Abstract
Background and Objectives
Due to a reduction in the availability of prescription opioids in the U.S., the potential transition from prescription opioids to heroin is a public health concern. We assessed trajectories of both nonmedical prescription opioid (NMPO) and heroin use from adolescence (age 18) to adulthood (age 50) and how these trajectories were associated with substance use disorder (SUD) in adulthood (age 35 to 50).
Methods
National sample of 26,569 individuals from eleven cohorts of U.S. high school seniors (1976–1986) who were followed until age 50 (2008–2018). The analysis focuses on respondents who engaged in past-year NMPO and heroin use. Outcomes included the endorsement of two or more SUD symptoms.
Results
Among NMPO users, 7.5% had used heroin by the age of 50. The latent profile analyses assessing individuals who reported both NMPO and heroin use during the 32-year study period found four unique trajectory groups: (1) “age 18 concurrent use” (81.2%); (2) “mid-30s NMPO-to-heroin use transition” (10.7%); (3) age 19/20 NMPO-to-heroin use transition, followed by 40s heroin-to-NMPO use transition (4.3%); and (4) “mid-20s NMPO-to-heroin use transition” (3.7%). Respondents in the “mid-30s NMPO-to-heroin use transition” trajectory group had the highest odds of indicating two or more SUD symptoms between ages 35–50.
Conclusion and Scientific Significance
This is the first study to assess NMPO and heroin use trajectories among a national probability-based sample followed from age 18 to 50. The findings suggest that prescription opioid misuse is a risk factor in the development of SUDs and has a long-term impact.
INTRODUCTION
The prescribing of opioid medications and nonmedical prescription opioid (NMPO) use increased significantly in the U.S. for over two decades. However, prescribing and NMPO use have declined in recent years [1–7]. Given the reduction in the availability of prescription opioids in the U.S., public health concerns have shifted to the potential transition from prescription opioid to heroin use. In particular, due to the high potential of people developing opioid use disorders on prescription opioids, the risk for these people to use other more available opioids such as heroin to avoid withdrawal increases when access to prescription opioids is reduced [8–12]. While the vast majority of NMPO use does not lead to heroin use, heroin incidence and prevalence rates in the U.S. have increased and are significantly greater among those who report prior NMPO use [11,13–15]. Among those who initiated heroin use in the past year, 80% reported prior NMPO use, while only 1% of those who initiated NMPO use in the past year had reported prior heroin use [11].
These patterns indicate that NMPO use during adolescence and young adulthood may signal a heroin use trajectory for select individuals during a critical developmental period. Indeed, the mean age of initiation of opioids is 16 years of age (i.e. 16.6) in the U.S., while the mean age of initiating heroin use in the U.S. is 17 years of age (i.e., 17.7) among adolescents and emerging adults.[16] Moreover, a recent national multi-cohort longitudinal study found that 16.4% of adults who indicated NMPO use prior to age 18 (with no history of heroin use) eventually used heroin by the age of 35 [17]. Problematically, NMPO use often involves polysubstance use among adolescents; with nearly seven out of every ten nonmedical users of prescription opioids reporting co-ingestion of prescription opioids with other drugs [18–19]. Polysubstance use with prescription opioids during adolescence is highly concerning given that approximately one in every four U.S. young adults in the general population develop a substance use disorder (SUD) involving alcohol or other drugs [20–21].
Our understanding of NMPO and heroin use among adolescents and young adults aging into adulthood is hindered by a lack of large national prospective investigations that measure frequency of use [22–23]. Accordingly, the purpose of this study is to use a multi-cohort nationally representative longitudinal study to examine the unique trajectories of both NMPO and heroin use over a 32-year period from adolescence (age 18) to adulthood (age 50) (along with identifying factors in adolescence that could predict membership into the unique trajectory groups), and to assess how these trajectories are associated with SUD symptoms in later adulthood.
METHODS
Sample
This study uses national U.S. panel data from the Monitoring the Future (MTF) study [7]. Based on a three-stage sampling procedure, MTF has surveyed nationally representative samples of approximately 17,000 U.S. high school seniors each year since 1975 using classroom-administered questionnaires. Approximately 2,400 high school seniors (modal age 18) were randomly selected each year for biennial follow-ups and surveyed using mailed questionnaires through age 30. One random half of each cohort started biennial surveys at age 19, and the other random half started at age 20. After age 30, respondents were surveyed every five years at ages 35, 40, 45, and 50.
