Abstract
BACKGROUND
Liposomal bupivacaine is claimed by the manufacturer to provide analgesia for up to 72 h postoperatively.
OBJECTIVES
To compare the postoperative analgesic efficacy of liposomal bupivacaine versus long-acting local anaesthetics for peripheral nerve or field blocks.
DESIGN
A systematic review and meta-analysis with trial sequential analysis.
DATA SOURCES
MEDLINE, Embase and Web of Science, among others, up to June 2022.
ELIGIBILITY CRITERIA
We retrieved randomised controlled trials comparing liposomal bupivacaine versus bupivacaine, levobupivacaine or ropivacaine for peripheral nerve and field blocks after all types of surgery. Our primary endpoint was rest pain score (analogue scale 0 to 10) at 24 h. Secondary endpoints included rest pain score at 48 and 72 h, and morphine consumption at 24, 48 and 72 h.
RESULTS
Twenty-seven trials including 2122 patients were identified. Rest pain scores at 24 h were significantly reduced by liposomal bupivacaine with a mean difference (95% CI) of -0.9 (-1.4 to -0.4), I2 = 87%, P < 0.001. This reduction in pain scores persisted at 48 h and 72 h with mean differences (95% CI) of -0.7 (-1.1 to -0.3), I2 = 82%, P = 0.001 and -0.7 (-1.1 to -0.3), I2 = 80%, P < 0.001, respectively. There were no differences in interval morphine consumption at 24 h (P = 0.15), 48 h (P = 0.15) and 72 h (P = 0.07). The quality of evidence was moderate.
CONCLUSIONS
There is moderate level evidence that liposomal bupivacaine reduces rest pain scores by 0.9 out of 10 units, when compared with long-acting local anaesthetics at 24 hours after surgery, and by 0.7 up to 72 hours after surgery.
KEY POINTS
Liposomal bupivacaine is claimed by the manufacturer to provide analgesia for up to 72 h postoperatively.
We undertook a systematic review and meta-analysis with trial sequential analysis to compare the postoperative analgesic efficacy of liposomal bupivacaine versus long-acting local anaesthetics for peripheral nerve and field blocks.
We analysed all randomised controlled trials comparing liposomal bupivacaine versus bupivacaine, levobupivacaine or ropivacaine for peripheral nerve blocks after all types of surgery.
Liposomal bupivacaine statistically reduces pain scores at 24, 48 and 72 postoperative hours, but without clinical relevance.
Introduction
Optimal pain control in the postoperative period remains challenging.1 When long-acting local anaesthetic molecules are combined with pharmacological adjuncts such as dexamethasone, the duration of analgesia after administration of local anaesthetics near peripheral nerves may last up to 24 h.2–4 To prolong the analgesia beyond 24 h, insertion of a perineural catheter with a continuous infusion of local anaesthetics is a frequently used option. However, the procedure is time-consuming, requires specific technical skills and necessitates a complex and costly logistic organisation for follow-up, while, at the same time, the catheters are prone to migration, spontaneous dislodgement, and leakage, leading to a nonnegligible rate of secondary failure.5
Another option to prolong analgesia beyond 24 h is the injection of liposomal bupivacaine: the manufacturer claims an efficacy lasting up to 72 h. Liposomal bupivacaine is a sustained-release multivesicular formulation of bupivacaine, which is currently approved by the United States Food and Drug Administration for wound infiltration and interscalene brachial plexus block,6 and by the European Medicines Agency for brachial plexus, femoral nerve and field blocks.7
Two previous meta-analyses investigated the analgesic efficacy of liposomal bupivacaine when compared with an active drug group for peripheral nerve blocks, but were limited by two factors.8,9 First, these two publications did not include articles comparing liposomal bupivacaine with levobupivacaine or ropivacaine,8,9 and second, one of them did not include studies investigating field blocks and included only five controlled trials published as full-text.8
As additional studies have been published since these two meta-analyses, and as bupivacaine, levobupivacaine and ropivacaine are all long-acting local anaesthetics with similar durations of analgesia, we undertook this systematic review and meta-analysis with trial sequential analysis with the objective of providing a more comprehensive understanding of the postoperative analgesic efficacy of liposomal bupivacaine for peripheral nerve and field blocks when compared with long-acting local anaesthetics.
Materials and methods
Literature search and inclusion criteria
This investigation was conducted following the recommended process from the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) statement10 and was prospectively registered through the International Prospective Register of Systematic Reviews (registration number CRD42021291745).
The following electronic sources were searched up to 30 June 2022: MEDLINE, Embase, CINHAL, Cochrane Central Register of Controlled Clinical Trials, Web of Science and ProQuest Theses. The intervention search terms applied were Liposome bupivacaine OR Liposomal bupivacaine OR liposome OR Exparel. Among others, the following words were searched as keywords: Liposom∗, Lipob∗. Deduplication of the retrieved records was done manually. Population limits were then applied including Clinical trials OR Random allocation OR Therapeutic use. Details of this literature search are provided in the supplementary document 1.
Search results were independently screened by two authors (AN and MG) using the title and the abstract. Only randomised controlled trials on adult patients were included, without language restriction. The full texts of potentially eligible articles were subsequently evaluated for inclusion. Discrepancies were resolved by discussion until consensus was reached or, if needed, involvement of the senior author (EA). Finally, after compiling the results of the above search, the authors independently reviewed the references from all included trials for any applicable articles that were not captured by the described approach.
Population
The meta-analysis addresses adult patients undergoing any type of surgery with a peripheral nerve or field block.
Intervention and comparator
Only randomised controlled trials investigating pain outcomes and comparing liposomal bupivacaine with any type of long-acting local anaesthetic (bupivacaine, levobupivacaine, ropivacaine), combined or not with perineural adjuncts, were included in this meta-analysis.
Outcomes
Defined outcomes were extracted from each article following our routine approach previously described in meta-analyses on acute postoperative pain.11,12 The primary outcome was the pain score at rest at 24 postoperative hours. Secondary outcomes were rest pain scores at 2, 48 and 72 postoperative hours; interval morphine consumption at 24, 48 and 72 postoperative hours; presence of nausea or vomiting at 24, 48 and 72 postoperative hours; and hospital length of stay. Additional outcomes were incidences of LAST (local anaesthetic systemic toxicity) and nerve injury.
Trial characteristics
Extracted trial characteristics were: type of surgery; technique of peripheral nerve or field block; anaesthetic strategy; concentration, volume and type of local anaesthetic administered; postoperative analgesic regimen; and whether a conflict of interest was declared or not (study sponsored by the industry or one of the authors received honorarium from Pacira BioSciences, Pacira BioSciences. (USA).
Rating of the studies
The Cochrane Collaboration's Risk of Bias Tool13 was applied to each randomised trial in order to evaluate the methodologic quality. Two authors (AN and MG) independently reviewed and scored the items from this tool for each trial. The senior author (EA) adjudicated disagreements during the initial assessment.
