Abstract
Vasospastic angina (VSA) refers to chest pain experienced as a consequence of myocardial ischaemia caused by epicardial coronary spasm, a sudden narrowing of the vessels responsible for an inadequate supply of blood and oxygen. Coronary artery spasm is a heterogeneous phenomenon that can occur in patients with non-obstructive coronary arteries and obstructive coronary artery disease, with transient spasm causing chest pain and persistent spasm potentially leading to acute myocardial infarction (MI). VSA was originally described as Prinzmetal angina or variant angina, classically presenting at rest, unlike most cases of angina (though in some patients, vasospasm may be triggered by exertion, emotional, mental or physical stress), and associated with transient electrocardiographic changes (transient ST-segment elevation, depression and/or T-wave changes). Ischaemia with non-obstructive coronary arteries (INOCA) is not a benign condition, as patients are at elevated risk of cardiovascular events including acute coronary syndrome, hospitalization due to heart failure, stroke and repeat cardiovascular procedures. INOCA patients also experience impaired quality of life and associated increased healthcare costs. VSA, an endotype of INOCA, is associated with major adverse events, including sudden cardiac death, acute MI and syncope, necessitating the study of the most effective treatment options currently available. The present literature review aims to summarize current data relating to the diagnosis and management of VSA and provide details on the sequence that treatment should follow.
Keywords: acetylcholine, coronary artery spasm, ischaemia with non-obstructive coronary arteries, vasospastic angina
Plain language summary
Diagnosis and treatment of epicardial coronary artery spasm
Vasospastic angina (VSA) refers to chest pain experienced as a consequence of a sudden narrowing of the epicardial coronary arteries. VSA can occur in patients with non-obstructive coronary arteries and obstructive coronary artery disease, with transient spasm causing chest pain and persistent spasm potentially leading to acute myocardial infarction. Reduced blood and oxygen supply in patients with non-obstructive coronary arteries is not a benign condition, as patients are at elevated risk of adverse cardiovascular events. These patients also experience impaired quality of life and associated increased healthcare costs. This review aims to summarise current data relating to the diagnosis of VSA and provides details on treatment strategies.
Introduction
Ischaemic heart disease remains the leading cause of death and lost life years in adults globally. 1 Up to 40% of patients with a positive non-invasive stress test undergoing elective coronary angiography exhibit no evidence of obstructive epicardial coronary arteries. 2 This frequency is notably higher among women (around 65%) compared to men (30–50%), and it is estimated to progressively increase in prevalence with the use of non-invasive imaging tests. 3 In the context of the chronic coronary syndrome (CCS), non-obstructive coronary arteries encompass a broad spectrum of clinical phenotypes ranging from angina with non-obstructive coronary arteries (ANOCA) to ischaemia with non-obstructive coronary arteries (INOCA). The latter stems from the mismatch between myocardial oxygen demands and blood supply and it is demonstrable in up to 28% of patients with angina symptoms and functionally non-obstructive coronary artery disease (CAD). 4
INOCA is not a benign condition and has a relevant influence on patients’ lives, affecting their home and social lives with >70% reporting an impact on mental health. 5 Furthermore, an increased risk for major adverse outcomes in this population is reported, predominantly represented by hospitalization for recurrent angina, heart failure or both. 6 Clinical research in these scenarios has recently garnered significant interest, leading to improved definition, prognostic stratification and pathophysiological classification to prevent misdiagnosis and under treatment. Large vessel coronary spasms, also known as vasospastic angina (VSA), are responsible for a significant proportion of INOCA cases. 7 Some reports suggest that coronary vasospasm is responsible for up to 40% of cases of stable angina with non-obstructive coronaries. 8 One study reported 54% of 304 patients experiencing angina to have <50% stenosis, with vasospasm elicited in 66% of these using acetylcholine (ACh). 7
Coronary spasm can also occur within the microvasculature and is referred to as microvascular spasm, presumably occurring at the level of the pre-arteriolar and arteriolar vessels. It should be differentiated from VSA based on the underlying pathophysiological mechanism and the involved vascular compartment. Coronary microcirculation dysfunction (CMD), which is a reduced vasodilatory ability of coronary microcirculation, is the other major endotype of INOCA. The present narrative review provides an overview of VSA summarizing the current available data on pathophysiology, diagnostic algorithm and management strategies according to the latest research evidence.
Pathophysiology and risk factors of VSA
Chest pain secondary to VSA typically occurs at rest given the fact that it is not associated with increased oxygen demand but it may also be induced by exertion during a ‘hot phase’ in which the exercise-related catecholamine release may trigger an angina episode. 9 It might be characterized by a circadian pattern (typically awakening in the early hours of the morning) and be suddenly precipitated by stress or hyperventilation after a quiescent period (cold phase). A diurnal circadian rhythm in VSA symptoms has been described since the late 1950s. 9 A significantly higher number of nocturnal or early morning attacks are reported compared to the rest of the day, even though it is often not clinically apparent. 10 A potential mechanism underlying the cyclic symptoms occurrence is the circadian variation in the tone of coronaries. 11 VSA is related to the epicardial segments’ hyperreactivity to vasoconstrictive stimuli, which occurs spontaneously but may also be induced to establish the diagnosis. 8 The abnormal response of the vascular smooth muscle cells (VSMCs) is entrenched by the process of inflammation and fibrocellular proliferation. VSMC contraction is initiated by Ca2+/calmodulin-activated myosin light chains (MLC) kinase which leads to phosphorylation of the regulatory MLC. Both the increased Ca2+ inflow and Ca2+ hypersensitivity of contractile proteins in the VSMCs have been proposed to represent the fundamental mechanism responsible for coronary artery hypercontraction. 12 The role of endothelium in the physiological regulation of coronary vascular tone is fundamental, mainly through the release of vasodilators, such as nitric oxide (NO). Accordingly, vasodilation may be impaired by significant endothelial damage, thus favouring spasms in response to vasoconstrictor stimuli. Several vasoactive stimuli (e.g. ACh, histamine, serotonin) cause vasodilation through NO release by the endothelium, but, at the same time, they may cause vasoconstriction through direct VSMC stimulation. In the case of endothelial dysfunction, their release in the vessel wall can lead to constriction. 13
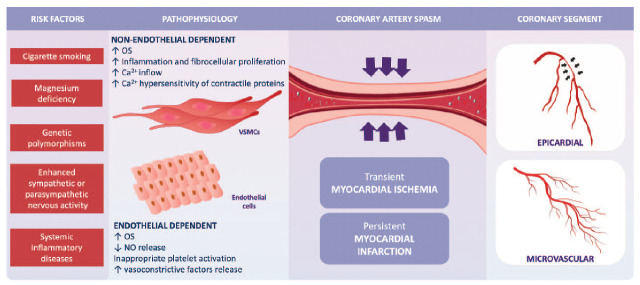
Both genetic and nonbiological factors contribute to the pathophysiology of vasospasm. Genetic factors may encompass reduced endothelial NO production, upregulation of alpha-adrenergic receptors and inappropriate platelet activation increasing endothelial production of vasoconstrictive factors, resulting in vasoconstriction. Low-grade inflammation, an altered autonomic nervous system response and oxidative stress (OS) may also be genetically influenced contributors 14 (Figure 1). In particular, OS activates perivascular cells, macrophages and fibroblasts, which induces the production and release of radical oxygen species (ROS). This triggers VSMC proliferation and migration with subsequent induction of adhesion molecules. Activation of protein kinase C also occurs and inflammatory processes are activated at the level of gene transcription by upregulation of the transcription nuclear factor kB located in the cytoplasm of lymphocytes, monocytes, endothelial cells and VSMCs, thus activating interleukins, interferon, tumour necrosis factor-α and adhesion molecules. 12 Cigarette smoking has been reported as the most important risk factor for VSA, 15 unlike hypertension and diabetes, and the relationship with dyslipidaemia remains unclear. 16 Association of VSA with migraine and Raynaud’s phenomenon has been reported.17,18
Figure 1.
