Abstract
The objective of this study was to assess the feasibility of developing and applying a laboratory tool that can provide three-dimensional product structural information during freeze-drying and which can accurately characterize the collapse temperature (Tc) of pharmaceutical formulations designed for freeze-drying. A single-vial freeze dryer coupled with optical coherence tomography freeze-drying microscopy (OCT–FDM) was developed to investigate the structure and Tc of formulations in pharmaceutically relevant products containers (i.e., freeze-drying in vials). OCT–FDM was used to measure the Tc and eutectic melt of three formulations in freeze-drying vials. The Tc as measured by OCT–FDM was found to be predictive of freeze-drying with a batch of vials in a conventional laboratory freeze dryer. The freeze-drying cycles developed using OCT–FDM data, as compared with traditional light transmission freeze-drying microscopy (LT-FDM), resulted in a significant reduction in primary drying time, which could result in a substantial reduction of manufacturing costs while maintaining product quality. OCT–FDM provides quantitative data to justify freeze-drying at temperatures higher than the Tc measured by LT-FDM and provides a reliable upper limit to setting a product temperature in primary drying.
Keywords: lyophilization, freeze-drying, protein formulation, proteins, microscopy
INTRODUCTION
The collapse temperature (Tc) of an amorphous or partially amorphous formulation is an important parameter to consider for the development of a lyophilization cycle. The Tc is recognized as the maximum allowable temperature during freeze-drying of an amorphous product.1 At temperatures above the Tc, during primary drying, loss of freeze-dried cake structure is observed. This loss of structure frequently results in loss of product elegance, high residual water in the final product, possible product degradation (depending on degradation pathways), prolonged reconstitution times, and extended secondary drying times, all of which are either undesirable or unacceptable for product quality.1 Accurate measurement of the collapse is critical to freeze-drying process development. Because a 1°C temperature increase during primary drying can generally result in an approximate 10% reduction in primary drying time, it is of interest to optimize primary drying temperature at a value close to (but not exceeding) the Tc.2
The Tc can be estimated by light transmission freeze-drying microscopy (LT-FDM) and differential scanning calorimetry (DSC). LT-FDM is performed using a low-temperature freeze-drying stage. A small volume of solution (1–2 μL) is cooled to −40°C and frozen between two microscope cover slips. Pressure is lowered and the temperature is raised, typically at a ramp rate of 1°C/min, to initiate sublimation. As the temperature rises, a change in the structure of the freeze-dried solid signals the collapse event. Some evidence suggests that the Tc in a product vial may be different than in a thin film because of differences in ice nucleation causing differences in dry layer resistance and drying rate.1 The Tc is a dynamic event, which is dependent on viscous flow of the product; therefore, if drying occurs before the significant viscous flow occurs, the cake will not collapse even if the temperature is above the glass transition temperature (Tg′) of the solute phase. LT-FDM of a thin film may not be representative of these dynamic processes. As collapse in a thin film can often occur over a range of temperatures, how this range applies to collapse in a vial is not obvious. Both theoretical and experimental evidence suggest that freeze-drying in a vial is sufficiently different than in the two–dimensional (2D) sample in the freeze-drying microscope. The effective Tc for a product in a vial may be several degrees higher than found in the conventional freeze-drying microscopy.1
Differential scanning calorimetry is used to determine the Tg′ of the maximally freeze concentrated solution and is also used to estimate the Tc.1 The Tg′ is normally 1°C–3°C lower than the Tc; however, differences of 5°C–10°C have been reported, particularly in formulations that have high protein concentration.3 Using Tg′ as an estimate for the Tc generally results in a shelf temperature during primary drying that is more conservative than required, extending the freeze-drying time unnecessarily.
Several authors have reported on freeze-drying above the Tc as measured by LT-FDM and DSC. Most authors have found that protein stability is not affected by freeze-drying above the Tc,4-10 although decreased long-term stability has been reported for one protein system.11 For formulations that remain partially crystalline, it is known that the bulking agent (e.g., mannitol) may act as a scaffold for the amorphous product and complete cake collapse will be avoided.5,9,10 Fully amorphous formulations may undergo the collapse more readily than a partially crystalline formulation. However, even when the Tc measured by LT-FDM is exceeded during primary drying of a 100% amorphous product, the collapse of the lyophilized cake may not occur.4,7 This has been demonstrated for high protein concentration formulations in which viscous flow may be limited during freeze-drying presumably because of rapid secondary drying, which drives the Tg′ up more rapidly than the solute phase will undergo significant flow, preventing the collapse above the “measured” Tc.7,12 Therefore, as collapse may not occur for some formulations during a freeze-drying cycle, the Tc measured in a thin film by LT-FDM may not be relevant for all formulations.
Here, we introduce a new technique to estimate the Tc for freeze-drying in vials (or other product containers) by the use of a single-vial freeze dryer (SVFD) coupled with optical coherence tomography (OCT) for three-dimensional (3D) imaging. This technique is intended to circumvent the problems with DSC and LT-FDM by determining the Tc of a formulation freeze-drying in a vial, just as in an actual production operation.
Optical coherence tomography is a high-resolution optical imaging technique. OCT is the optical analog of ultrasound and has been historically used in the medical field for imaging of tissue samples.13 Crosssectional imaging is enabled by the measurement of the magnitude and echo time delay of backscattered light from the sample volume. Image resolutions of 1–15 μm can be achieved, and imaging can be performed in situ and in real time. The incident light beam is directed at the object to be imaged, and the time delay and magnitude of backscattered or back-reflected light are measured. The beam is scanned in the transverse direction, and rapid successive axial measurements are performed. The result is a 2D data set, which represents the optical reflection or backscattering in a cross-sectional plane through the material. Depth profiling is used to generate 3D images of the object.13
The goal of this study was to assess the feasibility of developing and applying a laboratory tool, which efficiently and accurately characterizes the Tc of pharmaceutical formulations designed for freeze-drying. A SVFD coupled with OCT–FDM is described here as a tool to investigate the structure and the Tc of formulations in pharmaceutically relevant products containers (i.e., freeze-drying in vials).
MATERIALS AND METHODS
Materials
Bovine serum albumin (BSA) was used as a model protein (Fract V; Fisher Sci, Pittsburgh, Pennsylvania). Solutions of BSA were dialyzed in 2 mM phosphate buffer. Sucrose and sodium chloride (NaCl) were used as obtained (Sigma Chemical, St. Louis, Missouri). Concentration of BSA was verified by UV analysis at 254 nm.
Freeze-Drying Microscopy
Traditional LT-FDM was performed using an FDCS-196 stage (Linkam, Tadworth, Surrey, UK) and optical microscope (Zeiss, Jena, Germany) equipped with a color video camera. One drop (1–2 μL) of solution was placed between two glass cover slips and the following procedure was used;
ramp at 10°C/min to −40°C and hold for 5 min,
pull vacuum to <100 mTorr,
ramp at 1°C/min until collapse is observed.
