Abstract
PURPOSE
The Response Assessment in Neuro-Oncology (RANO) criteria for high-grade gliomas (RANO-HGG) and low-grade gliomas (RANO-LGG) were developed to improve reliability of response assessment in glioma trials. Over time, some limitations of these criteria were identified, and challenges emerged regarding integrating features of the modified RANO (mRANO) or the immunotherapy RANO (iRANO) criteria.
METHODS
Informed by data from studies evaluating the different criteria, updates to the RANO criteria are proposed (RANO 2.0).
RESULTS
We recommend a standard set of criteria for both high- and low-grade gliomas, to be used for all trials regardless of the treatment modalities being evaluated. In the newly diagnosed setting, the postradiotherapy magnetic resonance imaging (MRI), rather than the postsurgical MRI, will be used as the baseline for comparison with subsequent scans. Since the incidence of pseudoprogression is high in the 12 weeks after radiotherapy, continuation of treatment and confirmation of progression during this period with a repeat MRI, or histopathologic evidence of unequivocal recurrent tumor, are required to define tumor progression. However, confirmation scans are not mandatory after this period nor for the evaluation of treatment for recurrent tumors. For treatments with a high likelihood of pseudoprogression, mandatory confirmation of progression with a repeat MRI is highly recommended. The primary measurement remains the maximum cross-sectional area of tumor (two-dimensional) but volumetric measurements are an option. For IDH wild-type glioblastoma, the nonenhancing disease will no longer be evaluated except when assessing response to antiangiogenic agents. In IDH-mutated tumors with a significant nonenhancing component, clinical trials may require evaluating both the enhancing and nonenhancing tumor components for response assessment.
CONCLUSION
The revised RANO 2.0 criteria refine response assessment in gliomas.
BACKGROUND
Gliomas are the most common type of malignant primary brain tumor.1 Despite extensive research, progress in developing effective therapies has been unacceptably slow.2-4 The Response Assessment in Neuro-Oncology (RANO) Working Group published response criteria for high-grade gliomas (RANO-HGG) in 2010,5 and low-grade gliomas (RANO-LGG) in 2011,6 on the basis of consensus recommendations to improve the reliability and comparability of response assessments across clinical trials and to help identify more effective therapies. These criteria have been widely accepted and incorporated into most glioma clinical trials over the past decade. Over time, concerns regarding challenges of differentiating pseudoprogression secondary to radiochemotherapy and immunotherapies from true disease progression have led to the introduction of modifications of these criteria, including the Modified RANO Criteria (mRANO)7 and the Immunotherapy RANO Criteria (iRANO),8 to potentially address these issues. The original RANO-HGG criteria anticipated these challenges and included language recommending that if there is uncertainty regarding progression, the patient may continue treatment and undergo repeat imaging to confirm progression before necessitating coming off study. The mRANO criteria differs from RANO-HGG in using the first postradiotherapy magnetic resonance imaging (MRI) instead of the postsurgical MRI as the baseline, and mandates a repeat MRI to confirm progression.7 The iRANO criteria use the postoperative MRI as the baseline, similar to RANO-HGG. However, within the first 6 months of initiating an immunotherapy, repeat scans over a 3-month period are recommended to confirm disease progression before patients are taken off study.8 RANO-HGG, mRANO, and iRANO have been used in different clinical trials, leading to variability in response assessments and uncertainty about which set of criteria to use. There is a need for updated response criteria for glioma trials based upon clinical validation of the various criteria.
CONTEXT
Key Objective
Response Assessment in Neuro-Oncology (RANO) 2.0 is an update of the response criteria for gliomas in adults on the basis of data from evaluation of the original RANO criteria and variations such as the modified RANO criteria (mRANO) and the immunotherapy RANO criteria (iRANO).
Knowledge Generated
RANO 2.0 recommends a standard set of criteria for both high- and low-grade gliomas, to be used for all trials regardless of the treatment modalities being evaluated. Response criteria for contrast-enhancing tumors, non–contrast-enhancing tumors, and tumors with both enhancing and nonenhancing components are proposed, in addition to other guidance to improve the assessment of response and progression in glial tumors.
Relevance (J.P.S. Knisely)
-
RANO 2.0 provides unified, standardized guidelines for glioma response assessments that are applicable for all low- and high-grade tumors. Subclassifications for enhancing or nonenhancing tumors or for the type of antitumor therapy employed are no longer needed.*
*Relevance section written by JCO Associate Editor Jonathan P.S. Knisely, MD.
Previous response criteria for gliomas have been based primarily on expert recommendations. The RANO working group felt it was important to update the response criteria informed by available data. The following recommendations for RANO 2.0 have been guided in part by the results of a study comparing RANO-HGG, mRANO, and iRANO in 526 patients with newly diagnosed glioblastoma and 580 patients with recurrent glioblastoma treated with both conventional therapies and on clinical trials (see Data Supplement, Supplement and Fig S1 for summary [online only]).9 This study found no difference in progression-free survival (PFS), PFS at 6 months (PFS6), or in the Spearman correlation between PFS and survival between RANO-HGG, mRANO, and iRANO in either newly diagnosed or recurrent glioblastoma. Using a repeat scan to confirm progression appeared to be helpful in the first 3 months after radiotherapy, given the poor correlation between progression and survival during this period but the use of confirmation scans at any other time point, regardless of the treatment modality, was not helpful. Measuring nonenhancing progression also did not increase the correlation to overall survival, even in patients receiving bevacizumab.9 These data support findings from other studies that also questioned the value of assessing nonenhancing progression in patients with glioblastoma.7,10,11
In 2021, the WHO published a revised classification of CNS tumors.12-14 As a result, the traditional distinction of high-grade gliomas as enhancing tumors evaluated by RANO-HGG, and low-grade gliomas as nonenhancing tumors evaluated by RANO-LGG, has become less clear. For example, IDH wild-type astrocytoma with molecular features of glioblastomas may be nonenhancing, while enhancement can occur in high-grade IDH-mutated gliomas. Given this revised pathologic classification, the RANO Working Group felt that instead of separate RANO-HGG and RANO-LGG criteria, a single unified set of response criteria for all gliomas would be more appropriate.
The following sections outline recommendations for updated response criteria from the RANO Working Group, RANO 2.0 intended to be used for glioblastomas, all grades of IDH-mutated gliomas, and other glial tumors, regardless of the specific therapies being evaluated (see the Data Supplement [Supplement] for process of developing RANO 2.0).
METHOD OF MEASUREMENT
Contrast-enhanced MRI is the most sensitive and reproducible method of assessment of brain tumors.15 The same imaging protocol should be used to characterize each identified and reported lesion at baseline and across all subsequent imaging time points to ensure that the assessment of interval appearance or disappearance of lesions, or changes in size are not affected by scan parameters such as slice thickness. The standardized brain tumor imaging protocol (BTIP) should be used to reduce variability (Data Supplement, Table S1).16 Importantly, more advanced validated sequences can be added, if necessary, and integrated into the BTIP protocol. Ideally, to reduce variability, patients should be imaged on the same MRI scanner, or at least with the same magnet strength, for the duration of their study participation.