Risk factors and pathophysiology of vasospasm.
Ca2+, calcium; NO, nitric oxide; OS, oxidative stress; VSMCs, vascular smooth muscle cells.
Microvascular spasm
The diagnosis of microvascular spasm is based upon the provocative spasm testing (see below), where ACh provokes chest pain and ischaemic electrocardiogram (ECG) changes in the absence of large vessel spasm, that is, a presumptive conclusion that the microvasculature is responsible for the provoked myocardial ischaemia. Based on the diagnostic criteria, microvascular spasm is diagnosed in the absence of coronary artery (large vessel) spasm although potentially the two entities may coexist. 19 However, whether the extensive published literature in patients with VSA (i.e. large vessel spasm) applies to patients with microvascular spasm is unknown and until further research is available, this established literature on large vessel spasm should be considered only in reference to the latter.
With this important consideration, microvascular spasm is attributed to an increased release of vasoconstrictive substances, associated with an increased susceptibility of VSMCs or an abnormal activity of sympathetic tone. 20 Furthermore, until recently, there have been no therapies evaluated for microvascular spasms and the optimal management strategy for this condition necessitates further studies. Microvascular spasms and CMD are conventionally grouped as causes of microvascular angina to distinguish the involved distal segments from larger vessels.
Prognosis of coronary artery spasm
Timely recognition of symptoms and suspicion of coronary artery spasm may prevent the occurrence of adverse events such as sudden cardiac death (SCD), MI and syncope. 21 Patients suffering from VSA frequently experience recurrent angina during follow-up, ranging from 10% to 53%, 22 a characteristic in line with the results of the CorMicA (CORonary Microvascular Angina) trial. 23
Patients with epicardial and microvascular spasms have an impaired prognosis compared to individuals without spasms, even though the prevalence of cardiovascular mortality seems low between the two entities. 24 Conversely, nonfatal-MI significantly occurs more often in epicardial spasm patients (4%) compared with the microvascular spasm group (0.7%) and ANOCA patients without coronary artery spasm. 24 In patients with VSA, more than half of all reported major adverse cardiovascular events (MACE), including cardiac death, nonfatal-MI, unstable angina and hospitalization, is driven by hospitalization for unstable angina ranging from 52% to 90% of MACE. 22
Provocative testing
Gold-standard testing for coronary vasospasm uses angiography and spasm-inducing pharmacological stimuli such as ACh and ergonovine, both of which have a high sensitivity and specificity to confirm the presence of epicardial vasospasm after exclusion of flow-limiting obstructive CAD and other forms of microvascular dysfunction. ACh acts on the muscarinic cholinergic receptors and ergonovine acts on the serotonin receptors in VSMCs. Spasms caused by ACh have been reported to be distal and diffuse, whereas those caused by ergonovine are proximal and focal. 25 The muscarinic ACh receptors (mAChRs) are important for vascular homeostasis. Their activation at the endothelial level leads to NO-mediated vasodilatation, whereas activation of mAChRs on the VSMCs causes vasoconstriction. Depending on the integrity of the endothelium and the reactivity of the VSMCs, the net effect of ACh administration could be vasodilatation or vasoconstriction. 26 Due to its short half-life, ACh can be administered directly into the coronary arteries. According to the 2019 European Society of Cardiology (ESC) guidelines on CCS, ACh provocative testing may be considered to assess coronary spasm (IIa recommendation) 27 in patients with suspected VSA. It is characterized by high sensitivity and specificity (90% and 99%, respectively). 23 ACh use is preferred over ergonovine because the latter is more likely to provoke extended vasospasm. 28 Alternatively, non-pharmacological stimuli include hyperventilation and cold pressor testing, albeit characterized by low sensitivity. Other simultaneous assessment modalities include symptom monitoring and electrocardiography.
A positive provocation test comprises reproduction of usual angina, ischaemic ECG changes (ST-segment depression or elevation ⩾0.1 mV or new negative U waves) in at least two contiguous leads, and ⩾90% vasoconstriction. 29 Microvascular spasm is characterized by a lumen reduction <90%, symptoms and ECG changes usually occur with a lower dose of ACh. 30 However, there is a lack of a standardized recommended practical ACh protocol and instead, varies according to different centre’s experiences. 21
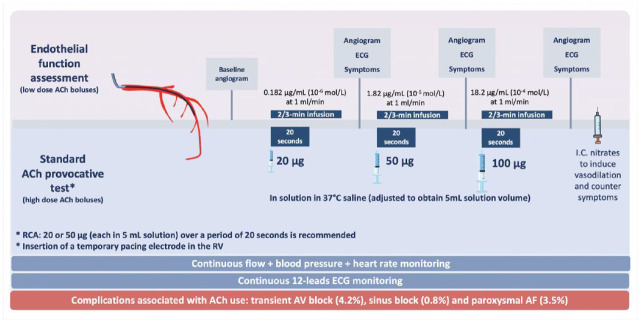
Intracoronary (IC) ACh provocative testing was first established in Japan. Rapid high-dose ACh bolus infusions have been validated and detailed in VSA guidelines. 19 The procedure consists of sequential injections of 20, 50 or 100 μg of ACh in solution in 37°C physiological saline (adjusted to obtain 5 mL solution volume for each quantity of ACh) into the left coronary artery over a period of 20 s. Coronary angiography is performed 1 min after the start of each injection. A reduced dosage (20 or 50 μg, each in 5 mL solution) over a period of 20 s is recommended to assess the right coronary artery (RCA). 19 After the ACh bolus, IC nitroglycerin is administered to assess basal epicardial artery tone (Figure 2).
Figure 2.
Validated original provocative ACh testing for VSA assessment (high-dose ACh boluses) and endothelial function assessment test (low-dose ACh boluses). Several alternatives to the original ACh test have been adopted in recent years, although not validated as the standard protocol.
ACh, acetylcholine; AF, atrial fibrillation; AV, atrioventricular; IC, intracoronary; LCA, left coronary arteries; RCA, right coronary artery; RV, right ventricle; VSA: vasospastic angina; µg, micrograms.
In recent years, several alternatives to the original test have been adopted in different centres, although not validated as the standard protocol,31,32 such as hybrid approaches using high-dose ACh administered over 2–3 min (slow bolus) and not including the 100 μg infusion in case of significant bradycardia. Considering the very short half-life of ACh, these methods could result in reduced ACh plasma concentrations compared to the conventional 20-s rapid-bolus administration, 33 and subsequently, uncertain diagnostic assessment. 34
On the other hand, endothelial function studies use low-dose ACh infusion (generally 10−6 mol/L = 0.18 µg/min and 10−5 mol/L = 1.8 µg/min) administered over 2–3 min. In this case, vasoconstriction is representative of endothelial dysfunction, whereas coronary vasodilation corresponds to intact endothelium-dependent vasodilation 35 (Figure 2).
Complication rates associated with ACh use are quite low and it encompasses transient atrioventricular block (4.2%), sinus block (0.8%) and paroxysmal atrial fibrillation (AF) (3.5%), preventable by insertion of a temporary pacemaker prior to ACh injection of the RCA. However, some operators prefer to avoid RCA testing or administer the ACh slower, leading to the unreliability of the findings.