Data extraction
The texts, tables or images from the included trials were assessed to extract the number of participants, number of events, means, standard deviations, standard error of means and 95% confidence intervals (CIs). If an included trial did not indicate the sample size or failed to describe the results as a mean and standard deviation or standard error of the mean and 95% CI, we attempted to contact the corresponding author three times via e-mail. We requested access to the missing data or alternately to the complete dataset, and if we were unable to obtain these additional elements, we employed the median and interquartile range as approximations of the mean and standard deviation, by estimating the mean as equivalent to the median, and the standard deviation as the interquartile range divided by 1.35 or the range divided by 4.13 If needed, data were extracted from figures using Plotdigitizer (https://plotdigitizer.com). All opioids were converted to equianalgesic intravenous (i.v.) morphine doses (i.v. morphine 10 mg = oral morphine 30 mg = i.v. tramadol 100 mg = i.v. pethidine 75 mg = i.v. fentanyl 100 μg = i.v. nalbuphine 10 mg = oral hydrocodone 30 mg = oral oxycodone 30 mg = oral codeine 165 mg).2 For pain scores reported on a 0 to 10 verbal, visual or numeric rating scale, we accepted these as analogue data for the purpose of statistical evaluation. When maximum and minimum pain scores were given, we elected to include the minimum pain score. Finally, the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group system was used to evaluate the quality of evidence for each reported outcome.
Statistical analysis
All meta-analyses were conducted using the Review Manager (RevMan, Computer program, version 5.4, The Cochrane Collaboration, 2020). For continuous data, this software estimates the weighted mean differences, and similarly the risk ratio for categorical data between groups, with an overall estimate of the pooled effect. If two or more included trials presented an outcome, we conducted a meta-analysis. We set predetermined limits for low (25 to 49%), moderate (50 to 74%) and high (≥75%) heterogeneity based on the calculated I2 coefficient.14 A random effects model was employed when heterogeneity was found to be moderate or high; otherwise, a fixed effects model was applied.15
To account for potential contributors to heterogeneity, we performed subgroup analyses for our primary outcome (rest pain score at 24 postoperative hours) according to the type of block (peripheral nerve versus field blocks), the nerve block technique (ultrasound versus nerve stimulation), the dose of liposomal bupivacaine administered (doses ≤ 133 mg versus doses from 134 to 266 mg), the comparator (bupivacaine, levobupivacaine or ropivacaine), the combination or not with adjuncts other than epinephrine, the anaesthetic strategy (general versus spinal anaesthesia or no additional anaesthesia), the presence of baseline analgesia defined as the prescription of two nonopioid analgesics and whether a conflict of interest was declared or not.
The risk of publication bias associated with the primary outcome was estimated by drawing a funnel plot of the mean difference standard error of rest pain score at 24 postoperative hours (y-axis) as a function of the mean difference of rest pain score at 24 postoperative hours (x-axis)16 and confirmed with Duval and Tweedie's trim and fill test.17 This assessment was performed using Comprehensive Meta-analysis Version 2 software (Biostat, Englewood, New Jersey, USA). Finally, trial sequential analysis was performed on the primary outcome to confirm whether firm evidence was reached or not (TSA software version 0.9.5.10 Beta; Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark).
We present results as the mean difference or relative risk with 95% confidence interval and a two-sided P value less than 0.05 was deemed to be significant.
Results
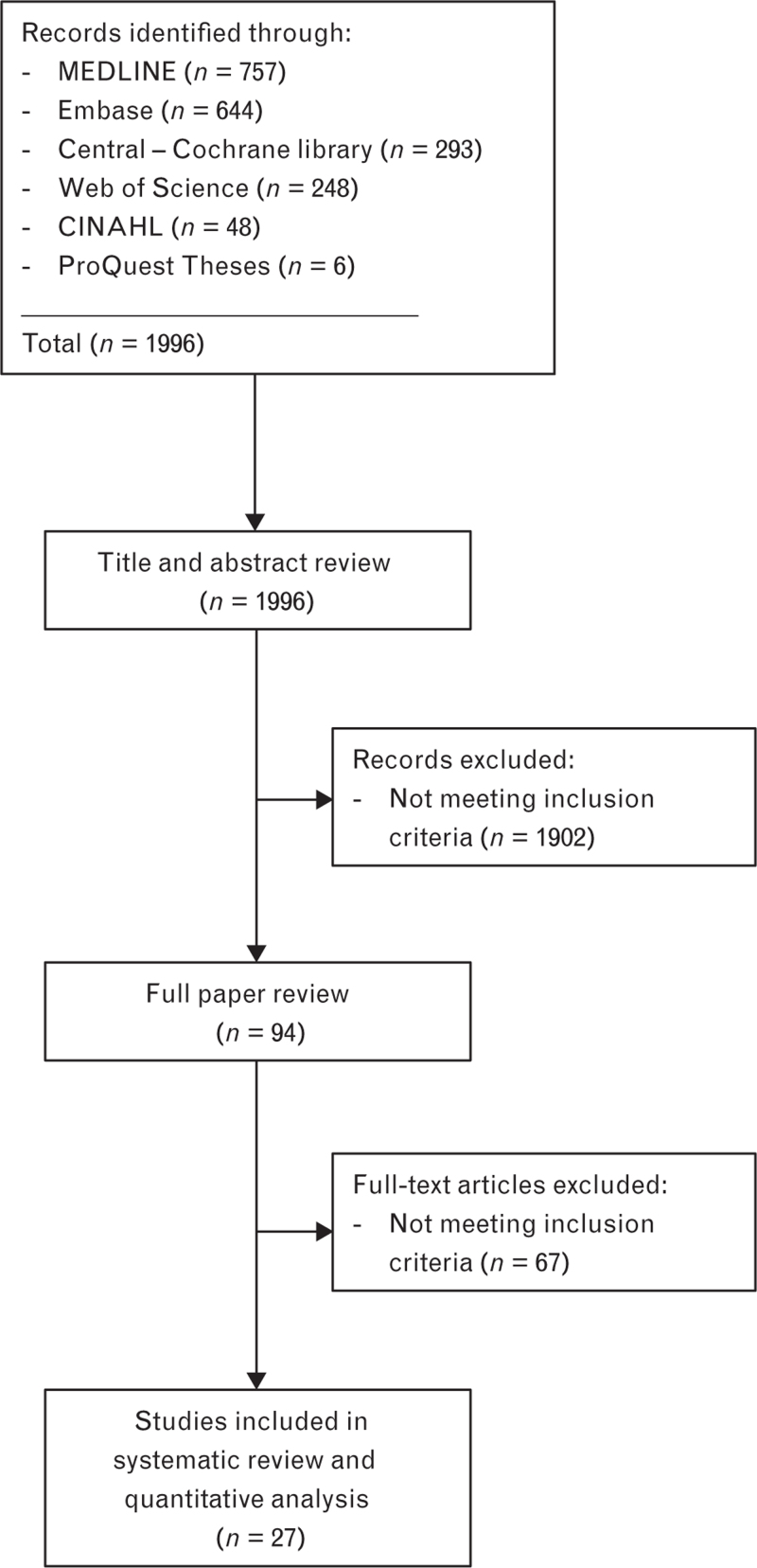
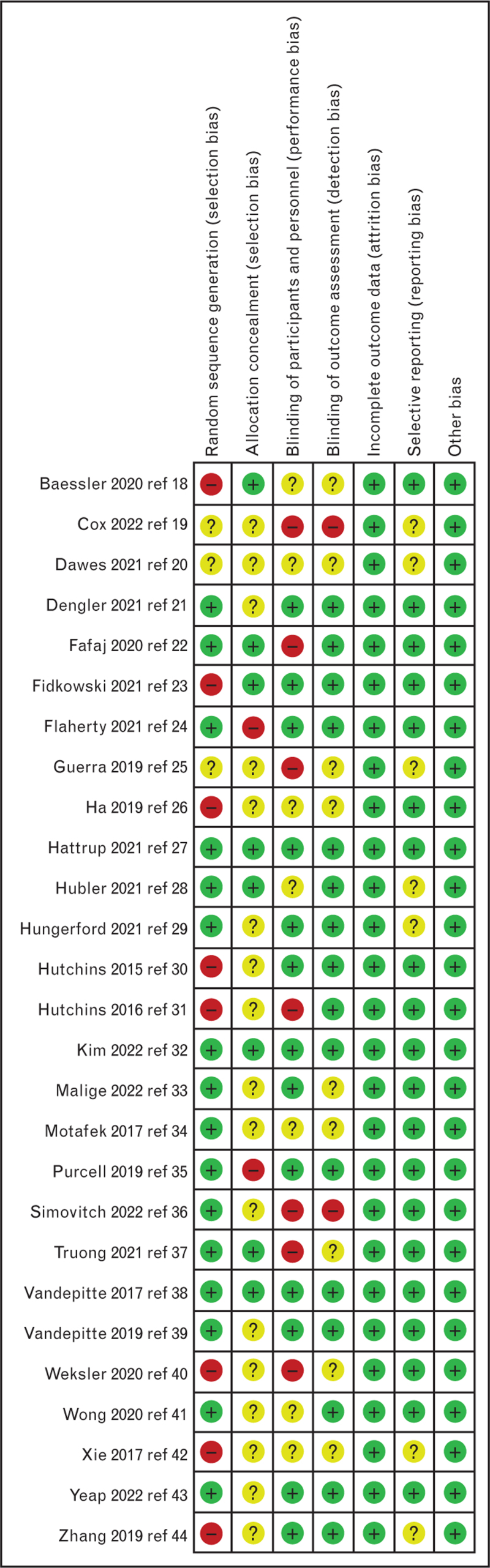
Of the 1996 trials identified from the literature search, 27 met the inclusion criteria,18–44 accounting for a total of 2122 patients. Figure 1 presents the PRISMA flow diagram showing the literature search results and Fig. 2 summarises the risk of bias of the different trials. Seventeen authors were contacted18,21–24,26–28,30,31,34,35,37–40,43 and five provided additional data.18,21,23,26,27
Fig. 1.
