Abstract
Clostridium difficile toxin A is associated with enterocolitis in animals and humans. However, the mechanisms of its secretory and damaging effects are not totally understood. In this work, we examined the intestinal secretion of electrolytes and water caused by supernatants from macrophages stimulated with toxin A in rabbit ileal mucosa mounted in Üssing chambers. We also investigated the mechanism by which the intestinal secretory factor (ISF) is released from stimulated macrophages. Supernatants from macrophages stimulated with toxin A caused potent intestinal secretion (change in short-circuit current [ΔIsc], 76 μA · cm−2; P < 0.01). The release of the ISF was pertussis toxin sensitive (reduction, 61%; P < 0.01) and was also reduced (P < 0.05) by a protein synthesis inhibitor (67%), protease inhibitors (57%), a phospholipase A2 inhibitor (54%), a cyclo-oxygenase inhibitor (62%), a dual cyclo- and lipoxygenase inhibitor (48%), a platelet-activating factor (PAF) receptor antagonist (55%), and tumor necrosis factor alpha (TNF-α) synthesis inhibitors (48%). However, this release was not inhibited by a lipo-oxygenase inhibitor. Monoclonal anti-interleukin 1β (IL-1β) but not anti-IL-1α antibody blocked (72%; P < 0.01) the secretory action of the ISF, as did recombinant human IL-1 receptor antagonist (80%; P < 0.01). High levels of IL-1β (3,476 pg/ml) were detected by an enzyme-linked immunosorbent assay in the above supernatants. Furthermore, the addition of IL-1β to the serosal side caused a potent secretory effect (ΔIsc, 80 μA · cm−2; P < 0.01). These results show that macrophages stimulated with toxin A release an ISF capable of provoking intestinal secretion. The regulation of this factor is dependent upon the activation of the G protein. In addition, prostaglandins, PAF, and TNF-α are involved in the release of the ISF. We conclude that IL-1β is probably the ISF released by macrophages in response to toxin A.
Antibiotic-associated diarrhea and pseudomembranous colitis are superinfections often associated with cytotoxigenic Clostridium difficile (24, 45). This organism produces an enterotoxin, toxin A (TxA) (308 kDa), and a cytotoxin, toxin B (279 kDa) (2, 15), which mediate diarrhea and colitis in humans as well as in experimental animals (24, 38).
The remarkable secretory and inflammatory responses produced by C. difficile are due in part to TxA (21, 27, 28, 32). Since both TxA and toxin B cause potent acute neutrophil migration in the rat peritoneal cavity model (42, 48), it seems likely that toxin B also participates in the intestinal inflammatory reaction produced by C. difficile. The striking secretory response to TxA in ligated rabbit ileal loops is comparable to that caused by cholera toxin (28, 52). However, the mediators and mechanisms of the putative TxA-induced intestinal damage and secretion are poorly understood.
Several reports have shown that TxA stimulates the secretion of a protein-rich, hemorrhagic fluid in ligated ileal loops and elicits in the mucosa an acute inflammatory response characterized by infiltration of the lamina propria with polymorphonuclear leukocytes, hemorrhagic edema, ulceration, and epithelial cell necrosis (27–29, 32, 39, 51).
Lima et al. (27) demonstrated that the inoculation of isolated rabbit intestinal loops with C. difficile TxA resulted in potent intestinal secretion of electrolytes and water, followed by early diffuse mononuclear cell infiltration into the lamina propria and the surface epithelium. In addition, several reports have shown that the intestinal secretory and damaging effects of TxA can be blocked by phospholipase A2 and cyclo-oxygenase inhibitors as well as by platelet-activating factor (PAF) receptor antagonists (16, 19).
We demonstrated previously that the in vivo neutrophil migration induced by TxA and toxin B is mediated by the release of chemotactic factors, such as leukotrienes and cytokines (interleukin 1 [IL-1] and tumor necrosis factor alpha [TNF-α]), from resident macrophages (42, 48). In addition, high doses of TxA were found to exert a potent, direct chemoattractant action on human neutrophils in vitro and to stimulate the release of cytokines from human monocytes (18, 31).
These studies suggest that the mechanism by which TxA induces intestinal secretion may be due in part to an indirect action mediated by the stimulation of resident immune cells, such as macrophages, present in the lamina propria of the intestine. The aims of the present study were (i) to determine the secretory effects of supernatants from TxA-stimulated macrophages on isolated rabbit ileal mucosa, (ii) to investigate the mechanisms involved in the release of the intestinal secretory factor (ISF) by macrophages stimulated with TxA, and (iii) to characterize the macrophage-derived mediator involved in the potent secretory effects of TxA.
MATERIALS AND METHODS
Purification of TxA.
C. difficile (VPI 10463) was grown anaerobically in dialysis tubing suspended in brain heart infusion broth as described previously (49). TxA was purified by ammonium sulfate precipitation, ion-exchange chromatography on DEAE–Sepharose CL-60, and precipitation at pH 5.6. TxA prepared as described above was homogeneous, as shown by crossed immunoelectrophoresis and polyacrylamide gel electrophoresis.
Macrophage cultures.
