Abstract
Background:
Out-of-hospital ventricular fibrillation (VF) cardiac arrest is a leading cause of death. Quantitative analysis of the VF electrocardiogram (ECG) can predict patient outcomes and could potentially enable a patient-specific, guided approach to resuscitation. However, VF analysis during resuscitation is confounded by cardiopulmonary resuscitation (CPR) artifact in the ECG, challenging continuous application to guide therapy throughout resuscitation. We therefore sought to design a method to predict VF shock outcomes during CPR.
Methods:
Study data included 4577 5-second VF segments collected during and without CPR prior to defibrillation attempts in N=1151 arrest patients. Using training data (460 patients), an algorithm was designed to predict the VF shock outcomes of defibrillation success (return of organized ventricular rhythm) and functional survival (Cerebral Performance Category 1–2). The algorithm was designed with variable-frequency notch filters to reduce CPR artifact in the ECG based on real-time chest compression rate. Ten ECG features and three dichotomous patient characteristics were then developed to predict outcomes. These variables were combined using support vector machines and logistic regression. Algorithm performance was evaluated by area under the receiver operating characteristic curve (AUC) to predict outcomes in validation data (691 patients).
Results:
AUC (95% Confidence Interval) for predicting defibrillation success was 0.74 (0.71–0.77) during CPR and 0.77 (0.74–0.79) without CPR. AUC for predicting functional survival was 0.75 (0.72–0.78) during CPR and 0.76 (0.74–0.79) without CPR.
Conclusion:
A novel algorithm predicted defibrillation success and functional survival during ongoing CPR following VF arrest, providing a potential proof-of-concept towards real-time guidance of resuscitation therapy.
Keywords: Ventricular fibrillation, cardiac arrest, resuscitation, electrocardiogram, defibrillation, cardiopulmonary resuscitation, algorithm, machine learning
Introduction
Out-of-hospital cardiac arrest (OHCA) results in over 300,000 deaths each year in the United States.[1] OHCA is commonly caused by ventricular fibrillation (VF), an electrically-disorganized ventricular arrhythmia which causes ineffective mechanical contraction and circulatory collapse.[1,2] Current treatment protocol for VF OHCA includes high-quality cardiopulmonary resuscitation (CPR) briefly interrupted every two minutes for rhythm analysis and defibrillation shock but otherwise performed continuously until return of spontaneous circulation.[3,4] Overall survival from witnessed VF OHCA is approximately 30%, though there is a nearly ten-fold variability in survival across regional emergency medical services systems.[5,6] This range in outcomes suggest that VF OHCA is a potentially modifiable condition for which modifications to current protocols may be beneficial and merit exploration.
Observational studies have indicated that VF OHCA is a heterogeneous and dynamic entity, suggesting that the current uniform treatment approach may not optimally target case-specific physiology.[2,7–9] VF resuscitation may instead benefit from a targeted strategy that delivers selected medication, defibrillation, and CPR with dosage, sequence, and timing tailored to the individual patient.[10–13] A challenge to such a strategy is accurate real-time assessment of patient status to guide their immediate treatment. However, there is now increasing evidence that real-time myocardial physiology during VF can be assessed using the electrocardiogram (ECG).[14,15] Specifically, VF ECG characteristics have been shown to predict the likelihood that a defibrillation shock will produce the near-term outcome of an organized rhythm and even the long-term clinical outcome of survival.[16–19] The application of such information to inform a patient-specific profile of medication, shock, and CPR care could dramatically alter our current ‘one-size-fits-all’ approach to resuscitation and its consequent outcome.
During active resuscitation, chest compression artifact during CPR obscures the ECG signal and can confound ECG analysis.[20–22] CPR would therefore need to be interrupted to allow accurate real-time estimation of physiologic status using VF ECG characteristics, but even brief CPR interruption undermines forward blood flow and reduces the likelihood of successful resuscitation.[23,24] Ideally, the ECG would be assessed continuously during CPR and used to inform time-sensitive treatment choices throughout resuscitation.[25,26] Such a strategy could potentially enable real-time titration of CPR and medication based on a patient’s status, advancing an approach for VF treatment whereby CPR is interrupted only once a shock is likely to produce organized rhythm and survival.
Therefore, in the current investigation we sought to develop an algorithm to predict the short- and long-term outcomes of VF shock without requiring CPR interruption. The proposed algorithm could potentially provide the basis to inform patient-specific VF treatment decisions during resuscitation while supporting the best practice of high-quality continuous CPR.
Methods
Data
Study Design, Population, and Setting
The investigation was a cohort study of out-of-hospital cardiac arrests presenting to emergency medical services (EMS) with an initial rhythm of VF from 2005–2015 in King County, WA (excluding Seattle) that had complete defibrillator recordings. The cohort included patients treated with Lifepak 12, Lifepak 15 (Physio-Control, Redmond, WA), HeartStart FR3, and HeartStart MRx (Philips Healthcare, Bothell, WA) biphasic defibrillators. Patients were excluded if they were treated by a public access or police defibrillator prior to EMS arrival or were <18 years in age.
In accordance with applicable regulations, the Research Review Committee of King County Public Health and the Institutional Review Board at the University of Washington Human Subjects Division approved the study and waiver of informed consent.
Data Resource
The study system maintains a clinical registry that uses data resources including the dispatch recording, EMS written reports, hospital records, death certificates, and the defibrillator recording. These data sources are systematically abstracted to determine OHCA circumstances, patient demographics, prehospital care, and clinical outcomes. The registry is organized according to the Utstein template.[27]
Study Outcomes
We evaluated both short- and long-term patient outcomes. Short-term outcome was defibrillation success, defined as the return of an organized rhythm with distinct QRS complexes at a rate of >12 per minute after a defibrillation attempt (Figure 1). Successful long-term outcome was survival with functional neurologic status, defined as survival to hospital discharge with a Cerebral Performance Category of 1 or 2.[28]
Figure 1. Examples of VF Segment Collection with and without CPR.
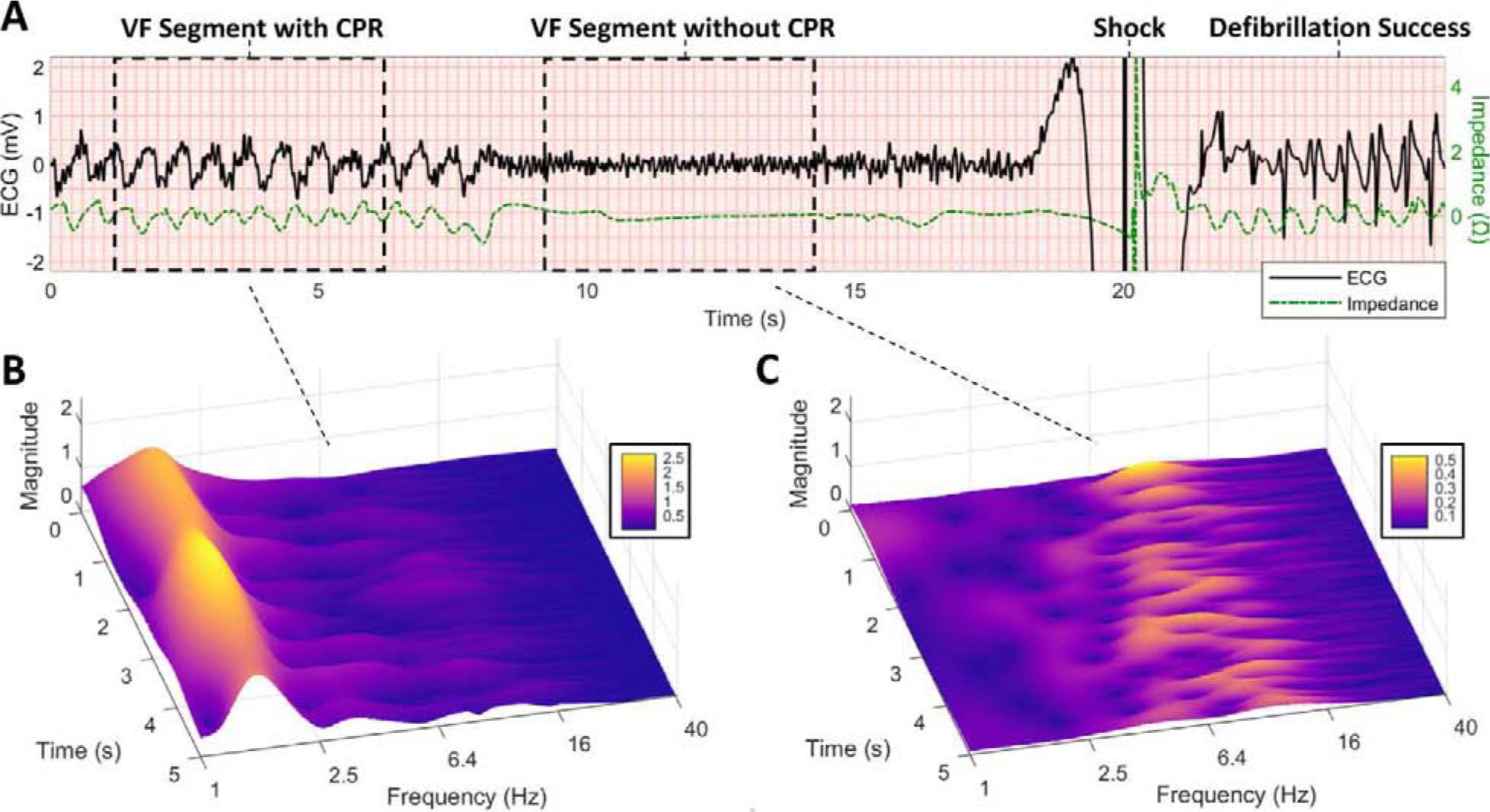
(A) Example of adjacent 5-s VF ECG segments collected during CPR and without CPR prior to defibrillation shock. CPR is confirmed by chest compression oscillations in the concurrent impedance channel. Defibrillation success is confirmed by termination of VF and return of organized ventricular rhythm (QRS complexes) following shock. (B) Scalogram of VF during CPR illustrates dominant CPR fundamental at approximately 1.6 Hz with transient compression artifacts extending to higher frequencies. (C) Scalogram of VF without CPR illustrates a relatively high dominant VF frequency (ranging from approximately 6–12 Hz in this example), which may suggest an energetic myocardial metabolic substrate amenable to defibrillation. (CPR = cardiopulmonary resuscitation, ECG = electrocardiogram, VF = ventricular fibrillation.)
