Abstract
Background
Xijiao Dihuang decoction (XJDHT), a traditional Chinese medicine, is widely used to treat patients with sepsis. However, the mechanisms underlying the effects of XJDHT on cardiac dysfunction have yet to be fully elucidated. The present study evaluated the potential utility of XJDHT in protecting against sepsis-induced cardiac dysfunction and myocardial injury.
Methods
The mice were randomly divided into 3 groups and administered Lipopolysaccharide (LPS,10 mg/kg) or equivalent saline solution (control) and treated with XJDHT (10 g/kg/day) or saline by gavage for 72 hours. XJDHT was dissolved in 0.9% sodium chloride and administered at 200 μL per mouse. Transthoracic echocardiography, RNA-seq, TUNEL assays and hematoxylin and eosin (H&E) staining of cardiac tissues were performed.
Results
Treatment with XJDHT significantly enhanced myocardial function and attenuated pathological change, infiltration of inflammatory cells, levels of TNF-α, IL-1β and expression of TLR4 and NF-κB in mice with sepsis. RNA sequencing and Kyoto Encyclopedia of Genes and Genomes pathway analyses identified 531 differentially expressed genes and multiple enriched signaling pathways including the PI3K/AKT pathway. Further, XJDHT attenuated cardiac apoptosis and decreased Bax protein expression while increasing protein levels of Bcl-2, PI3K, and p-AKT in cardiac tissues of mice with sepsis.
Conclusion
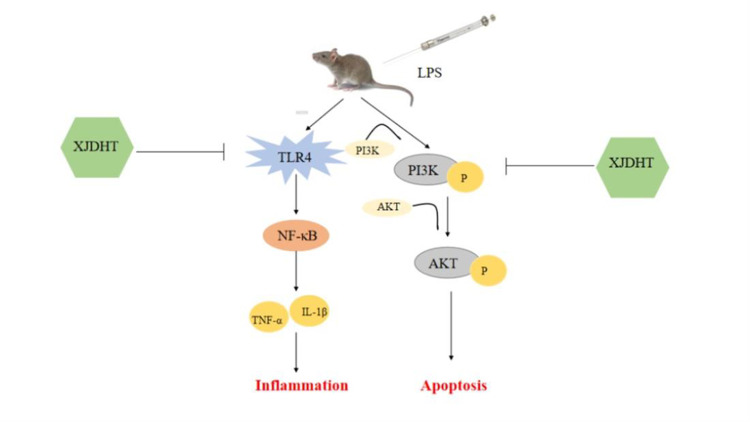
In summary, XJDHT improves cardiac function in a murine model of sepsis by attenuating cardiac inflammation and apoptosis via suppressing the TLR4/NF-κB pathway and activating the PI3K/AKT pathway.
Keywords: XJDHT, sepsis, inflammation, apoptosis, TLR4/NF-κB, PI3K/AKT pathway
Graphical Abstract
Introduction
Sepsis is a deleterious systemic host response to infection or injury which may progress to severe sepsis and septic shock. Multiple organ dysfunction is a hallmark of sepsis and a significant cause of death in intensive care units (ICU).1–3 Myocardial dysfunction is a recognized complication of sepsis with 20%–60% of patients with sepsis reported to develop myocardial dysfunction associated with an increased mortality rate of up to 70%.4–6 Accordingly, there is a clinical need for effective pharmacological treatments for protecting against sepsis-induced cardiac dysfunction and myocardial injury.
Numerous studies have shown that inflammatory response plays essential roles in the development of cardiac dysfunction in sepsis.7,8 When inflammatory response occurs after sepsis, proinflammatory cytokines or chemotactic cytokines, such as IL-6, TNF-α, IL-1β and MCP1 were released provoking further cardiac injury and cardiac dysfunction.9,10 Growing studies have demonstrated that TLR4, one of the major TLRs expressed by myocardial cells, was enhanced significantly in the myocardium after sepsis and particularly implicated in proinflammatory response by activating NF-κB pathway, leads to the transcription of inflammation cytokines, including TNF-α and IL-6, which further promotes inflammation,11 suggest that targeting TLR4/NF-κB pathway-modulated inflammation can be a novel strategy for sepsis induced cardiac dysfunction treatment.
Many previous studies have reported that large numbers of myocardial cells undergo apoptosis in response to sepsis, thereby contributing to the development of cardiac dysfunction.12–14 Activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway has been shown to attenuate cardiac apoptosis.15 The PI3K/AKT pathway can be activated by external stimuli leading to phosphorylation of downstream signaling molecules, including AKT, and alteration in gene expression, thereby regulating cardiac apoptosis.16 Accordingly, it has been hypothesized that promotion of the PI3K/AKT pathway may protect against sepsis-induced cardiac dysfunction.
Xijiao Dihuang decoction (XJDHT) is a well-known traditional Chinese medicine used for the treatment of sepsis. XJDHT consists of four herbs and one protein; Rehmannia, Peony, Cortex Moudan, and Cornu Bubali.17,18 Previous study has reported that XJDHT significantly improved survival in a rat model of sepsis by down-regulating IL-6 and suppressing the expressions of p65 and HIF-1α.18 XJDHT improves the prognosis of sepsis by suppressing aerobic glycolysis via suppression of the TLR4/HIF-1α/PKM2 signaling pathway.18 However, the mechanisms underlying the effect of XJDHT on sepsis-induced cardiac dysfunction have yet to be fully elucidated. Accordingly, the present study used RNA sequencing in a murine model of sepsis to investigate the mechanisms underlying the protective effects of XJDHT in sepsis-induced cardiac dysfunction.
