Abstract
The increased intestinal absorption induced by epidermal growth factor (EGF) is associated with diffuse lengthening of brush border microvilli. The aim of this study was to examine the in vivo effects of oral administration of EGF during infection with enteropathogenic Escherichia coli. New Zealand White rabbits (4 weeks old) received orogastric EGF daily starting 3 days prior to infection with enteropathogenic E. coli RDEC-1 and were compared with sham-treated infected animals and uninfected controls. Weight gain, food intake, fecal E. coli, and stool consistency were assessed daily. On day 10, segments of jejunum, ileum, proximal, and distal colon were assessed for gram-negative bacterial colonization, disaccharidase activities, and epithelial ultrastructure. Effects of EGF on E. coli RDEC-1 proliferation were studied in vitro. E. coli RDEC-1 caused diarrhea and reduced weight gain. Seven days postinfection, the small and large intestines were colonized with numerous bacteria, brush border microvilli were disrupted, and maltase and sucrase activities were significantly reduced in the jejunum. Daily treatment with EGF prevented the occurrence of diarrhea and reduction of weight gain. These effects were associated with significant inhibition of E. coli colonization in the small and large intestine, improved jejunal maltase and sucrase activities and reduced microvillous injury. EGF did not affect the proliferation of E. coli in vitro. The findings suggest that EGF protects the gastrointestinal tract against colonization by enteropathogenic E. coli.
Epidermal growth factor (EGF), a 53-amino-acid polypeptide, is synthesized in the salivary glands and kidneys, and, to a lesser extent, in the lactating mammary glands, small intestine, liver, and pancreas (2, 34). EGF has been implicated in the regulation of a number of physiological and pathophysiological processes, including cellular proliferation (44), wound repair (3), neoplasia (1), and gastrointestinal maturation (4, 30). Studies in vivo and in vitro have shown that in the intestine, EGF stimulates DNA synthesis and ornithine decarboxylase (12), accelerates brush border disaccharidase maturation (13, 15), and up-regulates absorption of amino acids and carbohydrates (4, 19, 37). EGF exerts its bioactivities in the small as well as the large intestine (22). In the small intestine, the EGF-induced enhancement of nutrient transport is associated with an overall increase in brush border surface area (17, 30). A closely related peptide which also binds to the EGF receptor, transforming growth factor alpha, does not produce this effect, implying that the biological effects of EGF are ligand specific (18).
The interaction of pathogens with the enteric brush border membrane of the host plays a seminal role in the pathogenesis of diarrhea in a number of viral and bacterial infections. Although the plasmid-encoded AF/R1 pilus adhesion of RDEC-1 binds to a transmembrane receptor that is distinct from the EGF receptor (36), Salmonella typhimurium, reovirus, and vaccinia virus appear to initiate cellular invasion, at least in part, by binding to the EGF receptor (11, 14, 38). EGF has been shown to promote mucosal healing in experimental colitis and gastroduodenal ulcers in rats, and a recent report suggested that oral EGF administration may accelerate intestinal recovery in piglets infected with rotavirus (24, 35, 46). A number of studies suggest that luminal EGF is a potent stimulant for mucosal repair in the damaged gastrointestinal tract (32). However, the effects of EGF on epithelial colonization by an enteric pathogen have not been assessed.
Enteropathogenic E. coli infections are thought to be the leading cause of death from bacterium-mediated diarrheal disease worldwide, and symptoms are most severe in young children from developing countries (25). Enteropathogenic E. coli is an example of a pathogen that induces diarrhea by mechanisms distinct from enterotoxin production or tissue invasion. The infection produces attaching-effacing lesions characterized by loss of microvillous structures at sites of bacterial attachment (25). This injury is associated with brush border enzyme deficiencies, sodium malabsorption, and chloride secretion which, combined, are responsible for the malabsorptive-secretory diarrhea seen in enteropathogenic E. coli infections (28, 40, 41). Malabsorptive diarrhea implicating diffuse injury to the epithelial brush border also results from infections with other enteropathogens, including Giardia sp. and Yersinia enterocolitica, as well as in intestinal anaphylaxis and Crohn’s disease (5, 6, 8, 10).
Clearly, EGF has numerous physiological benefits on the intestinal mucosa and profound effects on epithelial turnover and microvillous ultrastructure. The aim of this study was to examine the effects of oral EGF administration on intestinal colonization with enteropathogenic E. coli.
MATERIALS AND METHODS
Bacterial strain.
Noninvasive, attaching-effacing E. coli O15 (RDEC-1) obtained from the American Type Culture Collection was used in all experiments. This strain causes diarrhea in rabbits without producing heat-labile or heat-stable enterotoxins, similarly to enteropathogenic E. coli in humans (25). Stock cultures of RDEC-1 bacteria were stored at −70°C in tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.) coated onto Microbank porous beads (Pro-Labs Diagnostics, Richmond Hill, Ontario, Canada). At the time of study, one bead was deposited into TSB, E. coli was allowed to grow overnight, and late-log-phase bacteria were suspended in 10% sodium bicarbonate in phosphate-buffered saline (PBS) at a concentration of 2 × 106 CFU/ml as determined by spectrophotometry (405 nm).
Animal model.
