Abstract
Background:
Cough hypersensitivity is an important part of the neurophysiology of cough, which presents with increased cough response to a lower level of stimuli or triggers. Classification of stimuli might bring about additional insight into the underlying mechanisms and management.
Objectives:
This study investigated the profile of cough triggers in chronic cough patients and their relationship with capsaicin cough sensitivity.
Design:
This was a cross-sectional observational study.
Methods:
We enrolled patients with different causes of chronic cough from 2006 to 2021. Cough triggers were defined as cough response to chemical triggers, mechanical triggers, meal triggers, or thermal trigger. Cough sensitivity to capsaicin was evaluated by the capsaicin challenge test, which was expressed as the lowest concentration of capsaicin inducing 5 or more coughing (C5).
Results:
Among 1211 patients with chronic cough, 1107 (91.4%) patients reported at least one cough trigger. Chemical triggers (66.9%) were the most common cough triggers, followed by thermal exposure (50.6%), mechanical triggers (48.2%), and meal triggers (21.2%). There was no difference in the proportion of chemical triggers among different etiologies. Patients with refractory chronic cough reported the highest prevalence of cough triggers (97.1%). A higher number of meal triggers (34.9%) was associated with gastroesophageal reflux-related cough, and meal triggers and mechanical triggers were more common in refractory chronic cough. Among 254 patients who completed capsaicin challenge test, both the number of total triggers and the number of chemical triggers had a significant but mild correlation with capsaicin cough sensitivity.
Conclusion:
Cough hypersensitivity as reflected by a variety of cough triggers is a common feature in chronic cough patients, but different etiologies present specific profiles of cough triggers, which could not be evaluated comprehensively by capsaicin cough sensitivity.
Keywords: capsaicin cough sensitivity, chronic cough, cough hypersensitivity, cough trigger, refractory chronic cough
Introduction
Chronic cough (CC), defined as a cough lasting longer than 8 weeks, 1 is a frequent condition in general practitioners’ and respiratory specialists’ clinics. It could cause severe impairment of quality of life, limitation of activities, and depression. 2
Cough hypersensitivity is an important part of the neurophysiology of cough, presented with enhanced cough in response to endogenous and exogenous stimuli. This heightened cough sensitivity was observed in both pulmonary and extrapulmonary conditions. 2 To assess the sensitivity of the cough, challenges with inhaled cough-provoking stimuli such as capsaicin, citric acid, or allyl isothiocyanate are exploited,3–5 among which capsaicin is the most widely used chemical but has its limitations. Although the average sensitivity to capsaicin is increased in CC than in healthy control, there is substantial overlap in the data distributions between these two groups. 6 What’s more, capsaicin is a selective agonist of transient receptor potential vanilloid 1 (TRPV1) while cough hypersensitivity is associated with central and/or peripheral hypersensitivity, 2 which deprives capsaicin challenge test of reflecting completely the cough sensitivity. Thus, additional tools to assess cough sensitivity are needed.
It has been widely accepted that an exaggerated cough response to daily cough triggers is another sign of cough hypersensitivity. Previous studies found that cough hypersensitivity among different causes seems to be slightly different, indicated by various responsiveness to stimuli. Cough response to ‘cold air’ is associated with a diagnosis of asthma,7,8 and unexplained CC presents a higher number of positive responses to cough triggers. 9 Nonetheless, these results were derived from studies with either inaccurate diagnoses based on symptoms or small sample sizes. Therefore, a comprehensive description of cough response to cough triggers in CC patients with different etiologies is necessary.
Recently, cough hypersensitivity syndrome has been redefined as a disorder characterized by troublesome coughing that is often triggered by low levels of thermal, mechanical, or chemical exposure. 2 A classification of cough triggers can bring about additional insight into the underlying mechanisms and different types of management. However, the definition of different kinds of exposure hasn’t been well defined. In addition, the whole picture of cough hypersensitivity to this newly defined classification of exposure is still poorly understood and warrants validation in different populations.
Therefore, the present study is aimed to investigate the profile of classified triggers in different causes of CC. Besides, we explore the association between cough sensitivity to cough triggers and capsaicin.
Methods
This was a cross-sectional observational study. We recruited the consecutive and unselected CC patients who underwent full investigations and treatment at the First Affiliated Hospital of Guangzhou Medical University from 2006 to 2021 in this study. All these patients with CC had undergone thorough diagnostic and treatment workflow as previously described.1,10,11 A standard questionnaire was used to record demographics, clinical features, cough triggers, laboratory results, response to therapy, follow-up, and final diagnosis for CC patients. The inclusion criteria were as follows: (1) aged 14 years or more; (2) cough as the sole or predominant symptom lasting for at least 8 weeks; and (3) without overt identifiable abnormalities on chest X-ray. Patients were excluded under the following conditions: (1) without full investigations or loss of follow-up to make a definite diagnosis; and (2) incomplete record of cough triggers.
Cough triggers were classified as chemical triggers, mechanical triggers, thermal trigger, and meal triggers. The definitions of different kinds of triggers are displayed in Table 1. Chemical triggers were a range of irritant environmental chemicals that could cause inflammation or tissue damage, including dust, cigarette smoke, and cooking fumes. Mechanical triggers were defined as factors associated with a change of tension of the larynx or intrathoracic pressure, including talking, exercise, and supine position. Thermal trigger in this study was recorded if cough was induced by cold air. Cough that occurs during meals, within 2 h after meals, or after drinking alcohol was defined as the response to meal triggers.
Table 1.
