Abstract
Objective:
Loss-of-control (LOC) eating is associated with eating disorders and obesity, and thus it is imperative to understand its momentary risk factors in order to improve intervention efforts. Negative affect has been proposed as a momentary risk factor for LOC eating, but the evidence for its effects in children and adolescents is mixed. Short sleep duration (which is very common in youth), may be one variable that moderates the relation between negative affect and subsequent LOC eating. As such, we aimed to examine the moderating role of within-person sleep duration on the momentary relations between negative affect and subsequent LOC eating.
Method:
We recruited children (N=30) with overweight/obesity ages 8–14, who completed a 2-week ecological momentary assessment protocol assessing negative affect and LOC eating several times per day, while also wearing a sleep actigraphy device and completing sleep diaries.
Results:
Consistent with hypotheses, within-person sleep duration moderated the next-day momentary relation between within-person negative affect and LOC eating, such that shorter sleep duration strengthened the positive relation between negative affect and loss-of-control eating.
Conclusions:
Results suggest that, in children and adolescents, fluctuations in sleep duration may influence susceptibility to losing control over eating after experiencing negative affect. Future research should further investigate other metrics of sleep disturbance as they relate to emotion regulation and LOC eating. Such research will set the stage for augmenting pediatric interventions to better target maintenance factors for LOC eating.
Introduction
Loss-of-control (LOC) eating, defined as the feeling that one cannot stop eating once started, is the hallmark feature of binge eating, a key behavior that can drive both eating disorders and obesity. LOC eating often develops during childhood and early adolescence, and LOC eating during this period predicts negative health (e.g., excess weight gain; Fisher & Birch, 2002; Tanofsky-Kraff et al., 2009) and psychological outcomes (Tanofsky-Kraff et al., 2011). In order to improve outcomes, research is needed to understand the maintenance factors of LOC eating among youth, particularly the momentary or daily factors that may increase susceptibility to LOC eating.
Negative affect has been proposed as a momentary variable that predicts LOC eating, as eating can serve a negative reinforcement function (i.e., temporarily relieving or distracting from negative affect; Berg et al., 2015). This model has been mostly supported in the adult obesity and binge eating literature (Engel et al., 2016). For example, studies using ecological momentary assessment (EMA; a method of repeated sampling that allows for near real-time data collection in one’s natural environment) have demonstrated that negative affect increases in the moments prior to a binge eating episode (Haedt-Matt & Keel, 2011), and, in some studies, decreases following the episode (Berg et al., 2017; Berg et al., 2015). However, these findings largely have not been replicated in pediatric samples. In fact, three separate studies have shown no momentary effects of negative affect on subsequent likelihood of LOC eating (Goldschmidt et al., 2018; Hilbert, Rief, Tuschen-Caffier, de Zwaan, & Czaja, 2009; Ranzenhofer et al., 2014). One reason why these relationships have not been consistently observed in pediatric samples may be a lack of consideration of moderating variables that may strengthen or weaken the momentary relations between negative affect and LOC in youth specifically.
One such factor that is likely to impact the relation between negative affect and LOC eating in youth is sleep. Sleep duration is increasingly understood as a factor that impacts appetite and the ability to regulate negative mood (Baum et al., 2014; Cappuccio et al., 2008). A growing body of research suggests that shortened sleep duration impacts one’s next-day ability to downregulate negative emotions adaptively and effectively (Anderson & Platten, 2011; Gruber, Cassoff, Frenette, Wiebe, & Carrier, 2012; Zhang, Lau, & Hsiao, 2019), and may increase engagement in impulsive behaviors (Liu et al., 2020). Indeed, several studies have indicated that sleep restriction predicts next-day responsivity to food cues (Cedernaes et al., 2014) and overall propensity to dysregulated eating (Hart et al., 2013; Jensen et al., 2019; Sauchelli et al., 2016; Weiss et al., 2010). Taken together, the literature suggest that decreased sleep duration could impact a child’s capacity for adaptive emotion regulation, therefore increasing the likelihood of LOC eating as a method for coping with negative affect. In other words, sleep duration is likely to moderate the next-day relation between negative affect and subsequent LOC eating, such that less sleep would strengthen the link between negative affect and LOC.