Materials and Methods
Reagents
Lipopolysaccharide (LPS) was purchased from Sigma (St. Louis, MO, United States). Isoflurane was obtained from Shenzhen Ruiwode Life Technology Co., Ltd. ELISA kits for TNF-α (MM-0132M2), cTnI (MM-61550R1), and IL-1β (MM-0040M2) were purchased from Jiangsu Meimian industrial Co., Ltd. (Yancheng, China). PI3K (Ab140307) and Phospho-Akt (Ab8933) were purchased from Abcam (Cambridge Science Park, England). TLR4 (Ab13556), NF-κB (#8242, CST), Bax (#41162, CST), Bcl-2 (#4223, CST),GAPDH (#5174, CST) antibodies were purchased from CST (Cell Signaling Technology, United States). Hematoxylin was purchased from Solarbio Technology (Beijing, China). Phosphate buffer was purchased from Maixin Biotechnology (Fuzhou, Fujian, China). Transferase dUTP nick end labeling (TUNEL) Apoptosis Detection Kits (cat. no. MK1025) were purchased from Boster Biological Technology Co, Ltd. (Pleasanton, CA, USA).
Preparation of XJDHT
XJDHT was obtained from the Department of Pharmacy at the People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine. XJDHT was dissolved in 0.9% sodium chloride and administered at a final concentration of 10g/kg/day (200 μL per mouse). The concentration of XJDHT was calculated according to “Pharmacological Experimental Methodology”.
Animals
Male C57BL/6 mice aged 8 weeks were purchased from SLAC Laboratory Animal Technology Co. Ltd. (Shanghai, China). Mice were housed at a constant temperature (23°C ± 1°C) and humidity (50%–60%) under a 12-h light/dark cycle with free access to standard food and water. Mice were intraperitoneally administered 10 mg/kg LPS to establish a model of septic myocardial injury. Mice in the LPS+XJDHT group were administered XJDHT at a concentration of 10 g/kg (dissolved in double distilled water) daily by gavage, whereas mice in the control and LPS groups were administrated an equal volume of double distilled water by gavage for 3 days. Mice were randomly divided into three groups as follows: control (Control+NS group) (n=5), septic myocardial injury (LPS+NS group) (n=5), and XJDHT combined with LPS (LPS+XJDHT group) (n=5).
Cardiac Echocardiography
Transthoracic echocardiography was performed using a Vevo 2100 Ultrasound machine (VisualSonics, Toronto, Ontario, Canada). Briefly, mice were anesthetized with isoflurane then placed in dorsal recumbency on a heated platform while maintaining a heart rate of 400 to 450 beats per minute. Images were acquired in the two-dimensional mode under the parasternal long-axis view. The left ventricular ejection fraction (EF) and left ventricular fractional shortening (FS) were recorded. Image analysis was performed using Vevo Strain Software (Vevo LAB 1.7.1, FUJIFILM VisualSonics, Inc.).
Sample Collection
At the end of each experiment, mice were euthanized, and hearts were harvested and weighed to calculate the ratio of heart weight to body weight. Next, hearts were cut into halves widthwise with one half of the heart tissue washed with saline, fixed in 4% paraformaldehyde for 48 h, and embedded in paraffin for histological processing. The remaining tissues were frozen at −80°C before further use for RNA-seq analysis and Western blotting.
Hematoxylin-Eosin Staining
Heart tissue was embedded in paraffin and cut into 4-μm-thick sections. Paraffin sections were stained with hematoxylin and eosin (HE). Images were taken at x400 magnification using an optical microscope (Leica DM6000B, Leica Microsystems, Wetzlar, Germany). The criteria for cardiac involvement: abundant infiltration of inflammatory cells and healthy muscle tissue absent.
RNA-Seq Analysis
The complete RNA-seq datasets used in the present study are available from the Gene Expressing Omnibus database (accession number: GSE215955). RNA sequencing libraries were constructed using NEBNext Ultra RNA Library Prep kits according to the manufacturer’s protocol. Total mRNA was purified using oligo d(T) magnetic beads. Purified mRNA was fragmented to the target length. Then, cDNA was purified using 144 μL of AMPure XP Beads and repaired with 5′ phosphorylation and the addition of 3′ dA-tailed ends. Adapters were then ligated before amplification by polymerase chain reaction (PCR). Final libraries were quantified using an Agilent 2100 Bioanalyzer and KAPA library quantitative kits (KAPA Biosystem, Boston, USA). Sequencing of the transcriptome library was performed using an Illumina sequencing platform (Illumina, California, USA). Sequencing quality was assessed by FastQC (v0.11.2) to ensure the data quality was suitable for downstream analysis. Differentially expressed transcripts (DETs) between groups were analyzed using DESeq (v1.28.0). DETs were further analyzed using the database of Kyoto Encyclopedia of Genes and Genomes (KEGG).
ELISA Assays
Blood samples (1 mL) were collected from mice using a serum separator tube prior to euthanasia. Samples were allowed to clot at room temperature for 1 h before centrifugation at 3000 g for 10 min. Serum was then transferred to a new tube and stored at −80°C before further use. Serum TNF-α, cTnI, and IL-1β levels were measured using TNF-α, cTnI, andIL-1β ELISA kits (MEIMIAN, China).
Terminal Deoxynucleotidyl TUNEL Staining
TUNEL assays were used to detect cardiomyocyte apoptosis. Heart sections were incubated in proteinase K working solution at 37°C in a humidified atmosphere for 15 min. A total of 50 µL TUNEL reaction mixture was subsequently added to each sample, and sections were placed in a wet box for 60 min at 37°C in the dark. Sections were then rinsed three times with phosphate buffered saline (PBS) before the addition of 50 μL inverting peroxidase and incubation at 37°C for 30 min. Sections were again rinsed three times with PBS before the addition of 100 μL diaminobenzidine substrate. Sections were then examined using an optical microscope (Leica, Germany). Images were captured at x400 magnification. CVF was assessed by microscopic image analysis software (Motic Med 6.0).