Data from 11 cohorts (1976 through 1986) who were surveyed at modal ages 18 (baseline/12th grade) and ten follow-ups (19/20, 21/22, 23/24, 25/26, 27/28, 29/30, 35, 40, 45, and 50) made up the analytic sample (n = 26,569). The baseline student response rates ranged from 77% to 86%. Most non-response was due to the respondent being absent; less than 1% refused to participate. The MTF panel oversamples individuals who use drugs from the 12th-grade sample to secure a population that reports drug use into adulthood. The overall weighted retention rate for the longitudinal sample from baseline to age 50 was 52%. To help correct for attrition bias, we incorporated attrition weights to account for respondent characteristics associated with non-response at follow-up. The MTF project design, protocol, and sampling methods are described in greater detail elsewhere [6–7].
Measures
Past-year nonmedical prescription opioid (NMPO) use was measured at baseline and each follow-up with identical questions to assess past-year NMPO use (i.e., “…taken any…on your own—that is, without a doctor telling you to take them?”). Respondents were provided a list of several generic and brand name examples for prescription opioids (e.g., hydrocodone, oxycodone, codeine). The response scales for the questions ranged from (0) No occasions to (6) 40 or more occasions. This measure was treated as an ordinal/continuous variable in the analyses to assess mean frequency.
Past-year heroin use was assessed by asking respondents at baseline and each follow-up about “how many occasions (if any) they used heroin during the last 12 months?”. Response scales for the questions ranged from (0) No occasions to (6) 40 or more occasions. This measure was treated as an ordinal/continuous variable in the analyses to assess mean frequency.
Substance use disorder (SUD) symptoms were measured at ages 35, 40, 45, and 50 with several questions based on the DSM criteria for alcohol use disorder (AUD), cannabis use disorder (CUD), and other drug use disorder (ODUD). Fifteen items were used to characterize eight of the 11 DSM-5 symptom criteria that define AUD, CUD, and ODUD: (1) Substance use resulting in a failure to fulfill major role obligations; (2) Continued substance use when physically hazardous; (3) Continued substance use despite persistent or recurrent interpersonal or social problems; (4) Tolerance; (5) Withdrawal; (6) Persistent desire or unsuccessful efforts to cut down substance use; (7) Health-related issue(s) due to substance use; and (8) Craving. The criteria were summed (eight symptoms total and specific to AUD [0–8 AUD symptoms], CUD [0–8 CUD symptoms] and ODUD [0–8 ODUD symptoms]) to obtain an overall number of criteria endorsed in the past five years. Although these measures of SUD symptoms do not yield a clinical diagnosis, the items are consistent with the way SUD has been measured in other large-scale surveys [24–26] and reflects DSM-IV and DSM-5 SUD symptoms [27–28]. We followed recommended practice that any use disorder (including mild, moderate, or severe) was indicated by meeting two or more of the criteria (i.e., two or more symptoms specific to AUD [2+ AUD symptoms], CUD [2+ UD symptoms] and ODUD [2+ ODUD symptoms]), resulting in estimates closely resembling other national estimates for a similar age group [29–30]. Moreover, a composite SUD score was created by determining if any respondent indicated two or more AUD symptoms, two or more CUD symptoms or two or more ODUD symptoms.
Sociodemographic variables and substance use behaviors at baseline included: sex, race/ethnicity, U.S. Census geographic region, urbanicity based on metropolitan statistical area, parental education, college aspirations while in 12th grade, average grade in high school, cohort year, past 30-day cigarette use, past two-week binge drinking, and past 30-day marijuana use (see Table 1 for sociodemographic characteristics of the sample).
Table 1.
Sample characteristics at age 18 for full sample (n = 26,569)
| % (n) | Missing % (n) | |
|---|---|---|
| Sex | ||
| Male | 49.2% (13073) | |
| Female | 50.8% (13496) | |
| Race/ethnicity | ||
| White | 79.3% (21082) | |
| Black | 10.7% (2846) | |
| Hispanic | 3.6% (946) | |
| Other | 6.4% (1701) | |
| GPA | ||
| B- or higher | 69.8% (17917) | |
| C+ or lower | 30.2% (7735) | |
| Parents level of education | ||
| Less than a college degree | 64.7% (16458) | |
| College degree or higher | 35.3% (8964) | |
| Cigarette Use (past 30-day) | ||
| No | 60.5% (15761) | |
| Yes | 39.5% (10295) | |
| Binge drink (past two-week) | ||
| No | 52.3% (13098) | |
| Yes | 47.7% (11952) | |
| Marijuana (past 30-day) | ||
| No | 57.4% (14733) | |
| Yes | 42.6% (10947) | |
| Urbanicity | ||
| Large MSA (Urban) | 26.6% (7072) | |
| Other MSA (Suburban) | 42.2% (11206) | |
| Non-MSA (Rural) | 31.2% (8297) | |
| U.S. Region | ||
| Northeast | 24.2% (6441) | |
| Midwest | 29.7% (7882) | |
| South | 29.9% (7937) | |
| West | 16.2% (4315) | |
| Cohort Year | ||
| 1976–1978 | 26.3% (6993) | |
| 1979–1981 | 27.7% (7353) | |
| 1982–1984 | 27.5% (7301) | |
| 1985–1986 | 18.5% (4928) |
Analysis
First, latent profile analysis (LPA) was used to create longitudinal profiles of respondents based on past-year NMPO and heroin use at each specific wave. Of note: only respondents who indicated both NMPO and heroin use during the study period—during the same wave or at separate waves—were included in the LPA (n = 444; 1.2%) to extract unique trajectories of NMPO use and heroin use in order to detect potential transitions (e.g., NMPO-to-heroin use). The LPA did not include respondents who indicated: no past-year NMPO and heroin use (n = 18,252; 83.2%), only NMPO use (n = 4993; 15.1%), and only heroin use (n = 127; 0.50%). The exploratory LPA (with no covariates) was conducted using Mplus (version 8.0), and model fit was compared across different class solutions (1–6 class solutions) for any past-year NMPO and heroin use across the eleven waves. Class membership was determined using a modal approach, which involved identifying the highest posterior predicted probability of class membership for each of the respondents based on the best-fitting model [31].