Differential Scanning Calorimetry
The glass transitions of the freeze concentrated solutions (Tg′) and freeze-dried powders (Tg) were determined using a TA instrument Q1000 (New Castle, Delaware). For the measurement of the Tg′, 10μL of solution in a sealed aluminum pan was equilibrated at −70°C and ramped to 20°C at 10°C/min. For the determination of Tg, approximately 5 mg of lyophilized powder was compacted into an aluminum pan and hermetically sealed in a dry glove bag (Relative Humidity < 5%). The samples were equilibrated at 0°C and heated at a rate of 2°C/min to 200°C with modulation amplitude and period of ±1°C and 100 s, respectively. The sharp change in heat capacity (endothermic shift in baseline) signals the glass transition. Samples runs were performed in duplicate.
Single-Vial Freeze Dryer OCT–FDM
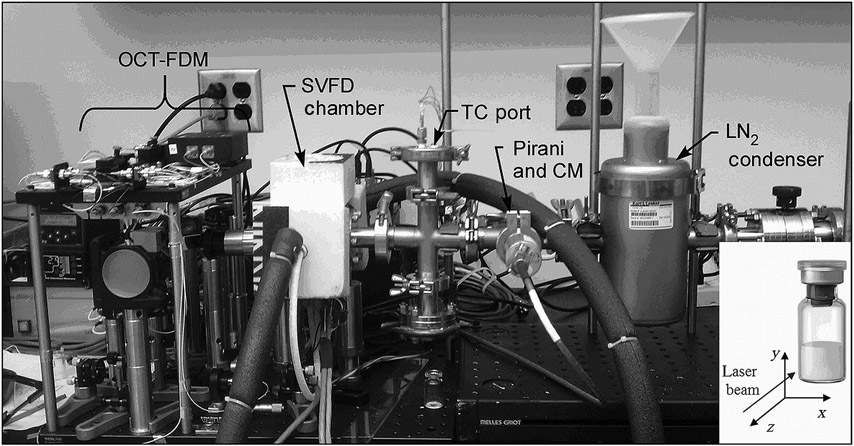
A SVFD equipped with OCT–FDM was designed and built for this study (Fig. 1).13 The freeze dryer consists of a chamber that holds a single 5 mL vial on a shelf, which is cooled using a thermoelectric cooler (TEC). A recirculating chiller flowing an ethylene glycol solution at −8°C was used to remove the heat from the back (hot) side of the TEC. The temperature of the shelf was measured using a type T thermocouple (TC), which enters the freeze-drying chamber through a port in the duct leading to the chamber. Two additional TCs were available at the port for the measurement of product temperature. TCs were calibrated using an ice-water suspension as a standard. A Pirani gauge and a capacitance manometer were connected through ports in the duct for pressure measurement. The condenser for the system consisted of a liquid-nitrogen-cooled cold trap. Noncondensable gas (air) was pumped from the product chamber using a vacuum pump (Hanning Elektro-Werke, Oerling-hausen, Germany) providing an ultimate low pressure of 2 mTorr. Custom control software was written to enable programmable temperature control of the SVFD and to synchronize shelf-temperature control and OCT image recording. This same software also recorded the temperature of the freeze dryer shelf and the product temperature within the vial. As pressure control is not believed to be important for the current mode of operation, where the product has near-perfect thermal contact with the shelf (using thermal grease between the bottom of the vial and the shelf), pressure control was not made available for the SVFD. Pressures range from 2 to 20 mTorr during a freeze-drying run.
Figure 1.
A photograph of the SVFD and OCT–FDM (TC, thermocouple; CM, capacitance manometer; LN, liquid nitrogen). (Inset) Coordinate system used to represent OCT–FDM data.
One side of the freeze-drying chamber was outfitted with a glass window to enable optical access for the OCT measurements. The following procedure was used for experiments in the SVFD OCT–FDM:
Spread heat transfer grease on the bottom of a freeze-drying vial containing the solution of interest.
Place the vial on the shelf, insert TCs into vials ensuring that one is touching the bottom, center of the vial.
Align the OCT imaging system by locating the vial edge.
Freeze the solution at a shelf temperature of −45°C.
After holding the temperature for 45 min at −45°C, pull vacuum on the system by starting the vacuum pump.
Fill the condenser chamber with liquid nitrogen.
After pressure in the chamber reached 20 mTorr, shut the butterfly valve and turn the vacuum pump off. Vacuum was maintained for all experiments. Water vapor vacuum pumping was accomplished by the liquid-nitrogen-cooled cold trap located upstream of the valve.
Raise temperature in steps to begin primary drying and collect OCT data.
The OCT–FDM was used to scan approximately 0.75 mm (width) × 4 mm (height) × 1.5 mm (depth into vial). The axial resolution was approximately 7.5μm, and the lateral resolution was approximately 6.9μm.13
Batch Freeze-Drying
Freeze-drying experiments were performed in a Lyostar II freeze dryer (SP Industries, Stone Ridge, New York). Two formulations were freeze-dried: 5% sucrose and 25% BSA–75% sucrose (5% total solids) at a high and low shelf temperatures based on collapse as measured from LT-FDM and OCT–FDM. The freezing step for each run was the same. The following protocol was used for freezing:
cool to 5°C at 1°C/min, hold for 15 min,
cool to −5°C at 1°C/min, hold for 15 min,
cool to −45°C at 1°C/min, hold for at least 1 h.
Primary drying shelf temperatures and pressures were determined according to either LT-FDM or OCT–FDM to demonstrate differences in process times and product quality results. For the low-temperature sucrose run, the shelf-temperature set point was −24°C, and the pressure was 65 mTorr. For the high-temperature sucrose run, the shelf-temperature set point was −8°C while the pressure was set at 65 mTorr. For the low-temperature BSA—sucrose run, the shelf temperature was set at −22°C with a pressure of 65 mTorr. The high-temperature BSA–sucrose formulation was run at a shelf temperature of 50°C and chamber pressure of 300 mTorr. The shelf temperature was set to 25°C for secondary drying of sucrose and 40°C for secondary drying of the low-temperature BSA–sucrose cycle. The shelf temperature for the high-temperature BSA–sucrose cycle remained at 50°C for secondary drying.
Specific Surface Area
Brunauer–Emmett–Teller (BET) specific surface area (SSA) analyzer Flowsorb II (Micromeritics Instrument Corporation, Norcross, Georgia) was used to measure the SSA of the freeze-dried samples. The instrument was calibrated with 100% krypton at room temperature, and the atmospheric pressure was recorded before the measurement. Each sample was degassed at 35°C under an atmosphere of 0.1 mol % krypton in helium for at least 4 h, and single-point adsorption was carried out at liquid-nitrogen temperature during which krypton was adsorbed, while helium served as the carrier gas. This was followed by desorption of adsorbed gas by immersing the sample holder in a water bath held at room temperature. Samples were performed in triplicate.
Residual Water
Residual water was determined by using Karl Fisher residual moisture analyzer (KF756; Metrohm, Riverview, FL). Freeze-dried cakes were reconstituted with 4 mL of dry methanol, and 0.5 mL of the solution was injected into the analyzer. Dry methanol blanks were run and were subtracted from sample titrant volumes. Weights of the solid were recorded to calculate the residual water in each vial. Three determinations were performed for each batch freeze-drying run.