Immunohistochemical Staining
Myocardial tissue was incubated at 60°C for 2 h. Tissues were deparaffinized and dehydrated with xylene and ethanol before treatment with sodium citrate and 3% hydrogen peroxide to inhibit endogenous peroxidase activity. Tissues were then incubated with 5% BSA at 37°C for 1 h. Tissues were incubated with 100 μL primary antibody in a humidified chamber at 4°C overnight. The following day, tissues were incubated with the corresponding secondary antibody for 1 h at 37°C. Finally, sections were incubated with diaminobenzidine as a substrate for 2 min. Six samples were randomly selected from each group, and five fields of view were randomly selected at x400 magnification using an optical microscope (Leica DM6000B, Leica Microsystems, Wetzlar, Germany).
Western Blot Analysis
The heart tissue was lysed with Western & IP cell lysis buffer (Beyotime, Shanghai, China) supplemented with phosphatase inhibitors and PMSF (Amresco, Solon, Ohio, USA) for 30 min on ice, centrifuged at 12,000 g for 15 min at 4°C, supernatants collected and total protein was measured using the BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). Equal amounts of protein were separated on 10% sodium dodecyl sulfate polyacrylamide gel and then transferred onto a 0.45μm PVDF membrane (AmershamHybond, GE Healthcare, München, Germany). Membranes were then blocked with TBST containing 0.5% bovine serum albumin (BSA; Amresco, Solon, Ohio, USA) for 2 h at room temperature and then incubated with the primary antibodies: rabbit anti-TLR4 (1:1000 dilution; Abcam, USA), rabbit anti-NF-κb (1:1000 dilution; CST, USA), rabbit anti-Bax (1:1000 dilution; CST, USA), rabbit anti-Bcl-2 (1:1000 dilution; CST, USA), rabbit anti-PI3K (1:1000 dilution; Abcam, USA), rabbit anti-p-AKT (1:1000 dilution; Abcam, USA), rabbit anti-GAPDH (1:5000 dilution; CST, USA) overnight at 4°C. After washing with TBST, membranes were incubated with goat anti-rabbit secondary antibody at room temperature for 1 h and washed with TBST. Target proteins were detected using ECL kits (Thermo Fisher Scientific). The protein levels were analyzed with Image J. GAPDH protein expression was used as an internal control.
Statistical Analysis
All data obtained from three repeat experiments results were expressed as the mean ± SD. Normality of data was determined using the Shapiro–Wilk test. The independent Student’s t-test was performed to compare differences between two groups. One-way analysis of variance was used to compare differences between three or more groups when data were normally distributed. Bonferroni correction was used when the variance was chi-square, and the Games–Howell test was used when the variance was not chi-square. The nonparametric Kruskal–Wallis test was used to compare differences between groups when data were not normally distributed using pairwise comparisons. All statistical analyses were performed using SPSS software (version 26.0, SPSS, Inc., Chicago, USA). P-values less than 0.05 were considered statistically significant.
Results
XJDHT Prevents Sepsis-Induced Cardiac Dysfunction
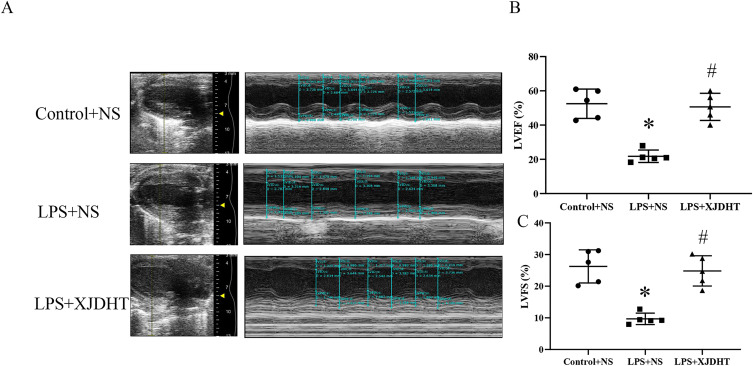
We first evaluated the effects of XJDHT on sepsis-induced cardiac dysfunction. Mice were treated with PBS or XJDHT for 72 h after the administration of LPS at a dose of 10 mg/kg. Echocardiography demonstrated significant decreases in both EF and FS in the LPS+NS group (Figure 1A–C; P < 0.05 vs Control+NS group). However, XJDHT treatment significantly increased EF and FS compared with the LPS+NS group (Figure 1A–C; P < 0.05 vs LPS+NS group). These results indicate that treatment with XJDHT improves cardiac function in response to sepsis-induced cardiac dysfunction.
Figure 1.
XJDHT prevents sepsis-induced cardiac dysfunction. Cardiac function in mice administered LPS (LPS, 10 mg/kg) or equivalent saline solution (control) then treated with XJDHT (10g/kg/d) or saline by gavage for 72 h (n = 5). (A) Representative echocardiographic images. (B) Ejection fraction (EF). (C) Fractional shortening (FS). All values are presented as the mean ± SD; *P < 0.05 LPS+NS vs control group, #P < 0.05 LPS+XJDHT vs LPS+NS group.