Second, multinomial logistic regression was used to examine how several key sociodemographic characteristics and substance use behaviors at baseline were associated with each trajectory group defined in the LPA; relative risk ratios (RRR) and 95% confidence intervals were reported using the “no use group” as the reference. Moreover, separate groups were also included in this analysis for respondents who indicated NMPO use only and heroin use only during the study period.
Third, descriptive statistics and adjusted odds ratios (AOR) were generated in Stata to examine the association between the trajectory groups and prevalence of two or more SUD symptoms between ages 35 and 50 (i.e., AUD, CUD, ODUD, any SUD). Logistic regression models were fitted using the generalized estimating equations (GEE) methodology[32] with an exchangeable correlation structure to assess the association between the trajectory groups and the past five-year prevalence of SUD symptoms during this 15-year period in middle adulthood (while accounting for the key control variables from step two above). Both AORs and 95% confidence intervals were reported in the GEE models.
All analyses incorporated survey weights provided by the MTF study to account for differential probabilities of selection into the sample in estimation and variance estimation. The LPA estimated in Mplus used full information likelihood estimation; only respondents who completed at least one follow-up were included. With respect to assessing the association between trajectory groups and SUDs, sample sizes varied across analyses due to missing items. All descriptive and multivariate analyses were conducted using STATA 17.0 (Stata Corp, College Station, Texas) and were weighted to adjust for differential attrition at age 50 [28]. The University of Michigan Institutional Review Board deemed this study exempt as deidentified data was used.
RESULTS
NMPO and heroin use trajectories from late adolescence to age 50
The analysis found that 7.5% of the respondents who indicated NMPO use reported heroin use by the age of 50, while 70.3% of those who reported heroin use indicated NMPO use. Out of the full sample, 1.2% (n = 444) indicated both NMPO use and heroin use between ages 18–50. The LPA (based on these 444 individuals) indicated that a four-class solution for the 11 waves assessing frequency of past-year NMPO and heroin use was the best fitting model. Model fit was assessed using the Bayesian Information Criterion (BIC), Lo-Mendell-Rubin adjusted likelihood ratio test (LRT), and entropy scores. The four-class (compared between a one- and six-class solution) solution was selected given the highest entropy score (entropy=0.949); the BIC = 20964.84 and AIC = 20860.63 for the four-class solution was smaller when compared to the three-class solution (versus BIC = 21310.87 and AIC = 21227.87 for a three-class solution) [30]. It should be noted that the Vuong-Lo-Mendell-Rubin Likelihood Ratio Test (VLMR = 512.78, p=.165) or LRT test (LRT=509.44, p=.168) when comparing the four-class and three-class solutions were not statistically significant; this may be due to the relatively small sample size of individuals reporting both NMPO and heroin use during the study period. Based on the latent profile analyses for the age 50 sample, the most prevalent trajectory group was “age 18 concurrent use” (81.2%), followed by “mid-30s NMPO-to-heroin use transition” (10.7%), “age 19/20 NMPO-to-heroin use transition, followed by 40s heroin-to-NMPO transition” (4.3%), and “mid-20s NMPO-to-heroin use transition” (3.7%). (see Figure 1).
Figure 1.