Scanning Electron Microscopy
Freeze-dried powders were investigated using a Philips ESEM 2020 (Phillips, Amsterdam, The Netherlands) equipped with an EDAX CDU/SUTW thin window, energy-dispersive X-ray system (Z > 4 detection).
Reconstitution Time
Freeze-dried powders were reconstituted in 2 mL of filtered water. Samples were gently swirled, and reconstitution time was recorded after the last drop of water was added to the vial. Three determinations were performed for each batch freeze-drying run.
RESULTS AND DISCUSSION
Studies with a Sucrose Formulation: Comparisons Between Conventional FDM, DSC, and OCT
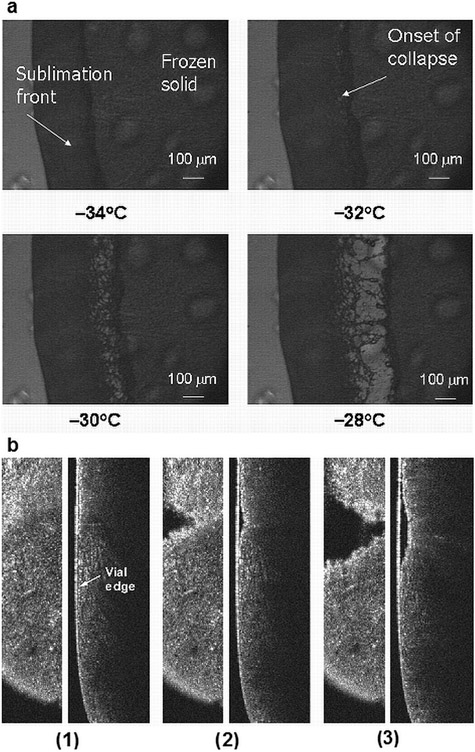
A 5% sucrose solution was used as a model formulation to investigate the Tc using LT-FDM, DSC, and OCT–FDM. The Tc for a 5% sucrose solution was found to be −32°C using LT-FDM (Fig. 2a).
Figure 2.
(a) Light transmission freeze-drying microscopy of a 5% sucrose solution. (b) OCT–FDM data for 0.6 mL of a 5% sucrose solution, (left) x–y cross section, (right) y–z cross section. (1) −30.5°C (step 3), (2) −28.9°C (step 4), and (3) −16.6°C (step 5) (video 1: x–y cross section and video 2: y–z cross section).
Figure 2a shows the intact sublimation front at −34°C. The onset of collapse is observed at −32°C. Complete loss of structure is observed at −28°C. A temperature ramping procedure was used to determine the Tc by LT-FDM; different procedures, such as step changes, may result in a more narrow difference between the onset of collapse and complete loss in structure. The Tg′ of a 5% sucrose solution was measured as −34°C using DSC (data not shown).
5% Sucrose: Initial Studies Showing Collapse
Optical coherence tomography–FDM was used to study the collapse of a 5% sucrose solution in a 5-mL freeze-drying vial using a solution volume of 0.6 mL. The shelf temperature was held at −45°C for 45 min and then raised to −40°C for 5 min. Vacuum was pulled and the temperature was raised by 2°C every 6 min to −10°C to facilitate sublimation. OCT measurements at each temperature step were completed in 5 min. The product temperature was recorded using the computer-controlled data acquisition system monitoring a TC located in the bottom center of the vial.
The coordinate system used to describe the OCT data is shown in Figure 1, inset. 2D images of the frozen solution, freeze-dried solid, and the sublimation front are visible using OCT–FDM (Fig. 2b). The reported temperatures are from the TC located in the bottom center of the vial. The intact frozen solid and sublimation front are shown in Figure 2b, frame 1 at −30.5°C. At −28.9°C (Fig. 2b, frame 2), we observed gaps in the solid in the x–y cross section (en face view as would be expected in a “melt” or collapse). In the y–z view, the solid appears to be separating from the vial wall. At −16.6°C (frame 3), the gap in the solid has expanded, indicating significant changes in the product structure. In the video of the OCT–FDM for this formulation, there is complete separation of the freeze-dried solid from the frozen solid, signifying complete collapse of the formulation. Video 1 (see Supplementary Material) shows the collapse in the x–y direction, whereas video 2 (see Supplementary Material) shows the collapse in the y–z direction. Using these data, the onset of collapse is interpreted as −28.9°C, the temperature when gaps in the cake are first observed. Although the shelf temperature in steps 4 and 5 differs by only 2°C, we observe that the product temperature increases dramatically after the onset of collapse. The experiment was repeated twice and this temperature increase occurred in each run after the onset of collapse. The reason for this increase is not obvious and is discussed below.
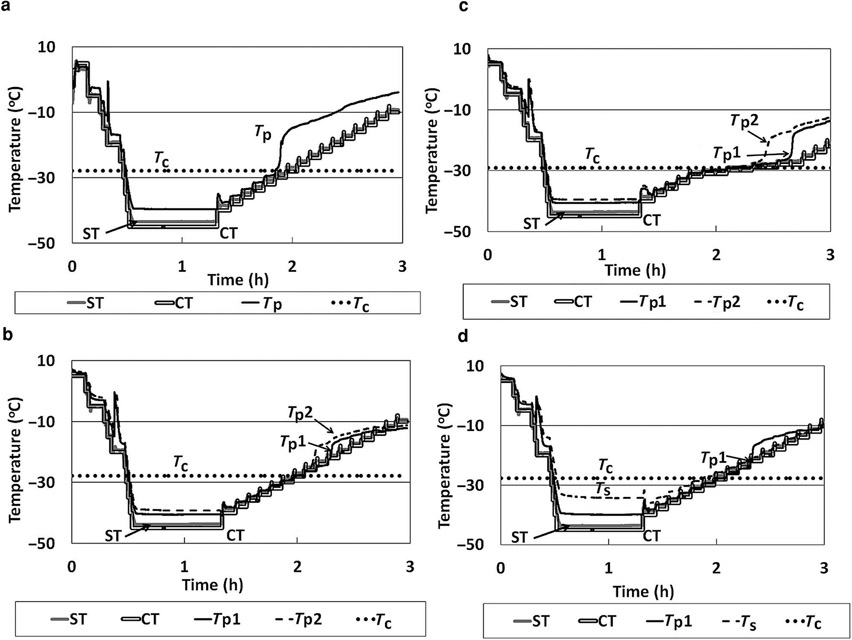
The temperature profile of the OCT–FDM for 5% sucrose is shown in Figure 3a. The product temperature closely corresponds to the shelf temperature throughout the freezing step and during early portions of the primary drying step. It is clear in the temperature profile that the temperature of the frozen solid diverges from (i.e., becomes greater than) the shelf temperature shortly after the onset of collapse. This type of temperature trend normally indicates loss of contact of the temperature sensor with the subliming ice, at least with conventional freeze-drying, but this interpretation may not be valid in the present situation. We return to this issue later. For three independent runs (n = 3), the mean Tc of 0.6 mL of a 5% sucrose solution was found to be −28.4 ± 0.8°C. Therefore, the Tc observed using OCT–FDM is 3.6°C higher than that found using LT-FDM, which is qualitatively consistent with theoretical expectations.1
Figure 3.