ECG Data
We sought to combine clinical and ECG characteristics to achieve prediction of patient outcomes. When available in continuous defibrillator recordings, we collected one 5-s VF ECG segment during chest compressions followed by an adjacent 5-s segment without compressions prior to a shock (Figure 1). ECG segments were collected concurrently with transthoracic impedance (TTI) to confirm CPR status.[29] ECG data were collected at sampling rates ranging from 125 – 250 Hz and TTI data were collected at rates ranging from 61 – 200 Hz. All signals sampled at rates lower than 250 Hz were resampled to 250 Hz (Supplementary Material A). Data and signal processing were performed with MATLAB 2018 (MathWorks, Natick, MA) and RStudio 1.1 (RStudio, Boston, MA).
Algorithm Description
An overview of the proposed algorithm is shown in Figure 2.
Figure 2. Algorithm Overview.
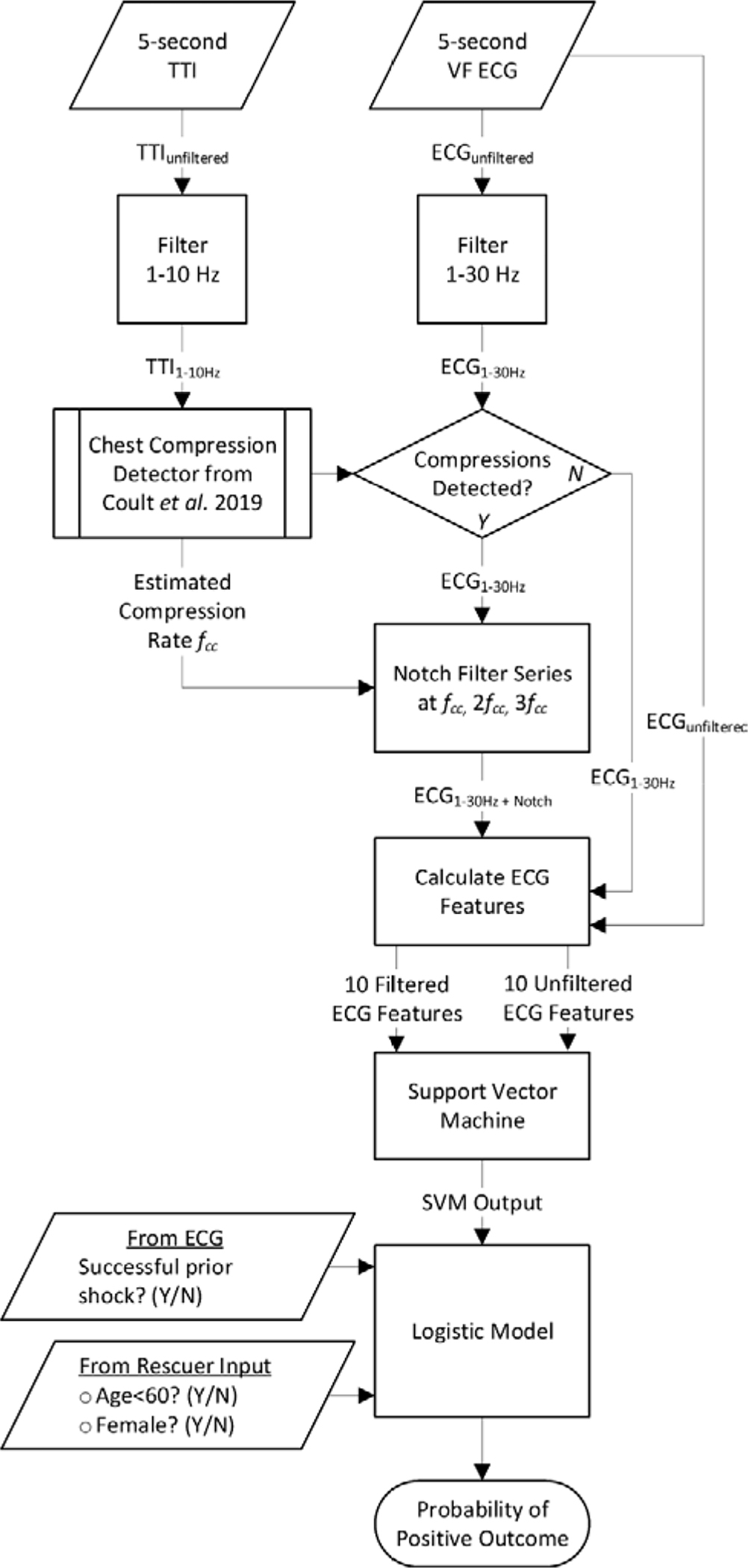
The algorithm accepts a 5-s VF ECG input segment, a concurrent 5-s TTI input segment, and three dichotomous inputs. ECG features are calculated in parallel from filtered and unfiltered ECGs and incorporated with dichotomous variables to predict shock outcome. (CC = chest compressions, CPR = cardiopulmonary resuscitation, ECG = electrocardiogram, fcc = chest compression frequency, SVM = support vector machine, TTI = transthoracic impedance.)
ECG Filtering
During CPR, chest compression artifact in the ECG is generally concentrated near a fundamental frequency of approximately 2 Hz but can produce transient and harmonic artifacts up to 20 Hz.[20,30] Since VF commonly has a dominant frequency between 3–8 Hz which may overlap compression harmonic frequencies, ECG filtering to reduce chest compression artifact may also attenuate essential VF frequency content.[31] Therefore, in the current algorithm, filtered and unfiltered ECG signals were analyzed in parallel to take potential advantage of artifact reduction in filtered data as well as increased VF content in unfiltered data.
To apply filtering, ECGs were first bandpass-filtered using a 1–30 Hz 4th-order Butterworth filter to reduce drift, ventilation artifact, and high-frequency transient and power noise. If chest compressions were automatically detected in the corresponding TTI signal using a previously-validated compression detection method [29], the bandpass-filtered ECG was also processed with a variable-frequency notch filter to reduce CPR artifact. We designed the notch filter with a series of three 2nd-order Butterworth band-stop filters to reduce the fundamental and first two harmonic compression frequencies based on the real-time estimated chest compression rate (Figure 3, Supplementary Material A). All filters were applied with a forwards-backwards implementation for linear phase.
Figure 3. Filter Example.
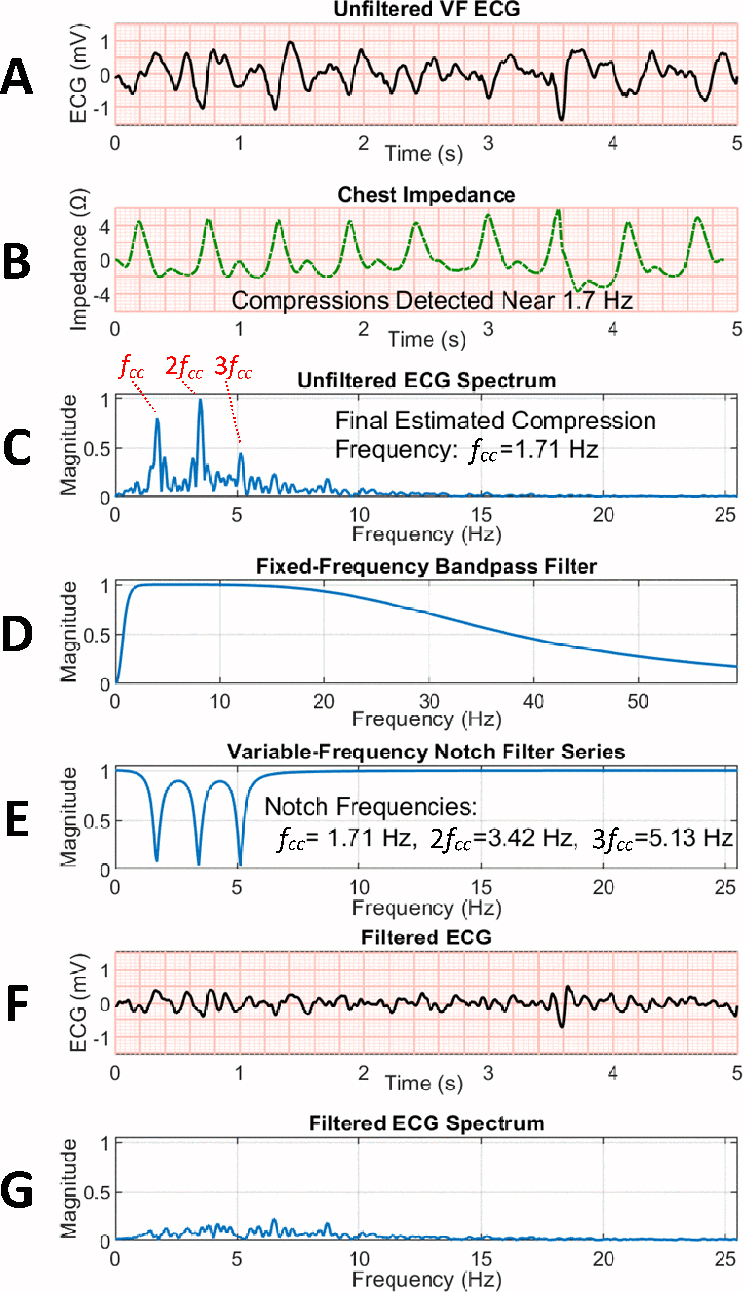
(A) Unfiltered VF ECG segment during chest compressions. (B) Compressions are detected in the concurrent transthoracic chest impedance signal. (C) Normalized spectrum of ECG during compressions, with compression fundamental and two harmonics visible. (D) 1–30 Hz bandpass filter. (E) Notch filter series based on estimated compression fundamental frequency fcc. (F) Filtered ECG following bandpass and notch filtering. (G) Spectrum of filtered ECG confirms removal of the majority of compression fundamental and first two harmonics. (ECG = electrocardiogram, VF = ventricular fibrillation.)
