Abstract
A broad variety of e-liquids are used by e-cigarette consumers. Additives to the e-liquid carrier solvents, propylene glycol and glycerol, often include flavorants and nicotine at various concentrations. Flavorants in general have been reported to increase toxicant formation in e-cigarette aerosols, yet there is still much that remains unknown about the effects of flavorants, nicotine, and flavorants + nicotine on harmful and potentially harmful constituents (HPHCs) when aerosolizing e-liquids. Common flavorants benzaldehyde, vanillin, benzyl alcohol, and trans-cinnamaldehyde have been identified as some of the most concentrated flavorants in some commercial e-liquids, yet there is limited information on their effects on HPHC formation. E-liquids containing flavorants + nicotine are also common, but the specific effects of flavorants + nicotine on toxicant formation remain understudied. We used 1H NMR spectroscopy to evaluate HPHCs and herein report that benzaldehyde, vanillin, benzyl alcohol, trans-cinnamaldehyde, and mixtures of these flavorants significantly increased toxicant formation produced during e-liquid aerosolization compared to unflavored e-liquids. However, e-liquids aerosolized with flavorants + nicotine decreased the HPHCs for benzaldehyde, vanillin, benzyl alcohol, and a “flavorant mixture” but increased the HPHCs for e-liquids containing trans-cinnamaldehyde compared to e-liquids with flavorants and no nicotine. We determined how nicotine affects the production of HPHCs from e-liquids with flavorant + nicotine versus flavorant, herein referred to as the “nicotine degradation factor”. Benzaldehyde, vanillin, benzyl alcohol, and a “flavorant mixture” with nicotine showed lower HPHC levels, having nicotine degradation factors <1 for acetaldehyde, acrolein, and total formaldehyde. HPHC formation was most inhibited in e-liquids containing vanillin + nicotine, with a degradation factor of ~0.5, while trans-cinnamaldehyde gave more HPHC formation when nicotine was present, with a degradation factor of ~2.5 under the conditions studied. Thus, the effects of flavorant molecules and nicotine are complex and warrant further studies on their impacts in other e-liquid formulations as well as with more devices and heating element types.
Graphical Abstract
1. INTRODUCTION
Electronic cigarettes (e-cigarettes) continue to be popular in the United States despite a limited understanding of their toxicity. As of 2020, ~20% of high school students reported using e-cigarettes.1 Despite their prevalence, the potential harmfulness of specific e-cigarettes and components still needs to be assessed. Variables such as device types, coil resistances, device wattages, e-liquid compositions, and vaping patterns can impact the degree to which e-cigarettes may be harmful. Aspects of e-cigarettes that can expose consumers to potential harm include the production of carbonyls during vaping,2 e-liquid components (e.g., flavorants),3 and the release of metals mostly from e-cigarette heating coils.4 Herein, we analyze the impact of individual e-liquid components (i.e., nicotine and common flavorants) on carbonyl production during vaping.
E-liquids typically contain a fluid—made up of propylene glycol (PG) and/or glycerol (GL), nicotine, and flavorants—that can be aerosolized during vaping. Some degradation can occur when vaping the PG and GL solvent and consequently produce harmful and potentially harmful constituents (HPHCs) as reported by Jensen et al.5 Li et al.6 found that aerosolizing different PG:GL mol ratios (i.e., 100:0, 70:30, 50:50, 30:70, and 0:100) produced varying levels of carbonyls with high performance liquid chromatography-high resolution mass spectrometry (HPLC-HRMS). We chose a 50:50 PG:GL mol ratio as the standard for this study. The levels of these components, which generally encompass the majority of the HPHCs, can be compared to assess the effect of a particular chemical on degradation.
The addition of flavorants to e-liquids can produce higher levels of HPHCs as well as novel flavorant toxicants.7,8 Furthermore, Gillman et al.9 and Khlystov and Samburova10 reported that vaping flavored commercial e-liquids, which contain a mix of flavorants, can increase the formation of aldehydes compared to vaping unflavored e-liquids. Triacetin (a flavor enhancer) was shown by Vreeke et al.11 to enhance the levels of degradation products. Sweeteners (e.g., sucralose) are also common additives, and sucralose was shown to increase the HPHC aldehyde levels in aerosols, as compared to aerosols from unflavored e-liquids.12,13 Thus, the effect of individual flavorants on toxicant formation needs to be assessed further, in particular, for the most common and most concentrated molecules in e-liquids.
Vanillin (vanilla flavor), benzyl alcohol (cherry/fruity/floral flavor), benzaldehyde (cherry/fruity/nutty flavor), and trans-cinnamaldehyde (cinnamon flavor) are among the most popular flavorants in commercial e-liquids as reported by Behar et al.14 Trans-cinnamaldehyde is one of the most concerning flavorants analyzed as it is typically present at high concentrations in cinnamon-flavored e-liquids and has been linked with cytotoxicity,15 adverse effects on cardiovascular function during early development of zebrafish embryos,16 impairment of respiratory immune cell function,17 disruption of mitochondrial function and inhibition of bioenergetic processes,18 and oxidative stress in human osteoblast-like cells.19 Benzaldehyde is present in many e-liquids and is especially concentrated in cherry-flavored e-liquids, despite being known to cause respiratory tract irritation.20 Multiple flavorants, including ethyl maltol, ethyl vanillin, and citral have been found to promote free radical formation during vaping.21
Despite the prevalence of nicotine in e-liquids, there is limited information about the effect of nicotine on flavorant and PG + GL degradation. Talih et al.22 theorized that e-cigarette consumers may be exposed to greater levels of carbonyls when vaping e-liquids with lower nicotine concentrations due to possible self-regulated nicotine dosing (i.e., vaping more overall in order to achieve a particular total nicotine intake). Baker et al.23 conducted a study that showed that consumers self-regulated (“titrated”) their nicotine intake when provided with a lower nicotine e-liquid to achieve a particular total nicotine dose, which was independent of flavorants.