The model used in this study has been described by Cantey and Blake (7). Human recombinant EGF (Austral Biologicals, San Ramon, Calif.) was used in all experiments. A number of reports have established that human EGF is cross-reactive in several other species, including rabbits (4, 13, 17, 19, 22, 24, 29, 30, 35). Weanling 4-week-old New Zealand White rabbits (mean weight, 709 ± 28 g) were assigned to three experimental groups: (i) infected treated (given 60 μg of orogastric EGF in 5 ml of sterile PBS daily for 10 days and inoculated with 107 E. coli RDEC in 5 ml of 10% NaHCO3 on day 3 [n = 9]); (ii) infected untreated (given sterile PBS daily and inoculated with 107 E. coli RDEC on day 3 [n = 10]); and (iii) controls (given sterile PBS daily and 10% NaHCO3 on day 3 [n = 9]). All inocula were delivered orogastrically with a 5-in. blunt feeding needle. Rabbits were housed individually at 24°C, 40% humidity, and 12-h:12-h photoperiods, and they received water and pellets (Unifeed, Calgary, Alberta, Canada) ad libitum.
Weight gain, food intake (calculated from the weight of chow remaining after each day of feeding), and signs of diarrhea were assessed daily. Diarrhea was recorded as present when either perineal soiling could be seen or soft and unformed stool was recovered from rectal swabs. All observations were recorded between 9:00 and 11:00 a.m. to minimize diurnal variations. Fecal passage of E. coli RDEC-1 was assessed from daily rectal swabs plated onto MacConkey agar (Difco). Fecal shedding was recorded as positive when lactose-fermenting gram-negative bacteria were recovered on MacConkey plates after overnight incubation at 37°C or as negative when no lactose-fermenting bacteria grew on MacConkey plates after overnight incubation. Animals were killed on day 10 (i.e., 7 days after E. coli infection or sham inoculation) by an intraperitoneal overdose of sodium pentobarbital (Euthanyl; MTC Pharmaceuticals, Cambridge, Ontario, Canada). Segments (total of 12 cm) of proximal jejunum (2 cm distal to the ligament of Treitz), ileum (12 cm proximal to the ileocecal junction), proximal colon (distal to the appendix), and distal colon (15 cm proximal to the rectum) were removed and prepared for measurement of disaccharidase activity and mucosal wet weights, bacterial counts, and transmission electron microscopy. All experimental procedures were carried out according to the guidelines of the University of Calgary Committee on Animal Care and Use and the Canadian Council of Animal Care.
Bacterial recovery.
Bacterial recovery was assessed from quantitative cultures performed on intestinal segments flushed with sterile PBS and homogenized in sterile PBS. Viable lactose-fermenting E. coli cells from intestinal homogenates were counted by serial dilution and culture on MacConkey agar for 18 h at 37°C. Bacterial colonization was expressed as log10 CFU per centimeter of gut. The difference between bacterial numbers in segments from EGF-treated and untreated animals was calculated and expressed as a percentage of the average number of microorganisms found in the samples from the infected untreated group. This number was referred to as E. coli clearance.
Mucosal disaccharidases.
Mucosa was scraped with a glass slide, weighed, homogenized in 2.5 mM EDTA, and frozen at −70°C until analyzed for enzyme activities. To assess the effects of the various experimental treatments on overall mucosal content per unit of intestinal length, weights of mucosal scrapings (i.e., mucosal wet weights) were expressed in milligrams per centimeter of gut. Maltase and sucrase were measured by the method of Dahlqvist (9). Protein concentration was assessed by a Bradford protein assay (Bio-Rad Laboratories, Hercules, Calif.), and enzyme activities were expressed as units per gram of protein.
Transmission electron microscopy.
Immediately after removal, intestinal tissues for transmission electron microscopy were immersed into 5% glutaraldehyde in Sorensen’s phosphate buffer, cut into 1-mm cubes, and stored in the fixative overnight at 4°C. Specimens were washed in buffer, postfixed in 1% OSO4, dehydrated in ethanol, cleared with propylene oxide, and embedded in Spurr’s low-viscosity medium (J.B. EM Services Inc., Dorval, Quebec, Canada). Sections (90 nm) were stained with saturated uranyl acetate in 50% ethanol, followed by 0.4% lead citrate (43). Specimens were examined on a Hitachi 7000 transmission electron microscope at 80 kV. To standardize observations, small intestinal villi were assessed from midvillus areas only, as determined by a low-magnification observation of an entire villus. To avoid observer bias, micrographs were coded before ultrastructural assessment. For each intestinal region, observations were generated from three to five grids obtained from each of five tissue blocks.
Direct effects of EGF on bacteria in vitro.
The effects of EGF on bacterial growth were determined in vitro. In a series of three experiments, log-phase E. coli RDEC-1 bacteria (103 CFU/ml) were added in duplicate to wells on a 96-well plate containing TSB, with either no EGF (control) or various concentrations of EGF (0.1, 1, 10, and 100 μM), in a total volume of 100 μl/well. These concentrations were chosen to reflect the variable physiological range of EGF levels that may be encountered by enteric bacteria in vivo (16). At 1-h intervals (0 to 5 h postinoculation), viable E. coli cells in each well were counted by serial dilution and culture on MacConkey agar plates for 18 h at 37°C. Bacterial numbers were expressed as log10 CFU per milliliter.
Statistical analysis.