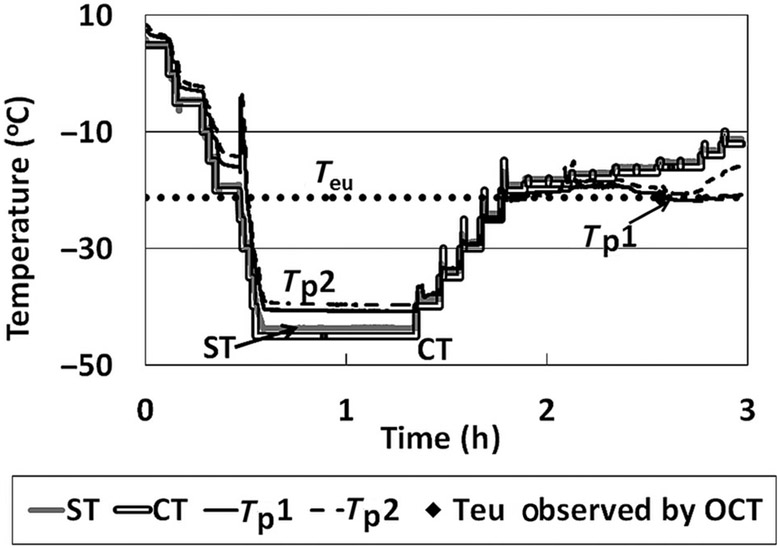
(a) Temperature data for OCT–FDM of a 5% sucrose solution, 0.6 mL. (b) Temperature data for OCT–FDM of a 5% sucrose solution, 1.25 mL. (c) Temperature data for OCT–FDM of a 5% sucrose solution, 1.25 mL slow ramp rate. (d) Temperature data for OCT–FDM of a 5% sucrose solution, 1.25 mL TC on side of the vial. ST, shelf temperature; CT, commanded temperature; Tp, product temperature; Tp1, product temperature 1; Tp2, product temperature 2; and Ts, temperature side of vial.
Impact of Protocol Variation on Collapse Observation with 5% Sucrose: Fill Depth Variation
The solution volume used for OCT–FDM experiments was increased from 0.6 to 1.25 mL. The depth of 0.6 mL solution in a 5 mL vial was low (~5 mm), which made TC placement challenging. The volume was increased to ensure good contact between the frozen solution and TC throughout the entire experiment. By increasing the solution volume, a second TC reading was also possible. TC 1 was placed on the bottom center of the vial, whereas TC 2 was placed 3 mm above TC 1. The shelf temperature was held at −45°C for 45 min and then raised to −40°C for 5 min. Vacuum was pulled and the temperature was raised by 2°C every 6 min to −10°C to facilitate sublimation. The onset of collapse was determined as described above. The Tc for 1.25 mL of a 5% sucrose solution was found to be −28.9°C, which is in close agreement with the previous measurements. Videos 3 and 4 show the x–y and y–z views of the collapse (see Supplementary Material). Product shrinkage from the wall is visible, followed by complete collapse. The temperature profile for the experiment is shown in Figure 3b. Similar to the experiments with a smaller solution volume (and depth), the product temperature and the shelf temperature diverge after the onset of collapse. This observation indicates that the occurrence of this event is not dependent on the solution volume used. The experiment was repeated, and the onset of collapse was found to be −27.5°C.
Impact of Protocol Variation on Collapse Observation with 5% Sucrose: Variation of Rate of Temperature Increase
To further study a 5% sucrose solution using the OCT–FDM, the freeze-drying recipe was changed such that close to the Tc the effective ramp rate was reduced to 1°C/step rather than 2°C/step as used in the previous experiments and held for 10 min. The solution volume was 1.25 mL. Using this procedure, the Tc was found to be −30.0°C. Videos 5 and 6 show the collapse in the x–y and y–z directions (see Supplementary Material). The temperature profile is shown in Figure 3c. Similar to the experiments performed at the faster ramp rate, the product temperature diverges from the shelf temperature after the onset of collapse. Therefore, this temperature event that occurs after the collapse does not appear to be dependent on ramp rate.
Impact of Protocol Variation on Collapse Observation with 5% Sucrose: Measurement of Glass Temperature
Lastly, in an effort to further study the abrupt product temperature increase that occurs after collapse, OCT–FDM was used to measure the Tc of 5% sucrose solution (1.25 mL) with a TC placed at the bottom center of the vial and a TC adhered to the outside of the vial. This was performed in an effort to evaluate the effect of radiation from the glass window of the freeze-drying chamber on drying. It is possible that radiation from the uncooled freeze-drying chamber wall could cause radial drying of the cake. This could potentially cause the cake to dry from the side of the vial through the bottom of the cake to the center where the temperature measurement takes place. If this was to occur, separation of the frozen system from the TC would cause the temperature deviation seen in the temperature profiles of the SVFD. The shelf temperature was held at −45°C for 45 min and then raised to −40°C for 5 min. Vacuum was pulled and the temperature was raised by 2°C every 6 min to −10°C to facilitate sublimation. The onset of collapse was found to be −28.7°C, very similar to previous experiments. Videos 7 and 8 show the x–y and y–z views of the collapse (see Supplementary Material). The temperature profile is shown in Figure 3d. As shown in the temperature profile, the deviation of the product temperature from the shelf temperature occurs at approximately −24°C. The temperature of the side of the vial follows the shelf temperature and product temperature within about 1° for the duration of the experiment, which is almost exactly the same difference as we observe for vials in the center of an array, attributable to lack of perfect contact of the external TC with the product, suggesting that radial gradients in product temperature and drying rate are likely not the cause for the abrupt product temperature deviation. Moreover, we do measure the correct eutectic temperature for NaCl. Radial gradients in product temperature will be considered for future studies. We tentatively conclude that there may be several causes for the product temperature deviation from the shelf temperature, including heating by conduction from ambient temperatures through the TC, resulting in loss of thermal contact between the ice and the TC, thereby causing the measured temperature to abruptly rise. Also, increased cake resistance because of collapse resulting in heat accumulation in the solid may also be an explanation, although not a likely cause in view of the fact that at the solute concentrations used here, the usual result of collapse is reduced resistance. Further experimentation is needed to verify the true cause.
A total of seven experiments using 5% sucrose were performed using the OCT–FDM. The average Tc was found to be −28.4 ± 0.9°C. The standard deviation indicates that the Tc as measured by OCT–FDM is a precise measurement. The Tc measured by OCT–FDM is 3.6°C higher than the Tc measured by LT-FDM. A freeze-drying cycle based on this Tc would reduce the length of primary drying time by about 45% compared with a cycle based on the Tc as measured by LT-FDM or DSC.