PRISMA flow diagram showing literature search results. Twenty-seven randomised controlled trials were included in the analysis.
Fig. 2.
Cochrane collaboration risk of bias summary: evaluation of bias risk items for each included study. Green circle, low risk of bias; red circle, high risk of bias; yellow circle, unclear risk of bias.
Trial characteristics
Table 1 presents the trial characteristics. Eleven trials included patients undergoing orthopaedic surgery,18,20,24,27–29,32,33,35,36,38 six others included patients undergoing breast or gynaecological surgery,21,23,26,30,34,44 six trials included patients undergoing abdominal surgery22,25,31,37,41,43 and four trials included patients who underwent other types of surgery.19,39,40,42 Fifteen trials investigated the efficacy of liposomal bupivacaine on peripheral nerve blocks,18–21,24,27–29,32,33,36,38–40,42 while 12 explored its efficacy on field blocks.22,23,25,26,30,31,34,35,37,41,43,44 Nerve block were performed under ultrasound guidance in 19 trials 18,20,22–24,27–36,38,39,43,44 and following anatomic landmarks in four studies19,21,25,42; in another four studies,26,37,40,41 the surgeon performed the block under direct vision. Liposomal bupivacaine doses ranged from 6539 to 266 mg.22,23,25,26,28,33,35,37,41–44 Of note, 12 trials21,24,27,29,32,33,35–39,43 combined liposomal bupivacaine with bupivacaine, six23,26,30,31,42,44 with 0.9% saline, and four with both. Plain bupivacaine was the active comparator in all trials, except four where ropivacaine was administered20,33,36,42; no trials injected levobupivacaine. The local anaesthetic was mixed with dexamethasone in four studies,18,32,36,43 with epinephrine in two studies34,40 and with both in another study.37 The peripheral nerve block was combined with a general anaesthesia in 23 studies18–27,30–32,34–38,40–44 and with a spinal anaesthesia in two studies29,33; the anaesthetic strategy was not specified in two trials.28,39 Fourteen trials prescribed a multimodal analgesic regimen for the postoperative period.20–22,24,29,32,33,36–41,43 Finally, nine trials declared a conflict of interest.22,24,27,29–31,38–40
Table 1.
Study characteristics
| Intervention | ||||||||
| Reference | Group (n) | Surgery | Peripheral nerve block, technique | Anaesthetic strategy | Liposomal bupivacaine | Long-acting local anaesthetics | Postoperative analgesic regimen | Minimal clinically important difference defined by the authors |
| Baessler et al.18 | Liposomal (n = 26) Control (n = 26) | Rotator cuff repair | Interscalene brachial plexus block, US | General anaesthesia | 1 : 1.5 : 0.5 Liposomal bupivacaine, bupivacaine 0.5% and normal saline, 30 ml, with dexamethasone 4 mg | Bupivacaine 0.5%, 30 ml, with dexamethasone 4mg | Oxycodone | 1.4 units in rest pain score (time interval not specified) |
| Cox et al.19 | Liposomal (n = 24) Control (n = 24) | Ocular evisceration | Retrobulbar nerve block, landmark | General anaesthesia | Liposomal bupivacaine, 10 ml | Bupivacaine 0.75%, 6 ml | Acetaminophen, oxycodone | Not specified |
| Dawes et al.20 | Liposomal (n = 56) Control (n = 58) | Total shoulder arthroplasty | Interscalene brachial plexus block, US | General anaesthesia | Liposomal bupivacaine, 6 ml | Ropivacaine 0.5%, 20 ml | Acetaminophen, NSAID, tramadol | 1.4 units in rest pain score (time interval not specified) |
| Dengler et al.21 | Liposomal (n = 60) Control (n = 60) | Posterior colporraphy | Pudendal nerve block, landmark | General anaesthesia | 1 : 1 Liposomal bupivacaine / bupivacaine 0.25%, 20 ml | Bupivacaine 0.25%, 20 ml | Acetaminophen, NSAID, oxycodone | Not specified |
| Fafaj et al.22 | Liposomal (n = 57) Control (n = 55) | Abdominal wall reconstruction | TAP block, US or direct visualisation by surgeon | General anaesthesia | 1 : 3:2 Liposomal bupivacaine, bupivacaine 0.2% and normal saline, 120 ml | 1 : 1 Bupivacaine 0.25%, and normal saline 120 ml | Acetaminophen, gabapentin, oxycodone, PCA of hydromorphone | 30% reduction in opioid consumption at 72 h |
| Fidkowski et al.23 | Liposomal (n = 27) Control (n = 25) | Open abdominal hysterectomy | TAP block, US | General anaesthesia | 1 : 2 Liposomal bupivacaine and normal saline, 60 ml | Bupivacaine 0.25%, 60 ml | NSAID, oral opioid, PCA of morphine | 35% reduction in opioid consumption at 72 h |
| Flaherty et al.24 | Liposomal (n = 35) Control (n = 35) | Rotator cuff repair | Interscalene brachial plexus block, US | General anaesthesia | 1 : 1 Liposomal bupivacaine / bupivacaine 0.5%, 20 ml | Bupivacaine 0.5%, 20 ml | Acetaminophen, NSAID, oxycodone | 1.4 units in rest pain score (time interval not specified) |
| Guerra et al.25 | Liposomal (n = 50) Control (n = 50) | Laparoscopic colorectal surgery | TAP block, landmark | General anaesthesia | 1 : 2:1 Liposomal bupivacaine, bupivacaine 0.2% and normal saline, 80 ml | Bupivacaine 0.25%, 80 ml | Hydromorphone, morphine | Not specified |
| Ha et al.26 | Liposomal (n = 22) Control (n = 22) | Breast reconstruction | TAP block, direct visualisation by surgeon | General anaesthesia | 2 : 1 Liposomal bupivacaine and normal saline, 30 ml | Bupivacaine 0.25%, 30 ml | Oxycodone, hydromorphone | 20 mg in opioid consumption (time interval not specified) |
| Hattrup et al.27 | Liposomal (n = 52) Control (n = 52) | Total shoulder arthroplasty | Interscalene brachial plexus block, US | General anaesthesia | 1 : 1.5 Liposomal bupivacaine, bupivacaine 0.375%, 25 ml | Bupivacaine 0.5%, 20 ml | Acetaminophen, oxycodone, tramadol, fentanyl | 2 units in rest pain score (time interval not specified) |