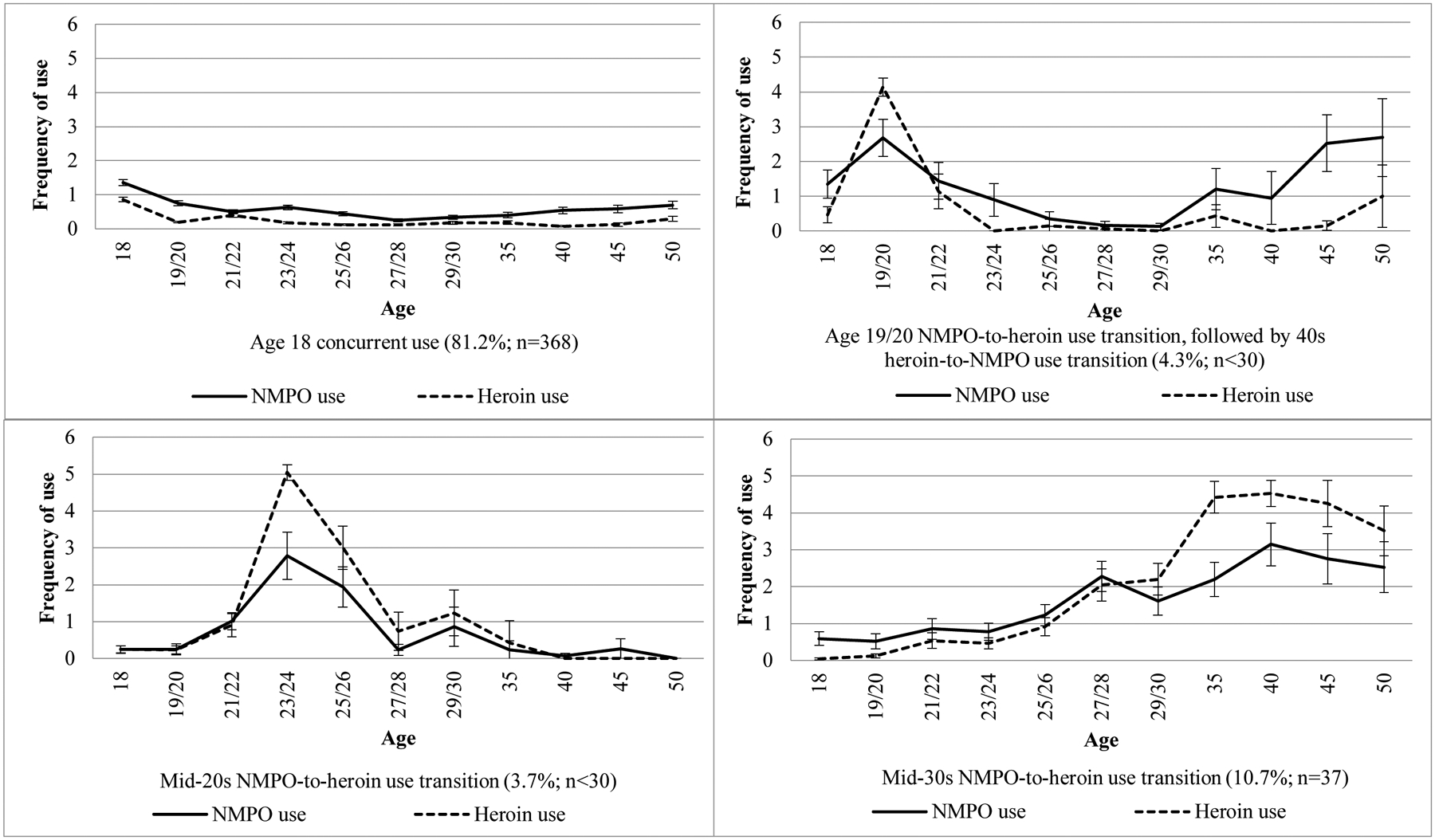
Trajectories of nonmedical prescription opioid use and heroin use between ages 18 and 50 from a latent profile analysis (n=444)
Notes: All percentages use attrition weights at age 50. All 95% confidence intervals are based on standard errors using Taylor series linearization. Response scales for past-year nonmedical prescription opioid use and heroin use ranged from (0) No occasions to (6) 40 or more occasions. NMPO = nonmedical prescription opioid. NMPO Use and Heroin Use among the Full Sample: No use (i.e., no NMPO use or heroin use), 83.2% (n=18252); NMPO use only, 15.1% (n=4993); Heroin use only, 0.5% (n=127); Both NMPO and heroin use, 1.2% (n=444).
Factors associated with NMPO and heroin use trajectory membership
As shown in Table 2, several baseline measures were significantly associated with trajectory group membership. In particular, the expected risk of being in any the four trajectory groups (when compared to the “no use” group) was substantially higher for respondents who indicated either cigarette or marijuana use at age 18. Moreover, the expected risk of being in the “age 18 concurrent use” trajectory group was lower among respondents who were female and from later cohorts.
Table 2.
