Abstract
Maternal attachment security is an important predictor of caregiving behavior. However, little is known regarding the neurobiological mechanisms by which maternal attachment security influences processing of infant cues, a critical component of caregiving. We examined whether differences in attachment security, measured by the Adult Attachment Interview, might relate to maternal neural responses to infant cues using event-related potentials (ERPs). Secure (n=35) and insecure (n=24) mothers viewed photographs of infant faces and heard recordings of infant vocalizations (derived from their own infant and an unfamiliar infant) while electroencephalography was simultaneously recorded. We examined initial processing of infant faces (N170) and cries (N100), and attentional allocation to infant faces and cries (P300). At early stages of processing, secure mothers were significantly faster than insecure mothers to orient to infant cries (N100), and significantly faster to structurally encode their own infant’s face (N170). At later stages of processing, secure mothers were faster than insecure mothers to attend to infant faces (P300). These differences may be mechanistically important in our understanding the role of attachment in shaping neural processing of infant cues and highlight the relevance of social neuroscientific approaches in examining clinically relevant aspects of attachment security.
Keywords: infant cues, mothers, adult attachment, ERP, social neuroscience
Adult attachment, as defined as the presence or absence of an organized and consistent valuing of attachment relationships, is an important predictor of caregiving. Consistently, mothers with secure attachment demonstrate greater behavioral sensitivity to their infants’ needs (Pederson, Gleason, Moran, & Bento, 1998; Ward & Carlson, 1995), reflective functioning (i.e., ability to understand others’ and one’s own behavior in terms of underlying thoughts and feelings; Slade, Grienenberger, Bernbach, Levy, & Locker, 2005), and mind-mindedness (i.e., attunement to infants’ internal thoughts and feelings; Meins, 2013; Riva Crugnola, Ierardi, & Canevini, 2018) as compared to mothers who are classified as insecure. Maternal sensitivity, reflective functioning, and mind-mindedness have captured researchers’ attention for decades given that they may explain ‘the transmission gap’ of attachment security from one generation to the next (Meins, 1999; Pederson et al., 1998; Slade et al., 2005; Van IJzendoorn, 1995). Although these constructs are key mediators in the well-documented relationship between mothers’ adult attachment representations and their infants’ attachment behaviors, they are not the only factors involved (De Wolff & Van IJzendoorn, 1997), suggesting the relevance of investigating how maternal attachment relates to other aspects of the caregiving system, including potential underlying neurobiological mechanisms that might facilitate responding to infant cues.
Despite numerous empirical studies demonstrating the importance of maternal attachment in the manifestation of caregiving, little is known regarding the neurobiological mechanisms by which maternal attachment influences processing of infant cues, a critical component of caregiving. In contrast to fMRI, which has limited temporal resolution, the event-related potential (ERP) method allows researchers to study the timing and strength of rapidly occurring neural responses to infant cues, thus providing detailed data related to the various stages of infant cue processing (Maupin, Hayes, Mayes, & Rutherford, 2015). ERP methods involve time-locking scalp-recorded electrocortical activity to a task or stimulus presentation across multiple trials and generating waveforms with clearly defined positive (P) and negative (N) peaks. These peaks may be interpreted as indices of different stages of stimulus processing, including perception, cognition, and attention, across different sensory modalities (e.g., visual, auditory).
Integrating data regarding both strength (i.e., amplitude) and speed (i.e., latency) of ERP responses to various stimuli may provide insight into the efficiency of neural processing. Although ERP studies often use the terms efficiency and speed interchangeably, others have suggested that the efficiency of neural processing actually encompasses both how fast and how much neural activity occurs in response to a stimulus or task (Ansari & Derakshan, 2011a). In other words, in addition to the speed of neural response, neural efficiency is also dependent upon the degree of neural resources elicited to meet a given task demand (Neubauer & Fink, 2009). Importantly, Rösler, Heil, and Röder (1997) assert that larger amplitudes are markers of greater neural resource allocation, whereas smaller amplitudes reflect less resource allocation. Collectively, shorter latencies and smaller amplitudes may therefore represent more efficient processing, whereas longer latencies and greater amplitudes may represent less efficient processing (Ansari & Derakshan, 2011a, 2011b; Daffner et al., 2011; Espinet, Anderson, & Zelazo, 2012; Judah, Grant, Mills, & Lechner, 2013; van Dinteren, Arns, Jongsma, & Kessels, 2014). Overall, ERP methods are thus well-suited for use in caregiving research given that optimal, sensitive caregiving may be characterized by efficient parental responding (Ainsworth, Blehar, Waters, & Wall, 1978; Beebe, 1982; Beebe et al., 2010; Bretherton, 2013; Van Ijzendoorn, Goldberg, Kroonenberg, & Frenkel, 1992), and given that “some of the most important mother-infant nonverbal communicative events occur in less than a second” (Beebe, 1982; p. 169).
A long line of maternal ERP research has examined the N100, N170, and P300 (each described below) to elucidate how mothers perceive and attend to caregiving-related stimuli. At early stages, the N100 (i.e., a negative waveform peak occurring at approximately 100 ms following the onset of a sound) is employed to study mothers’ initial auditory processing of infant vocalizations given that crying is a primary method of infant communication and proximity maintenance (Bowlby, 1969), and mothers’ ability to register and orient to this cue is vital to initiating caregiving responses. Recently postpartum women demonstrated stronger N100 responses to infant vocalizations compared to non-pregnant women, women not caring for young children, and women not employed in childcare, suggesting that infant cries are particularly salient to new mothers (Purhonen, Valkonen-Korhonen, & Lehtonen, 2008). The N170 (i.e., a negative waveform peak occurring at approximately 170 ms following the presentation of a facial stimulus) has been used to study mothers’ early visual processing given its specificity to facial structural encoding (Eimer & Holmes, 2007), another integral component of understanding infants’ needs. Relative to fathers and non-parents, mothers demonstrate significantly greater N170 responses to infant faces, suggesting heightened visual responsivity that is specific to parental status and gender (Proverbio, Brignone, Matarazzo, Del Zotto, & Zani, 2006). In experiments using infant faces with varying emotional expressions, regardless of infant familiarity, mothers showed greater N170 responses to sad infant faces, as compared to happy and neutral faces (Doi & Shinohara, 2012). In contrast, however, other research suggests that although various maternal clinical and demographic characteristics impact neural response to infant faces, maternal N170 responses are not necessarily modulated by infant emotional expressions (Maupin, Rutherford, Landi, Potenza, & Mayes, 2019; Rutherford, Byrne, et al., 2017; Rutherford, Maupin, Landi, Potenza, & Mayes, 2017; Rutherford, Maupin, & Mayes, 2019).