Herein, we used 1H NMR spectroscopy to analyze the aerosols produced by vaping PG + GL e-liquids without and with flavorants and flavorants + nicotine. The HPHC levels in these aerosol samples were compared with those from unflavored e-liquids to determine the effects of these common e-liquid additives individually and together.
2. MATERIALS AND METHODS
2.1. Materials.
USP grade PG, USP grade GL, benzaldehyde (>99%), and styrene (>99%) were purchased from Sigma-Aldrich (st. Louis, MO). (S)-(−)-nicotine (99%) and vanillin (>99%) were purchased from Alfa Aesar (Ward Hill, MA). Benzyl alcohol (>99%), trans-cinnamaldehyde (>98%), and trans-cinnamic acid (>99.8%) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). “Unicorn Blood” with 6 mg/mL nicotine was purchased online from Fuzion Vapor. The commercial e-liquid “Unicorn Blood” was chosen because it contains nicotine and sucralose (which we have previously shown leads to increased production of carbonyl degradants).12 The procedure we used to aerosolize the “Unicorn Blood” e-liquid with a refillable tank e-cigarette is given in the caption of Figure S1. Benzene (>99.7%) was purchased from EMD Millipore Corporation (Billerica, MA). Toluene (>99%) was obtained from Mallinckrodt Chemicals (Phillipsburg, NJ). DMSO-d6 (D 99.9%) and D2O (D 99.9%) were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA).
2.2. Methods.
2.2.1. Vaping Experiments.
E-liquid stock containing equimolar quantities of PG and GL was prepared. Aliquots of this stock were then combined with either 2.5 mg/mL benzaldehyde, 31 mg/mL vanillin, 39 mg/mL benzyl alcohol, 39 mg/mL trans-cinnamaldehyde, 155 mg/mL trans-cinnamaldehyde, or a “flavorant mixture” (0.025 mg/mL benzaldehyde, 7.75 mg/mL vanillin, 9.75 mg/mL benzyl alcohol, 39 mg/mL trans-cinnamaldehyde). Lastly, aliquots of the PG + GL + flavorant mixtures were combined with 6 mg/mL nicotine. The concentrations of flavorants were selected based upon commercial e-liquid values reported by Behar et al.14 The chosen nicotine concentration is common and within the range of observed values (0–60 mg/mL) in commercial e-liquids.24 All ratios were verified by 1H NMR spectroscopy.
E-liquids with flavorants were vaped in the following order: PG + GL, PG + GL + flavorant, PG + GL + flavorant+6 mg/mL nicotine, and then PG + GL. The initial and final aerosolized PG + GL degradation levels were compared to demonstrate that the sequence of vaping experiments did not damage the coil in each series, which would have been shown by significantly increased degradation in the final PG + GL aerosol versus initial. The second PG + GL condition was aerosolized last for every experiment except for one trial with 2.5 mg/mL benzaldehyde, 31 mg/mL vanillin, 39 mg/mL benzyl alcohol, and 155 mg/mL trans-cinnamaldehyde as the flavorant. Each experiment was repeated with 3 separate coils of the same type/brand. In addition, a set of e-liquids without flavorants were vaped in the following order: PG + GL, PG + GL+6 mg/mL nicotine, and PG + GL.
Devices used, setup, collection methods including the sample puff protocol, and NMR parameters were detailed previously.25–27 The power button was pressed 1 s prior to the start of each puff and followed the CORESTA puff protocol.25 All samples were collected using a Kangertech Subtank Mini (equipped with a 1.2 Ω coil) attached to a KBOX Mini (Kangertech, Shenzen, China) using 22 W.
New coils were conditioned with 10 puffs at 26 W prior to first time use per previous methods.26 Ten or 20 “wicking puffs” at 22 W were done using each new e-liquid condition prior to sample collection. Samples (3 puffs/sample) were generated using 22 W and collected as described elsewhere.5,12,28 When the e-liquid was changed during an experiment, the tank was emptied of e-liquid and dried using lint-free tissues prior to refilling the tank with the new e-liquid. Between experiments, coils were washed with methanol and dried using a vacuum oven at room temperature. All aerosolized samples were evaluated by 1H NMR spectroscopy within 1 h of collection. The aerosol and e-liquid composition samples were prepared in DMSO-d6, then analyzed using a 600 MHz Bruker AVANCE III NMR spectrometer using either 16 or 64 scans, a 30° observation pulse, and a 3 s relaxation delay at 25 °C.
2.2.2. Identification of Degradation Products Derived from Flavorants.
To identify substances unambiguously, vaped PG + GL + 155 mg/mL trans-cinnamaldehyde + 6 mg/mL nicotine was independently spiked with toluene, styrene, and benzaldehyde; PG + GL+155 mg/mL trans-cinnamaldehyde was spiked with cinnamic acid; and PG + GL + 2.5 mg/mL benzaldehyde + 6 mg/mL nicotine was spiked with benzene (data not shown) to identify if the spiked substance was formed upon aerosolization. The amount of each degradation product in the aerosol samples was determined by comparing the integrations from the spiked and original samples.
3. RESULTS AND DISCUSSION
3.1. Percentage of Aerosol Collected.
The percentage of the aerosol collected in the sample vial (%-collected) was calculated for each sample by dividing the absolute value of the change in the collected vial mass by the absolute value of the change in e-cigarette tank mass and multiplying by 100 to generate a percent. Values were then averaged for each condition, and the standard deviation (SD) was calculated. The average % aerosol collected ± SD from the samples generated in each experiment are shown in Tables 1 and S1.
Table 1.