XJDHT Attenuates Inflammation and Pathological Changes of Heart in Sepsis Mice
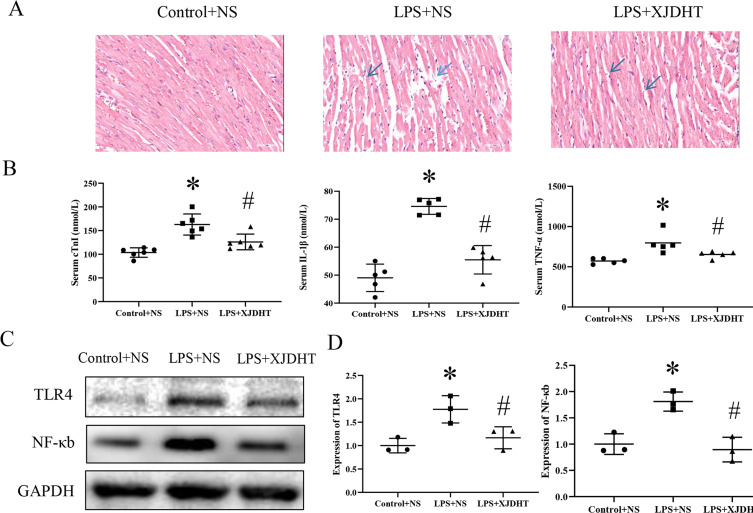
To further investigate the cardiac protective effect of XJDHT, hematoxylin and eosin (H&E) staining of cardiac tissues was performed. Histological analyses demonstrated that myocardial cells in the LPS treatment group were disordered and enlarged with increased inflammatory cell infiltration and myocardial death. These pathological changes were reversed in the XJDHT treatment group (Figure 2A). As the pro-inflammatory state promoted by sepsis is known to lead to cardiac dysfunction. We then measured serum levels of inflammatory cytokines. As shown in Figure 2B, LPS significantly increased serum levels of TNF-α, cTnI, and IL-1β in the LPS+NS group; however, XJDHT treatment significantly reduced serum levels of inflammatory markers (Figure 2B; P < 0.05 vs LPS+NS group). Furthermore, as shown in Figure 2C and D, the protein expression of TLR4 and NF-κB was significantly upregulated in cardiac tissues of LPS+NS group, while it was obviously downregulated after XJDHT treatment (Figure 2C and D, P < 0.05 vs LPS+NS group). These results demonstrate that XJDHT treatment prevented cardiac injury and inflammation partly via inhibited TLR4/NF-κB pathways.
Figure 2.
XJDHT improves sepsis-induced myocardial pathological changes. (A) Hematoxylin and eosin staining (n = 5; scale bar = 50 µm; magnification, x400). All values are presented as the mean ± SD; *P < 0.05 LPS+NS vs control group; #P < 0.05 LPS+NS + XJDHT vs LPS group. (B) ELISA demonstrated reduced serum levels of TNF-α, cTnI, and IL-1β following treatment with XJDHT. (C and D) Western blotting to determine levels of TLR4 and NF-κb after XJDHT treatment. *P < 0.05 LPS+NS vs control group; #P < 0.05 LPS+NS + XJDHT vs LPS group.
Genome-Wide Gene Expression Profiling of Cardiac Tissues from Mice with Sepsis After XJDHT Treatment
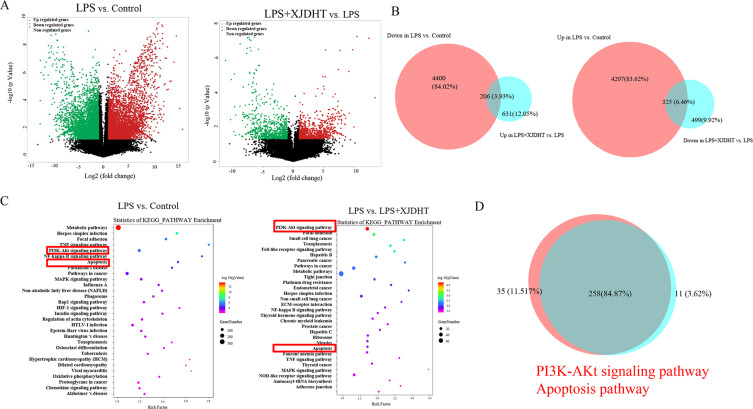
To investigate the mechanisms underlying the cardioprotective effects of XJDHT, RNA sequencing was used to identify genome-wide alterations in sepsis and in response to treatment with XJDHT. We performed two comparisons (Control+NS vs LPS+NS and LPS+NS vs LPS+XJDHT) to identify gene alterations following treatment with XJDHT. Integrative analysis identified 325 up-regulated transcripts in the LPS+NS group which were down-regulated in LPS+XJDHT group and 206 down-regulated transcripts in the LPS+NS group that were up-regulated after XJDHT treatment (Figure 3A and B). Moreover, KEGG pathway analysis of DEGs revealed that genes involved in the PI3K/AKT signaling pathway and cell apoptosis were enriched (Figure 3C and D). As a result of these findings, we performed further experiments to determine whether the regulatory effects of XJDHT on cardiac apoptosis are mediated through the PI3K/AKT signaling pathway.
Figure 3.
Genome-wide gene expression profiling of cardiac tissues from mice with sepsis after XJDHT treatment. (A) Volcano plots comparing gene expression profiles (fold change ≥ 2, P < 0.05). (B) Overlapping areas represent genes under-expressed in the LPS group but over-expressed in the LPS + XJDHT group (n = 206) and genes over-expressed in the LPS group but under-expressed in the LPS + XJDHT group (n = 324). (C) KEGG pathway enrichment analysis of differentially expressed transcripts for LPS+NS vs control and LPS+XJDHT vs LPS+NS. (D) Overlapping KEGG pathways for LPS+NS vs control and LPS+XJDHT vs LPS+NS.