Results were expressed as means ± standard error of the mean (SEM) and compared by one-way analysis of variance, followed by Student-Newman-Keuls test for multiple-comparison analysis. P <0.05 levels were considered significant.
RESULTS
Clinical observations.
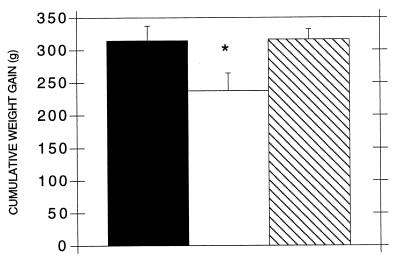
Four of ten infected untreated animals showed signs of diarrhea. Rabbits from the infected EGF-treated and control groups remained asymptomatic. Cumulative weight gain from days 1 to 7 postinfection or sham inoculation was significantly lower in infected untreated animals than in controls (Fig. 1). Weight gain in infected EGF-treated animals was significantly higher than in infected untreated animals and not different from that in controls (Fig. 1). Food intake was not significantly different between any group at any day of the study (data not shown). E. coli was isolated from rectal swabs of infected untreated and treated animals within 24 to 72 h of infection, and E. coli excretion in the stool persisted for the duration of the study. E. coli was not detected in controls. Postmortem, three infected untreated animals, but none from the other groups, showed evidence of a fluid-filled bowel.
FIG. 1.
Cumulative weight gain in control (■), infected untreated (□), or infected EGF-treated ( ) rabbits from days 1 to 7 after infection with E. coli RDEC-1 or sham inoculation. ∗, P < 0.05 versus control and EGF-treated rabbits. Values are means ± SEM.
) rabbits from days 1 to 7 after infection with E. coli RDEC-1 or sham inoculation. ∗, P < 0.05 versus control and EGF-treated rabbits. Values are means ± SEM.
Bacterial recovery.
Viable E. coli counts obtained in intestinal specimens from untreated and EGF-treated animals after 7 days of infection are illustrated in Table 1. Bacterial numbers in the jejunum, ileum, and proximal colon of treated rabbits were significantly lower than in untreated animals. Highest bacterial clearance in EGF-treated animals was observed in the jejunum and ileum (92.0 and 94.7%, respectively [Table 1]). Differences in bacterial numbers recovered from distal colon samples failed to reach statistical significance between untreated and treated animals. No E. coli organisms were recovered from any intestinal sample in control rabbits.
TABLE 1.
Effects of EGF on live bacterial recovery from intestinal mucosal homogenates of rabbits infected for 7 days with E. coli RDEC-1
| Group (n) | Mean live bacterial recovery (log10 CFU/cm of gut) ± SEMa
|
|||
|---|---|---|---|---|
| Jejunum | Ileum | Proximal colon | Distal colon | |
| Infected untreated (10) | 4.19 ± 0.26 | 6.16 ± 0.26 | 4.92 ± 0.29 | 5.16 ± 0.19 |
| Infected EGF treated (9) | 3.10 ± 0.41* | 4.88 ± 0.23** | 4.01 ± 0.16* | 4.50 ± 0.28 |
| % Clearanceb | 92.0 | 94.7 | 85.5 | 78.0 |
Calculated from serial dilutions of homogenates plated onto MacConkey agar. No bacteria were recovered from any of the nine control rabbits. ∗, P < 0.05 untreated compared with EGF treated; ∗∗, P < 0.01 untreated compared with EGF treated.
Calculated from absolute numbers of bacteria recovered per centimeter of gut as (total number of bacteria in untreated sample/total number of bacteria in EGF-treated sample)/total number of bacteria in untreated sample.
Disaccharidases and mucosal weights.
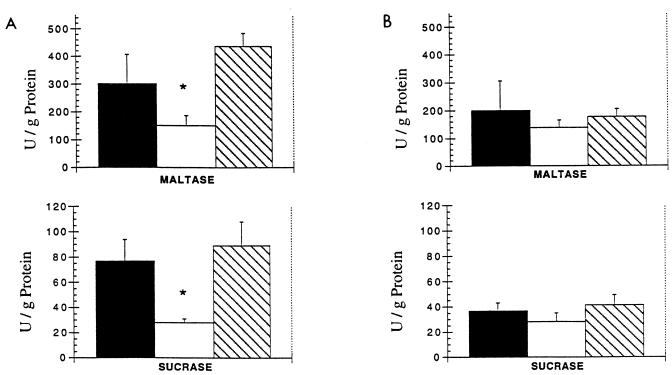
Mucosal maltase and sucrase activities are illustrated in Fig. 2. Maltase and sucrase activities were significantly reduced in the jejunum of untreated animals infected for 7 days with E. coli compared to values obtained from controls. Jejunal enzyme activities in infected animals treated with EGF were significantly higher than in infected untreated animals and were not different from activities in controls. In the ileum, control disaccharidase levels were lower than in the jejunum and enzyme activities were not significantly affected by experimental treatments. Protein contents in mucosal homogenates were not different between any experimental groups at any site (data not shown). Mucosal wet weights of jejunal, ileal, and proximal and distal colonic tissue are shown in Table 2. Wet weights did not differ significantly between any groups in any of the intestinal segments.
FIG. 2.
Maltase and sucrase activities in jejunum (A) and ileum (B) of control (■), infected untreated (□), or infected EGF-treated ( ) rabbits 7 days after infection with E. coli RDEC-1 or sham inoculation. ∗, P < 0.05 versus control and EGF-treated rabbits. Values are means ± SEM.