| Hubler et al.28 | Liposomal (n = 31) Control (n = 32) | Total knee arthroplasty | Adductor canal block, US | Not specified | Liposomal bupivacaine, 20 ml | Bupivacaine 0.5%, 20 ml | NSAID, oxycodone, hydromorphone | 0.9 units in rest pain score (time interval not specified) |
| Hungerford et al.29 | Liposomal (n = 46) Control (n = 54) | Total knee arthroplasty | Adductor canal block, US | Spinal anaesthesia | 1 : 1.5 Liposomal bupivacaine, bupivacaine 0.5%, 25 ml | Bupivacaine 0.5%, 25 ml | Acetaminophen, NSAID, oxycodone | 1 unit in rest pain score (time interval not specified) |
| Hutchins et al.30 | Liposomal (n = 28) Control (n = 30) | Robotic assisted hysterectomy | TAP block, US | General anaesthesia | 1 : 2 Liposomal bupivacaine and normal saline, 30 ml | Bupivacaine 0.25%, 30 ml | Hydromorphone, fentanyl | Not specified |
| Hutchins et al.31 | Liposomal (n = 30) Control (n = 29) | Laparoscopic nephrectomy | TAP block, US | General anaesthesia | 1 : 2 Liposomal bupivacaine and normal saline, 30 ml | Bupivacaine 0.25%, 30 ml | NSAID, oxycodone, hydromorphone, morphine, fentanyl | Not specified |
| Kim et al.32 | Liposomal (n = 55) Control (n = 56) | Shoulder surgery | Interscalene brachial plexus block, US | General anaesthesia | 2 : 1 Liposomal bupivacaine and bupivacaine 0.5%, 15 ml | Bupivacaine 0.5%, 15 ml, with dexamethasone 4 mg | Acetaminophen, NSAID, oxycodone, tramadol, hydromorphone | 1.3 units in rest pain score (time interval not specified) |
| Malige et al.33 | Liposomal (n = 50) Control (n = 50) | Total knee arthroplasty | Adductor canal block, US | Spinal anaesthesia | 2 : 0.5 Liposomal bupivacaine, bupivacaine 0.5%, 25 ml | Ropivacaine 0.2%, 25 ml | Acetaminophen, gabapentin, hydromorphone, methocarbamol | 28% reduction in rest pain score (time interval not specified) |
| Motakef et al.34 | Liposomal (n = 12) Control (n = 12) | Breast reconstruction | Interpectoral plane block, US | General anaesthesia | Liposomal bupivacaine, 10 ml | Bupivacaine 0.25% with epinephrine 5 μg ml−1, 20 ml | Acetaminophen, hydrocodone, hydromorphone | Not specified |
| Purcell et al.35 | Liposomal (n = 33) Control (n = 37) | Hip arthroscopy | Fascia iliaca block, US | General anaesthesia | 1 : 1 Liposomal bupivacaine, and bupivacaine 0.5%, 40 ml | Bupivacaine 0.25%, 20 ml | Oxycodone, NSAID, opioid | 2 units in rest pain score (time interval not specified) |
| Simovitch et al.36 | Liposomal (n = 45) Control (n = 44) | Arthroscopic rotator cuff | Interscalene brachial plexus block, US | General anaesthesia | 1 : 1 Liposomal bupivacaine, and bupivacaine 0.5%, 20 ml | Ropivacaine 0.5%, 30 ml, with dexamethasone 8 mg | Acetaminophen, NSAID, oxycodone/acetaminophen, hydrocodone/acetaminophen | 1 unit in rest pain score (time interval not specified) |
| Truong et al.37 | Liposomal (n = 51) Control (n = 50) | Minimal invasive colorectal surgery | TAP block, direct visualisation per surgery | General anaesthesia | 2 : 1 Liposomal bupivacaine and bupivacaine 0.5%, 30 ml | 1 : 1 Bupivacaine 0.25% with epinephrine 2.5 μg ml−1, 30 ml, with dexamethasone 8 mg | Acetaminophen, NSAID, oxycodone, gabapentin, tramadol, opioid | 15 mg in opioid consumption at 48 h |
| Vandepitte et al.38 | Liposomal (n = 26) Control (n = 24) | Major shoulder surgery | Interscalene brachial plexus block, US | General anaesthesia | 2 : 1 Liposomal bupivacaine and bupivacaine 0.25%, 15 ml | Bupivacaine 0.25%, 15 ml | Acetaminophen, NSAID, dexamethasone, tramadol | 3 units in rest pain score (time interval not specified) |
| Vandepitte et al.39 | Liposomal (n = 16) Control (n = 16) | Dupuytren contracture release | Median and ulnar nerve blocks at the forearm, US | Not specified | 2 : 1 Liposomal bupivacaine and bupivacaine 0.5%, 7.5 ml | Bupivacaine 0.5%, 7.5 ml | Acetaminophen, NSAID, tramadol | Not specified |
| Weksler et al.40 | Liposomal (n = 25) Control (n = 25) | Video-assisted thoracic surgery | Port site infiltration and intercostal nerve block, direct visualisation by surgeon | General anaesthesia | Liposomal bupivacaine, 10 ml | Bupivacaine with epinephrine 5 μg ml−1, 10 ml, concentration not specified | Acetaminophen, NSAID, oxycodone, PCA of morphine or hydromorphone | 25% in opioid consumption during hospital length of stay |
| Wong et al.41 | Liposomal (n = 75) Control (n = 73) | Bariatric surgery | TAP block, direct visualisation by surgeon | General anaesthesia | 2 : 3:10 Liposomal bupivacaine, bupivacaine 0.25% and normal saline,150 ml | 1 : 2 Bupivacaine 0.25% with normal saline, 150 ml | Acetaminophen, NSAID, morphine, PCA of fentanyl | Not specified |
| Xie et al.42 | Liposomal (n = 40) Control (n = 47) | Penile prothesis | Dorsale penile and penile ring block, landmark | General anaesthesia | 2 : 1 Liposomal bupivacaine and normal saline, 30 ml | Ropivacaine 0.5%, 30 ml | Acetaminophen, oxycodone, morphine | Not specified |
| Yeap et al.43 | Liposomal (n = 38) Control (n = 40) | Colorectal surgery | Quadratus lumborum block, US | General anaesthesia | 1 : 2 Liposomal bupivacaine and bupivacaine 0.125%, 60 ml, with dexamethasone 4 mg | Bupivacaine 0.25%, 60 ml, with dexamethasone 4 mg | Acetaminophen, gabapentin, oxycodone, hydromorphone | 60% in opioid consumption (time interval not specified) |
| Zhang et al.44 | Liposomal (n = 43) Control (n = 43) | Unilateral mastectomy | Interpectoral plane block, US | General anaesthesia | 2 : 3 Liposomal bupivacaine, and normal saline, 50 ml | Bupivacaine 0.5%, 30 ml | Not specified | Not specified |
Iv, intravenous; PCA, patient-controlled analgesia; TAP, transversus abdominis plane; US, ultrasound.