Average % Aerosol Collected for Trials 1–3 for Each Vaping Experiment
| average % aerosol collected ± standard deviation |
||||||
|---|---|---|---|---|---|---|
| PG + GL | PG + GL + flavorant | PG + GL + flavorant + 6 mg/mL nicotine | PG + GL | overall | ||
| benzaldehyde (2.5 mg/mL) | trial 1 | 46 ± 3 | 59 ± 8 | 52 ± 2 | 62 ± 14 | 55 ± 7 |
| trial 2 | 52 ± 5 | 52 ± 6 | 47 ± 6 | 44 ± 3 | 49 ± 4 | |
| trial 3 | 62 ± 18 | 50 ± 19 | 53 ± 10 | NAa | 55 ± 6 | |
| vanillin (31 mg/mL) | trial 1 | 49 ± 2 | 47 ± 11 | 58 ± 4 | 48 ± 6 | 50 ± 5 |
| trial 2 | 61 ± 10 | 57 ± 6 | 60 ± 5 | 53 ± 3 | 58 ± 3 | |
| trial 3 | 60 ± 19 | 32 ± 4 | 46 ± 3 | NAa | 46 ± 14 | |
| Benzyl alcohol (39 mg/mL) | trial 1 | 48 ± 6 | 53 ± 3 | 50 ± 4 | 46 ± 3 | 49 ± 3 |
| trial 2 | 45 ± 12 | 52 ± 3 | 56 ± 5 | 47 ± 5 | 40 ± 5 | |
| trial 3 | 43 ± 6 | 40 ± 15 | 37 ± 1 | NAa | 40 ± 3 | |
| trans-cinnamaldehyde (39 mg/mL) | trial 1 | 59 ± 2 | 57 ± 8 | 49 ± 4 | 60 ± 4 | 56 ± 5 |
| trial 2 | 60 ± 1 | 42 ±6 | 44 ± 6 | 56 ± 5 | 50 ± 9 | |
| trial 3 | 64 ± 3 | 55 ± 3 | 39 ± 7 | 49 ± 17 | 52 ± 10 | |
| trans-cinnamaldehyde (155 mg/mL) | trial 1 | 76 ± 14 | 45 ± 30 | 20 ± 2 | 56 ± 15 | 49 ± 23 |
| trial 2 | 82 ± 33 | 32 ± 17 | 9 ± 1 | 51 ± 15 | 44 ± 31 | |
| trial 3 | 48 ± 4 | 11 ± 2 | 8 ± 1 | NAa | 23 ± 22 | |
| “flavorant mixture”b | trial 1 | 41 ± 7 | 59 ±2 | 62 ± 3 | 71 ± 4 | 58 ± 13 |
| trial 2 | 56 ± 14 | 55 ± 3 | 52 ± 10 | 52 ± 4 | 54 ± 2 | |
| trial 3 | 64 ± 5 | 55 ± 3 | 57 ± 3 | 39 ± 8 | 54 ± 11 | |
Not able to be analyzed; PG + GL was not aerosolized again after the + 6 mg/mL nicotine addition.
Flavorant mixture = 0.025 mg/mL benzaldehyde; 7.75 mg/mL vanillin; 9.75 mg/mL benzyl alcohol; 39 mg/mL trans-cinnamaldehyde.
The average % aerosol collected was similar for most of the flavorants when comparing trials 1–3 (each trial represents a different coil). However, there was a notable decrease in the average % aerosol collected when vaping PG + GL e-liquids containing 155 mg/mL trans-cinnamaldehyde compared to the initial aerosolized PG + GL for each trial (Table 1). The average % aerosol collected decreased less when e-liquids contained 39 mg/mL trans-cinnamaldehyde instead of 155 mg/mL trans-cinnamaldehyde (Table 1). This is similar to the report by Duell et al.,5,12,28 stating that the addition of sucralose (a flavorant enhancer) to e-liquids also can alter the % aerosol collected compared to the PG + GL only conditions. Aldehydes can polymerize, form hemiacetals, and/or form acetals in the PG + GL mixture, which could alter the particulate matter (PM) and gas phase fractions in the aerosol, thereby causing variations in the % aerosol collected.29,30
3.2. Flavorants and Flavorant + Nicotine Effects on Degradation.
The levels of propanal, acetaldehyde, glycolaldehyde, acrolein, formaldehyde, formaldehyde hemiacetals at about 6.2 ppm, total multiple formaldehyde-addition products (sum of 5.8 + 5.3+5.1 ppm MAPs), and total formaldehyde (sum of formaldehyde + formaldehyde hemiacetal + total MAPs) were determined in aerosol samples by integrating their respective peaks relative to the 3-proton PG methyl peak (divided by 3 to represent 1 proton) in the 1H NMR spectra (Figures 1 and 2). While mass spectrometry (MS) methods may be more generally available in labs working on e-cigarettes, NMR spectroscopy may allow detection and quantitation of species that are not directly amenable to MS. For example, Salamanca et al.31 compared the total formaldehyde levels (formaldehyde + formaldehyde hemiacetals in their study) in aerosolized equimolar PG + GL e-liquids using 2,4-dinitrophenylhydrazine (DNPH) derivatization by HPLC with direct analysis of aerosols by NMR spectroscopy. They found that formaldehyde hemiacetals detected by NMR make up a considerable fraction of the total formaldehyde levels produced upon e-liquid aerosolization. However, the total formaldehyde levels were significantly underestimated using derivatization.31,32
Figure 1.