At later stages of neural processing, the P300 (i.e., a positive waveform peak occurring at approximately 300 ms following the onset of a sound or presentation of a visual stimulus) may be used to measure attentional allocation and engagement to visual and auditory caregiving-related stimuli. One study reported that, as compared to fathers and non-parents, mothers demonstrated greater P300 responses to infant faces, particularly distressed infant faces (Proverbio et al., 2006). Similarly, in other studies of mothers only, mothers’ demonstrated the greatest Late Positive Potential responses (LPP, a positive waveform peak occurring at approximately 300–600 ms following stimulus presentation) to distressed infant faces, followed by happy and neutral infant faces (Bernard, Simons, & Dozier, 2015; Kuzava, Nissim, Frost, Nelson, & Bernard, 2019). In contrast, other studies of mothers have found that infant emotional expression does not have a main effect on maternal P300 responses to infant faces (Maupin et al., 2019; Rutherford, Byrne, et al., 2017; Rutherford, Maupin, et al., 2017; Rutherford et al., 2019). When familiarity of the infant face is introduced as a factor, however, mothers show greater and faster P300 responses when viewing their own infant’s sad face, as compared to their own infant’s happy and neutral faces and unknown infant faces, suggesting that mothers’ own infant’s negative emotional states are most salient (Doi & Shinohara, 2012). These findings have been shown to extend to both biological and foster/adoptive mothers, with both groups showing significantly greater P300 responses to pictures of their own child relative to images of unfamiliar children or adults (Grasso, Moser, Dozier, & Simons, 2009). With regard to auditory stimuli, one study found that mothers also exhibit greater P300 responses to infant cries relative to a neutral tone (Maupin et al., 2019). Although this literature is mixed, overall, given that mothers of young children (as compared to non-caregivers and fathers) demonstrate greater P300 responses to infant emotions, particularly their own infant’s emotions, it is suggested that the early stage of motherhood may be associated with increased salience of infant cues, sensitive encoding of infant emotions, attention to infant distress, and acuity for detecting personal significance (Grasso et al., 2009; Proverbio et al., 2006).
Emerging ERP research has begun to examine the role of attachment security in neural processing of social stimuli in non-parent samples (Ma, Ran, Chen, Ma, & Hu, 2017; Mark, Geurdes, & Bekker, 2012; Zhang, Li, & Zhou, 2008; Zilber, Goldstein, & Mikulincer, 2007). Several ERP studies employing odd-ball experimental tasks have also examined the impact of attachment security on neural response to infant cues in maternal samples. Notably, these studies have yielded mixed results, likely for multiple reasons, including varying measurement of attachment, variable sample sizes, and differing statistical approaches. Consistent with an efficiency model (i.e., smaller amplitudes/shorter latencies vs. larger amplitudes/longer latencies) of neural processing, however, these studies have shown that secure mothers are generally more efficient (i.e., smaller amplitudes) at processing infant cues as compared to insecure mothers. When shown unfamiliar infant faces and instructed to focus on negative emotional cues, insecure mothers (as categorized by the Adult Attachment Interview, AAI; George, Kaplan, & Main, 1996) demonstrated greater N170 amplitudes than secure mothers (Leyh, Heinisch, Behringer, Reiner, & Spangler, 2016). One small study utilizing the Adult Attachment Projective (George & West, 2003) similarly found that, compared to secure mothers, insecure mothers demonstrate greater N170 amplitudes in response to unfamiliar infant faces (Fraedrich, Lakatos, & Spangler, 2010). The authors of these studies suggested that secure mothers employ a lesser degree of neural resources at this early stage of perceptual processing, whereas insecure mothers require greater resources to structurally encode infant emotional faces.
This pattern may persist at later stages of processing, though results regarding the P300 are variable. When presented with unfamiliar infant faces, secure mothers demonstrated greater P300 amplitudes than insecure mothers (Fraedrich et al., 2010; Leyh et al., 2016). In contrast, consistent with an efficiency model, when presented with images of one’s own infant’s face, insecure mothers (as measured by the Attachment Script Assessment; Waters & Rodrigues-Doolabh, 2004) exhibited greater P300 responses to their own infant’s distressed face as compared to their own infants’ happy face (Groh & Haydon, 2018). Also consistent with an efficiency model, compared to secure mothers, insecure mothers demonstrated delayed P300 latencies in response to unfamiliar faces (Leyh et al., 2016). Collectively, some argue that infant distress is particularly salient for insecure mothers (Groh & Haydon, 2018), whereas others suggest that secure mothers allocate more attentional resources to caregiving-relevant stimuli, thereby enabling adaptive caregiving (Fraedrich et al., 2010). Though results have been variable, this literature suggests that early experiences impact mothers’ adult attachment representations, which in turn may shape how the maternal brain is activated by infant cues.
To our knowledge, no studies have examined how maternal attachment as measured by the AAI relates to both the strength and timing of mothers’ neural processing of own and unknown infant faces and cries. Notably, prior studies regarding attachment and maternal ERPs most often report amplitude but not latency findings, thereby representing a gap in the literature, particularly in light of the utility of ERP methods to elucidate the temporal characteristics of maternal neural responses and thus informing our understanding of the efficiency of infant cue processing. Thus, our study utilized ERP methods to determine whether the amplitude and/or latency of mothers’ neural responses to both own and unfamiliar infant cues differ as a function of attachment security. Given findings suggesting that, relative to insecure attachment, secure attachment is associated with decreased neural activation (Fraedrich et al., 2010; Groh & Haydon, 2018; Leyh et al., 2016) and physiological reactivity (Groh & Roisman, 2009) in response to infant affective cues, we hypothesized that secure mothers would demonstrate attenuated N170 and P300 amplitudes to infant faces and attenuated N100 and P300 amplitudes to infant cries, whereas insecure mothers would demonstrate greater amplitudes. To date, only one study (Leyh et al., 2016) has reported latency effects as a function of maternal attachment security. Similar to this prior work, and consistent with an efficiency model of neural processing, we hypothesized that secure mothers would demonstrate faster latencies, whereas insecure mothers would demonstrate delayed latencies in response to infant faces and cries. Further, we anticipated that these effects would be exaggerated for own infant cues in secure mothers. Overall, findings such as these may inform current knowledge related to the mechanisms underlying the manifestation of caregiving and the transmission of attachment across generations.
Methods
Participants
Fifty-nine postpartum (M=8.17 months, SD=2.29 months) mothers (aged 18 to 43 years; M=29.33 years, SD=5.82 years) were recruited from a diverse inner city population as part of a larger study. Given the impact of substance use on mothers’ neural responses to infant cues (Lowell et al., 2020; Rutherford, Maupin, et al., 2017), participants identified as substance-using as part of the larger study were excluded. Participants were analyzed in groups based on their attachment classifications as measured by the Adult Attachment Interview (AAI; Crittenden, 2004; George et al., 1996; Hesse, 2008). Our samples of secure (n=35) and insecure (n=24) were comparable to those in previous neuroimaging work utilizing the AAI (e.g., Strathearn, Fonagy, Amico, & Montague, 2009). Of the 59 mothers included in our sample, 12 mothers had a secondary AAI designation of unresolved/disorganized; however, they were analyzed based on their primary AAI classification of secure (n=5) or insecure (n=7). Preliminary analyses indicated that there were no AAI group differences with respect to demographic variables (Table 1). Yale University’s Human Investigation Committee approved all procedures prior to recruitment.
Table 1.
Characteristics of Secure and Insecure Mothers
| Secure Mothers (n=35) | Insecure Mothers (n=24) | |
|---|---|---|
|
| ||
| Maternal age (in years) | 30.50 (4.46) | 27.67 (7.10) |
| Maternal race/ethnicity | ||
| African American | 10 | 16 |
| European American | 11 | 5 |
| Hispanic/Latina | 9 | 3 |
| Asian American | 3 | 0 |
| Other | 1 | 0 |
| Prefer not to answer | 1 | 0 |
| Annual family income | ||
| Below $15,000 | 11 | 11 |
| $15,000-$30,000 | 3 | 6 |
| $30,000-$45,000 | 3 | 3 |
| $45,000-$70,000 | 11 | 1 |
| $70,000-$100,000 | 1 | 1 |
| Above $100,000 | 3 | 1 |
| Did not report | 3 | 1 |
| Parity | ||
| Primiparous | 18 | 13 |
| Multi-parous | 16 | 11 |
| Did not report | 1 | 0 |
| Infant age (in months) | 8.11 (1.82) | 8.25 (2.86) |
| Infant sex | ||
| Male | 12 | 13 |
| Female | 22 | 11 |
| Did not report | 1 | 0 |
Note: Chi square analyses and independent samples t-tests indicated no differences in any demographic variables as a function of attachment security.