Attenuation of Sepsis-Induced Myocardial Cell Apoptosis by XDJHT is Partially Mediated by Enhancing of the PI3K/AKT Signaling Pathway
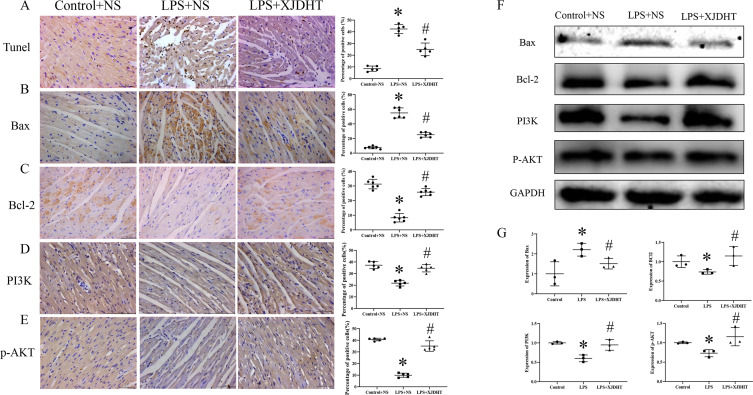
We next used TUNEL assays to detect apoptosis of myocardial cells. We observed increased numbers of apoptotic cells in cardiac tissues of the LPS+NS group (Figure 4A; P < 0.05 vs Control+NS group), while XJDHT treatment markedly reduced the number of apoptotic cardiomyocytes (Figure 4A; P < 0.05 vs LPS+NS group). Cardiac Bax expression levels were increased in mice with sepsis (Figure 4B; P < 0.05 vs Control+NS group), while expression levels of anti-apoptotic proteins such as Bcl2 were decreased (Figure 4C; P < 0.05 vs Control+NS group). Interestingly, XJDHT treatment was associated with up-regulation of anti-apoptotic factors and down-regulation of pro-apoptotic proteins in cardiac tissues from mice with sepsis (Figure 4B and C; P < 0.05 vs LPS+NS group). Furthermore, XJDHT treatment increased PI3K and p-AKT levels (Figure 4D and E; P < 0.05 vs LPS+NS group). We utilized Western blotting to determine levels of PI3K, p-AKT, Bax and Bcl-2 after XJDHT treatment in myocardial tissue (Figure 4F and G). Data demonstrated that XJDHT could reduce the up-regulation of Bax caused by LPS and increase the expressions of Bcl-2, PI3K and p-AKT (Figure 4F and G; P < 0.05 vs LPS+NS group). Collectively, these data demonstrate that XJDHT treatment effectively attenuates sepsis-induced myocardial cell apoptosis by enhancing the PI3K/AKT signaling pathway.
Figure 4.
Attenuation of sepsis-induced myocardial cell apoptosis by XDJHT is partially mediated by suppression of the PI3K/AKT signaling pathway. (A) Apoptosis detected by TUNEL assay. (B-E) Immunohistochemical staining for Bax, Bcl2, PI3K and p-AKT in myocardial tissue. Scale bar: 50 μm. All values are presented as the mean ± SD; *P < 0.05 for LPS+NS vs control group, #p < 0.05 for LPS+XJDHT vs LPS+NS group.(F and G) Western blotting to determine levels of Bax,Bcl-2,PI3K and p-AKT after XJDHT treatment in myocardial tissue.*P < 0.05 LPS+NS vs control group; #P < 0.05 LPS+NS + XJDHT vs LPS group.
Discussion
The findings of the present study demonstrate that treatment with XJDHT improves cardiac function in a mice model of sepsis by attenuating cardiac inflammation and apoptosis via suppression TLR4/NF-κB and activating the PI3K/AKT pathway. We used LPS induction to establish a model of sepsis-induced myocardial dysfunction to evaluate the effect of XJDHT on cardiac injury in vivo. We found XJDHT improves cardiac function and reduces myocardial pathological damage, inflammation and cardiomyocyte apoptosis. Furthermore, we used RNA-seq to identify DETs and enriched signaling pathways, with the PI3K/AKT signaling pathway found to be enriched in LPS mice relative to control mice and in LPS+XJDHT mice relative to LPS mice. Therefore, our study provides evidence that XJDHT exertsanti-inflammation and anti-apoptotic effects and improves LPS-induced myocardial dysfunction by regulating the TLR4/NF-κB and PI3K/AKT pathway.
Sepsis has a rapid onset and is associated with a high rate of mortality. Growing evidence suggests that most patients with septic shock suffer from myocardial depression.19 The mechanisms underlying myocardial depression during sepsis predominantly include mitochondrial dysfunction,20,21 cell death (necrosis and apoptosis),22,23 and inflammation.24,25 LPS is highly pathogenic and can induce severe sepsis,26–29 accordingly, LPS is often used to induce myocardial injury in animal models. Previous studies have posited that LPS can activate the apoptotic signaling pathway in cardiomyocytes.30–32 Accordingly, the identification of compounds that prevent LPS-induced apoptosis leading to protection of cardiomyocytes may facilitate the development of novel treatments for severe sepsis. Accordingly, our study established an LPS-induced endotoxemia heart injury model in C57BL/6J mice.
XJDHT was first depicted in the medical classic; “Beiji Qianjin Yaofang”. As an antipyretic agent, XJDHT exhibits potent anti-inflammation effects and can decrease the expression of adhesion molecules by vascular endothelial cells.23,33,34 Previous study demonstrated that XJDHT reduces the release of inflammatory cytokines such as IL-6 and improves survival in rats with sepsis via regulation of the HIF-1α signaling pathway.18 Recent studies have reported that XJDHT suppresses aerobic glycolysis by down-regulating the TLR4/HIF-1α/PKM2 signaling pathway, therebyimproving prognosis in sepsis.17 In the LPS mouse model, a dose of 10 g/kg of XJDHT is administered by gavage for three days. We measured left ventricular EF and FS values by echocardiography and observed that XJDHT treatment for three days reduced LPS-induced cardiac dysfunction in mice. H&E staining of myocardial tissue demonstrated that XJDHT reduced the degree of myocardial injury compared with the LPS group.