ECG Features
Prior studies have proposed quantitative measures of the VF ECG to assess myocardial metabolism and predict patient outcomes.[14,32] These measures quantify features such as VF amplitude and frequency.[32–38] However, such features are designed to analyze ECGs without CPR, and are thus confounded by chest compression artifact.[25,39] Prior investigation has also suggested that combinations of multiple features may improve prognostic performance versus using a single feature.[39,40]
We therefore designed ten novel features of the VF ECG to quantify characteristics associated with prognosis while remaining robust against potential chest compression artifact (Figure 4). Two features per domain were included from domains previously demonstrated to predict outcomes: ECG amplitude [33], entropy [41,42], scalogram energy [32,43], dominant frequency [34,35,44], and Fourier spectrum characteristics [45]. Features were each empirically designed with variable parameters to allow optimization for use during chest compressions, and were only retained if their performance on training data was significantly predictive of survival.
Figure 4. Examples of features from filtered ECGs with CPR versus without CPR.
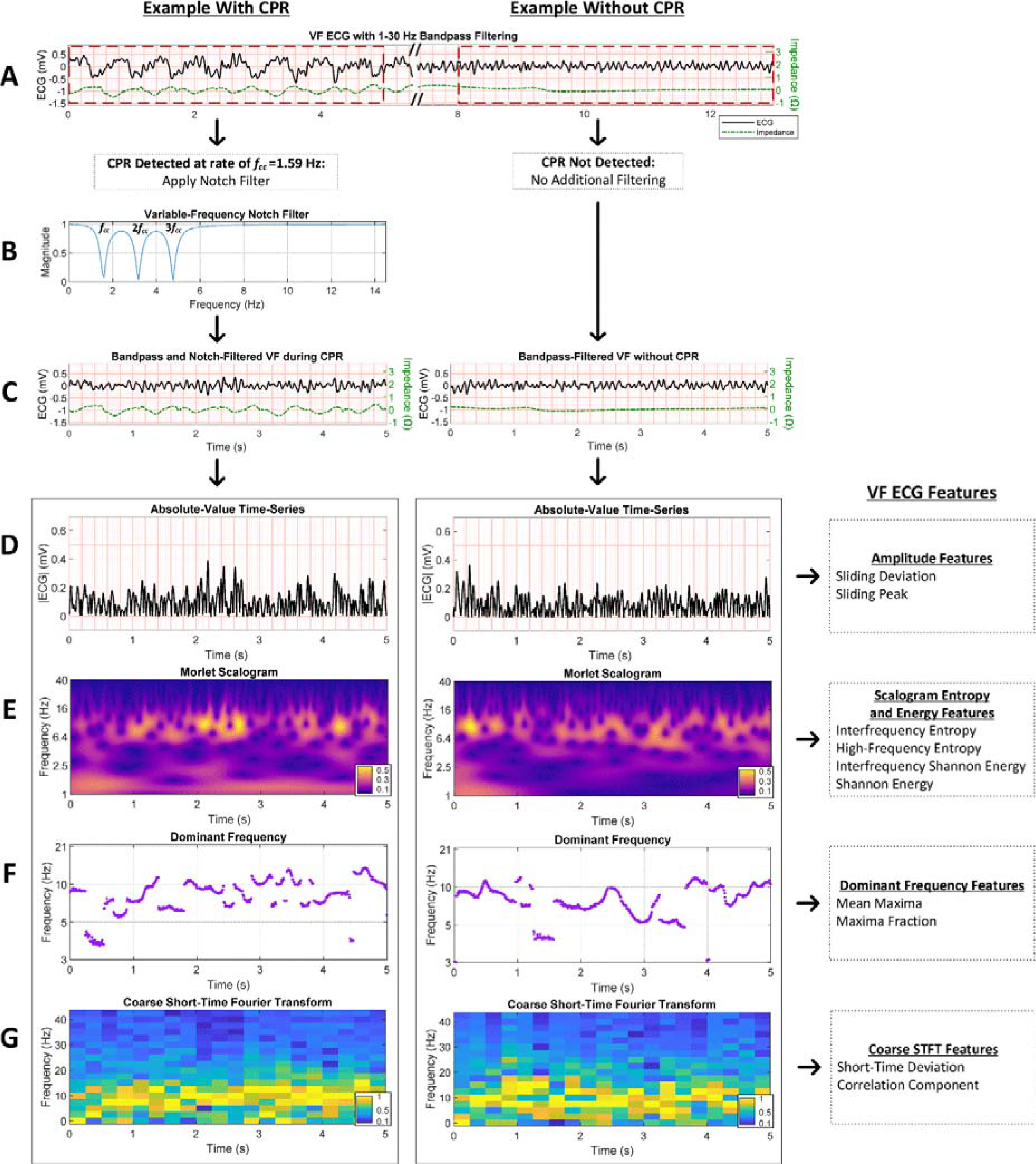
(A) Adjacent VF ECG segments after filtering with 1–30 Hz bandpass filter but prior to CPR detection and potential notch filtering. (B) When chest compressions are detected in the concurrent transthoracic impedance signal, a series of notch filters based on estimated compression rate fcc are applied to the ECG to attenuate the compression fundamental and first two harmonic frequencies. (C) VF ECGs with CPR versus without CPR after filtering. (D) Amplitude features are calculated from the absolute time-series of the ECG. (E) Time-frequency features are calculated from the Morlet wavelet-based scalogram of the ECG. (F) Dominant frequency features are calculated from the frequencies of greatest magnitude in the wavelet scalogram. (G) Coarse STFT features are calculated from the STFT of the ECG calculated using non-overlapping box windows. (CPR = cardiopulmonary resuscitation, ECG = electrocardiogram, STFT = short-time Fourier transform, VF = ventricular fibrillation.)
Specifically, to quantify ECG amplitude while remaining robust against low-frequency artifact and intermittent transient noise, Sliding Deviation and Sliding Peak were defined as the median of the absolute standard deviation and peak amplitudes, respectively, within variable-length time windows (Supplementary Material B). To quantify the entropy in the ECG scalogram while mitigating the effects of CPR artifact, Interfrequency Entropy and High-Frequency Entropy (Supplementary Material C) were defined as the median Shannon entropies of specific scalogram frequency bands. To describe the energy in the ECG scalogram while reducing confounding by CPR, the Shannon Energy and Interfrequency Shannon Energy (Supplementary Material C) were defined as the median of Shannon-transformed magnitudes at each frequency band and at each time step in the scalogram, respectively, within variable frequency limits. To represent the dominant ventricular fibrillation frequency, Mean Maxima was defined as the average dominant scalogram frequency within variable frequency limits, while Maxima Fraction was defined as the proportion of time the dominant scalogram frequency exceeds a frequency threshold indicative of good prognosis (Supplementary Material D). Finally, as a measure of spectral variation and self-similarity in the short-time Fourier transform, the Short-Term Deviation was defined as the median of standard deviations at each time step, while Correlation Component was calculated using principal component analysis of correlations between frequency bands (Supplementary Material E).
Support Vector Machine
We used support vector machine classifiers to combine individual ECG features into a single continuous output.[46,47] The classifiers were of the form , where is an −dimensional input of ECG features, are model parameters, is a constant, and support vectors with associated class assignments are generated from training data. The selected gaussian kernel function represents similarity between arbitrary inputs and given kernel size . Support vector machine output values were mapped to continuous probabilities using a sigmoid transformation of the form given trained parameters .[48]
Dichotomous Patient Variables
We considered basic patient characteristics for use in the algorithm that would be available in a time-sensitive acute clinical setting.[49] Prior investigation has suggested a relationship between the interaction of age and sex with arrest outcome.[50,51] Therefore in the current investigation, we included the basic demographic characteristics of age (dichotomized at <60 years and denoted Age) and female sex (denoted Sex) as well as their interaction. Additionally, evidence indicates that short-term response to prior shock is associated with response to subsequent shocks.[9,52,53] Hence we also included whether the prior shock resulted in return of organized rhythm (denoted Prior ROR), when applicable. We assumed that automated inclusion of prior organized rhythm in a prognostic algorithm is feasible given recent advancements in rhythm classification during CPR.[26,54]
Logistic Model
We used logistic regression to generate a probability of good prognosis for each 5-s input segment (Figure 2). The logistic model incorporated the variables described above to predict outcome of VF shock, and was of the form:
where represents the probability of successful outcome, the logistic function represents the current shock cycle number, is the output from the support vector machine, , and are dichotomous patient characteristic variables, and are the logistic model parameters.
Training of Features and Models
We used training data to develop and optimize the proposed algorithm (Figure 5). To optimize the ten novel ECG features for use on both filtered and unfiltered ECGs, we selected parameters (such as frequency limits and sliding window size) to maximize the area under the receiver operating characteristic curve (AUC) on training data for each feature. We then trained a support vector machine to combine all 20 ECG features calculated in parallel from filtered and unfiltered ECGs. Support vector machine kernel size and box constraint hyperparameters were selected by performing a grid search of 5-fold cross-validation error versus hyperparameter values on training data. In order to improve support vector machine generalizability, we intentionally underfit the parameters by allowing increased kernel size and reduced box constraint during grid search while maintaining a cross-validation error within an empirically-defined tolerance of the absolute minimum error (see Supplementary Material F).[39] The support vector machine output and dichotomous patient characteristics were combined to predict patient outcome using a logistic model which was trained using maximum likelihood estimation on the training data. Individual predictors for the logistic model were characterized by univariate odds ratios.
Figure 5: Training and Validation Procedure.