Multinomial logistic regression: baseline predictors of nonmedical prescription opioid use and heroin use over 32 years (ages 18–50; n = 20,575)
| NMPO use only | Heroin use only | Age 18 concurrent use | Age 19/20 NMPO-to-heroin use transition, followed by 40s heroin-to-NMPO use transition | Mid-20s NMPO-to-heroin use transition” | Mid-30s NMPO-to-heroin use transition | |
|---|---|---|---|---|---|---|
| RRR(95% CI) | RRR(95% CI) | RRR(95% CI) | RRR(95% CI) | RRR(95% CI) | RRR(95% CI) | |
| Sex | ||||||
| Male | Reference | Reference | Reference | Reference | Reference | Reference |
| Female | .958(.878, 1.04) | .779(.472, 1.28) | .612 *** (.456, .819) | .571(.192, 1.69) | .593(.207, 1.69) | .920(.341, 2.48) |
| Race | ||||||
| White | Reference | Reference | Reference | Reference | Reference | Reference |
| Non-White | .513 *** (.447, .590) | 1.87 * (1.08, 3.24) | .865(.584, 1.28) | .332(.059, 1.84) | .271(.058, 1.25) | .542(.187, 1.56) |
| GPA | ||||||
| B- or higher | Reference | Reference | Reference | Reference | Reference | Reference |
| C+ or lower | .987(.894, 1.09) | .875(.515, 1.48) | 1.12(.841, 1.49) | 1.63(.684, 3.87) | 2.13(.787, 5.78) | .783(.302, 2.02) |
| Parents Level of Education | ||||||
| Less than a college degree | Reference | Reference | Reference | Reference | Reference | Reference |
| College degree or higher | 1.17 *** (1.07, 1.28) | 1.24(.712, 2.16) | 1.03(.778, 1.38) | 1.27(.497, 3.25) | 1.31(.496, 3.49) | .940(.383, 2.30) |
| Cigarette Use (past 30-day) | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 1.53 *** (1.39, 1.68) | 1.54(.889, 2.67) | 1.69 *** (1.24, 2.31) | 2.89 * (1.17, 7.18) | 6.70 ** (1.58, 28.4) | 2.64(.915, 7.63) |
| Binge drink (past two-week) | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 1.43 *** (1.30, 1.57) | 1.36(.771, 2.41) | 2.46 *** (1.74, 3.49) | .984(.320, 3.03) | 1.36(.471, 3.93) | 1.98(.621, 6.33) |
| Marijuana (past 30-day) | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 2.90 *** (2.62, 3.20) | 6.07 *** (3.08, 11.9) | 5.99 *** (4.09, 8.78) | 9.66 *** (2.97, 31.4) | 2.77(.476, 16.2) | 12.1 * (1.64, 89.4) |
| Urbanicity | ||||||
| Urban | Reference | Reference | Reference | Reference | Reference | Reference |
| Suburban | 1.06(.952, 1.18) | .811(.456, 1.44) | .873(.616, 1.23) | 2.62(.595, 11.5) | .345(.109, 1.09) | .889(.295, 2.67) |
| Rural | .935(.831, 1.05) | .788(.413, 1.50) | .951(.661, 1.36) | 2.38(.660, 8.63) | .430(.093, 1.97) | .464(.147, 1.45) |
| U.S. Region | ||||||
| Northeast | Reference | Reference | Reference | Reference | Reference | Reference |
| Midwest | 1.03(.922, 1.15) | .838(.431, 1.63) | .925(.651, 1.31) | .050 ** (.006, .413) | .533(.115, 2.46) | .219 * (.067, .720) |
| South | .967(.855, 1.09) | 1.11(.463, 1.71) | 1.29(.877, 1.92) | .707(.244, 2.04) | .444(.093, 2.11) | .421(.124, 1.42) |
| West | 1.65 *** (1.44, 1.89) | 1.78(.893, 3.55) | 1.61 * (1.01, 2.57) | .110 * (.013, .915) | 1.82(.341, 9.69) | .548(.158, 1.90) |
| Cohort Year | ||||||
| 1976–1978 | Reference | Reference | Reference | Reference | Reference | Reference |
| 1979–1981 | .945(.846, 1.05) | .864(.464, 1.61) | 523 *** (.373, .723) | .785(.252, 2.44) | 1.04(.294, 3.70) | .516(.215, 1.24) |
| 1982–1984 | .995(.889, 1.11) | .889(.463, 1.71) | .726(.507, 1.03) | .368(.093, 1.45) | .106 * (.013, .839) | .407(.131, 1.26) |
| 1985–1986 | 1.09(.965, 1.24) | .863(.435, 1.71) | .559 * (.356, .877) | .468(.090, 2.43) | .188(.022, 1.58) | .705(.181, 2.74) |
Notes: RRR = relative risk ratios.
p<.05.
p<.01.
p<.001 (all statistically significant values are in bold).
The “No use” group is the reference for the multinomial logistic regression. Unweighted samples sizes are provided (listwise deletion was used to handle missing data across the age 18 predictors). All provided estimates use weights to adjust for attrition at age 50. Models control for sex, race/ethnicity, parents level of education, urbanicity, U.S. region, cohort year, average GPA in their senior year, college aspirations in their senior year, past 30-day cigarette use in their senior year, past two-week binge drinking in their senior year, and past 30-day marijuana use in their senior year.