Two-Dimensional Versus Volumetric Assessment
As with RANO-HGG, the product of the maximal cross-sectional diameters of the enhancing lesions will be used to determine the size of contrast-enhancing lesions. As with RANO-LGG, the maximal cross-sectional T2-weighted fluid-attenuated inversion recovery (FLAIR) diameters will be used to determine the size of non–contrast-enhancing lesions.5,6
Despite growing interest in replacing two-dimensional measurements with volumetric analysis to provide more accurate assessment of tumor size, studies to date have not demonstrated a conclusive benefit of volumetric analysis over two-dimensional measurement17-19 with rare exceptions,20 and there remain challenges with limitations of the software used for volumetric analysis, their availability, as well as the added costs, complexity, and logistical challenges. Two-dimensional tumor measurement will remain the recommended primary measurement, but volumetric measurements can be used if available. Importantly, for any specific trial, two-dimensional or volumetric measurements should be prespecified for all treated patients to optimize consistency. The proposed cutoff for partial response (PR) will be a 65% reduction in volume to be consistent with the 50% reduction in area with the current criteria, and 40% increase in volume will constitute progression to be consistent with the 25% increase in area (Data Supplement, Table S2).21
Definitions
Measurable and Nonmeasurable Disease
Measurable disease is defined as contrast-enhancing or non–contrast-enhancing lesions with clearly defined margins by MRI scan, with both perpendicular diameters on a single slice of at least 10 mm, visible on two or more slices that are preferably, at most, 4 mm apart with 0-mm interslice gap. The plane of lesion measurement in 2D (axial, coronal, or sagittal) should be chosen based on the plane with the largest lesion extent. Volumetrically, measurable disease in 3D will be defined as having contrast-enhancing or nonenhancing disease of at least 1 cm in all three orthogonal directions. Although not recommended, in the event the MRI is performed with thicker slices, the size of a measurable lesion at baseline for both perpendicular measurements should be two times the slice thickness and interslice gap (eg, if the slice thickness is 5 mm with 1.5-mm interslice gap, the minimum tumor size on both perpendicular dimensions should be 13 mm).7 Measurement of tumor around a cyst or surgical cavity remains challenging. Such lesions should generally be considered nonmeasurable unless there is a nodular component measuring ≥10 × 10 mm in diameter. The cystic or surgical cavity should not be measured in determining therapeutic response.
Nonmeasurable disease remains defined as either unidimensionally measurable lesions, masses with unclear margins, or lesions with maximal perpendicular diameters <10 mm. Patients without measurable disease, such as those who have undergone a gross total resection, cannot exhibit a response to subsequent treatment and can only achieve stable disease (SD) as their best radiologic outcome, assuming treatment is started before there is radiologic evidence of new tumor growth. Therefore, only patients with measurable disease can be included in the assessment of overall response rate, while patients without measurable disease may be included in assessments for other outcomes such as time-to-event end points (eg, PFS or survival) and clinical functioning.
Algorithms for determining measurable and nonmeasurable disease are included in the Data Supplement (Fig S2).7
Target Lesions
When multiple measurable lesions exist, at least two and no more than three lesions should be identified as target lesions for studies evaluating either enhancing or nonenhancing tumors. For studies evaluating both enhancing and nonenhancing tumors, a maximum of two measurable enhancing and two measurable nonenhancing lesions can be identified as target lesions. The enhancing lesion(s) can be in the nonenhancing tumor. The sum of the products of the perpendicular diameters of these lesions should be determined. Generally, the largest enlarging lesion(s) should be selected. Emphasis should also be placed on lesions that allow reproducible repeated measurements. Occasionally, the largest lesions may not lend themselves to reproducible measurements, and the next largest lesions that can be measured reproducibly should be selected. For patients with multiple lesions, those that are increasing in size should be selected as target lesions, regardless of their relative size. The other lesions will be considered nontarget and should be recorded but not integrated into the total lesion size calculation (Data Supplement, Fig S2).
Baseline MRI
The immediate postoperative MRI scan, obtained within 48 hours of surgery, has been used as the baseline MRI in most response criteria for newly diagnosed gliomas, including RANO-HGG5 and iRANO.8 By contrast, the mRANO criteria7 recommend using the first postradiotherapy MRI as the baseline for newly diagnosed gliomas to reduce the impact of the increased contrast enhancement from pseudoprogression after radiochemotherapy,22-25 and address the challenges associated with immediate postoperative scans including the presence of postoperative changes (blood products and edema), and variability in corticosteroid dosing, timing of the scans, and imaging techniques used. In support of this approach, Youssef et al9 showed greater correlation between PFS and OS when the postradiotherapy scan was used as a baseline compared with the postoperative scan, although this difference was not statistically significant. Given these factors, if a patient is sufficiently stable clinically, we recommend using the postradiotherapy MRI scan, performed around 4 weeks (21-35 days) from the end of radiotherapy, as the baseline scan in newly diagnosed gliomas for comparison with future imaging studies. Patients who deteriorate significantly before the postradiotherapy baseline MRI can be taken off study for clinical progression. For patients with newly diagnosed glioma not undergoing radiotherapy, the postsurgery, pretreatment MRI will be used as the baseline. The pretreatment MRI will also be used for patients with recurrent glioma as the baseline. Ideally, baseline scans should be performed as close as possible to the initiation of therapy with an interval not exceeding 14 days, especially for glioblastomas.26
Although the immediate postoperative MRI will no longer be used as the baseline for response assessment, this scan still has value in detecting postsurgical complications and determining the extent of resection, which has prognostic implications.27
Criteria for Entry on to Clinical Trials for Recurrent/Progressive Disease
We propose that a 25% increase in the sum of the products of perpendicular diameters of the lesions, or a 40% increase in volume, or a new measurable lesion, while on stable or increasing doses of corticosteroids should be required for enrollment onto clinical trials for recurrent disease. Clinical deterioration or increase in corticosteroid dosing alone is not sufficient to indicate progressive disease (PD) for entry into trials.
Given the challenges of determining radiologic progression, we recommend routine collection of all neuroimaging studies for at least 3 months for recurrent glioblastomas and CNS WHO grade 4 IDH-mutated astrocytomas, and 12 months for CNS WHO grade 2 and 3 IDH-mutated gliomas and other glial tumors, before enrollment to allow for confirmation of progression. Although not mandatory for clinical trial participation, such guidance will help diminish the likelihood of premature or inaccurate progression determination and subsequent inappropriate clinical trial enrollment.
Since the incidence of pseudoprogression is high in the first 12 weeks after chemoradiotherapy for glioblastomas (occurring in up to 30%-40% of patients),9,25,28,29 and there is poor correlation between radiologic changes and PD and survival during this period,9 we propose that if a patient with concern for radiologic progression during this period is clinically stable, a repeat MRI should be performed (eg, at 4- or 8-week intervals) to confirm progression (additional 25% or more increase in area or 40% increase or more in volume compared with previous scan) before necessitating a patient coming off study. If follow-up imaging supports true tumor progression, the date of progression should be backdated to the time of the scan when progression was first measured. Patients who develop progression in the first 12 weeks after completion of radiotherapy should be excluded from clinical trials for recurrent disease unless the progression is clearly outside the radiation field (eg, beyond the high-dose region or 80% isodose line) or there is pathologic confirmation of disease progression.30 We recognize the limited reliability of pathology currently in differentiating progression from pseudoprogression,31,32 and a RANO working group is currently addressing this issue. Advanced imaging techniques such as diffusion MRI, dynamic susceptibility contrast (perfusion) MRI,33 and amino acid positron emission tomography imaging34 may help differentiate progression from pseudoprogression but require further validation before formal incorporation into RANO 2.0.
For IDH-mutated gliomas and other glial tumors, the time course for pseudoprogression can extend well beyond 3 months.35 For these tumors, we recommend confirming progression in the first 3 months after completion of radiotherapy and optional confirmation of progression at later time points before entry into trials for recurrent tumors.
