Abstract
Background
Campylobacter species are the most predominant bacterial agents to cause diarrhea in under-five children. It poses a serious challenge to public health worldwide with ongoing acquisition of resistance to different antimicrobials with multiple patterns. Thus, this study aimed to determine the prevalence, and antimicrobial resistance of Campylobacter species, and associated factors among under-five children with diarrhea in selected public health facilities.
Methods
A cross-sectional study was conducted among under-five children with diarrhea using convenient sampling. Health facilities were selected using a simple random sampling method. The stool samples collected from 214 study participants were transported and processed following standard microbiological protocols. Campylobacter isolates were identified using Gram staining, biochemical test, serological test, and aerobic growth at 25°C. Antimicrobial susceptibility profiles of isolates were performed using the Kirby–Bauer method. Data were analyzed using SPSS ver. 25.0. Association between variables was assessed using Chi-square test and Logistic regression, with P ≤ 0.05.
Results
The subject's mean age was 31.3 (±3.9) months. Of the 214 samples cultured, 14 (6.5%) of them were positive for Campylobacter species with 95% CI (3.3–10.3). Out of the isolated species, 12 (85.7%) were Campylobacter jejuni /Campylobacter coli and 2 (14.3%) were other Campylobacter species. Bottle feeding and history of direct contact to domestic animals were associated with Campylobacter species (AOR=5.13, CI=1.21–21.6, p=0.026 and AOR=4.93, CI=1.33–18.17, P=0.016), respectively. Campylobacter isolates were highly resistant to ciprofloxacin 5 (35.7%), and tetracycline 3 (21.4%).
Conclusion
A higher incidence of Campylobacter species was obtained in children who were bottle-fed and who had a history of direct contact with domestic animals. The isolates were highly resistant to ciprofloxacin and tetracycline. These findings indicate that special attention is needed for better management of Campylobacter drug resistance in under-five children. To enhance and support our current findings, further research using molecular techniques is needed to identify the resistant and virulent genes of the bacterial isolates.
Keywords: Campylobacter species, drug susceptibility, gastroenteritis, Mekelle city, prevalence, risk factors, under-five children
Introduction
Diarrhea is the passage of high fluid content of stool or an abnormal increase in daily stool frequency.1 Diarrheal illness is a major health problem in the world, principally in low- and middle-income countries.2,3 It is the second most important infectious cause of death, accounting for 9.2% of deaths in under-five children across the globe.1,4 Of these, 90% of the death rate among under-five children due to diarrhea happens in low- and middle-income countries,5 and 46% in African continents.6
Acute gastroenteritis (AGE) is the irritation of the mucous membranes covering the digestive tract that leads to vomiting and diarrhea.7 Globally, AGE affects an estimated 2.5 billion and kills nearly 1.5 million children under five years old each year.8 AGE contributes to the mortality of under-five children by about 5–20% and 5–35% in developed and developing countries, respectively.8,9 About 10–20% of all AGE are caused by acute bacterial gastroenteritis. Campylobacter is one of the most recognized etiologies of AGE.10,11 Moreover, it is a principal cause of food-borne infection.12 Campylobacter jejuni is the most commonly isolated Campylobacter species, which causes human gastroenteritis.13,14
Campylobacter is an animal pathogen that commonly inhabits the intestine of numerous animal species. Campylobacter infection is mostly a foodborne illness that leads to inflammation of the human intestine.15,16 Ingestion of contaminated uncooked food (eg milk), drinking polluted water, contact with domestic animals and diarrheal persons, poor personal hygiene, and improper use of latrine are the key risk factors of Campylobacter species.16
In several countries, Campylobacter antimicrobial resistance is common and leads to traveler’s infection.17 In the United States of America, about 23% and 2% of Campylobacter species are resistant to ciprofloxacin and azithromycin, respectively. In 2017 WHO reported that Campylobacter species were recorded as one of six high-priority antibiotic-resistant pathogens.18,19 The main causes for the development of antimicrobial resistance are prescribing and use of ineffective antimicrobials (accounts for about 50%), irrational use of antimicrobials, the person-to-person spread of bacterial-resistant strains,20 and common use of antimicrobials as treatment for both animals and humans.21–23
In Ethiopia, diarrheal diseases are the major causes of infant and child mortality and morbidity.24 In Ethiopia, around 39 million diarrheal episodes occur every year, of which 230 thousand children die because of it.25 The prevalence of Campylobacter species among under-five children with diarrhea is around 14.5% of which Campylobacter jejuni and Campylobacter coli account for 71.1% and 21.1%, respectively.26–28
Although Campylobacter diarrheal disease among under-five years of age is a huge problem in Ethiopia, particularly in Tigray, to our knowledge to date, few studies have been conducted to look into the prevalence, antimicrobial resistance, and associated factors with the incidence of campylobacteriosis. Thus, this study was conducted to determine the prevalence of Campylobacter species and associated factors among children under-five years of age who visited Mekelle General Hospital, Mekelle Health Center, Semen Health Center, and Kasech Asfaw Health Center. This study will help to recognize the prevalence, antimicrobial resistance, and risk factors of diarrheal diseases that enable the concerned bodies to develop appropriate interventions.