Apparatus and Stimuli
Auditory and visual stimuli were presented to participants in a sound-attenuated room with low ambient light. Stimuli were presented using a Pentium IV computer running E-Prime 1.2 software (Schneider, Eschman, & Zuccolotto, 2002), and using a 51cm color monitor (75Hz, 1024×768 resolution). Continuous EEG was recorded using Net Station 4.2.1 with a 250Hz sampling rate and high impedance amplifiers (0.1Hz high pass, 100Hz low pass). A 128 Ag/AgCl electrode net (Electrical Geodesics, Inc.) was soaked in a warm potassium chloride solution, and net electrodes were spaced evenly and symmetrically on each participant’s head to cover the scalp (i.e., nasion to inion, left ear to right ear). The Cz electrode was used for reference during EEG recording, and electrode impedances were kept <40kΩ.
Infant cry stimuli included 20 audio samples: 10 cries from the participants’ own infant, and 10 cries from an unfamiliar infant. Each audio sample was approximately 2 seconds. Each cry stimulus was presented 4 times. Infant face stimuli included 40 images: 20 photographs of the participants’ own infant (10 happy, 10 sad), and 20 photographs of an unfamiliar infant (10 happy, 10 sad). Each face stimulus was presented 4 times. Smiling infant faces were elicited by video-recording play interactions using a variety of age-appropriate toys, whereas cries and sad faces were elicited by a brief maternal separation. Still images and audio clips were selected from these recordings. Infant facial expressions were classified by trained research staff into two affect groups (happy/sad), validated in previous neuroimaging research (Kim et al., 2017). Coders utilized the procedure described in Cole, Barrett, and Zahn-Waxler (1992), where happy infant faces were those with the corner of the lips up, cheeks raised, crinkled eyes, and smooth forehead, whereas sad infant faces were those with the corners of the mouth turned down, the lower lip pushed out, the inner eyebrows raised or lowered, and drooped eyes. Infant faces were closely cropped from the still images to remove all extraneous visual information and presented on a black background. The size of the infant faces averaged approximately 11×11cm. Unfamiliar infant face stimuli were matched for participants’ own infant’s sex (if distinguishable), race, and age. Viewing distance was approximately 70cm.
Measures
The Adult Attachment Interview (AAI; Crittenden, 2004; George et al., 1996; Hesse, 2008) was used to assess mothers’ attachment security. Interview questions focus on an individual’s memories and affect associated with day-to-day interactions with caregivers (e.g., bedtime/comfort/illness), as well as less common experiences (e.g., abuse/loss/trauma) throughout childhood. The AAI classifies an adult’s attachment security based on the characteristics of the language used within their narrative discourse rather than on the content of the experiences described. Individuals are categorized in one of three categories: secure-autonomous, insecure-dismissing, insecure-preoccupied, or cannot classify (Main, Hesse, & Goldwyn, 2008). An unresolved/disorganized designation may additionally be assigned to individuals who demonstrate a unique pattern of disorganized lapses in their descriptions of attachment relationships, particularly following the experience of trauma or loss. AAIs in this study were coded by Deanne Dozier, a coder who has completed reliability certification with Dr. Mary Main’s laboratory. An additional coder was not employed to double-code transcripts; therefore, interrater reliability was not conducted. In this study, insecure-dismissing (n=19), insecure-preoccupied (n=4), and cannot classify (n=1) individuals were grouped as insecure, consistent with prior literature (Leyh et al., 2016; Main & Goldwyn, 1995; Riem, Bakermans-Kranenburg, van IJzendoorn, Out, & Rombouts, 2012).
Procedure
Prior to the experimental trials, participants completed 12 practice trials to become familiarized with the apparatus and experimental set-up. Trials consisted of a centrally-presented fixation cross (2000ms), followed by stimulus presentation (cries 2000ms; faces 500ms), and a blank screen in a passive viewing/listening paradigm. The inter-trial interval and fixation cross presentation were jittered (1400–2000ms) to avoid possible expectancy effects related to stimulus onset. The experiment consisted of 4 blocks of 60 trials (240 experimental trials total), wherein infant face and cry stimuli were randomly presented. The task took approximately 30 minutes.
Analysis
Net Station 4.5 was used to pre-process electroencephalography (EEG) data, which were first digitally filtered (30Hz low-pass filter). Data were then segmented into 1 second epochs (100ms pre- and 900ms post-stimulus onset). Ocular Artifact Removal (OAR; Gratton, Coles, & Donchin, 1983), using a blink slope threshold of 14μV/ms, was applied to data for all participants. Net Station artifact detection was set to 200μV for bad channels, 150μV for eye-blinks, and 150μV for eye-movements. Spline interpolation was used to replace channels with artifacts in more than 40% of trials. Following artifact detection, all EEG data were re-referenced to the average reference of all electrodes and baseline corrected to the 100ms interval pre-stimulus onset. Lastly, the EEG data were averaged across stimulus conditions for each participant. At completion of pre-processing, there were on average 32.87 trials per condition across all participants (SD=6.32 trials), with no attachment security difference in trial numbers [t<1.54, p>.13].
All ERPs were visually inspected in the grand-averaged data and verified for each participant. The N100 (60ms–160ms) was assessed and averaged at 10 central electrode sites (CZ,6,7,13,31,32,81,106,107,113), consistent with prior N100 cry perception research (Maupin et al., 2019; Purhonen et al., 2001; Purhonen et al., 2008). The N170 (135ms–202ms) was assessed and averaged at 6 electrode sites over the left (58,59,64,65,69,70) and right (90,91,92,95,96,97) posterior scalp sites, consistent with prior N170 studies (McPartland, Dawson, Webb, Panagiotides, & Carver, 2004; Noll, Mayes, & Rutherford, 2012). The P300 for infant faces (250ms–600ms) and infant cries (250ms–800ms) was assessed at 6 electrode sites that clustered around Pz (54,61,62,68,79,80), consistent with existing literature (Grasso et al., 2009; Maupin et al., 2019; Proverbio et al., 2006; Rutherford, Maupin, et al., 2017). For topographic plots, please see Supplementary Materials. Data analysis was conducted separately for each component; mean amplitude analysis was used to examine strength of neural response, and latency-to-peak was used to examine timing of neural response. Effect sizes are presented as partial eta-squared (ηp2), where .01 represents a small effect size, .06 a medium effect size, and .14 a large effect size (Cohen, 1988). Cohen’s d is used to present effect sizes of t-tests, where .20 represents a small effect size, .50 a medium effect size, and .80 a large effect size (Cohen, 1988).