Rat peritoneal macrophages were collected with RPMI medium 4 days after the intraperitoneal injection of thioglycolate (3% [wt/vol], 10 ml) and placed in plastic tissue culture dishes as previously described (41). After incubation at 37°C in a 5% CO2 atmosphere for 1.5 h, the nonadherent cells were removed by washing the dishes three times with RPMI medium. The cellular pattern was based on the cellular morphology analyzed by optical microscopy. The percentage of macrophages (purity) was calculated from the total number of different cells present in the culture. The total cells, consisting of 95% macrophages, were incubated at 37°C in a 5% CO2 atmosphere for 1 h in fresh medium (control), in medium containing only TxA (10−6 M), or in medium containing TxA (10−6 M) and different pharmacological inhibitors. The supernatants were discarded and, after additional washing, the cells were incubated for a further 2 h with 1.0 ml of RPMI medium without toxin or drugs. Cell-free incubation medium was obtained by centrifugation (300 × g, 5 min), and 1.0 ml of supernatant adjusted for 1.3 × 107 cells/ml by use of a Neubauer chamber was tested on portions of isolated rabbit ileum mounted in Üssing chambers as described below. Macrophage viability was determined by trypan blue exclusion as described elsewhere (25, 30). Macrophage viability ranged from 89 to 97% in the different experimental protocols.
Üssing chamber experiments.
New Zealand White rabbits (1.5 to 2.0 kg) of both sexes were sacrificed, and the distal ileum was removed, stripped of the serosa, and mounted between Lucite half chambers (cross-sectional area, 1 cm2). The tissue was bathed on both sides with Ringer solution containing 5 mM glucose on the serosal side and 5 mM mannitol on the mucosal side. The preparation was aerated with 95% O2 and 5% CO2 at 37°C. The composition of the Ringer solution (pH 7.4) (in milliequivalents per liter) was as follows: Na+, 145; K+, 4.6; Ca2+, 3.4; Mg2+, 0.8; Cl−, 119; HCO3−, 25; and SO4−, 0.8. The electrical apparatus for supplying the short-circuit current (Isc) and for measuring the potential difference (PD) was similar to that described by Schultz and Zalusky (44). The tissues were equilibrated for at least 30 min prior to the addition of a test agent. The supernatant (1.0 ml) and drugs were added to the serosal bathing solution, and the electrical parameters, such as PD, Isc, and tissue resistance (defined as the change in Isc [ΔIsc] divided by the change in PD; Ohm’s law) (11, 34), were measured at 10-min intervals for 90 min.
IL-1β assay.
The medium from cultured cells was obtained as described above and stored at −70°C until used. The IL-1β concentration was determined with an enzyme-linked immunosorbent assay (ELISA) specific for rat IL-1β (Biosource International, Camarillo, Calif.) in accordance with the manufacturer’s instructions.
Drugs.
Purified pertussis toxin (PTx) preparations were obtained from Erik Hewlett (Clinical Pharmacology, School of Medicine, University of Virginia, Charlottesville). Briefly, wild-type PTx was purified from supernatants of Bordetella pertussis 165 cultures by hydroxylapatite chromatography and fetuin affinity chromatography and stored at 4°C. A noncatalytic mutant of PTx (9K/129G; PTxm) was prepared at Chiron Bioscience (Siena, Italy) and was kindly provided by Rino Rappuoli. This mutant contains two amino acid substitutions in the S1 subunit (an Arg→Lys substitution at position 9 and a Glu→Gly substitution at position 129) which abolish the enzymatic activity of the subunit (4, 37).
BN 52021 was obtained from Institute Pasteur, Paris, France. Thioglycolate was obtained from Difco Laboratories, Detroit, Mich. Phenylmethylsulfonyl fluoride (PMSF), trypsin inhibitor, cycloheximide, quinacrine, indomethacin, nordihydroguaiaretic acid (NDGA), pentoxifylline, RPMI medium, recombinant human IL-1β (rhIL-1β), anti-IL-1α and anti-IL-1β monoclonal antibodies, and trypan blue were purchased from Sigma Chemical Company, St. Louis, Mo. MK 886 was obtained from Merck Sharp & Dohme, Rahway, N.J. Dexamethasone was obtained from Merck Sharp & Dohme, São Paulo, São Paulo, Brazil. Thalidomide was obtained from ICN Biomedical Inc., Aurora, Ohio. Recombinant human IL-1 receptor antagonist (IL-1ra) was obtained from Bachem Bioscience Inc., King of Prussia, Pa.
Statistical analysis.
The statistical significance of the differences between various groups was assessed by use of analysis of variance (ANOVA) (Bonferroni or Dunn method), and the results are presented as the mean ± standard error of the mean (SEM). A P value of ≤0.05 was considered to indicate statistical significance. n indicates the total number of Üssing chambers containing rabbit ileum from at least four different animals.
RESULTS
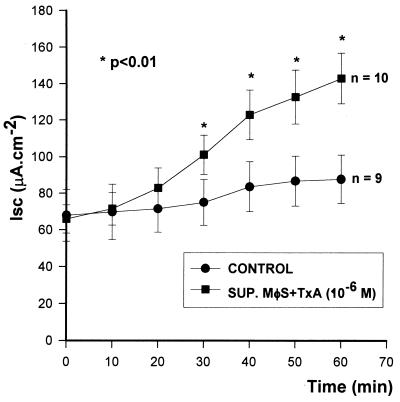
Supernatants from macrophages stimulated with TxA (10−6 M) caused potent secretion by rabbit ileum in the Üssing chambers (ΔIsc ringer [control], 66 ± 7.7, versus ΔIsc SUP. MφS + TxA, 142 ± 13.8 μA · cm−2; n = 10; P < 0.01); the results were dose (data not shown) and time (Fig. 1) dependent. Supernatants from macrophages not treated with TxA did not change the Isc (ΔIsc ringer [control], 68 ± 14.2, versus ΔIsc SUP.MφS + RPMI [control], 87 ± 13.1 μA · cm−2; n = 9; P > 0.05) (Fig. 1). Pure TxA (10−6 M) added directly to Üssing chambers did not cause intestinal secretion (60 ± 5.3 versus 62 ± 6.8 μA · cm−2; n = 5; P > 0.05) or tissue damage (data not shown).