In a recent study by Montone et al., a previous history of paroxysmal AF, moderate-to-severe left ventricular diastolic dysfunction and higher QT dispersion at baseline ECG have been demonstrated to be the only predictors of complications during the ACh provocative test. At a medium- to long-term follow-up, the occurrence of complications during the ACh test was not associated with a worse prognosis. 36
The detection of the mixed forms including both spasm disorders (epicardial and microvascular) is currently limited to cases in which microvascular spasm occurs at lower ACh doses than epicardial spasm. Thus, the coexisting mechanisms may be underdiagnosed. The concept of rechallenging coronary arteries (ACh rechallenge) with ACh immediately after IC administration of an antivasospastic substance has been proposed to detect coexisting different spasm endotypes. In a recent study, to assess the reinducibility of microvascular and epicardial spasm, ACh rechallenge was systematically performed 3 min after IC injection of nitroglycerin by readministration of the spasm provocation dose into the previously spastic coronary artery. ACh rechallenge may unmask combined spasm disorders. In 48% of patients with epicardial spasms, ACh rechallenge revealed coexisting nitroglycerin-persistent microvascular spasms, demonstrating that nitroglycerin is much more effective in preventing epicardial spasms than microvascular spasms. 37 These findings require further investigation.
Diagnosis
International standardized criteria have been established by the Coronary Vasomotion Disorders International Study (COVADIS) group. For a definitive diagnosis of VSA, the following criteria are required: (1) nitrate-responsive angina symptoms with at least one of the following (a) rest angina, (b) diurnal variation in symptoms, (c) hyperventilation-induced angina or (d) symptom improvement with calcium-channel blockers (CCBs); (2) transient ischaemic ECG changes during spontaneous symptoms; (3) documented coronary artery spasm (>90% constriction) occurring spontaneously or in response to provocation testing (with pain and ischaemic ECG changes). If only two of these criteria are met, the patient is diagnosed with ‘suspected VSA’.
Evidence supports sex-related differences in the vasomotor response to provocative testing, with a higher prevalence of epicardial spasms among men and of microvascular spasms among women. 38 Moreover, ethnic differences exist as a predisposing factor. Several studies have reported a higher prevalence of VSA in Asian populations. 39 History of associated symptoms such as migraine, Raynaud’s phenomenon and Kounis syndrome may increase suspicion of a diagnosis of VSA. 40
Management overview
Studies on VSA therapy are limited by small sample sizes, as detailed in a recent meta-analysis and systematic review. 41 Well-designed clinical trials to guide future research and clinical recommendations are still needed.
In the emergency setting, VSA management adheres to current guidelines on acute coronary syndrome (ACS) based on the initial clinical presentation at the emergency care. 42 Electrocardiographic normalization of ST-segment elevation associated with symptom resolution secondary to nitro-glycerine administration is highly suggestive of coronary spasm.42,43 In case of a previously established diagnosis of VSA, a detailed review of the past medical history could guide physicians in the emergency department to administer short-acting nitrates or optimize pharmacological treatment to prevent recurrences and subsequent readmissions to emergency care.
VSA management should be based on a patient-centred approach and a multidisciplinary approach may be required. Holistic, patient-centred care should be considered to enable patients with VSA to be able to self-manage their VSA as much as possible. Current management options for VSA consist of lifestyle modifications, risk factors management and pharmacological strategies (Table 1). Pharmacological treatment includes CCBs, nitrates, nicorandil, statins and renin–angiotensin system (RAS) inhibitors (Figure 3).
Table 1.
Pharmacological strategies for VSA management.
| Class of drugs | Mechanisms of action | Drugs | Dosage | Special recommendations | |
|---|---|---|---|---|---|
| CCBs | ↓ Spontaneous and inducible coronary spasm via vascular smooth muscle relaxation ↓ O2 demand |
Non-dihydropyridine | Verapamil | 240 mg SR (single or divided doses) daily | In severe VSA consider high dosages of CCBs (2 × 200 mg diltiazem daily) or a combination of both non-dihydropyridine with dihydropyridine CCBs |
| Diltiazem | 90 mg twice daily or 120–360 mg (single or divided doses) | ||||
| Dihydropyridine | Nifedipine | 5 mg 3 times/day | |||
| SR: 10 mg twice/day (up to 30 mg/day) | |||||
| Amlodipine | 5–10 mg once a day | ||||
| Nitrates | ↓ Spontaneous and inducible coronary spasm via large epicardial vasodilation ↓ O2 demand |
Short-acting nitrates | Glyceryl trinitrate | 300 µg if needed | Efficacy of short-acting nitrates might vary, and repeated administration is often needed |
| Isosorbide mononitrate XL | 30 mg daily | ||||
| Isosorbide dinitrate | 30–120 mg daily | ||||
| Long-acting nitrates | Isosorbide mononitrate SR | 25 mg to a maximum of 120 mg daily | |||
| Isosorbide dinitrate SR | 40 mg once daily | ||||
| Potassium channel activator | Coronary microvascular dilatory effect | Nicorandil | 10–20 mg once daily | Consider patients experiencing VSA and still symptomatic despite CCBs followed by nitrates | |
CCBs, calcium-channel blockers; SR, slow release; VSA, vasospastic angina.
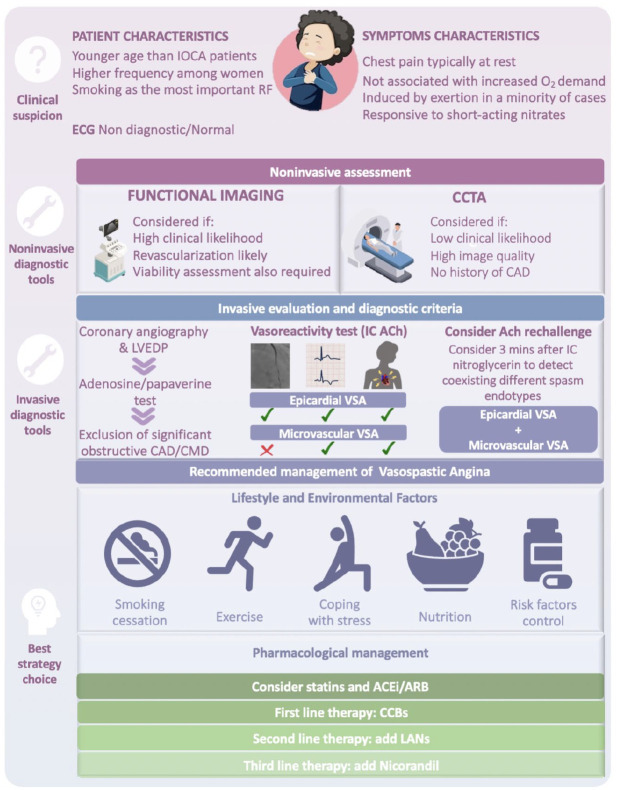
Figure 3.
Summary of diagnostic and therapeutic recommendations for vasospastic angina.
ACEi, angiotensin-converting enzyme inhibitor; ACh, acetylcholine; ARB, Angiotensin II receptor blocker; CAD, coronary artery disease; CCBs, calcium-channel blockers; CCTA, coronary computed tomography angiography; CMD, coronary microvascular dysfunction; IC, intracoronary; IOCA, ischaemia with obstructive coronary arteries; LANs, long-acting nitrates; LVEDP, left ventricle end diastolic pressures; RF, risk factor; VSA, vasospastic angina.
Management of lifestyle and environmental factors
Managing lifestyle factors is the first recommended intervention. Evidence supports its importance in improving health outcomes in VSA, as acknowledged and recommended by the 2020 Expert Consensus Document for INOCA and the 2019 ESC guidelines for CCS.21,27 Smoking cessation is particularly favourable, as smoking cigarettes is a well-known risk factor for vasospasm 16 (especially in young women) due to its harmful effect on endothelium and NO bioavailability. 15 Avoidance of excessive mental or emotional stress and fatigue is also recommended. 21 It is important for patients to identify their personal triggers so they can manage them. Keeping a diary of symptoms, activity and response to medication can be a useful tool. In addition, current recommendations include control of blood pressure, body weight, lipid, cholesterol, glucose levels through regular exercise and consumption of a balanced, healthy diet, avoiding excessive sodium intake, particularly in older individuals with hypertension. 21 These factors should be monitored and managed as they may worsen the prognosis of patients with VSA either directly or through increasing the likelihood of developing comorbid atherosclerosis. Known triggers of vasospasm should also be avoided. These include recreational drugs (amphetamines, cocaine, ecstasy, alcohol), sympathomimetic agents (adrenaline and noradrenaline), chemotherapy agents (5-fluorouracil, capecitabine, vascular endothelial growth factor inhibitors), ergot derivatives (e.g. ergonovine in obstetrics, 44 ergotamine, triptans in migraine) and beta-blockers (BBs).45,46 Eventually, the awareness that during a ‘hot phase’ the amount of exercise needs to be reduced may prevent an acute episode. There needs to be more training and education for cardiac rehabilitation practitioners to be able to advise VSA patients on how to safely exercise.