To test this model, we conducted a secondary data analysis of an EMA study of children and young adolescents (ages 8–14) with overweight or obesity (Goldschmidt et al., 2018) during which participants also wore an actigraphy device that measured sleep duration. In the original study, no direct links were observed between sleep duration and next-day LOC eating (Goldschmidt et al., 2020). In the present study, we examined sleep duration (i.e., total sleep time; TST – partitioned into between and within-person effects) as a moderator of the relation between momentary levels of negative affect and subsequent LOC eating. We hypothesized that lower within-person TST (i.e., lower sleep duration in a night relative to one’s typical amount of sleep) would strengthen (i.e., moderate) the relationship between next day, within-person negative affect and risk for subsequent LOC eating.
Methods
Participants and procedures
Participants were children and young adolescents with overweight or obesity who were enrolled in a study examining several factors associated with eating behavior in the natural environment (Goldschmidt et al., 2018). Participants were recruited from two academic institutions in Chicago, IL via advertisements, direct pediatrician referrals, and phone logs from previous studies. To be included, participants had to have a body mass index (BMI; kg/m2) ≥ 85th age- and sex-adjusted percentile and be between the ages of 8–14 years. Exclusion criteria were: medical conditions or medications known to influence sleep, weight, or appetite (e.g., sleep apnea); eating disorders other than binge eating disorder (as assessed by diagnostic items from the Child Eating Disorder Examination; Bryant-Waugh, Cooper, Taylor, & Lask, 1996); inability to read and understand English fluently; and concurrent treatment for overweight/obesity. Caregivers of interested participants completed a phone screen and eligible participants and their caregiver(s) attended a baseline study visit. In total, 92 participants were screened via phone, 44 of whom presented to the research sites for a baseline evaluation, and 30 of whom provided adequate EMA data (e.g., at least 7 days of EMA recording) and sufficient sleep data to be considered in the current analyses. At the baseline assessment, participants provided written informed assent/consent, had their height and weight measured, and completed interviews and questionnaires assessing sleep, eating patterns, and psychosocial functioning. Participants and their caregivers were then trained to complete the EMA, actigraphy, and sleep diary protocols.
Participants were asked to complete EMA recordings after each eating occasion (event-contingent); before bedtime (interval-contingent); and at 3–5 semi-random times throughout the day (signal-contingent). EMA recordings were collected when participants were not in school, such that signal-contingent prompts occurred between 7:00–8:00am, 3:00–4:00pm, and 6:00–7:00pm on weekdays and every 2–3 h between 8:00am-9:00pm on the weekends. In each EMA recording, participants were instructed to report on characteristics of any recent eating episode since the last survey. This combination of signal-, event-, and interval-contingent recordings has been successfully implemented in previous EMA studies of children with overweight/obesity (Hilbert et al., 2009; Ranzenhofer et al., 2014). A 1-day practice period during which adherence was ≥70% made children eligible to initiate the 14-day study period. These data were not used in statistical analyses to reduce concerns about the effect of immediate reactivity to self-monitoring. Two participants were excluded based on low adherence during the 1-day practice period.
Participants were instructed wear wrist actigraphy monitors (Actiwatch 2, Respironics/Phillips, Bend, OR) continuously on the non-dominant wrist throughout the 14-day protocol. For each night of actigraphy data collection, participants were instructed to mark the time they began falling asleep and the time they awakened by pressing an event marker button on the actigraphy monitor, which is a standard procedure. These data were supplemented by daily sleep diaries to confirm time in bed and time out of bed, which created windows for which to examine actigraphy sleep data (see “Measures” section for more details).