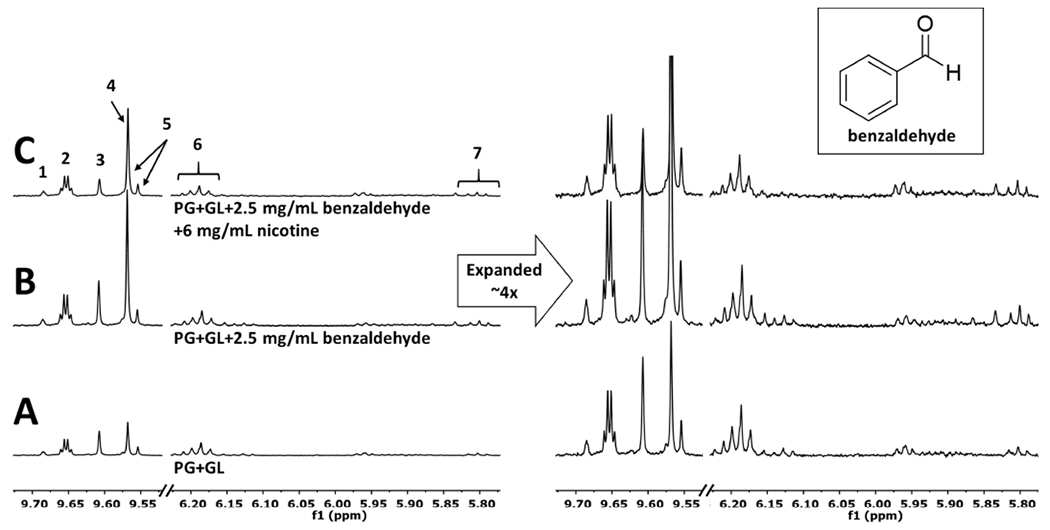
1H NMR spectra for aerosolized (A) PG + GL, (B) PG + GL + 2.5 mg/mL benzaldehyde, and (C) PG + GL + 2.5 mg/mL benzaldehyde + 6 mg/mL nicotine show the enhancing effects of benzaldehyde and inhibitory effects of nicotine on the degradation levels relative to the PG + GL. The intensities were normalized to the PG methyl resonance at ~1.05 ppm. The samples (3 puffs each) were aerosolized at 22 W using a 1.2 Ω coil and the CORESTA puff method. 1 = propanal; 2 = acetaldehyde; 3 = glycolaldehyde; 4 = formaldehyde; 5 = acrolein; 6 = formaldehyde hemiacetals; 7 = 5.8 ppm multiple addition product (MAP).
Figure 2.

1H NMR spectra for the aerosolized (A) PG + GL, (B) PG + GL + 155 mg/mL trans-cinnamaldehyde, and (C) PG + GL + 155 mg/mL trans-cinnamaldehyde+6 mg/mL nicotine illustrate the enhancing effects of trans-cinnamaldehyde and nicotine on the levels of degradation relative to PG + GL. The samples (3 puffs each) were generated at 22 W using a 1.2 Ω coil and the CORESTA puff method. The intensities were normalized to the PG methyl resonance at ~1.05 ppm. 1 = propanal; 2 = acetaldehyde; 3 = glycolaldehyde; 4 = formaldehyde; 5 = acrolein; 6 = trans-cinnamaldehyde-acetal peaks overlapped the formaldehyde hemiacetals; 7 = styrene; 8 = 5.8 ppm MAP.
The MAP peaks are from formaldehyde-releasing agents, similar to the formaldehyde hemiacetals identified by Jensen et al.,28 that are formed by the addition of formaldehyde to glycerol and exhibit triplets at 5.8, 5.3, and 5.1 ppm (Figures 3, S1, and S2). The 5.8 (Figure S1) and 5.3 ppm peaks are found when vaping GL but not when vaping PG (with no GL). The peak at ~5.1 ppm appears to correspond to a product (again hemiacetal-like) from either solvent. Both disappear when D2O is added, consistent with hemiacetal –CH2–OH resonances. Because the 6.2 ppm region is from the single addition products,5,28 we provisionally assign these 5.8, 5.3, and 5.1 ppm to MAP resonances, from both PG and GL (at 5.1 ppm) and from GL (at 5.3 and 5.8 ppm). Consistent with these assignments, the homonuclear correlation spectroscopy (COSY) and total correlation spectroscopy (TOCSY) from an aerosolized “Unicorn Blood” e-liquid sample shows connectivities to upfield doublets that become singlets when the hemiacetal –CH2–OH is exchanged by D2O to form –CH2–OD (Figure S2). An aerosolized “Unicorn Blood” sample was collected and analyzed because the sucralose-containing commercial e-liquid has been shown to produce high levels of HPHCs (including MAPs).12 The high concentration of MAPs made the connectivities easier to observe on the COSY and TOCSY. We were unable to determine the integration of the formaldehyde hemiacetals, and consequently the total formaldehyde, for e-liquids containing trans-cinnamaldehyde due to peak overlap from the PG- and GL-trans-cinnamaldehyde acetals (Figure 1).
Figure 3.

1H NMR spectrum for vaped pure glycerol in DMSO-d6. These peaks labeled “L” are labile, in the context of e-liquids, hemiacetal –CH2–OH resonances that are coupled to upfield doublets (see Figure S2), which become singlets when the –CH2–OH is exchanged to form –CH2–OD, as discussed in the text. The triplet in the 6.2 ppm region has already been identified as hemiacetals from PL and GL.5,28
The % values, relative to the remaining PG peak, for the degradation products are shown in Table S3. The effects of additives on HPHC formation in aerosolized PG + GL e-liquids were evaluated by comparing the degradation levels of acetaldehyde, acrolein, and total formaldehyde for e-liquids without (set to 1) versus with flavorant and flavorant + nicotine (Tables 2–4). The concentrations of flavorants used were chosen based on the upper limit of values observed in commercial e-liquids.13 Vaping e-liquids with the addition of each flavorant resulted in increased amounts of acetaldehyde, acrolein, and total formaldehyde relative to PG + GL (Tables 2–4). We also compared the HPHCs in aerosols produced from the initial and final PG + GL only e-liquids to assess coil changes that may have occurred during the vaping process. Individual flavorants in e-liquids could thermally degrade to contribute to the levels of HPHC formation. Trans-cinnamaldehyde (an α,β-unsaturated aldehyde) could undergo nucleophilic attack at the β–carbon to produce acrolein similar to trans-2-hexenal.33 However, specific degradation of flavorants will be limited by the amount of flavorant present, which is typically small relative to PG and GL.