FIG. 1.
Intestinal secretory effect of supernatants (SUP.) from macrophages (MφS) stimulated with TxA. The supernatants were tested on rabbit ileum mounted in Üssing chambers. The results represent the mean ± SEM of the number (n) of preparations tested. ∗, the P value of the indicated data versus the control was determined by ANOVA (Bonferroni or Dunn method).
Subscripts are used in the reporting of ΔIsc data for inhibitor assays: SUP. MφS, supernatants from macrophages; CHX, cycloheximide; PROT I, protease inhibitors; DEXA, dexamethasone; QUINAC, quinacrine; INDO, indomethacin; PTF, pentoxifylline; THALID, thalidomide; RPMI, RPMI medium; and Ringer, Ringer solution. Other subscripts are as defined earlier. ΔIsc is reported in microamps · centimeter−2.
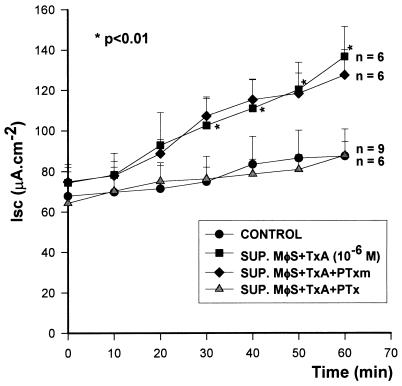
The intestinal secretory activity produced by supernatants from TxA (10−6 M)-stimulated macrophages was blocked by pretreatment of the macrophages with active PTx (100 ng/ml) (ΔIscSUP. MφS + TxA, 62 ± 7.6; ΔIscSUP. MφS + TxA + PTx, 24 ± 6.6 [n = 6]; P < 0.01). On the other hand, PTxm (100 ng/ml) did not alter this secretory activity (ΔIscSUP. MφS + TxA, 62 ± 7.6; ΔIscSUP. MφS + TxA + PTxm, 52 ± 8.7 [n = 6]; P > 0.05) (Fig. 2).
FIG. 2.
Effect of B. pertussis toxins on the release of ISF. PTx or PTxm was added to the incubation medium of macrophages (MφS) before and during stimulation with TxA. The cells were washed and incubated for a further 2 h without stimulation. The supernatants (SUP.) were subsequently tested on rabbit ileum in vitro. The results represent the mean ± SEM of the number (n) of preparations tested. ∗, the P value for SUP. MφS + TxA versus the control or SUP. MφS + TxA + PTx was determined by ANOVA (Bonferroni or Dunn method). The P value for SUP. MφS + TxA versus SUP. MφS + TxA + PTxm was not significant (Bonferroni or Dunn method).
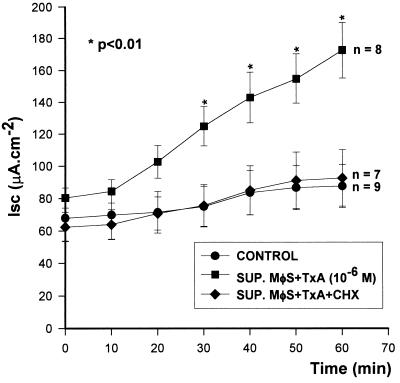
The release of ISF by TxA (10−6 M)-stimulated macrophages was also significantly blocked by the addition of cycloheximide (10 μM), a protein synthesis inhibitor, to the culture medium (ΔIscSUP. MφS + TxA, 91 ± 15.2 [n = 8]; ΔIscSUP. MφS + TxA + CHX, 30 ± 8.9 [n = 7]; P < 0.01) (Fig. 3).
FIG. 3.
Protein synthesis and release of ISF. Cycloheximide (CHX) was added to the incubation medium of macrophages (MφS) before and during stimulation with TxA. The cells were washed and incubated for a further 2 h without stimulation. The supernatants (SUP.) were subsequently tested on rabbit ileum in vitro. The results represent the mean ± SEM of the number (n) of preparations tested. ∗, the P value for SUP. MφS + TxA versus the other two groups was determined by ANOVA (Bonferroni or Dunn method).
The inclusion of protease inhibitors, such as trypsin inhibitor and PMSF, during the last 2 h of incubation inhibited the intestinal secretion produced by ISF present in supernatants from TxA-stimulated macrophages (ΔIscSUP. MφS + TxA, 91 ± 15.2 [n = 8]; ΔIscSUP. MφS + TxA + PROT I, 39 ± 7.4 [n = 7]; P < 0.01) (Fig. 4).
FIG. 4.
Effect of protease inhibitors (PROT I) on the release of ISF. Macrophages (MφS) were stimulated with TxA and then washed and incubated for a further 2 h in the presence of protease inhibitors (trypsin inhibitor and PMSF). The inhibitors were maintained in the incubation medium throughout the experiment. The resulting supernatants (SUP.) were tested on rabbit ileum. The results represent the mean ± SEM of the number (n) of preparations tested. ∗, the P value for SUP. MφS + TxA versus the other two groups was determined by ANOVA (Bonferroni or Dunn method).
