srikantiah et al., 2004; Braden, 2006; Voetsch et al., 2009).
The estimated overall incidence of NTS infections has not changed much in the last two decades and remains well above the national goal (Crim et al., 2014, 2015; Office of Disease Prevention and Health Promotion, 2018). Having a good estimate of the percentage of illnesses from foodborne transmission can aid in designing interventions to reduce incidence and to measure the effectiveness of interventions. To obtain such an estimate, we need to understand the transmission modes by serotypes. Because no single data source provides this information, we combined data from two surveillance systems to develop a quantitative measure of a serotype’s association with foodborne transmission.
Methods
We examined 18 years of data, from 1998 to 2015, reasoning that NTS epidemiology is informed by both common and uncommon serotypes and that accumulating sufficient person-time to capture the occurrence of uncommon serotypes requires longer observational periods. Due to a lack of empirical information, we made the assumption that the dominant transmission pathways for each serotype did not change during this study period.
Data sources
Laboratory-based Enteric Disease Surveillance system.
The Laboratory-based Enteric Disease Surveillance (LEDS) system is a passive, national surveillance system for enteric diseases cases that are culture confirmed and reported by public health laboratories; reported information includes Salmonella serotype (Centers for Disease Control and Prevention, 2011). Serotypes with fewer than 30 cases reported in the study period were excluded to reduce the effect of differential reporting by serotype.
Foodborne Disease Outbreak Surveillance System.
CDC’s Foodborne Disease Outbreak Surveillance System (FDOSS) is part of the National Outbreak Reporting System, which conducts surveillance for foodborne, waterborne, environmental, person-to-person, and animal contact outbreaks in the United States. FDOSS is the subset of this system focused solely on foodborne outbreaks. Local, state, and territorial public health departments detect and investigate foodborne disease outbreaks, and voluntarily submit reports to FDOSS. Salmonella is considered the confirmed etiology of an outbreak when the same serotype is isolated from two or more ill persons, or when the bacterium is isolated from an epidemiologically implicated food (Centers for Disease Control and Prevention, 2015). Suspected etiologies are those that do not meet the confirmed criteria (Centers for Disease Control and Prevention, 2015). We selected all confirmed and suspected Salmonella foodborne outbreaks, and excluded from the analysis outbreaks caused by more than one pathogen or more than one Salmonella serotype to avoid additional assumptions about the assignment and weighting of these outbreaks, and to be consistent with previous work (Interagency Food Safety Analytics Collaboration, 2017). We used the number of laboratory-confirmed outbreak-associated illnesses in this analysis.
U.S. Census population data.
The annual estimated population size from 1998 to 2015 provided by the U.S. Census Bureau was used in calculating incidence (U.S. Census Bureau, 2017).
Data analysis
The annual incidence rate for each serotype was calculated using the number of laboratory-confirmed cases in LEDS system and the U.S. population estimates. Annual incidence rates were averaged for each serotype to yield an overall incidence.
Using LEDS system and FDOSS laboratory-confirmed illnesses, we calculated the proportion of the total number of isolates identified as each serotype in each system during the study period. We then computed a foodborne relatedness (FBR) measure to rank serotypes by the strength of their association with foodborne transmission:
where i indexes serotype. Higher FBR measures suggest that the serotype is more likely to be associated with foodborne transmission, and lower measures indicate that the serotype may be more associated with other transmission routes (i.e., waterborne, person-to-person, animal contact, or environmental). We established two thresholds for presenting FBRs, which would limit to the FBRs that change less than a specified absolute difference if one case were to be added to the FDOSS data for that serotype. The thresholds were <0.01 (i.e., no change in the last decimal place provided) for Table 1 and <0.1 (i.e., no change in the first decimal place provided) for the Supplementary Table S1. We performed a subanalysis comparing the FBR measure for cases of those younger than 5 years to those 5 years of age and older using the reported proportion of cases in an outbreak belonging to each age group. We used a Bayesian bootstrap sampling distribution based on 10,000 replications of a two-stage resampling of years and reports within a year to calculate the mean and confidence interval (5th and 95th percentiles) for the FBR measure for each serotype. We used R 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria) for data analysis.