Primary outcome
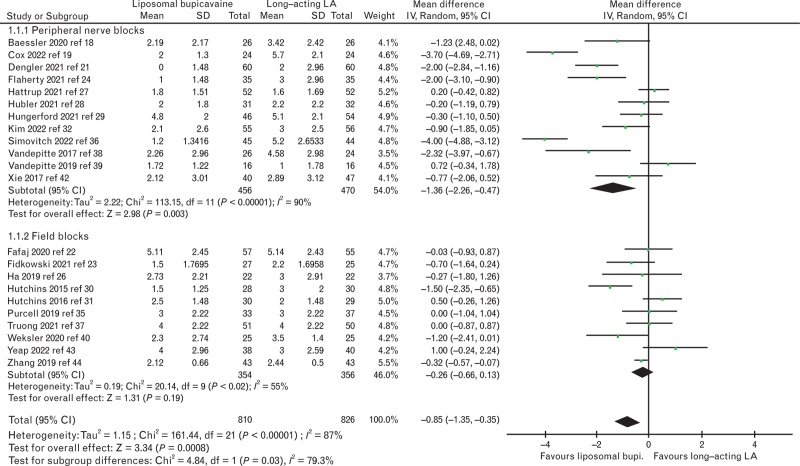
On the basis of 22 trials with a total of 1636 patients,18,19,21–24,26–32,35–40,42–44 the pain score at rest at 24 postoperative hours was significantly reduced in patients receiving liposomal bupivacaine. For both types of block combined, the mean difference (95% CI) was -0.9 (-1.4 to -0.4), I2 = 87%, P < 0.001. When analysed by subgroup, there was no difference in the field block group; there was a significant difference between the block groups (P = 0.03, Fig. 3). There was also a subgroup difference in doses of liposomal bupivacaine administered with a higher mean difference in the low-dose group (up to 133 mg: mean difference (95% CI) of -1.3 (-2.2 to -0.5), I2 = 91%, P = 0.002; 134 to 266 mg: mean difference (95% CI) of -0.3 (-0.5 to -0.1), I2 = 0%, P = 0.01; subgroup difference: P = 0.01). On the contrary, there was no subgroup difference when our primary outcome was analysed according to the nerve block technique (P = 0.29), type of local anaesthetics (P = 0.28), the combination or not with adjuncts (P = 0.46), the anaesthetic strategy (P = 0.05), the presence of baseline analgesia (P = 0.61) or whether a conflict of interest was declared or not (P = 0.35). The trial sequential analysis indicated that firm evidence was reached regarding the superiority of liposomal bupivacaine over the other long-acting local anaesthetics (supplementary Figure 1). The funnel plot reveals absence of risk of publication for the primary outcome (supplementary Figure 2), confirmed with the Duval and Tweedie's Trim and Fill test; with a random effects model: this test calculated the point estimate for the combined studies to be -0.40 (95% CI: -0.64 to -0.16); using Trim and Fill, these values were unchanged, suggesting that no studies are missing.
Fig. 3.
Rest pain score at 24 postoperative hours in patients receiving liposomal bupivacaine or long-acting local anaesthetics (bupivacaine, ropivacaine) analysed according to peripheral nerve versus field blocks. LA, local anaesthetics.
Secondary outcomes
Pain scores at rest at 48 and 72 postoperative hours were also significantly reduced, as opposed to the majority of the other secondary outcomes (Table 2). Two trials looked at the incidence of LAST and reported none23,39; no trials investigated the incidence of nerve injury.
Table 2.
Secondary pain-related outcomes
| Total number of patients | |||||||
| Outcome | Number of trials | References | Liposomal Bupivacaine |
Long-acting local anaesthetics | Mean difference [95% CI] or relative risk [95%CI] | I2 (%) | P value for overall effect |
| Rest pain score at 2 postoperative hours (analogue scale 0 to 10) | 15 | Baessler et al.,18 Cox et al., Fafaj et al.,22 Flaherty et al.,24 Ha et al.,26 Hutchins et al.,30 Hutchins et al.,31 Kim et al.,32 Purcell et al.,35 Simovitch et al.,36 Truong et al.,37 Weksler et al.,40 Xie et al.,42 Yeap et al.,43 Zhang et al.44 | 552 | 563 | -0.3 [-0.7 to 0.1] | 70 | 0.19 |
| Rest pain score at 48 postoperative hours; analogue scale 0 to 10 | 24 | Baessler et al.,18 Cox et al.,19 Dengler et al.,21 Fafaj et al.,22 Fidkowski et al.,23 Flaherty et al.,24 Ha et al.,26 Hattrup et al.,27 Hubler et al.,28 Hungerford et al.,29 Hutchins et al.,30 Hutchins et al.,31 Kim et al.,32 Purcell et al.,35 Simovitch et al.,36 Truong et al.,37 Vandepitte et al.,3830Vandepitte et al.,39 Weksler et al.,40 Wong et al.,41 Xie et al.,42 Yeap et al.,43 Zhang et al.44 | 871 | 888 | -0.7 [-1.1 to -0.3] | 82 | 0.001 |
| Rest pain score at 72 postoperative hours; analogue scale 0 to 10 | 21 | Baessler et al.,18 Cox 2022,19 Dengler 2021,21 Fafaj 2020,22 Fidkowski 2021,23 Flaherty 2021,24 Ha 2019,26 Hattrup 2021,27 Hubler 2021,28 Hungerford 2021,29 Hutchins 2015,30 Hutchins 201631, Kim 2022,32 Purcell 2019,35 Simovitch 2022,36 Truong 2021,37 Vandepitte 2017,38 Vandepitte 2019,39 Xie 2017,42 Yeap 2022,43 Zhang 201944 | 775 | 795 | -0.7 [-1.1 to -0.3] | 80 | <0.001 |
| Interval iv morphine consumption at 0 to 24 postoperative hours (mg) | 17 | Baessler et al.,18 Dawes et al.,20 Dengler et al.,21 Fafaj et al.,22 Fidkowski et al.,23 Flaherty et al.,24 Hubler et al.,28 Hungerford et al.,29 Hutchins et al.,30 Hutchins et al.,31 Kim et al.,32 Purcell et al.,35 Simovitch et al., Truong et al.,37 Vandepitte et al.,38 Weksler et al.,40 Xie et al.42, Yeap et al.43 | 694 | 720 | -2.5 [-5.8 to 0.9] | 97 | 0.13 |
| Interval iv morphine consumption at 24 to 48 postoperative hours (mg) | 14 | Dawes et al.,20 Fafaj et al.,22 Fidkowski et al.,23 Flaherty et al.,24 Hubler et al.,28 Hungerford et al.,29 Hutchins et al.,30 Hutchins et al.,31 Kim et al.,32 Purcell et al.,35 Simovitch et al., Truong et al.,37 Vandepitte et al.,38 Weksler et al.,40 Yeap et al.43 | 570 | 583 | -2.4 [-5.9 to 0.8] | 95 | 0.14 |
| Interval iv morphine consumption at 48 to 72 postoperative hours (mg) | 13 | Baessler et al.,18 Fafaj et al.,22 Fidkowski et al.,23 Flaherty et al.,24 Hubler et al.,28 Hungerford et al.,29 Hutchins et al.,30 Hutchins et al.,31 Kim et al.,32 Purcell et al.,35 Simovitch et al., Truong et al.,37 Vandepitte et al.38, Yeap et al.43 | 528 | 539 | -1.7 [-3.6 to 0.2] | 86 | 0.06 |
| Presence of nausea or vomiting at 24 postoperative hours | 2 | Hubler et al.28, Yeap et al.43 | 69 | 72 | 1.1 [0.5 to 2.1]a | 0 | 0.86 |
| Presence of nausea or vomiting at 48 postoperative hours | 2 | Hubler et al.28, Yeap et al.43 | 69 | 72 | 0.9 [0.4 to 2.0]a | 12 | 0.81 |
| Presence of nausea or vomiting at 72 postoperative hours | 6 | Hubler et al.,28 Hutchins et al.,30 Hutchins et al.,31 Motafek et al.,34 Vandepitte et al.39, Yeap et al.43 | 155 | 159 | 0.6 [0.3 to 0.9]a | 29 | 0.02 |
| Hospital length of stay (h) | 16 | Dawes et al.,20 Dengler et al.,21 Fafaj et al.,22 Fidkowski et al.,23 Guerra et al.,25 Ha et al.,26 Hattrup et al.,27 Hubler et al., Hungerford et al.,29 Hutchins et al.,30 Hutchins et al.,31 Purcell et al.,35 Truong et al.,37 Weksler et al.,40 Wong et al.,41 Zhang et al. | 686 | 695 | -0.6 [-1.4 to 0.3] | 48 | 0.20 |
CI, confidence interval; iv, intravenous.