Materials and Methods
Study Area
The study was conducted in Mekelle city, which is the regional capital city of Tigray Mekelle city found around 780 km drive north of the national capital city, Addis Ababa. Geographically it is located between 130 24’30“ to 130 36’ 52“ Latitude and 390 25’30” to 390 38’33” Longitude. Its altitude ranges from 2200 m above sea level. According to the Central Statistical Agency of Tigray, Mekelle has an estimated total population of 537,822. Mekelle owns one specialized hospital, two general hospitals, one primary hospital, and five health centers.25,29 The study was conducted among randomly selected four public health facilities through a simple random sampling (lottery) method due to resource scarcity to encompass all.
Study Design and Period
A cross-sectional study design was used to conduct this research.
Study Population
All under-five children with diarrhea who visited any one of the four health facilities in Mekelle were used as study population.
Inclusion and Exclusion Criteria
All under-five children (0–60 months) with diarrhea and their parents or caregivers who were willing to provide written and oral consent to participate their child in the study were included, whereas severely ill under-five children were excluded.
Sampling Technique and Sample Size Determination
A convenient sampling technique was employed for the patient that fulfilled the inclusion criteria during the study period. Based on a previous prevalence rate of 16.7% from Jimma, Ethiopia,30 a 95% confidence interval, and a 5% margin of error, the sample size was determined using a single population proportion formula:
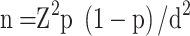 |
From this calculation, about 214 children were included in the study of which 60 were from Mekelle General Hospital, 62 from Mekelle Health Center, 39 from Semen Health Center, and 52 from Kasech Asfaw Health Center.
Study Variables
The prevalence of Campylobacter species and the status of antimicrobial resistance were taken as outcome variables, whereas socio-demographic variables (age, sex, residence, and educational and occupational status of caregivers), environmental variables (water source, exposure to domestic animals, contact history with a diarrheal person), and behavioral variables (hand washing before feeding the child, cleaning the child after defecation, caregiver relation to the child, latrine usage, bottle feeding, nutritional status, consuming of raw animal product, eg milk) were taken as determinant factors.
Data Collection Procedure
After obtaining written consent from the parents/caregivers, socio-demographic, environmental, behavioral, and clinical data were collected using a pre-structured questionnaire with the help of trained clinicians on how to collect the data for 1 day. About 5 mL of fresh diarrheal stool specimen free of urine and soil contamination was collected using leak-proof stool containers (Figure 1). The specimen was transported with Cary Blair transport medium to Mekelle University, Ayder Comprehensive Specialized Hospital, Medical Microbiology laboratory in an ice-cold box within 4 h of collection and processed immediately.30
Figure 1.
Schematic illustration of the experimental work flow.
The specimen was inoculated on a Modified Charcoal Cefoperazone Deoxycholate Agar (MCCDA) (OXOID, CM0739) with supplements and kept in a 2.5 L anaerobic jar. Campy-Gen gas generating kit (5% O2 and 10% CO2) (Oxoid CN0025A) was inserted to maintain the micro-aerophilic condition. The jars were incubated at a temperature of 42°C for 48 h.30 The presence or absence of bacterial growth on the agar media was observed. Campylobacter species were identified using colony appearance on the culture medium (moist, creamy-grey, and flat-spreading), Gram stain, serological test (dry spot Campylobacter test (OXOID, Basing stoke Hampshire, England), biochemical test (oxidase positive, catalase positive), and growth at 25°C on sheep blood agar.19 Cephalothin (30 µg) and nalidixic acid (30 µg) both obtained from OXOID were used for the susceptibility test. Campylobacter species sensitive to nalidixic acid but resistant to cephalothin were considered Campylobacter jejuni or Campylobacter coli, while strains that are resistant to both drugs were considered other Campylobacter species).7,30,31
Antimicrobial Susceptibility Testing
It was performed using the disk diffusion method. In brief, 3–5 morphologically identical bacterial colonies were picked from the culture and suspended in 5 mL of 0.85% normal saline. The turbidity of the broth culture was adjusted to 0.5 McFarland standards and measured by a McFarland densitometer (DEN-1B).
A sterile cotton swab was dipped into bacterial suspension. An excess amount of the suspension was removed and streaked on the entire surface of Muller Hinton Agar supplemented with 5% sheep blood agar (SBA). The inoculated plates were left at room temperature for 3–5 min to dry.32 According to CLSI, a set of antimicrobial discs were then delivered onto the inoculated surface of the Muller-Hinton plate with ciprofloxacin (5 µg), erythromycin (30 µg), and tetracycline (30 µg). Then the plate was incubated at micro-aerophilic atmospheric conditions, at 42°C for 24 h.33 After 24 h incubation, a clear zone of inhibition was measured (diameter in mm) using a straight-line ruler. The zone of inhibition was read and interpreted using an interpreting chart and determined as susceptible, intermediate, and resistant.
Quality Control
To ensure the reliability of the information, the questionnaire was initially prepared in English and then translated into a local language (Tigrinya). Each questionnaire was checked for its completeness and consistency. All specimens were properly labeled parallel to the questionnaire. During sample collection, handling, transportation, processing, and interpretation, standard laboratory guidelines were followed. Expiry date, damage and storage problem of media, and antimicrobial disc were checked. The sterility of the prepared media was checked by incubating, 5% of the media for 24 h. A known isolate of Campylobacter jejuni was used for MCCDA media and E. coli ATCC 25922 reference strains for broth culture media and discs31
Data Management and Statistical Analysis
Data were analyzed using SPSS version 25.0. Descriptive analyses such as frequencies and mean were computed. Initially, the association between each exposure and the presence of infection was assessed using the chi-square test. To determine independent risk factors, bivariate logistic regression analysis (COR) was employed to measure the strength of the association without reducing the confounding effect, and P≤ 0.3 was made eligible in the multivariate logistic regression (AOR) to reduce the confounding effect. P ≤ 0.05 was used to declare it statistically significant.