Table 2.
Levels of the Degradation Product Acetaldehyde, Normalized to the Amount Formed by Aerosolization of Equimolar PG + GL, Followed by Sequential Addition of the Indicated Flavorants, Then 6 mg/mL Nicotine
| acetaldehyde (normalized relative to that formed by PG + GL only) ± standard deviation |
|||||||
|---|---|---|---|---|---|---|---|
| benzaldehyde (2.5 mg/mL) |
vanillin (31 mg/mL) |
benzyl alcohol (39 mg/mL) |
trans-cinnamaldehyde (39 mg/mL) |
trans-cinnamaldehyde (155 mg/mL) |
“flavorant mixture”a | ||
| trial 1 | PG + GL | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.4 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| +flavorant | 1.6 ± 0.2 | 2.1 ± 0.2 | 6.8 ± 0.6 | 62.6 ± 2.6 | 48.6 ± 12.4 | 17.2 ± 0.5 | |
| +6 mg/mL nicotine | 1.3 ± 0.1 | 0.9 ± 0.2 | 4.9 ± 0.4 | 196.8 ± 4.6 | 164.8 ± 25.5 | 13.5 ± 1.0 | |
| trial 2 | PG + GL | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.7 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| +flavorant | 1.6 ± 0.2 | 2.8 ± 0.4 | 9.1 ± 0.7 | 128.1 ± 16.8 | 4.3 ± 0.2 | 20.8 ± 2.1 | |
| +6 mg/mL nicotine | 1.1 ± 0.1 | 0.9 ± 0.2 | 7.4 ± 0.4 | 227.6 ± 10.8 | 14.9 ± 0.7 | 14.9 ± 1.8 | |
| trial 3 | PG + GL | 1.0 ± 0.3b | 1.0 ± 0.2b | 1.0 ± 0.4b | 1.0 ± 0.6 | 1.0 ± 0.1b | 1.0 ± 0.7 |
| +flavorant | 1.7 ± 0.1 | 2.0 ± 0.5 | 39.3 ± 2.9 | 267.3 ± 7.0 | 5.5 ± 0.5 | 8.8 ± 0.7 | |
| +6 mg/mL nicotine | 1.1 ± 0.1 | 1.1 ± 0.2 | 28.4 ± 4.2 | 503.6 ± 21.2 | 9.5 ± 1.9 | 3.6 ± 0.5 | |
Flavorant mixture = 0.025 mg/mL benzaldehyde; 7.75 mg/mL vanillin; 9.75 mg/mL benzyl alcohol; 39 mg/mL trans-cinnamaldehyde.
PG + GL was not aerosolized again after the + 6 mg/mL nicotine addition.
Table 4.
Levels of the Degradation Product Total Formaldehydea, Normalized to the Amount Formed by Aerosolization of Equimolar PG + GL, Followed by Sequential Addition of the Indicated Flavorants, Then 6 mg/mL Nicotine
| total formaldehydea (normalized relative to that formed by PG + GL only) ± standard deviation |
|||||||
|---|---|---|---|---|---|---|---|
| benzaldehyde (2.5 mg/mL) |
vanillin (31 mg/mL) |
benzyl alcohol (39 mg/mL) |
trans-cinnamaldehyde (39 mg/mL) |
trans-cinnamaldehyde (155 mg/mL) |
“flavorant mixture”b | ||
| trial 1 | PG + GL | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | NAd | NAd | NAd |
| +flavorant | 1.6 ± 0.2 | 1.7 ± 0.4 | 3.2 ± 0.3 | NAd | NAd | NAd | |
| +6 mg/mL nicotine | 1.2 ± 0.1 | 0.9 ± 0.1 | 2.7 ± 0.1 | NAd | NAd | NAd | |
| trial 2 | PG + GL | 1.0 ± 0.1 | 1.0 ± 0.3 | 1.0 ± 0.3 | NAd | NAd | NAd |
| +flavorant | 1.6 ± 0.1 | 1.7 ± 0.2 | 6.4 ± 0.6 | NAd | NAd | NAd | |
| +6 mg/mL nicotine | 0.8 ± 0.1 | 0.5 ± 0.2 | 5.4 ± 0.4 | NAd | NAd | NAd | |
| trial 3 | PG + GL | 1.0 ± 0.2c | 1.0 ± 0.3c | 1.0 ± 0.1c | NAd | NAc,d | NAd |
| +flavorant | 1.6 ± 0.1 | 1.6 ± 0.2 | 22.6 ± 1.1 | NAd | NAd | NAd | |
| +6 mg/mL nicotine | 0.8 ± 0.2 | 0.9 ± 0.2 | 18.5 ± 2.8 | NAd | NAd | NAd | |
Formaldehyde + formaldehyde hemiacetal + total MAPs; total MAPs = 5.8 + 5.3+5.1 ppm multiple addition products.
Flavorant mixture = 0.025 mg/mL benzaldehyde; 7.75 mg/mL vanillin; 9.75 mg/mL benzyl alcohol; 39 mg/mL trans-cinnamaldehyde.
PG + GL was not aerosolized again after the + 6 mg/mL nicotine addition.
Not able to be analyzed due to peak overlap.