Definition of Radiologic Response and Progression
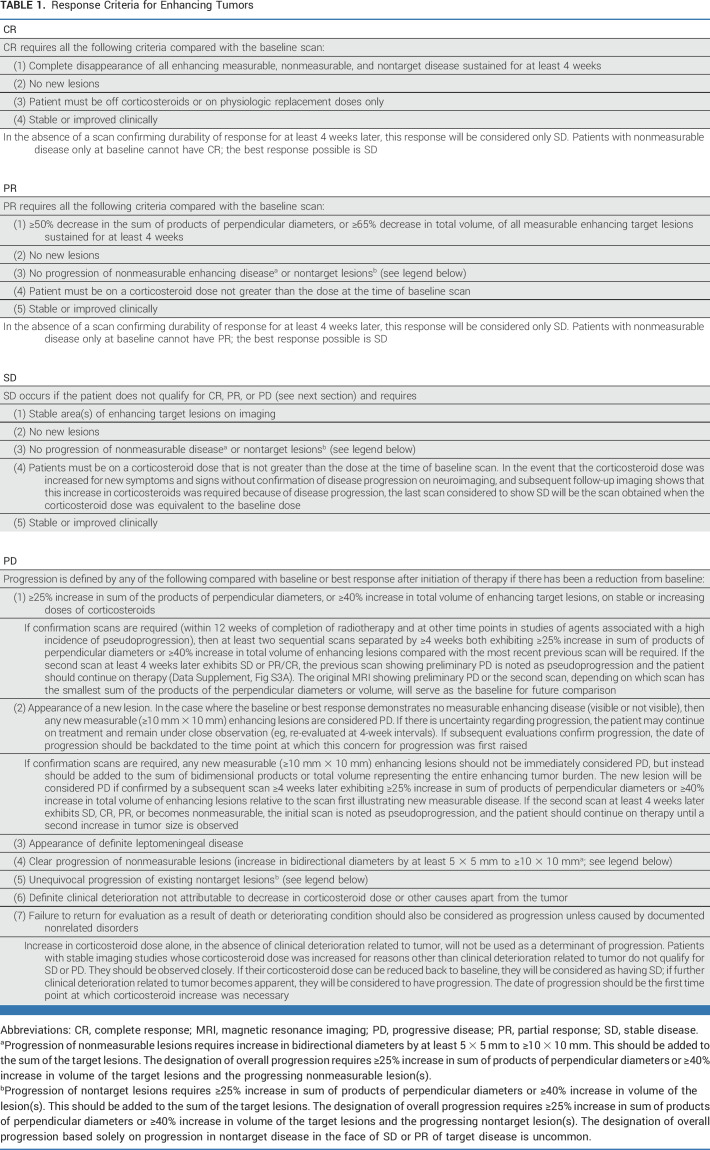
The radiologic response must be determined by comparison to the tumor measurement obtained at the pretreatment baseline or the first postradiotherapy scan for patients with newly diagnosed glioma undergoing radiotherapy. PR is defined as ≥50% decrease, compared with baseline, in the sum of products of perpendicular diameters of all measurable target lesions, or ≥65% reduction in volume, sustained for at least 4 weeks with stable or decreasing corticosteroid doses. PD is defined as ≥25% increase in the sum of products of perpendicular diameters of all measurable target lesions or ≥40% in volume compared with the smallest tumor measurements at either pretreatment baseline or after initiation of therapy. The appearance of a new measurable lesion will also constitute progression unless per-protocol confirmation of progression is required, in which case it should be added to the sum of the existing target lesions, and progression only occurs if there is ≥25% increase in area or ≥40% increase in volume on repeat imaging. The steroid dose must also be considered (Tables 1-3).
TABLE 1.
Response Criteria for Enhancing Tumors
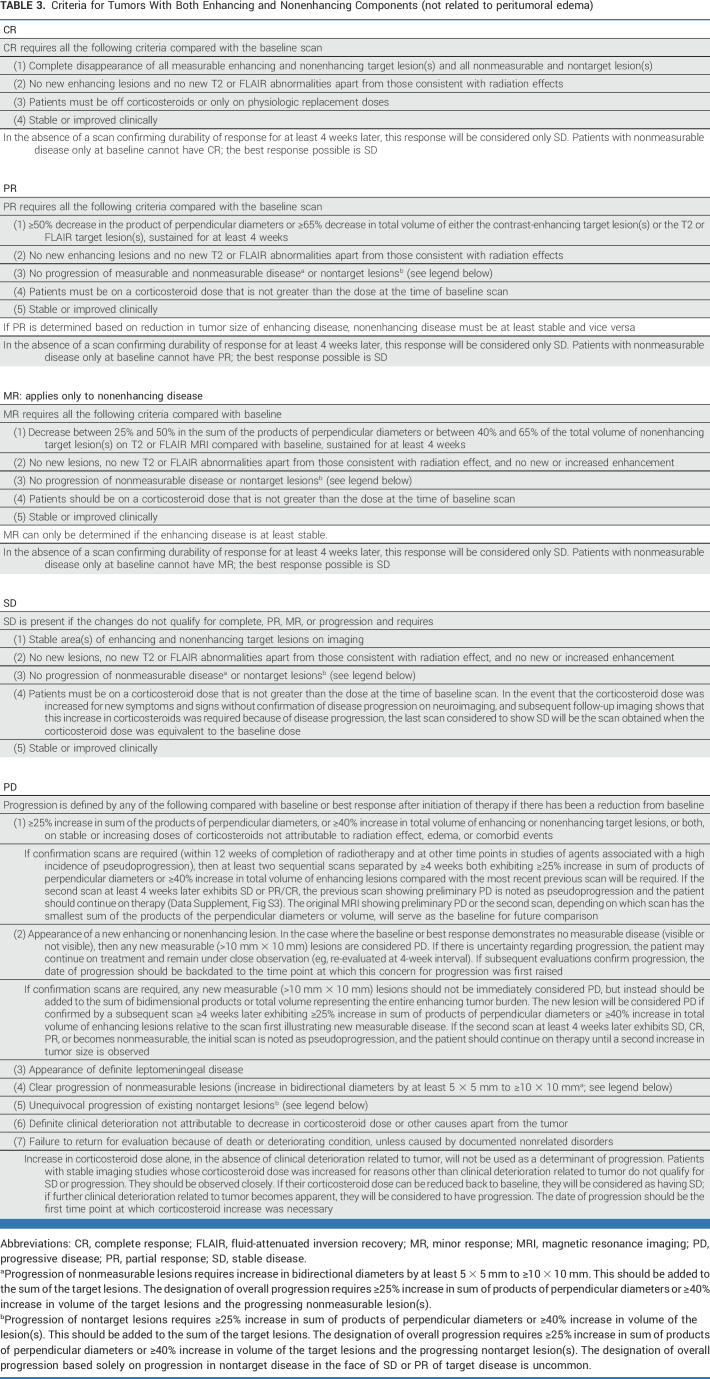
TABLE 3.
Criteria for Tumors With Both Enhancing and Nonenhancing Components (not related to peritumoral edema)
Occasionally, unequivocal progression of a nonmeasurable lesion (lesion increasing by at least 5 × 5 mm and becoming measurable [≥10 × 10 mm]) or a nontarget lesion (25% increase in area or 40% increase in volume) may occur. These lesions should be added to the sum of the existing target lesions. These changes would qualify as progression if the total sum of the products of the perpendicular diameters exceeds ≥25% increase in area or ≥40% in volume and require discontinuation of therapy, even in the setting of SD or PR in the target lesions (Tables 1-3).