Participants received $50 for the intake assessment; $50 for completion of the 14-day protocol; and up to $50 for daily assessments prorated according to degree of response to random signals ($1 for each response to a total of 50 semi-random signals over the course of the 14-day protocol). Study procedures were approved by the appropriate Institutional Review Boards.
Measures
Baseline measures
Height and weight were measured via stadiometer and calibrated digital scale, respectively. BMI z-score was calculated using CDC growth charts (CDC, 2000). Demographic data were reported by children and caregivers.
EMA
At eating episode recording, participants reported on the type of eating episode they experienced (meal, snack, or binge). Ratings for LOC eating (“While you were eating, did you feel a sense of loss of control?“; “While you were eating, did you feel that you could not stop eating once you had started?“; “While you were eating, did you feel like you could not resist eating?“; “While you were eating, did you feel like a car without brakes, you just kept eating and eating?“) were made on a 1- to 5-point Likert-type scale (1 = “no, not at all,” and 5 = “yes, extremely”). The four items assessing LOC eating were summed to form a total score (range = 4–20) based on their high internal consistency (α = 0.91). The Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) was used to assess current negative mood state. The PANAS is a widely used and well-validated measure (Laurent et al., 1999) that has been used in numerous EMA studies in children (Hilbert et al., 2009; Ranzenhofer et al., 2014). Each negative affect item (e.g., afraid, upset) was rated on a 5-point scale (“1” = “Not at all”; “5” = “Extremely”) and summed to form a composite negative affect scale (range = 0–50).
Actigraphy
Actigraphy is a well-validated measure of sleep and has been validated against the “gold standard” polysomnography, with agreement rates exceeding 90% for minute-by-minute sleep–wake identification (Ancoli-Israel et al., 2003; Sadeh & Acebo, 2002). Actigraphy data were collected continuously and stored in 30-s epochs. Time in bed began with the first minute of a 10-minute bout in which the activity counts were all zero and wake-up time was the first minute of a 10-minute bout in which the activity counts were all greater than zero (Sadeh, Sharkey, & Carskadon, 1994). The “medium” threshold (meaning that the number of activity counts required to be considered “awake” was 40, as compared to 20 for the “low” threshold and 80 for the “high” threshold) was used for sleep-wake detection in Philips Actiware v 6.0.7 software. TST measurement was constrained to nighttime sleep. Sleep intervals were defined as the period between time-to-bed and wake time. Sleep diaries were used to inform coding of sleep and wake times during preliminary actigraphy analyses, such that diary data informed the identification of boundaries for when sleep started and ended. Participants (with caregiver assistance when necessary) were instructed to indicate the start and end time of all sleep episodes. Actigraphy and sleep diary data were used in conjunction to determine TST.
Statistical Analyses
Descriptive data and bivariate correlations were examined for demographic, EMA, and actigraphy variables. EMA data were matched to actigraphy-measured sleep duration (i.e., TST) on the previous night. Participants were considered an outlier if mean TST was > 2 SD above or below the sample mean. One participant met this criteria; analyses were run without this outlier and results were equivalent, so results including the outlier are presented throughout for consistency. To evaluate primary study aims, generalized linear mixed models (GLMMs) examined previous night’s TST as a moderator of momentary associations between EMA-measured negative affect and LOC eating on the next day. In each GLMM, the fixed effects of TST and negative affect were separated into within-person (person-mean centered) and between-person (grand-mean centered) components. Within-person associations indicated the degree to which changes in daily TST or momentary negative affect (relative to an individual’s average level of these variables) were related to LOC. In order to establish temporal relationships between negative affect and LOC eating, within-person effects of negative affect were lagged from the preceding EMA signal but not lagged across day. Therefore, each GLMM included fixed effects of previous night’s TST and negative affect (within- and between-person components) and the two-way interactions of within-person negative affect and previous night’s TST (within- and between-person components) as predictors of LOC eating. Given that the primary research question focused on moderating effects of sleep on momentary (within-person) associations between negative affect and LOC eating, interactions with between-person negative affect components were not evaluated. Age, z-BMI, and gender were included as covariates, and random intercepts were included to model variability in outcomes. GLMMs specified an AR1 covariance structure a gamma link function due to non-normal distributions of outcome variables. Analyses were conducted using SPSS version 27.