NaCl Formulation Comparison Between Conventional FDM and OCT: Validation of Temperature Measurement Via Characterization of Eutectic Melt
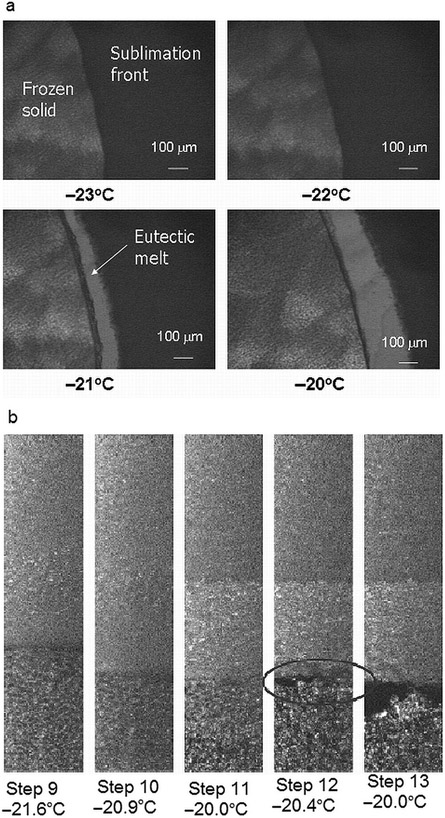
Collapse of a freeze-dried product is not a thermodynamic event. Many factors may affect the measurement of the collapse such as method of measurement, temperature ramping, resolution of the microscope, and so on. To verify the accuracy of the OCT–FDM temperature measurement, the eutectic melt of a 5% NaCl solution was measured. Because this event is a thermodynamic event, it will occur at the same temperature, regardless of the method used. The eutectic melt of NaCl is reported to be −21.1°C.14 LT-FDM was used to measure the eutectic melt, which occurred at −21°C (Fig. 4a).
Figure 4.
(a) Light transmission freeze-drying microscopy of a 5% NaCl solution. (b) OCT–FDM of the eutectic melt of a 5% NaCl solution, x–y direction (video 9—x–y cross section, video 10—y–z cross section).
The eutectic melt of a 5% NaCl solution was measured using OCT–FDM in the SVFD. A solution volume of 1.25 mL was used. The shelf temperature was held at −45°C for 45 min and then raised to −40°C for 5 min. Vacuum was pulled and the temperature was raised by 2°C every 6 min to −10°C. The eutectic melt temperature was determined in the same manner as the Tc. OCT–FDM shows gaps in the frozen solution at −20.4°C (Fig. 4b). This was taken as the eutectic melt temperature. Videos 9 and 10 show the x–y and y–z views of the melt (see Supplementary Material).
The experiment to measure the eutectic melt of a 5% NaCl solution using the OCT–FDM was repeated using a procedure in which the temperature ramp rate was reduced close to the eutectic melt temperature. The shelf temperature was held at −45°C for 45 min and then raised to −40°C for 5 min. Vacuum was pulled, and the temperature was raised by 5°C every 6 min for four steps and then raised by 1°C and held for 6 min for the subsequent steps until the melt was observed.
The eutectic melt was observed at −21.2°C (videos 11 and 12, x–y and y–z views; Supplementary Material). This value is in excellent agreement with literature observations and the LT-FDM measurements, indicating the accuracy of OCT–FDM temperature measurement. The temperature profile for this experiment is shown in Figure 5.
Figure 5.
Temperature data for OCT–FDM of a 5% NaCl solution. ST, shelf temperature; CT, commanded temperature; Tp1, product temperature 1; Tp2, product temperature 2; and Teu, eutectic melt temperature.
Studies with a Disaccharide-Based Protein Formulation, BSA–Sucrose: Comparisons Between LT-FDM and OCT–FDM
The Tc of a BSA–sucrose formulation (25% BSA, 75% sucrose, 5% total solids) was measured using DSC, LT-FDM, and OCT–FDM. The Tg′, which is used as an estimate for Tc, was found to be −28°C by DSC (data not shown). The onset of collapse for BSA–sucrose was found to be −28°C by LT-FDM (Fig. 6a). Complete collapse is observable at temperatures above −28°C.
Figure 6.
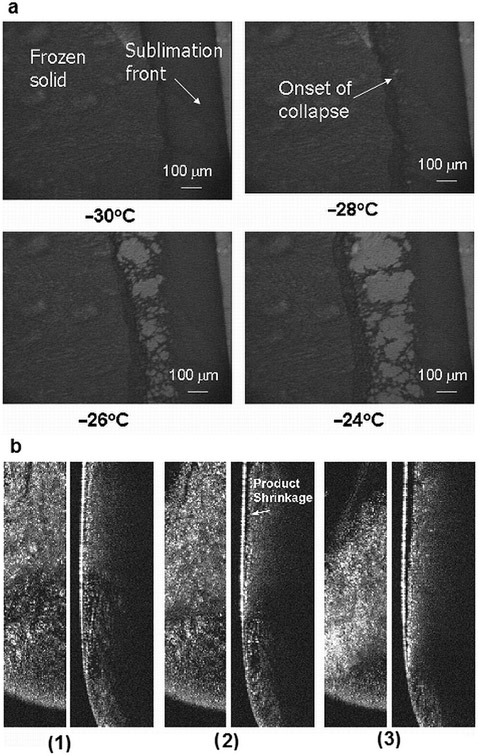
(a) Light transmission freeze-drying microscopy of a 5% BSA–sucrose solution. (b) OCT–FDM for BSA–sucrose formulation. (1) −24.3°C, (2) −22.3°C, and (3) −15.2°C. (Left) x–y view, (right) y–z view (video 13-–x–y cross section, video 14—y–z cross section).
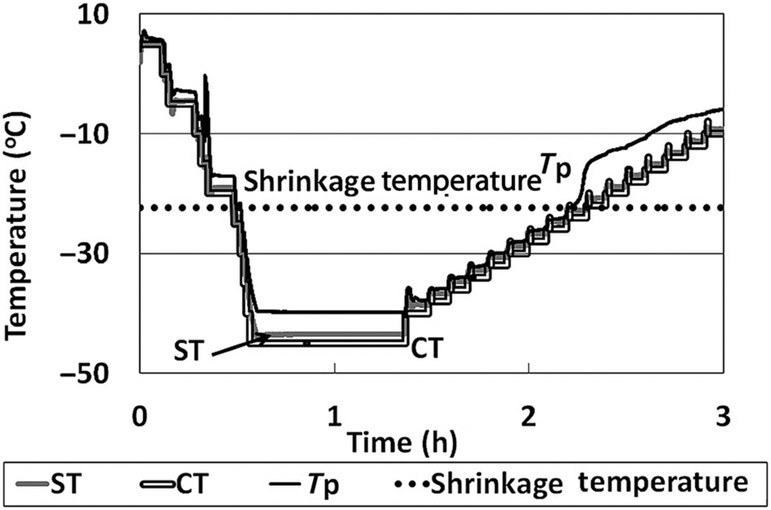
For the OCT–FDM experiments, the shelf temperature was held at −45°C for 45 min and then raised to −40°C for 5 min. Vacuum was pulled, and the temperature was raised by 2°C every 6 min to −10°C to facilitate sublimation. A solution volume of 0.6 mL was used for the experiments. Unexpectedly, the BSA–sucrose formulation did not exhibit a collapse as measured by OCT–FDM (Fig. 6b; videos 13 and 14, Supplementary Material). The solid appeared to slightly pull away from the vial wall beginning at −22.3°C (Fig. 6b, frame 2), which would be considered to be product shrinkage. As the product temperature increases, the product continues to pull away from the vial wall (Fig. 6b, frame 3). The temperature profile for this formulation in the SVFD is similar to the sucrose-only formulation. The product temperature deviates from the shelf temperature shortly after the temperature where the initial product shrinkage is observed (Fig. 7).