NMPO and heroin use trajectory groups and SUD symptoms between ages 35–50
Table 3 shows that when compared to the “no use” group, respondents who only engaged in NMPO use, the “age 18 concurrent use” group, and the “mid-30s NMPO-to-heroin use transition” group were associated with increased odds of indicating two or more AUD, CUD, ODUD, and any SUD symptoms between ages 35 to 50, when controlling for background characteristics, other drug use covariates, and the time of survey collection. Respondents who only engaged in heroin use and the “age 19/20 NMPO-to-heroin use transition, followed by 40s heroin-to-NMPO use transition” group were associated with increased odds of indicating two or more CUD and ODUD symptoms between ages 35 to 50 when compared to the “no use” group. The odds of indicating two or more SUD or ODUD symptoms was higher among the “mid-30s NMPO-to-heroin use transition” group when compared to the “no use” group, “NMPO use only” group, “heroin use only” group, “age 18 concurrent use” group, and “mid-20s NMPO-to-heroin use transition” group.
Table 3.
Generalized estimating equations logistic regression: nonmedical prescription opioid and heroin use trajectory groups and substance use disorder symptoms (ages 18–50)
| AUD 2+ | CUD 2+ | ODUD 2+ | Any SUD 2+ | |
|---|---|---|---|---|
| n = 14,878 | n = 14,684 | n = 14,840 | n = 14,927 | |
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| NMPO & Heroin Use Groups – Time Invariant | ||||
| No use | Reference | Reference | Reference | Reference |
| NMPO use only | 2.00 *** (1.84, 2.18) | 3.53 *** (3.02, 4.11) | 5.27 *** (4.40, 6.30) | 2.30 *** (2.11, 2.51) |
| Heroin use only | 1.28(.809, 2.03) | 2.76 ** (1.33, 5.72) | 4.09 *** (1.97, 8.46) | 1.22(.768, 1.95) |
| Age 18 concurrent use peak | 2.06 *** (1.61, 2.65) | 3.45 *** (2.46, 4.83) | 8.49 *** (5.69, 12.6) | 2.27 *** (1.75, 2.93) |
| Age 19/20 NMPO-to-heroin use transition, followed by 40s heroin-to-NMPO use transition | 3.29(.732, 14.8) | 5.13 * (1.21, 21.7) | 10.1 *** (2.63, 38.6) | 4.29(.853, 21.6) |
| Mid-20s NMPO-to-heroin use transition | .989(.281, 3.48) | 1.94(.365, 10.3) | 13.8***(4.59, 41.7) | 1.72(.530, 5.61) |
| Mid-30s NMPO-to-heroin use transition | 5.79 *** (2.06, 16.3) | 9.25 *** (4.53, 18.9) | 210.7 *** (77.4, 573.1) | 26.3 *** (8.44, 82.2) |
| Time – Time Varying | ||||
| Time – continuous (0 = age 35, 1 = 40, 2 = 45, 3 = 50) | .831 *** (.815, .848) | .691 *** (.662, .722) | .805 *** (.767, .844) | .837 *** (.821, .853) |
Notes: NMPO = nonmedical prescription opioid.
p<.05.
p<.01.
p<.001 (all statistically significant values are in bold).
Unweighted samples sizes are provided (listwise deletion was used to handle missing data across the age 18 predictors and age 35 to 50 substance use disorder outcomes). All provided estimates use weights to adjust for attrition at age 50. Models control for the following time invariant variables: sex, race/ethnicity, parents level of education, urbanicity, U.S. region, cohort year, average GPA in their senior year, past 30-day cigarette use in their senior year, past two-week binge drinking in their senior year, and past 30-day marijuana use in their senior year.
DISCUSSION
This is the first prospective study to assess NMPO and heroin use trajectories among a national probability-based sample followed from age 18 to 50. This study found that 92.5% of the respondents who indicated NMPO use had never used heroin by the age of 50, while 70.3% of those reporting heroin use also indicated NMPO use. Our results confirm prior research: the majority of individuals who have misused opioids never initiate heroin, while the majority people who use heroin have misused prescription opioids at some point during their life [9,11,15,17]. However, this study also found that individuals who engaged in only NMPO use with no history of heroin use by the time they reached age 50 still had at least two times greater odds of indicating two or more SUD symptoms during middle adulthood (when compared to their peers who never engaged in NMPO or heroin use). These findings suggest that any prescription opioid misuse is a significant risk factor in the development of SUDs and has a long-term impact [33].