As a major component of gram-negative bacterial cell walls, LPS promotes the synthesis and release of inflammatory mediators such as TNF-α, IL-1β, and IL-6.35 Numerous studies have reported that pro-inflammatory mediators, such as TNF-α, IL-1β, and IL-6, are involved in cardiac dysfunction.36 For example, TNF-α was found to induce myocardial depression by modulating the inflammatory response.37,38 Further, IL-6 knockout mice have reduced levels ofinflammation and apoptosis and improved contractile function after sepsis compared with wild‑type mice.39 Therefore, we measured serum levels of inflammatory cytokines in LPS-treated mice using ELISA. We observed significantly increased serum levels of TNF-α and IL-6 after LPS administration for 72 h, with reduced serum levels of TNF-α and IL-6 following treatment with XJDHT. As the key role of TLR4/NF-κB pathway in sepsis inflammation, we also found that XJDHT treatment inhibited TLR4/NF-κB pathways.
We next explored the mechanisms underlying the protective effects of XJDHT against LPS-induced myocardial injury. We observed enrichment of apoptosis and PI3K/AKT-related pathways by KEGG pathway analysis of DEGs. The PI3K/AKT is a classical signaling pathway that regulates intracellular processes including cell survival, metabolism, and apoptosis.40 Chen et al demonstrated that PI3K/AKT/mTOR signaling is significantly altered in sepsis myocardial injury.41 Shang X et al found that resveratrol activates the PI3K/AKT signaling pathway and inhibits the NF-κB signaling pathway during myocardial injury in a rat model of sepsis.42 However, the mechanisms responsible for the increased apoptosis observed in LPS-induced cardiac dysfunction have yet to be fully elucidated. In the present study, XJDHT reduced the number of apoptotic cardiomyocytes and increased PI3K and p-AKT levels, indicating that XJDHT exerts a protective effect on LPS-induced myocardial injury by regulating the PI3K/AKT pathway. Studies have demonstrated that when Bax is highly expressed in cells, the cells are sensitive to death signals, which promote cell apoptosis.43,44 When Bcl-2 is highly expressed, it form heterodimers with Bax to inhibit cell apoptosis.43,44 Therefore, the intracellular Bcl-2/Bax ratio plays an important role in determining the sensitivity of cell apoptosis. PI3K/AKT activation further upregulates Bcl-2 expression and downregulates Bax expression, which plays an important regulatory role in resisting cardiomyocyte apoptosis.45 Our study confirmed that XJDHT further inhibits Bax expression and upregulates Bcl-2 expression by regulating the PI3K/AKT pathway, thereby inhibiting the occurrence of cardiomyocyte apoptosis caused by sepsis. However, further studies are required to determine the specific mechanisms by which XJDHT treatment regulates the PI3K/AKT pathway.
The present study provides preliminary evidence that XJDHT is protective against LPS-induced cardiac dysfunction in vivo. Accordingly, XJDHT may have efficacy as a pharmacological intervention for the treatment of cardiac insufficiency in patients with sepsis. However, in future studies, we intend to perform a series of experiments to explore the XJDHT’s effect on sepsis-mediated damage to other organs.
Conclusions
In summary, the present study demonstrates that XJDHT has notable benefits in preventing sepsis-induced cardiac dysfunction. The potential mechanisms of XJDHT in sepsis-induced cardiac injury may be due to its anti-inflammation and anti-apoptotic effects mediated by suppression TLR4/NF-κB pathway and enhancing PI3K/AKT signaling pathways. Future studies should explore the effect of XJDHT on cardiac remodeling and the PI3K/AKT signaling pathway in vitro in order to more precisely understand the contribution of each therapeutic component of the formulation to its overall mechanism of action.
Funding Statement
Present study was supported by the Natural Science Foundation of Fujian Province (2022J01846); Medical Innovation Project of Fujian Health and Family Planning Commission (2020CXB032); The Special Project of Fujian National Clinical Research Base of Traditional Chinese Medicine (JDZX201902).
Data Sharing Statement
The following RNA sequencing supporting information can be downloaded at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE215955.
Ethics Statement
All animal experiments were approved by the Animal Protection and Utilization Committee of the Institute at Fujian University of Traditional Chinese Medicine (No. FJTCM IACUC 2021133) and executed in stringent accordance with the “Guide for the Care and Use of Laboratory Animals” and “Principles for the Utilization and Care of Vertebrate Animals”.
Disclosure
Wei Li, Mingrui Lin and Jiapeng Li are co-first authors for this study. The author reports no conflicts of interest in this work.