Results
N100 (Infant Cries)
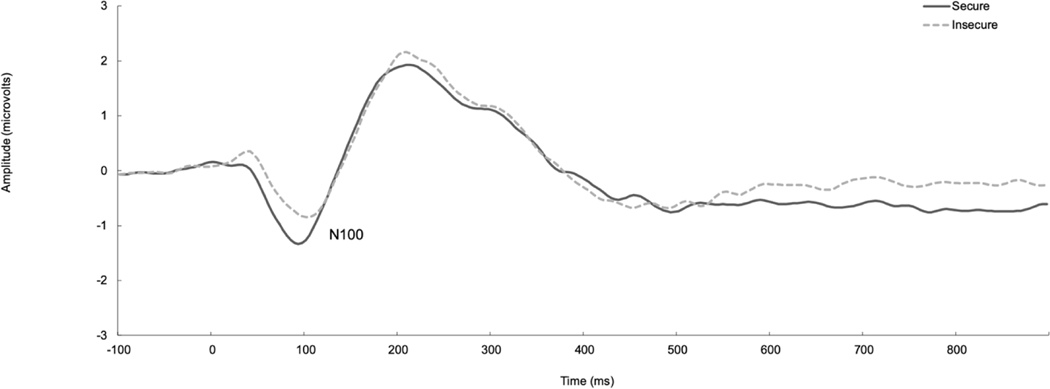
Grand averaged data averaged across infant cry familiarity for the N100 as a function of attachment classification are presented in Figure 1. A repeated-measures ANOVA specifying infant cry familiarity (own/unfamiliar) and participant attachment classification (secure/insecure) was conducted to examine mothers’ N100 responses to infant cries. With respect to N100 amplitude, there was a main effect for cry familiarity [F(1,57)=8.33, p=.005, ηp2=.13]. Specifically, regardless of attachment classification, mothers demonstrated larger N100 responses to unfamiliar infant cries (M=−0.76, SD=0.98) as compared to their own infant’s cries (M=−0.46, SD=0.78). In contrast, there was no main effect of attachment classification [F(1,57)=1.00, p=.32, ηp2=.02], nor was there an interaction between cry familiarity and attachment classification on N100 amplitude [F(1,57)=1.09, p=.30, ηp2=.02].
Figure 1.
Grand-averaged ERP waveforms representing the N100 response to infant cries in secure and insecure mothers. Data are averaged across own/unfamiliar infant cries.
With respect to N100 latency, there was a main effect for attachment classification [F(1,57)=5.36, p=.02, ηp2=.09]. Specifically, regardless of cry familiarity, secure mothers were faster to respond to infant cries (M=96.75, SD=20.62) as compared to insecure mothers (M =110.76, SD=25.72). In contrast, there was no main effect for cry familiarity on N100 latency, nor was there an interaction between cry familiarity and attachment classification on N100 latency [F’s<1].
N170 (Infant Faces)
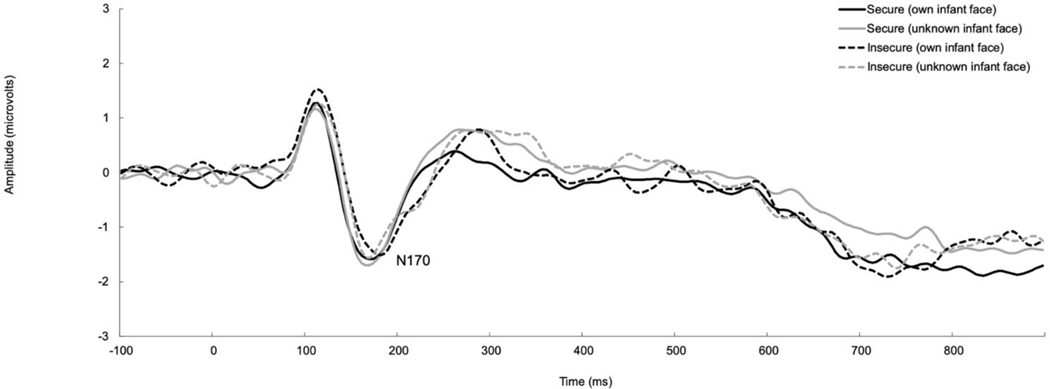
Grand-averaged data for infant faces averaged across left and right hemisphere electrodes for the N170 as a function of attachment classification are presented in Figure 2. A repeated-measures ANOVA specifying infant facial expression (happy/sad), familiarity (own/unfamiliar), hemisphere (left/right), and participant attachment classification (secure/insecure) was conducted to examine mothers’ N170 responses to infant faces. With respect to N170 amplitude, there was a main effect of hemisphere [F(1,57)=8.68, p=.005, ηp2=.13], where mothers showed significantly greater N170 responses in the right hemisphere (M=−1.48, SD=1.58) as compared to the left hemisphere (M=−1.01, SD=1.64). In contrast, there was no main effect of attachment classification [F<1], facial expression, or familiarity, nor were there other significant interactions among these variables on N170 amplitude [F’s<3.47, p’s>.07].
Figure 2.
Grand-averaged ERP waveforms representing the N170 response to infant faces in secure and insecure mothers. Data are averaged across hemispheres.
With respect to N170 latency, there was a significant interaction between facial familiarity and attachment classification [F(1,57)=4.81, p=.03, ηp2=.08]. Post-hoc paired samples t-tests revealed that secure mothers showed no differences in their N170 latency based on familiarity [t<1], whereas insecure mothers showed a delayed N170 latency in response to their own infant’s face (M=172.77, SD=20.37) as compared to an unfamiliar infant’s face (M=167.06, SD=17.17) regardless of facial expression [t(23)=2.17, p=.04, d=.30]. There was no main effect of attachment classification [F<1.02], infant facial expression, infant facial familiarity, or hemisphere on N170 latency, nor were there other significant interactions among these variables [F’s<3.03, p’s>.09].
P300 (Infant Cries)
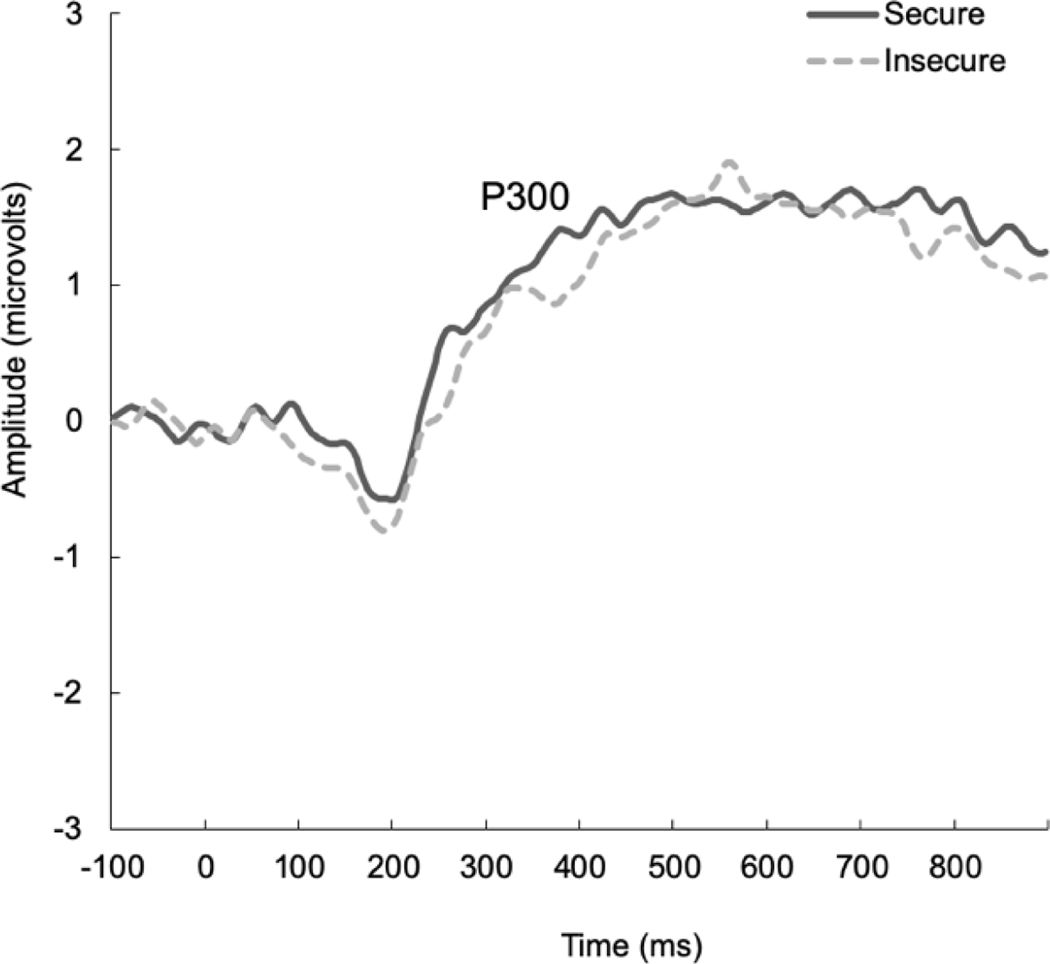
Grand-averaged data averaged across the central electrode sites for infant cries for the P300 as a function of attachment classification are presented in Figure 3. A repeated-measures ANOVA specifying infant cry familiarity (own/unfamiliar) and participant attachment classification (secure/insecure) was conducted to examine mothers’ P300 responses to infant cries. With respect to P300 amplitude, there was no main effect of attachment classification [F<1], nor was there a main effect of cry familiarity, or an interaction between these variables [F’s<1]. With respect to P300 latency, there was no main effect of attachment classification [F<1], nor was there a main effect of cry familiarity or an interaction between these variables [F’s<2.13, p’s>.15].