) rabbits 7 days after infection with E. coli RDEC-1 or sham inoculation. ∗, P < 0.05 versus control and EGF-treated rabbits. Values are means ± SEM.
TABLE 2.
Effects of EGF on mucosal wet weights in the small and large intestines of rabbits infected with E. coli RDEC-1
| Sample (n) | Mean mucosal wet wt (mg/cm) ± SEa
|
|||
|---|---|---|---|---|
| Jejunum | Ileum | Proximal colon | Distal colon | |
| Control (9) | 121.6 ± 18.3 | 110.2 ± 8.8 | 228.2 ± 18.0 | 52.1 ± 3.6 |
| Infected untreated (10) | 115.3 ± 10.6 | 93.8 ± 9.8 | 195.4 ± 13.5 | 53.8 ± 2.9 |
| Infected EGF treated (9) | 156.5 ± 9.6 | 112.4 ± 9.3 | 233.7 ± 17.5 | 67.9 ± 7.8 |
Differences between experimental groups did not reach statistical significance in any segment of the intestine.
Electron microscopy.
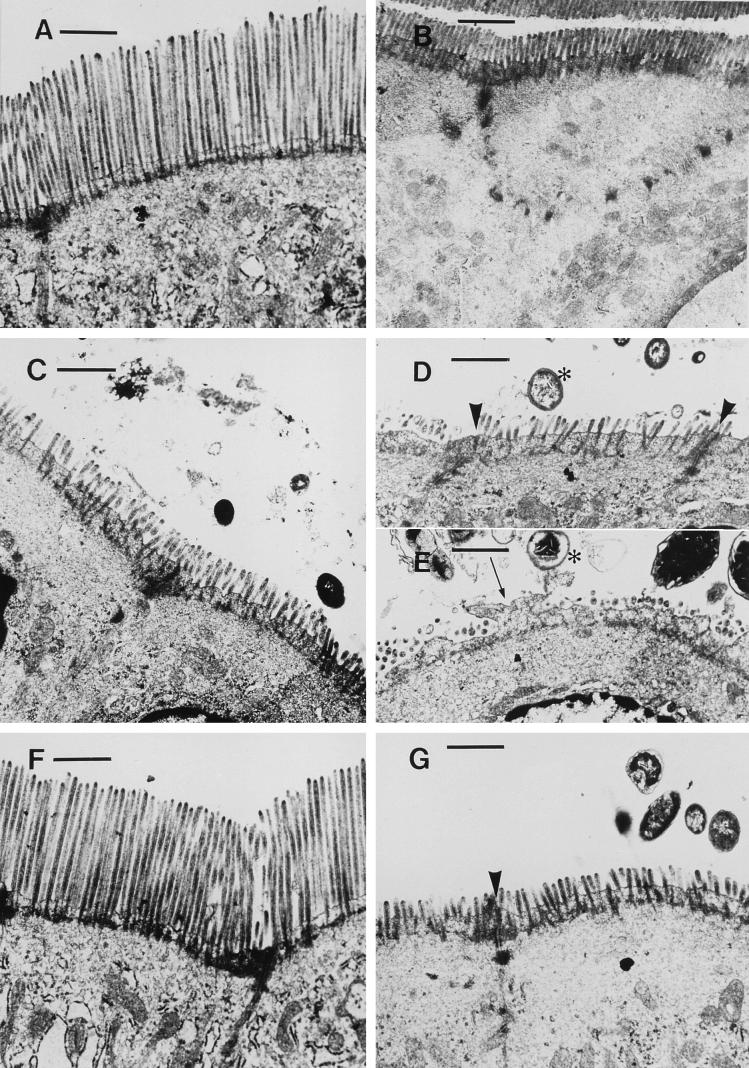
Jejunal, ileal, and proximal and distal colonic samples from three animals in each group were assessed with transmission electron microscopy. Representative results from these observations are illustrated in Fig. 3. After 7 days of E. coli infection, numerous gram-negative bacteria were observed in the small and large intestines of infected animals. Bacteria were seen in close association with microvilli, and a number of enterocytes exhibited apical pseudopods devoid of microvilli. Adding to this alteration to the apical membrane, microvilli were shortened along the entire epithelial surface or, in some areas, totally deleted. In samples from EGF-treated rabbits, gram-negative bacteria were found less frequently, and brush border abnormalities were less apparent than in untreated animals. Overall, epithelial ultrastructure in EGF-treated rabbits was similar to that observed in controls (Fig. 3).
FIG. 3.
Representative transmission electron micrographs (at the same magnification) from jejunum (A, C, and F) and proximal colon (B, D, E, and G) of control (A and B), infected untreated (C to E), or EGF-treated (F and G) rabbits 7 days after infection with E. coli RDEC-1 or sham inoculation. In tissues from infected untreated animals, ultrastructural observations revealed colonization by gram-negative bacteria (∗), localized deletion of individual microvilli, formation of apical pseudopods devoid of microvilli on enterocytes (arrow), and generalized shortening of microvilli along the entire epithelial surface in the jejunum (C). Bacteria did not invade the epithelium and did not compromise the integrity of junctional complexes (arrowheads). In specimens from the EGF-treated group, gram-negative bacteria were seen less frequently, and disruptions to the epithelial brush border were inhibited. In the jejunum of EGF-treated infected rabbits (F), microvillous length was restored to that observed in tissues from controls (A). Bar = 1 μm.