Results
A total of 30 participants (56.7% female; 60.0% African American; 20.0% White; 13.3% Hispanic; 3.3% Asian; 3.3% not identifying) provided sufficient actigraphy data to match with EMA data. These participants completed an average of 43.57±19.51 EMA signals over an average of 13.73±2.16 EMA days, and provided an average of 11.13±3.76 days of actigraphy data. A total of 346 data points were available for analyses (given that GLMMs were conducted at the day level). Descriptive statistics and bivariate correlations among continuous variables are displayed in Table 1, and GLMM results are shown in Table 2. With respect to covariates, age was negatively associated LOC eating (B= −.06, SE=.02, p=.016), such that younger participants reported higher levels of LOC. Girls also reported greater LOC eating severity compared to boys (B= −.18, SE=.09, p=.040). Random intercept effects were significant across GLMMs (ps=.002 to .020), indicating there was significant variability in outcomes across participants.
Table 1.
Descriptive statistics and Pearson correlations (N=30)
| z-BMI | Age | TST | NA | LOC eating | |
|---|---|---|---|---|---|
|
| |||||
| z-BMI | - | ||||
| Age | 0.06 | - | |||
| TST | 0.22 | −0.17 | - | ||
| NA | −0.05 | 0.23 | 0.22 | - | |
| LOC eating | 0.00 | −.37* | −0.01 | 0.08 | - |
| Mean | 2.08 | 11.00 | 6.95 | 12.58 | 4.72 |
| SD | 0.44 | 1.89 | 1.62 | 2.26 | 1.56 |
| Minimum | 1.15 | 8.00 | 0.42 | 10.00 | 4.00 |
| Maximum | 2.78 | 14.00 | 9.78 | 21.91 | 10.36 |
Note. BMI=body mass index; TST=total sleep time; NA=negative affect; LOC=loss of control. Variables measured via ecological momentary assessment and actigraphy were aggregated within persons and reflect individual means across the study.
p<.05.
p<.01.
Table 2.
Generalized linear mixed model results for LOC eating
| B | SE | t | p | Lower CI | Upper CI | |
|
|
||||||
| Intercept | 1.61 | 0.07 | 24.06 | <0.001 | 1.48 | 1.74 |
| Gender | −0.18 | 0.09 | −2.06 | 0.040 | −0.35 | −0.01 |
| z-BMI | 0.09 | 0.10 | 0.94 | 0.349 | −0.10 | 0.29 |
| Age | −0.06 | 0.02 | −2.42 | 0.016 | −0.11 | −0.01 |
| TST (between) | −0.03 | 0.03 | −1.10 | 0.271 | −0.09 | 0.02 |
| TST (within) | −0.01 | 0.01 | −1.33 | 0.185 | −0.03 | 0.01 |
| NA (between) | 0.02 | 0.02 | 1.18 | 0.237 | −0.02 | 0.07 |
| NA (within) | <−0.01 | <0.01 | −0.46 | 0.649 | −0.01 | 0.01 |
| TST (between) × NA within) | <-0.01 | <0.01 | −0.97 | 0.332 | −0.01 | <0.01 |
| TST (within) × NA (within) | −0.01 | <0.01 | −2.25 | 0.025 | −0.01 | <-0.01 |
| B | SE | Z | p | Lower CI | Upper CI | |
|
|
||||||
| Random intercept | 0.05 | 0.01 | 3.08 | 0.002 | 0.02 | 0.09 |
A significant interaction between within-person negative affect and within-person TST emerged in the GLMM predicting LOC eating severity (B= −.01, SE<.01, p=.025). As shown in Figure 1, following nights characterized by lower TST (relative to one’s average), higher momentary negative affect was related to higher subsequent LOC eating severity scores. Conversely, this association was attenuated following nights characterized by higher TST.