Figure 3.
Grand-averaged ERP waveforms representing the P300 response to infant cries in secure and insecure mothers. Data are averaged across own/unfamiliar infant cries.
P300 (Infant Faces)
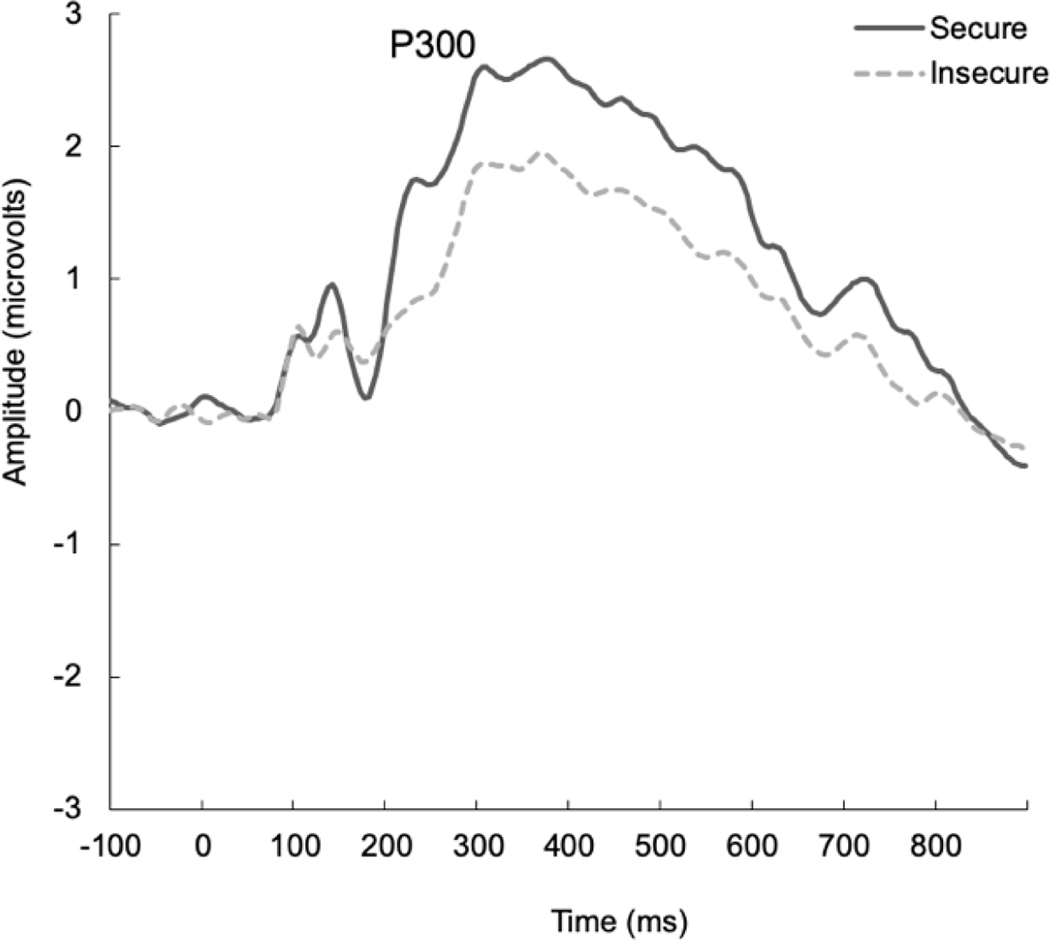
Given that parity has been shown to impact maternal P300 response to infant faces (Maupin et al., 2019; Rutherford et al., 2019), analyses controlled for parity. Grand-averaged data averaged across the central electrode sites for infant faces for the P300 as a function of attachment classification are presented in Figure 4. A repeated-measures ANOVA specifying infant facial expression (happy/sad), familiarity (own/unfamiliar), and participant attachment classification (secure/insecure) was conducted to examine mothers’ P300 responses to infant faces. With respect to P300 amplitude, there was a main effect of familiarity [F(1,57)=11.60, p =.001, ηp2=.17]. Specifically, regardless of attachment classification, mothers demonstrated significantly greater P300 responses to their own infant’s face (M=2.16, SD=1.68) as compared to an unfamiliar infant’s face (M=1.77, SD=1.59). There was no main effect of attachment classification [F(1,57)=2.85, p=.10, ηp2=.05] or facial expression on P300 amplitude, nor were there interactions among these variables [F’s<3.37, p’s>.07].
Figure 4.
Grand-averaged ERP waveforms representing the P300 response to infant faces in secure and insecure mothers. Data are averaged across own/unfamiliar infant faces
With respect to P300 latency in response to infant faces, there was a significant main effect of attachment classification [F(1,55)=4.44, p=.04, ηp2=.08]. Specifically, regardless of facial expression or familiarity, secure mothers were significantly faster to allocate attention to infant faces (M=388.98, SD=62.28) as compared to insecure mothers (M=421.90, SD=66.67). There were no main effects of facial expression or familiarity on P300 latency, nor were there interactions among these variables [F’s<2.53, p’s>.12].