Direct effects of EGF on bacteria in vitro.
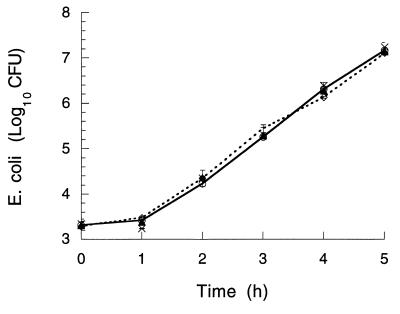
To assess whether the EGF-induced bacterial clearance observed in vivo was due to an antibacterial effect of EGF, growth of E. coli RDEC-1 was assessed in vitro in the presence or absence of EGF. In growth medium without EGF, bacterial numbers increased by 4 logs in 5 h (Fig. 4). No difference in bacterial proliferation was observed when 0.1, 1.0, 10, or 100 μM EGF was added to the medium (Fig. 4).
FIG. 4.
In vitro proliferation of E. coli RDEC-1 in the absence (○) or presence (•) of 10 μM EGF added to the medium. Similar results were obtained when 0.1, 1, or 100 μM EGF was added to the preparations (data not shown). Values are means ± SEM from three experiments measured in duplicate for each group. No significant difference was observed between values of each group at any time.
DISCUSSION
This study used an animal model of rabbits infected with enteropathogenic E. coli to examine the potential benefits of oral EGF administration in the management of gastrointestinal infections. Compared to control animals, untreated rabbits infected with E. coli showed signs of diarrhea and reduced weight gain. After 7 days of infection, high numbers of bacteria colonized the small and large intestine of these animals. Infection was associated with a diffuse disruption to brush border microvilli, and maltase and sucrase activities were significantly impaired in the jejunum. Oral treatment with EGF prevented diarrhea and reduction in weight gain. EGF-treated animals also demonstrated significant bacterial clearance in the small intestine and proximal colon (up to 94.7% bacteria cleared versus untreated). Future studies will assess whether the failure of EGF to significantly reduce bacterial colonization in the distal colon may have resulted at least in part from decreased luminal concentrations of unbound EGF as the peptide transits through the entire length of the gastrointestinal tract. Administration of EGF also prevented the brush border injury and reversed the impairment of jejunal maltase and sucrase activities seen in infected rabbits. Epithelial ultrastructure and disaccharidase activities in EGF-treated infected animals did not differ from control values. EGF alone did not affect multiplication or survival of live bacteria in vitro. The results suggest that EGF administration may be useful in the control of intestinal infection with E. coli and that the benefits of EGF are not due to a direct antibacterial effect.
Results from this study confirm the previous clinical and pathological observations on the disease induced in rabbits orally infected with attaching-effacing E. coli RDEC-1 (7, 25). In the present study, 4 of 10 animals developed diarrhea. Other authors previously reported 100% rates of diarrhea in animals subjected to similar experimental conditions (45). The fact that these authors assigned a diarrheal score to an animal when soft pellets were found in its cage, while the present experimental design required unformed stool from rectal swabs or perineal soiling, may explain this discrepancy. These experiments also demonstrate that orogastric administration of EGF (100 μg/kg of body weight daily) prevents bacterial colonization and the subsequent establishment of disease in weanling rabbits. The dosage of EGF used in these experiments is consistent with EGF concentrations delivered to intestinal segments in previous studies (17, 19, 29) and is within the highly variable physiological concentrations of EGF measured in the mature intestine during feeding (16). Following a meal, EGF concentrations in the jejunum can exceed 100 ng/ml (16). In normal weanling mammals, intestinal EGF is significantly lower, and the amount of EGF produced in the salivary glands is known to increase after puberty (23, 27). A recent report demonstrated that oral EGF increases lactase activity in the mid-small intestine of neonatal piglets infected with rotavirus (46). In that study, however, little or no improvement was measured in alkaline phosphatase, leucine aminopeptidase, or maltase activities and, most notably, EGF treatment did not prevent the occurrence of diarrhea in rotavirus-infected piglets (46). Moreover, the effects of EGF administration on viral colonization were not measured. The present report demonstrates for the first time that EGF administration inhibits intestinal colonization by a pathogen and prevents the subsequent development of diarrhea.
The diarrhea seen during infection with enteropathogenic E. coli is a result of both chloride secretion and sodium malabsorption (5, 28, 41). EGF has the ability to inhibit chloride secretion and stimulate sodium absorption by alterating brush border phosphatidylinositol 3-kinase (21, 42). Furthermore, reduction of brush border microvillus height is a direct cause of malabsorptive diarrhea in a number of intestinal diseases (5, 6, 8, 10, 25). Previous reports have described that E. coli RDEC-1 induces a characteristic attaching-effacing lesion on the epithelial brush border (7, 25). In the present study, jejunal colonization with E. coli RDEC-1 was associated with generalized disruption of the microvillous brush border, as characterized with formation of apical microvilli-free pseudopods on enterocytes and focal deletion of individual microvilli. The present report adds to the spectrum of E. coli RDEC-1-mediated injury a diffuse shortening of brush border microvilli. In the jejunum, these alterations to brush border microvilli were associated with significant impairment of disaccharidase activities. The findings suggest that oral administration of EGF reduces bacterial colonization of the intestinal epithelium, which in turn prevents the brush border lesions associated with enteropathogenic E. coli infections in rabbits.