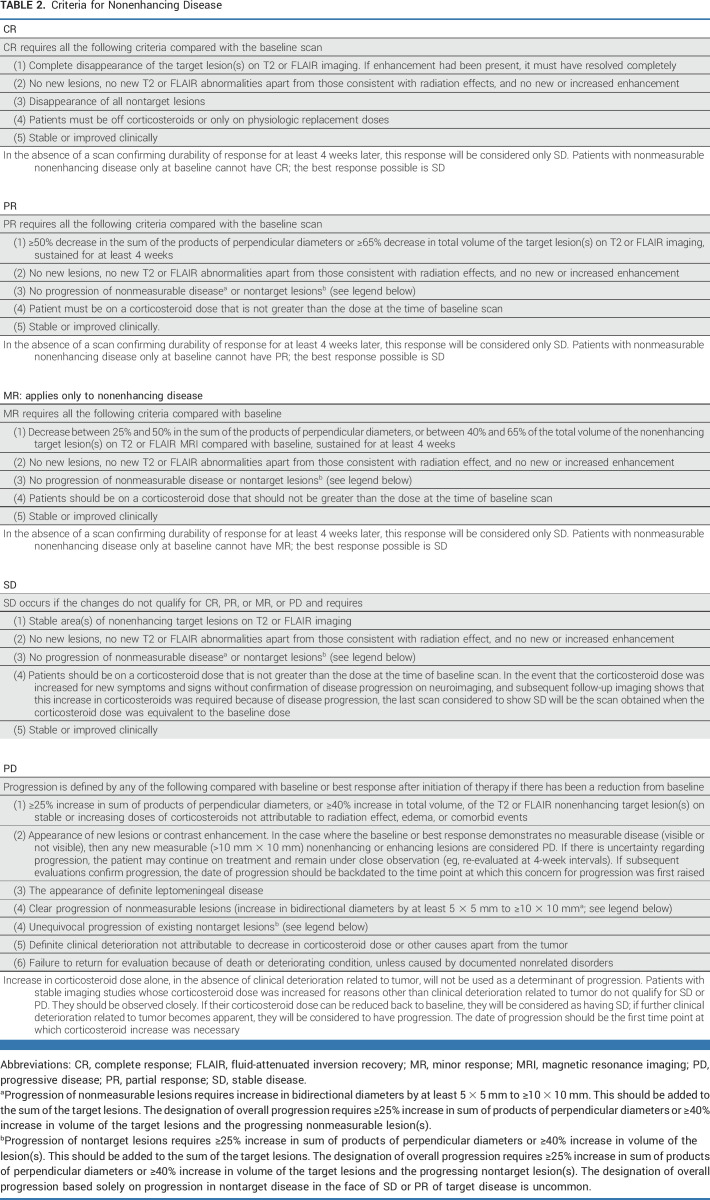
TABLE 2.
Criteria for Nonenhancing Disease
Contrast-enhancing disease should be measured for IDH wild-type glioblastoma, and T2/FLAIR should be measured for IDH-mutated nonenhancing gliomas and for the uncommon nonenhancing glioblastomas. For tumors with a mixture of contrast-enhancing and non–contrast-enhancing components, both enhancing and nonenhancing disease can be measured. However, measuring the contrast-enhancing disease only is also acceptable if it is the lesion(s) determining progression for study entry (Tables 1-3).
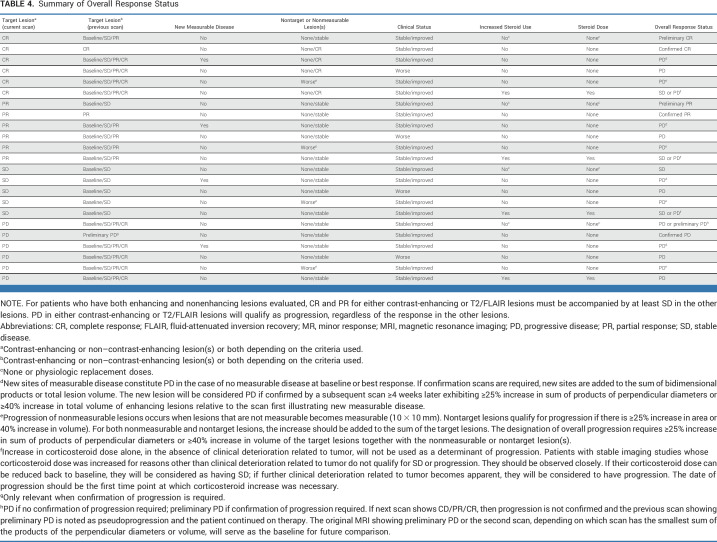
Tables 1-3 summarize the response criteria. Table 4 summarizes the overall response status. The Data Supplement (Figs S3A-S3D) provides algorithms for determining response. The Data Supplement (Table S3) summarizes the differences between RANO 2.0 and previous response criteria.
TABLE 4.
Summary of Overall Response Status
Neuroimaging for Confirmation of Progression
For most situations, confirmation of progression as required by mRANO and recommended by iRANO is not necessary to determine progression.9 The original RANO criteria allow for the optional continuation of treatment and confirmation of progression if radiologic changes are equivocal. In these situations, it is recommended that patients should be observed closely but continue treatment, for example, for another 1-2 treatment cycles. If the subsequent scans confirm progression, the date of progression should be backdated to the time initial tumor progression was noted. Continuation of treatment for equivocal changes may especially be needed for small tumors since measurement errors are magnified. An exception is in the first 12 weeks after completion of radiochemotherapy in IDH wild-type glioblastoma, when pseudoprogression is most commonly observed.28 In this period, if imaging studies show worsening, for clinically stable patients, a repeat MRI should be performed to confirm progression before taking a patient off study (Tables 1 and 3 and Data Supplement, Figs S3A and S3D). We recognize that there may be therapies that are especially likely to be associated with high rates of pseudoprogression or radiation necrosis (eg, intratumoral therapies such as oncolytic viruses). For clinical trials of such agents, mandatory confirmation of progression with follow-up MRI can be considered.
In general, pseudoprogression is largely associated with changes in contrast enhancement. Therefore, confirmation of progression is recommended in selected cases evaluated with the criteria for contrast-enhancing tumors but is unnecessary in assessing nonenhancing tumors.
Evaluation of Nonenhancing Progression in Patients With Glioblastoma
The original RANO-HGG criteria were developed partly to address the challenges posed by therapies that reduce vascular permeability, including bevacizumab. This reduction in vascular permeability can give rise to pseudoresponses and nonenhancing tumor progression.5 RANO-HGG incorporated a qualitative evaluation of T2/FLAIR changes into the response criteria to address the issue of nonenhancing progression. Specifically, patients on antiangiogenic agents with stable or decreased contrast enhancement with progression of T2/FLAIR abnormality were determined to have progressed.5 Although up to 40% of patients treated with bevacizumab develop nonenhancing progression,36 and its evaluation results in shorter PFS,37 most patients develop enhancing progression within 1-2 months after nonenhancing progression, resulting in no increase in correlation of PFS with survival.9,10,37 Furthermore, nonenhancing progression is challenging to distinguish from other causes of increased T2/FLAIR signal, including radiation changes, postsurgical changes, edema, or corticosteroid dosing changes. T2/FLAIR signal abnormality is also difficult to measure reliably, contributing to increased workload and cost.38 Some treatments such as immunotherapies or intratumoral therapies can be associated with increased peritumoral edema and T2/FLAIR changes on MRI independent of tumor progression. Given the limited value of evaluating nonenhancing progression in enhancing glioblastoma, we recommend removing it from the criteria for determining progression from most trials. Evaluation of T2/FLAIR changes remains of value for evaluation of IDH-mutated gliomas with a nonenhancing component and in trials evaluating agents anticipated to significantly affect vascular permeability.
Assessment of Clinical Deterioration
Clinical deterioration remains an important component for response assessment, particularly for determining progression. Although the determination of clinical deterioration continues to be left to the treating physician's discretion, it is recommended that a decline in KPS for at least 7 days from 100 or 90 to 70 or less, a decline in KPS of at least 20 points from 80 or less, or a decline in KPS from any baseline to 50 or less be considered neurologic deterioration unless attributable to comorbid events or changes in corticosteroid dose. Similarly, a decline in the Eastern Cooperative Oncology Group and WHO performance scores from 0 or 1 to 2 or 2 to 3 would be considered neurologic deterioration.
The Neurologic Assessment in Neuro-Oncology (NANO) scale was developed as a simple, standardized tool providing a quantifiable evaluation of nine relevant neurologic domains to determine neurologic function (Data Supplement, Fig S4).39 This tool is being used increasingly in clinical trials to assess response but formal incorporation into the RANO criteria will require further validation studies.
Advanced Imaging Techniques
There is increasing evidence that advanced imaging techniques including perfusion imaging (dynamic susceptibility contrast or dynamic contrast-enhanced MRI), diffusion imaging, magnetic resonance spectroscopy, and amino acid positron emission tomography may help predict tumor response or allow the differentiation of pseudoprogression from progression.33,40-42 There is also increasing interest in the use of automated assessment of response and the use of artificial intelligence.43,44 Some of these techniques are undergoing validation studies and may eventually be incorporated into the RANO criteria.