These are relative risk (95% confidence interval).
Quality of evidence
According to the GRADE system, the quality of evidence was moderate for our primary and secondary outcomes (Table 3).
Table 3.
Quality of evidence assessment for each outcome
| Quality assessment | Summary of findings | |||||||
| Outcome | Limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Total number of participants | Conclusion | Quality of evidence (GRADE) |
| Rest pain score at 2 postoperative hours (analogue scale, 0–10) | No major limitationsa | Serious inconsistencyb | No serious indirectnessc | No serious imprecisiond | No publication bias | 1115 | No difference between groups | Moderate quality (⊕⊕⊕O)e |
| Rest pain score at 24 postoperative hours (analogue scale, 0–10) | No major limitationsa | Serious inconsistencyb | No serious indirectnessc | No serious imprecisiond | No publication bias | 1636 | Reduced rest pain score in liposomal bupivacaine group | Moderate quality (⊕⊕⊕O)e |
| Rest pain score at 48 postoperative hours (analogue scale, 0–10) | No major limitationsa | Serious inconsistencyb | No serious indirectnessc | No serious imprecisiond | No publication bias | 1759 | Reduced rest pain score in liposomal bupivacaine group | Moderate quality (⊕⊕⊕O)e |
| Rest pain score at 72 postoperative hours (analogue scale, 0–10) | No major limitationsa | Serious inconsistencyb | No serious indirectnessc | No serious imprecisiond | No publication bias | 1570 | Reduced rest pain score in liposomal bupivacaine group | Moderate quality (⊕⊕⊕O)e |
| Interval iv morphine equivalent consumption at 0–24 postoperative hours | No major limitationsa | Serious inconsistencyb | No serious indirectnessc | No serious imprecisiond | No publication bias | 1414 | No difference between groups | Moderate quality (⊕⊕⊕O)e |
| Interval iv morphine equivalent consumption at 24–48 postoperative hours | No major limitationsa | Serious inconsistencyb | No serious indirectnessc | No serious imprecisiond | No publication bias | 1153 | No difference between groups | Moderate quality (⊕⊕⊕O)e |
| Interval iv morphine equivalent consumption at 48–72 postoperative hours | No major limitationsa | Serious inconsistencyb | No serious indirectnessc | No serious imprecisiond | No publication bias | 1067 | No difference between groups | Moderate quality (⊕⊕⊕O)e |
| Presence of nausea or vomiting at 24 postoperative hours | No major limitationsa | No serious inconsistency | No serious indirectnessc | No serious imprecisiond | No publication bias | 141 | No difference between groups | Moderate quality (⊕⊕⊕O)f |
| Presence of nausea or vomiting at 48 postoperative hours | No major limitationsa | No serious inconsistency | No serious indirectnessc | No serious imprecisiond | No publication bias | 141 | No difference between groups | Moderate quality (⊕⊕⊕O)f |
| Presence of nausea or vomiting at 72 postoperative hours | No major limitationsa | No serious inconsistency | No serious indirectnessc | No serious imprecisiond | No publication bias | 314 | Presence of nausea or vomiting reduced in liposomal bupivacaine group | Moderate quality (⊕⊕⊕O)f |
| Hospital length of stay (hours) | No major limitationsa | Serious inconsistencyb | No serious indirectnessc | No serious imprecisiond | No publication bias | 1381 | No difference between groups | Moderate quality (⊕⊕⊕O)e |
As only a limited number of studies suffered from a high-risk of bias, we estimated there is no major limitation.
I2 above 50%.
Consistent definition of the reported outcome.
No serious imprecision as the clinical decision would not be modified whether the upper of lower boundary limit of the confidence interval represented the truth.
We rated down the quality of evidence for serious inconsistency.
We rated down for limitations, as six trials or less reported this outcome.
Discussion
This systematic review and meta-analysis with trial sequential analysis explored the analgesic efficacy of liposomal bupivacaine for peripheral nerve blocks when compared with any long-acting local anaesthetics. On the basis of the analysis of 27 randomised controlled trials representing a total of 2122 patients, we established that liposomal bupivacaine statistically decreased pain scores at rest at 24, 48 and 72 postoperative hours, but without an impact on interval morphine consumption or postoperative nausea and vomiting. The significant difference in the presence of nausea or vomiting at 72 postoperative hours is based on six trials and 314 patients and might represent a type I error in the setting of an equivalent opioid consumption between groups. Our subgroup analysis according to the type of block revealed that there is no significant difference in pains scores at rest when liposomal bupivacaine is compared with long-acting local anaesthetics for field blocks. Firm evidence is reached according to the trial sequential analysis and the quality of evidence is moderate for all outcomes following the GRADE assessment system.
Some discussion of the small mean difference in pain scores at rest (< one unit) between groups at 24, 48 and 72 postoperative hours is required. Twenty years ago, the question of what represents a minimal clinically important difference in pain scores was investigated.45,46 After examining 2724 patients, Farrar et al.45 concluded that a reduction of 30% or two points on an 11-unit pain score scale represents a clinically important difference among patients suffering from medical conditions, while Cepeda et al.46 stated that a difference of 1.3 units or a 20% reduction is clinically relevant after investigating 700 patients who underwent all types of surgery. More recently, after enrolling 224 patients undergoing different types of surgery, Myles et al.47 determined that a one-unit difference in pain score is a relevant improvement in contemporary practice. In this meta-analysis, the mean difference between groups is consistently less than one unit up to postoperative hour 72 and thus does not reach the threshold of clinical relevance: and there was no reduction in opioid consumption. These facts question the administration of liposomal bupivacaine in the clinical practice, especially in light of the high cost of the medication: liposomal bupivacaine is 100 times more expensive than bupivacaine.48
Of note, among the different peripheral nerve blocks, liposomal bupivacaine is approved in adults for the interscalene brachial plexus block by the United States Food and Drug Administration,6 and for brachial plexus, femoral nerve and field blocks by the European Medicines Agency,7 which means that administration of liposomal bupivacaine for other peripheral nerve blocks represent an off-label route of administration. This raises questions about the use of liposomal bupivacaine for brachial plexus blocks, as the neural structures of this plexus are more prone to injury secondary to its elevated ratio of neural/connective tissue when compared with more distal nerves.49 Despite our willingness to report the incidence of nerve injury, we were unable to draw any conclusion since this outcome was not sought by the included trials.