Another important finding from this study is that the majority of the respondents reporting both NMPO and heroin use peaked at age 18 and typically only engaged in experimental use (i.e. 1–2 occasions during a 12-month span). However, these respondents still had greater odds of indicating two or more SUD symptoms involving other substance classes in middle adulthood. Prior research has found that experimental NMPO use during adolescence is not generally associated with greater odds of indicating SUDs in early adulthood [33]. However, the present study demonstrates that those who report experimental NMPO and heroin use must be viewed from a polysubstance use perspective and should be considered as high risk for the development of later SUD symptoms. The majority of U.S. adults with a past-year DSM-5 opioid use disorder also meet criteria for at least one other DSM-5 substance use disorder [34].These findings reinforce the importance of clinicians screening for a range of substances and referring individuals for more comprehensive substance use assessment when individuals report both NMPO and heroin use, even when such use is experimental.
We also found evidence of a small high-risk group of individuals that appear to transition from NMPO use to heroin use later in middle adulthood. In particular, the respondents in the “Mid 30s NMPO-to-heroin use transition” group had the highest risk of indicating all forms of SUD symptoms between age 35 and 50. This is a small but clinically important subgroup to identify and distinguish from other subgroups due to its heightened risk for SUD symptoms. These late peak subgroups with higher frequency of NMPO and heroin use indicate that the transition from NMPO to heroin use in middle adulthood may be less associated with experimental use, and more likely associated with substance-related problems, such as SUD symptoms and possibly overdose. Unintentional overdose is a leading cause of mortality among individuals who inject drugs [9]. The higher frequency of NMPO use and heroin use in the later peak subgroups reinforces the importance of overdose training for the social networks of individuals who inject opioids.
Limitations
We note limitations in our study that call for future work. First, self-reporting bias is a potential issue. Although self-report substance use data have been found to be reliable and valid, under- or over-reporting of substance use does occur [35]. Second, attrition in the MTF panel is differential with respect to substance use, indicating that individuals who use drugs are less likely to remain in the sample. Although this is addressed by using attrition weights, our findings likely reflect conservative estimates of NMPO and heroin use and associated substance-related problems. Third, we did not examine medical use of prescription opioids or the full array of SUD symptoms during the study period; more longitudinal research is needed to examine how medical use is linked to subsequent NMPO use, heroin use, and SUD symptoms. Fourth, the MTF does use a school-based sample and misses adolescents who have dropped out of high school, truant, incarcerated (subpopulations more likely to have an SUD) or those who are homeschooled (a subpopulation less likely to have an SUD). However, it should be noted that the MTF panel purposely oversamples heavy drug users during the 12th grade to help capture a risker subset of adolescents to follow into adulthood. Finally, while the sample size of respondents who indicated transitions from NMPO use to heroin use was relatively small, these subgroups represent individuals who are infrequently studied and at significantly greater risk of developing SUD symptoms.
CONCLUSIONS
This study fills an important gap by assessing longitudinal transitions between NMPO use and heroin use, as well as the associated substance-related problems, over a 32-year period using a large multi-cohort U.S. panel study. While NMPO use has declined in recent years, the increase in prescription opioid use and misuse during the past two decades has had a long-term impact on the substance use behaviors in the U.S. population. We must continue to examine trajectories of prescription opioid and heroin use, and how these trajectories are associated with later SUDs among U.S. adults. Such studies can guide clinical screening and intervention, as well as prevention and secondary intervention efforts, to avoid short- and long-term health consequences related to prescription opioid and heroin use.
ACKNOWLEDGMENTS
This study was supported by grant R01DA031160 the National Institutes of Health (NIH), National Institute on Drug Abuse (NIDA).