There was a decrease in HPHCs for e-liquids containing benzaldehyde, vanillin, benzyl alcohol, and a “flavorant mixture” when aerosolized with 6 mg/mL nicotine versus without nicotine (Tables 2–4). The basicity of nicotine would decrease the HPHCs in aerosols from e-liquids with benzaldehyde, vanillin, benzyl alcohol, and a “flavorant mixture” if the primary thermal degradation mechanism is acid-catalyzed. For example, sucralose and triacetin could thermally degrade into hydrochloric acid and acetic acid that were shown to enhance degradation levels, respectively.11,12 However, the degradation levels increased in aerosolized trans-cinnamaldehyde-containing PG + GL e-liquids (39 and 155 mg/mL trans-cinnamaldehyde) with 6 mg/mL nicotine versus without (Tables 2–4). Trans-cinnamaldehyde can initially be oxidized to produce acids that promote the neutralization of nicotine and promote degradation during aerosolization. Friedman et al.34 showed that trans-cinnamaldehyde in food products and essential oils can be oxidized with heat to produce benzaldehyde and glyoxal. Yu et al.35 used gas chromatography–mass spectrometry (GC–MS) to identify oxidation products from trans-cinnamaldehyde, finding acetaldehyde, benzaldehyde, benzoic acid, and cinnamic acid as some of the main oxidation products.
The effect of nicotine on degradation in aerosolized e-liquids with flavorants was determined by dividing the degradation levels of “PG + GL + flavorant+6 mg/mL nicotine” by “PG + GL + flavorant” in Tables 2–4. The average values (±SD) for the “nicotine degradation factors” are shown in Table 5. The nicotine degradation factors for acetaldehyde, acrolein, and total formaldehyde were similar for each flavorant (Table 5). E-liquids with benzaldehyde, vanillin, benzyl alcohol, and the “flavorant mixture” had nicotine degradation factors less than 1 (where 1 = no observed effect), thereby inhibiting HPHC formation (Table 5). Vanillin was the flavorant that generated toxicants that were most inhibited by a nicotine degradation factor of ~0.5 (Table 5). E-liquids with the greatest promoted toxicant formation contained 39 and 155 mg/mL trans-cinnamaldehyde and had nicotine degradation factors of 2.3 and 2.9, respectively (Table 5).
Table 5.
Average Nicotine Degradation Factors (Levels of Degradation of +6 mg/mL nicotine/+flavorant) for Acetaldehyde, Acrolein, and Total Formaldehydea
| average nicotine degradation factor (+6 mg/mL nicotine/+flavorant) ± standard deviation |
|||||||
|---|---|---|---|---|---|---|---|
| no flavorantb | benzaldehyde (2.5 mg/mL) |
vanillin (31 mg/mL) |
benzyl alcohol (39 mg/mL) |
trans-cinnamaldehyde (39 mg/mL) |
trans-cinnamaldehyde (155 mg/mL) |
“flavorant mixture”c | |
| acetaldehyde | 1.0 ± 0.1 | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.1 | 2.3 ± 0.8 | 2.9 ± 1.0 | 0.6 ± 0.2 |
| acrolein | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.5 ± 0.2 | 0.8 ± 0.1 | 2.3 ± 0.9 | 2.9 ± 0.8 | 0.6 ± 0.2 |
| total formaldehydea | 1.0 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.2 | NAd | NAd | NAd |
Formaldehyde + formaldehyde hemiacetal + total MAPs; total MAPs = 5.8 + 5.3 + 5.1 ppm multiple addition products.
The nicotine degradation factor was calculated by dividing the + 6 mg/mL nicotine by PG + GL.
Flavorant mixture = 0.025 mg/mL benzaldehyde; 7.75 mg/mL vanillin; 9.75 mg/mL benzyl alcohol; 39 mg/mL trans-cinnamaldehyde.
Not able to be analyzed due to peak overlap.
The interactions of nicotine with the e-cigarette solvents, flavorants, and metal coil could further alter toxicant formation upon aerosolization. Son et al.36 found that hydroxyl radical levels were slightly higher in aerosolized GL and PG + GL e-liquids when the nicotine concentration was higher; aerosolized PG e-liquids had higher hydroxyl radical levels when the nicotine concentration was lower. Bhagwat et al.37 observed an increase in lipid peroxidation products when rat brain tissues were exposed to chronic levels of nicotine (1.6 mg/kg/day) daily for a 10 day period, indicating that nicotine had oxidative properties. However, Linert et al.38 found that nicotine could be an antioxidant with its ability to bind Fe2+ and reduce transferrin-mediated Fe uptake in rat brain tissue. The role of nicotine as a prooxidant or antioxidant in flavored and unflavored e-liquids during aerosolization is unknown and requires further study.
The effect of 6 mg/mL nicotine on toxicant formation was determined by aerosolizing e-liquids containing PG + GL, followed by PG + GL + 6 mg/mL nicotine, and PG + GL (to compare the final and initial degradation levels). The average % aerosol collected for each trial is shown in Table S1, and the HPHC levels produced upon aerosolization are shown in Table S3. The degradation levels for acetaldehyde, acrolein, and total formaldehyde were similar in aerosolized e-liquids with and without nicotine (Table S2). The average nicotine degradation factor (degradation levels of “PG + GL + 6 mg/mL nicotine” divided by the average initial “PG + GL”) was 1, which indicates nicotine had no effect on the HPHCs formed upon aerosolization (Table 5).
We analyzed the composition of e-liquids containing benzaldehyde, vanillin, and trans-cinnamaldehyde over time and observed that the composition changed as determined by 1H NMR. The e-liquids with aldehyde flavorants formed acetals with PG and GL, as indicated by the new peaks in the aged trans-cinnamaldehyde e-liquids (Figure S3). Erythropel et al.29 reported that trans-cinnamaldehyde, benzaldehyde, and vanillin form and reach equilibrium with PG-acetal conversions up to ~92% in 1 day, ~95% in 5 days, and ~40% in 7 days, respectively. We did not observe a difference in the HPHCs produced from aerosolized e-liquids with aldehyde flavorants before and after they reached equilibrium with their respective PG-acetals, which is consistent with the values reported by Erythropel et al.29 The PG-flavorant acetals had a similar effect as the parent flavorant on HPHCs produced upon e-liquid aerosolization under our conditions. Similar to what was reported by Erythropel et al.,39 we noticed that the PG- and GL-flavorant acetals carried over into the aerosols. The differences in degradation levels from each trial with flavorants were more likely associated with the quality of the coil used in each experiment12 instead of acetal versus aldehyde presence in the e-liquid.