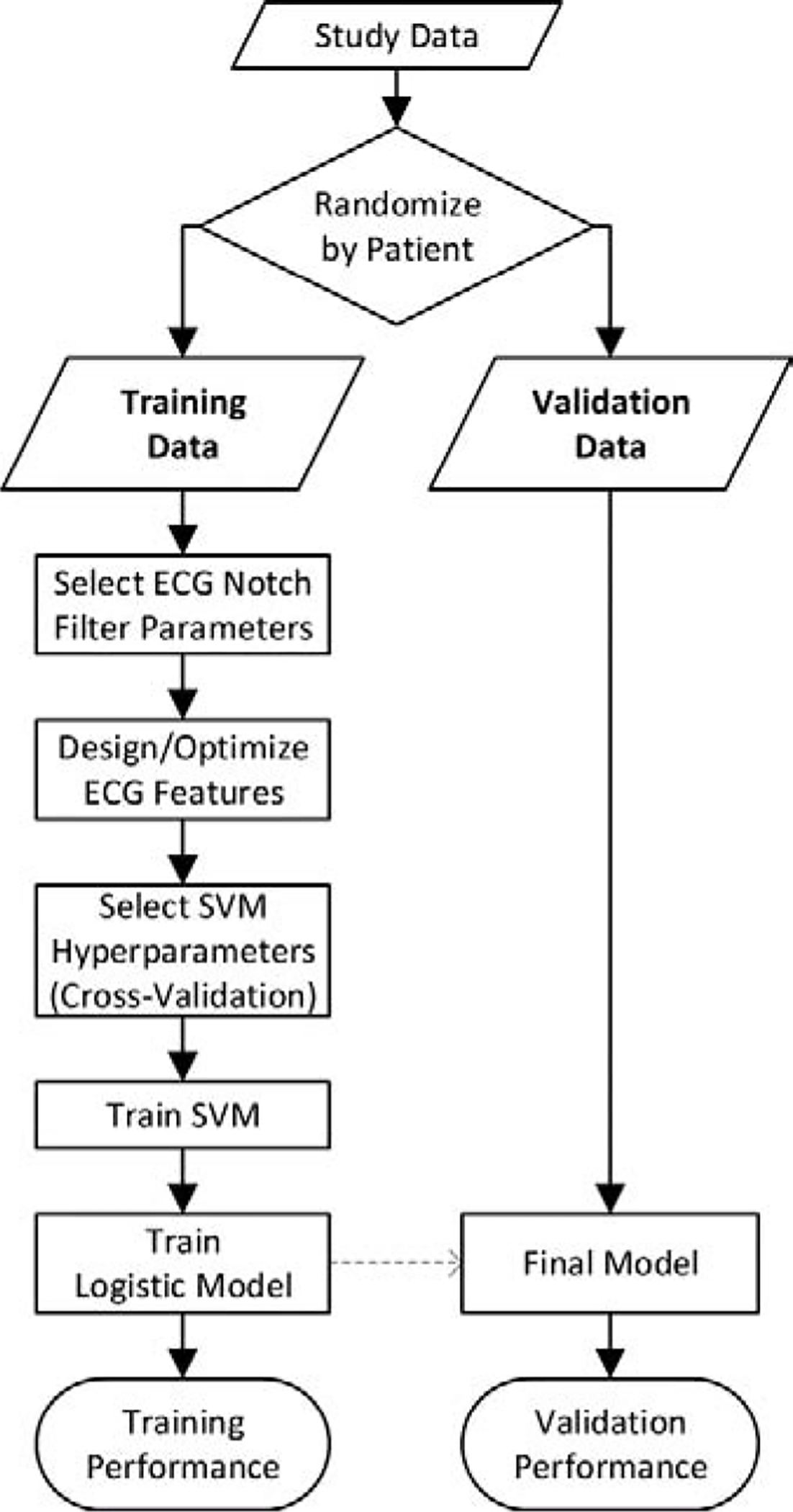
The use of training and validation data for development and evaluation of the proposed method is illustrated. (ECG = electrocardiogram, SVM = support vector machine)
The algorithm was designed to operate during different CPR states (i.e. with and without chest compressions) and to predict multiple patient outcomes (i.e. defibrillation success and functional survival). Therefore, four versions of the algorithm were trained depending on CPR state and predicted outcome, each with a separate ECG feature parameter set, support vector machine model, and logistic model.
Algorithm Performance
We sought to assess the algorithm’s prognostic performance and determine whether prediction of patient outcomes using the algorithm was greater versus leading VF prognostic methods described in prior investigations. For comparison against the current algorithm, we selected the best-performing method from a recent comprehensive benchmark of VF waveform measures, denoted the SVM24 method.[39] SVM24 was calculated as described in prior study as the support vector machine combination of 24 previously-described features of the ECG. For further comparison against the current algorithm, we also selected a method well-validated in literature, the Amplitude Spectrum Area (AMSA).[55] We selected a version of AMSA from recent study calculated as the frequency-weighted sum of discrete Fourier transform magnitudes from 1–26 Hz.[56]
Algorithm performance was characterized using validation data. We compared AUC values for prediction of short-term and long-term patient outcomes, both with and without CPR artifact, for the current algorithm versus SVM24 and AMSA using the DeLong method.[57] An alpha of 0.05 (adjusted for eight comparisons to 0.0063 using the Bonferroni correction) was used to determine the statistical significance of AUC differences. Confidence intervals for AUC values were calculated by stratified bootstrapping.
We sought to further illustrate potential of the proposed algorithm to monitor patient prognostic status and predict likelihood of positive outcomes in the presence of CPR artifact. Using validation data, we grouped the predicted outputs during chest compressions into four equally-spaced probability quartiles. To evaluate the algorithm’s ability to stratify patients into these four different prognostic groups, we compared the predicted rates of positive outcome versus true rates of positive outcome within each probability quartile.
Results
The study cohort was comprised of 1151 patients divided randomly into 40% training (N=460) and 60% validation (N=691) groups (Table 1). There were 868 and 991 training ECG segments collected with and without chest compressions, respectively, and 1264 and 1454 validation ECG segments with and without chest compressions, respectively. There were more ECG segments collected without compressions primarily because in some cases there was not a full 5-s period of CPR between defibrillator electrode placement and initial shock.
Table 1:
Patient characteristics
| Patients, n | 1151 |
| Female, n(%) | 265(23.0) |
| Age, median (IQR) | 61(52, 72) |
| Cardiac etiology, n(%) | 1080(93.8) |
| Location, n(%) | |
| Home | 707(61.4) |
| Public | 399(34.7) |
| Nursing Home | 45(3.9) |
| Arrest before EMS arrival, n(%) | 1093(95.0) |
| Witnessed, n(%) | 885(76.9) |
| Bystander cardiopulmonary resuscitation, n(%) | 833(72.4) |
| EMS Response (minutes), median (IQR) | 5(4, 6) |
| Total shocks, median (IQR) | 3(2, 6) |
| Return of spontaneous circulation at end of EMS care, n(%) | 817(71.0) |
| Admit to hospital, n(%) | 810(70.4) |
| Survive to hospital discharge, n(%) | 524(45.5) |
| Survive with cerebral performance category 1 or 2, n(%) | 471(40.9) |
(EMS = emergency medical services, IQR = interquartile range)
Of the individual predictors used in the logistic model, univariate odds ratios for and were significantly predictive of the short-term outcome of return of rhythm. Likewise, univariate odds ratios for , , , and the interaction of and were significantly predictive of the long-term outcome of functional survival (Table 2).
Table 2:
Odds ratios for predictors in logistic model
| Odds Ratios for Defibrillation Success | Odds Ratios for Functional Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Training | Validation | Training | Validation | |||||
| Predictor | −CPR | +CPR | −CPR | +CPR | −CPR | +CPR | −CPR | +CPR |
| Age (<60) | 1.0 (0.78–1.3) | 0.86 (0.66–1.1) | 1.1 (0.89–1.4) | 1.1 (0.84–1.3) | 1.7 (1.3–2.2)* | 1.8 (1.4–2.2)* | 1.8 (1.5–2.3)* | 1.7 (1.4–2.2)* |
| Sex (Female) | 1.2 (0.85–1.6) | 1.3 (0.89–1.8) | 1.3 (1.0–1.7) | 1.3 (1.0–1.8) | 1.0 (0.76–1.4) | 1.2 (0.87–1.7) | 1.1 (0.88–1.4) | 1.0 (0.80–1.4) |
| Age × Sex | 1.5 (0.9–1.4) | 1.2 (0.75–2.0) | 1.4 (1.0–2.0) | 1.5 (1.0–2.2) | 2.3 (1.5–3.6)* | 2.5 (1.6–4.1)* | 1.9 (1.4–2.7)* | 1.8 (1.2–2.5) |
| Prior ROR | 4.6 (3.2–6.6)* | 4.2 (3.0–5.9)* | 5.8 (4.2–4.9)* | 5.5 (4.1–7.4)* | 2.8 (2.0–4.1)* | 2.3 (1.6–3.2)* | 2.2 (1.7–3.0)* | 2.3 (1.7–3.0)* |
| SVM | 2.4 (2.0–2.7)* | 2.2 (1.8–2.5)* | 2.6 (2.3–3.0)* | 2.1 (1.9–2.4)* | 2.7 (2.3–3.1)* | 2.3 (2.0–2.7)* | 2.7 (2.4–3.1)* | 2.7 (2.3–3.1)* |
Univariate odds ratios (95% confidence interval) of successful outcome are presented for each predictor included in the final logistic regression model. Odds ratios for continuous variables are standardized. Prior ROR only includes segments prior to shocks 2–4. (−CPR = ECG segments without chest compressions, +CPR = ECG segments with chest compressions, Prior ROR = prior return of organized rhythm, SVM = support vector machine output).
univariate odds significantly greater than 1 after Bonferroni correction (p<0.0012)
Without CPR, the algorithm predicted both short-term and long-term outcomes (Table 3, Figure 6). Compared to the prior published methods of AMSA and SVM24 without CPR on validation data, the algorithm achieved significantly greater prediction of the short-term outcome of return of rhythm: AUC was 0.77 for the current algorithm versus 0.74 for AMSA (p<0.001 for difference) and 0.75 for SVM24 (p=0.005 for difference). The algorithm also achieved significantly greater prediction of the long-term outcome of functional survival versus AMSA but not SVM24: AUC was 0.76 for the current algorithm versus 0.74 for AMSA (p<0.001 for difference) and 0.75 for SVM24 (p=0.21 for difference).