The definition of different kinds of cough triggers.
| Variables | Definition | Cough triggers |
|---|---|---|
| Chemical triggers | A range of irritant environmental chemicals and mediators that could cause inflammation or tissue damage | Dust, cigarette smoke, cooking fumes |
| Mechanical triggers | Factors associated with change of tension of larynx or intrathoracic pressure | Talking, exercise, supine position |
| Thermal trigger | Factors relating to temperature | Cold air |
| Meal triggers | Factors relating to eating or drinking alcohol | Cough during meals, cough within 2 h after meals, cough after drinking alcohol |
Cough reflex sensitivity to capsaicin was tested in a subset of patients. These subjects were participants in one of our previous studies investigating the phenotypes of cough hypersensitivity (ClinicallTrials.gov NCTO2591550). 12 Participants exhaled to functional residual capacity and then inhaled through the mouthpiece for 1 s. Each participant inhaled capsaicin solution of increasing concentrations by a single breath in the dosimeter method at 1-minute intervals. Record the frequency of coughs during the first 30 seconds after inhalation. The lowest concentrations of capsaicin that evoked two (C2) or five (C5) coughs were recorded. Cough hypersensitivity to capsaicin was suggested when C5 was 62.5 μmol/L or less.
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 13
Statistical analyses
Sample size and power were determined by power analysis using PASS (version 15.0, NCSS, LLC.). Data were expressed as frequency (percentage) or median (interquartile range). Shapiro–Wilk test was used to evaluate the normality. Statistical comparisons between groups were performed with one-way ANOVA for normally distributed data, Kruskal–Wallis tests for skewed data, and χ2 tests or Fisher exact tests for proportions data, followed by post hoc tests with Bonferroni correction for multiple comparisons. A logistic regression test was used to identify triggers associated with the corresponding diagnosis. Variables whose p-value <0.1 in univariable models were put into the multivariable model. Multiple logistic regression analysis was conducted with the method of enter. The Spearman rank correlation test was performed to assess the correlation between the number of cough triggers and capsaicin cough sensitivity. Data were analyzed with SPSS Statistics version 25.0 (IBM Corp, Armonk, NY, USA) and R programming language.
Results
Clinical features of patients
A total of 1211 patients were enrolled in the study. The demographic characteristics are shown in Table 2. The median age of all patients was 40.0 (IQR 31.0–52.0) years. The number of females (644, 53.2%) was roughly equal to that of males (567, 46.8%). The median course of the disease was 24.0 (IQR 9.3–82.5) months. Among the 1211 patients, 240 (19.8%) were diagnosed as asthma, 277 (22.9%) as EB, 90 (7.4%) as upper airway cough syndrome (UACS), 189 (15.6%) as gastroesophageal reflex-related cough (GERC), 71 (5.9%) as atopic cough (AC), 206 (17.0%) as RCC, and 138 (11.4%) as other causes. Pharyngeal symptoms were observed in most subjects (85.2%), including tickle in the throat (47.2%), frequent throat clearing (36.3%), pharyngeal foreign body sensation (28.1%), tickle below the throat (26.7%), and mucus adhesion to the throat (24.1%).
Table 2.
Prevalence of cough triggers in different causes among 1211 subjects with chronic cough.
| Variables | Total (n = 1211) | Asthma (n = 240) | EB (n = 277) | UACS (n = 90) | GERC (n = 189) | AC (n = 71) | Other Cause (n = 138) | RCC (n = 206) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Female, n (%) | 644 (53.2) | 173 (72.1)* | 140 (50.5) $ | 40 (44.4) $ | 84 (44.4) $ | 31 (43.7) $ | 81 (58.7)* $ | 95 (46.1) $ | <0.001 |
| Age, Median (Q1, Q3) | 40.0 (31.0, 52.0) | 45.0 (34.8, 56.0)* | 39.0 (31.0, 52.0)* $ | 37.0 (29.0, 47.0) $ | 38.0 (30.0, 49.0) $ | 39.0 (30.0, 49.5)* $ | 38.0 (30.0, 52.0)* $ | 39.0 (30.0, 50.0) $ | <0.001 |
| Duration month, Median (Q1, Q3) | 24.0 (9.3, 82.5) | 24.0 (9.8, 80.0)* $ ‡ | 24.0 (6.0, 84.0)* ‡ | 24.0 (6.3, 72.0)* $ ‡ | 36.0 (12.0, 84.0) $ ‡ | 36.0 (12.0, 84.0)* $ ‡ | 18.0 (5.3, 57.0)* | 36.0 (12.0, 96.0) $ | <0.001 |
| Triggers, n (%) | 1107 (91.4) | 208 (86.7)* | 244 (88.1)* | 85 (94.4)* $ | 176 (93.1)* $ | 66 (93.0)* $ | 128 (92.8)* $ | 200 (97.1) $ | 0.001 |