Pharmacological management of VSA
Calcium-channel blockers
CCBs include dihydropyridines (nifedipine, amlodipine and benidipine) and non-dihydropyridines (verapamil and diltiazem). They prevent calcium ion (Ca2+) entry into VSMCs by blocking L-type voltage-gated channels, causing vasodilation. CCBs effectively prevent vasospasm and are the recommended first-line treatment for VSA.21,27 A previous study demonstrated a reduction in the mean weekly rate of anginal attacks from 16 to 2 in patients with coronary artery spasms treated with nifedipine, with a marked decrease in glyceryl trinitrate (GTN) requirement. Complete control was achieved in 63% and the frequency of angina was reduced by at least half in 87% of patients. Long-acting nifedipine is also effective in reducing coronary spasms after percutaneous coronary intervention (PCI). 47 In severe VSA, high dosages of CCBs (2 × 200 mg diltiazem daily) may be needed or even a combination of both non-dihydropyridine with dihydropyridine CCBs. 21 A recent study showed patients with VSA receiving second-generation CCBs (amlodipine and benidipine) to experience a significantly lower incidence of ACS compared to first-generation CCBs (diltiazem and nifedipine). Moreover, benidipine showed significantly better control of angina symptoms compared with diltiazem for 3 years. 48 Diltiazem, however, remains the most widely used CCB for VSA. 49 In the recent efficacy of diltiazem to improve coronary vasomotor dysfunction in angina and non-obstructive coronary arteries (EDIT-CMD) trial, diltiazem up to 360 mg/once daily failed to improve coronary function testing, microvascular dysfunction, symptoms or quality of life after 6 weeks in 85 patients suffering from angina with non-obstructive CAD. 50 Previous studies had demonstrated a favourable effect of diltiazem as opposed to EDIT-CMD. However, the results from these trials were based on different populations and no distinction between different vasomotor dysfunction endotypes.9,51 CCBs may aggravate ECG abnormalities in Brugada syndrome, so these patients require careful ECG monitoring. 52
Nitrates
Nitrates dilate coronary arteries, increase perfusion of ischaemic myocardium, and decrease ventricular filling pressure by systemic venodilation, which decreases myocardial oxygen demand. For treatment of acute attacks, short-acting nitrates, namely sublingual GTN and isosorbide dinitrate (ISDN), can effectively alleviate symptoms within 1–3 min. 53 Extensive evidence suggests that CCBs improve the efficacy of nitrates, this synergistic effect promoting combination therapy with these drugs. 54 However, the efficacy of short-acting nitrates might vary, and repeated administration is often needed. Modified, extended-release, long-acting nitrates (LANs) are recommended by current ESC guidelines 27 and by the Expert Consensus Document on INOCA 21 alongside CCBs for chronic treatment of VSA. VSA patients with refractory symptoms requiring regular admissions to the hospital for treatment with IV GTN can benefit from an agreed codesigned care plan. While effective for symptomatic management of VSA, the long-term prognostic benefit of nitrates is not clear. The prognostic impact of vasodilator therapy has been investigated in the multicentre, prospective Variant Angina-Korea (VA-KOREA) registry. The study population was divided into four groups: no vasodilators, non-nitrate vasodilators, conventional nitrates and a combination of both. The results showed no difference in the primary endpoint (a composite of cardiac death, ACS and new-onset arrhythmia at 2 years) between the vasodilator group and no-vasodilator group. Moreover, the risk of ACS occurrence was significantly higher in the conventional nitrate [hazard ratio (HR), 2.49; 95% confidence interval (CI), 1.01–6.14; p = 0.047] and combination groups (HR, 3.34; 95% CI, 1.15–9.75; p = 0.027) compared with the no-vasodilator group, especially in low-risk patients. 55 Takahashi et al. investigated the use of GTN, isosorbide mononitrate and ISDN (as well as nicorandil, a vasodilatory drug with properties like that of a nitrate) in 1429 patients with VSA. These drugs were used in combination with a CCB in >90% of the patients. Over a 32-month median follow-up period, there was no evidence of improvement in long-term prognosis associated with the use of a LAN. 56
The potential for tachyphylaxis, which is the effectiveness of LANs decreasing with repeated exposure due to tolerance affecting NO signalling and vasodilation, is acknowledged. A nitrate-free interval of 12–14 h between morning and evening dose is therefore necessary to counteract this. 57
For these reasons, it is recommended that LANs be regarded as second-line treatment, generally in combination with CCBs, in VSA that proves refractory to CCB monotherapy. When prescribed alongside a CCB for VSA prophylaxis, administration of the LAN should be timed such that its effects cover periods of high likelihood of vasospasm occurrence. 58 Nitrates are typically well-tolerated drugs. Headache is reported as their main side effect, followed by flushing, light-headedness, orthostatic hypotension and syncope.
Research investigating the role of nitrates is limited to ascertained cases of epicardial spasm, and most data do not discern between different involved segments. Therefore, the related efficacy and long-term outcomes of patients undergoing nitrates administration may not apply to microvascular spasms.
Beta-adrenergic receptor antagonists
Evidence suggests that BBs that do not possess alpha1-adrenergic antagonist activity may be detrimental in VSA by exacerbating coronary epicardial vasospasm, 59 provoking attacks. This is through the antagonism of beta2-adrenergic receptors (responsible for peripheral vasodilation), leading to unopposed activation of alpha1-adrenergic receptors, which causes an enhanced vasoconstrictive response from sympathetic stimulation. 59 BBs are therefore best avoided in VSA and if absolutely indicated, agents with mixed alpha1 and beta-adrenergic activity should be considered (i.e. labetalol or carvedilol), as they may result in overall vasodilation. 58
Evidence suggests that BBs alone and in combination with CCBs are less effective in reducing vasospasm compared to monotherapy with a CCB, with some patients treated with BB monotherapy experiencing worsening symptoms.47,60 A randomized trial of 52 patients provides evidence for the potential importance of BB administration after drug-eluting stent (DES) implantation to reduce the risk of MACE without any difference in reducing coronary artery spasm at 9 months of angiographic follow-up. 61
Statins
β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase inhibitors inhibit the formation of atherosclerotic plaques and reduce endothelial dysfunction, exerting pleiotropic effects such as antioxidant activity, which may help attenuate vasoconstriction. 62 Since endothelial dysfunction promotes coronary vasospasm, statins may aid prevention.62,63 Some studies found that the addition of a statin significantly reduced vasospasm, angina attacks, the incidence of MACE and concluded that their addition reduced the risk of myocardial infarction (MI) with non-obstructive coronary arteries mediated by coronary vasospasm more greatly than CCB monotherapy.62,64 Tani et al. 63 concluded that improved lipid metabolism by statin addition may help to inhibit vascular contractility. Recent evidence showed a correlation between statin therapy and a lower rate of cardiovascular events in VSA patients free of significant stenosis. 64 Their anti-inflammatory properties may be effective and safe in VSA and the Expert Consensus Document on INOCA recommends their use to reduce endothelial dysfunction. 21 However, conflicting evidence regarding their effectiveness in terms of prognosis requires further, larger-scale, double-blind randomized trials.63,65 The Women’s IschemiA Trial to Reduce Events in Non-ObstRuctIve CORonary Artery Disease Study (WARRIOR, NCT03417388) is currently enrolling patients in a multicentre, prospective, randomized blinded outcome evaluation, to evaluate intensive statin and angiotensin-converting enzyme inhibitors (ACEi)/Angiotensin II receptor blocker (ARB) therapy and usual care on MACE in symptomatic women with INOCA. 66
Alpha-adrenergic receptor antagonists
Conflicting evidence, lack of large-scale trials using alpha1 receptor blockers in VSA and unavailability of selective peripheral alpha2 inhibitors make it difficult to determine the potential usefulness of alpha blockers in treating VSA.67,68 Some small-scale trials and evidence reviews suggest that alpha-blockers such as phenoxybenzamine, prazosin, guanethidine and clonidine may be effective in reducing epicardial spasm and relieving angina. 68 Other small-scale trials found that prazosin provided no reduction in symptoms, GTN requirement or transient ST-segment changes, even at increased doses. 67 Therefore, their use in VSA is not recommended by current guidelines. Whether these agents are effective in microvascular spasms is unknown.