Table 3:
AUC values
| AUC for Predicting Defibrillation Success | AUC for Predicting Functional Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Training | Validation | Training | Validation | |||||
| −CPR | +CPR | −CPR | +CPR | −CPR | +CPR | −CPR | +CPR | |
| Study algorithm | 0.76 (0.72–0.79) | 0.74 (0.71–0.77) | 0.77 (0.74–0.79) | 0.74 (0.71–0.77) | 0.78 (0.75–0.81) | 0.75 (0.72–0.79) | 0.76 (0.74–0.79) | 0.75 (0.72–0.78) |
| SVM24 (Coult 2019) [39] | 0.72 (0.69–0.75) | 0.69 (0.65–0.72) | 0.75 (0.72–0.77) | 0.70 (0.67–0.73) | 0.75 (0.72–0.78) | 0.73 (0.70–0.77) | 0.75 (0.73–0.78) | 0.75 (0.72–0.78) |
| AMSA1–26 Hz (Firoozabadi 2013) [56] | 0.71 (0.68–0.74) | 0.66 (0.62–0.70) | 0.74 (0.71–0.76) | 0.66 (0.63–0.69) | 0.74 (0.71–0.77) | 0.70 (0.66–0.73) | 0.74 (0.71–0.76) | 0.70 (0.67–0.73) |
AUC values (95% confidence interval) for prediction of patient outcomes are presented for training and validation datasets. (AMSA = amplitude spectrum area [56] method, AUC = area under the receiver operating characteristic curve, −CPR = ECG segments without chest compressions, +CPR = ECG segments with chest compressions, SVM24 = support vector machine combination of 24 previously-published features [39].)
Figure 6. Specific examples of predicted outcomes versus true outcomes.
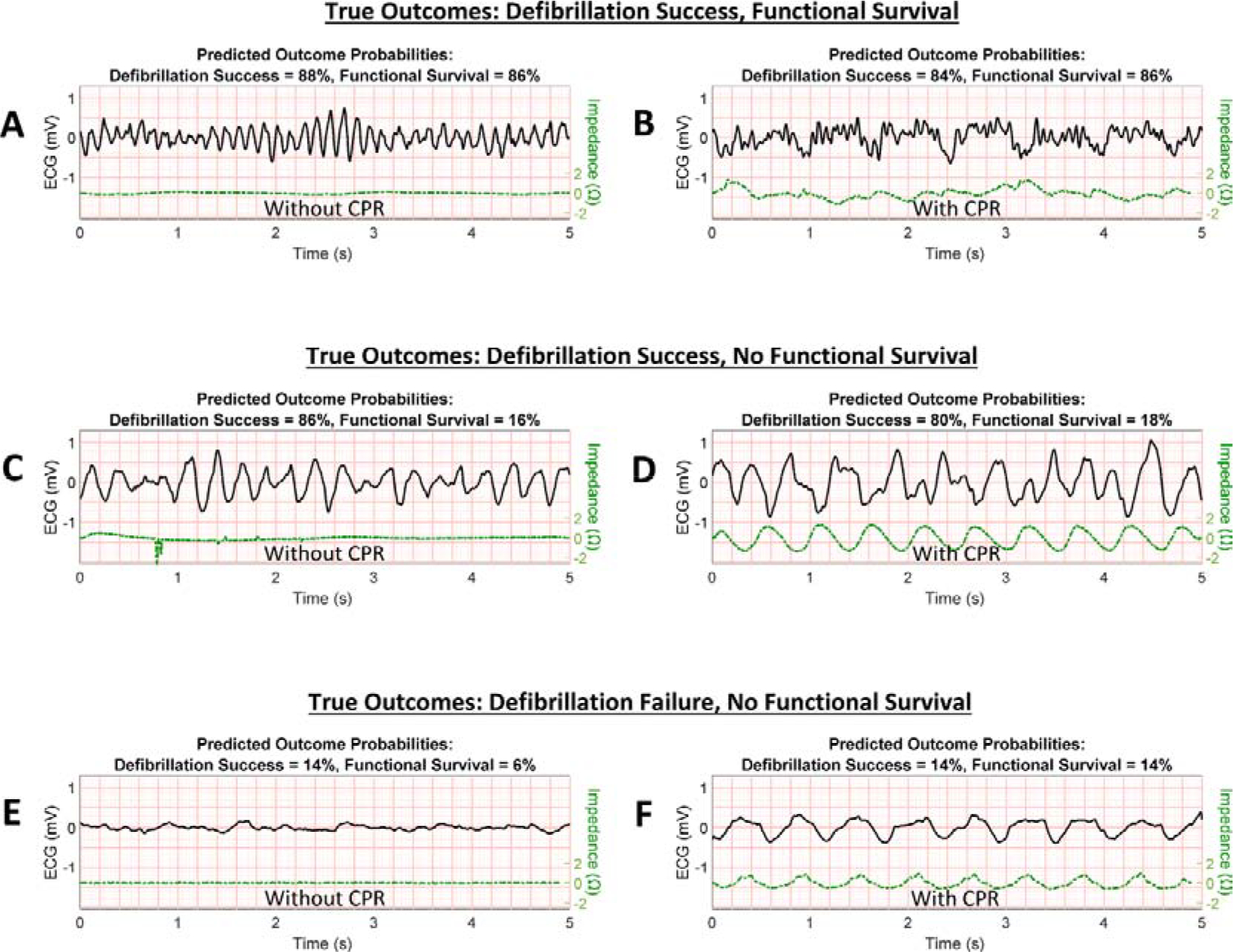
Selected VF ECG validation segment examples from six patients are displayed with algorithm-predicted probabilities for defibrillation success (return of organized rhythm following shock) and functional survival (survival with Cerebral Performance Category of 1 or 2). VF examples with good prognosis (actual outcomes of successful defibrillation and functional survival) are shown without CPR (A) and during CPR (B). VF examples with mixed prognosis (actual outcomes of successful defibrillation but no functional survival) are shown in (C) without CPR and (D) during CPR. VF examples with poor prognosis (actual outcomes of unsuccessful defibrillation and no functional survival) are shown in (E) without CPR and (F) during CPR. (CPR = cardiopulmonary resuscitation, ECG = electrocardiogram, VF = ventricular fibrillation.)
The algorithm also predicted outcomes in the presence of CPR artifact (Table 3, Figure 6). Compared to AMSA and SVM24 during CPR on validation data, the algorithm achieved significantly greater prediction of return of rhythm: AUC was 0.74 for the current algorithm versus 0.66 for AMSA (p<0.001 for difference) and 0.70 for SVM24 (p<0.001 for difference). The algorithm also achieved significantly greater prediction of functional survival versus AMSA but not SVM24: AUC was 0.75 for the current algorithm versus 0.70 for AMSA (p<0.001 for difference) and 0.75 for SVM24 (p=0.60 for difference).
During CPR, the true rates of successful outcomes were similar to those predicted by the algorithm (Figure 7). For example, the group of n=501 validation samples with low predicted probabilities of survival in the lowest quartile (0–0.25) had a true observed survival outcome rate within this range (0.18), and the group n=92 samples with predicted probabilities of survival within the highest quartile (0.75–1) had a true survival outcome rate within this range (0.82).
Figure 7. Predicted outcomes versus true outcomes.
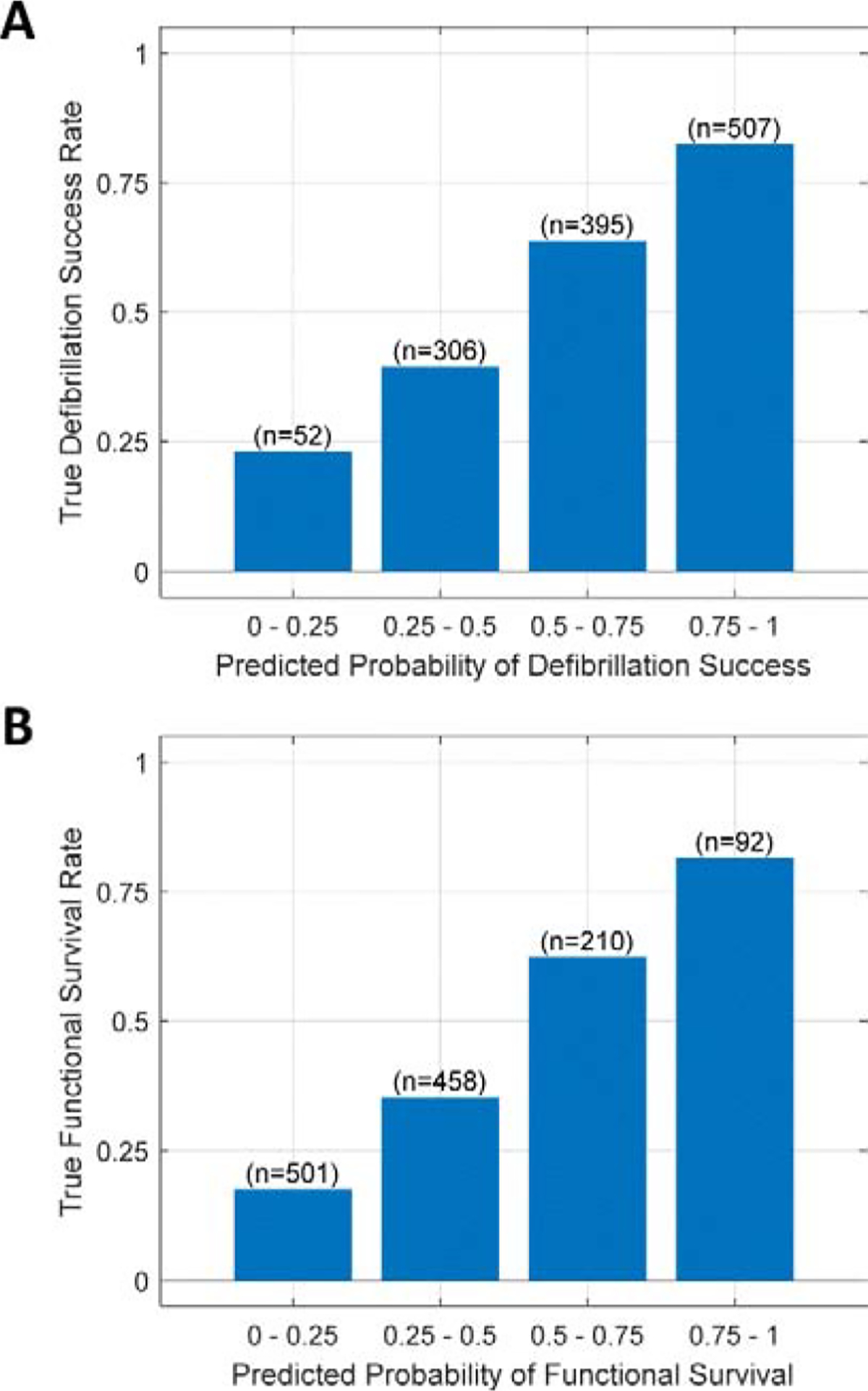
True rates of positive outcome are shown within each quartile of predicted probabilities of positive outcome, using validation data during CPR. (A) The true rates of defibrillation success were within the predicted probability quartiles for defibrillation success. (B) The true rates of functional survival were within to the predicted probability quartiles for functional survival. (n = number of validation segments within each predicted probability decile)
Discussion
Summary
In this retrospective cohort investigation of VF OHCA, a novel algorithm applied with and without ongoing CPR demonstrated improved prediction of the short-term outcome of defibrillation success compared to leading prior methods. Prediction of functional survival was also similar to the best-performing prior method. These results suggest that the current algorithm is an iterative step forward towards the goal of uninterrupted monitoring of patient physiologic status to enable a patient-specific approach to resuscitation.