Figure 7.
Temperature profile for the OCT–FDM of BSA–sucrose formulation: a system that freeze dries without collapse by OCT but with collapse with conventional FDM.
Product shrinkage is not normally considered unacceptable in a freeze-dried product and is not expected to impact any critical product quality attributes. The OCT–FDM results are unexpected given that a product collapse for the BSA–sucrose formulation is clearly observed using LT-FDM, as well at a clear Tg′ at the same temperature. The experiment was repeated, and again collapse was not observed in any temperature range studied (up to ≈−10°C). Product shrinkage was observed at −24.6°C. These data suggest that freeze-drying of this formulation may be executed at a temperature much higher than the Tc as measured by LT-FDM (−28°C), which would result in a much shorter primary drying cycle.
The collapse temperatures as measured by the OCT–FDM and LT-FDM were used to develop primary drying cycles for sucrose and BSA–sucrose formulations in an effort to compare the measured collapse temperatures with collapse behavior in vials freeze-dried in a laboratory freeze dryer.
Batch Freeze-Drying: Cycles Developed Using Conventional FDM and OCT–FDM
Sucrose and BSA–sucrose formulations were freeze-dried in a Lyostar II freeze dryer (SP Industries) utilizing one full tray of vials. Two freeze-drying runs were performed for each formulation: a high-temperature run developed using data from the OCT–FDM and a low-temperature run developed using data from the conventional FDM.
Sucrose Freeze-Drying Runs
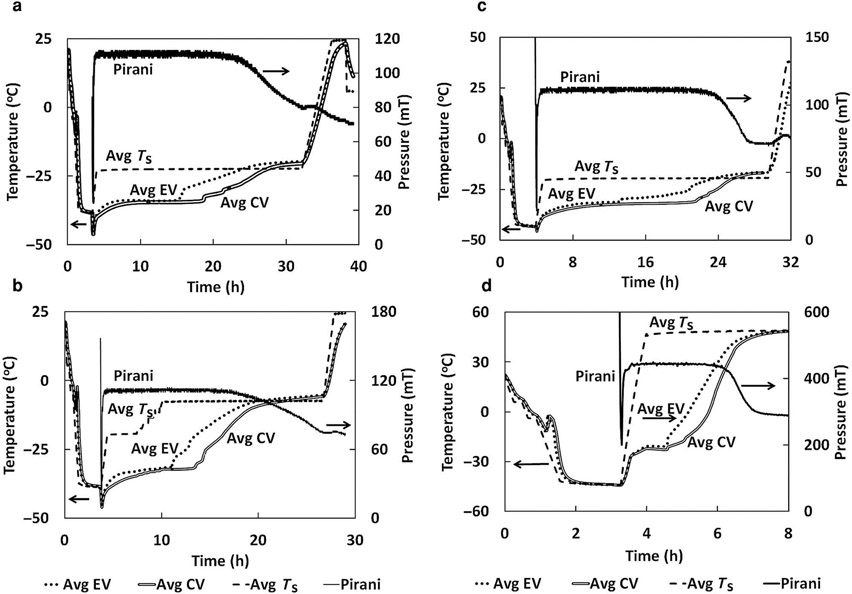
As shown in Table 1, the high-temperature freeze-drying cycle for sucrose was run with an average product temperature of −32°C, the onset of collapse as measured by conventional LT-FDM. The maximum product temperature that was observed in this freeze-drying run was −31°C, which is slightly above the Tc for a 5% sucrose formulation as measured by LT-FDM (−32°C) but below the OCT–FDM value of −28°C. The maximum product temperature in the low-temperature cycle was −34°C, below the collapse temperatures measured by OCT–FDM and LT-FDM. The high-temperature cycle resulted in a primary drying time reduction of approximately 20% (Figs. 8a and 8b).
Table 1.
Batch Freeze-Drying Results for Sucrose and BSA–Sucrose Formulations
| Formulation | Maximum Tp Primary Drying (°C) |
Average Tp Primary Drying (°C) |
Tg (°C) | Residual Water (%) | SSA (m2/g) |
|---|---|---|---|---|---|
| Sucrose high temperature | −31 | −32 | 53.6 ± 1.4 | 3.1 ± 0.5 | 0.778 ± 0.117 |
| Sucrose low temperature | −34 | −34 | 65.5 ± 0.85 | 1.8 ± 0.3 | 0.875 ± 0.176 |
| BSA–Sucrose high temperature | −22 | −23 | 65.6 ± 1.2 | 1.7 ± 0.2 | 0.138 ± 0.011 |
| BSA–sucrose low temperature | −34 | −34 | 72.2 ± 2.3 | 1.2 ± 0.4 | 0.760 ± 0.030 |
No collapse observed for any of the freeze-drying runs.
Tp, product temperature.
Figure 8.
Temperature and pressure data from freeze-drying runs. (a) Sucrose low-temperature cycle. (b) Sucrose high-temperature cycle. (c) BSA–sucrose low-temperature cycle. (d) BSA–sucrose high-temperature cycle (EV, edge vial, CV, center vial, Ts, shelf temperature).
Macroscopically, vials from both the high- and low-temperature runs show no indication of collapse; freeze-dried cake structures remained intact for both batch runs (Fig. 9a). Minor shrinkage of the freeze-dried cakes was observed for both cycles. However, scanning electron microscopy (SEM) images show only very slight differences in pore structure and morphology. The material that was freeze-dried at a higher shelf temperature appears to perhaps have more heterogeneous, larger pores than the material freeze-dried below the LT-FDM Tc, but the differences are very minor. These results are not exclusive to this formulation. Freeze-drying above and below the Tc as measured by LT-FDM has previously been shown to exhibit different morphology by SEM,15-18 with the structural changes shown by freeze-drying above the LT-FDM Tc often being termed “microcollapse.”