Ethical Considerations
The study was approved by the institutional review committee of Mekelle University, College of Health Sciences (Ref. #: MU-CHS-1482/2020). All the methods were performed based on relevant national, international, and scientific guidelines and regulations. Besides, our study was carried out by the code of ethics of the World Medical Association (Declaration of Helsinki) for experiments in humans. Before data collection, the objective of the study was explained to each parent/caregiver of the study participants and informed consent and assent were collected. All information, samples, and experimental results obtained from the parents were kept confidential thoroughly and used for the specified objectives of the study only. Finally, the specimens were discarded following infection prevention guidelines.
Operational Definitions
Mal nourished: considers children who have deficiency or imbalance in their intake of nutrients, leading to negative health outcome manifested as under nutrition including stunting, wasting, or being underweight or over nutrition example obesity with regard to their age. All these was measured through anthropometric measurements of weight for height (wasting), low height for (stunting) and low weight for age (underweight).
Well nourished: incorporates children with appropriate balance of essential nutrients, optimal growth, and development and overall health. Anthropometric measurements were also used to evaluate it.
Results
Socio-Demographic Characteristics
In the present study, a total of 214 (125 male and 89 female) under-five children with diarrhea were recruited (Table 1). About 39 (18.2%), 52 (24.2%), 60 (28%), and 62 (28.9%) study participants were obtained from Semen, Kasich Asfaw, Mekelle health centers, and Mekelle General Hospital, respectively. The median age of the study participants was obtained to be 30 months. Whereas the mean was 31.3 (±13.9) with 95% CI (29.3–32.9) and ranges of 1 to 59 months. The majority of participants (85 (39.7%)) were belonged to the 12–23-month group.
Table 1.
Baseline Characteristics of the Under-Five Children with Diarrhea
| Variable | Category | Frequency | Percent |
|---|---|---|---|
| Age (Months) | <12 | 20 | 9.3 |
| 12–23 | 85 | 39.7 | |
| 24–35 | 49 | 22.9 | |
| 36–47 | 32 | 15.0 | |
| 48–59 | 28 | 13.1 | |
| Sex | Male | 125 | 58.4 |
| Female | 89 | 41.6 | |
| Residence | Rural | 50 | 23.3 |
| Urban | 164 | 76.6 | |
| Caregiver relation to child | Mother | 28 | 13.1 |
| Father and Mother | 186 | 86.9 | |
| Educational status of the caregiver | Illiterate | 20 | 9.3 |
| Primary level | 56 | 26.2 | |
| Secondary level | 78 | 36.4 | |
| College and above | 60 | 28.0 | |
| Occupational status | Labor day | 69 | 32.2 |
| Farmer | 43 | 20.1 | |
| Merchant | 61 | 28.5 | |
| Governmental employed | 41 | 19.2 |
Prevalence of Campylobacter Species
In the present study, the overall prevalence of Campylobacter species was 14 (6.5%) with 95% CI (3.3–10.3). Of the 14 isolates, 12 (85.7%) were Campylobacter jejuni / Campylobacter coli and 2 (14.3%) were other Campylobacter species. The prevalence of Campylobacter species between males and females was 9 (7.2%) and 5 (5.6%), respectively, which was statistically insignificant (p=0.64). The prevalence of Campylobacter species among the age group of less than 12 months, rural residents, children caring by mother only, caregivers who had secondary educational level, and caregivers who were governmentally employed were detected at (2/20, 10.0%), (8/50, 16%), (2/28, 7.1%), (7/78, 9%), and (3/41, 7.3%), respectively (Table 2).
Table 2.
Bivariate and Multivariable Analysis of the Socio-Demographic Characteristics and Prevalence of Culture-Confirmed Campylobacter Species Among Under-Five Children with Diarrhea
| Variable | Category | Total | Campylobacter Species | COR (95%) | AOR (95%) | |
|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | |||||
| Age (Months) | <12 | 20 | 2 (10) | 18 (90.0) | 0.40 (0.024–6.79) | |
| 12–23 | 85 | 7 (8.2) | 78 (91.7) | 0.30 (0.03–3.01) | ||
| 24–35 | 49 | 1 (2.0) | 48 (97.9) | 0.49 (0.04–4.89) | ||
| 36–47 | 32 | 2 (6.2) | 30 (93.7) | 0.36 (0.03–3.66) | ||
| 48–59 | 28 | 2 (7.1) | 27 (96.4) | 1 | ||
| Sex | Male | 125 | 9 (7.2) | 116 (92.8) | 1.30 (0.42–4.03) | |
| Female | 89 | 5 (5.6) | 84 (94.4) | 1 | ||
| Residence | Rural | 50 | 8 (16.0) | 42 (84.0) | 5.0 (1.65–15.24) | 2.18 (0.57–8.23)* |
| Urban | 164 | 6 (3.7) | 158 (96.3) | 1 | ||
| Caregiver relation to child | Mother | 28 | 2 (7.1) | 26 (92.9) | 1.11 (0.23–5.26) | |
| Father and Mother | 186 | 12 (6.5) | 174 (93.5) | 1 | ||
| Educational status of the caregiver | Illiterate | 20 | 1 (5.0) | 19 (95.0) | 0.65 (0.05–7.63) | |
| Primary level | 56 | 4 (7.1) | 52 (92.9) | 0.44 (0.07–2.54) | ||
| Secondary level | 78 | 7 (9.0) | 71 (91.0) | 0.35 (0.07–1.74) | ||
| College and above | 60 | 2 (3.3) | 58 (96.7) | 1 | ||
| Occupational status | Labor day | 69 | 4 (5.80) | 65 (94.20) | 1.28 (0.27–6.04) | |
| Farmer | 43 | 3 (7.0) | 40 (93.00) | 1.05 (0.20–5.54) | ||
| Merchant | 61 | 4 (6.6) | 57 (93.4) | 1.12 (0.23–5.310) | ||
| Governmental employed | 41 | 3 (7.3) | 38 (92.70) | 1 | ||
Note: *Statistically Significant Association.