Table 1.
Mean Annual Incidence of the 20 Most Common Salmonella Serotypes, Percentage of Outbreak-Associated Illnesses That Were Foodborne for Each Serotype, Percentage of All Cases Reported to Laboratory-Based Enteric Disease Surveillance System, and Bootstrapped Foodborne Relatedness Measures, 1998–2015, United States
| Serotype | Incidence per 100,000 populationa | Percentage of outbreak-associated illnesses that were foodborneb | Percentage of confirmed cases reported to LEDSa | Bootstrapped FBR measurec (90% confidence interval, ranked high to low) | |
|---|---|---|---|---|---|
| 1 | Saintpaul | 0.25 | 7.45 | 1.99 | 2.14 (0.43–9.86) |
| 2 | Heidelberg | 0.51 | 6.55 | 4.00 | 1.61 (0.93–2.58) |
| 3 | Berta | 0.09 | 1.22 | 0.68 | 1.48 (0.43–4.34) |
| 4 | Poona | 0.11 | 3.57 | 0.84 | 1.37 (0.12–14.59) |
| 5 | Enteritidis | 2.23 | 21.04 | 17.49 | 1.22 (0.93–1.56) |
| 6 | Newport | 1.27 | 11.69 | 9.99 | 1.16 (0.75–1.71) |
| 7 | Montevideo | 0.31 | 3.10 | 2.45 | 1.14 (0.48–2.54) |
| 8 | Braenderup | 0.20 | 1.86 | 1.60 | 1.10 (0.51–2.16) |
| 9 | Agona | 0.15 | 1.51 | 1.19 | 1.08 (0.17–3.26) |
| 10 | I 4,[5],12:i:- | 0.36 | 3.39 | 2.81 | 1.06 (0.28–2.71) |
| 11 | Hadar | 0.10 | 0.84 | 0.78 | 1.01 (0.29–2.13) |
| 12 | Muenchen | 0.28 | 2.69 | 2.21 | 0.86 (0.22–3.25) |
| 13 | Javiana | 0.63 | 4.17 | 4.91 | 0.79 (0.36–1.58) |
| 14 | Typhimurium | 2.18 | 13.72 | 17.10 | 0.77 (0.52–1.18) |
| 15 | Infantis | 0.24 | 1.19 | 1.91 | 0.61 (0.31–1.06) |
| 16 | Thompson | 0.18 | 0.81 | 1.41 | 0.57 (0.28–0.99) |
| 17 | Oranienburg | 0.22 | 1.03 | 1.74 | 0.56 (0.18–1.17) |
| 18 | Paratyphi B variant L(+) tartrate(+) | 0.13 | 0.23 | 1.04 | 0.20 (0.02–0.53) |
| 19 | Bareilly | 0.10 | 0.11 | 0.78 | 0.13 (0.02–0.32) |
| 20 | Mississippi | 0.15 | 0.01 | 1.20 | 0.01 (0.00–0.03) |
Calculated from the LEDS data.
Calculated from the Foodborne Disease Outbreak Surveillance System data.
Bootstrapped FBR measures cannot be directly calculated from the ratio of the crude percentages of outbreak-associated illnesses that were foodborne and confirmed cases reported to LEDS. Bootstrapped FBR measure estimates and 90% confidence intervals were calculated from 10,000 replications of a two-stage sample process.
FBR, foodborne relatedness; LEDS, Laboratory-based Enteric Disease Surveillance.
Results
During 1998–2015, health departments reported 692,012 laboratory-confirmed NTS infections, and we excluded from our analyses 4397 cases, which were attributed to more than one serotype or a serotype with <30 cases in the study period. The highest mean annual incidence of infections was observed for serotypes Enteritidis (120,291 cases; incidence 2.23 per 100,000), Typhimurium (117,562; 2.18), Newport (68,668; 1.27), and Javiana (33,741; 0.63) (Table 1). The 20 serotypes included in Table 1 caused 76.1% (523,318/687,615) of Salmonella infections and 86.2% (26413/30,644) of Salmonella outbreak-associated illnesses.