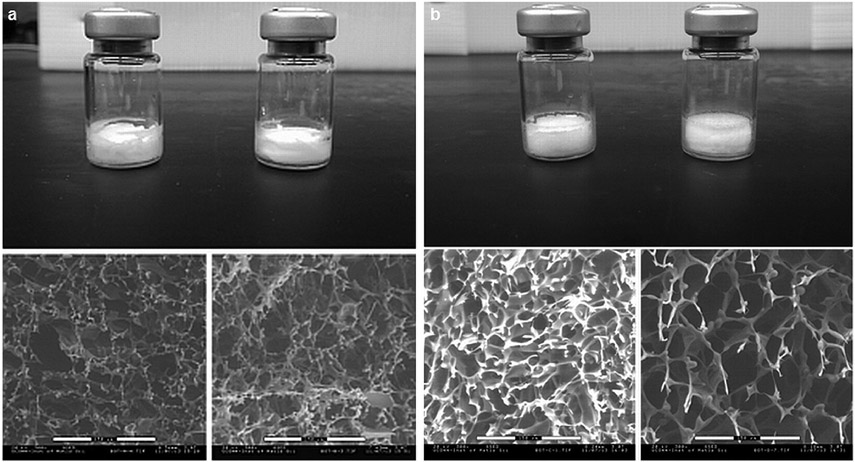
Figure 9.
(a) 5% Sucrose, top: freeze-dried product in vials of 5% sucrose; left—sample lyophilized at −34°C product temperature, right—sample lyophilized at −31°C maximum product temperature; bottom: SEM images (300×) of 5% sucrose. Left—Sample lyophilized at −34°C maximum product temperature, right—sample lyophilized at −31°C maximum product temperature. (b) BSA–sucrose, top: freeze-dried product in vials of BSA–sucrose; left—sample lyophilized at −34°C maximum product temperature, right—sample lyophilized at −22°C maximum product temperature, bottom: SEM images (300×) of BSA-sucrose, left—sample lyophilized at −34°C product temperature, right—sample lyophilized at −22°C maximum product temperature.
The sucrose formulation freeze-dried at a higher shelf temperature had a lower Tg than the material freeze-dried at a lower shelf temperature (Table 1). This difference is consistent with the differences in residual water (Table 1). It is well known that as the residual water increases, the Tg will decrease for the same formulation.17,19,20 Here, the secondary drying process was quite “mild,” and lower water contents could easily be achieved by higher temperatures and longer times in secondary drying. These physical attributes may or may not impact product stability. Thus, so far, no generalizations have been made on the impact on stability of freeze-drying near or above the Tc. Stability has been evaluated on a case by case basis,4,9,17,19,21 with the result generally (but not always) being that stability is not affected. Reconstitution times for samples freeze-dried above and below the LT-FDM Tc were the same. Freeze-dried sucrose samples were reconstituted in 5 s, almost immediately on exposure to water. The SSA of the freeze-dried powders was not significantly different. The main point for this study, however, is that the OCT–FDM studies are in agreement with batch freeze-drying data, that is, collapse or no collapse is correctly predicted by the OCT–FDM, but not by the LT-FDM. Even with a maximum product temperature above the LT-FDM Tc, no evidence of macroscopic collapse was observed for a 5% sucrose formulation.
BSA–Sucrose Freeze-drying Runs
The BSA–sucrose formulation was freeze-dried such that the average product temperature, −23°C, for the high-temperature cycle was well above the Tc as measured by conventional FDM, −28°C (Table 1). The low-temperature cycle had an average product temperature of −34°C. The freeze-drying cycle developed using OCT–FDM data resulted in an 80% reduction in primary drying time (Figs. 8c and 8d). This is a huge reduction in processing time, which could result in a significant reduction of manufacturing costs. As shown in Figure 9b, although product shrinkage was observed for both freeze-drying cycles, there was no evidence of collapse in either cycle, high and low temperature. The material that was freeze dried at a higher shelf temperature appears to have larger pores than the material freeze dried below the LT-FDM Tc. As mentioned earlier, freeze-drying above and below the Tc as measured by LT-FDM has previously been shown to exhibit different morphology by SEM,15-18 with the structural changes shown by freeze-drying above the LT-FDM Tc being termed “microcollapse.” From the SEM and surface area data presented here, it is probable that some “microcollapse” occurred. Microcollapse in this case did not result in full collapse and the product was still considered “pharmaceutically elegant.” Although in the high-temperature cycle, maximum product temperatures were 6°C above the Tc measured by LT-FDM, the freeze-dried cake remained intact, pharmaceutically elegant, and visually essentially identical to the product produced by the low-temperature cycle. These results are in exact agreement with OCT–FDM data, which show no collapse for this formulation in the temperature range tested. Again, the conventional technique, the Tc measured by LT-FDM, does not agree with batch freeze-drying data, but OCT-FDM is in agreement with batch freeze-drying in a vial, which will scale to full-scale manufacturing.
Surface area was significantly lower for the BSA—sucrose formulation freeze-dried at the high shelf temperature than at the lower shelf temperature. This indicates that microcollapse may have occurred during freeze-drying, as viscous flow would be expected to change pore structure and decrease surface area.1 Microcollapse is suggested to occur when freeze-drying is performed between the onset of collapse as measured, Ton, and full collapse, Tfc.3,4,17 The onset of collapse for this formulation was observed at −28°C, whereas full collapse was observed at −24°C (Fig. 6). The maximum temperature observed during freeze-drying for the high-temperature cycle was −22°C, which is even above the “full Tc” for this formulation, demonstrating that OCT–FDM gives much better prediction of batch freeze-drying behavior (i.e., collapse) in vials than LT-FDM. Such a decrease in surface area is generally accompanied with an increase in residual moisture because of the slower water desorption rate during secondary drying.20 However, here, increased residual moisture was not observed for the high-temperature cycle as the moisture content for the high- and low-temperature cycles are not significantly different (p > 0.05). The Tg values of the BSA–sucrose powders freeze-dried above and below the LT-FDM collapse were also not statistically different (p > 0.05). Many authors have reported freeze-drying in the microcollapse regime without detriment.4,6,15,16,18,19,22,23 Although microcollapse or some viscous flow of the freeze concentrate, likely occurred for the high-temperature freeze-drying cycle, it did not have an effect on the macroscopic structure of the freeze-dried cake, which remained pharmaceutically elegant. Reconstitution times for samples dried above and below the Tc as measured by LT-FDM were the same. For each cycle, high and low temperature, freeze-dried powders were reconstituted in approximately 10 s. In short, freeze-drying a full 6°C above the LT-FDM Tc did not change any of the critical quality attributes measured. Note that although it is instructive to compare SSA and SEM, neither is a critical quality attribute.
In summary, freeze-drying cycles were developed using collapse temperatures as measured by LT-FDM and OCT–FDM. Maximum product temperatures observed for the higher temperature cycles were above collapse temperatures as measured by LT-FDM. No collapse was observed in any of the batch freeze-drying cycles, which is in agreement with OCT–FDM data but not in agreement with LT-FDM. Freeze-drying cycles based on OCT–FDM data, rather than LT-FDM data, resulted in significant reduction in primary drying times, which would be expected to result in considerable manufacturing cost savings.