Treatment with dexamethasone (10 μM) or quinacrine (10 μM) 30 min before and also during the stimulation of macrophages with TxA significantly blocked the release of ISF (ΔIscSUP. MφS + TxA, 91 ± 15.2 [n = 8]; ΔIscSUP. MφS + TxA + DEXA, 36 ± 7.1 [n = 7]; P < 0.01; ΔIscSUP. MφS + TxA + QUINAC, 42 ± 6.1 [n = 6]; P < 0.05). Similarly, indomethacin (10 μM), NDGA (1 μM), and BN 52021 (10 μM) also reduced the ability of the supernatants to cause intestinal secretion (ΔIscSUP. MφS + TxA, 91 ± 15.2 [n = 8]; ΔIscSUP. MφS + TxA + INDO, 35 ± 6.1 [n = 6]; P < 0.01; ΔIscSUP. MφS + TxA + NDGA, 47 ± 3.6 [n = 5]; P < 0.05; ΔIscSUP. MφS + TxA + BN 52021, 41 ± 9.7 [n = 6]; P < 0.05). On the other hand, the specific lipo-oxygenase inhibitor MK 886 (10 μM) did not change the intestinal secretory activity of the supernatants (ΔIscSUP. MφS + TxA, 91 ± 15.2 [n = 8]; ΔIscSUP. MφS + TxA + MK 886, 81 ± 18.1 [n = 8]; P > 0.05) (Table 1).
TABLE 1.
Effects of potential pharmacological inhibitors on the release of ISF
| Exptl protocola | nb | ΔIsc (μA · cm−2) (mean ± SEM) | Inhibition (%) | Pc | Pharmacology |
|---|---|---|---|---|---|
| SUP. MφS + RPMI medium | 9 | 20 ± 3.1 | <0.01 | RPMI medium | |
| SUP. MφS + TxA (1 μM) | 8 | 91 ± 15.2 | TxA from C. difficile | ||
| SUP. MφS + TxA + dexamethasone (10 μM) | 7 | 36 ± 7.1 | 60 | <0.01 | Phospholipase A2 and cytokine synthesis inhibitor |
| SUP. MφS + TxA + quinacrine (10 μM) | 6 | 42 ± 6.1 | 54 | <0.05 | Phospholipase A2 inhibitor |
| SUP. MφS + TxA + indomethacin (10 μM) | 6 | 35 ± 6.1 | 62 | <0.01 | Cyclo-oxygenase inhibitor |
| SUP. MφS + TxA + MK 886 (10 μM) | 8 | 81 ± 18.1 | NS | NS | Lipo-oxygenase inhibitor |
| SUP. MφS + TxA + NDGA (1 μM) | 5 | 47 ± 3.6 | 48 | <0.05 | Dual cyclo- and lipo-oxygenase inhibitor |
| SUP. MφS + TxA + BN 52021 (10 μM) | 6 | 41 ± 9.7 | 55 | <0.05 | PAF antagonist |
| SUP. MφS + TxA + pentoxifylline (500 μM) | 6 | 47 ± 7.2 | 48 | <0.05 | TNF-α synthesis inhibitor |
| SUP. MφS + TxA + thalidomide (15 μM) | 6 | 48 ± 11.2 | 47 | <0.05 | TNF-α synthesis inhibitor |
Each experiment was repeated at least four times. SUP. MφS, supernatants from macrophages.
Total number of Üssing chambers containing rabbit ileum.
Statistical significance of the SUP. MφS+TxA group compared to the other experimental groups. NS, not significant (P > 0.05).
Pentoxifylline (500 μM) and thalidomide (15 μM), both of which inhibit the synthesis of TNF-α, partial-ly blocked the release of ISF (ΔIscSUP. MφS + TxA, 91 ± 15.2 [n = 8]; ΔIscSUP. MφS + TxA + PTF, 47 ± 7.2 [n = 6]; ΔIscSUP. MφS + TxA + THALID, 48 ± 11.2 [n = 6]; P < 0.05) (Table 1).
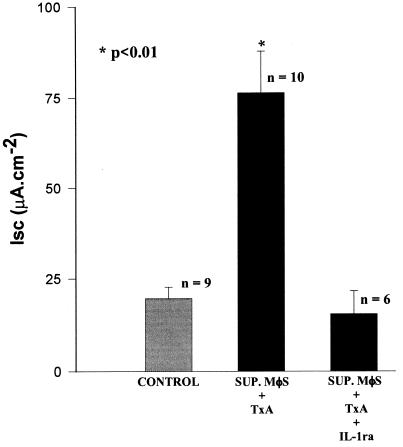
Pretreatment of the rabbit ileum with IL-1ra (4.5 μM) for 30 min inhibited the intestinal secretion caused by supernatants from TxA-stimulated macrophages (ΔIscSUP. MφS + TxA, 76 ± 8.4 [n = 10]; ΔIscSUP. MφS + TxA + IL-1ra, 15 ± 6.2 [n = 6]; ΔIscSUP. MφS + RPMI (CONTROL), 20 ± 3.14 [n = 9]; P < 0.001) (Fig. 5).
FIG. 5.
Inhibition of intestinal secretion by IL-1ra. Rabbit ileum was treated with IL-1ra for 30 min before the addition of supernatants (SUP.) from TxA-stimulated macrophages (MφS). Intestinal secretion was evaluated as described in Materials and Methods. The results represent the mean ± SEM of the number (n) of preparations tested. ∗, the P value for SUP. MφS + TxA versus the other two groups was determined by ANOVA (Bonferroni or Dunn method).