Background
During VF, the myocardium is in a metabolically-demanding state concurrent with a complete interruption of coronary blood flow. These conditions rapidly deplete myocardial high-energy phosphate concentrations, reducing action potential conduction velocity and contractile function.[14,58,59] Successful restoration of organized rhythm following defibrillation therefore becomes increasingly unlikely as VF persists over time.[60–62] However, during resuscitation, sustained high-quality CPR can counteract the effects of prolonged VF and improve myocardial amenability to defibrillation by partially restoring coronary blood flow.[7,61,63] Therefore, rather than prioritizing early defibrillation, patients with a metabolically-compromised myocardium may instead benefit from a period of CPR to improve the myocardial substrate before a shock.[11,12] However, attempts to prioritize CPR using presumed myocardial status (e.g. based on EMS response time) have shown only a marginal survival benefit.[64] Instead there may be greater potential to improve VF OHCA survival by tailoring resuscitation therapy based on a direct assessment of patient physiology.
Measures of the VF ECG waveform predict defibrillation outcomes, are associated with myocardial metabolic substrate concentrations, and change dynamically to reflect patient response to alterations in treatment.[14,59,65–67] VF ECG waveform measures have thus been proposed as a means to provide real-time prioritization of shock versus CPR throughout resuscitation.[16–18] However, as with most ECG-based algorithms, these measures have been designed to analyze ECGs free of chest compression artifact.[20] This characteristic limits their application to few intermittent assessments that interrupt CPR, challenging the potential of such measures to continuously monitor patient status.[25] Indeed, recent prospective clinical investigation of a VF waveform measure applied during a singular CPR interruption prior to shock did not demonstrate a significant benefit to survival.[67] Ideally, prognostic evaluation would instead be performed continuously during CPR throughout resuscitation. Such uninterrupted evaluation could provide a continuous measure of patient myocardial status and trajectory during VF, potentially enabling real-time titration of CPR and medication to achieve the best possible defibrillation outcome.[10,25]
Algorithm Performance
We therefore sought to design an algorithm to predict patient outcomes both during and without CPR, and to determine whether performance was improved compared to leading prior predictive methods. In recent investigation, we conducted a benchmark of existing VF prognostic methods during CPR and identified a best-performing method (termed the SVM24) among the 27 previously-published approaches tested.[39] When compared to the SVM24 method, the current algorithm exhibited significantly-improved prediction of defibrillation success. However, performance for predicting functional survival was only marginally improved versus SVM24.
We also compared the current algorithm to a classical method – the AMSA – which has been well-validated in retrospective human studies and is currently undergoing prospective investigation for clinical application.[55,56,68,69] Compared to AMSA, the current study algorithm demonstrated a significant AUC increase on validation data both with and without CPR.
Algorithm Design
We hypothesize that the observed performance of the current algorithm was enabled primarily through three attributes which distinguish it from the SVM24 and AMSA methods: Parallel use of filtered and unfiltered ECGs, design and combination of ECG features, and inclusion of dichotomous patient characteristics.
First, the algorithm analyzed filtered and unfiltered ECGs in parallel. ECG filtering improved amplitude-based features susceptible to ECG fluctuations caused by chest compression artifact and high-frequency noise, such as the Sliding Peak feature. On the other hand, use of unfiltered ECG data was generally more suitable for time-frequency features, such as the Shannon Energy feature. During CPR, such time-frequency features may not require notch filtering at estimated compression frequencies due to an inherent ability to ignore lower-frequency CPR artifact using variable frequency cutoffs, and may also utilize high-frequency VF content indicative of good prognosis that might otherwise be attenuated by bandpass filtering.
Second, ECG features were designed to quantify multiple prognostic qualities of the VF waveform related to myocardial metabolic status, such as amplitude, entropy, high-frequency energy, and dominant frequency.[32–38,46,47] This collection of ECG features was then combined using machine learning models trained to predict different resuscitation outcomes during each CPR state. We hypothesize that separately training models to predict specific outcomes during specific CPR states enabled improved performance over use of a single model for all conditions, since different VF waveform features are affected differently by CPR artifact and may also differ in their indication of specific resuscitation outcomes.[22,62] For instance, with regards to the effects of CPR, VF energy as described by the Interfrequency Shannon Energy feature was most predictive during CPR when calculated from frequencies above 13 Hz (hence avoiding most lower-frequency compression artifact), but was most predictive without CPR when calculated from frequencies above 5 Hz (thus including increased VF frequency content). As another example with regards to specific resuscitation outcome, the amplitude-based feature Sliding Deviation was generally most predictive of defibrillation success, whereas the time-frequency feature Interfrequency Shannon Energy was generally most predictive of functional survival (Supplementary Material G). Thus, use of separately-trained features and models to predict different resuscitation outcomes during both CPR states may enable simultaneous prediction of different outcomes, even in cases with disparate prognoses (e.g. positive short-term prognosis but negative long-term prognosis, such as in Figure 6c–d).
Third, use of dichotomized variables such as Prior ROR improved prognostic performance over that of the ECG alone, with an observed validation AUC increase (versus ECG-only prediction) ranging from 0.01–0.04 overall depending on the predicted outcome (Supplementary Material G). Consistent with prior investigations, we observed a significant improvement in prognostic prediction by including response to prior shocks, and only a marginal improvement by including patient demographic variables.[50,52,53]
Clinical Implications
The current algorithm demonstrates potential to estimate probability of successful outcomes during CPR. These prognostic probabilities could potentially be used to monitor patient status and observe real-time effects from CPR and medications on the myocardial substrate during resuscitation. For instance, a prognostic indicator corresponding to the current predicted probability quartile (e.g. Figure 7) could be displayed to rescuers to inform real-time treatment decisions, such as determining when CPR and vasopressor medications have improved shock-resistant myocardium enough to enable successful defibrillation.[25] Therefore while prospective clinical investigation is required to determine how the current algorithm might actually be applied in practice, the results of the present study suggest potential to improve the current one-size-fits-all protocol for OHCA resuscitation by enabling a more patient-specific approach.
Limitations
The investigation used data collected from four different device models with unique hardware-based filtering bandwidths and sampling rates, which may reduce overall AUC for prognostic analysis when analyzed uniformly across devices.[39] However, the current results highlight the cross-platform applicability of the algorithm. While the algorithm outperformed prior methods, overall AUC was not optimal, hence the algorithm’s utility in clinical practice is uncertain. The algorithm incorporated dichotomous patient variables presumed available to rescuers, and would thus require rescuer input to evaluate prognosis. However, a reduced version of the algorithm based solely on the ECG was also significantly predictive of outcomes (Supplementary Material H). The results of the current study were retrospective, and the potential for improved patient care using a prognostic algorithm is hypothetical. Prospective evaluation in a clinical setting would ultimately be required to determine whether a prognostic algorithm could improve patient survival.
Conclusion
A novel prognostic algorithm demonstrated a significant improvement in prediction of defibrillation success versus best-performing published methods regardless of ongoing CPR. These results may support a more tailored, patient-specific approach to resuscitation that could potentially direct treatment based on a patient’s real-time prognostic status. Future investigation may seek to improve these prognostic methods by analyzing continuous ECG data rather than isolated segments as in the current study, and by incorporating larger datasets to enable deep learning methods for development of improved ECG features.
Supplementary Material
Ventricular fibrillation measures predict cardiac arrest resuscitation outcomes
Measures could be used to guide dosage and timing of resuscitation therapies
During resuscitation, chest compressions reduce the accuracy of current measures
We designed a method to predict resuscitation outcomes during chest compressions
We observed improved prediction of defibrillation success versus current measures
Acknowledgements
This work was supported in part by grants provided to the University of Washington by the Washington Research Foundation, the Washington State Life Sciences Discovery Fund, Philips Healthcare, and the National Institute for Biomedical Imaging and Bioengineering of the NIH (T32EB001650). The function for comparing correlated receiver operating characteristic curves by the DeLong method was provided by John W Pickering (University of Otago, Christchurch, New Zealand). Formatting assistance was provided by Diya Sashidhar (University of Washington, Seattle, WA, USA). Additional references are provided for suggested reading in Supplementary Material I.
Role of the Funding Source
C.L., an employee of Philips Healthcare, contributed concepts related to ECG filtering. The funding organizations otherwise had no participation in the collection or analysis of study data and were not involved in generation or interpretation of results, in the drafting of the manuscript, or the decision to submit the work for publication. The content of this work is solely the responsibility of the authors and does not necessarily represent the views of Public Health – Seattle & King County or the funding organizations.
Footnotes
Conflicts of Interest
C.L. is an employee of Philips Healthcare, a medical device company that manufactured the defibrillators used to collect a portion of the study data. The other authors have no conflicts to declare.