Discussion
This study utilized ERP methods to understand whether maternal attachment relates to the strength and timing of neural responses to own and unfamiliar infant faces and cries. To our knowledge, this is the first study to use the AAI in conjunction with ERPs in response to both own and unfamiliar infant faces and cries in the investigation of how maternal attachment impacts the strength and timing of mothers’ neural responses to these salient infant cues. Our findings indicate that, at a neural level, secure and insecure mothers process infant cues differently. Specifically, we found that in comparison to secure mothers who did not show differences in the speed of their facial structural encoding (N170) based on infant familiarity, insecure mothers were slower to structurally encode their own infant’s face than an unfamiliar infant’s face. Secure mothers were also faster than insecure mothers to orient to infant cries (N100) and attend to infant faces (P300). With the exception of the N170, these latency effects occurred independently of infant cue familiarity. Notably, in our study, secure and insecure mothers differed in terms of the speed (i.e., latency) with which they process infant cues, rather than in the strength (i.e., amplitude) of their neural responses. Our work thus adds to the growing literature in this area, given that very few prior studies report latency results despite latency of the maternal neural response having implications for intuitive caregiving behavior extending to child outcomes.
Overall, our findings show that at both early and late processing stages, mothers with secure attachment representations exhibit faster neural processing of auditory and visual infant cues as compared to insecure mothers. The pattern of delayed latency effects exhibited by insecure mothers mirrors those documented in other clinical populations. For example, relative to non-smoking mothers, tobacco-smoking mothers demonstrate N170 responses to infant faces that are slower in speed but equivalent in strength (Rutherford, Maupin, et al., 2017). Behavioral experiments have also yielded comparable results, with mothers classified as unresolved/disorganized (i.e., a severe form of attachment insecurity) demonstrating longer latencies in their behavioral responses to threat stimuli relative to neutral stimuli, whereas mothers with organized attachments showed comparable latencies regardless of stimulus valence (Atkinson et al., 2009).
There are several possible interpretations regarding the delayed neural responses demonstrated by insecure mothers in our study. First, the overall slowing of neural processing may reflect decreased salience of caregiving cues as evidenced by previous findings that insecure mothers evidence attenuated activation in brain reward regions upon viewing images of their own infant’s face and show a diminished peripheral oxytocin response while interacting with their infant (Strathearn et al., 2009). The delays noted in our study may also be associated with insecure mothers’ documented difficulty with emotional awareness within themselves and their children (DeOliveira, Moran, & Pederson, 2005) and delayed behavioral response latency to attachment-related words on an emotional Stroop task (Atkinson et al., 2009). Additionally, the majority of our insecure sample of mothers were categorized as insecure-dismissing, an attachment classification characterized by a tendency to avoid and disengage from emotionally-arousing relational stimuli (Main et al., 2008). The delayed ERP latencies observed in our study may be neural markers of such avoidance.
Although visual inspection of Figures 1 and 4 suggest that attachment security may modulate ERP amplitudes, these analyses did not reach statistical significance and yielded only small effect sizes. Consequently, we did not replicate findings that insecure mothers showed greater ERPs in response to infant faces (Fraedrich et al., 2010; Groh & Haydon, 2018), or had greater physiological responses to infant cries (Groh & Roisman, 2009). The reasons for these apparent discrepancies may be related to study design rather than the validity of any study’s findings. For instance, compared to our passive viewing paradigm, prior ERP studies examining maternal attachment (Fraedrich et al., 2010; Groh & Haydon, 2018; Leyh et al., 2016) utilized oddball tasks in which participants were instructed to focus on certain stimuli and indicate deviant stimuli quickly and accurately. The value of the passive viewing task may more closely represents mothers’ natural experiences of infant cue perception, and permitted examination of ways in which different ERP components unfold over time.
Our ERP latency findings, in conjunction with previous research documenting that insecure mothers show greater ERP amplitudes than secure mothers, are indicative of an efficiency model of infant cue detection and processing. We suggest that secure mothers may respond in a more efficient manner (i.e., speedier, less resource-heavy) to attachment-related cues which may be associated with adaptive caregiving. Conversely, insecure mothers may be engaging in a less efficient pattern (i.e., slower, more resource-heavy) of orienting, encoding, and attending to infant cues, which may have the potential to negatively impact caregiving. This proposed efficiency model challenges the adage that “bigger is better” when it comes to maternal neural response. Given that secure mothers (who exhibit smaller and faster neural responses to infant cues) show greater maternal sensitivity, reflective functioning, and mind-mindedness, and are more likely to have secure infants as compared to insecure mothers (Pederson et al., 1998; Riva Crugnola et al., 2018; Shah, Fonagy, & Strathearn, 2010; Slade et al., 2005; Ward & Carlson, 1995), it may be that the ideal pattern of maternal neural activation is characterized less by the strength of the neural response and more by the optimal balance of the degree and timing of the neural response. Indeed, there are cases in which greater neural activation may be less than ideal, such as in previous research evidencing that insecure mothers demonstrate greater amygdala activation than secure mothers when hearing infant cries (Riem et al., 2012), thereby suggesting heightened threat detection, fear, and aversion (Davis & Whalen, 2001). This increase in neural activation in conjunction with the delayed neural activation found in our study provides additional evidence for the notion that insecure mothers may process attachment-related stimuli less efficiently than secure mothers, though future research is needed to advance this proposed infant cue efficiency model. If the efficiency of infant cue perception and processing extends to caregiving behaviors (such as more attuned infant mirroring; Kim et al., 2014) and ultimately child outcomes, this will provide insight into the mechanisms involved in the intergenerational transmission of attachment and thus elucidate important targets for interventions.
Interestingly, our study did not replicate previous findings that maternal neural responses (N170, P300) are modulated by infant facial expression (Doi & Shinohara, 2012; Proverbio et al., 2006). The discrepancy between such findings and the null results documented in our study may be a function of experimental design. Specifically, studies reporting an impact of infant facial expression on maternal neural response involved an emotion recognition task wherein mothers were instructed to actively categorize infant emotions during each experimental trial. In contrast, our null findings are consistent with the null results of previous works employing passive viewing experimental paradigms similar to our study (Malak, Crowley, Mayes, & Rutherford, 2015; Maupin et al., 2019; Noll et al., 2012; Rutherford, Byrne, et al., 2017; Rutherford, Maupin, et al., 2017; Rutherford et al., 2019). It may be that the intentional directing of attention toward the emotional content of infant faces as required by some experiments could account for findings that distressed infant faces elicited greater neural responses than neutral or happy faces, whereas in the context of a more naturalistic experience of passively viewing infant faces, mothers encode (N170) and attend (P300) to infant faces of all emotions similarly regardless of affect.
This is a preliminary study that requires replication and extension. Future research should utilize larger samples of mothers to increase generalizability of results and increase statistical power. Although there were no statistically significant differences between attachment groups on sociodemographic characteristics in our sample, future research including larger samples would allow for adequate power to determine if sociodemographic variables interact with attachment security, infant face and cry familiarity, and infant facial expression to impact ERP responses. Increasing sample size could also allow for comparisons to be made between groups of insecure mothers, which may be worthwhile given the differences between insecure-dismissing individuals (characterized by avoidance of threat stimuli) and insecure-preoccupied individuals (characterized by increased engagement with threat stimuli; Main et al., 2008; Mark et al., 2012). Further, although our AAI’s were coded by a certified highly reliable coder, future studies may also consider double-coding AAI’s to ensure reliability. Including non-social as well as social stimuli (infant and adult faces) will be important to understand the specificity of these findings to infant cues. Additionally, to examine whether the ERP latency differences observed in our study act mechanistically in the transmission of attachment security, future research should incorporate measures of infant attachment in addition to other measures of caregiver characteristics, including reflective functioning and maternal sensitivity. With regard to our ERP analysis, although the latency-to-peak approach is widely employed in maternal ERP studies, criticism of this approach exists given variable reliability when trial numbers are few (Huffmeijer, Bakermans-Kranenburg, Alink, & Van IJzendoorn, 2014), and future research may also consider the use of alternative ERP latency measures such as percent-area latency or percent-amplitude latency to further enhance reliability (Liesefeld, 2018).