Abbreviations: N, Number; COR, Crud odds Ratio; AOR, Adjusted Odds Ratio.
Possible Risk Factors Associated with Campylobacter Species
From the risk factors of campylobacteriosis, children who used bottle feeding and had a history of animal contact (cat, dog, hen, and pigeon) had a chance of getting high risk of Campylobacteriosis and also was statistically significant association (AOR=5.13, CI=1.21–21.6, p=0.026 and AOR=4.93, CI=1.33–18.17, P=0.016), respectively (Table 3).
Table 3.
Bivariate and Multivariate Analysis of Potential Predictors of Campylobacter Species Among Under-Five Children with Diarrhea
| Variable | Category | Total | Campylobacter Species | COR (95%) | AOR (95%) | |
|---|---|---|---|---|---|---|
| Positive (N (%)) | Negative (N (%)) | |||||
| Nutritional Status of the child | Mal-nourished | 14 | 2 (14.3) | 12 (85.7) | 2.61 (0.52–13.0) | 2.07 (0.29–14.5) |
| Well-nourished | 200 | 12 (6.0) | 188 (94.0) | 1 | ||
| Bottle feeding | Yes | 97 | 11 (11.3) | 86 (88.70) | 4.86 (1.31–17.95) | 5.13 (1.21–21.6)** |
| No | 117 | 3 (2.60) | 114 (97.40) | 1 | ||
| Water source | Well | 33 | 2 (6.1) | 31 (93.9) | 1.10 (0.23–5.24) | |
| Spring | 16 | 1 (6.2) | 15 (93.8) | 1.07 (0.12–8.87) | ||
| Pipe | 165 | 11 (6.7) | 154 (93.3) | 1 | ||
| Animal contact status | Yes | 77 | 10 (13.0) | 67 (87.0) | 4.96 (1.50–16.41) | 4.93 (1.33–18.17)** |
| No | 137 | 4 (2.9) | 133 (97.1) | 1 | ||
| Poultry farm | Yes | 37 | 6 (16.2) | 31 (83.8) | 4.08 (1.32–12.60) | 2.02 (0.55–7.47)* |
| No | 177 | 8 (4.5) | 169 (95.5) | 1 | ||
| Child Contact with a diarrheal person | Yes | 24 | 3 (12.5) | 21 (87.5) | 2.32 (0.60–9.00) | 0.52 (0.08–3.16) |
| No | 190 | 11 (5.8) | 179 (94.2) | 1 | ||
| Defecation practice | Open defecation | 13 | 1 (7.7) | 12 (92.3) | 1.20 (0.14–10.0) | |
| Toilet | 201 | 13 (6.5) | 188 (93.5) | 1 | ||
| Clean the child after defecation | Not at all | 22 | 1 (4.5) | 21 (95.5) | 1.55 (0.19–12.57) | |
| Yes sometimes | 18 | 1 (5.6) | 17 (94.4) | 1.25 (0.15–10.28) | ||
| Yes, always | 174 | 12 (6.9) | 162 (93.1) | 1 | ||
| The habit of hand wash | Not at all | 62 | 4 (6.5) | 58 (93.5) | 1.10 (0.30–3.93) | |
| Yes, sometimes | 53 | 3 (5.7) | 50 (94.3) | 1.26 (0.314–5.12) | ||
| Yes, always | 99 | 7 (7.1) | 92 (92.9) | 1 | ||
| Cleaning agents for utensils | Use water only | 60 | 4 (6.7) | 56 (93.3) | 1.21 (0.208–7.11) | |
| Watery soap | 129 | 8 (6.2) | 121 (93.8) | 1.31 (0.26–6.59) | ||
| Hypochlorite | 25 | 4 (6.7) | 21 (84) | 1 | ||
| Consuming raw milk | Yes | 8 | 1 (12.5) | 7 (87.5) | 2.12 (0.24–18.56) | |
| No | 206 | 13 (6.3) | 193 (93.7) | 1 | ||
Notes: **Significant at p.value<0.001, *Significant at p.value<0.05.
Abbreviations: N, Number; COR, Crude odds Ratio; AOR, Adjusted Odds Ratio.
Antimicrobial Susceptibility Tests
Based on CLSI 2015, three antimicrobial agents were tested using the disc diffusion method. Among the Campylobacter isolated strains, a higher resistance was observed for ciprofloxacin 5(35.7%). Lower resistance was shown to tetracycline 3(21.4%) and no resistance for erythromycin (Table 4).
Table 4.