Reports have indicated that in some species, including humans, EGF receptors on enterocytes are located on the basolateral membrane only (33), while they may be found on the apical membrane in others (20). Regardless, the EGF receptor is known to be involved in the initial attachment of some viral and bacterial organisms onto their target cells. Binding to the EGF receptor and activation of its intrinsic tyrosine kinase are required for cellular entry by reovirus (38). Further, occupancy of the EGF receptor on fibroblasts by synthetic peptides of the EGF family inhibits vaccinia virus infection in a dose-dependent fashion (11). Other studies have shown that invasion of epithelial cells by S. typhimurium involves binding to the EGF receptor and subsequent phosphorylation of its intrinsic tyrosine kinase (14). In contrast, it has been demonstrated that E. coli RDEC-1 binds, via AF-R1 pili, to an enterocytic membrane glycoprotein receptor complex that is distinct from the EGF receptor (36). This observation suggests that the EGF-mediated inhibition of intestinal colonization by E. coli seen in the present study implicates mechanisms which are distinct from competitive binding with the EGF receptor.
Cytokines may directly affect microorganisms. In addition to the classical antiviral effects of interferon, it has been shown that tumor necrosis factor alpha may be lethal to Pneumocystis carinii and capable of altering virulence properties of gram-negative bacteria (26, 31). As results from this study demonstrated that oral EGF reduces the colonization by pathogenic E. coli in the gut, further experiments sought to assess whether EGF had direct antibacterial effects. EGF alone did not affect E. coli proliferation in vitro, which implies that this peptide improves bacterial clearance via mechanisms which are independent of a direct bactericidal or bacteriostatic effect.
The potential value of using EGF as a therapeutic tool to treat gastrointestinal injury has been raised previously. EGF may accelerate healing of gastric ulcers and colitis (24, 35); systemic administration of EGF successfully treated children with necrotizing enteritis (39); and there is experimental evidence to support a therapeutic role for EGF in the management of short gut syndrome (29). Together, these observations suggest that EGF may be a potent stimulant for gut repair. The present study provides evidence that oral EGF therapy may also inhibit colonization of the intestinal tract by pathogens and hence be useful in the control of infectious diarrhea.
In summary, the findings demonstrate that oral EGF administration inhibits the production of diarrhea and reduction in weight gain seen in weanling rabbits infected with attaching-effacing E. coli. In the intestine, EGF significantly reduces epithelial colonization by this pathogen, decreases brush border injury, and suppresses disaccharidase impairment. The mechanisms whereby EGF induces these effects have yet to be uncovered, but findings reported herein indicate that they may operate independently from competitive binding to the EGF receptor or a direct antibacterial effect of the polypeptide. Adding to the well-described role of EGF as a repair and maturation stimulant in the gut, these observations suggest a role for EGF in protecting the gastrointestinal tract from colonization by bacterial pathogens.
ACKNOWLEDGMENTS
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada, the Medical Research Council of Canada, and the Alberta Agricultural Research Institute. A. Buret is funded by the Margaret Gunn Endowment for Animal Health Research.
We thank Alex Chin, Dorota Kamieniecki, and Wayne Jansen for technical assistance.
REFERENCES
- 1.Bans-Schlegel S P, Quintero J. Human esophageal carcinoma cells have fewer but higher affinity epidermal growth factor receptors. J Biol Chem. 1986;261:4359–4362. [PubMed] [Google Scholar]
- 2.Barnard J A, Beauchamp R D, Russell W E, Dubois R N, Coffey R J. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995;108:564–580. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 3.Brown G L, Nanney L B, Griffen J, Cramer A B, Yancey J M, Curtsinger L J, Holtzin L, Schultz G S, Jurkiewicz M J, Lynch J B. Enhancement of wound healing by topical treatment with epidermal growth factor. Lancet. 1989;ii:76–79. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- 4.Buchmiller T L, Shaw K S, Chopourian H L, Lloyd K C K, Gregg J P, Rivera F A, Lam M L, Diamond J M, Fonkalsrud E W. Effect of transamniotic administration of epidermal growth factor on fetal rabbit small intestinal nutrient transport and disaccharidase development. J Pediatr Surg. 1993;28:1239–1244. doi: 10.1016/s0022-3468(05)80305-7. [DOI] [PubMed] [Google Scholar]
- 5.Buret A, Hardin J A, Olson M E, Gall D G. Pathophysiology of small intestinal malabsorption in gerbils (Meriones unguiculatus) infected with Giardia lamblia. Gastroenterology. 1993;103:506–513. doi: 10.1016/0016-5085(92)90840-u. [DOI] [PubMed] [Google Scholar]