References
- 1.Wen R, Liu YP, Tong XX, et al. Molecular mechanisms and functions of pyroptosis in sepsis and sepsis-associated organ dysfunction. Front Cell Infect Microbiol. 2022;12:962139. doi: 10.3389/fcimb.2022.962139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang N, Liu YJ, Yang C, et al. Review of research progress on the role of the effective components of traditional Chinese medicine in sepsis with multiple organ dysfunction. Heliyon. 2023;9(11):e21713. doi: 10.1016/j.heliyon.2023.e21713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skei NV, Nilsen TIL, Mohus RM, et al. Trends in mortality after a sepsis hospitalization: a nationwide prospective registry study from 2008 to 2021. Infection. 2023;51(6):1773–1786. doi: 10.1007/s15010-023-02082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun M, Zhao H, Jin Z, et al. Silibinin protects against sepsis and septic myocardial injury in an NR1H3-dependent pathway. Free Radic Biol Med. 2022;187:141–157. doi: 10.1016/j.freeradbiomed.2022.05.018 [DOI] [PubMed] [Google Scholar]
- 5.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116(7):793–802. doi: 10.1161/CIRCULATIONAHA.106.678359 [DOI] [PubMed] [Google Scholar]
- 6.Jeong HS, Lee TH, Bang CH, et al. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: a comparative retrospective study. Medicine. 2018;97(13):e0263. doi: 10.1097/MD.0000000000010263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong X, Lu L, Wang Z, et al. Irisin attenuates sepsis-induced cardiac dysfunction by attenuating inflammation-induced pyroptosis through a mitochondrial ubiquitin ligase-dependent mechanism. Biomed Pharmacother. 2022;152:113199. doi: 10.1016/j.biopha.2022.113199 [DOI] [PubMed] [Google Scholar]
- 8.Wei A, Liu J, Li D, et al. Syringaresinol attenuates sepsis-induced cardiac dysfunction by inhibiting inflammation and pyroptosis in mice. Eur J Pharmacol. 2021;913:174644. doi: 10.1016/j.ejphar.2021.174644 [DOI] [PubMed] [Google Scholar]
- 9.Zhao X, Zhang S, Shao H. Dexpanthenol attenuates inflammatory damage and apoptosis in kidney and liver tissues of septic mice. Bioengineere. 2022;13(5):11625–11635. doi: 10.1080/21655979.2022.2070585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L, Jin L, Luo Y, et al. Shenfu injection attenuates cardiac dysfunction and inhibits apoptosis in septic mice. Ann Transl Med. 2022;10(10):597. doi: 10.21037/atm-22-836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XS, Wang SH, Liu CY, et al. Losartan attenuates sepsis-induced cardiomyopathy by regulating macrophage polarization via TLR4-mediated NF-κB and MAPK signaling. Pharmacol Res. 2022;185:106473. doi: 10.1016/j.phrs.2022.106473 [DOI] [PubMed] [Google Scholar]
- 12.Wu M, Huang Z, Huang W, et al. microRNA-124-3p attenuates myocardial injury in sepsis via modulating SP1/HDAC4/HIF-1α axis. Cell Death Discov. 2022;8(1):40. doi: 10.1038/s41420-021-00763-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang T, Peng D, Shi W, et al. IL-6/STAT3 Signaling Promotes Cardiac Dysfunction by Upregulating FUNDC1-Dependent Mitochondria-Associated Endoplasmic Reticulum Membranes Formation in Sepsis Mice. Front Cardiovasc Med. 2022;8:790612. doi: 10.3389/fcvm.2021.790612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi Z, Wang R, Liao R, et al. Corrigendum: neferine Ameliorates Sepsis-Induced Myocardial Dysfunction Through Anti-Apoptotic and Antioxidative Effects by Regulating the PI3K/AKT/mTOR Signaling Pathway. Front Pharmacol. 2022;13:913778. doi: 10.3389/fphar.2022.913778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Yan M, Yang R, et al. Adiponectin Attenuates Lipopolysaccharide-induced Apoptosis by Regulating the Cx43/PI3K/AKT Pathway. Front Pharmacol. 2021;12:644225. doi: 10.3389/fphar.2021.644225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi Z, Wang R, Liao R, et al. Neferine Ameliorates Sepsis-Induced Myocardial Dysfunction Through Anti-Apoptotic and Antioxidative Effects by Regulating the PI3K/AKT/mTOR Signaling Pathway. Front Pharmacol. 2021;12:706251. doi: 10.3389/fphar.2021.706251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu J, Zhang L, Cheng L, et al. Xijiao Dihuang decoction improves prognosis of sepsis via inhibition of aerobic glycolysis. Biomed Pharmacother. 2020;129:110501. doi: 10.1016/j.biopha.2020.110501 [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Yan J, Yan J, et al. Network pharmacology based research into the effect and mechanism of Xijiao Dihuang decoction against sepsis. Biomed Pharmacother. 2020;122:109777. doi: 10.1016/j.biopha.2019.109777 [DOI] [PubMed] [Google Scholar]
- 19.Xu Q, Xiong H, Zhu W, et al. Small molecule inhibition of cyclic GMP-AMP synthase ameliorates sepsis-induced cardiac dysfunction in mice. Life Sci. 2020;260:118315. doi: 10.1016/j.lfs.2020.118315 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Jasper H, Toan S, et al. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biol. 2021;45:102049. doi: 10.1016/j.redox.2021.102049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Zhang Z. Sepsis-induced myocardial dysfunction: the role of mitochondrial dysfunction. Inflamm Res. 2021;70(4):379–387. doi: 10.1007/s00011-021-01447-0 [DOI] [PubMed] [Google Scholar]
- 22.Chauin A. The Main Causes and Mechanisms of Increase in Cardiac Troponin Concentrations Other Than Acute Myocardial Infarction (Part 1): physical Exertion, Inflammatory Heart Disease, Pulmonary Embolism, Renal Failure, Sepsis. Vasc Health Risk Manag. 2021;17:601–617. doi: 10.2147/VHRM.S327661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng N, Jian Z, Zhu W, et al. KLF13 overexpression protects sepsis-induced myocardial injury and LPS-induced inflammation and apoptosis. Int J Exp Pathol. 2023;104(1):23–32. doi: 10.1111/iep.12459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonucci E, Fiaccadori E, Donadello K, et al. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014;29(4):500–511. doi: 10.1016/j.jcrc.2014.03.028 [DOI] [PubMed] [Google Scholar]