Code Availability
MATLAB code for demonstration of the method on example data is available at https://github.com/jcoult/vf-outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018;137:E67–492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- [2].Ten Tusscher KHWJ, Hren R, Panfilov AV. Organization of Ventricular Fibrillation in the Human Heart. Circ Res 2007;100:e87–101. doi: 10.1161/CIRCRESAHA.107.150730. [DOI] [PubMed] [Google Scholar]
- [3].Kleinman ME, Brennan EE, Goldberger ZD, Swor RA, Terry M, Bobrow BJ, et al. Part 5: Adult Basic Life Support and Cardiopulmonary Resuscitation Quality. Circulation 2015;132:S414–35. doi: 10.1161/CIR.0000000000000259. [DOI] [PubMed] [Google Scholar]
- [4].Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: Adult Advanced Cardiovascular Life Support. Circulation 2015;132:S444–64. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- [5].Rea TD, Eisenberg MS, Sinibaldi G, White RD. Incidence of EMS-treated out-of-hospital cardiac arrest in the United States. Resuscitation 2004;63:17–24. doi: 10.1016/j.resuscitation.2004.03.025. [DOI] [PubMed] [Google Scholar]
- [6].Abrams HC, McNally B, Ong M, Moyer PH, Dyer KS. A composite model of survival from out-of-hospital cardiac arrest using the Cardiac Arrest Registry to Enhance Survival (CARES). Resuscitation 2013;84:1093–8. doi: 10.1016/j.resuscitation.2013.03.030. [DOI] [PubMed] [Google Scholar]
- [7].Weisfeldt ML, Becker LB. Resuscitation After Cardiac Arrest: A 3-Phase Time-sensitive Model. JAMA 2002;288:3035–8. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- [8].Nash MP, Mourad A, Clayton RH, Sutton PM, Bradley CP, Hayward M, et al. Evidence for Multiple Mechanisms in Human Ventricular Fibrillation. Circulation 2006;114:536–42. doi: 10.1161/CIRCULATIONAHA.105.602870. [DOI] [PubMed] [Google Scholar]
- [9].Bhandari S, Doan J, Blackwood J, Coult J, Kudenchuk P, Sherman L, et al. Rhythm profiles and survival after out-of-hospital ventricular fibrillation cardiac arrest. Resuscitation 2018;125:22–7. doi: 10.1016/j.resuscitation.2018.01.037. [DOI] [PubMed] [Google Scholar]
- [10].Chalkias A, Arnaoutoglou E, Xanthos T. Personalized physiology-guided resuscitation in highly monitored patients with cardiac arrest—the PERSEUS resuscitation protocol. Heart Fail Rev 2019;24:473–80. doi: 10.1007/s10741-019-09772-7. [DOI] [PubMed] [Google Scholar]
- [11].Cobb LA, Fahrenbruch CE, Walsh TR, Copass MK, Olsufka M, Breskin M, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA 1999;281:1182–8. [DOI] [PubMed] [Google Scholar]
- [12].Wik L, Hansen TB, Fylling F, Steen T, Vaagenes P, Auestad BH, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation. JAMA 2003;289:1389–95. [DOI] [PubMed] [Google Scholar]
- [13].Morgan RW, French B, Kilbaugh TJ, Naim MY, Wolfe H, Bratinov G, et al. A quantitative comparison of physiologic indicators of cardiopulmonary resuscitation quality: Diastolic blood pressure versus end-tidal carbon dioxide. Resuscitation 2016;104:6–11. doi: 10.1016/j.resuscitation.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Salcido DD, Menegazzi JJ, Suffoletto BP, Logue ES, Sherman LD. Association of intramyocardial high energy phosphate concentrations with quantitative measures of the ventricular fibrillation electrocardiogram waveform. Resuscitation 2009;80:946–50. doi: 10.1016/j.resuscitation.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [15].Olasveengen TM, Eftestøl T, Gundersen K, Wik L, Sunde K. Acute ischemic heart disease alters ventricular fibrillation waveform characteristics in out-of hospital cardiac arrest. Resuscitation 2009;80:412–7. doi: 10.1016/j.resuscitation.2009.01.012. [DOI] [PubMed] [Google Scholar]
- [16].Callaway CW, Menegazzi JJ. Waveform analysis of ventricular fibrillation to predict defibrillation. Curr Opin Crit Care 2005;11:192–9. [DOI] [PubMed] [Google Scholar]
- [17].Strohmenger H-U. Predicting defibrillation success. Curr Opin Crit Care 2008;14:311–6. doi: 10.1097/MCC.0b013e3282fc9a9c. [DOI] [PubMed] [Google Scholar]
- [18].Li Y, Tang W. Optimizing the timing of defibrillation: the role of ventricular fibrillation waveform analysis during cardiopulmonary resuscitation. Crit Care Clin 2012;28:199–210. doi: 10.1016/j.ccc.2011.10.013. [DOI] [PubMed] [Google Scholar]
- [19].Coult J, Sherman L, Kwok H, Blackwood J, Kudenchuk PJ, Rea TD. Short ECG segments predict defibrillation outcome using quantitative waveform measures. Resuscitation 2016;109:16–20. doi: 10.1016/j.resuscitation.2016.09.020. [DOI] [PubMed] [Google Scholar]
- [20].Ruiz de Gauna S, Irusta U, Ruiz J, Ayala U, Aramendi E, Eftestøl T. Rhythm Analysis during Cardiopulmonary Resuscitation: Past, Present, and Future. Biomed Res Int 2014;2014:1–13. doi: 10.1155/2014/386010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li Y, Tang W. Techniques for artefact filtering from chest compression corrupted ECG signals: Good, but not enough. Resuscitation 2009;80:1219–20. doi: 10.1016/j.resuscitation.2009.09.003. [DOI] [PubMed] [Google Scholar]
- [22].Fitzgibbon E, Berger R, Tsitlik J, Halperin HR. Determination of the noise source in the electrocardiogram during cardiopulmonary resuscitation. Crit Care Med 2002;30:S148–53. [DOI] [PubMed] [Google Scholar]
- [23].Yu T, Weil MH, Tang W, Sun S, Klouche K, Povoas H, et al. Adverse Outcomes of Interrupted Precordial Compression During Automated Defibrillation. Circulation 2002;106:368–72. doi: 10.1161/01.CIR.0000021429.22005.2E. [DOI] [PubMed] [Google Scholar]
- [24].Cheskes S, Schmicker RH, Christenson J, Salcido DD, Rea T, Powell J, et al. Perishock Pause. Circulation 2011;124:58–66. doi: 10.1161/CIRCULATIONAHA.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Affatato R, Li Y, Ristagno G. See through ECG technology during cardiopulmonary resuscitation to analyze rhythm and predict defibrillation outcome. Curr Opin Crit Care 2016;22:199–205. doi: 10.1097/MCC.0000000000000297. [DOI] [PubMed] [Google Scholar]
- [26].Kwok H, Coult J, Drton M, Rea TD, Sherman L. Adaptive rhythm sequencing: A method for dynamic rhythm classification during CPR. Resuscitation 2015;91:26–31. doi: 10.1016/j.resuscitation.2015.02.031. [DOI] [PubMed] [Google Scholar]
- [27].Cummins RO, Chamberlain D a, Abramson NS, Allen M, Baskett PJ, Becker L, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke. Circulation 1991;84:960–75. doi: 10.1161/01.CIR.84.2.960. [DOI] [PubMed] [Google Scholar]
- [28].Ajam K, Gold LS, Beck SS, Damon S, Phelps R, Rea TD. Reliability of the Cerebral Performance Category to classify neurological status among survivors of ventricular fibrillation arrest: a cohort study. Scand J Trauma Resusc Emerg Med 2011;19:38. doi: 10.1186/1757-7241-19-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Coult J, Blackwood J, Rea TD, Kudenchuk PJ, Kwok H. A Method to Detect Presence of Chest Compressions During Resuscitation Using Transthoracic Impedance. IEEE J Biomed Heal Informatics 2020;24:768–74. doi: 10.1109/JBHI.2019.2918790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gong Y, Chen B, Li Y. A Review of the Performance of Artifact Filtering Algorithms for Cardiopulmonary Resuscitation. J Healthc Eng 2013;4:185–202. doi: 10.1260/2040-2295.4.2.185. [DOI] [PubMed] [Google Scholar]
- [31].Strohmenger HU, Lindner KH, Brown CG. Analysis of the ventricular fibrillation ECG signal amplitude and frequency parameters as predictors of countershock success in humans. Chest 1997;111:584–9. [DOI] [PubMed] [Google Scholar]
- [32].Endoh H, Hida S, Oohashi S, Hayashi Y, Kinoshita H, Honda T. Prompt prediction of successful defibrillation from 1-s ventricular fibrillation waveform in patients with out-of-hospital sudden cardiac arrest. J Anesth 2011;25:34–41. doi: 10.1007/s00540-010-1043-x. [DOI] [PubMed] [Google Scholar]
- [33].Weaver WD, Cobb LA, Dennis D, Ray R, Hallstrom AP, Copass MK. Amplitude of ventricular fibrillation waveform and outcome after cardiac arrest. Ann Intern Med 1985;102:53–5. [DOI] [PubMed] [Google Scholar]
- [34].Brown C, Dzwonczyk R. Estimating the duration of ventricular fibrillation. Ann Emerg Med 1989;18:1181–5. [DOI] [PubMed] [Google Scholar]
- [35].Watson JN, Uchaipichat N, Addison PS, Clegg GR, Robertson CE, Eftestol T, et al. Improved prediction of defibrillation success for out-of-hospital VF cardiac arrest using wavelet transform methods. Resuscitation 2004;63:269–75. doi: 10.1016/j.resuscitation.2004.06.012. [DOI] [PubMed] [Google Scholar]
- [36].Chicote B, Irusta U, Aramendi E, Alcaraz R, Rieta J, Isasi I, et al. Fuzzy and Sample Entropies as Predictors of Patient Survival Using Short Ventricular Fibrillation Recordings during out of Hospital Cardiac Arrest. Entropy 2018;20:591. doi: 10.3390/e20080591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Watson JN, Addison PS, Clegg GR, Steen PA, Robertson CE. Wavelet transform-based prediction of the likelihood of successful defibrillation for patients exhibiting ventricular fibrillation. Meas Sci Technol 2005;16:L1–6. doi: 10.1088/0957-0233/16/10/L01. [DOI] [Google Scholar]