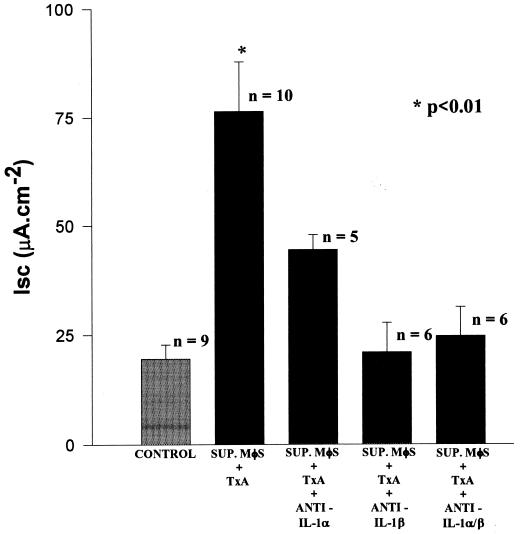
Similarly, the intestinal secretory activity of the supernatants was inhibited by preincubation with an anti-IL-1β monoclonal antibody (250 μg/ml) (ΔIscSUP. MφS + TxA, 76 ± 11.4 [n = 10]; ΔIscSUP. MφS + TxA + anti-IL-1β, 21 ± 6.8 [n = 6]; ΔIscSUP. MφS + RPMI (CONTROL), 20 ± 3.1 [n = 9]; P < 0.01). In contrast, an anti-IL-1α monoclonal antibody (250 μg/ml) had no significant effect on the secretory activity of the supernatants (ΔIscSUP. MφS + TxA, 76 ± 11.4 [n = 10]; ΔIscSUP. MφS + TxA + anti-IL-1α, 44 ± 3.5 [n = 5]; ΔIscSUP. MφS + RPMI (CONTROL), 20 ± 3.1 [n = 9]; P > 0.05). The combined effect of anti-IL-1α and anti-IL-1β antibodies (250 μg/ml each) on the intestinal secretory activity was not statistically different from that observed with the anti-IL-1β antibody alone (ΔIscSUP. MφS + TxA, 76 ± 11.4 [n = 10]; ΔIscSUP. MφS + TxA + anti-IL-1α and -β, 25 ± 6.6 [n = 6]; P < 0.01) (Fig. 6).
FIG. 6.
Influence of anti-IL-1α and/or anti-IL-1β antibodies on intestinal secretion induced by supernatants (SUP.) from macrophages (MφS) stimulated with TxA. Supernatants of macrophages stimulated with TxA were incubated with either anti-IL-1α or anti-IL-1β antibodies or a combination of both for 30 min. The supernatants, together with the respective antibodies, were subsequently tested on rabbit ileum. The results represent the mean ± SEM of the number (n) of preparations tested. ∗, the P value for SUP. MφS + TxA versus SUP. MφS + TxA + anti-IL-1β, SUP. MφS + TxA + anti-IL-1α/β, or the control was determined by ANOVA (Bonferroni or Dunn method). The P value for SUP. MφS + TxA versus SUP. MφS + TxA + anti-IL-1α was not significant (Bonferroni or Dunn method).
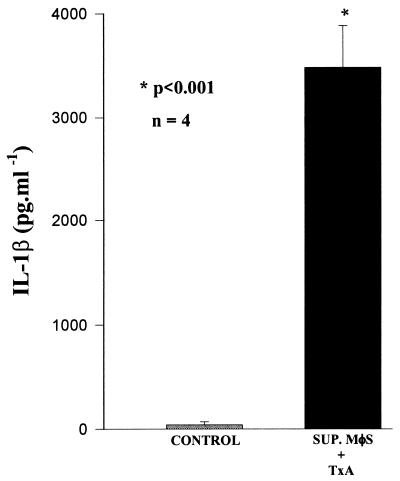
High levels of IL-1β were detected by the ELISA in supernatants of macrophages stimulated with TxA (3,476 ± 200 pg/ml; the level in the control was 40 ± 14.5 pg/ml; n = 4; P < 0.001) (Fig. 7). Furthermore, the addition of rhIL-1β (10−7 M) to the serosal side of the Üssing chambers caused a potent secretory effect (ΔIscIL-1β, 80 ± 12.0 [n = 6]; ΔIscRinger, 28 ± 4.9 [n = 8]; P < 0.01).
FIG. 7.
Detection of IL-1β in supernatants (SUP.) from macrophages (MφS) stimulated with TxA. The concentration of IL-1β present in supernatants from macrophages stimulated with TxA was determined by an ELISA. The results represent the mean ± SEM of the number (n) of samples measured in duplicate. ∗, the P value for SUP. MφS + TxA versus SUP. MφS + RPMI medium (control) was determined by ANOVA (Bonferroni or Dunn method).
DISCUSSION
C. difficile is a major cause of antibiotic-associated inflammatory diarrhea and pseudomembranous colitis, principally through the action of TxA and toxin B (29, 38, 40). The injection of TxA into the intestine produces an acute inflammatory response, with the activation of macrophages and mast cells and the mobilization of neutrophils (39, 40, 52). Two hours after the inoculation of this toxin into the intestinal loop of rabbits, there is a large infiltration of mononuclear cells into the lamina propria, with the consequent destruction of the intestinal mucosa (27).
We demonstrated recently that C. difficile TxA is a potent inducer of neutrophil migration in the rat peritoneal cavity and air pouch. This migration was dependent on the release of IL-1β, TNF-α, and leukotrienes by the macrophages (42).
Macrophages are considered to be one of the most important cell types in inflammatory processes. By acting as alarm cells, these phagocytes signal the presence of foreign bodies through the elaboration and release of several substances, including cytokines and arachidonic acid metabolites (17, 26, 35). In order to examine whether macrophages are important in the enterotoxic effects of TxA, we evaluated the ability of this enterotoxin to stimulate the release of ISF from these cells in vitro.
Macrophages stimulated with TxA released a factor that activated intestinal secretory activity, as shown by changes in ion transport based on Isc analysis of rabbit ileal segments. These results confirmed an involvement of macrophages in the intestinal secretory response to TxA.