Health departments reported 2533 foodborne outbreaks and 39,801 outbreak-associated illnesses caused by Salmonella during 1998–2015. Of the 2533 outbreaks, 2096 (82.78%) were caused by a confirmed (1960) or suspected (136) single serotype, which also had ≥30 cases in LEDS system, meeting criteria for inclusion, and resulting in 30,644 total outbreak-associated cases. Of 82 serotypes included, the 5 most common were Enteritidis (21.0% of outbreak-associated illnesses), Typhimurium (13.7%), Newport (11.7%), Saintpaul (7.5%), and Heidelberg (6.6%) (Table 1).
Among the 20 serotypes included in Table 1, Saintpaul, Heidelberg, and Berta had the highest FBR measures; Mississippi, Bareilly, and Paratyphi B variant L(+) tartrate(+) had the lowest (Supplementary Table S1). The age analysis showed that the calculated FBR measure was lower for children younger than 5 years compared with those older, and for nine serotypes, the age differences were significant (Enteritidis, Typhimurium, Newport, Muenchen, Javiana, Infantis, Oranienburg, Braenderup, and Thompson) (Fig. 1).
FIG. 1.
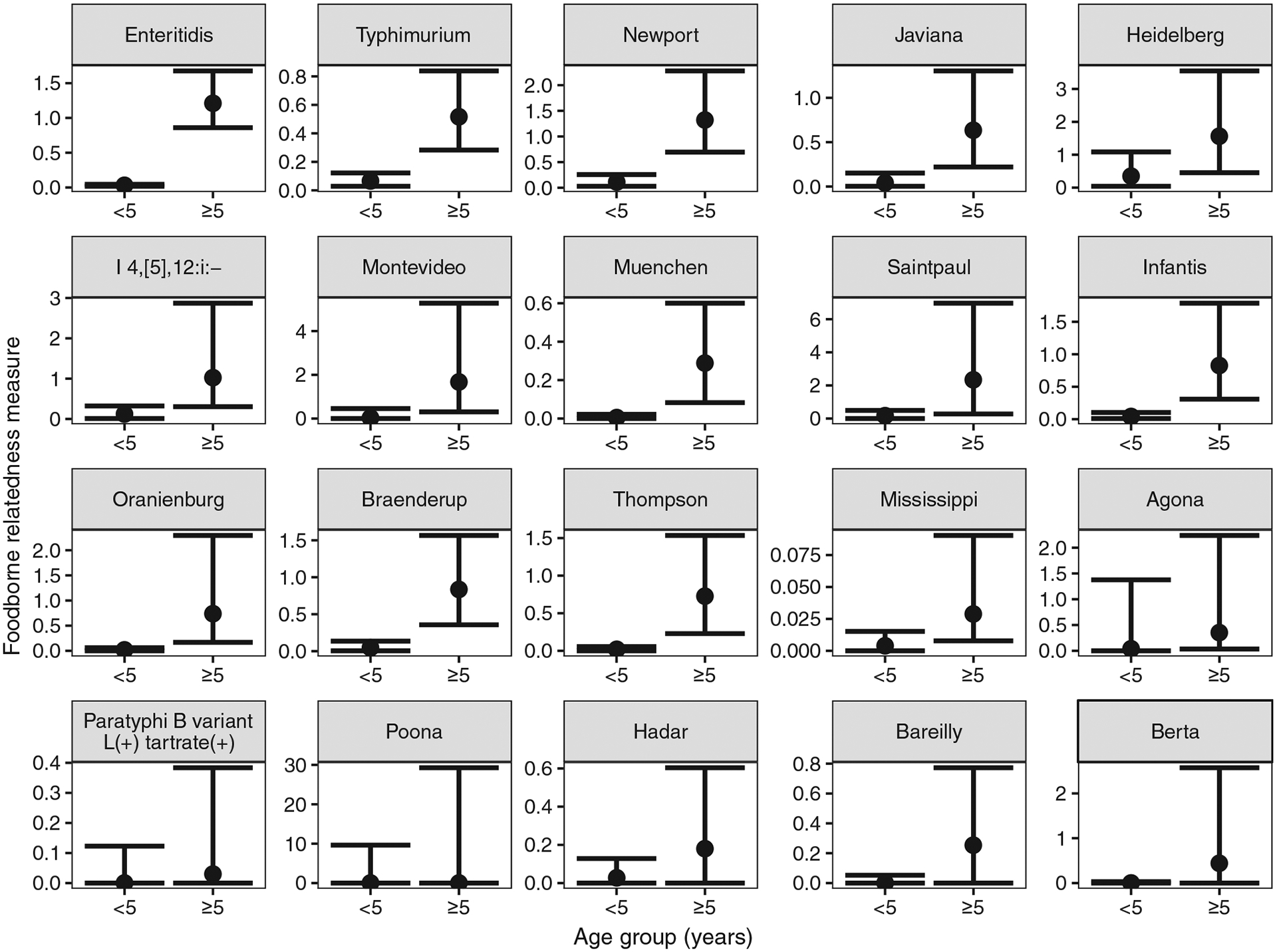
FBR measures (point estimate and 90% confidence interval) for the 20 most common Salmonella serotypes ascertained from Laboratory-based Enteric Disease Surveillance data and ordered from highest to lowest overall FBR, by age group (<5 and ≥5 years), 1998–2015, United States. Note: y-axis changes per panel due to large range of FBR values. FBR, foodborne relatedness.
Discussion
Information from outbreak investigations can be used to assess transmission routes of Salmonella. In defining the FBR measure, we have provided a route to better understand the relative importance of the foodborne pathway at the serotype level. This method provides a quantitative way to estimate the relative likelihood that illnesses caused by particular serotypes were transmitted by food. Furthermore, these methods can likely be extended to other pathogens and their subtypes to help better understand the relative contribution of specific transmission modes, which should allow the development of more tailored approaches to illness prevention.