| Number of triggers, Median (Q1, Q3) | 3 (1.0, 4.0) | 2 (1.0, 4.0)* | 2 (1.0, 4.0)* | 3 (2.0, 4.0)*, $ | 3 (2.0, 4.5) $ | 3 (2.0, 4.0)* $ | 3 (2.0, 4.0)* $ | 3 (1.0, 5.0) $ | <0.001 |
| Chemical triggers, n (%) | 810 (66.9) | 149 (62.1) | 180 (65.0) | 60 (66.7) | 131 (69.3) | 48 (67.6) | 94 (68.1) | 148 (71.8) | 0.440 |
| Dust, n (%) | 473 (39.1) | 79 (32.9)* | 96 (34.7)* $ | 39 (43.3)* $ | 76 (40.2)* $ | 28 (39.4)* $ | 69 (50.0) $ | 86 (41.7)* $ | 0.025 |
| Cooking fumes, n (%) | 575 (47.5) | 116 (48.3) | 122 (44.0) | 43 (47.8) | 93 (49.2) | 35 (49.3) | 53 (38.4) | 113 (54.9) | 0.096 |
| Cigarette smoke, n (%) | 543 (44.8) | 95 (39.6) | 124 (44.8) | 38 (42.2) | 93 (49.2) | 35 (49.3) | 59 (42.8) | 99 (48.1) | 0.416 |
| Number of chemical triggers, Median (Q1, Q3) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 3.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 2.0 (0.0, 2.0) | 0.293 |
| Mechanical triggers, n (%) | 584 (48.2) | 102 (42.5)* | 117 (42.2)* | 45 (50.0)* $ | 110 (58.2) $ | 28 (39.4)* $ | 69 (50.0)* $ | 113 (54.9)* $ | 0.002 |
| Talking, n (%) | 405 (33.4) | 55 (22.9)* | 75 (27.1)* $ | 32 (35.6)* $ ‡ | 77 (40.7) ‡ | 20 (28.2)* $ ‡ | 55 (39.9) $ ‡ | 91 (44.2) ‡ | < 0.001 |
| Exercise, n (%) | 222 (18.3) | 51 (21.2)* | 40 (14.4)* | 8 (8.9)* | 39 (20.6)* | 11 (15.5)* | 33 (23.9)* | 40 (19.4)* | 0.034 |
| Supine position, n (%) | 186 (15.4) | 44 (18.3)* $ | 42 (15.2)* $ | 20 (22.2) $ | 27 (14.3)*, $ | 3 (4.2)* | 16 (11.6)* $ | 34 (16.5)* $ | 0.035 |
| Number of mechanical triggers, Median (Q1, Q3) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0)* | 0.0 (0.0, 1.0)* | 0.5 (0.0, 1.0)* | 1.0 (0.0, 1.0)* | 0.0 (0.0, 1.0)* | 1.0 (0.0, 1.0)* | 1.0 (0.0,1.0)* | 0.003 |
| Meal triggers, n (%) | 257 (21.2) | 31 (12.9)* | 38 (13.7)* | 23 (25.6)* $ ‡ | 66 (34.9)‡ | 14 (19.7)* $ ‡ | 22 (15.9)* | 63 (30.6) $ ‡ | <0.001 |
| During meal, n (%) | 99 (8.2) | 14 (5.8) | 17 (6.1) | 10 (11.1) | 23 (12.2) | 4 (5.6) | 11 (8.0) | 20 (9.7) | 0.143 |
| After meal, n (%) | 139 (11.5) | 13 (5.4)* | 17 (6.1)* | 12 (13.3)* $ ‡ | 45 (23.8)‡ | 8 (11.3)* $ ‡ | 9 (6.5)* $ | 35 (17.0) $ ‡ | <0.001 |
| Alcohol, n (%) | 56 (4.6) | 8 (3.3)* | 8 (2.9)* | 6 (6.7)* | 7 (3.7)* | 4 (5.6)* | 4 (2.9)* | 19 (9.2)* | 0.032 |
| Number of meal triggers, Median (Q1, Q3) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0)* | 0.0 (0.0, 0.0)* | 0.0 (0.0, 1.0)* $ | 0.0 (0.0, 1.0) $ | 0.0 (0.0, 0.0)* $ | 0.0 (0.0, 0.0)* | 0.0 (0.0, 1.0) $ | <0.001 |
| Thermal trigger-Cold air, n (%) | 613 (50.6) | 126 (52.5) | 138 (49.8) | 49 (54.4) | 93 (49.2) | 39 (54.9) | 62 (44.9) | 106 (51.5) | 0.749 |
| Other triggers, n (%) | 271 (22.4) | 47 (19.6)* $ | 35 (12.6) $ | 14 (15.6)* $ | 46 (24.3)* ‡ | 14 (19.7)* $ ‡ | 40 (29.0)* ‡ | 75 (36.4) ‡ | <0.001 |
| Pharyngeal symptoms, n (%) | 1032 (85.2) | 189 (78.8)* | 254 (91.7) $ | 86 (95.6) $ | 168 (88.9)* $ | 56 (78.9)* | 106 (76.8)* | 173 (84.0)* $ | <0.001 |
| Tickle in the throat, n (%) | 572 (47.2) | 106 (44.2) | 138 (49.8) | 50 (55.6) | 95 (50.3) | 28 (39.4) | 56 (40.6) | 99 (48.1) | 0.169 |
| Tickle below the throat, n (%) | 323 (26.7) | 58 (24.2)* | 88 (31.8)* | 33 (36.7)* | 43 (22.8)* | 13 (18.3)* | 35 (25.4)* | 53 (25.7)* | 0.039 |
| Pharyngeal foreign body sensation, n (%) | 340 (28.1) | 49 (20.4)* $ | 45 (16.2) $ | 36 (40.0) ‡ | 61 (32.3)* ‡ | 21 (29.6)* $ ‡ | 44 (31.9)* ‡ | 84 (40.8) ‡ | <0.001 |
| Frequent throat clearing, n (%) | 440 (36.3) | 65 (27.1)* | 83 (30.0)* | 56 (62.2) $ | 86 (45.5) $ ‡ | 28 (39.4)* $ ‡ | 43 (31.2)* ‡ | 79 (38.3)* ‡ | <0.001 |
| Mucus adhesion to the throat, n (%) | 292 (24.1) | 38 (15.8)* | 34 (12.3)* | 32 (35.6) $ | 55 (29.1) $ | 17 (23.9)* $ | 47 (34.1) $ | 69 (33.5) $ | <0.001 |
| Total (n = 235) | Asthma (n = 40) | EB (n = 52) | UACS (n = 16) | GERC (n = 33) | AC (n = 14) | Other Cause (n = 24) | CRC (n = 56) | ||
| logC5, Median (Q1, Q3) | 1.8 (1.2, 2.7) | 1.8 (1.2, 2.2) | 2.3 (1.4, 3.0) | 2.1 (1.5, 2.7) | 1.8 (0.9, 2.4) | 2.0 (1.6, 2.6) | 1.5 (0.5, 2.1) | 1.8 (1.2, 2.5) | 0.055 |
| Cough hypersensitivity, n (%) | 128 (54.5) | 24 (60.0) | 22 (42.3) | 5 (31.3) | 20 (60.6) | 7 (50.0) | 17 (70.8) | 33 (58.9) | 0.097 |
Data are presented as frequency (percentage) or median (interquartile range). p Values for the post hoc test were adjusted with the Bonferroni method.