To explore a possible role for the inhibitory G-protein (Gi) pathway of the adenylyl cyclase system, we studied the actions of PTx and PTxm. The biological effects of PTx result from the action of the S1 subunit. This subunit has ADP-ribosyltransferase activity that catalyzes the transfer of ADP-ribose from NAD to a regulatory GTP-binding protein (G protein) in eukaryotic cells (4, 20). The intestinal secretory activity of rabbit ileum exposed to macrophage supernatants was completely inhibited by PTx. In contrast, PTxm, which is devoid of enzymatic activity, did not alter this activity.
Certain macrophage-derived mediators, such as IL-1 (8, 9), TNF-α (23), PAF (22), leukotrienes (46, 47), and prostaglandins (6), can cause intestinal secretion. Therefore, pharmacological screening was used in an attempt to investigate if one of these products was the ISF present in the supernatants of TxA-stimulated macrophages.
Cycloheximide, a classic inhibitor of mRNA (36), significantly inhibited the release of ISF, indicating that this factor is dependent upon protein synthesis. This finding was confirmed by the observation that dexamethasone, a compound known to block the transcription of mRNA for several cytokines, including TNF-α, IL-1, and IL-8, in macrophages (1, 3), potently inhibited the formation of ISF.
The ability of quinacrine, a phospholipase A2 inhibitor, to partially block the secretory activity of the supernatants indicated that arachidonic acid metabolites are involved in the release of ISF. This fact was confirmed by the inhibition observed with dexamethasone which, in addition to its effects on mRNA transcription, also markedly inhibits eicosanoid production through the formation of lipocortins. The latter also block the activity of phospholipase A2 (1). Indomethacin, a cyclo-oxygenase inhibitor, and NDGA, a dual cyclo- and lipo-oxygenase inhibitor, reduced the intestinal secretory activity in the supernatants of TxA-stimulated macrophages. In contrast, MK 886, a specific lipo-oxygenase inhibitor, had no significant effect on this activity. The PAF receptor antagonist BN 52021 only partially inhibited the release of ISF. These results indicate that prostaglandins and PAF, but not leukotrienes, probably play an important role in the release of ISF.
Pretreating the macrophages with pentoxifylline and thalidomide, both of which are directly related to TNF-α synthesis (33), resulted in partial inhibition of the release of ISF. This finding suggests that TNF-α may have a role as an amplifying element in the release of ISF.
Preincubation of the supernatants with anti-IL-1β monoclonal antibody resulted in 72% inhibition of intestinal secretion. Nevertheless, anti-IL-1α monoclonal antibody had no significant effect. The combination of anti-IL-1α and anti-IL-1β monoclonal antibodies did not inhibit the intestinal secretory activity to a greater extent than that observed with anti-IL-1β antibody alone. These experiments demonstrated the importance of IL-1β in the intestinal secretion caused by supernatants of TxA-stimulated macrophages. IL-1ra (10, 12, 13, 50) completely blocked the secretory response of the supernatants, confirming that IL-1 was the ISF present in the supernatants studied.
The addition of rhIL-1β (10−7 M) to the serosal side of the Üssing chambers provoked potent intestinal secretion similar to that seen with the conditioned supernatants from macrophages. Furthermore, Chiossone et al. (9) found that the addition of rhIL-1α or rhIL-1β to rabbit ileum on the serosal side of Üssing chambers provoked intestinal secretion in a dose-dependent manner, with the maximum effect occurring at a dose of 5 ng.
The presence of protease inhibitors during the final stage of incubation neutralized the intestinal secretion caused by the conditioned supernatants. This finding indicates that proteolytic activity is needed during the formation of ISF. In this regard, IL-1β is initially synthesized as a precursor molecule, with a molecular mass of 31 kDa, and later cleaved by intra- and extracellular proteases to yield the mature form, with a molecular mass of 17.5 kDa (12, 14). The synthesis and release of IL-1 can be blocked by cyclo-oxygenase and dual cyclo-oxygenase–lipo-oxygenase inhibitors but not by specific lipo-oxygenase inhibitors (12). In addition, IL-1 is capable of stimulating the production by endothelial cells of PAF, which can in turn regulate the synthesis of IL-1, IL-2, and TNF-α (5, 7, 43).
The detection of IL-1β in the supernatants of TxA-stimulated macrophages by an ELISA further confirmed IL-1β to be the ISF.
In conclusion, macrophages stimulated with TxA release a factor capable of provoking intestinal secretion in vitro. The regulation of this factor is dependent on the activation of the G protein. In addition, cyclo-oxygenase products, PAF, and TNF-α are involved in the release of ISF (Fig. 8). Furthermore, the data demonstrate that IL-1β is the ISF.
FIG. 8.
Model for how PTx, arachidonic acid metabolites, TNF-α, and PAF modulate the synthesis or secretion of IL-1β in macrophages stimulated by TxA from C. difficile. In this model, TxA interacts with a G-protein-linked receptor present in the macrophage membrane and induces the release of inflammatory mediators, such as prostaglandins (PGs), PAF, and TNF-α. These mediators might stimulate the macrophages to secrete IL-1β in an autocrine manner. In addition, proteolytic activity is needed during the formation of IL-1β. This scheme also shows that potential pharmacological blockers, such as cyclo-oxygenase products, PAF, TNF-α, and protease inhibitors, could affect the synthesis and release of this cytokine.
ACKNOWLEDGMENTS
This study was supported in part by the National Institute of Allergy and Infectious Diseases (ICIDR grant PO1-AI-26512 and TRMC grant 2P50 AI30639 from the National Institutes of Health) and by CNPq grant 521015/94. M. F. G. Rocha is the recipient of a fellowship from CAPES, Brasília, Brazil.
We thank Stephen Hyslop (University of Campinas, Campinas, São Paulo, Brazil) for editing the manuscript.