Our findings are supported by case–control studies that have identified risk factors for sporadic Salmonella infection. For example, Heidelberg has the second highest FBR measure among the 20 most common serotypes. Studies have identified eating eggs prepared outside the home, consuming chicken nuggets or strips, and consuming undercooked eggs as risk factors for sporadic Heidelberg infection (Hennessy et al., 2004; Currie et al., 2005). In contrast, sporadic infections caused by Typhimurium, with an FBR measure that ranked 14th among the 20 serotypes, were not only associated with the consumption of many foods but also with other routes of transmission, such as playing in a sandbox for children 4–12 years of age and contact with animals, particularly ill farm animals (Hedberg et al., 1993; Wall et al., 1994; Doorduyn et al., 2006). Likewise, Javiana had a relatively low FBR measure, which is supported by case–control studies that found contact with amphibians, reptiles, and their environment, and consumption of well water to be important risk factors for these infections (Srikantiah et al., 2004; Clarkson et al., 2010).
The method of ranking Salmonella serotypes according to the strength of their association with foodborne transmission is sensitive to relatively small variations in the incidence or in the percentage of outbreak-associated illnesses that are foodborne. To mitigate this limitation, we used a long series of years and reported the FBR measure for those serotypes that were minimally sensitive to the effect of changes in case counts. We also assumed the proportion of foodborne outbreak-associated illnesses caused by each serotype is representative of its association with foodborne transmission. The bootstrap resampling provides a measure of uncertainty by which we can judge whether any two FBR measures are statistically different. We did not account for variations in outbreak detection and reporting that may influence the observed distribution of serotypes in outbreaks. Factors that influence whether a foodborne outbreak is detected and investigated may affect the likelihood that the food causing illness in the population will be implicated in the outbreak, and could bias the FBR measure for serotypes more associated with these foods.