A subset of different causes of chronic cough categories whose column data do not differ significantly from each other at the 0.05 level.
A subset of different causes of chronic cough categories whose column data do not differ significantly from each other at the 0.05 level.
A subset of different causes of chronic cough categories whose column data do not differ significantly from each other at the 0.05 level.
AC, atopic cough; EB, eosinophilic bronchitis; GERC, gastroesophageal reflex-related cough; RCC, refractory chronic cough; UACS, upper airway cough syndrome.
The prevalence of cough triggers in different causes of CC
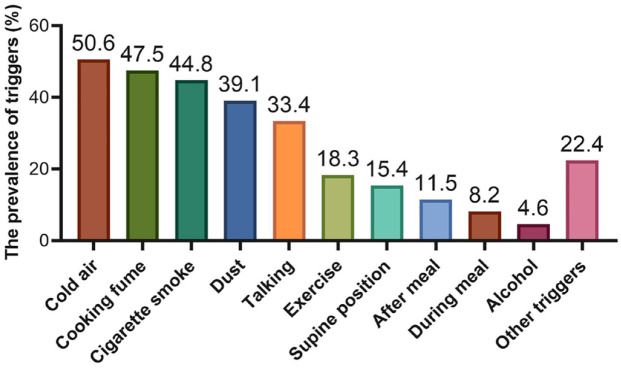
The prevalence of cough triggers in all patients and different causes of CC are shown in Table 2, Figures 1, and 2. A total of 1107 (91.4%) patients reported at least one cough trigger. Regarding the specific triggers, the most common cough triggers were cold air (50.6%), cooking fumes (47.5%), cigarette smoke (44.8%), and dust (39.1%). The least common trigger in this study was alcohol, with 4.6% of patients coughing after they drank alcohol (Figure 1). When cough triggers were categorized as chemical, mechanical, meal, and thermal triggers, chemical triggers were the most frequent triggers for cough, occurring in 66.9% of participants, followed by thermal exposure (50.6%), mechanical triggers (48.2%), and meal triggers (21.2%).
Figure 1.
Proportion of triggers in patients with chronic cough.
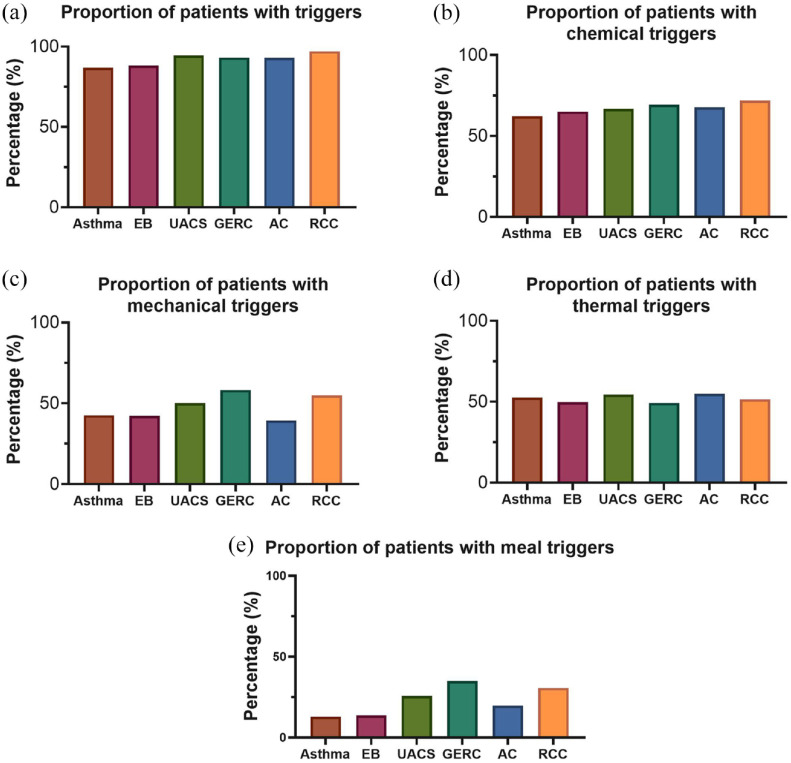
Figure 2.
The proportion of hypersensitivity to different triggers in chronic cough patients. (a) at least one triggers; (b) chemical triggers; (c) mechanical triggers; (d) thermal trigger; (e) meal triggers.
The prevalence of cough triggers was highest among subjects with RCC (200, 97.1%), followed by patients with UACS (85, 94.4%) and GERC (176, 93.1%). Mechanical triggers (110, 58.2%) and meal triggers (66, 34.9%) were most reported in GERC patients, followed by RCC, with the second highest prevalence of mechanical triggers (113, 54.9%), and meal triggers (63, 30.6%). There was no significant difference among different causes of CC in terms of chemical triggers and thermal trigger (Table 2).