Antimicrobial Susceptibility Pattern of Campylobacter Species
| Drug | Interpretive Category | Campylobacter Species | ||
|---|---|---|---|---|
| C. jejuni /coli | Other Species | Total N (%) | ||
| Erythromycin | S | 12 (85.7%) | 2 (14.3%) | 14 (100%) |
| R | 0 (0%) | 0 (0%) | 0 (0%) | |
| Ciprofloxacin | S | 7 (77.8%) | 2 (22.2%) | 9 (64.3%) |
| R | 5 (32.2%) | 0 (0%) | 5 (35.7%) | |
| Tetracycline | S | 9 (81.8%) | 2 (18.2%) | 11 (78.6%) |
| R | 3 (100.0%) | 0 (0%) | 3 (21.4%) | |
Note: The Interpretive category and breakpoint are performed according to CLSI-2015 (M45, 3rd edn).
Abbreviations: S, Sensitive; R, Resistance.
Discussion
In this study, we found that 6.5% of under-five children with diarrhea were confirmed to have an infection with Campylobacter species. Participants having history of contact with domestic animals and bottle feeding were more likely to be infected with Campylobacteriosis.
The present prevalence of Campylobacter species infection rate in under-five diarrheal children was 6.5% with 95% CI (3.3–10.3); comparable to the prevalence in developing countries, which ranges from “5–20%.”11 Compared with other previous studies, the prevalence of the present study is lower than other studies conducted in Ethiopia like Dembia (12.7%)11 Hawassa (12.7%)32 Gondar (15.4%)31 and Jimma (16.7%).18 This prevalence was also lower than in previous studies done in African and other countries Egypt (42%),19 Liberia 44.9%,34 Uganda (9.3%),35 Tanzania (9.7%),36 Cameroon (9.6%),27 Bangladesh (23.71%),37 Thailand (13%),23 Brazil (9.9%),38 Poland (9.6%),10 while our study showed similar prevalence with studies reported in Brazil (6.8%)38 and Iran (8%).21 However, a lower prevalence was reported in Sudan (2%),26 Zambia (93.5%),39 Nigeria (90.5%),40 and Burkina Faso (92.3%).18 These variations could be owing to demographic, geographic location, study period, study design, isolation method, and sample size difference between these studies.31
Bottle feeding and animal contact (cat, dog, and hen) showed a statistically significant association with the recovery rate of Campylobacter species. The risk of acquiring Campylobacter through bottle feeding and animal contact was five and four-fold higher than those who did not, respectively. The increment of risk in bottle feeding may be due to the use of unpasteurized milk, low-level sterilization of the bottles, and storage of cooked food for later use and the increasing risk in animal contact may be due to direct contact with domestic animals, which are the main modes of transmission to humans.41
According to WHO 2017 report, Campylobacter species are recorded as one of the six great priority antibiotic-resistant pathogens. Effective antibiotic therapy is critical for people with severe or prolonged Campylobacter infection, for the elderly, young, and immune-compromised. According to the CDC, the recommended antimicrobial treatment for Campylobacter species is Macrolides (erythromycin), Fluor quinolones (ciprofloxacin), and tetracycline.42,43 In this study, all of the isolated Campylobacter species (100%) were sensitive to erythromycin, which is similar to studies done in Uganda35 and Poland, however, differing from the studies elsewhere with various resistance patterns. Briefly, in Iran (2.9%),21 Hawassa (55%),32 Jimma, (18.4%),30 Gondar (27.7%),31 and Bahirdar (17.6%),44 respectively. In the current study, the resistance of the Campylobacter species against ciprofloxacin was 35.7%, which is lower resistance than studies conducted in Iran, 61.7%45 in Poland (65.2%),46 Gondar (84%),31 but higher resistance than Uganda (5%),47 Hawassa (18.8%),18 Jimma (15.8%),48 and Bahirdar (12.5%).49 Moreover, the resistance to tetracycline was 21.4%, which is relatively comparable with a study conducted in Iran (20.5%)45 and Bahirdar (22.2%),49 but lower resistance than a study reported from Poland (39.1%),46 Jimma (39.5%),48 Gondar (56.8%),31 and Hawassa (31.1%).18 However, higher resistance than study conducted in Uganda (5%).47
The variation might be due to uncontrolled use of antibiotics such as self-medication and access without prescription, interpretive criteria of antimicrobial susceptibility tests for Campylobacter, ineffective prescribed antimicrobials, feeding of transfer contaminated food, water, and milk are the main causes for the development of antimicrobial resistance.35,50–52
Limitation of the Study
The primary limitations of our study were its cross-sectional study design and small calculated sample size. We have missed incorporating breastfeeding and breastfeeding duration-related characteristics; this is also another limitation from our study. The additional limitation of this study was the inability to differentiate C. jejuni from C. coli due to the unavailability of the Hippurate hydrolysis test and advanced molecular gene sequencers.
Conclusion
The prevalence of Campylobacter species among the under-five children with diarrhea at the selected health facilities is slightly high. These Campylobacter isolates showed higher resistance. Campylobacter species were significantly associated with the use of bottle feeding and being with a history of animal contact. To reduce/eliminate the incidence of Campylobacter species; uninterrupted assessment and monitoring of the prevalence and antimicrobial susceptibility of Campylobacter species in health-care facilities and community is needed. Besides, proper sterilization of bottle feeding or drinking properly pasteurized milk is recommended to prevent Campylobacter infections. In addition to culture, to prescribe recommended drugs according to the accurate results a molecular study is mandatory to identify genes responsible for drug resistance and virulence process mainly aimed at reduction of drug-resistant strain transmission through selective treatment by emphasizing sensitive drugs like that of erythromycin in the treatment guidelines. It is also high time to setting different intervention modalities such as health education and health promotion intervention for mothers/caretakers who are poor and less educated.