In summary, we outline updated response criteria from the RANO Working Group, RANO 2.0. These criteria will be used for all grades of glial tumors regardless of the IDH mutational status or the specific therapies being evaluated. As with the previous criteria, this represents a work in progress and future updates will incorporate novel developments, advanced imaging techniques, and end points as they become validated.
ACKNOWLEDGMENT
The authors gratefully acknowledge the support of David Arons and the National Brain Tumor Society (NBTS), the American Brain Tumor Association, the Society for Neuro-Oncology, and feedback from Joohee Sul from NBTS and Amy Barone from the Food and Drug Administration.
Patrick Y. Wen
Consulting or Advisory Role: AstraZeneca, Vascular Biogenics, VBI Vaccines, Bayer, Karyopharm Therapeutics, ElevateBio, Integral Health, Prelude Therapeutics, Novocure, Mundipharma, Black Diamond Therapeutics, Day One Biopharmaceuticals, Sapience Therapeutics, Nuvation Bio, Celularity, Novartis, Merck, Boston Pharmaceuticals, Chimerix, Servier, Insightec, Novocure, Sagimet Biosciences, Boehringer Ingelheim, Servier, Genenta Science, Prelude Therapeutics, GlaxoSmithKline
Research Funding: AstraZeneca (Inst), Merck (Inst), Novartis (Inst), Oncoceutics (Inst), Lilly (Inst), Beigene (Inst), Kazia Therapeutics (Inst), MediciNova (Inst), Vascular Biogenics (Inst), VBI Vaccines (Inst), Puma Biotechnology (Inst), Celgene (Inst), Bayer (Inst), Nuvation Bio (Inst), Chimerix (Inst), Karyopharm Therapeutics (Inst), Servier (Inst)
Martin van den Bent
Employment: AstraZeneca
Consulting or Advisory Role: Boehringer Ingelheim, carthera, Genenta Science, Nerviano Medical Sciences, Chimerix, AstraZeneca, Servier, Roche, Incyte, Fore Biotherapeutics
Research Funding: AbbVie (Inst)
Timothy F. Cloughesy
Leadership: Katmai Pharmaceuticals
Stock and Other Ownership Interests: Katmai Pharmaceuticals, Chimerix, Erasca, Inc
Consulting or Advisory Role: Roche/Genentech, Tocagen, VBL Therapeutics, Novartis, Merck, Boehringer Ingelheim, KIYATEC, Bayer, DelMar Pharmaceuticals, QED Therapeutics, Amgen, Katmai Pharmaceuticals, Global Coalition for Adaptive Research, Inovio Pharmaceuticals, Sapience Therapeutics, SonaCare Medical, SERVIER, Lista, Chimerix
Patents, Royalties, Other Intellectual Property: US Provisional Application No.: 62/819,322 Title: Compositions and Methods for Treating Cancer Filing Date: March 15, 2019 Inventor(s): David A. Nathanson et al. FH Reference No.: UCH-17760 (32246-17760) Your Reference No.: (UCLA 2019-630-1) US
Other Relationship: Global Coalition for Adaptive Research, Break Through Cancer
Benjamin M. Ellingson
Consulting or Advisory Role: Siemens, Medicenna, MedQIA, Imaging Endpoints, Neosoma, Kazia Therapeutics, VBL Therapeutics, Global Coalition for Adaptive Research, Servier, Janssen, Chimerix, Sumitomo Dainippon Pharma Oncology, ImmunoGenesis, Ellipses Pharma, Monteris Medical, Alpheus Medical
Research Funding: Siemens, Janssen
Travel, Accommodations, Expenses: Siemens
Michael Weller
Honoraria: Novocure, Bayer, Pierre Fabre
Consulting or Advisory Role: Curevac, Medac, Novartis, Orbus Therapeutics, Philogen, Roche, Sandoz, Janssen, Seagen, LEO Pharma, Bayer
Research Funding: Quercegen Pharmaceuticals (Inst), Versameb (Inst)
Evanthia Galanis
Consulting or Advisory Role: KIYATEC, Karyopharm Therapeutics (Inst), Boston Scientific (Inst), Servier (Inst)
Research Funding: MedImmune (Inst), Servier (Inst), Celgene (Inst), Denovo Biopharma (Inst), Denovo Biopharma (Inst)
Daniel P. Barboriak
Uncompensated Relationships: Blue Earth Diagnosticds
John de Groot
Employment: Alaunos Therapeutics, Brii Biosciences
Stock and Other Ownership Interests: WuXi Biologics, Alaunos Therapeutics, Brii Biosciences
Consulting or Advisory Role: Merck, Mundipharma Research, Bioasis Technologies, InSightec, Samus Therapeutics, Karyopharm Therapeutics, Cure Brain Cancer Foundation, Sapience Therapeutics, Monteris Medical, Kintara Therapeutics, Kazia Therapeutics, CarThera, Sumitomo Dainippon Pharma Oncology, VBI Vaccines, Chimerix, Aucentra Therapeutics, Midatech Pharma, Servier, Telix Pharmaceuticals, Alpha Pharmaceutical
Research Funding: CarThera (Inst), Haihe Pharmaceutical (Inst), Taiho Pharmaceutical (Inst)
Other Relationship: VBI Vaccines, Chimerix
Raymond Huang
Consulting or Advisory Role: Agios, Nuvation Bio, Nuvation Bio, Vysioneer
Research Funding: Agios, Bristol Myers Squibb
Andrew B. Lassman
Honoraria: Clinical Education Alliance
Consulting or Advisory Role: Sapience Therapeutics, Orbus Therapeutics, Novocure, Vivacitas Oncology, Chimerix, Affinia Therapeutics, Neuron-D, Leal Therapeutics, AlphaDetail, AlphaSights, Bluestar Bioadvisors, Clarion Healthcare, ExpertConnect, Gerson Lehrman Group, Guidepoint Global, IP2IPO, KJT, Tavistock Life Sciences, techspert.io, BioClinica, RadMD, Global Coalition for Adaptive Research
Research Funding: AbbVie, Genentech/Roche, Aeterna Zentaris, VBI Vaccines, Pfizer, Karyopharm Therapeutics, Bayer, QED Therapeutics, Orbus Therapeutics, BMS, Chimerix, NextSource, DelMar, Polaris (Inst), Kintara (Inst) Pharmaceuticals, Corden, Kazia Therapeutics, Servier, Biohaven Pharmaceuticals, Vigeo Therapeutics, Incyte, Abbott Laboratories
Travel, Accommodations, Expenses: AbbVie, Chimerix, Karyopharm Therapeutics, Pfizer, Bridgebio, VBI Vaccines, Helsinn Healthcare, Foundation Medicine, Servier, Anheart Therapeutics
Minesh Mehta
Stock and Other Ownership Interests: Chimerix
Consulting or Advisory Role: Mevion Medical Systems, ZappRx, Xoft, Kazia Therapeutics, Novocure
Patents, Royalties, Other Intellectual Property: WARF patent 14/934,27, Topical Vasoconstritor Preparations and Methods for Protecting Cells During Cancer Chemotherapy and Radiotherapy
Uncompensated Relationships: Xcision Medical Systems
Matthias Preusser
Honoraria: Roche, GlaxoSmithKline, Bayer, Bristol Myers Squibb, Novartis, Gerson Lehrman Group, CMC Contrast, Mundipharma, BMJ Journals, MedMedia, AstraZeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra Pharmaceuticals, Gan & Lee, Servier
Consulting or Advisory Role: Roche, Bristol Myers Squibb, Novartis, Gerson Lehrman Group, CMC Contrast, GlaxoSmithKline, Mundipharma, AbbVie, Bayer, AstraZeneca, Lilly, Daiichi Sankyo/Astra Zeneca, Sanofi, Merck Sharp & Dome, Tocagen, Adastra Pharmaceuticals, Gan & Lee, Servier
Research Funding: Roche (Inst), GlaxoSmithKline (Inst), Boehringer Ingelheim (Inst), Merck Sharp & Dohme, Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Roche, GlaxoSmithKline, Bristol Myers Squibb, MSD, Mundipharma, Servier
Rifaquat Rahman
Consulting or Advisory Role: Beijing Saint Lucia Consulting
Research Funding: Bristol Myers Squibb (Inst), Puma Biotechnology (Inst), Lilly (Inst)
Roger Stupp
Stock and Other Ownership Interests: Alpheus Medical, CarThera
Consulting or Advisory Role: Celgene, Northwest Biotherapeutics, Zai Lab, InSightec, Celularity, CranioVation, Triact Therapeutics, Hemispherian, GT Medical Technologies, Novocure, AstraZeneca, Boston Scientific, Carthera, The Lockwood Group, Boston Biomedical, Novartis, AimedBio