Aspirin
Acetylsalicylic acid (ASA), also known as aspirin, at high doses (>325 mg daily) may aggravate coronary vasospasm through the inhibition of prostacyclin, a vasodilator. 69 Low doses of ASA (<100 mg daily) could provide benefits in VSA due to the blockade of thromboxane A2, a vasoconstrictor. 69 A study found that while diffuse vasospasm was less frequently observed in an ASA group versus a non-ASA group, low-dose aspirin did not affect cardiovascular events in patients with non-obstructive coronary arteries. 70 Lim et al. 71 associated aspirin use with a greater risk of MACE and rehospitalization requiring angiography in VSA patients without significant coronary artery stenosis, corroborating its potential unsafety in INOCA due to VSA. 71 More large-scale studies concluded that in VSA patients without significant concomitant coronary artery stenosis, aspirin may provide no benefit regarding MACE or cardiac death. 72 Aspirin should therefore be reserved for VSA with concomitant obstructive CAD. 70
Nicorandil
Nicorandil has dual properties of a nitrate and adenosine triphosphate-sensitive K+ channel agonist. The nicorandil-induced vasodilation results from the NO synthesis and hyperpolarization across VSMC membranes, leading to reduced opening of voltage-gated Ca2+ channels. 73 Nicorandil also stimulates guanylate cyclase to increase the formation of cyclic guanosine monophosphate (cGMP). cGMP activates protein kinase G, decreasing Rho-kinase activity and subsequently the Ca2+ sensitivity of the VSMCs. Small-scale trials have shown nicorandil to effectively treat and prevent vasospasm as well as reduce the frequency of ST-segment elevation on ECG, with one study even concluding nicorandil to be at least as effective at preventing vasospasm as nifedipine. 74 Currently, it should only be considered in patients experiencing VSA and who are still symptomatic despite treatment with CCBs followed by nitrates.
Renin–angiotensin system inhibitors
ACEi blocks the activity of angiotensin-converting enzyme, which converts angiotensin I to angiotensin II and hydrolyses bradykinin. Therefore, ACEi decreases the synthesis of angiotensin II, a vasoconstrictor, and increases the level of bradykinin, a peptide vasodilator. Similarly, angiotensin II receptor blockers (ARBs) block the arteriolar constriction through the inhibition of angiotensin II receptor type 1 (AT1). ACEi/ARBs can be easily combined with CCBs and they have a relevant role in the improvement of coronary flow reserve and small vessel remodelling associated with a workload reduction. 21
In an observational, propensity-matched study of 3349 patients with coronary artery spasm without significant stenosis, the 5-year incidence of recurrent angina, total death and total MACE were significantly lower in a group receiving an ACEi or ARB compared to those who were not. 75 RAS inhibitors should be considered in VSA management for their potential long-term prevention of cardiovascular events.
Rho-kinase inhibitors
Rho-kinase is an enzyme with different functions including cellular contraction, motility and inflammatory mediation. It enhances MLC phosphorylation, leading to a modulation of Ca2+ sensitivity of MLC in VSMCs. 76 OS with a consequent increase in intracellular ROS concentration promotes activation of the Rho-kinase pathway, leading to an impairment of NO-mediated vasodilation and enhanced endothelin-1 vasoconstriction activity. Higher levels of Rho-kinase activity are associated with smoking and levels are lowered by statins. High Rho-kinase activity in circulating leucocytes is associated with increased angina frequency and worse prognosis in terms of cardiac events. 77 A recent study distinguished two groups of patients with epicardial spasms: a diffuse spasm (⩾2 segments) group and a focal spasm (1 segment) group. The former was positively correlated with Rho-kinase activation. Therefore, a Rho-kinase inhibitor would hold promise for a risk-reduction strategy for diffuse spasm. 78 IC infusion of fasudil, a selective Rho-kinase inhibitor, has effectively prevented coronary vasospasm and ameliorated myocardial ischaemia attributable to spasm of coronary microvasculature. 79 Unlike conventional therapy, these drugs inhibit one specific mechanism of vasospasm. 58 Furthermore, larger-scale trials are necessary to investigate the effects of Rho-kinase inhibitors in treating VSA.
Phosphodiesterase 3 inhibitor
Cilostazol is a selective phosphodiesterase 3 inhibitor that acts as an antiplatelet and vasodilator by increasing intracellular cyclic adenosine monophosphate and coronary NO production. Early positive results from studies showed it to decrease the severity and frequency of VSA episodes. 80 Its efficacy and safety in VSA have been studied in the STELLA trial (Study to evaluaTe the Efficacy and safety of Pletaal ciLostazoL in subjects with vAsospastic angina). The relative reduction of chest pain frequency during the last week of the double-blind period compared to the 1-week period before randomization was significantly greater in the cilostazol group compared with the placebo group (−66.5 ± 88.6% versus −17.6 ± 140.1%, respectively, p = 0.009). Cilostazol was also favoured by the secondary endpoints, including a change in the frequency of chest pain (−3.7 ± 0.5 versus −1.9 ± 0.6, respectively, p = 0.029), a change in the chest pain severity scale (−2.8 ± 0.4 versus −1.1 ± 0.4, respectively, p = 0.003) and the proportion of chest pain-free patients (76.0% versus 33.3%, respectively, p = 0.003). 81 These findings have been confirmed in real-world observational studies of refractory VSA patients. 82
Other agents
Some evidence suggests that magnesium 83 or antioxidants 84 (vitamin C and E) deficiency may exacerbate VSA, suggesting that supplementation may be useful. However, more research is needed since symptoms reported by patients undergoing additional intake are controversial. Amiodarone, levosimendan, bosentan (an endothelin receptor antagonist), ranolazine (a late-sodium current inhibitor), L-arginine, ketanserin and stellate ganglion blockade are among other drugs and methods tried in VSA management but without significant evidence to support their use. Promising but very limited evidence exists surrounding denopamine, a selective beta1-adrenoceptor agonist to potentially treat refractory VSA with INOCA. Evidence for and against these agents is sparse and more evidence is required from large-scale randomized trials before recommendations can be made.