By the time consumers purchase e-liquids flavored with aldehydes, the PG- and GL-flavorant acetals likely reach equilibrium. The PG- and GL-flavorant acetals can have different toxicological properties than the individual solvents and flavorants. Jabba et al.40 reported that PG-flavorant acetals were cytotoxic to pulmonary epithelial cells and hindered mitochondrial function generally more than the parent flavorants. According to the results reported herein, consumers can also be exposed to higher levels of carbonyls when vaping flavored e-liquids compared to unflavored e-liquids,7,8,10 although consumers who vape flavored e-liquids with nicotine can be exposed to higher or lower amounts of carbonyls compared to flavored e-liquids without nicotine, depending on the specific flavorants. El-Hellani et al.41 and Reilly et al.42 inferred that nicotine did not affect carbonyl and oxidant production, but under our conditions, we found that nicotine can promote, inhibit, or have no effect on HPHC formation, depending on the conditions including the identities of the flavorants.
3.3. Toxicological Implications of Degradation Products Derived from Flavorants.
Aerosolized PG + GL e-liquids containing trans-cinnamaldehyde were individually spiked with benzaldehyde, trans-cinnamic acid, toluene, and styrene in order to confirm the identities of the unknown peaks in the 1H NMR spectra. Also, benzene was identified as a degradation product in aerosolized e-liquids containing benzaldehyde with and without nicotine, which is consistent with what was reported by Pankow et al.43 As noted above, Yu et al.35 identified benzaldehyde and trans-cinnamic acid as oxidation products of trans-cinnamaldehyde, and Li et al.44 identified styrene and toluene as pyrolysis products of trans-cinnamaldehyde.
The presence of benzaldehyde, trans-cinnamic acid, toluene, and styrene in aerosolized trans-cinnamaldehyde-containing PG + GL e-liquids (39 and 155 mg/mL trans-cinnamaldehyde) with and without 6 mg/mL nicotine was identified based on NMR chemical shifts and peak splitting. Benzaldehyde, toluene, and styrene were individually spiked into NMR samples containing aerosolized e-liquids with 155 mg/mL trans-cinnamaldehyde and 6 mg/mL nicotine (Figures S4–S6). Trans-cinnamic acid was spiked into NMR samples containing aerosolized e-liquids with 155 mg/mL trans-cinnamaldehyde (Figure S7). The benzaldehyde, toluene, and styrene resonances were not present in the previous aerosolized PG + GL or unvaped trans-cinnamaldehyde-containing e-liquid samples, indicating that they were formed during aerosolization (Figures S4–S6). The trans-cinnamic acid peaks were not observed in the aerosolized PG + GL but were observed in the unvaped trans-cinnamaldehyde-containing e-liquid, and then formed ~2× more during aerosolization (Figure S7). We estimated that 1 × 10−4, 3 × 10−4, 0.05, and 0.02 mg/puff benzaldehyde, trans-cinnamic acid, toluene, and styrene were formed in each aerosol, respectively, under our conditions (Figures S4–S7).
The Environmental Protection Agency’s (EPA) Integrated Risk Information System (IRIS) determined that the no-observed-adverse-effect level (NOAEL) for toluene was 46 mg/m3 per day (1.9 mg/m3 per 1 h) for human subjects.45 The physiological daily inhalation rate (PDIR) of 17.48 m3/day (0.73 m3/h) for 23–30 year old males was used to estimate the breath volume.46 The IRIS limit per hour for toluene would be 1.40 mg/h based on the chosen inhalation rate. The e-cigarette used in this study produced 0.05 mg/puff toluene at 22 W at a flow rate of 18.3 mL/s. Kosmider et al.47 found that the average number of puffs per day for 24 adult e-cigarette consumers was 156 puffs/day (~7 puffs/h). At 7 puffs/h, the rate of toluene inhalation would be 0.35 mg/h, which does not exceed the IRIS limit and does not account for any aerosol exhaled.
The EPA determined that the Acute Exposure Guideline Level (AEGL-1) for nondisabling effects of styrene inhalation in the central nervous system of humans was 85 mg/m3 per hour.48 Using the PDIR for 23–30 year old males of 17.48 m3/day (0.73 m3/h) yields an AEGL-1 limit of 62.1 mg/h.46 If 156 puffs/day47 (7 puffs/h) were inhaled using the e-cigarette and e-liquid in this study at 22 W, a flow rate of 18.3 mL/s, and 0.02 mg/puff, the consumer would inhale styrene at a rate of 0.14 mg/h. Under our conditions, the levels of styrene inhaled do not exceed the AEGL-1 limit (also assuming that no aerosol is exhaled). The consumer could be exposed to higher concentrations of toluene and styrene by vaping with a higher power setting (>22 W)41 and/or having a higher concentration of trans-cinnamaldehyde (>155 mg/mL) in the e-liquid.42 Inhaling any styrene and/or toluene is concerning due to classifications as a Group 2A probable human carcinogen and nervous system depressant, respectively.45,49
Yu et al.35 found that the oxidation of trans-cinnamaldehyde to benzaldehyde formed more readily at higher temperatures and involved oxidative cleavage; however, the oxidation of trans-cinnamaldehyde to trans-cinnamic acid was less dependent on temperature than the formation of benzaldehyde. The trans-cinnamaldehyde in e-liquids underwent partial oxidation during storage at room temperature resulting in trans-cinnamic acid formation (Figure S7). Li et al. reported that toluene and styrene were produced upon the pyrolysis of trans-cinnamaldehyde44 and proposed seven possible pathways for styrene to form, many of which begin with the H radical addition to or abstraction from trans-cinnamaldehyde (Figures S4 and S5). Toluene and styrene were previously identified as degradation products from e-cigarettes through GC–MS analysis by others, but conversion to toluene or styrene from cinnamaldehyde was not reported.50 The presence of benzaldehyde, trans-cinnamic acid, styrene, and toluene in aerosolized e-liquids with trans-cinnamaldehyde shows that trans-cinnamaldehyde underwent oxidation and free radical cleavage during thermal degradation.