Figure 1.
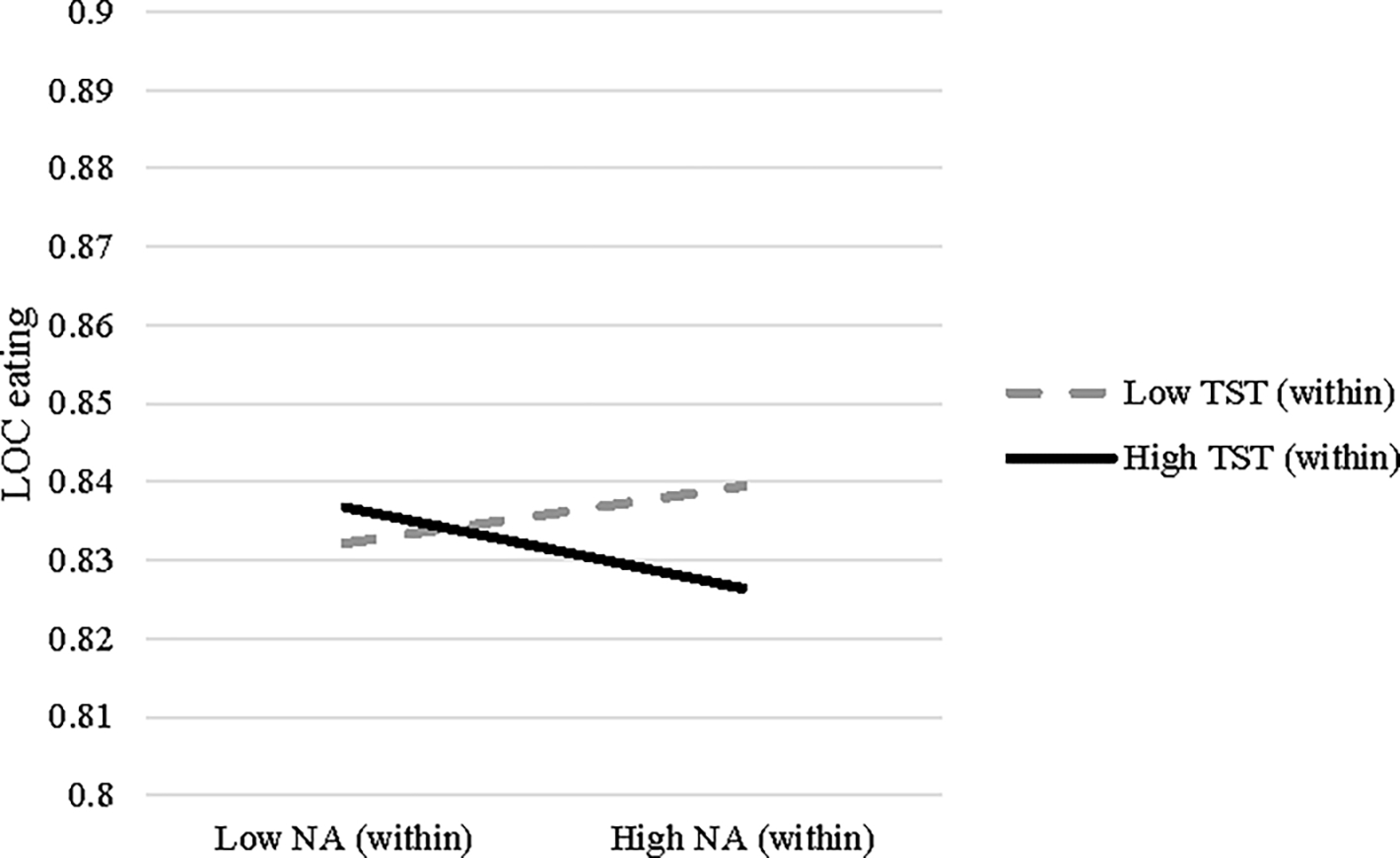
Two-way interaction of momentary (within-person) negative affect (NA) and daily (within-person) total sleep time (TST) predicting loss of control (LOC) eating. High and low values reflect 1 SD above and below individual means, respectively.