Percutaneous coronary intervention
Medical therapy optimization represents the best therapeutic indication for VSA and PCI of spasmodic segments should be strongly discouraged. Endothelial dysfunction after stent implantation could lead to serious cardiac events such as MI, fatal arrhythmias and SCD. 85 Possible mechanisms underlying endothelial dysfunction after DES implantation include delays in endothelial repair due to antiproliferative drugs, the direct effects of polymers or drugs and mechanical injury. DES impairs the endothelial function of the coronary artery distal to the stent, potentially promoting the risk of ischaemia and coronary occlusion. 86 However, vasomotor disorders may coexist with obstructive CAD, and PCI could be required in the presence of significant obstructive CAD. A recent prospective, open-label, randomized trial (D5 study) investigated the degree of endothelial dysfunction caused by treatment of de novo coronary lesion with a drug-coated balloon (DCB) as compared to new-generation DES implantation. In the distal segment, epicardial vasoconstriction after ACh infusion was less pronounced in the DCB arm than in the DES arm (percentage of change in vessel diameter with low-dose ACh: 6 ± 13% versus −3 ± 18%, p = 0.060; high-dose ACh: −4 ± 17% versus −21 ± 29%, p = 0.035), with between-group differences of 9% (95% CI: 0–20) after low-dose ACh infusion and 17% (95% CI: 1–32) after high-dose infusion. This study demonstrated better preservation of endothelial vasomotor activity after treatment with DCB than with DES. 87
Implantable cardioverter defibrillator
Patients with coronary vasospasm are at increased risk of potentially lethal ventricular arrhythmias (VA). 88 Implantable cardioverter defibrillator (ICD) implantation is indicated in VSA in anyone with resuscitated cardiac arrest with normal angiographic findings due to a greater risk of further arrhythmias and death.88,89 The long-term prognosis of VSA patients who have survived a sudden cardiac arrest (SCA) is worse than in other patients with VSA. 90 Since medical intervention and vasodilator drugs might not be sufficiently protective, according to the latest European guidelines for VA and the prevention of SCD, placement of an ICD should be considered in SCA survivors with VSA (class of recommendation IIa, level of evidence C). 91 There is limited data regarding ICD implantation in patients with specific risk factors (e.g. smoking, multivessel disease) but who have not experienced SCD, so further trials are required to determine appropriateness in this group.
Surgery
Surgical management of VSA has seen widely variable results, possibly largely due to the broad range of degrees of arterial obstruction in patients studied.92,93 Success rates are lower than in treating classic angina, with clinical benefit in less than 50% of patients 92 and higher rates of operative mortality, postoperative MI, graft occlusion and recurrent chest pain.92,93 Efficacy is greater in cases of significant fixed atherosclerotic lesions, particularly in single vessel disease, rather than in unobstructed arteries. 93 In conclusion, surgery should be particularly avoided in VSA with non-obstructive coronary arteries.89,92
Role of patient support groups
Initially, INOCA patients experience diagnostic uncertainty despite the persistence of symptoms adversely impacting their lives, and in 34.4% of cases for ⩾3 years, before an INOCA diagnosis. Moreover, 77.8% of patients are told their symptoms are not cardiac. 5 This leads to seeing different clinicians before a correct diagnosis is established and less than a third of INOCA patients undergo complete invasive functional assessment to determine the best optimal medical therapies. 5 This group of patients requires empathetic and compassionate care since many could develop a distrust of healthcare professionals and poor experience of care. Clinical staff need to be aware of the psychological impact of VSA pain on their patients. Without timely adequate management, coronary vasospasm can lead to depression, anxiety, distress and even post-traumatic distress disorder. These conditions can increase an individual’s cardiovascular risk further and need to be identified quickly and treated appropriately. The task force of the latest ESC 2023 guidelines on ACS drew up, for the first time, a new section based on patient perspectives to address a patient-centric multidisciplinary approach taking into account the physical and psychosocial needs of patients. 42 In the INOCA setting, this concept has already been introduced in recent years through the institution of specific patient support groups due to unmet needs. Particularly, INOCA International and International Heart Spasms Alliance are forerunners of a patient-led initiative supported by an International Medical Advisory Board. The main purpose is mutual sharing of knowledge to raise awareness amongst patients and physicians, thus understanding real-life conditions, and improving outcomes and quality of life. The mission is carried out through surveys, periodic meetings and shared experiences to prevent misdiagnosis and to ameliorate physical, mental and social health quality of life in this particular setting.
Conclusion
Coronary artery spasm is a heterogeneous phenomenon that can occur in patients with both non-obstructive coronary arteries and obstructive CAD. While there is considerable data for many treatment options for VSA, there remains a lack of research surrounding the efficacy of certain agents and their applicability to microvascular spasms. More evidence is required, which would further strengthen the current recommendations. The present review summarizes the underlying pathophysiological mechanisms leading to VSA and guides the recommended diagnostic approach and therapeutic management based on the existing guidelines, the Experts Consensus Document and the best available evidence.
Acknowledgments
None.
Footnotes
ORCID iD: Vijay Kunadian  https://orcid.org/0000-0003-2975-6971
https://orcid.org/0000-0003-2975-6971
Contributor Information
Kenny Jenkins, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK.
Graziella Pompei, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK; Cardiovascular Institute, Azienda Ospedaliero-Universitaria di Ferrara, Cona, Italy.
Nandine Ganzorig, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK.
Sarah Brown, Cardiovascular Care Partnership, British Cardiovascular Society, London, UK.
John Beltrame, Basil Hetzel Institute for Translational Health Research, Adelaide Medical School, University of Adelaide and Royal Adelaide Hospital and The Queen Elizabeth Hospital, Adelaide, SA, Australia.
Vijay Kunadian, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University Medical School, 4th Floor William Leech Building, Newcastle upon Tyne NE2 4HH, UK; Cardiothoracic Centre, Freeman Hospital, Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne, UK.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Kenny Jenkins: Writing – original draft; Writing – review & editing.
Graziella Pompei: Writing – original draft; Writing – review & editing.
Nandine Ganzorig: Writing – review & editing.
Sarah Brown: Writing – review & editing.
John Beltrame: Visualization; Writing – review & editing.
Vijay Kunadian: Conceptualization; Supervision; Validation; Writing – review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Roth GA, Mensah GA, Fuster V. The global burden of cardiovascular diseases and risks: a compass for global action. J Am Coll Cardiol 2020; 76: 2980–2981. [DOI] [PubMed] [Google Scholar]
- 2. Patel MRP, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010; 362: 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pepine CJ. ANOCA/INOCA/MINOCA: open artery ischemia. Am Heart J Plus 2023; 26: 20230125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee SH, Shin DL, Lee JM, et al. Clinical relevance of ischemia with nonobstructive coronary arteries according to coronary microvascular dysfunction. J Am Heart Assoc 2022; 11: e025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gulati M, Khan N, George M, et al. Ischemia with no obstructive coronary artery disease (INOCA): a patient self-report quality of life survey from INOCA international. Int J Cardiol 2023; 371: 28–39. 20220923. [DOI] [PubMed] [Google Scholar]