- 6.Buret A, O’Loughlin E V, Curtis G, Gall D G. Effect of acute Yersinia enterocolitica infection on small intestinal ultrastructure. Gastroenterology. 1990;98:1401–1407. doi: 10.1016/0016-5085(90)91068-h. [DOI] [PubMed] [Google Scholar]
- 7.Cantey J R, Blake R K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977;135:454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- 8.Curtis G H, Patrick M K, Catto-Smith A G, Gall D G. Intestinal anaphylaxis in the rat: effect of chronic antigen exposure. Gastroenterology. 1990;98:1558–1566. doi: 10.1016/0016-5085(90)91090-s. [DOI] [PubMed] [Google Scholar]
- 9.Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem. 1964;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak A M. Ultrastructural pathology in Crohn’s disease. In: Goebell H, Peskar B M, Malchow H, editors. Inflammatory bowel disease—basic research and clinical implications. Lancaster, England: MTP Press Ltd.; 1988. pp. 3–42. [Google Scholar]
- 11.Eppstein D A, Marsh Y V, Schreiber A B, Newman S R, Todaro G J, Nestor J J. Epidermal growth factor receptor occupancy inhibits vaccinia virus infection. Nature. 1985;318:663–664. doi: 10.1038/318663a0. [DOI] [PubMed] [Google Scholar]
- 12.Fehlman E J, Anres D, Grossman M I. Epidermal growth factor stimulates ornithine decarboxylase activity in the digestive tract of mouse. Proc Soc Exp Biol Med. 1978;159:400–402. doi: 10.3181/00379727-159-40357. [DOI] [PubMed] [Google Scholar]
- 13.Foltzer-Jourdaine C, Garaud J C, Nsi-Emvo E, Raul F. Epidermal growth factor and the maturation of intestinal sucrase in suckling rats. Am J Physiol. 1993;265:G459–G466. doi: 10.1152/ajpgi.1993.265.3.G459. [DOI] [PubMed] [Google Scholar]
- 14.Galan J E, Pace J, Hayman M J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992;357:588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- 15.Goodlad R A, Raja K B, Peters T J, Wright N A. Effects of urogastrone-epidermal growth factor on intestinal brush border enzymes and mitotic activity. Gut. 1991;32:994–998. doi: 10.1136/gut.32.9.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregory H. In vivo aspects of urogastrone-epidermal growth factor. J Cell Sci. 1985;3:11–17. doi: 10.1242/jcs.1985.supplement_3.2. [DOI] [PubMed] [Google Scholar]
- 17.Hardin J A, Buret A, Meddings J B, Gall D G. Effect of epidermal growth factor on enterocyte brush border surface area. Am J Physiol. 1993;264:G312–G318. doi: 10.1152/ajpgi.1993.264.2.G312. [DOI] [PubMed] [Google Scholar]
- 18.Hardin J A, Gall D G. The effect of TGFα on intestinal solute transport. Regul Pept. 1992;39:169–176. doi: 10.1016/0167-0115(92)90538-6. [DOI] [PubMed] [Google Scholar]
- 19.Hardin J A, Wong J K, Cheeseman C I, Gall D G. Effect of luminal epidermal growth factor on enterocyte glucose and proline transport. Am J Physiol. 1996;271:G509–G515. doi: 10.1152/ajpgi.1996.271.3.G509. [DOI] [PubMed] [Google Scholar]
- 20.Kelly D, McFayden M, King T P, Morgan P J. Characterization and localization of the epidermal growth factor receptor in the jejunum of the neonatal and weaned pig. Reprod Fertil Dev. 1992;4:183–189. doi: 10.1071/rd9920183. [DOI] [PubMed] [Google Scholar]
- 21.Khurana S, Nath S K, Levine S A, Bowser J M, Tse C M, Cohen M, Donowitz M. Brush border phosphatidylinositol 3-kinase mediates epidermal growth factor stimulation of intestinal NaCl absorption and Na+/H+ exchange. J Biol Chem. 1996;271:9919–9927. doi: 10.1074/jbc.271.17.9919. [DOI] [PubMed] [Google Scholar]
- 22.Kissmeyer-Nielsen P, Vinter-Jensen L, Smerup M. Effects of longterm epidermal growth factor treatment on the normal rat colon. Gut. 1996;38:582–586. doi: 10.1136/gut.38.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koldovsky O, Britton J, Davis D, Davis T, Grimes J, Kong W, Rao R, Schaudies P. The developing gastrointestinal tract and milk-borne epidermal growth factor. In: Mestecki J, editor. Immunology of milk and the neonate. New York, N.Y: Plenum Press; 1991. pp. 99–105. [DOI] [PubMed] [Google Scholar]
- 24.Konturek S J, Dembinski A, Warzecha Z, Brzozowski T, Gregory H. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology. 1988;94:1300–1307. doi: 10.1016/0016-5085(88)90667-1. [DOI] [PubMed] [Google Scholar]
- 25.Law D. Adhesion and its role in the virulence of enteropathogenic Escherichia coli. Clin Microbiol Rev. 1994;7:152–173. doi: 10.1128/cmr.7.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo G, Niesel D W, Shaban R A, Grimm E A, Klimpel G R. Tumor necrosis factor alpha binding to bacteria: evidence for a high-affinity receptor and alteration of bacterial virulence properties. Infect Immun. 1993;61:830–835. doi: 10.1128/iai.61.3.830-835.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marti U, Burwen S J, Jones A L. Biological effects of epidermal growth factor, with emphasis on the gastrointestinal tract and liver: an update. Hepatology. 1989;9:126–138. doi: 10.1002/hep.1840090122. [DOI] [PubMed] [Google Scholar]