Acknowledgments
The authors would like to thank Tigray Health Research Institute and Mekelle University for their financial support. Moreover, the authors would like to acknowledge all staff of Dr. Tewelde Health Science College, Ayder Specialized Comprehensive Hospital, and Mekelle General Hospital for their genuine cooperation.
Funding Statement
This study was funded by Mekelle University. Dr. Tewelde College of Health Sciences also provided the reagents required to perform the respective laboratory investigations. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
AGE, Acute Gastroenteritis; ATCC, American Type Culture Collection; CDC, Centers for Disease Control and Prevention; EU/EEA, European Union/European Economic Area; CLSI, Clinical and Laboratory Standards Institute; GBS, Guillain Barre Syndrome; IBD, Inflammatory Bowel Disease; MCCDA, Modified Charcoal Cefoperazone Deoxycholate Agar; MHA, Muller Hinton Agar; NCCLS, National Committee for Clinical Laboratory Standards; ReA; SBA, Reactive Arthritis; Sheep Blood Agar; WHO, World Health Organization.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available because of the sensitive nature of the data but are accessible from the corresponding author at a reasonable request.
Ethical Approval and Consent Given to the Patients
The Ethical Review Committee of the College of Health Sciences of Mekelle University approved the study. All patients were included after providing written informed consent and/or assent as appropriate. For illiterate patients, data collectors read the informed consent for each respondent and confirmed the willingness of the patients by signing the informed consent sheet. Moreover, the confidentiality of the patient’s information was safeguarded.
Disclosure
The authors declare that there is no competing interest in this work.
References
- 1.Debalkie G, Id D, Id YY, Aleminew W, Id YA. Diarrhea and associated factors among under five children in sub-Saharan Africa: evidence from demographic and health surveys of 34 sub-Saharan countries. PLoS One. 2021;24(9):1–13. doi: 10.1371/journal.pone.0257522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merid MW, Alem AZ, Chilot D, Belay DG. Impact of access to improved water and sanitation on diarrhea reduction among rural under ‑ five children in low and middle ‑ income countries: a propensity score matched analysis. Trop Med Health. 2023;51(1):1–10. doi: 10.1186/s41182-023-00525-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girmay AM, Gari SR, Alemu BM, Martin R, Gebreyohannes Gebremariam A. Diarrheal disease and associated behavioural factors among food handlers in Addis Ababa, Ethiopia. AIMs Public Heal. 2020;7(1):100–113. doi: 10.3934/publichealth.2020010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolde D, Tilahun GA, Kotiso KS, Medhin G, Eguale T. The burden of diarrheal diseases and its associated factors among under-five children in Welkite Town: a community based cross-sectional study. Int J Public Health. 2022;67:1–9. doi: 10.3389/ijph.2022.1604960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon ET, Gari SR, Alemu BM, Solomon ET. Prevalence and risk factors of diarrhea among children less than five years of age in the rural suburbs of Dire Dawa, Eastern Ethiopia; Robust Poisson Regression Analysis. Afr Health Sci. 2022;22(4):653–663. doi: 10.4314/ahs.v22i4.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gessesse DN, Tarekegn AA. Prevalence and associated factors of diarrhea among under-five children in the Jawi district, Awi Zone Ethiopia, 2019. Community based comparative cross-sectional study. Front Pediatr. 2022;10:1–9. doi: 10.3389/fped.2022.890304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazaheri M, Rezaei MH, Aalinezhad M, Sharif MR, Akhavan T. Clinical and Laboratory Characteristics of Pediatric Campylobacter spp. Acute Gastroenteritis. Arch Pediatr Infect Dis. 2016;4(4):1–6. doi: 10.5812/pedinfect.35730.Research [DOI] [Google Scholar]
- 8.Cai H, Shao Y, Yu W. Prevalence and associated factors of acute gastroenteritis in children and adolescents aged from 6 to 17 years old: a cross- - sectional study based on the National Health and Nutrition Examination Survey database 1999 – 2018. BMJ Open. 2023;13(2):1–8. doi: 10.1136/bmjopen-2022-068319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pakhtunkhwa K, Khan MA. Epidemiological studies on gastroenteritis in children in the Bannu district, Khyber Pakhtunkhwa, Pakistan. J Public Heal from Theory to Pract. 2023;31:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, Lee SA, Xue J, Riordan SM, Zhang L. Global epidemiology of campylobacteriosis and the impact of COVID-19. Front Cell Infect Microbiol. 2022;12:1–37. doi: 10.3389/fcimb.2022.979055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epps SVR, Harvey RB, Hume ME, Phillips TD, Anderson RC, Nisbet DJ. Foodborne Campylobacter: infections, Metabolism, Pathogenesis and Reservoirs. Int J Environ Res Public Health. 2013;10(12):6292–6304. doi: 10.3390/ijerph10126292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samie A, Moropeng RC, Tanih NF, et al. Epidemiology of Campylobacter infections among children of 0 – 24 months of age in South Africa. Arch Public Health. 2022;80(1):107. doi: 10.1186/s13690-022-00850-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nigusu Y, Abdissa A, Tesfaw G, Kim H-Y. Campylobacter gastroenteritis among under-five children in Southwest Ethiopia. Gut Pathog. 2022;14(1):1–11. doi: 10.1186/s13099-022-00517-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hankel J, Gibson T, Skov J, et al. Monitoring of Campylobacter jejuni in a chicken infection model by measuring specific volatile organic compounds and by qPCR. Sci Rep. 2022;12(1):1–11. doi: 10.1038/s41598-022-15863-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serwecińska L. Antimicrobials and Antibiotic-Resistant Bacteria. Water. 2020;12(12):3313. doi: 10.3390/w12123313 [DOI] [Google Scholar]