- 6. Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012; 33: 734–744. [DOI] [PubMed] [Google Scholar]
- 7. Ong P, Athanasiadis A, Borgulya G, et al. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol 2012; 59: 655–662. [DOI] [PubMed] [Google Scholar]
- 8. Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation 2014; 129: 1723–1730. [DOI] [PubMed] [Google Scholar]
- 9. Prinzmetal M, Kennamer R, Merliss R, et al. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med 1959; 27: 375–388. [DOI] [PubMed] [Google Scholar]
- 10. Waters DD, Miller DD, Bouchard A, et al. Circadian variation in variant angina. Am J Cardiol 1984; 54: 61–64. [DOI] [PubMed] [Google Scholar]
- 11. Guo YF, Stein PK. Circadian rhythm in the cardiovascular system: chronocardiology. Am Heart J 2003; 145: 779–786. [DOI] [PubMed] [Google Scholar]
- 12. Miwa K, Fujita M, Sasayama S. Recent insights into the mechanisms, predisposing factors, and racial differences of coronary vasospasm. Heart Vessels 2005; 20: 1–7. [DOI] [PubMed] [Google Scholar]
- 13. Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation 2011; 124: 1774–1782. [DOI] [PubMed] [Google Scholar]
- 14. Picard F, Sayah N, Spagnoli V, et al. Vasospastic angina: a literature review of current evidence. Arch Cardiovasc Dis 2019; 112: 44–55. [DOI] [PubMed] [Google Scholar]
- 15. Takaoka K, Yoshimura M, Ogawa H, et al. Comparison of the risk factors for coronary artery spasm with those for organic stenosis in a Japanese population: role of cigarette smoking. Int J Cardiol 2000; 72: 121–126. [DOI] [PubMed] [Google Scholar]
- 16. Sato K, Kaikita K, Nakayama N, et al. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: analysis of a single-center study over 20 years. J Am Heart Assoc 2013; 2: e000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura Y, Shinozaki N, Hirasawa M, et al. Prevalence of migraine and Raynaud’s phenomenon in Japanese patients with vasospastic angina. Jpn Circ J 2000; 64: 239–242. [DOI] [PubMed] [Google Scholar]
- 18. Miller D, Waters DD, Warnica W, et al. Is variant angina the coronary manifestation of a generalized vasospastic disorder? N Engl J Med 1981; 304: 763–766. [DOI] [PubMed] [Google Scholar]
- 19. Hokimoto S, Kaikita K, Yasuda S, et al. JCS/CVIT/JCC 2023 guideline focused update on diagnosis and treatment of vasospastic angina (coronary spastic angina) and coronary microvascular dysfunction. Circ J 2023; 87: 879–936. [DOI] [PubMed] [Google Scholar]
- 20. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol 2018; 72: 2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on coronary pathophysiology & microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020; 41: 3504–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woudstra J, Vink CEM, Schipaanboord DJM, et al. Meta-analysis and systematic review of coronary vasospasm in ANOCA patients: prevalence, clinical features and prognosis. Front Cardiovasc Med 2023; 10: 1129159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol 2018; 72: 2841–2855. [DOI] [PubMed] [Google Scholar]
- 24. Seitz A, Gardezy J, Pirozzolo G, et al. Long-term follow-up in patients with stable angina and unobstructed coronary arteries undergoing intracoronary acetylcholine testing. JACC Cardiovasc Interv 2020; 13: 1865–1876. [DOI] [PubMed] [Google Scholar]
- 25. Hwang D, Park SH, Koo BK. Ischemia with nonobstructive coronary artery disease: concept, assessment, and management. JACC Asia 2023; 3: 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980; 288: 373–376. [DOI] [PubMed] [Google Scholar]
- 27. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407–477. [DOI] [PubMed] [Google Scholar]
- 28. Kunadian V, Raharjo DE. Breaking down the barriers in the management of INOCA: how can we do better in the diagnosis of coronary vasomotor disorders? EuroIntervention 2022; 17: 1201–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017; 38: 2565–2568. [DOI] [PubMed] [Google Scholar]
- 30. Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018; 250: 16–20. [DOI] [PubMed] [Google Scholar]
- 31. Montone RA, Niccoli G, Fracassi F, et al. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J 2018; 39: 91–98. [DOI] [PubMed] [Google Scholar]
- 32. Ong P, Athanasiadis A, Sechtem U. Intracoronary acetylcholine provocation testing for assessment of coronary vasomotor disorders. J Vis Exp 2016; 114: 54295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015; 131: 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheikh AR, Zeitz C, Beltrame JF. Letter by Sheikh et al regarding article, ‘Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease’. Circulation 2015; 132: e242. [DOI] [PubMed] [Google Scholar]
- 35. Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 1986; 315: 1046–1051. [DOI] [PubMed] [Google Scholar]
- 36. Montone RA, Rinaldi R, Del Buono MG, et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and non-obstructive coronary arteries. EuroIntervention 2022; 18: e666–e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seitz A, Feenstra R, Konst RE, et al. Acetylcholine rechallenge: a first step toward tailored treatment in patients with coronary artery spasm. JACC Cardiovasc Interv 2022; 15: 65–75. [DOI] [PubMed] [Google Scholar]
- 38. Montone RA, Niccoli G, Russo M, et al. Clinical, angiographic and echocardiographic correlates of epicardial and microvascular spasm in patients with myocardial ischaemia and non-obstructive coronary arteries. Clin Res Cardiol 2020; 109: 435–443. [DOI] [PubMed] [Google Scholar]
- 39. Shantsila E, Wrigley B, Shantsila A, et al. Ethnic differences in macrovascular and microvascular function in systolic heart failure. Circ Heart Fail 2011; 4: 754–762. [DOI] [PubMed] [Google Scholar]
- 40. Nguyen TH, Ong GJ, Girolamo OC, et al. Angina due to coronary artery spasm (variant angina): diagnosis and intervention strategies. Expert Rev Cardiovasc Ther 2021; 19: 917–927. [DOI] [PubMed] [Google Scholar]
- 41. Beltrame JF, Tavella R, Jones D, et al. Management of ischaemia with non-obstructive coronary arteries (INOCA). BMJ 2021; 375: e060602. [DOI] [PubMed] [Google Scholar]
- 42. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023; 44: 3720–3826. [DOI] [PubMed] [Google Scholar]
- 43. Henrikson CA, Howell EE, Bush DE, et al. Chest pain relief by nitroglycerin does not predict active coronary artery disease. Ann Intern Med 2003; 139: 979–986. [DOI] [PubMed] [Google Scholar]
- 44. Jang SK, Berlacher K, Hauspurg A. Post-partum myocardial ischemia due to intramuscular methylergonovine-induced coronary vasospasm: case report. BMC Cardiovasc Disord 2023; 23: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kalsner S. Cocaine sensitization of coronary artery contractions: mechanism of drug-induced spasm. J Pharmacol Exp Ther 1993; 264: 1132–1140. [PubMed] [Google Scholar]
- 46. Beltrame JF. Management of vasospastic angina. Heart 2022; 109: 70–77. [DOI] [PubMed] [Google Scholar]
- 47. Antman E, Muller J, Goldberg S, et al. Nifedipine therapy for coronary-artery spasm. Experience in 127 patients. N Engl J Med 1980; 302: 1269–1273. [DOI] [PubMed] [Google Scholar]
- 48. Kim SE, Jo SH, Han SH, et al. Comparison of calcium-channel blockers for long-term clinical outcomes in patients with vasospastic angina. Korean J Intern Med 2021; 36: 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim HL. A new perspective on calcium channel blockers in vasospastic angina. Korean J Intern Med 2021; 36: 63–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jansen TPJ, Konst RE, de Vos A, et al. Efficacy of diltiazem to improve coronary vasomotor dysfunction in ANOCA: the EDIT-CMD randomized clinical trial. JACC Cardiovasc Imaging 2022; 15: 1473–1484. [DOI] [PubMed] [Google Scholar]
- 51. Pesola A, Lauro A, Gallo R, et al. Efficacy of diltiazem in variant angina. Results of a double-blind crossover study in CCU by Holter monitoring. The possible occurrence of a withdrawal syndrome. G Ital Cardiol 1987; 17: 329–339. [PubMed] [Google Scholar]