Discussion
The present study was the first to examine sleep duration as a moderator of momentary relationships between negative affect and subsequent LOC eating in children and young adolescents with overweight or obesity. Results supported the hypothesis that less sleep on a given night (relative to one’s usual amount of sleep) was associated with more severe LOC following momentary negative affect on the next day. This finding is especially notable given that there are no main effects of negative affect on LOC eating in this sample (Goldschmidt et al., 2018). In addition, longer sleep duration (relative to one’s usual amount) was found to be protective against LOC eating following momentary negative affect, such that a longer night’s sleep made LOC less likely as negative affect increased.
Our findings are overall consistent with a small but growing body of literature demonstrating that sleep disturbance may impact affect and affect regulation in individuals with LOC eating (Cerolini, Ballesio, Ferlazzo, Lucidi, & Lombardo, 2020). A distinct feature of the present study is that we examined within-person sleep, i.e., amount of sleep relative to one’s typical amount, which is distinct from raw amount of sleep. The importance of a “shift” from one’s typical sleep pattern (e.g., sleep variability) has been documented in the broader sleep literature as a negative predictor of several outcomes, such as impaired neurocognitive development in adolescence (Telzer, Goldenberg, Fuligni, Lieberman, & Gálvan, 2015), depression (Suh et al., 2012), excess caloric intake (He et al., 2015). Future eating disorder research should aim to compare the predictive ability of within- versus between-person levels of sleep disruption. Additionally, future research is needed regarding how variables such as sleep and negative affect develop and interact with each other over the course of adolescence into adulthood. For example, children with greater sleep disturbance may be a group vulnerable to higher levels of negative affect (and thus, more frequent subsequent binge eating), which over time could develop into a more severe disordered presentation in adulthood. Given these findings, it is possible that a behavioral intervention meant to reduce sleep variability in particular could improve emotion regulation capacities and in turn, reduce the likelihood of engaging in maladapting coping such as LOC eating. Research suggests that individuals with LOC or binge eating experience greater difficulties with emotion regulation compared to weight matched peers (Leehr et al., 2015); it is possible that longer sleep duration improves emotion regulation to a degree that decreases risk for LOC eating.
There were several important strengths in the current study. First, we used EMA, which allows for understanding temporality of relationships as well as examination of within-person relationships. Second, we used a combination of accelerometry and sleep diaries to objectively measure sleep duration. We also recruited a racially diverse community sample, which allows for greater generalizability. However, these findings must also be interpreted within the context of several important limitations. First, the sample was relatively small, limiting our statistical power. Second, linear mixed models do not allow for the calculation of effect sizes for interaction effects. As such, effects may be small, limiting clinical significance, although even small changes in LOC eating could have large effects on distress and impairment. Third, while EMA confers significant advantages over standard retrospective self-report measures, it still relies on self-report, therefore making it prone to several forms of bias. Relatedly, while children had completed assessments of loss-of-control eating prior to the EMA protocol and parents/children were generally oriented to the assessment protocols, they were not instructed explicitly on how LOC is defined on the EMA, which may have led to inconsistencies in how LOC items were interpreted by participants. Further, there may have been unmeasured individual differences in the extent to which parents helped their children complete the EMA and ASA24 protocols. Lastly, we captured the 8–14 age group, but sleep disruption (as well as LOC eating) may be most prevalent in later adolescence, and as such, future research should examine these relationships in an older group of adolescents.