This meta-analysis contains several weaknesses. First, we were confronted with an elevated heterogeneity coefficient in our primary outcome that we could not explain with our hypotheses and different subgroup analyses. Indeed, differences in the types of blocks, doses of liposomal bupivacaine administered, adjuncts used, nerve block technique, types of local anaesthetics, anaesthetic strategy or prescription of baseline analgesia were parameters that only partially reduced the heterogeneity. Other factors might impact this heterogeneity such as the spread of the local anaesthetics, which is difficult to statistically assess, especially when it is not described in the different articles. Second, we included seven trials18,32,34,36,37,40,43 that combined long-acting local anaesthetic with a perineural adjunct such as epinephrine or dexamethasone; however, as the analgesic duration of such a combination would last maximum 24 h, we do not believe it reduces the impact of our findings. Finally, none of the trials compared liposomal bupivacaine with levobupivacaine; however, it is doubtful that results would differ from current comparators such as bupivacaine or ropivacaine.
To conclude, there is moderate level evidence that liposomal bupivacaine reduces rest pain scores by 0.9 out of 10 units, when compared with long-acting local anaesthetics at 24 hours after surgery, and by 0.7 up to 72 hours after surgery.
Supplementary Material
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: we are grateful to Mrs Cécile Jaques and Jolanda Elmers (Medical Library, Research and Education Department, Lausanne University Hospital, Switzerland) for their assistance in the literature search.
Financial support and sponsorship: this work was supported by departmental funding (Department of Anaesthesia, University Hospital of Lausanne, Lausanne, Switzerland).
Conflicts of interest: EA has received grants from the Swiss Academy for Anaesthesia Research (SACAR), Lausanne, Switzerland (no grant numbers attributed), from B. Braun Medical AG, Sempach, Switzerland (no grant numbers attributed) and from the Swiss National Science Foundation to support his clinical research. EA has also received an honorarium from B. Braun Medical AG Switzerland, from Sintetica Ltd UK and MSD AG Switzerland. SG has received an honorarium from MSD AG Switzerland. No conflict of interest declared by the other authors.
Presentation: ESAIC 2022, Milano, Italy.
This manuscript was handled by Esther M. Pogatzki-Zahn.
Footnotes
Supplemental digital content is available for this article.
References
- 1.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016; 17:131–157. [DOI] [PubMed] [Google Scholar]
- 2.Baeriswyl M, Kirkham KR, Jacot-Guillarmod A, et al. Efficacy of perineural vs systemic dexamethasone to prolong analgesia after peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth 2017; 119:183–191. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht E, Kern C, Kirkham KR. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia 2015; 70:71–83. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht E, Reynvoet M, Fournier N, et al. Dose-response relationship of perineural dexamethasone for interscalene brachial plexus block: a randomised, controlled, triple-blind trial. Anaesthesia 2019; 74:1001–1008. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht E, Chin KJ. Advances in regional anaesthesia and acute pain management: a narrative review. Anaesthesia 2020; 75: (Suppl 1): e101–e110. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022496s035lbl.pdf [Accessed 24 July 2022]. [Google Scholar]
- 7.European Medicines Agency. European Medicines Agency. https://www.ema.europa.eu/en/documents/product-information/exparel-liposomal-epar-product-information_en.pdf. [Accessed 16 December 2022]. [Google Scholar]
- 8.Hussain N, Brull R, Sheehy B, et al. Perineural liposomal bupivacaine is not superior to nonliposomal bupivacaine for peripheral nerve block analgesia. Anesthesiology 2021; 134:147–164. [DOI] [PubMed] [Google Scholar]
- 9.Dinges H-C, Wiesmann T, Otremba B, et al. The analgesic efficacy of liposomal bupivacaine compared with bupivacaine hydrochloride for the prevention of postoperative pain: a systematic review and meta-analysis with trial sequential analysis. Reg Anesth Pain Med 2021; 46:490–498. [DOI] [PubMed] [Google Scholar]
- 10.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of healthcare interventions: checklist and explanations. Ann Intern Med 2015; 162:777–784. [DOI] [PubMed] [Google Scholar]
- 11.Grape S, Kirkham KR, Albrecht E. Transversus abdominis plane block versus local anaesthetic wound infiltration for analgesia after caesarean section: a systematic review and meta-analysis with trial sequential analysis. Eur J Anaesth 2022; 39:244–251. [DOI] [PubMed] [Google Scholar]
- 12.Grape S, Kirkham KR, Albrecht E. The analgesic efficacy of transversus abdominis plane block vs. wound infiltration after inguinal and infra-umbilical hernia repairs: a systematic review and meta-analysis with trial sequential analysis. Eur J Anaesth 2022; 39:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochrane Handbook for systematic reviews of interventions. https://training.cochrane.org/handbook/current. [Accessed 10 March 2022]. [Google Scholar]
- 14.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Me 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 15.Choi SW, Lam DMH. Heterogeneity in meta-analyses. Comparing apples and oranges? Anaesthesia 2017; 72:532–534. [DOI] [PubMed] [Google Scholar]
- 16.Carlisle JB. Systematic reviews: how they work and how to use them. Anaesthesia 2007; 62:702–707. [DOI] [PubMed] [Google Scholar]
- 17.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 18.Baessler AM, Moor M, Conrad DJ, et al. Single-shot liposomal bupivacaine reduces postoperative narcotic use following outpatient rotator cuff repair: a prospective, double-blinded, randomized controlled trial. J Bone Joint Surg 2020; 102:1985–1992. [DOI] [PubMed] [Google Scholar]
- 19.Cox SG, Vicinanzo MG, Jacobs SM, et al. Liposomal bupivacaine suspension for pain control following ocular evisceration surgery. Ophthalmic Plast Reconstr Surg 2022; 38:263–265. [DOI] [PubMed] [Google Scholar]
- 20.Dawes AM, Spencer CC, Guisse N, et al. A comparison of liposomal bupivacaine to standard ropivacaine used in interscalene blocks for patients undergoing total shoulder arthroplasty. J Shoulder Elbow Surg 2021; 31:117–124. [Google Scholar]
- 21.Dengler KL, Craig ER, DiCarlo-Meacham AM, et al. Preoperative pudendal block with liposomal and plain bupivacaine reduces pain associated with posterior colporrhaphy: a double-blinded, randomized controlled trial. Am J Obstet Gynecol 2021; 225:556e1–556e10. [DOI] [PubMed] [Google Scholar]
- 22.Fafaj A, Krpata DM, Petro CC, et al. The efficacy of liposomal bupivacaine on postoperative pain following abdominal wall reconstruction: a randomized, double-blind placebo-controlled trial. Ann Surg 2022; 276:224–232. [DOI] [PubMed] [Google Scholar]