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
References
- 1.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. Rockville, Md.: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, 2019. (HHS Publication No. PEP19–5068, NSDUH Series H-54). (https://www.samhsa.gov/data/) [Google Scholar]
- 2.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015;372:241–8. [DOI] [PubMed] [Google Scholar]
- 3.Fortuna RJ, Robbins BW, Caiola E, Joynt M, Halterman JS. Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics 2010;126:1108–16. [DOI] [PubMed] [Google Scholar]
- 4.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician 2010;13:401–35. [PubMed] [Google Scholar]
- 5.McCabe SE, West BT, Veliz P, McCabe VV, Stoddard SA, Boyd CJ. Trends in medical and nonmedical use of prescription opioids among US adolescents: 1976–2015. Pediatrics 2017;139:e20162387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future national survey results on drug use, 1975–2019: volume I, secondary school students. Ann Arbor, Mi.: Institute for Social Research, The University of Michigan, 2020. [Google Scholar]
- 7.Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the Future national survey results on drug use, 1975–2019: volume II, college students and adults ages 19–60. Ann Arbor, Mi.: Institute for Social Research, The University of Michigan, 2020. [Google Scholar]
- 8.Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA 2014;312:118–9. [DOI] [PubMed] [Google Scholar]
- 9.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016;374:154–63. [DOI] [PubMed] [Google Scholar]
- 10.Kanouse AB, Compton P. The epidemic of prescription opioid abuse, the subsequent rising prevalence of heroin use, and the federal response. J Pain Palliat Care Pharmacother 2015;29:102–14. [DOI] [PubMed] [Google Scholar]
- 11.Muhuri PK, Gfroerer JC, Davie C. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Review, Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 12.Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend 2003;69:215–32. [DOI] [PubMed] [Google Scholar]
- 13.Lipari RN, Hughes A. The NSDUH report: trends in heroin use in the United States: 2002 to 2013. The CBHSQ report. Rockville, Md.: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, 2015. [PubMed] [Google Scholar]
- 14.Martins SS, Sarvet A, Santaella-Tenorio J, Saha T, Grant BF, Hasin DS. Changes in US lifetime heroin use and heroin use disorder: prevalence from the 2001–2002 to 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCabe SE, Boyd CJ, Cranford JA, Teter CJ. Motives for nonmedical use of prescription opioids among high school seniors in the United States: self-treatment and beyond. Arch Pediatr Adolesc Med 2009;163:739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alcover KC, Thompson CL. Patterns of Mean Age at Drug Use Initiation Among Adolescents and Emerging Adults, 2004–2017. JAMA Pediatr. 2020;174(7):725–727. doi: 10.1001/jamapediatrics.2019.6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCabe SE, Boyd CJ, Evans-Polce RJ, McCabe VV, Schulenberg JE, Veliz PT. Pills to powder: a 17-year transition from prescription opioids to heroin among us adolescents followed into adulthood [published online ahead of print, 2020 Sep 24]. J Addict Med 2020; 10.1097/ADM.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCabe SE, Cranford JA, Morales M, Young A. Simultaneous and concurrent polydrug use of alcohol and prescription drugs: prevalence, correlates, and consequences. J Stud Alcohol. 2006;67(4):529–537. doi: 10.15288/jsa.2006.67.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCabe SE, West BT, Teter CJ, Boyd CJ. Co-ingestion of prescription opioids and other drugs among high school seniors: results from a national study. Drug Alcohol Depend 2012;126:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 2007;64:830–42. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593–602. [DOI] [PubMed] [Google Scholar]
- 22.Young AM, Glover N, Havens JR. Nonmedical use of prescription medications among adolescents in the United States: a systematic review. J Adolesc Health 2012;51:6–17. [DOI] [PubMed] [Google Scholar]
- 23.Compton WM, Lopez MF. Pathways to heroin use: commentary on McCabe et al [published online ahead of print, 2020 Sep 24]. J Addict Med 2020; 10.1097/ADM.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 24.Harford TC, Muthén BO. The dimensionality of alcohol abuse and dependence: a multivariate analysis of DSM-IV symptom items in the National Longitudinal Survey of Youth. J Stud Alcohol 2001;62:150–7. [DOI] [PubMed] [Google Scholar]
- 25.Muthén BO. Psychometric evaluation of diagnostic criteria: application to a two-dimensional model of alcohol abuse and dependence. Drug Alcohol Depend 1996;41:101–12. [DOI] [PubMed] [Google Scholar]
- 26.Nelson CB, Heath AC, Kessler RC. Temporal progression of alcohol dependence symptoms in the U.S. household population: results from the National Comorbidity Survey. J Consult Clin Psychol 1998;66:474–83. [DOI] [PubMed] [Google Scholar]
- 27.Merline A, Jager J, Schulenberg JE. Adolescent risk factors for adult alcohol use and abuse: stability and change of predictive value across early and middle adulthood. Addiction 2008;103:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulenberg JE, Patrick ME, Kloska DD, Maslowsky J, Maggs JL, O’Malley PM. Substance use disorder in early midlife: a national prospective study on health and well-being correlates and long-term predictors. Subst Abuse 2016;9:41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013. [Google Scholar]
- 30.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015;72:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins LM, Lanza ST. Latent class and latent transition analysis: with applications in the social, behavioral, and health sciences. Hoboken, Nj.: John Wiley & Sons, Inc., 2010. [Google Scholar]
- 32.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 33.McCabe SE, Veliz PT, Boyd CJ, Schepis TS, McCabe VV, Schulenberg JE. A prospective study of nonmedical use of prescription opioids during adolescence and subsequent substance use disorder symptoms in early midlife. Drug Alcohol Depend 2019;194:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCabe SE, West BT, Jutkiewicz EM, Boyd CJ. Multiple DSM-5 substance use disorders: a national study of US adults. Hum Psychopharmacol 2017;32: 10.1002/hup.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston LD, O’Malley PM. Issues of validity and population coverage in student surveys of drug use. NIDA Res Monogr 1985;57:31–54 [PubMed] [Google Scholar]