The univariate logistic regression analysis investigating cough triggers as risk factors of different causes of CC are shown in Supplemental Table S1. Multivariate analysis showed that a higher number of meal triggers was positively associated with GERC (OR: 1.770, 95% CI: 1.340–2.330, p < 0.001) and RCC (OR: 1.480, 95% CI: 1.120–1.960, p = 0.005) but negatively associated with a diagnosis of asthma (OR: 0.620, 95% CI: 0.430–0.890, p = 0.010) and EB (OR: 0.570, 95% CI: 0.410–0.800, p = 0.001), corrected by age and sex. In addition, RCC patients presented with higher sensitivity to mechanical triggers (OR: 1.230, 95% CI: 1.030–1.480, p = 0.022) in multivariate analysis (Tables 3 and 4 and Supplemental Tables S2 and S3).
Table 3.
Univariate and multivariable logistic regression analysis of triggers for GERC.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Characteristics | OR | 95% CI | p | OR | 95% CI | p |
| Sex | 0.660 | 0.483–0.902 | 0.009 | 0.730 | 0.530–1.010 | 0.054 |
| Age | 0.986 | 0.975–0.999 | 0.028 | 0.990 | 0.980–1.000 | 0.182 |
| Number of chemical triggers | 1.068 | 0.933–1.223 | 0.341 | – | – | – |
| Number of mechanical triggers | 1.159 | 0.964–1.393 | 0.117 | – | – | – |
| Number of meal triggers | 1.870 | 1.427–2.450 | <0.001 | 1.770 | 1.340–2.330 | <0.001 |
| Thermal trigger-Cold air | 0.935 | 0.686–1.276 | 0.672 | – | – | – |
CI, confidence interval; GERC, gastroesophageal reflex-related cough; OR, odds ratio.
Table 4.
Univariate and multivariable logistic regression analysis of triggers for RCC.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Characteristics | OR | 95% CI | p | OR | 95% CI | p |
| Sex | 0.711 | 0.526–0.960 | 0.026 | 0.720 | 0.530–0.990 | 0.041 |
| Age | 0.990 | 0.979–1.002 | 0.102 | 0.990 | 0.980–1.010 | 0.326 |
| Number of chemical triggers | 1.130 | 0.992–1.289 | 0.067 | 1.090 | 0.950–1.260 | 0.223 |
| Number of mechanical triggers | 1.251 | 1.050–1.491 | 0.012 | 1.230 | 1.030–1.480 | 0.022 |
| Number of meal triggers | 1.647 | 1.260–2.153 | <0.001 | 1.480 | 1.120–1.960 | 0.005 |
| Thermal trigger-Cold air | 1.041 | 0.771–1.405 | 0.792 | – | – | – |
CI, confidence interval; OR, odds ratio; RCC, refractory chronic cough.
Demographic profiles of cough triggers in CC
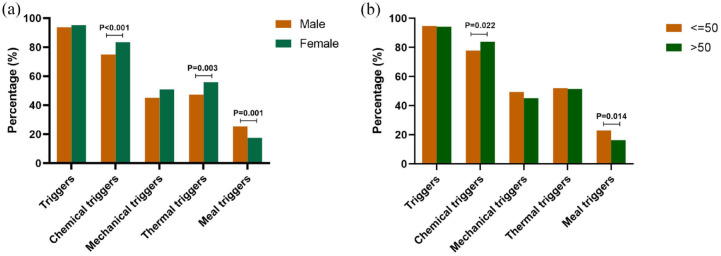
As is shown in Figure 3, chemical triggers (83.4% versus 75.0%; p < 0.001) and thermal trigger (55.9% versus 47.3%; p = 0.003) were reported more frequently in females than in males. Cough associated with meals was more common in males than in females (25.4% versus 17.5%; p = 0.001). Both the proportions of all triggers and mechanical triggers showed no significant sex difference (p > 0.05). The prevalence of cough triggers in different age groups is presented in Supplemental Figure S1. Age was dichotomized as younger than 50 years versus older than 50 years according to the obvious difference in the prevalence of specific kinds of cough triggers in these two groups. In terms of age, patients older than 50 years were more sensitive to chemical triggers (83.9% versus 77.8%; p = 0.022) but less sensitive to meal triggers (16.4% versus 23.0%; p = 0.014) than those younger than 50 years (Figure 3).
Figure 3.
(a) Sex and (b) age differences of triggers among chronic cough patients.
Association between cough triggers and capsaicin cough sensitivity
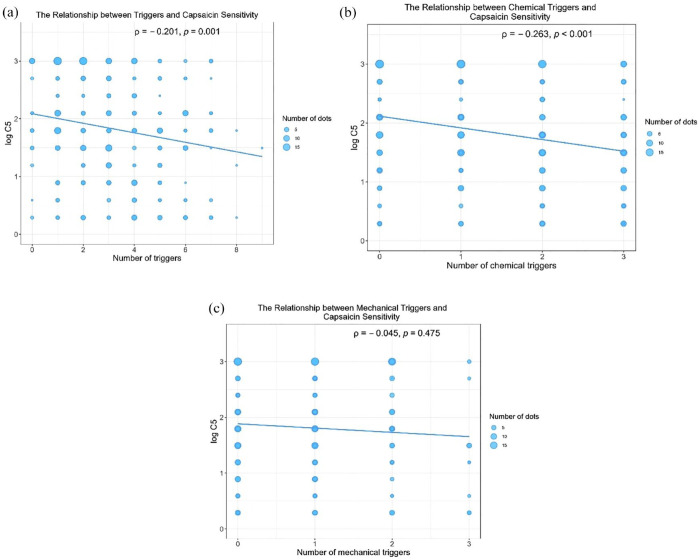
Among the 235 patients who completed the capsaicin cough challenge test with a single cause of cough, 128 (54.5%) reached C5, exhibiting hypersensitivity to capsaicin. No significant difference was observed in cough sensitivity (log C5 value) between patients with CC of different causes (Table 2). As shown in Supplemental Table S4, female patients showed higher cough sensitivity to capsaicin than male patients (64.1% versus 35.9%, p < 0.001), and patients with heightened cough sensitivity to capsaicin had older age (p = 0.044). Among the 254 patients who completed capsaicin cough challenge test with either single or multiple causes of cough, both the number of all triggers (ρ = −0.201, p = 0.001) and the number of chemical triggers (ρ = −0.263, p < 0.001) had a significant but low correlation with capsaicin cough sensitivity, whereas the number of mechanical triggers (ρ = −0.045, p = 0.475) was not associated with capsaicin cough sensitivity (Figure 4). In addition, patients with at least one trigger showed significantly lower logC5 (1.8 versus 2.4; p = 0.049), indicating higher cough sensitivity (Supplemental Table S5). In the analysis of the correlation between specific triggers and capsaicin cough sensitivity, dust (ρ = −0.213, p = 0.001), cooking fume (ρ = −0.177, p = 0.005) cigarette smoke (ρ = −0.207, p = 0.001) were associated with increased LogC5, while other triggers were not associated with capsaicin cough sensitivity (Supplemental Table S6).