- [38].Neurauter A, Eftestøl T, Kramer-Johansen J, Abella BS, Wenzel V, Lindner KH, et al. Improving countershock success prediction during cardiopulmonary resuscitation using ventricular fibrillation features from higher ECG frequency bands. Resuscitation 2008;79:453–9. doi: 10.1016/j.resuscitation.2008.07.024. [DOI] [PubMed] [Google Scholar]
- [39].Coult J, Blackwood J, Sherman L, Rea TD, Kudenchuk PJ, Kwok H. Ventricular Fibrillation Waveform Analysis During Chest Compressions to Predict Survival From Cardiac Arrest. Circ Arrhythm Electrophysiol 2019;12:1–10. doi: 10.1161/CIRCEP.118.006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ivanović MD, Ring M, Baronio F, Calza S, Vukčević V, Hadžievski L, et al. ECG derived feature combination versus single feature in predicting defibrillation success in out-of-hospital cardiac arrested patients. Biomed Phys Eng Express 2018;5:015012. doi: 10.1088/2057-1976/aaebec. [DOI] [Google Scholar]
- [41].Chicote B, Irusta U, Alcaraz R, Rieta J, Aramendi E, Isasi I, et al. Application of Entropy-Based Features to Predict Defibrillation Outcome in Cardiac Arrest. Entropy 2016;18:313. doi: 10.3390/e18090313. [DOI] [Google Scholar]
- [42].Rosso OA, Blanco S, Yordanova J, Kolev V, Figliola A, Schürmann M, et al. Wavelet entropy: a new tool for analysis of short duration brain electrical signals. J Neurosci Methods 2001;105:65–75. doi: 10.1016/S0165-0270(00)00356-3. [DOI] [PubMed] [Google Scholar]
- [43].Watson JN, Addison PS, Clegg GR, Holzer M, Sterz F, Robertson CE. A novel wavelet transform based analysis reveals hidden structure in ventricular fibrillation. Resuscitation 2000;43:121–7. [DOI] [PubMed] [Google Scholar]
- [44].Martin DR, Brown CG, Dzwonczyk R. Frequency analysis of the human and swine electrocardiogram during ventricular fibrillation. Resuscitation 1991;22:85–91. doi: 10.1016/0300-9572(91)90067-9. [DOI] [PubMed] [Google Scholar]
- [45].Eftestøl T, Sunde K, Ole Aase S, Husøy JH, Steen PA. Predicting Outcome of Defibrillation by Spectral Characterization and Nonparametric Classification of Ventricular Fibrillation in Patients With Out-of-Hospital Cardiac Arrest. Circulation 2000;102:1523–9. doi: 10.1161/01.CIR.102.13.1523. [DOI] [PubMed] [Google Scholar]
- [46].Howe A, Escalona OJ, Di Maio R, Massot B, Cromie N a, Darragh KM, et al. A support vector machine for predicting defibrillation outcomes from waveform metrics. Resuscitation 2014;85:343–9. doi: 10.1016/j.resuscitation.2013.11.021. [DOI] [PubMed] [Google Scholar]
- [47].Shandilya S, Ward K, Kurz M, Najarian K. Non-linear dynamical signal characterization for prediction of defibrillation success through machine learning. BMC Med Inform Decis Mak 2012;12:116. doi: 10.1186/1472-6947-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Platt JC. Probabilistic Outputs for Support Vector Machines and Comparisons to Regularized Likelihood Methods. Adv Large Margin Classif 1999;10:61–74. [Google Scholar]
- [49].Rea TD, Cook AJ, Stiell IG, Powell J, Bigham B, Callaway CW, et al. Predicting Survival After Out-of-Hospital Cardiac Arrest: Role of the Utstein Data Elements. Ann Emerg Med 2010;55:249–57. doi: 10.1016/j.annemergmed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- [50].Gundersen K, Kvaløy JT, Kramer J, Eftestøl T. Identifying approaches to improve the accuracy of shock outcome prediction for out-of-hospital cardiac arrest. Resuscitation 2008;76:279–84. doi: 10.1016/j.resuscitation.2007.07.019. [DOI] [PubMed] [Google Scholar]
- [51].Ng YY, Wah W, Liu N, Zhou SA, Ho AFW, Pek PP, et al. Associations between gender and cardiac arrest outcomes in Pan-Asian out-of-hospital cardiac arrest patients. Resuscitation 2016;102:116–21. doi: 10.1016/j.resuscitation.2016.03.002. [DOI] [PubMed] [Google Scholar]
- [52].Coult J, Kwok H, Sherman L, Blackwood J, Kudenchuk PJ, Rea TD. Ventricular fibrillation waveform measures combined with prior shock outcome predict defibrillation success during cardiopulmonary resuscitation. J Electrocardiol 2018;51:99–106. doi: 10.1016/j.jelectrocard.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].He M, Lu Y, Zhang L, Zhang H, Gong Y, Li Y. Combining Amplitude Spectrum Area with Previous Shock Information Using Neural Networks Improves Prediction Performance of Defibrillation Outcome for Subsequent Shocks in Out-Of-Hospital Cardiac Arrest Patients. PLoS One 2016;11:e0149115. doi: 10.1371/journal.pone.0149115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liu C, Gehman S, Jorgenson D, Lyster T, Coult J, Eisenberg M, et al. A shock advisory algorithm to analyse through chest compressions for reducing interruptions during cardiopulmonary resuscitation. Resuscitation 2017;118:e6. doi: 10.1016/j.resuscitation.2017.08.026. [DOI] [Google Scholar]
- [55].Marn-Pernat A, Weil MH, Tang W, Pernat A, Bisera J. Optimizing timing of ventricular defibrillation. Crit Care Med 2001;29:2360–5. [DOI] [PubMed] [Google Scholar]
- [56].Firoozabadi R, Nakagawa M, Helfenbein ED, Babaeizadeh S. Predicting defibrillation success in sudden cardiac arrest patients. J Electrocardiol 2013;46:473–9. doi: 10.1016/j.jelectrocard.2013.06.007. [DOI] [PubMed] [Google Scholar]
- [57].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988;44:837. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- [58].Klabunde RE. Cardiovascular Physiology Concepts. 2nd ed. 2012.
- [59].Kern KB, Garewal HS, Sanders AB, Janas W, Nelson J, Sloan D, et al. Depletion of myocardial adenosine triphosphate during prolonged untreated ventricular fibrillation: effect on defibrillation success. Resuscitation 1990;20:221–9. doi: 10.1016/0300-9572(90)90005-Y. [DOI] [PubMed] [Google Scholar]
- [60].Eilevstjønn J, Kramer-Johansen J, Sunde K. Shock outcome is related to prior rhythm and duration of ventricular fibrillation. Resuscitation 2007;75:60–7. doi: 10.1016/j.resuscitation.2007.02.014. [DOI] [PubMed] [Google Scholar]
- [61].Niemann JT, Cairns CB, Sharma J, Lewis RJ. Treatment of prolonged ventricular fibrillation. Immediate countershock versus high-dose epinephrine and CPR preceding countershock. Circulation 1992;85:281–7. doi: 10.1161/01.CIR.85.1.281. [DOI] [PubMed] [Google Scholar]
- [62].Jin D, Dai C, Gong Y, Lu Y, Zhang L, Quan W, et al. Does the choice of definition for defibrillation and CPR success impact the predictability of ventricular fibrillation waveform analysis? Resuscitation 2017;111:48–54. doi: 10.1016/j.resuscitation.2016.11.022. [DOI] [PubMed] [Google Scholar]
- [63].Berg RA, Hilwig RW, Kern KB, Ewy GA. Precountershock cardiopulmonary resuscitation improves ventricular fibrillation median frequency and myocardial readiness for successful defibrillation from prolonged ventricular fibrillation: A randomized, controlled swine study. Ann Emerg Med 2002;40:563–70. doi: 10.1067/mem.2002.129866. [DOI] [PubMed] [Google Scholar]
- [64].Meier P, Baker P, Jost D, Jacobs I, Henzi B, Knapp G, et al. Chest compressions before defibrillation for out-of-hospital cardiac arrest: A meta-analysis of randomized controlled clinical trials. BMC Med 2010;8:52. doi: 10.1186/1741-7015-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Schoene P, Coult J, Murphy L, Fahrenbruch C, Blackwood J, Kudenchuk P, et al. Course of quantitative ventricular fibrillation waveform measure and outcome following out-of-hospital cardiac arrest. Hear Rhythm 2014;11:230–6. doi: 10.1016/j.hrthm.2013.10.049. [DOI] [PubMed] [Google Scholar]
- [66].Eftestol T Effects of Interrupting Precordial Compressions on the Calculated Probability of Defibrillation Success During Out-of-Hospital Cardiac Arrest. Circulation 2002;105:2270–3. doi: 10.1161/01.CIR.0000016362.42586.FE. [DOI] [PubMed] [Google Scholar]
- [67].Freese JP, Jorgenson DB, Liu P-Y, Innes J, Matallana L, Nammi K, et al. Waveform Analysis–Guided Treatment Versus a Standard Shock-First Protocol for the Treatment of Out-of-Hospital Cardiac Arrest Presenting in Ventricular Fibrillation. Circulation 2013;128:995–1002. doi: 10.1161/CIRCULATIONAHA.113.003273. [DOI] [PubMed] [Google Scholar]
- [68].ClinicalTrials.gov. Real Time Amplitude Spectrum Area to Guide Defibrillation (AMSA): NCT03237910 2017. https://clinicaltrials.gov/ct2/show/NCT03237910 (accessed July 12, 2019).
- [69].Ristagno G, Mauri T, Cesana G, Li Y, Finzi A, Fumagalli F, et al. Amplitude spectrum area to guide defibrillation. Circulation 2015;131:478–87. doi: 10.1161/CIRCULATIONAHA.114.010989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