- 52. Yoshikawa T, Izumi C, Kaitani K, et al. A case of Brugada syndrome coexisting with vasospastic angina: caution should be taken when using calcium channel blockers. J Cardiol Cases 2011; 4: e143–e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lanza GA, Shimokawa H. Management of coronary artery spasm. Eur Cardiol 2023; 18: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Conti CR, Hill JA, Feldman RL, et al. Isosorbide dinitrate and nifedipine in variant angina pectoris. Am Heart J 1985; 110: 251–256. [DOI] [PubMed] [Google Scholar]
- 55. Lim Y, Kim MC, Ahn Y, et al. Prognostic impact of chronic vasodilator therapy in patients with vasospastic angina. J Am Heart Assoc 2022; 11: e023776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takahashi J, Nihei T, Takagi Y, et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: multicentre registry study of the Japanese coronary spasm association. Eur Heart J 2014; 36: 228–237. [DOI] [PubMed] [Google Scholar]
- 57. Munzel T, Gori T. Nitrate therapy and nitrate tolerance in patients with coronary artery disease. Curr Opin Pharmacol 2013; 13: 251–259. [DOI] [PubMed] [Google Scholar]
- 58. Harris JR, Hale GM, Dasari TW, et al. Pharmacotherapy of vasospastic angina. J Cardiovasc Pharmacol Ther 2016; 21: 439–451. [DOI] [PubMed] [Google Scholar]
- 59. Ajani AE, Yan BP. The mystery of coronary artery spasm. Heart Lung Circ 2007; 16: 10–15. [DOI] [PubMed] [Google Scholar]
- 60. Kook H, Hong SJ, Yang KS, et al. Comparison of nebivolol versus diltiazem in improving coronary artery spasm and quality of life in patients with hypertension and vasospastic angina: a prospective, randomized, double-blind pilot study. PLoS One 2020; 15: e0239039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sawano M, Katsuki T, Kitai T, et al. Beta blockers versus calcium channel blockers for provocation of vasospastic angina after drug-eluting stent implantation: a multicentre prospective randomised trial. Open Heart 2020; 7: e001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yasue H, Mizuno Y, Harada E, et al. Effects of a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, fluvastatin, on coronary spasm after withdrawal of calcium-channel blockers. J Am Coll Cardiol 2008; 51: 1742–1748. [DOI] [PubMed] [Google Scholar]
- 63. Tani S, Nagao K, Anazawa T, et al. Treatment of coronary spastic angina with a statin in addition to a calcium channel blocker: a pilot study. J Cardiovasc Pharmacol 2008; 52: 28–34. [DOI] [PubMed] [Google Scholar]
- 64. Ishii M, Kaikita K, Sato K, et al. Impact of statin therapy on clinical outcome in patients with coronary spasm. J Am Heart Assoc 2016; 5: e003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Park SJ, Park H, Kang D, et al. Association of statin therapy with clinical outcomes in patients with vasospastic angina: data from Korean health insurance review and assessment service. PLoS One 2019; 14: e0210498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Handberg EM, Merz CNB, Cooper-Dehoff RM, et al. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J 2021; 237: 90–103. [DOI] [PubMed] [Google Scholar]
- 67. Winniford MD, Filipchuk N, Hillis LD. Alpha-adrenergic blockade for variant angina: a long-term, double-blind, randomized trial. Circulation 1983; 67: 1185–1188. [DOI] [PubMed] [Google Scholar]
- 68. Tzivoni D, Keren A, Benhorin J, et al. Prazosin therapy for refractory variant angina. Am Heart J 1983; 105: 262–266. [DOI] [PubMed] [Google Scholar]
- 69. Miwa K, Kambara H, Kawai C. Effect of aspirin in large doses on attacks of variant angina. Am Heart J 1983; 105: 351–355. [DOI] [PubMed] [Google Scholar]
- 70. Kim MC, Ahn Y, Park KH, et al. Clinical outcomes of low-dose aspirin administration in patients with variant angina pectoris. Int J Cardiol 2013; 167: 2333–2334. [DOI] [PubMed] [Google Scholar]
- 71. Lim AY, Park TK, Cho SW, et al. Clinical implications of low-dose aspirin on vasospastic angina patients without significant coronary artery stenosis; a propensity score-matched analysis. Int J Cardiol 2016; 221: 161–166. [DOI] [PubMed] [Google Scholar]
- 72. Lin Y, Chen Y, Yuan J, et al. Impact of aspirin use on clinical outcomes in patients with vasospastic angina: a systematic review and meta-analysis. BMJ Open 2021; 11: e048719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nakae I, Matsumoto T, Horie H, et al. Effects of intravenous nicorandil on coronary circulation in humans: plasma concentration and action mechanism. J Cardiovasc Pharmacol 2000; 35: 919–925. [DOI] [PubMed] [Google Scholar]
- 74. Lablanche JM, Bauters C, Leroy F, et al. Prevention of coronary spasm by nicorandil: comparison with nifedipine. J Cardiovasc Pharmacol 1992; 20 Suppl 3: S82–S85. [DOI] [PubMed] [Google Scholar]
- 75. Choi BG, Jeon SY, Rha SW, et al. Impact of renin-angiotensin system inhibitors on long-term clinical outcomes of patients with coronary artery spasm. J Am Heart Assoc 2016; 5: e003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shimokawa H, Sunamura S, Satoh K. RhoA/Rho-kinase in the cardiovascular system. Circ Res 2016; 118: 352–366. [DOI] [PubMed] [Google Scholar]
- 77. Nihei T, Takahashi J, Hao K, et al. Prognostic impacts of Rho-kinase activity in circulating leucocytes in patients with vasospastic angina. Eur Heart J 2017; 39: 952–959. [DOI] [PubMed] [Google Scholar]
- 78. Nishimiya K, Suda A, Fukui K, et al. Prognostic links between OCT-delineated coronary morphologies and coronary functional abnormalities in patients with INOCA. JACC Cardiovasc Interv 2021; 14: 606–618. [DOI] [PubMed] [Google Scholar]
- 79. Masumoto A, Mohri M, Shimokawa H, et al. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002; 105: 1545–1547. [DOI] [PubMed] [Google Scholar]
- 80. Yoo SY, Song SG, Lee JH, et al. Efficacy of cilostazol on uncontrolled coronary vasospastic angina: a pilot study. Cardiovasc Ther 2013; 31: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shin ES, Lee JH, Yoo SY, et al. A randomised, multicentre, double blind, placebo controlled trial to evaluate the efficacy and safety of cilostazol in patients with vasospastic angina. Heart 2014; 100: 1531–1536. [DOI] [PubMed] [Google Scholar]
- 82. Lin R, Tavella R, Beltrame J. Impact of cilostazol therapy in refractory vasospastic angina. Heart Lung Circ 2022; 31: S206. [Google Scholar]
- 83. Satake K, Lee JD, Shimizu H, et al. Relation between severity of magnesium deficiency and frequency of anginal attacks in men with variant angina. J Am Coll Cardiol 1996; 28: 897–902. [DOI] [PubMed] [Google Scholar]
- 84. Hirashima O, Kawano H, Motoyama T, et al. Improvement of endothelial function and insulin sensitivity with vitamin C in patients with coronary spastic angina: possible role of reactive oxygen species. J Am Coll Cardiol 2000; 35: 1860–1866. [DOI] [PubMed] [Google Scholar]
- 85. Brott BC, Anayiotos AS, Chapman GD, et al. Severe, diffuse coronary artery spasm after drug-eluting stent placement. J Invasive Cardiol 2006; 18: 584–592. [PubMed] [Google Scholar]
- 86. Luscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115: 1051–1058. [DOI] [PubMed] [Google Scholar]
- 87. Kawai T, Watanabe T, Yamada T, et al. Coronary vasomotion after treatment with drug-coated balloons or drug-eluting stents: a prospective, open-label, single-centre randomised trial. EuroIntervention 2022; 18: e140–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Matsue Y, Suzuki M, Nishizaki M, et al. Clinical implications of an implantable cardioverter-defibrillator in patients with vasospastic angina and lethal ventricular arrhythmia. J Am Coll Cardiol 2012; 60: 908–913. [DOI] [PubMed] [Google Scholar]
- 89. Slavich M, Patel RS. Coronary artery spasm: current knowledge and residual uncertainties. Int J Cardiol Heart Vasc 2016; 10: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ahn JM, Lee KH, Yoo SY, et al. Prognosis of variant angina manifesting as aborted sudden cardiac death. J Am Coll Cardiol 2016; 68: 137–145. [DOI] [PubMed] [Google Scholar]
- 91. Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022; 43: 3997–4126. [DOI] [PubMed] [Google Scholar]
- 92. Gaasch WH, Lufschanowski R, Leachman RD, et al. Surgical management of Prinzmetal’s variant angina. Chest 1974; 66: 614–621. [DOI] [PubMed] [Google Scholar]
- 93. Pasternak RC, Thibault GE, Savoia M, et al. Chest pain with angiographically insignificant coronary arterial obstruction. Clinical presentation and long-term follow-up. Am J Med 1980; 68: 813–817. [DOI] [PubMed] [Google Scholar]