In summary, this study examined ERP responses to infant cues exhibited by secure and insecure mothers. Overall, secure mothers were faster to process both infant faces and cries as compared to insecure mothers. These differences may be mechanistically important to our understanding of the role of attachment in shaping neural processing of infant cues. Together, these results have implications for caregiving in light of prior work demonstrating that ERPs are related to maternal sensitivity and reflective functioning. Additionally, our findings highlight a role for social neuroscientific approaches in studying the transmission of attachment security by examining neurobiological mechanisms that underscore infant cue perception.
Supplementary Material
Funding sources:
This work was supported by grants from the National Institutes of Health [R01 DA026437-08 and T32 DA019426]. Dr. Potenza’s involvement was supported through the National Center for Responsible Gaming Center of Excellence grant. The views presented in this manuscript are those of the authors and do not necessarily reflect those of the funding agencies.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest with respect to the content of this manuscript. Dr. Potenza has: consulted for and advised Rivermend Health, Opiant/Lightlake Therapeutics and Jazz Pharmaceuticals; received research support from the Mohegan Sun Casino and the National Center for Responsible Gaming; and consulted for legal and gambling entities on issues related to impulse-control disorders and addictions. The other authors report no disclosures.
References
- Ainsworth MDS, Blehar MC, Waters E, & Wall S. (1978). Patterns of attachment: A psychological study of the strange situation. Oxford, England: Lawrence Erlbaum. [Google Scholar]
- Ansari TL, & Derakshan N. (2011a). The neural correlates of cognitive effort in anxiety: Effects on processing efficiency. Biological psychology, 86(3), 337–348. [DOI] [PubMed] [Google Scholar]
- Ansari TL, & Derakshan N. (2011b). The neural correlates of impaired inhibitory control in anxiety. Neuropsychologia, 49(5), 1146–1153. [DOI] [PubMed] [Google Scholar]
- Atkinson L, Leung E, Goldberg S, Benoit D, Poulton L, Myhal N, … Kerr S. (2009). Attachment and selective attention: Disorganization and emotional Stroop reaction time. Dev Psychopathol, 21(1), 99–126. [DOI] [PubMed] [Google Scholar]
- Beebe B. (1982). Micro-timing in mother-infant communication. Nonverbal communication today, 33, 169–195. [Google Scholar]
- Beebe B, Jaffe J, Markese S, Buck K, Chen H, Cohen P, … Feldstein S. (2010). The origins of 12-month attachment: A microanalysis of 4-month mother–infant interaction. Attachment & Human Development, 12(1–2), 3–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Simons R, & Dozier M. (2015). Effects of an attachment-based intervention on child protective services–referred mothers’ event-related potentials to children’s emotions. Child Dev, 86(6), 1673–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. (1969). Attachment and loss: Vol. 1. Attachment. New York, NY: Basic Books. [Google Scholar]
- Bretherton I. (2013). Revisiting Mary Ainsworth’s conceptualization and assessments of maternal sensitivity-insensitivity. Attachment & Human Development, 15(5–6), 460–484. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.): Routledge. [Google Scholar]
- Cole PM, Barrett KC, & Zahn-Waxler C. (1992). Emotion displays in two-year-olds during mishaps. Child Dev, 63(2), 314–324. [PubMed] [Google Scholar]
- Crittenden PM (2004). Patterns of attachment in adulthood: A dynamic-maturation approach to analyzing the Adult Attachment Interview. Unpublished manuscript. [Google Scholar]
- Daffner KR, Chong H, Sun X, Tarbi EC, Riis JL, McGinnis SM, & Holcomb PJ (2011). Mechanisms underlying age-and performance-related differences in working memory. Journal of cognitive neuroscience, 23(6), 1298–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, & Whalen PJ (2001). The amygdala: Vigilance and emotion. Molecular psychiatry, 6(1), 13–34. [DOI] [PubMed] [Google Scholar]
- De Wolff MS, & Van IJzendoorn MH (1997). Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Dev, 68(4), 571–591. [PubMed] [Google Scholar]
- DeOliveira CA, Moran G, & Pederson DR (2005). Understanding the link between maternal adult attachment classifications and thoughts and feelings about emotions. Attachment & Human Development, 7(2), 153–170. [DOI] [PubMed] [Google Scholar]
- Doi H, & Shinohara K. (2012). Event-related potentials elicited in mothers by their own and unfamiliar infants’ faces with crying and smiling expression. Neuropsychologia, 50(7), 1297–1307. [DOI] [PubMed] [Google Scholar]
- Eimer M, & Holmes A. (2007). Event-related brain potential correlates of emotional face processing. Neuropsychologia, 45(1), 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinet SD, Anderson JE, & Zelazo PD (2012). N2 amplitude as a neural marker of executive function in young children: An ERP study of children who switch versus perseverate on the Dimensional Change Card Sort. Developmental Cognitive Neuroscience, 2, S49–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraedrich EM, Lakatos K, & Spangler G. (2010). Brain activity during emotion perception: the role of attachment representation. Attachment & Human Development, 12(3), 231–248. [DOI] [PubMed] [Google Scholar]
- George C, Kaplan N, & Main M. (1996). Adult attachment interview, third edition. Unpublised manuscript. Department of Psychology. University of California, Berkeley. [Google Scholar]
- George C, & West M. (2003). The Adult Attachment Projective: Measuring individual differences in attachment security using projective methodology. In Hilsenroth MJ & Sega D. (Eds.), Comprehensive Handbook of Psychological Assessment, Volume 2: Personality Assessment (Vol. 2). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Grasso DJ, Moser JS, Dozier M, & Simons R. (2009). ERP correlates of attention allocation in mothers processing faces of their children. Biological psychology, 81(2), 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology, 55(4), 468–484. [DOI] [PubMed] [Google Scholar]
- Groh AM, & Haydon KC (2018). Mothers’ neural and behavioral responses to their infants’ distress cues: The role of secure base script knowledge. Psychological science, 29(2), 242–253. [DOI] [PubMed] [Google Scholar]
- Groh AM, & Roisman GI (2009). Adults’ autonomic and subjective emotional responses to infant vocalizations: The role of secure base script knowledge. Dev Psychol, 45(3), 889. [DOI] [PubMed] [Google Scholar]
- Hesse E. (2008). The Adult Attachment Interview: Protocol, method of analysis, and empirical studies. In Cassidy J. & Shaver PR (Eds.), Handbook of attachment: Theory, research, and clinical applications (pp. 552–598). New York, NY: The Guilford Press. [Google Scholar]
- Huffmeijer R, Bakermans-Kranenburg MJ, Alink LR, & Van IJzendoorn MH (2014). Reliability of event-related potentials: The influence of number of trials and electrodes. Physiology & behavior, 130, 13–22. [DOI] [PubMed] [Google Scholar]
- Judah MR, Grant DM, Mills AC, & Lechner WV (2013). The neural correlates of impaired attentional control in social anxiety: An ERP study of inhibition and shifting. Emotion, 13(6), 1–11. [DOI] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Allen J, Martinez SR, Iyengar U, & Strathearn L. (2014). Mothers who are securely attached during pregnancy show more attuned infant mirroring at 7 months postpartum. Infant Behav Dev, 37(4), 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Iyengar U, Mayes LC, Potenza MN, Rutherford HJ, & Strathearn L. (2017). Mothers with substance addictions show reduced reward responses when viewing their own infant’s face. Human brain mapping, 38(11), 5421–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzava S, Nissim G, Frost A, Nelson B, & Bernard K. (2019). Latent profiles of maternal neural response to infant emotional stimuli: Associations with maternal sensitivity. Biological psychology, 143, 113–120. [DOI] [PubMed] [Google Scholar]