Research Funding: CarThera, Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: Methods of Determining Responsiveness to Chemotherapeutic Compounds for Cancer Therapy Type: Provisional Serial No. 63/202,761 Filed: June 23, 2021 (Inst)
Javier E. Villanueva-Meyer
Consulting or Advisory Role: GE Healthcare, MedQIA, Alpheus Medical
Research Funding: GE Healthcare
Wolfgang Wick
Consulting or Advisory Role: MSD Oncology (Inst), Roche/Genentech (Inst), SERVIER, GlaxoSmithKline
Research Funding: Roche (Inst), Apogenix (Inst), Pfizer (Inst)
David R. Macdonald
Research Funding: Celgene (Inst), Servier (Inst)
David A. Reardon
Honoraria: Merck, Novocure, Regeneron, Bristol Myers Squibb, Oncorus, Agenus, EMD Serono, Merck KGaA, Taiho Pharmaceutical, Advantagene, Bayer, DelMar Pharmaceuticals, Imvax, Medicenna, Sumitono Dainippon Pharma, Vivacitas Oncology, Anheart Therapeutics, Deciphera, Ellipses Pharma, Genenta Science, Inovio Pharmaceuticals, Kintara Therapeutics, Kintara Therapeutics, KIYATEC, NEUVOGEN, Taiho Pharmaceutical, Y-mAbs Therapeutics
Consulting or Advisory Role: Merck, Novocure, Regeneron, Bristol Myers Squibb, Oncorus, Agenus, EMD Serono, Merck KGaA, Taiho Pharmaceutical, Delmar Pharmaceuticals, Advantagene, Bayer, Imvax, Medicenna, Vivacitas Oncology, Anheart Therapeutics, Ellipses Pharma, Genenta Science, Kintara Therapeutics, Kiyatec, Agios
Research Funding: Celldex (Inst), Incyte (Inst), Agenus (Inst), EMD Serono (Inst), Acerta Pharma (Inst), Omniox, Enterome (Inst)
Michael A. Vogelbaum
Stock and Other Ownership Interests: Infusion Therapeutics
Honoraria: Plus Therapeutics, Chimerix
Consulting or Advisory Role: Olympus, Midatech Pharma
Research Funding: Infusion Therapeutics (Inst), Celgene (Inst), Oncosynergy (Inst), Denovo Biopharma (Inst)
Patents, Royalties, Other Intellectual Property: Patents for drug delivery devices for the brain
Susan M. Chang
Consulting or Advisory Role: AstraZeneca
No other potential conflicts of interest were reported.
Footnotes
P.Y.W., M.v.d.B., G.Y., T.F.C., B.M.E., M.A.V., and S.M.C. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Patrick Y. Wen, Martin van den Bent, Gilbert Youssef, Timothy F. Cloughesy, Benjamin M. Ellingson, Michael Weller, Mark R. Gilbert, Raymond Huang, Andrew B. Lassman, Minesh Mehta, Lalitha K. Shankar, Javier E. Villanueva-Meyer, David R. Macdonald, David A. Reardon, Michael A. Vogelbaum, Susan M. Chang
Administrative support: Patrick Y. Wen
Collection and assembly of data: Patrick Y. Wen, Martin van den Bent, Gilbert Youssef, Timothy F. Cloughesy, Benjamin M. Ellingson, Evanthia Galanis, Raymond Huang, Minesh Mehta, Javier E. Villanueva-Meyer, David A. Reardon, Susan M. Chang
Data analysis and interpretation: Patrick Y. Wen, Martin van den Bent, Gilbert Youssef, Timothy F. Cloughesy, Benjamin M. Ellingson, Michael Weller, Daniel P. Barboriak, John de Groot, Mark R. Gilbert, Raymond Huang, Minesh Mehta, Annette M. Molinaro, Matthias Preusser, Rifaquat Rahman, Lalitha K. Shankar, Roger Stupp, Javier E. Villanueva-Meyer, David A. Reardon, Michael A. Vogelbaum, Susan M. Chang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
RANO 2.0: Update to the Response Assessment in Neuro-Oncology Criteria for High- and Low-Grade Gliomas in Adults
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Patrick Y. Wen
Consulting or Advisory Role: AstraZeneca, Vascular Biogenics, VBI Vaccines, Bayer, Karyopharm Therapeutics, ElevateBio, Integral Health, Prelude Therapeutics, Novocure, Mundipharma, Black Diamond Therapeutics, Day One Biopharmaceuticals, Sapience Therapeutics, Nuvation Bio, Celularity, Novartis, Merck, Boston Pharmaceuticals, Chimerix, Servier, Insightec, Novocure, Sagimet Biosciences, Boehringer Ingelheim, Servier, Genenta Science, Prelude Therapeutics, GlaxoSmithKline
Research Funding: AstraZeneca (Inst), Merck (Inst), Novartis (Inst), Oncoceutics (Inst), Lilly (Inst), Beigene (Inst), Kazia Therapeutics (Inst), MediciNova (Inst), Vascular Biogenics (Inst), VBI Vaccines (Inst), Puma Biotechnology (Inst), Celgene (Inst), Bayer (Inst), Nuvation Bio (Inst), Chimerix (Inst), Karyopharm Therapeutics (Inst), Servier (Inst)
Martin van den Bent
Employment: AstraZeneca
Consulting or Advisory Role: Boehringer Ingelheim, carthera, Genenta Science, Nerviano Medical Sciences, Chimerix, AstraZeneca, Servier, Roche, Incyte, Fore Biotherapeutics
Research Funding: AbbVie (Inst)
Timothy F. Cloughesy
Leadership: Katmai Pharmaceuticals
Stock and Other Ownership Interests: Katmai Pharmaceuticals, Chimerix, Erasca, Inc
Consulting or Advisory Role: Roche/Genentech, Tocagen, VBL Therapeutics, Novartis, Merck, Boehringer Ingelheim, KIYATEC, Bayer, DelMar Pharmaceuticals, QED Therapeutics, Amgen, Katmai Pharmaceuticals, Global Coalition for Adaptive Research, Inovio Pharmaceuticals, Sapience Therapeutics, SonaCare Medical, SERVIER, Lista, Chimerix
Patents, Royalties, Other Intellectual Property: US Provisional Application No.: 62/819,322 Title: Compositions and Methods for Treating Cancer Filing Date: March 15, 2019 Inventor(s): David A. Nathanson et al. FH Reference No.: UCH-17760 (32246-17760) Your Reference No.: (UCLA 2019-630-1) US
Other Relationship: Global Coalition for Adaptive Research, Break Through Cancer
Benjamin M. Ellingson
Consulting or Advisory Role: Siemens, Medicenna, MedQIA, Imaging Endpoints, Neosoma, Kazia Therapeutics, VBL Therapeutics, Global Coalition for Adaptive Research, Servier, Janssen, Chimerix, Sumitomo Dainippon Pharma Oncology, ImmunoGenesis, Ellipses Pharma, Monteris Medical, Alpheus Medical
Research Funding: Siemens, Janssen
Travel, Accommodations, Expenses: Siemens
Michael Weller
Honoraria: Novocure, Bayer, Pierre Fabre
Consulting or Advisory Role: Curevac, Medac, Novartis, Orbus Therapeutics, Philogen, Roche, Sandoz, Janssen, Seagen, LEO Pharma, Bayer
Research Funding: Quercegen Pharmaceuticals (Inst), Versameb (Inst)
Evanthia Galanis
Consulting or Advisory Role: KIYATEC, Karyopharm Therapeutics (Inst), Boston Scientific (Inst), Servier (Inst)
Research Funding: MedImmune (Inst), Servier (Inst), Celgene (Inst), Denovo Biopharma (Inst), Denovo Biopharma (Inst)
Daniel P. Barboriak
Uncompensated Relationships: Blue Earth Diagnosticds
John de Groot
Employment: Alaunos Therapeutics, Brii Biosciences
Stock and Other Ownership Interests: WuXi Biologics, Alaunos Therapeutics, Brii Biosciences
Consulting or Advisory Role: Merck, Mundipharma Research, Bioasis Technologies, InSightec, Samus Therapeutics, Karyopharm Therapeutics, Cure Brain Cancer Foundation, Sapience Therapeutics, Monteris Medical, Kintara Therapeutics, Kazia Therapeutics, CarThera, Sumitomo Dainippon Pharma Oncology, VBI Vaccines, Chimerix, Aucentra Therapeutics, Midatech Pharma, Servier, Telix Pharmaceuticals, Alpha Pharmaceutical
Research Funding: CarThera (Inst), Haihe Pharmaceutical (Inst), Taiho Pharmaceutical (Inst)