CONCLUSIONS
In this study, we have demonstrated the use of OCT–FDM to measure the Tc and eutectic melt of three formulations in freeze-drying vials. The Tc as measured by OCT–FDM was predictive of freeze-drying with a batch of vials in a conventional laboratory freeze dryer. The freeze-drying cycles developed using OCT–FDM data resulted in a significant reduction in primary drying time, which could result in a significant reduction of manufacturing costs while maintaining product quality.
This imaging system is particularly useful for generating data to aid in efficient lyophilization process design, in which the primary drying product temperature is run as close to the actual Tc as possible. Other authors have reported freeze-drying above the Tc measured by LT-FDM without detriment to product quality.4-9 OCT–FDM provides quantitative data to justify freeze-drying at temperatures higher than the Tc measured by LT-FDM and an upper limit to setting a product temperature in primary drying. Because the OCT–FDM system provides in situ imaging of the product during freeze-drying, it may be useful to explore other applications of the technology such as investigation of ice structure during freeze-drying.
ACKNOWLEDGMENTS
The project described was supported by award number R43EB010317 from the National Institute of Biomedical Imaging and Bioengineering.
The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
Footnotes
Additional Supporting Information may be found in the online version of this article. Supporting Information
REFERENCE
- 1.Pikal MJ, Shah S. 1990. The collapse temperature in freeze drying: Dependence on measurement methodology and rate of water removal from the glassy phase. Int J Pharm 62(2–3):165–186. [Google Scholar]
- 2.Tang X, Pikal MJ. 2004. Design of freeze-drying processes for pharmaceuticals: Practical advice. Pharm Res 21(2):191–200. [DOI] [PubMed] [Google Scholar]
- 3.Meister E, Gieseler H. 2009. Freeze-dry microscopy of protein/sugar mixtures: Drying behavior, interpretation of collapse temperatures and a comparison to corresponding glass transition Data. J Pharm Sci 98(9):3072–3087. [DOI] [PubMed] [Google Scholar]
- 4.Colandene James D, Maldonado Linda M, Creagh Alma T, Vrettos John S, Goad Kenneth G, Spitznagel Thomas M. 2007. Lyophilization cycle development for a high-concentration monoclonal antibody formulation lacking a crystalline bulking agent. J Pharm Sci 96(6):1598–1608. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RE, Kirchhoff CF, Gaud HT. 2002. Mannitol–sucrose mixtures—Versatile formulations for protein lyophilization. J Pharm Sci 91(4):914–922. [DOI] [PubMed] [Google Scholar]
- 6.Lewis LM, Johnson RE, Oldroyd ME, Ahmed SS, Joseph L, Saracovan I, Sinha S. 2010. Characterizing the freeze-drying behavior of model protein formulations. AAPS PharmSciTech 11(4):1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel S. 2012. Peptalk. 2012: The protein science week. Coronado, California. [Google Scholar]
- 8.Schersch K, Betz O, Garidel P, Muehlau S, Bassarab S, Winter G. 2010. Systematic investigation of the effect of lyophilizate collapse on pharmaceutically relevant proteins I: Stability after freeze-drying. J Pharm Sci 99(5):2256–2278. [DOI] [PubMed] [Google Scholar]
- 9.Wang DQ, Hey JM, Nail SL. 2004. Effect of collapse on the stability of freeze-dried recombinant factor VIII and alpha-amylase. J Pharm Sci 93(5):1253–1263. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee K, Shalaev EY, Suryanarayanan R. 2005. Partially crystalline systems in lyophilization: II. Withstanding collapse at high primary drying temperatures and impact on protein activity recovery. J Pharm Sci 94(4):809–820. [DOI] [PubMed] [Google Scholar]
- 11.Passot Sp, Fonseca F, Barbouche N, Marin Ml, Alarcon-Lorca M, Rolland D, Rapaud M. 2007. Effect of product temperature during primary drying on the long-term stability of lyophilized proteins. Pharm Dev Technol 12(6):543–553. [DOI] [PubMed] [Google Scholar]
- 12.Johnson R, Lewis L. 2011. Freeze-drying protein formulations above their collapse temperatures: Possible issues and concerns. Am Pharm Rev 14(3):50–54. [Google Scholar]
- 13.Mujat M, Greco K, Galbally-Kinney KL, Hammer DX, Ferguson RD, Iftimia N, Mulhall P, Sharma P, Pikal MJ, Kessler WJ. 2011. Optical coherence tomography-based freeze-drying microscopy. Biomed Opt Express 3(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean J. 1985. Lange’s handbook of chemistry. 13th ed. New York: McGraw-Hill. [Google Scholar]
- 15.Parker A, Rigby-Singleton S, Perkins M, Bates D, Le Roux D, Roberts Clive J, Madden-Smith C, Lewis L, Teagarden Dirk L, Johnson Robert E, Ahmed Saleem S. 2010. Determination of the influence of primary drying rates on the microscale structural attributes and physicochemical properties of protein containing lyophilized products. J Pharm Sci 99(11):4616–4629. [DOI] [PubMed] [Google Scholar]
- 16.Overcashier DE, Patapoff TW, Hsu CC. 1999. Lyophilization of protein formulations in vials: Investigation of the relationship between resistance to vapor flow during primary drying and small-scale product collapse. J Pharm Sci 88(7):688–695. [DOI] [PubMed] [Google Scholar]
- 17.Schneid SC, Stärtzel PM, Lettner P, Gieseler H. 2011. Robustness testing in pharmaceutical freeze-drying: Interrelation of process conditions and product quality attributes studied for a vaccine formulation. Pharm Dev Technol 16(6):583–590. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RE, Oldroyd ME, Ahmed SS, Gieseler H, Lewis LM. 2010. Use of manometric temperature measurements (MTM) to characterize the freeze-drying behavior of amorphous protein formulations. J Pharm Sci 99(6):2863–2873. [DOI] [PubMed] [Google Scholar]
- 19.Schersch K, Betz O, Garidel P, Muehlau S, Bassarab S, Winter G. 2010. Systematic investigation of the effect of lyophilizate collapse on pharmaceutically relevant proteins I: Stability after freeze-drying. J Pharm Sci 99(5):2256–2278. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter JF, Pikal MJ, Chang BS, Randolph TW. 1997. Rational design of stable lyophilized protein formulations: Some practical advice. Pharm Res 14(8):969–975. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee K, Shalaev Evgenyi Y, Suryanarayanan R. 2005. Partially crystalline systems in lyophilization: II. Withstanding collapse at high primary drying temperatures and impact on protein activity recovery. J Pharm Sci 94(4):809–820. [DOI] [PubMed] [Google Scholar]
- 22.Barresi AA, Ghio S, Fissore D, Pisano R. 2009. Freeze drying of pharmaceutical excipients close to collapse temperature: Influence of the process conditions on process time and product quality. Dry Technol 27(6):805–816. [Google Scholar]
- 23.Fonseca F, Passot S, Cunin O, Marin M. 2004. Collapse temperature of freeze-dried Lactobacillus bulgaricus suspensions and protective media. Biotechnol Prog 20(1):229–238. [DOI] [PubMed] [Google Scholar]