- 28.Nath S K, Dechelotte P, Darmaun D, Gotteland M, Rongier M, Desjeux J F. [15N] and [14C] glutamine fluxes across rabbit ileum in experimental bacterial diarrhea. Am J Physiol. 1992;262:G312–G318. doi: 10.1152/ajpgi.1992.262.2.G312. [DOI] [PubMed] [Google Scholar]
- 29.O’Loughlin E V, Winter M, Shun A, Hardin J A, Gall D G. Structural and functional jejunal resection in rabbits: effect of epidermal growth factor. Gastroenterology. 1994;107:87–93. doi: 10.1016/0016-5085(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 30.Opleta-Madsen K, Meddings J B, Gall D G. Epidermal growth factor and postnatal development of intestinal transport and membrane structure. Pediatr Res. 1991;30:342–350. doi: 10.1203/00006450-199110000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Pesanti E L. Interaction of cytokines and alveolar cells with Pneumocystis carinii in vitro. J Infect Dis. 1991;163:611–616. doi: 10.1093/infdis/163.3.611. [DOI] [PubMed] [Google Scholar]
- 32.Playford R J. Peptides and gastrointestinal mucosal integrity. Gut. 1995;37:595–597. doi: 10.1136/gut.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Playford R J, Hanby A M, Gschmeissner S, Peiffer L P, Wright N A, McGarrity T. The epidermal growth factor receptor is present on the basolateral, but not apical, surface of enterocytes in the human gastrointestinal tract. Gut. 1996;39:262–266. doi: 10.1136/gut.39.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Playford R J, Wright N A. Why is epidermal growth factor present in the gut? Gut. 1996;38:303–305. doi: 10.1136/gut.38.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Procaccino F, Reinshagen M, Hoffman P, Zeek J M, Laksmanan J, McRoberts J A, Patel A, French S, Eysselein V E. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994;107:12–17. doi: 10.1016/0016-5085(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 36.Rafiee P, Leffler H, Byrd J C, Cassels F J, Boedeker E C, Kim Y S. A sialoglycoprotein complex linked to the microvillus cytoskeleton acts as a receptor for pilus (AF/R1) mediated adhesion of enteropathogenic Escherichia coli (RDEC-1) in rabbit small intestine. J Cell Biol. 1991;115:1021–1029. doi: 10.1083/jcb.115.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salloum R M, Stevens B R, Schultz G S, Souba W W. Regulation of small intestinal glutamine transport by epidermal growth factor. Surgery. 1993;113:552–559. [PubMed] [Google Scholar]
- 38.Strong J E, Tang D, Lee P W K. Evidence that the epidermal growth factor receptor on host cells confers reovirus infection efficiency. Virology. 1993;197:405–411. doi: 10.1006/viro.1993.1602. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan P B, Brueton M J, Tabara Z B, Goodlad R A, Lee C Y, Wright N A. Epidermal growth factor in necrotizing enteritis. Lancet. 1991;338:53–54. doi: 10.1016/0140-6736(91)90042-n. [DOI] [PubMed] [Google Scholar]
- 40.Tai Y H, Gage T P, McQueen C, Formal S B, Boedecker E C. Electrolyte transport in rabbit cecum. I. Effect of RDEC-1 infection. Am J Physiol. 1989;256:G721–G726. doi: 10.1152/ajpgi.1989.256.4.G721. [DOI] [PubMed] [Google Scholar]
- 41.Taylor C J, Hart A, Batt R M, McDougall C, McLean L. Ultrastructural and biochemical changes in human jejunal mucosa associated with enteropathogenic Escherichia coli (O111) infection. J Pediatr Gastroenterol Nutr. 1986;5:70–73. doi: 10.1097/00005176-198601000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Uribe J M, Keely S J, Traynor-Kaplan A E, Barrett K E. Phosphatidylinositol 3-kinase mediates the inhibitory effect of epidermal growth factor on calcium-dependent chloride secretion. J Biol Chem. 1996;271:26588–26595. doi: 10.1074/jbc.271.43.26588. [DOI] [PubMed] [Google Scholar]
- 43.Venable J H, Coggeshall R. A simplified lead citrate stain for use in electron microscopy. J Cell Biol. 1965;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker-Smith J A, Phillips A D, Walford N, Gregory H, Fitzgerald J D, MacCullagh K, Wright N A. Intravenous epidermal growth factor/urogastrone increases small intestinal cell proliferation in congenital microvillous atrophy. Lancet. 1985;ii:1239–1240. doi: 10.1016/s0140-6736(85)90762-7. [DOI] [PubMed] [Google Scholar]
- 45.Wolf M K, Andrews G P, Fritz D L, Sjogren R W, Jr, Boedecker E C. Characterization of the plasmid from Escherichia coli RDEC-1 that mediates expression of adhesin AF/R1 and evidence that AF/R1 pili promote but are not essential for enteropathogenic disease. Infect Immun. 1988;56:1846–1857. doi: 10.1128/iai.56.8.1846-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zijlstra R T, Odle J, Hall W F, Petschow B W, Gelberg H B, Litov R E. Effect of orally administered epidermal growth factor on intestinal recovery of neonatal pigs infected with rotavirus. J Pediatr Gastroenterol Nutr. 1994;19:382–390. doi: 10.1097/00005176-199411000-00003. [DOI] [PubMed] [Google Scholar]