Other Relationship: VBI Vaccines, Chimerix
Raymond Huang
Consulting or Advisory Role: Agios, Nuvation Bio, Nuvation Bio, Vysioneer
Research Funding: Agios, Bristol Myers Squibb
Andrew B. Lassman
Honoraria: Clinical Education Alliance
Consulting or Advisory Role: Sapience Therapeutics, Orbus Therapeutics, Novocure, Vivacitas Oncology, Chimerix, Affinia Therapeutics, Neuron-D, Leal Therapeutics, AlphaDetail, AlphaSights, Bluestar Bioadvisors, Clarion Healthcare, ExpertConnect, Gerson Lehrman Group, Guidepoint Global, IP2IPO, KJT, Tavistock Life Sciences, techspert.io, BioClinica, RadMD, Global Coalition for Adaptive Research
Research Funding: AbbVie, Genentech/Roche, Aeterna Zentaris, VBI Vaccines, Pfizer, Karyopharm Therapeutics, Bayer, QED Therapeutics, Orbus Therapeutics, BMS, Chimerix, NextSource, DelMar, Polaris (Inst), Kintara (Inst) Pharmaceuticals, Corden, Kazia Therapeutics, Servier, Biohaven Pharmaceuticals, Vigeo Therapeutics, Incyte, Abbott Laboratories
Travel, Accommodations, Expenses: AbbVie, Chimerix, Karyopharm Therapeutics, Pfizer, Bridgebio, VBI Vaccines, Helsinn Healthcare, Foundation Medicine, Servier, Anheart Therapeutics
Minesh Mehta
Stock and Other Ownership Interests: Chimerix
Consulting or Advisory Role: Mevion Medical Systems, ZappRx, Xoft, Kazia Therapeutics, Novocure
Patents, Royalties, Other Intellectual Property: WARF patent 14/934,27, Topical Vasoconstritor Preparations and Methods for Protecting Cells During Cancer Chemotherapy and Radiotherapy
Uncompensated Relationships: Xcision Medical Systems
Matthias Preusser
Honoraria: Roche, GlaxoSmithKline, Bayer, Bristol Myers Squibb, Novartis, Gerson Lehrman Group, CMC Contrast, Mundipharma, BMJ Journals, MedMedia, AstraZeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra Pharmaceuticals, Gan & Lee, Servier
Consulting or Advisory Role: Roche, Bristol Myers Squibb, Novartis, Gerson Lehrman Group, CMC Contrast, GlaxoSmithKline, Mundipharma, AbbVie, Bayer, AstraZeneca, Lilly, Daiichi Sankyo/Astra Zeneca, Sanofi, Merck Sharp & Dome, Tocagen, Adastra Pharmaceuticals, Gan & Lee, Servier
Research Funding: Roche (Inst), GlaxoSmithKline (Inst), Boehringer Ingelheim (Inst), Merck Sharp & Dohme, Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Roche, GlaxoSmithKline, Bristol Myers Squibb, MSD, Mundipharma, Servier
Rifaquat Rahman
Consulting or Advisory Role: Beijing Saint Lucia Consulting
Research Funding: Bristol Myers Squibb (Inst), Puma Biotechnology (Inst), Lilly (Inst)
Roger Stupp
Stock and Other Ownership Interests: Alpheus Medical, CarThera
Consulting or Advisory Role: Celgene, Northwest Biotherapeutics, Zai Lab, InSightec, Celularity, CranioVation, Triact Therapeutics, Hemispherian, GT Medical Technologies, Novocure, AstraZeneca, Boston Scientific, Carthera, The Lockwood Group, Boston Biomedical, Novartis, AimedBio
Research Funding: CarThera, Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: Methods of Determining Responsiveness to Chemotherapeutic Compounds for Cancer Therapy Type: Provisional Serial No. 63/202,761 Filed: June 23, 2021 (Inst)
Javier E. Villanueva-Meyer
Consulting or Advisory Role: GE Healthcare, MedQIA, Alpheus Medical
Research Funding: GE Healthcare
Wolfgang Wick
Consulting or Advisory Role: MSD Oncology (Inst), Roche/Genentech (Inst), SERVIER, GlaxoSmithKline
Research Funding: Roche (Inst), Apogenix (Inst), Pfizer (Inst)
David R. Macdonald
Research Funding: Celgene (Inst), Servier (Inst)
David A. Reardon
Honoraria: Merck, Novocure, Regeneron, Bristol Myers Squibb, Oncorus, Agenus, EMD Serono, Merck KGaA, Taiho Pharmaceutical, Advantagene, Bayer, DelMar Pharmaceuticals, Imvax, Medicenna, Sumitono Dainippon Pharma, Vivacitas Oncology, Anheart Therapeutics, Deciphera, Ellipses Pharma, Genenta Science, Inovio Pharmaceuticals, Kintara Therapeutics, Kintara Therapeutics, KIYATEC, NEUVOGEN, Taiho Pharmaceutical, Y-mAbs Therapeutics
Consulting or Advisory Role: Merck, Novocure, Regeneron, Bristol Myers Squibb, Oncorus, Agenus, EMD Serono, Merck KGaA, Taiho Pharmaceutical, Delmar Pharmaceuticals, Advantagene, Bayer, Imvax, Medicenna, Vivacitas Oncology, Anheart Therapeutics, Ellipses Pharma, Genenta Science, Kintara Therapeutics, Kiyatec, Agios
Research Funding: Celldex (Inst), Incyte (Inst), Agenus (Inst), EMD Serono (Inst), Acerta Pharma (Inst), Omniox, Enterome (Inst)
Michael A. Vogelbaum
Stock and Other Ownership Interests: Infusion Therapeutics
Honoraria: Plus Therapeutics, Chimerix
Consulting or Advisory Role: Olympus, Midatech Pharma
Research Funding: Infusion Therapeutics (Inst), Celgene (Inst), Oncosynergy (Inst), Denovo Biopharma (Inst)
Patents, Royalties, Other Intellectual Property: Patents for drug delivery devices for the brain
Susan M. Chang
Consulting or Advisory Role: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022;24:v1–v95. doi: 10.1093/neuonc/noac202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller JJ, Gonzalez Castro LN, McBrayer S, et al. Isocitrate dehydrogenase (IDH) mutant gliomas: A Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol. 2023;25:4–25. doi: 10.1093/neuonc/noac207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22:1073–1113. doi: 10.1093/neuonc/noaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 6. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): Assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12:583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 7. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14:307–320. doi: 10.1007/s13311-016-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015;16:e534–e542. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Youssef G, Rahman R, Bay C, et al. Evaluation of standard response assessment in neuro-oncology, modified response assessment in neuro-oncology, and immunotherapy response assessment in neuro-oncology in newly diagnosed and recurrent glioblastoma. J Clin Oncol. 2023;41:3160–3171. doi: 10.1200/JCO.22.01579. [DOI] [PubMed] [Google Scholar]
- 10. Gallego Perez-Larraya J, Lahutte M, Petrirena G, et al. Response assessment in recurrent glioblastoma treated with irinotecan-bevacizumab: Comparative analysis of the Macdonald, RECIST, RANO, and RECIST + F criteria. Neuro Oncol. 2012;14:667–673. doi: 10.1093/neuonc/nos070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boxerman JL, Ellingson BM. Response assessment and magnetic resonance imaging issues for clinical trials involving high-grade gliomas. Top Magn Reson Imaging. 2015;24:127–136. doi: 10.1097/RMR.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 12. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berger TR, Wen PY, Lang-Orsini M, et al. World Health Organization 2021 classification of central nervous system tumors and implications for therapy for adult-type gliomas: A review. JAMA Oncol. 2022;8:1493–1501. doi: 10.1001/jamaoncol.2022.2844. [DOI] [PubMed] [Google Scholar]