Figure 4.
The relationships between capsaicin sensitivity and the number of (a) triggers, (b) chemical triggers, and (c) mechanical triggers in patients who underwent capsaicin cough challenge test (n = 254).
Discussion
The study showed an overall cough hypersensitivity in terms of at least one cough trigger in CC patients in our cohort. Higher sensitivity to mechanical triggers and meal triggers was observed in RCC and meal triggers in GERC. Capsaicin cough sensitivity had a low correlation with cough sensitivity to chemical triggers but was not associated with mechanical triggers. This study, for the first time, roughly classified cough triggers in daily life according to the possible mechanism and investigated their profile in different causes of CC in a large sample size. In addition, the relationship between cough sensitivity to classified cough triggers and capsaicin was also studied, which supported the existence of heterogeneity in cough hypersensitivity.
Cough hypersensitivity, mediated by peripheral and/or central hypersensitivity, is an important part of the neurophysiology of cough. 2 In our study, more than 90.0% of patients reported at least one cough trigger, which also indicated that cough hypersensitivity is a general feature of CC. For neuropathic pain, the classification of allodynia or hyperalgesia according to different types of stimuli can bring about additional insight into the underlying pain mechanisms and different types of management. 14 By analogy with this, we classified cough triggers into chemical triggers, mechanical triggers, meal triggers, and thermal trigger. Chemical-triggers-induced cough is triggered by a range of irritant environmental chemicals and mediators causing airway inflammation or tissue damage, which might be mostly mediated by jugular vagal C-fibers. 15 In this study, cough sensitivity to chemical triggers was similar among all etiologies, and so was capsaicin sensitivity. As the receptor for capsaicin was TRPV1 in vagal pulmonary C-fiber sensory nerves, the analogous response of each etiology to chemical and capsaicin might indicate a common heightened activation of C-fiber in CC. Mechanical triggers, characterized by their responsiveness to touch-like mechanical stimuli and change of tension of the larynx or intrathoracic pressure, were speculated to be primarily associated with nodose vagal afferent Aδ fibers.16,17 RCC was more responsive to mechanical triggers than other causes in this study. Previous studies have also found increased sensitivity to talking, one of the mechanical triggers, in RCC. 9 These findings prompt possible sensitization of Aδ fiber in RCC. However, the value of Aδ fiber activation represented by mechanical triggers for the diagnosis and treatment of RCC needs further study. Meals-induced cough was denoted as factors relating to eating or drinking. Cough sensitivity to meal triggers was elevated in GERC compared to patients with other causes in this study. It was in line with one of our previous studies that cough triggered by meals might indicate GERC. 18 Generally considered, reflux-induced microaspiration or neuronal sensitization of esophageal-bronchial crosstalk account for cough in GERC. On the other hand, we found heightened cough sensitivity to meal triggers in RCC, which was consistent with a previous study. 9 The inclusion of refractory GERC in RCC might be partly responsible for this finding, 19 but more reasons involved remain uncertain. The significance of RCC patients’ hypersensitivity to meal triggers on the diagnosis and treatment of RCC needs further study. Thermal trigger in the present study was referred to as cold air. On one hand, dry air induces a transient change in osmolarity in the epithelial fluid, causing bronchoconstriction and coughing through the activation of mechanical receptors. On the other hand, cold stimuli to the skin and cross-talk of sensory afferents between the skin and airways in the brainstem could mediate cough.20–22 Previous studies showed that cold air was more related to asthma.8,23 However, inconsistent with this, cold air-induced cough did not differ significantly between etiologies in the present study. The discrepancy may be related to the enormously distinct climate where the studies were conducted. Central sensitization is also a key feature of cough hypersensitivity. 2 While central sensitization is understood to increase the responsiveness of neurons to stimuli, how this process specifically interacts with different triggers of cough is not well-defined. A more accurate and comprehensive classification of cough triggers and their prompting value in the diagnosis and treatment of different causes of CC demand further study.
A female and elder predominance in increased cough sensitivity to chemical triggers was observed in this study, which is consistent with higher capsaicin cough sensitivity in older females in previous studies.12,24,25 Higher sensitivity in females might be associated with genetically higher central sensitivity25,26 and enhanced activation of TRPV1 by estrogen hormone. 27 Regardless of sex, increased capsaicin cough sensitivity was observed in the older age group compared with younger patients. 28 The above studies combined account for a potential mechanism for the female and elder predominance in patients with CC. In contrast, cough induced by meals was more common in male and younger patients. Gastro-esophageal reflux disease was predominantly in males. 29 Also, acid-reflux-induced esophageal damage was more intensively observed in male rats than in female rats. 30 Therefore, age and sex differences should not be overlooked in the evaluation of cough hypersensitivity. The capsaicin challenge test has the same threshold for different sex and age groups. This may be the reason for the large overlap of cough sensitivity between patients with CC and healthy control. Thus, delineating abnormal ranges for different sex and age groups should be considered in assessing cough sensitivity more accurately.