- 25.Cuello F, Shankar-Hari M, Mayr U, et al. Redox state of pentraxin 3 as a novel biomarker for resolution of inflammation and survival in sepsis. Mol Cell Proteomics. 2014;13(10):2545–2557. doi: 10.1074/mcp.M114.039446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honda T, He Q, Wang F, et al. Acute and chronic remote ischemic conditioning attenuate septic cardiomyopathy, improve cardiac output, protect systemic organs, and improve mortality in a lipopolysaccharide-induced sepsis model. Basic Res Cardiol. 2019;114(3):15. doi: 10.1007/s00395-019-0724-3 [DOI] [PubMed] [Google Scholar]
- 27.de Pádua Lúcio K, Rabelo ACS, Araújo CM, et al. Morus nigraAnti-Inflammatory and Antioxidant Properties of Black Mulberry (Morus nigra L.) in a Model of LPS-Induced Sepsis. Oxid Med Cell Longev. 2018;2018:5048031. doi: 10.1155/2018/5048031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi S, Okada M, Ijiri E, et al. β3-Adrenergic receptor blockade reduces mortality in endotoxin-induced heart failure by suppressing induced nitric oxide synthase and saving cardiac metabolism. Am J Physiol Heart Circ Physiol. 2020;318(2):H283–H294. doi: 10.1152/ajpheart.00108.2019 [DOI] [PubMed] [Google Scholar]
- 29.Kumari A, Dash D, Singh R. Curcumin inhibits lipopolysaccharide (LPS)-induced endotoxemia and airway inflammation through modulation of sequential release of inflammatory mediators (TNF-α and TGF-β1) in murine model. Inflammopharmacology. 2017;25(3):329–341. doi: 10.1007/s10787-017-0334-3 [DOI] [PubMed] [Google Scholar]
- 30.Wu B, Ni H, Li J, et al. The Impact of Circulating Mitochondrial DNA on Cardiomyocyte Apoptosis and Myocardial Injury After TLR4 Activation in Experimental Autoimmune Myocarditis. Cell Physiol Biochem. 2017;42(2):713–728. doi: 10.1159/000477889 [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Lin C, Wang T, et al. Ergosterol Attenuates LPS-Induced Myocardial Injury by Modulating Oxidative Stress and Apoptosis in Rats. Cell Physiol Biochem. 2018;48(2):583–592. doi: 10.1159/000491887 [DOI] [PubMed] [Google Scholar]
- 32.Zhang T, Liu CF, Zhang TN, et al. Overexpression of Peroxisome Proliferator-Activated Receptor γ Coactivator 1-α Protects Cardiomyocytes from Lipopolysaccharide-Induced Mitochondrial Damage and Apoptosis. Inflammation. 2020;43(5):1806–1820. doi: 10.1007/s10753-020-01255-4 [DOI] [PubMed] [Google Scholar]
- 33.Liu R, Wang M, Duan JA. Antipyretic and antioxidant activities of the aqueous extract of Cornu bubali (water Buffalo horn). Am J Chin Med. 2010;38(2):293–306. doi: 10.1142/S0192415X10007853 [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Pei T, Mu J, et al. Systems Pharmacology Uncovers the Multiple Mechanisms of Xijiao Dihuang Decoction for the Treatment of Viral Hemorrhagic Fever. Evid Based Complement Alternat Med. 2016;2016:9025036. doi: 10.1155/2016/9025036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu N, Wang C, Dai X, et al. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J Ethnopharmacol. 2020;248:112361. doi: 10.1016/j.jep.2019.112361 [DOI] [PubMed] [Google Scholar]
- 36.Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 37.Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, et al. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7(3):163–183. doi: 10.2174/157340311798220494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jude B, Vetel S, Giroux-Metges MA, et al. Rapid negative inotropic effect induced by TNF-α in rat heart perfused related to PKC activation. Cytokine. 2018;107:65–69. doi: 10.1016/j.cyto.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Wang HY, Bassel-Duby R, et al. Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am J Physiol Heart Circ Physiol. 2007;292(5):H2408–16. doi: 10.1152/ajpheart.01150.2006 [DOI] [PubMed] [Google Scholar]
- 40.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13(3):195–203. doi: 10.1038/nrm3290 [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Liu P, Feng X, et al. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med. 2017;21(12):3178–3189. doi: 10.1111/jcmm.12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shang X, Lin K, Yu R, et al. Resveratrol Protects the Myocardium in Sepsis by Activating the Phosphatidylinositol 3-Kinases (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Pathway and Inhibiting the Nuclear Factor-κB (NF-κB) Signaling Pathway. Med Sci Monit. 2019;25:9290–9298. doi: 10.12659/MSM.918369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao W, Rao Z, Wu L, et al. Cariporide Attenuates Doxorubicin-Induced Cardiotoxicity in Rats by Inhibiting Oxidative Stress, Inflammation and Apoptosis Partly Through Regulation of Akt/GSK-3βand Sirt1 Signaling Pathway. Front Pharmacol. 2022;13:850053. doi: 10.3389/fphar.2022.850053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi C, Song M, Sun L, et al. Asiatic Acid Alleviates Myocardial Ischemia-Reperfusion Injury by Inhibiting the ROS-Mediated Mitochondria-Dependent Apoptosis Pathway. Oxid Med Cell Longev. 2022;2022:3267450. doi: 10.1155/2022/3267450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S, Peng J, Sherchan P, et al. TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2020;17(1):168. doi: 10.1186/s12974-020-01853-x [DOI] [PMC free article] [PubMed] [Google Scholar]