Overall, sleep duration appears to influence momentary relations between negative affect and LOC eating. Future research should investigate other metrics of sleep disturbance (e.g., sleep continuity or regularity) as they relate to emotion regulation and LOC eating.
Highlights.
The present study examined the moderating role of previous night sleep duration on the momentary relationship between negative affect and LOC eating in children and young adolescents.
Shorter previous night sleep duration strengthened the prospective relations between negative affect and subsequent LOC eating the next day.
Findings suggest that increasing sleep duration may improve LOC eating in response to negative affect.
Funding:
This research was funded by grants from the National Center for Advancing Translational Sciences (UL1-TR000430). Dr. Manasse is supported by an award from the National Institute of Health (K23DK124514).
References
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, & Pollak CP (2003). The role of actigraphy in the study of sleep and circadian rhythms. Sleep, 26(3), 342–392. [DOI] [PubMed] [Google Scholar]
- Anderson C, & Platten CR (2011). Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behavioural brain research, 217(2), 463–466. [DOI] [PubMed] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, & Beebe DW (2014). Sleep restriction worsens mood and emotion regulation in adolescents. Journal of Child Psychology and Psychiatry, 55(2), 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Cao L, Crosby RD, Engel SG, Peterson CB, Crow SJ, … Durkin N (2017). Negative affect and binge eating: Reconciling differences between two analytic approaches in ecological momentary assessment research. International Journal of Eating Disorders, 50(10), 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Crosby RD, Cao L, Crow SJ, Engel SG, Wonderlich SA, & Peterson CB (2015). Negative affect prior to and following overeating-only, loss of control eating-only, and binge eating episodes in obese adults. International Journal of Eating Disorders, 48(6), 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant-Waugh RJ, Cooper PJ, Taylor CL, & Lask BD (1996). The use of the eating disorder examination with children: A pilot study. International Journal of Eating Disorders, 19(4), 391–397. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala N-B, Currie A, Peile E, Stranges S, & Miller MA (2008). Meta-analysis of short sleep duration and obesity in children and adults. Sleep, 31(5), 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2000). Clinical Growth Charts. Retrieved from https://www.cdc.gov/growthcharts/clinical_charts.htm.
- Cedernaes J, Brandell J, Ros O, Broman JE, Hogenkamp PS, Schiöth HB, & Benedict C (2014). Increased impulsivity in response to food cues after sleep loss in healthy young men. Obesity, 22(8), 1786–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerolini S, Ballesio A, Ferlazzo F, Lucidi F, & Lombardo C (2020). Decreased inhibitory control after partial sleep deprivation in individuals reporting binge eating: preliminary findings. PeerJ, 8, e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SG, Crosby RD, Thomas G, Bond D, Lavender JM, Mason T, … Wonderlich SA (2016). Ecological momentary assessment in eating disorder and obesity research: a review of the recent literature. Current psychiatry reports, 18(4), 37. [DOI] [PubMed] [Google Scholar]
- Fisher JO, & Birch LL (2002). Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. The American Journal of Clinical Nutrition, 76(1), 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Evans EW, Saletin JM, O’Sullivan K, Koren D, Engel SG, & Haedt-Matt A (2020). Naturalistic, multimethod exploratory study of sleep duration and quality as predictors of dysregulated eating in youth with overweight and obesity. Appetite, 146, 104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Smith KE, Crosby RD, Boyd HK, Dougherty E, Engel SG, & Haedt-Matt A (2018). Ecological momentary assessment of maladaptive eating in children and adolescents with overweight or obesity. International Journal of Eating Disorders, 51(6), 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Cassoff J, Frenette S, Wiebe S, & Carrier J (2012). Impact of sleep extension and restriction on children’s emotional lability and impulsivity. Pediatrics, 130(5), e1155–e1161. [DOI] [PubMed] [Google Scholar]