The measure appears to be further validated by the age-specific analysis that consistently found lower FBR measures among those younger than 5 years compared with older persons, which supports previous evidence that infants and young children have a high proportion of salmonellosis resulting from nonfoodborne routes of transmission (Schutze et al., 1999; Jones et al., 2006). One could also consider other subanalyses of the FBR measure to examine regional differences, seasonal differences, changes over time, and other features that may help us better understand the sources of Salmonella serotypes.
Although we constructed the FBR measure using LEDS system and FDOSS laboratory-confirmed illnesses because of the availability of nationally collected data over a long series of comparable years by both systems, alternative data sources could be considered. For example, FoodNet’s active surveillance of both outbreak-associated illnesses reported to FDOSS and incidence by serotype could be used, but given that it is a smaller sample of the U.S. population, it would reduce the person-time available to characterize rarer serotypes with precision. Rather than using the number of laboratory-confirmed ill from FDOSS, the percentage of outbreaks or the total ill (including cases that were not confirmed by culture) could be considered numerators, but they would not as closely represent the surveillance catchment of the laboratory-confirmed illnesses reported by LEDS system. However, the method is flexible enough to accommodate other data sources that may be available in other areas of the world or that may be more appropriate, depending on the intended use of the measure.
To the best of our knowledge, this is the first attempt to develop a quantitative measure to rank Salmonella serotypes based on their relative likelihood of foodborne transmission. The FBR measure may allow the development of refined estimates of the number of Salmonella infections attributable to food. Furthermore, it may help determine Salmonella serotypes that are most likely to cause foodborne infection and the highest priorities for food safety interventions.
Supplementary Material
Acknowledgments
We thank state and local health departments and their public health laboratories for their contributions to FDOSS and LEDS system. We would also like to thank Kelly Barrett for her contributions to this work.
Funding Information
No funding was received for this article.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- Barton Behravesh C, Griffin PM. Salmonellosis. In Heymann DL, (ed.): Control of Communicable Diseases Manual. Washington, DC: American Public Health Association, 2015:532–539. [Google Scholar]
- Braden CR. Salmonella enterica serotype Enteritidis and eggs: A national epidemic in the United States. Clin Infect Dis 2006;43:512–517. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Salmonella surveillance overview. Atlanta, GA: National Enteric Disease Surveillance, US Department of Health and Human Services, 2011. [Google Scholar]
- Centers for Disease Control and Prevention. Guide to confirming a diagnosis in foodborne disease. 2015. Available at : www.cdc.gov/foodsafety/outbreaks/investigating-outbreaks/confirming_diagnosis.html, accessed April 1, 2018.
- Clarkson LS, Tobin-D’Angelo M, Shuler C, Hanna S, Benson J, Voetsch AC. Sporadic Salmonella enterica serotype Javiana infections in Georgia and Tennessee: A hypothesis-generating study. Epidemiol Infect 2010;138:340–346. [DOI] [PubMed] [Google Scholar]
- Crim SM, Griffin PM, Tauxe R, Marder EP, Gilliss D, Cronquist AB, Cartter M, Tobin-D’Angelo M, Blythe D, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Wolpert B, Henao OL. Preliminary incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2014. MMWR Morb Mortal Wkly Rep 2015;64:495–499. [PMC free article] [PubMed] [Google Scholar]
- Crim SM, Iwamoto M, Huang JY, Griffin PM, Gilliss D, Cronquist AB, Cartter M, Tobin-D’Angelo M, Blythe D, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Lance S, Tauxe R, Henao OL. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2013. MMWR Morb Mortal Wkly Rep 2014;63:328–332. [PMC free article] [PubMed] [Google Scholar]