- 23.Fidkowski CW, Choksi N, Alsaden M-R. A randomized-controlled trial comparing liposomal bupivacaine, plain bupivacaine, and the mixture of liposomal bupivacaine and plain bupivacaine in transversus abdominus plane block for postoperative analgesia for open abdominal hysterectomies. Can J Anesth 2021; 68:773–781. [DOI] [PubMed] [Google Scholar]
- 24.Flaherty JM, Berg AA, Harrison A, et al. Comparing liposomal bupivacaine plus bupivacaine to bupivacaine alone in interscalene blocks for rotator cuff repair surgery: a randomized clinical trial. Reg Anesth Pain Med 2022. [DOI] [PubMed] [Google Scholar]
- 25.Guerra L, Philip S, Lax EA, et al. Transversus abdominis plane blocks in laparoscopic colorectal surgery: better pain control and patient outcomes with liposomal bupivacaine than bupivacaine. Am Surg 2019; 85:1013–1016. [PubMed] [Google Scholar]
- 26.Ha AY, Keane G, Parikh R, et al. The analgesic effects of liposomal bupivacaine versus bupivacaine hydrochloride administered as a transversus abdominis plane block after abdominally based autologous microvascular breast reconstruction: a prospective, single-blind, randomized, controlled trial. Plast Reconstru Surg 2019; 144:35–44. [DOI] [PubMed] [Google Scholar]
- 27.Hattrup SJ, Chung AS, Rosenfeld DM, et al. Liposomal bupivacaine interscalene nerve block in shoulder arthroplasty is not superior to plain bupivacaine: a double-blinded prospective randomized control trial. J Shoulder Eldow Surg 2021; 30:587–598. [DOI] [PubMed] [Google Scholar]
- 28.Hubler CP, Bevil KM, Greiner JJ, et al. Liposomal bupivacaine versus standard bupivacaine in the adductor canal for total knee arthroplasty: a randomized, controlled trial. Orthopedics 2021; 44:249–255. [DOI] [PubMed] [Google Scholar]
- 29.Hungerford M, Neubauer P, Ciotola J, et al. Liposomal bupivacaine vs ropivacaine for adductor canal blocks in total knee arthroplasty: a prospective randomized trial. J Arthroplast 2021; 36:3915–3921. [DOI] [PubMed] [Google Scholar]
- 30.Hutchins J, Delaney D, Vogel RI, et al. Ultrasound guided subcostal transversus abdominis plane (TAP) infiltration with liposomal bupivacaine for patients undergoing robotic assisted hysterectomy: a prospective randomized controlled study. Gynecol Oncol 2015; 138:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchins JL, Kesha R, Blanco F, et al. Ultrasound-guided subcostal transversus abdominis plane blocks with liposomal bupivacaine vs. nonliposomal bupivacaine for postoperative pain control after laparoscopic hand-assisted donor nephrectomy: a prospective randomised observer-blinded study. Anaesthesia 2016; 71:930–937. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Liu J, Beathe JC, et al. Interscalene brachial plexus block with liposomal bupivacaine versus standard bupivacaine with perineural dexamethasone: a noninferiority trial. Anesthesiology 2022; 136:434–447. [DOI] [PubMed] [Google Scholar]
- 33.Malige A, Pellegrino AN, Kunkle K, et al. Liposomal bupivacaine in adductor canal blocks before total knee arthroplasty leads to improved postoperative outcomes: a randomized controlled trial. J Arthroplast 2022; 37:1549–1556. [DOI] [PubMed] [Google Scholar]
- 34.Motakef S, Wong WW, Ingargiola MJ, et al. Liposomal bupivacaine in implant-based breast reconstruction. Plast Reconstr Surg Glob Open 2017; 5:e1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purcell RL, Brooks DI, Steelman TJ, et al. Fascia iliaca blockade with the addition of liposomal bupivacaine versus plain bupivacaine for perioperative pain management during hip arthroscopy: a double-blinded prospective randomized control trial. Arthroscopy 2019; 35:2608–2616. [DOI] [PubMed] [Google Scholar]
- 36.Simovitch RW, Hernandez T, YaDeau JT, et al. Liposomal bupivacaine plus bupivacaine versus ropivacaine plus dexamethasone brachial plexus blockade for arthroscopic rotator cuff repair: an unblinded randomized controlled trial. J Bone Joint Surg Open Access 2022; 7:e21.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Truong A, Fleshner PR, Mirocha JM, et al. A prospective randomized trial of surgeon-administered intraoperative transversus abdominis plane block with bupivacaine against liposomal bupivacaine: the TINGLE Trial. Dis Colon Rectum 2021; 64:888–898. [DOI] [PubMed] [Google Scholar]
- 38.Vandepitte C, Kuroda M, Witvrouw R, et al. Addition of liposome bupivacaine to bupivacaine HCl versus bupivacaine HCl alone for interscalene brachial plexus Block in patients having major shoulder surgery. Reg Anesth Pain Med 2017; 42:334–341. [DOI] [PubMed] [Google Scholar]
- 39.Vandepitte CF, Van Boxstael S, Duerinckx JF, et al. Effect of bupivacaine liposome injectable suspension on sensory blockade and analgesia for Dupuytren contracture release. J Hand Surg Global e 2019; 1:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weksler B, Sullivan JL, Schumacher LY. Randomized trial of bupivacaine with epinephrine versus bupivacaine liposome suspension in patients undergoing minimally invasive lung resection. J Thorac Cardiovasc Surg 2021; 161:1652–1661. [DOI] [PubMed] [Google Scholar]
- 41.Wong KA, Cabrera AG, Argiroff AL, et al. Transversus abdominis plane block with liposomal bupivacaine and its effect on opiate use after weight loss surgery: a randomized controlled trial. Surg Obes Relat Dis 2020; 16:886–893. [DOI] [PubMed] [Google Scholar]
- 42.Xie D, Nicholson M, Azaiza M, et al. Effect of operative local anesthesia on postoperative pain outcomes of inflatable penile prosthesis: prospective comparison of two medications. Int J Impot Res 2018; 30:93–96. [DOI] [PubMed] [Google Scholar]
- 43.Yeap YL, Wolfe J, Stewart J, et al. Liposomal bupivacaine addition versus standard bupivacaine alone for colorectal surgery: a randomized controlled trial. Pain Manag 2022; 12:35–43. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Li J, Chen Z. The postoperative analgesia effect of liposome bupivacaine ultrasound-guided PECS blockade after total mammectomy. J Pract Med 2019; 35:3214–3217. [Google Scholar]
- 45.Farrar JT, Young JP, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94:149–158. [DOI] [PubMed] [Google Scholar]
- 46.Cepeda MS, Africano JM, Polo R, et al. What decline in pain intensity is meaningful to patients with acute pain? Pain 2003; 105:151–157. [DOI] [PubMed] [Google Scholar]
- 47.Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth 2017; 118:424–429. [DOI] [PubMed] [Google Scholar]
- 48.McCann ME. Liposomal bupivacaine: effective, cost-effective, or (Just) costly? Anesthesiology 2021; 134:139–142. [DOI] [PubMed] [Google Scholar]
- 49.Moayeri N, Bigeleisen PE, Groen GJ. Quantitative architecture of the brachial plexus and surrounding compartments, and their possible significance for plexus blocks. Anesthesiology 2008; 108:299–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.