- 16.Gao F, Tu L, Chen M, et al. Erythromycin resistance of clinical Campylobacter jejuni and Campylobacter coli in Shanghai, China. Front Microbiol. 2023;14(May). doi: 10.3389/fmicb.2023.1145581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lόpez-Vélez R, Lebens M, Bundy L, Barriga J, Steffen R. Bacterial travellers’ diarrhoea: a narrative review of literature published over the past 10 years. Travel Med Infect Dis. 2022;47:102293. doi: 10.1016/j.tmaid.2022.102293 [DOI] [PubMed] [Google Scholar]
- 18.Behailu Y, Hussen S, Alemayehu T, Mengistu M, Fenta DA, Algammal AM. Susceptibility patterns of Campylobacter infection among under-five children with diarrhea at Governmental Hospitals in Hawassa city, Sidama, Ethiopia. A cross-. PLoS One. 2022;17(5):1–19. doi: 10.1371/journal.pone.0266976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sainato R, Elgendy A, Kuroiwa J, Guerry P, Riddle MS, Porter CK. Epidemiology of campylobacter infections among children in Egypt. Am J Trop Med Hyg. 2018;98(2):581–585. doi: 10.4269/ajtmh.17-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mebrahtom S, Worku A, Gage DJ, Jaafar MH, Abdullah H. The risk of water, sanitation and hygiene on diarrhea-related infant mortality in eastern Ethiopia: a population-based nested case- control. BMC Public Health. 2022;22(1):1–14. doi: 10.1186/s12889-022-12735-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansarifar E, Riahi SM, Tasara T, Sadighara P, Zeinali T. Campylobacter prevalence from food, animals, human and environmental samples in Iran: a systematic review and meta ‑ analysis. BMC Microbiol. 2023;23(1):1–20. doi: 10.1186/s12866-023-02879-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mbindyo SN, Kitaa JMA, Abuom TO, et al. Molecular prevalence and risk factors of campylobacter infection in puppies in the Nairobi Metropolitan Region, Kenya. Vet Med Int. 2023;2023:1–7. doi: 10.1155/2023/8813405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charoenwat B, Suwannaying K, Paibool W, Laoaroon N, Sutra S, Thepsuthammarat K. Burden and pattern of acute diarrhea in Thai children under 5 years of age: a 5 ‑ year descriptive analysis based on Thailand National Health Coverage (NHC) data. BMC Public Health. 2022;22(1):1–10. doi: 10.1186/s12889-022-13598-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terefe Y, Deblais L, Ghanem M, Helmy YA. Co-occurrence of Campylobacter species in children from Eastern Ethiopia, and Their Association With Environmental Enteric Dysfunction, Diarrhea, and Host Microbiome. Front Public Health. 2020;8:1–16. doi: 10.3389/fpubh.2020.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiros M, Gebru SB, Tewelde B. Knowledge, attitude, practice and associated factors towards COVID-19 and its prevention measures among residents of Mekelle City, Tigray Region, Northern Ethiopia: a community-based cross sectional study. J Public Health. 2023;12:1–16. doi: 10.1007/s10389-023-01826-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeed A, Abd H, Sandstrom G. Microbial aetiology of acute diarrhoea in children under five years of age in Khartoum, Sudan. J Microbiol. 2015;64:432–437. doi: 10.1099/jmm.0.000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tambe AB, Nzefa LD, Nicoline NA. Childhood diarrhea determinants in Sub-Saharan Africa: a cross sectional study of Tiko-Cameroon. Challenges. 2015;6(2):229–243. doi: 10.3390/challe6020229 [DOI] [Google Scholar]
- 28.Schreyer ME, Olivero CR, Rossler E, et al. Current research in food science identified in a slaughterhouse in Argentina. Curr Res Food Sci. 2022;5:590–597. doi: 10.1016/j.crfs.2022.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahir M, Imam E, Hussain T. Evaluation of land use/land cover changes in Mekelle City, Ethiopia using Remote Sensing and GIS. Comput Ecol Softw. 2013;3(1):9–16. [Google Scholar]
- 30.Debelo M, Mohammed N, Tiruneh A, Tolosa T, Cartelle Gestal M. Isolation, identification and antibiotic resistance profile of thermophilic Campylobacter species from Bovine, Knives and personnel at Jimma Town Abattoir, Ethiopia. PLoS One. 2022;17(10):1–14. doi: 10.1371/journal.pone.0276625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lengerh A, Moges F, Unakal C, Anagaw B. Prevalence, associated risk factors and antimicrobial susceptibility pattern of Campylobacter species among under five diarrheic children at Gondar University Hospital, Northwest Ethiopia. BMC Pediatr. 2013;13(1):1–9. doi: 10.1186/1471-2431-13-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Getamesay M, Getenet B, Ahmed Z. Prevalence of Shigella, Salmonella and Campylobacter Species and their susceptibility patterns among under five children with diarrhea in Hawassa Town, South Ethiopia. Ethiop J Health Sci. 2014;2(7):101–108. doi: 10.4314/ejhs.v24i2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gajic I, Kabic J, Kekic D, et al. Antimicrobial susceptibility testing: a comprehensive review of currently used methods. Antibiotics. 