- 14. Horbinski C, Berger T, Packer RJ, et al. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat Rev Neurol. 2022;18:515–529. doi: 10.1038/s41582-022-00679-w. [DOI] [PubMed] [Google Scholar]
- 15. Ellingson BM, Wen PY, Cloughesy TF. Evidence and context of use for contrast enhancement as a surrogate of disease burden and treatment response in malignant glioma. Neuro Oncol. 2018;20:457–471. doi: 10.1093/neuonc/nox193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17:1188–1198. doi: 10.1093/neuonc/nov095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah GD, Kesari S, Xu R, et al. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro Oncol. 2006;8:38–46. doi: 10.1215/S1522851705000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gahrmann R, van den Bent M, van der Holt B, et al. Comparison of 2D (RANO) and volumetric methods for assessment of recurrent glioblastoma treated with bevacizumab-a report from the BELOB trial. Neuro Oncol. 2017;19:853–861. doi: 10.1093/neuonc/now311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galanis E, Buckner JC, Maurer MJ, et al. Validation of neuroradiologic response assessment in gliomas: Measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro Oncol. 2006;8:156–165. doi: 10.1215/15228517-2005-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellingson BM, Kim GHJ, Brown M, et al. Volumetric measurements are preferred in the evaluation of mutant IDH inhibition in non-enhancing diffuse gliomas: Evidence from a phase I trial of ivosidenib. Neuro Oncol. 2022;24:770–778. doi: 10.1093/neuonc/noab256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chappell R, Miranpuri SS, Mehta MP. Dimension in defining tumor response. J Clin Oncol. 1998;16:1234. doi: 10.1200/JCO.1998.16.3.1234. [DOI] [PubMed] [Google Scholar]
- 22. Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 23. Hagiwara A, Schlossman J, Shabani S, et al. Incidence, molecular characteristics, and imaging features of "clinically-defined pseudoprogression" in newly diagnosed glioblastoma treated with chemoradiation. J Neurooncol. 2022;159:509–518. doi: 10.1007/s11060-022-04088-3. [DOI] [PubMed] [Google Scholar]
- 24. Wick W, Chinot OL, Bendszus M, et al. Evaluation of pseudoprogression rates and tumor progression patterns in a phase III trial of bevacizumab plus radiotherapy/temozolomide for newly diagnosed glioblastoma. Neuro Oncol. 2016;18:1434–1441. doi: 10.1093/neuonc/now091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 26. Ellingson BM, Gerstner ER, Lassman AB, et al. Hypothetical generalized framework for a new imaging endpoint of therapeutic activity in early phase clinical trials in brain tumors. Neuro Oncol. 2022;24:1219–1229. doi: 10.1093/neuonc/noac086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karschnia P, Young JS, Dono A, et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro Oncol. 2023;25:940–954. doi: 10.1093/neuonc/noac193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shidoh S, Savjani RR, Cho NS, et al. Relapse patterns and radiation dose exposure in IDH wild-type glioblastoma at first radiographic recurrence following chemoradiation. J Neurooncol. 2022;160:115–125. doi: 10.1007/s11060-022-04123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boxerman JL, Zhang Z, Safriel Y, et al. Prognostic value of contrast enhancement and FLAIR for survival in newly diagnosed glioblastoma treated with and without bevacizumab: Results from ACRIN 6686. Neuro Oncol. 2018;20:1400–1410. doi: 10.1093/neuonc/noy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 31. Holdhoff M, Ye X, Piotrowski AF, et al. The consistency of neuropathological diagnoses in patients undergoing surgery for suspected recurrence of glioblastoma. J Neurooncol. 2019;141:347–354. doi: 10.1007/s11060-018-03037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Melguizo-Gavilanes I, Bruner JM, Guha-Thakurta N, et al. Characterization of pseudoprogression in patients with glioblastoma: Is histology the gold standard? J Neurooncol. 2015;123:141–150. doi: 10.1007/s11060-015-1774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fu R, Szidonya L, Barajas RF, Jr., et al. Diagnostic performance of DSC perfusion MRI to distinguish tumor progression and treatment-related changes: A systematic review and meta-analysis. Neurooncol Adv. 2022;4:vdac027. doi: 10.1093/noajnl/vdac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18:1199–1208. doi: 10.1093/neuonc/now058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van West SE, de Bruin HG, van de Langerijt B, et al. Incidence of pseudoprogression in low-grade gliomas treated with radiotherapy. Neuro Oncol. 2017;19:719–725. doi: 10.1093/neuonc/now194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nowosielski M, Wiestler B, Goebel G, et al. Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology. 2014;82:1684–1692. doi: 10.1212/WNL.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 37. Huang RY, Rahman R, Ballman KV, et al. The impact of T2/FLAIR evaluation per RANO criteria on response assessment of recurrent glioblastoma patients treated with bevacizumab. Clin Cancer Res. 2016;22:575–581. doi: 10.1158/1078-0432.CCR-14-3040. [DOI] [PubMed] [Google Scholar]
- 38. Nowosielski M, Ellingson BM, Chinot OL, et al. Radiologic progression of glioblastoma under therapy-an exploratory analysis of AVAglio. Neuro Oncol. 2018;20:557–566. doi: 10.1093/neuonc/nox162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nayak L, DeAngelis LM, Brandes AA, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: A tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19:625–635. doi: 10.1093/neuonc/nox029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Galldiks N, Langen KJ. Amino acid PET in neuro-oncology: Applications in the clinic. Expert Rev Anticancer Ther. 2017;17:395–397. doi: 10.1080/14737140.2017.1302799. [DOI] [PubMed] [Google Scholar]
- 41. Soni N, Ora M, Mohindra N, et al. Diagnostic performance of PET and perfusion-weighted imaging in differentiating tumor recurrence or progression from radiation necrosis in posttreatment gliomas: A review of literature. AJNR Am J Neuroradiol. 2020;41:1550–1557. doi: 10.3174/ajnr.A6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Strauss SB, Meng A, Ebani EJ, et al. Imaging glioblastoma posttreatment: Progression, pseudoprogression, pseudoresponse, radiation necrosis. Neuroimaging Clin N Am. 2021;31:103–120. doi: 10.1016/j.nic.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 43. Kickingereder P, Isensee F, Tursunova I, et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: A multicentre, retrospective study. Lancet Oncol. 2019;20:728–740. doi: 10.1016/S1470-2045(19)30098-1. [DOI] [PubMed] [Google Scholar]
- 44. Chang K, Beers AL, Bai HX, et al. Automatic assessment of glioma burden: A deep learning algorithm for fully automated volumetric and bidimensional measurement. Neuro Oncol. 2019;21:1412–1422. doi: 10.1093/neuonc/noz106. [DOI] [PMC free article] [PubMed] [Google Scholar]