- Haedt-Matt AA, & Keel PK (2011). Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychological Bulletin, 137(4), 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CN, Carskadon MA, Considine RV, Fava JL, Lawton J, Raynor HA, … Wing R (2013). Changes in children’s sleep duration on food intake, weight, and leptin. Pediatrics, 132(6), e1473–e1480. [DOI] [PubMed] [Google Scholar]
- He F, Bixler EO, Berg A, Kawasawa YI, Vgontzas AN, Fernandez-Mendoza J, … Liao D (2015). Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Medicine, 16(7), 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert A, Rief W, Tuschen-Caffier B, de Zwaan M, & Czaja J (2009). Loss of control eating and psychological maintenance in children: An ecological momentary assessment study. Behaviour Research and Therapy, 47(1), 26–33. [DOI] [PubMed] [Google Scholar]
- Jensen CD, Duraccio KM, Barnett KA, Carbine KA, Stevens KS, Muncy NM, & Kirwan CB (2019). Sleep duration differentially affects brain activation in response to food images in adolescents with overweight/obesity compared to adolescents with normal weight. Sleep, 42(4), zsz001. [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE Jr, Rudolph KD, Potter KI, Lambert S, … Gathright T (1999). A measure of positive and negative affect for children: scale development and preliminary validation. Psychological assessment, 11(3), 326. [Google Scholar]
- Leehr EJ, Krohmer K, Schag K, Dresler T, Zipfel S, & Giel KE (2015). Emotion regulation model in binge eating disorder and obesity-a systematic review. Neuroscience & Biobehavioral Reviews, 49, 125–134. [DOI] [PubMed] [Google Scholar]
- Liu RT, Steele SJ, Hamilton JL, Do QB, Furbish K, Burke TA, … Gerlus N (2020). Sleep and suicide: A systematic review and meta-analysis of longitudinal studies. Clinical psychology review, 101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzenhofer LM, Engel SG, Crosby RD, Anderson M, Vannucci A, Cohen LA, … Tanofsky-Kraff M (2014). Using ecological momentary assessment to examine interpersonal and affective predictors of loss of control eating in adolescent girls. International Journal of Eating Disorders, 47(7), 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, & Acebo C (2002). The role of actigraphy in sleep medicine. Sleep medicine reviews, 6(2), 113–124. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey M, & Carskadon MA (1994). Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep, 17(3), 201–207. [DOI] [PubMed] [Google Scholar]
- Sauchelli S, Jiménez-Murcia S, Fernández-García JC, Garrido-Sánchez L, Tinahones FJ, Casanueva FF, … De la Torre R (2016). Interaction Between Orexin-A and Sleep Quality in Females in Extreme Weight Conditions. European Eating Disorders Review, 24(6), 510–517. [DOI] [PubMed] [Google Scholar]
- Suh S, Nowakowski S, Bernert RA, Ong JC, Siebern AT, Dowdle CL, & Manber R (2012). Clinical significance of night-to-night sleep variability in insomnia. Sleep Medicine, 13(5), 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, … Yanovski SZ (2011). A prospective study of pediatric loss of control eating and psychological outcomes. Journal of abnormal psychology, 120(1), 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, & Yanovski JA (2009). A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. International Journal of Eating Disorders, 42(1), 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Goldenberg D, Fuligni AJ, Lieberman MD, & Gálvan A (2015). Sleep variability in adolescence is associated with altered brain development. Developmental cognitive neuroscience, 14, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology, 54(6), 1063. [DOI] [PubMed] [Google Scholar]
- Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, & Redline S (2010). The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep, 33(9), 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lau EYY, & Hsiao J. H. w. (2019). Using emotion regulation strategies after sleep deprivation: ERP and behavioral findings. Cognitive, Affective, & Behavioral Neuroscience, 19(2), 283–295. [DOI] [PubMed] [Google Scholar]