2022;11(4):1–26. doi: 10.3390/antibiotics11040427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paintsil EK, Ofori LA, Adobea S, et al. Prevalence and antibiotic resistance in Campylobacter spp. isolated from humans and food-producing animals in West Africa: a systematic review and meta-analysis. Pathogens. 2022;11(140):1–17. doi: 10.3390/pathogens11020140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nigusu Y, Abdissa A, Tesfaw G. Campylobacter gastroenteritis among under-five children in Southwest Ethiopia. Infect Drug Resist. 2022;15:2969–2979. doi: 10.2147/IDR.S354843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hugho EA, Kumburu HH, Amani NB, et al. Enteric pathogens detected in children under five years old admitted with Diarrhea in Moshi, Kilimanjaro, Tanzania. Pathogens. 2023;12(618):1–11. doi: 10.3390/pathogens12040618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pervin MK, Jhora ST, Paul S, Naher A, Sarkar D. Causative agents for diarrhoea in under 5 children in a tertiary care hospital. Bangladesh Med J Khulna. 2018;51(1–2):25–28. doi: 10.3329/bmjk.v51i1-2.40470 [DOI] [Google Scholar]
- 38.Aldo AML, Oliveira DB, Quetz JS, et al. Etiology and severity of diarrheal diseases in infants at the semiarid region of Brazil: a case- control study. PLoS Negl Trop Dis. 2019;13(2):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiyangi H, Muma JB, Malama S, et al. Identification and antimicrobial resistance patterns of bacterial enteropathogens from children aged 0 – 59 months at the University Teaching Hospital, Lusaka, Zambia: a prospective cross sectional study. BMC Infect Dis. 2017;17(1):1–9. doi: 10.1186/s12879-017-2232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiwok JC, Adebowale AS, Wilson I, Kancherla V, Umeokonkwo CD. Patterns of diarrhoeal disease among under-five children in Plateau State. BMC Public Health. 2021;21(1):1–9. doi: 10.1186/s12889-021-12110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansson I, Habib I, Lowman R, Engvall EO, Engvall EO. Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound Emerg Dis. 2018;65(1):30–48. doi: 10.1111/tbed.12870 [DOI] [PubMed] [Google Scholar]
- 42.Sithole V, Amoako DG, Luther A, et al. Characterization of Campylobacter spp. in Intensive Pig Production in South Africa. Pathogens. 2021;10(4):1–16. doi: 10.3390/pathogens10040439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehouse CA, Zhao S, Tate H. Antimicrobial resistance in campylobacter species: mechanisms and genomic epidemiology. In: Advances in Applied Microbiology. 1st ed. Vol. 100. Elsevier Inc.; 2018:1–47. doi: 10.1016/bs.aambs.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 44.Dar B. Prevalence and antimicrobial resistance of campylobacter isolates from humans. Foodborne Pathog Dis. 2010;7(6):7–10. [DOI] [PubMed] [Google Scholar]
- 45.Sharifi S, Bakhshi B, Najar-peerayeh S. Significant contribution of the CmeABC Efflux pump in high-level resistance to ciprofloxacin and tetracycline in Campylobacter jejuni and Campylobacter coli clinical isolates. Ann Clin Microbiol Antimicrob. 2021;20(1):1–9. doi: 10.1186/s12941-021-00439-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szczepanska B, Andrzejewska M, Spica D, Klawe JJ. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from children and environmental sources in urban and suburban areas. BMC Microbiol. 2017;17(1):1–9. doi: 10.1186/s12866-017-0991-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Se M, Joloba M, Kakooza A, Mulindwa K. Campylobacter spp among Children with acute diarrhea attending Mulago hospital in Kampala-Uganda. Afr Health Sci. 2009;9(3):201–205. [PMC free article] [PubMed] [Google Scholar]
- 48.Tafa B, Sewunet T, Tassew H, Asrat D. Isolation and Antimicrobial Susceptibility Patterns of Campylobacter Species among Diarrheic Children at Jimma, Ethiopia. Int J Bacteriol. 2014;2014:1–7. doi: 10.1155/2014/560617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ewnetu D, Mihret A. Prevalence and Antimicrobial Resistance of Campylobacter Isolates from Humans. Foodborne Pathog Dis. 2010;7(6):7–10. doi: 10.1089/fpd.2009.0433 [DOI] [PubMed] [Google Scholar]
- 50.Zachariah OH, Lizzy MA, Rose K, Angela MM. Multiple drug resistance of Campylobacter jejuni and Shigella isolated from diarrhoeic children at Kapsabet County referral hospital, Kenya. BMC Infect Dis. 2021;21(1):4–11. doi: 10.1186/s12879-021-05788-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mabilika RJ, Mpolya E, Shirima G. Prevalence and predictors of self-medication with antibiotics in selected urban and rural districts of the Dodoma region, Central Tanzania: a cross-sectional study. Antimicrob Resist Infect Control. 2022;11(1):1–9. doi: 10.1186/s13756-022-01124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simegn W, Moges G. Antibiotics self-medication practice and associated factors among residents in Dessie City, Northeast Ethiopia: community-based cross-sectional study. Patient Prefer Adherence. 2022;16:2159–2170. doi: 10.2147/PPA.S370925 [DOI] [PMC free article] [PubMed] [Google Scholar]