- Currie A, MacDougall L, Aramini J, Gaulin C, Ahmed R, Isaacs S. Frozen chicken nuggets and strips and eggs are leading risk factors for Salmonella Heidelberg infections in Canada. Epidemiol Infect 2005;133:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduyn Y, Van Den Brandhof WE, Van Duynhoven YT, Wannet WJ, Van Pelt W. Risk factors for Salmonella Enteritidis and Typhimurium (DT104 and non-DT104) infections in The Netherlands: Predominant roles for raw eggs in Enteritidis and sandboxes in Typhimurium infections. Epidemiol Infect 2006;134:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard-Petter J The chicken, the egg and Salmonella Enteritidis. Environ Microbiol 2001;3:421–430. [DOI] [PubMed] [Google Scholar]
- Hale CR, Scallan E, Cronquist AB, Dunn J, Smith K, Robinson T, Lathrop S, Tobin-D’Angelo M, Clogher P. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin Infect Dis 2012;54(Suppl 5):S472–S479. [DOI] [PubMed] [Google Scholar]
- Hedberg CW, David MJ, White KE, MacDonald KL, Osterholm MT. Role of egg consumption in sporadic Salmonella Enteritidis and Salmonella Typhimurium infections in Minnesota. J Infect Dis 1993;167:107–111. [DOI] [PubMed] [Google Scholar]
- Hennessy TW, Cheng LH, Kassenborg H, Ahuja SD, Mohle-Boetani J, Marcus R, Shiferaw B, Angulo FJ; Emerging Infections Program FoodNet Working Group. Egg consumption is the principal risk factor for sporadic Salmonella serotype Heidelberg infections: A case-control study in FoodNet sites. Clin Infect Dis 2004;38(Suppl 3):S237–S243. [DOI] [PubMed] [Google Scholar]
- Interagency Food Safety Analytics Collaboration. Foodborne illness source attribution estimates for 2013 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter using multi-year outbreak surveillance data, United States. Atlanta, GA and Washington, DC: U.S. Department of Health and Human Services, 2017. [Google Scholar]
- Jones TF, Ingram LA, Fullerton KE, Marcus R, Anderson BJ, McCarthy PV, Vugia D, Shiferaw B, Haubert N, Wedel S, Angulo FJ. A case-control study of the epidemiology of sporadic Salmonella infection in infants. Pediatrics 2006;118:2380–2387. [DOI] [PubMed] [Google Scholar]
- Mermin J, Hutwagner L, Vugia D, Shallow S, Daily P, Bender J, Koehler J, Marcus R, Angulo FJ; Emerging Infections Program FoodNet Working Group. Reptiles, amphibians, and human Salmonella infection: A population-based, case-control study. Clin Infect Dis 2004;38(Suppl 3):S253–S261. [DOI] [PubMed] [Google Scholar]
- Office of Disease Prevention and Health Promotion. Healthy People 2020. 2018. Available at: www.healthypeople.gov, accessed April 1, 2018.
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—Major pathogens. Emerg Infect Dis 2011;17:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze GE, Sikes JD, Stefanova R, Cave MD. The home environment and salmonellosis in children. Pediatrics 1999;103:E1. [DOI] [PubMed] [Google Scholar]
- Srikantiah P, Lay JC, Hand S, Crump JA, Campbell J, Van Duyne MS, Bishop R, Middendor R, Currier M, Mead PS, Molbak K. Salmonella enterica serotype Javiana infections associated with amphibian contact, Mississippi, 2001. Epidemiol Infect 2004;132:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. Population and Housing Unit Estimates Tables. 2017. Available at: www.census.gov/programs-surveys/popest/data/tables.2000.html, accessed January 1, 2018.
- Voetsch AC, Poole C, Hedberg CW, Hoekstra RM, Ryder RW, Weber DJ, Angulo FJ. Analysis of the FoodNet case-control study of sporadic Salmonella serotype Enteritidis infections using persons infected with other Salmonella serotypes as the comparison group. Epidemiol Infect 2009;137:408–416. [DOI] [PubMed] [Google Scholar]
- Wall PG, Morgan D, Lamden K, Ryan M, Griffin M, Threlfall EJ, Ward LR, Rowe B. A case control study of infection with an epidemic strain of multiresistant Salmonella Typhimurium DT104 in England and Wales. Commun Dis Rep CDR Rev 1994;4:R130–R135. [PubMed] [Google Scholar]
- Woodward DL, Khakhria R, Johnson WM. Human salmonellosis associated with exotic pets. J Clin Microbiol 1997;35: 2786–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