REFERENCES
- 1.Barnes P J, Adcock I. Antiinflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 2.Barroso L A, Wang S Z, Phelps C J, Johnson J L, Wilkins T D. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990;18:4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B, Krochin N, Milsark I W, Luedke C, Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986;232:977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- 4.Brito G A C, Souza M H L P, Melo-Filho A A, Hewlett E L, Lima A A M, Flores C A, Ribeiro R A. Role of pertussis toxin A subunit in neutrophil migration and vascular permeability. Infect Immun. 1997;65:1114–1118. doi: 10.1128/iai.65.3.1114-1118.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bussolino F, Camussi G, Baglione C. Synthesis and release of platelet-activating factor by human vascular endothelial cells treated with tumor necrosis factor or interleukin-1α. J Biol Chem. 1988;263:11856–11861. [PubMed] [Google Scholar]
- 6.Calderaro V, Giovane A, De Simone B, Camussi G, Rossiello R, Quagliuolo L, Servillo L, Taccone W, Giordano C, Balestrieri C. Arachidonic acid metabolites and chloride secretion in rabbit distal colonic mucosa. Am J Physiol. 1991;261:G443–G450. doi: 10.1152/ajpgi.1991.261.3.G443. [DOI] [PubMed] [Google Scholar]
- 7.Camussi G, Tetta C, Baglione C. The role of platelet-activating factor in inflammation. Clin Immunol Immunopathol. 1990;57:331–338. doi: 10.1016/0090-1229(90)90108-3. [DOI] [PubMed] [Google Scholar]
- 8.Chang E B, Musch M W, Mayer L. Interleukins 1 and 3 stimulate anion secretion in chicken intestine. Gastroenterology. 1990;98:1518–1524. doi: 10.1016/0016-5085(90)91084-j. [DOI] [PubMed] [Google Scholar]
- 9.Chiossone D C, Simon P L, Smith P L. Interleukin-1 effects on rabbit ileal mucosa ion transport in vitro. Eur J Pharmacol. 1990;180:217–228. doi: 10.1016/0014-2999(90)90305-p. [DOI] [PubMed] [Google Scholar]
- 10.Cominelli F, Nast C C, Clark B D, Schindler R, Lierena R, Eysselein V E, Thompson R C, Dinarello C A. Interleukin-1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Investig. 1990;86:972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dharmsathaphorn K, Madara L. Established intestinal cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990;192:354–389. doi: 10.1016/0076-6879(90)92082-o. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello C A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- 13.Dinarello C A, Thompson R C. Blocking IL-1: interleukin-1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello C A, Wolff S M. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 15.Dove C H, Wang S Z, Price S B, Phelps C J, Lyerly D M, Wilkins T D, Johnson J L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990;58:480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang G, Lima A A M, Thielman N M, Fonteles M C, Yotseff P, Lyerly D M, Guerrant R L. Role of phospholipase A2 in the histologic, epithelial and secretory responses to Clostridium difficile. Biomed J. 1994;1:1–5. [Google Scholar]
- 17.Ferreira S H. Are macrophages the body’s alarm cells? Agents Actions. 1980;10:229–230. [Google Scholar]
- 18.Flegel W A, Müller F, Daubener W, Fischer H G, Hadding U, Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun. 1991;59:3659–3666. doi: 10.1128/iai.59.10.3659-3666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonteles M C, Fang G, Thielman N M, Yotseff P S, Guerrant R L. Role of platelet activating factor in inflammatory and secretory effects of Clostridium difficile toxin A. J Lipid Med Cell Signal. 1995;11:133–143. doi: 10.1016/0929-7855(94)00033-9. [DOI] [PubMed] [Google Scholar]
- 20.Gierschik P. ADP-ribosylation of signal-transducing guanine nucleotide-binding proteins by pertussis toxin. Microbiol Immunol. 1992;175:69–96. doi: 10.1007/978-3-642-76966-5_4. [DOI] [PubMed] [Google Scholar]
- 21.Guerrant R L. Lessons from diarrheal diseases: demography to molecular pharmacology. J Infect Dis. 1994;169:1206–1218. doi: 10.1093/infdis/169.6.1206. [DOI] [PubMed] [Google Scholar]
- 22.Hanglow A C, Bienenstock J, Perdue M H. Effects of platelet-activating factor on ion transport in isolated rat jejunum. Am J Physiol. 1989;257:G845–G850. doi: 10.1152/ajpgi.1989.257.5.G845. [DOI] [PubMed] [Google Scholar]
- 23.Kandil H, Berschneider H, Argenzio A. TNF-α alters intestinal ion transport through a paracrine mechanism involving prostaglandins. Gastroenterology. 1992;102:A217. doi: 10.1136/gut.35.7.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly C P, Pothoulakis C, LaMont J T. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 25.Korzeniewsky C, Callewaert D M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 26.Laskin D L, Pendino K J. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol. 1995;35:655–677. doi: 10.1146/annurev.pa.35.040195.003255. [DOI] [PubMed] [Google Scholar]
- 27.Lima A A M, Innes D J, Chadee K, Lyerly D M, Wilkins T D, Guerrant R L. Clostridium difficile toxin A interactions with mucus and early sequential histopathologic effects in rabbit small intestine. Lab Investig. 1989;61:419–425. [PubMed] [Google Scholar]