In the present study, capsaicin cough sensitivity had a considerably low correlation with cough sensitivity to daily cough triggers, suggesting little overlap of mechanism in cough hypersensitivity. No significant difference in cough sensitivity to capsaicin and mechanical triggers was found in the present study, indicating that they portray different aspects of cough sensitivity. In addition, an observational study showed RCC presented with different patterns of response to stimuli mediated by Transient receptor potential vanilloid 1 and transient receptor potential ankyrin 1, with 10.7% of patients hypersensitive to both allyl isothiocyanate (AITC) and capsaicin, 11.9% responded only to capsaicin and 18.8% responded only to AITC. 5 All in all, the above results indicate the heterogeneity in cough hypersensitivity and the deficiency of capsaicin challenge tests in evaluating cough sensitivity. A more comprehensive and validated cough-specific measurement tool comprising every type of stimuli, for example, a questionnaire about cough hypersensitivity, should be developed to evaluate cough sensitivity in CC.
Limitations
There are some limitations in the present study. First, response to cough triggers in this study was recorded in the form of binary answer (yes/no) but not an incremental scale to quantify the degree, so it fails to investigate the severity of cough hypersensitivity to triggers. Second, trigger entries for each category may not be comprehensive and need further development, but it can still confirm the insufficient detection of cough sensitivity with capsaicin challenge tests alone. Third, as cough response to cough triggers in healthy volunteers was not investigated in this study, it failed to distinguish physiologic cough response from pathological cough response. Fourth, the patients in this study did not include those who had never visited a doctor due to cough, thus further studies which encompass these subjects are needed.
Despite these limitations, this study provides direction for future research on cough hypersensitivity. Utilization of comprehensive and validated measurement tools for cough sensitivity would be a key to the investigations. It is also worthwhile to investigate the mechanism underlying cough sensitivity to different types of triggers and their role in the management of responsive CC. Moreover, studies involving serial evaluation by these comprehensive and validated measurement tools can demonstrate the effect of a pathological condition or therapeutic intervention on cough reflex sensitivity.
Conclusion
Cough hypersensitivity to cough triggers is a common phenomenon in different causes of CC. However, GERC presents with higher sensitivity to meal triggers and RCC presents with both higher sensitivity to meal triggers and mechanical triggers. In illustrating demographic profiles of cough triggers, females and elders were more sensitive to chemical triggers while cough induced by meals were more common in males and the younger. Different patterns of cough response to cough triggers and capsaicin supported the existence of heterogeneity in cough pathways.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-2-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-3-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-4-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-5-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-6-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-jpg-7-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Acknowledgments
None.
Footnotes
ORCID iDs: Tingting Xu  https://orcid.org/0000-0003-0256-1234
https://orcid.org/0000-0003-0256-1234
Zhiyin Chen  https://orcid.org/0009-0003-4782-8737
https://orcid.org/0009-0003-4782-8737
Wenzhi Zhan  https://orcid.org/0000-0002-2093-4332
https://orcid.org/0000-0002-2093-4332
Fang Yi  https://orcid.org/0000-0003-4049-7245
https://orcid.org/0000-0003-4049-7245
Kefang Lai  https://orcid.org/0000-0001-7994-7296
https://orcid.org/0000-0001-7994-7296
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tingting Xu, Guangzhou Institute of Respiratory Health, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, the First Affiliated Hospital of Guangzhou Medical University.
Zhiyin Chen, Guangzhou Institute of Respiratory Health, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, the First Affiliated Hospital of Guangzhou Medical University.
Chen Zhan, Guangzhou Institute of Respiratory Health, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, the First Affiliated Hospital of Guangzhou Medical University.
Wenzhi Zhan, Guangzhou Institute of Respiratory Health, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, the First Affiliated Hospital of Guangzhou Medical University.
Fang Yi, Guangzhou Institute of Respiratory Health, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, the First Affiliated Hospital of Guangzhou Medical University.
Kefang Lai, Guangzhou Institute of Respiratory Health, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, the First Affiliated Hospital of Guangzhou Medical University, 151 Yanjiang Road, Guangzhou 510120, China.
Declarations
Ethics approval and consent to participate: The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (no. 2020150). Participants were given a detailed explanation of the purpose of the publication and its potential implications. All subjects gave verbal informed consent to participate. Verbal consent was documented in the participant’s study file along with the date and the name of the researcher who obtained the consent. Verbal consent was obtained for accessibility. As some CC patients were elderly or with reading disabilities, verbal consent is a more appropriate option. Also, since our data were collected in the clinic, verbal consent was obtained for effectively simplify procedures while still maintaining ethical standards.
Consent for publication: Verbal consent was obtained for accessibility and simplify procedures mentioned above. To obtain verbal consent, researchers read the contents of each participant’s study file to participants, informing them that their data would be used for analysis and publication. It was also clarified that no personal privacy information would be involved in the published material. Upon obtaining their consent, each instance of consent was meticulously recorded in a participant’s study file where we collected data, noting the date and the details of the consent provided. Additionally, the name of the researcher who obtained the consent was also recorded for reference. This process ensured transparency and respected the privacy and autonomy of the participants, aligning with ethical research practices.
Author contributions: Tingting Xu: Conceptualization; Formal analysis; Investigation; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Zhiyin Chen: Data curation; Formal analysis; Investigation; Resources; Validation; Writing – original draft; Writing – review & editing.