- Leyh R, Heinisch C, Behringer J, Reiner I, & Spangler G. (2016). Maternal attachment representation and neurophysiological processing during the perception of infants’ emotional expressions. PLoS One, 11(2), e0147294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesefeld HR (2018). Estimating the timing of cognitive operations with MEG/EEG latency measures: A primer, a brief tutorial, and an implementation of various methods. Frontiers in neuroscience, 12, 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell A, Maupin AN, Landi N, Potenza MN, Mayes LC, & Rutherford HJ (2020). Substance use and mothers’ neural responses to infant cues. Infant Ment Health J, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Ran G, Chen X, Ma H, & Hu N. (2017). Adult attachment styles associated with brain activity in response to infant faces in nulliparous women: an event-related potentials study. Front Psychol, 8, 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main M, & Goldwyn R. (1995). Interview-based adult attachment classifications: Related to infant-mother and infant-father attachment. Dev Psychol, 19, 227–239. [Google Scholar]
- Main M, Hesse E, & Goldwyn R. (2008). Studying differences in language usage in recounting attachment history: An introduction to the AAI. [Google Scholar]
- Malak SM, Crowley MJ, Mayes LC, & Rutherford HJ (2015). Maternal anxiety and neural responses to infant faces. Journal of Affective Disorders, 172, 324–330. [DOI] [PubMed] [Google Scholar]
- Mark RE, Geurdes FI, & Bekker MH (2012). Attachment styles are related to erps elicited to angry faces in an oddball paradigm. J. Behav. Brain Sci, 2, 128–140. [Google Scholar]
- Maupin AN, Hayes NJ, Mayes LC, & Rutherford HJ (2015). The application of electroencephalography to investigate the neural bases of parenting: a review. Parenting, 15(1), 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin AN, Rutherford HJ, Landi N, Potenza MN, & Mayes LC (2019). Investigating the association between parity and the maternal neural response to infant cues. Social neuroscience, 14(2), 214–225. [DOI] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, & Carver LJ (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 45(7), 1235–1245. [DOI] [PubMed] [Google Scholar]
- Meins E. (1999). Sensitivity, security and internal working models: Bridging the transmission gap. Attachment & Human Development, 1(3), 325–342. [DOI] [PubMed] [Google Scholar]
- Meins E. (2013). Sensitive attunement to infants’ internal states: Operationalizing the construct of mind-mindedness. Attachment & Human Development, 15(5–6), 524–544. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, & Fink A. (2009). Intelligence and neural efficiency. Neuroscience & Biobehavioral Reviews, 33(7), 1004–1023. [DOI] [PubMed] [Google Scholar]
- Noll LK, Mayes LC, & Rutherford HJ (2012). Investigating the impact of parental status and depression symptoms on the early perceptual coding of infant faces: an event-related potential study. Social neuroscience, 7(5), 525–536. [DOI] [PubMed] [Google Scholar]
- Pederson DR, Gleason KE, Moran G, & Bento S. (1998). Maternal attachment representations, maternal sensitivity, and the infant–mother attachment relationship. Dev Psychol, 34(5), 925. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Brignone V, Matarazzo S, Del Zotto M, & Zani A. (2006). Gender and parental status affect the visual cortical response to infant facial expression. Neuropsychologia, 44(14), 2987–2999. [DOI] [PubMed] [Google Scholar]
- Purhonen M, Kilpeläinen-Lees R, Pääkkönen A, Yppärilä H, Lehtonen J, & Karhu J. (2001). Effects of maternity on auditory event-related potentials to human sound. Neuroreport, 12(13), 2975–2979. [DOI] [PubMed] [Google Scholar]
- Purhonen M, Valkonen-Korhonen M, & Lehtonen J. (2008). The impact of stimulus type and early motherhood on attentional processing. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 50(6), 600–607. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, van IJzendoorn MH, Out D, & Rombouts SA (2012). Attachment in the brain: adult attachment representations predict amygdala and behavioral responses to infant crying. Attachment & Human Development, 14(6), 533–551. [DOI] [PubMed] [Google Scholar]
- Riva Crugnola C, Ierardi E, & Canevini MP (2018). Reflective functioning, maternal attachment, mind-mindedness, and emotional availability in adolescent and adult mothers at infant 3 months. Attachment & Human Development, 20(1), 84–106. [DOI] [PubMed] [Google Scholar]
- Rösler F, Heil M, & Röder B. (1997). Slow negative brain potentials as reflections of specific modular resources of cognition. Biological psychology, 45(1–3), 109–141. [DOI] [PubMed] [Google Scholar]
- Rutherford HJ, Byrne SP, Austin GM, Lee JD, Crowley MJ, & Mayes LC (2017). Anxiety and neural responses to infant and adult faces during pregnancy. Biological psychology, 125, 115–120. [DOI] [PubMed] [Google Scholar]
- Rutherford HJ, Maupin AN, Landi N, Potenza MN, & Mayes LC (2017). Current tobacco-smoking and neural responses to infant cues in mothers. Parenting, 17(1), 1–10. [Google Scholar]
- Rutherford HJ, Maupin AN, & Mayes LC (2019). Parity and neural responses to social and non-social stimuli in pregnancy. Social neuroscience, 14(5), 545–548. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, & Zuccolotto A. (2002). E-Prime: User’s guide: Psychology Software Incorporated. [Google Scholar]
- Shah PE, Fonagy P, & Strathearn L. (2010). Is attachment transmitted across generations? The plot thickens. Clinical Child Psychology and Psychiatry, 15(3), 329–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade A, Grienenberger J, Bernbach E, Levy D, & Locker A. (2005). Maternal reflective functioning, attachment, and the transmission gap: A preliminary study. Attachment & Human Devlopment, 7(3), 283–298. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, & Montague PR (2009). Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology, 34(13), 2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinteren R, Arns M, Jongsma ML, & Kessels RP (2014). P300 development across the lifespan: a systematic review and meta-analysis. PLoS One, 9(2), e87347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn MH (1995). Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychological bulletin, 117(3), 387. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, Goldberg S, Kroonenberg PM, & Frenkel OJ (1992). The relative effects of maternal and child problems on the quality of attachment: A meta-analysis of attachment in clinical samples. Child Dev, 63(4), 840–858. [DOI] [PubMed] [Google Scholar]
- Ward MJ, & Carlson EA (1995). Associations among adult attachment representations, maternal sensitivity, and infant-mother attachment in a sample of adolescent mothers. Child Dev, 66(1), 69–79. [DOI] [PubMed] [Google Scholar]
- Waters H, & Rodrigues-Doolabh L. (2004). Manual for decoding secure base narratives. Unpublished manuscript. State University of New York at Stony Brook. [Google Scholar]
- Zhang X, Li T, & Zhou X. (2008). Brain responses to facial expressions by adults with different attachment–orientations. Neuroreport, 19(4), 437–441. [DOI] [PubMed] [Google Scholar]
- Zilber A, Goldstein A, & Mikulincer M. (2007). Adult attachment orientations and the processing of emotional pictures–ERP correlates. Personality and Individual Differences, 43(7), 1898–1907. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.