- 28.Lima A A M, Lyerly D M, Wilkins T D, Innes D J, Guerrant R L. Effects of Clostridium difficile toxins A and B in rabbit small and large intestines in vivo and on cultured cells in vitro. Infect Immun. 1988;56:582–588. doi: 10.1128/iai.56.3.582-588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyerly D M, Krivan H C, Wilkins T D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall J S, Leal-Berumen I, Nielsen L, Glibetic M, Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not performed mediator release from rat peritoneal mast cells. J Clin Investig. 1996;97:1122–1128. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller P D, Pothoulakis C, Baeker T R, LaMont J T, Rothstein T L. Macrophage-dependent stimulation of T cell-depleted spleen cells by Clostridium difficile toxin A and calcium ionophore 1. Cell Immunol. 1990;126:153–163. doi: 10.1016/0008-8749(90)90308-e. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell T J, Ketley J M, Haslam S C, Stephen J, Burdon D W, Candy D C A, Daniel R. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut. 1986;27:78–85. doi: 10.1136/gut.27.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreira A L, Sampaio E P, Zmuidzinas A, Frindt P, Smith K A, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor α by enhancing mRNA degradation. J Exp Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nash S, Parkos C, Nusrat A, Delp C, Madara J L. In vitro model of intestinal crypt abscess—a novel neutrophil-derived secretagogue activity. J Clin Investig. 1991;87:1474–1477. doi: 10.1172/JCI115156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathan C F. Secretory products of macrophages. J Clin Investig. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohh M, Takei F. Regulation of ICAM-1 mRNA stability by cycloheximide: role of serine/threonine phosphorylation and protein synthesis. J Cell Biochem. 1995;59:202–213. doi: 10.1002/jcb.240590210. [DOI] [PubMed] [Google Scholar]
- 37.Pizza M, Covacci A, Bartoloni A, Perugine M, Nencione L, De Magistris M T, Villa L, Nucci D, Manetti R, Bugnoli M, Giovannoni F, Roberto O, Barbieri J T, Sato H, Rappuoli R. Mutants of pertussis toxin suitable for vaccine development. Science. 1989;246:497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- 38.Pothoulakis C. Pathogenesis of Clostridium difficile-associated diarrhoea. Eur J Gastroenterol Hepatol. 1996;8:1041–1047. doi: 10.1097/00042737-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Pothoulakis C, Castagliulo I, LaMont J T, Jaffer A, O’Keane J C, Snyder R M, Leeman S E. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pothoulakis C, LaMont J T. Clostridium difficile colitis and diarrhea. Gastroenterol Clin North Am. 1993;22:623–637. [PubMed] [Google Scholar]
- 41.Ribeiro R A, Flores C A, Cunha F Q, Ferreira S H. IL-8 causes in vivo neutrophil migration by a cell-dependent mechanism. Immunology. 1991;73:472–477. [PMC free article] [PubMed] [Google Scholar]
- 42.Rocha M F G, Maia M E T, Bezerra L R P S, Lyerly D M, Guerrant R L, Ribeiro R A, Lima A A M. Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: role of interleukin-1β, tumor necrosis factor alpha, and leukotrienes. Infect Immun. 1997;65:2740–2746. doi: 10.1128/iai.65.7.2740-2746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rola-Pleszcynski M, Pignol B, Pouliot C, Braquet P. Inhibition of human lymphocyte proliferation and IL-2 production by PAF (PAF-acether): reversal by specific antagonism, BN 52021. Biochem Biophys Res Commun. 1987;142:654–659. doi: 10.1016/0006-291x(87)91478-1. [DOI] [PubMed] [Google Scholar]
- 44.Schultz S G, Zalusky R. Ion transport in isolated rabbit ileum. Short-circuit current and Na fluxes. J Gen Physiol. 1964;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith P L, Chiossone D C, McCafferty G P. Characterization of LTC4 effects on rabbit ileal mucosa in vitro. Arch Pharmacol. 1990;341:94–100. doi: 10.1007/BF00195064. [DOI] [PubMed] [Google Scholar]
- 47.Smith P L, Montzka D P, McCafferty G P, Wasserman M A, Fondacaro J D. Effect of sulfidopeptide leukotrienes D4 and E4 on ileal ion transport, in vitro, in the rat and rabbit. Am J Physiol. 1988;255:G175–G183. doi: 10.1152/ajpgi.1988.255.2.G175. [DOI] [PubMed] [Google Scholar]
- 48.Souza M H L P, Melo-Filho A A, Rocha M F G, Lyerly D M, Cunha F Q, Lima A A M, Ribeiro R A. The involvement of macrophage-derived tumor necrosis factor and lipoxygenase products on the neutrophil recruitment induced by Clostridium difficile toxin B. Immunology. 1997;91:281–288. doi: 10.1046/j.1365-2567.1997.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan N M, Pellet S, Wilkins T D. Purification of toxins A and B of Clostridium difficile. Infect Immun. 1982;35:1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Towbin H, Schmitz A, Vanoostrum J, Seitz M, Dewald B, Zingel O, Motz J, Vosbeck K, Rordorf C. Monoclonal antibody based enzyme-linked and chemiluminescent assays for the human interleukin-1 receptor antagonist. Application to measure hIL-1ra levels in monocyte cultures and synovial fluids. J Immunol Methods. 1994;170:125–135. doi: 10.1016/0022-1759(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 51.Triadafilopoulos G, Pothoulakis C, LaMont J T. In vivo production of leukotriene B4 and prostaglandin E2 in experimental C. difficile colitis. Am J Gastroenterol. 1987;82:A950. [Google Scholar]
- 52.Triadafilopoulos G, Pothoulakis C, Weiss R, Giampaolo C, LaMont J T. Comparative study of Clostridium difficile toxin A and cholera toxin in rabbit ileum. Gastroenterology. 1989;97:1186–1192. doi: 10.1016/0016-5085(89)91689-2. [DOI] [PubMed] [Google Scholar]