Chen Zhan: Conceptualization; Data curation; Methodology; Writing – original draft; Writing – review & editing.
Wenzhi Zhan: Conceptualization; Data curation; Methodology; Resources; Validation; Writing – original draft; Writing – review & editing.
Fang Yi: Data curation; Validation; Writing – original draft; Writing – review & editing.
Kefang Lai: Conceptualization; Methodology; Project administration; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Reference
- 1. Lai KF, Chen RC, Liu CL, et al. [Etiology and a diagnostic protocol for patients with chronic cough]. Zhonghua Jie He He Hu Xi Za Zhi 2006; 29: 96–99. [PubMed] [Google Scholar]
- 2. Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014; 44: 1132–1148. [DOI] [PubMed] [Google Scholar]
- 3. Morice AH. The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung 2010; 188(Suppl 1): S87–S90. [DOI] [PubMed] [Google Scholar]
- 4. Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther 2011; 24: 267–271. [DOI] [PubMed] [Google Scholar]
- 5. Long L, Yao H, Tian J, et al. Heterogeneity of cough hypersensitivity mediated by TRPV1 and TRPA1 in patients with chronic refractory cough. Respir Res 2019; 20: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prudon B, Birring SS, Vara DD, et al. Cough and glottic-stop reflex sensitivity in health and disease. Chest 2005; 127: 550–557. [DOI] [PubMed] [Google Scholar]
- 7. Kanemitsu Y, Matsumoto H, Osman N, et al. ‘Cold air’ and/or ‘talking’ as cough triggers, a sign for the diagnosis of cough variant asthma. Respir Investig 2016; 54: 413–418. [DOI] [PubMed] [Google Scholar]
- 8. Koskela HO, Latti AM, Pekkanen J. Subfreezing air as a cough trigger and multiple triggers are strongly associated with the presence of asthma in chronic cough. Respir Med 2019; 153: 26–30. [DOI] [PubMed] [Google Scholar]
- 9. Won HK, Kang SY, Kang Y, et al. Cough-related laryngeal sensations and triggers in adults with chronic cough: symptom profile and impact. Allergy Asthma Immunol Res 2019; 11: 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006; 129(1 Suppl): 1S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asthma Workgroup of Chinese Society of Respiratory Diseases (CSRD) CMA. [Guidelines for diagnosis and management of cough (2015)]. Zhonghua Jie He He Hu Xi Za Zhi 2016; 39: 323–354. [Google Scholar]
- 12. Lai K, Long L, Yi F, et al. Age and sex distribution of chinese chronic cough patients and their relationship with capsaicin cough sensitivity. Allergy Asthma Immunol Res 2019; 11: 871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 14. Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 2014; 13: 924–935. [DOI] [PubMed] [Google Scholar]
- 15. Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev 2016; 96: 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mazzone SB, Undem BJ. Cough sensors. V. Pharmacological modulation of cough sensors. Handb Exp Pharmacol 2009: 99–127. [DOI] [PubMed] [Google Scholar]
- 17. Canning BJ, Mazzone SB, Meeker SN, et al. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 2004; 557: 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai K, Zhan W, Li H, et al. The predicative clinical features associated with chronic cough that has a single underlying cause. J Allergy Clin Immunol Pract 2021; 9: 426–432 e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lv HJ, Qiu ZM. Refractory chronic cough due to gastroesophageal reflux: definition, mechanism and management. World J Methodol 2015; 5: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plevkova J, Kollarik M, Poliacek I, et al. The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J Appl Physiol (1985) 2013; 115: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dong R, Zhang T, Wei W, et al. A cold environment aggravates cough hyperreactivity in guinea pigs with cough by activating the trpa1 signaling pathway in skin. Front Physiol 2020; 11: 833. DOI: 10.3389/fphys.2020.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koskela H, Tukiainen H. Facial cooling, but not nasal breathing of cold air, induces bronchoconstriction: a study in asthmatic and healthy subjects. Eur Respir J 1995; 8: 2088–2093. [DOI] [PubMed] [Google Scholar]
- 23. Matsumoto H, Tabuena RP, Niimi A, et al. Cough triggers and their pathophysiology in patients with prolonged or chronic cough. Allergol Int 2012; 61: 123–132. [DOI] [PubMed] [Google Scholar]
- 24. Song W-J, Kim J-Y, Jo E-J, et al. Capsaicin cough sensitivity is related to the older female predominant feature in chronic cough patients. Allergy Asthma Immunol Res 2014; 6: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morice AH, Jakes AD, Faruqi S, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J 2014; 44: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 26. Farrell MJ, Cole LJ, Chiapoco D, et al. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. Neuroimage 2012; 61: 1324–1335. [DOI] [PubMed] [Google Scholar]
- 27. Patberg KW. The female preponderance to cough hypersensitivity syndrome: another clue pointing to the role of TRPV1 in cough. Lung 2011; 189: 257–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morice AH. Chronic cough hypersensitivity syndrome. Cough 2013; 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Royston C, Bardhan KD. Adam, Eve and the reflux enigma: age and sex differences across the gastro-oesophageal reflux spectrum. Eur J Gastroenterol Hepatol 2017; 29: 634–639. [DOI] [PubMed] [Google Scholar]
- 30. Masaka T, Iijima K, Endo H, et al. Gender differences in oesophageal mucosal injury in a reflux oesophagitis model of rats. Gut 2013; 62: 6–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-2-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-3-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-4-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-5-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-6-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-jpg-7-tar-10.1177_17534666231225562 for Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough by Tingting Xu, Zhiyin Chen, Chen Zhan, Wenzhi Zhan, Fang Yi and Kefang Lai in Therapeutic Advances in Respiratory Disease