The presence of benzene was determined in the aerosolized benzaldehyde-containing PG + GL e-liquids based on the observed chemical shift (7.37 ppm) and peak shape consistent with that reported for benzene by Pankow et al.43 The peak was not observed in the unvaped e-liquid nor vaped samples of PG + GL. We calculated approximately 4 × 10−4 mg/puff of benzene in the aerosolized e-liquid with benzaldehyde. Benzene is carcinogenic to humans, and there is no safe level of exposure via inhalation according to the World Health Organization (WHO).51 Pankow et al. identified benzene as a degradation product of various e-liquid mixtures (including benzaldehyde-containing e-liquids) upon vaporization, and Namysl et al. identified benzene as a pyrolysis product of benzaldehyde.43,52
4. CONCLUSIONS
We found that benzaldehyde, vanillin, benzyl alcohol, and trans-cinnamaldehyde can enhance PG and GL degradation during vaping, consistent with other reports, including that e-liquids that contain greater concentrations of flavorants produce more HPHCs (as measured by carbonyl production).7,10,53 We also found that nicotine inhibited the levels of HPHC formation in the presence of benzaldehyde, vanillin, benzyl alcohol, and a “flavorant mixture” when aerosolized, as compared to flavored e-liquids without nicotine. However, nicotine enhanced the levels of degradation when added to e-liquids with low and high concentrations of trans-cinnamaldehyde (39 and 155 mg/mL, respectively), as compared to the same e-liquids without nicotine. The effects of other common flavorants with nicotine should also be explored because there is widespread use of many different flavorants and combinations thereof54,55 and because concentrations of nicotine in e-liquids can vary by brand and local regulations.54
Supplementary Material
Table 3.
Levels of the Degradation Product Acrolein, Normalized to the Amount Formed by Aerosolization of Equimolar PG + GL, Followed by Sequential Addition of the Indicated Flavorants, Then 6 mg/mL Nicotine
| acrolein (normalized relative to that formed by PG + GL only) ± standard deviation |
|||||||
|---|---|---|---|---|---|---|---|
| benzaldehyde (2.5 mg/mL) |
vanillin (31 mg/mL) |
benzyl alcohol (39 mg/mL) |
trans-cinnamaldehyde (39 mg/mL) |
trans-cinnamaldehyde (155 mg/mL) |
“flavorant mixture”a | ||
| trial 1 | PG + GL | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.4 | 1.0 ± 0.1 | 1.0 ± 0.6 |
| +flavorant | 1.5 ± 0.2 | 3.6 ± 0.3 | 6.4 ± 0.5 | 28.7 ± 0.5 | 66.1 ± 13.7 | 12.5 ± 0.3 | |
| +6 mg/mL nicotine | 1.3 ± 0.1 | 1.4 ± 0.2 | 4.8 ± 0.2 | 97.2 ± 3.9 | 212.1 ± 32.6 | 8.4 ± 0.9 | |
| trial 2 | PG + GL | 1.0 ± 0.1 | 1.0 ± 0.4 | 1.0 ± 0.5 | 1.0 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.3 |
| +flavorant | 1.5 ± 0.3 | 4.3 ± 0.9 | 7.6 ± 0.6 | 145.4 ± 9.9 | 8.8 ± 1.2 | 24.1 ± 2.7 | |
| +6 mg/mL nicotine | 1.2 ± 0.1 | 1.2 ± 0.5 | 6.4 ± 0.3 | 243.8 ± 21.3 | 31.3 ± 1.6 | 15.3 ± 3.0 | |
| trial 3 | PG + GL | 1.0 ± 0.3b | 1.0 ± 0.3b | 1.0 ± 0.3b | 1.0 ± 0.1 | 1.0 ± 0.2b | 1.0 ± 0.7 |
| +Flavorant | 1.6 ± 0.1 | 2.1 ± 0.6 | 36.7 ± 2.1 | 296.0 ± 19.1 | 8.6 ± 1.0 | 26.2 ± 3.2 | |
| +6 mg/mL | 1.3 ± 0.1 | 1.4 ± 0.2 | 27.6 ± 4.8 | 541.7 ± 19.2 | 17.0 ± 3.8 | 9.1 ± 1.4 | |
Flavorant mixture = 0.025 mg/mL benzaldehyde; 7.75 mg/mL vanillin; 9.75 mg/mL benzyl alcohol; 39 mg/mL trans-cinnamaldehyde.
PG + GL was not aerosolized again after the + 6 mg/mL nicotine addition.
ACKNOWLEDGMENTS
We thank Wentai Luo for useful discussions about this topic.
Funding
This work was supported by the U.S. National Institutes of Health, grant R01ES025257. Research reported was supported by the NIEHS and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
ABBREVIATIONS
- COSY
homonuclear correlation spectroscopy
- TOCSY
total correlation spectroscopy
- e-cigarette
electronic cigarette
- e-liquid
electronic cigarette liquid
- PG
propylene glycol
- GL
glycerol
- NMR
nuclear magnetic resonance
- HPHC
harmful and potentially harmful constituents
- PM
particulate matter
- HPLC-HRMS
high performance liquid chromatography-high resolution mass spectrometry
- MAP
multiple addition formaldehyde hemiacetal product
- MS
mass spectrometry
- DNPH
2,4-dinitrophenylhydrazine
- GC-MS
gas chromatography–mass spectrometry
- IRIS
Integrated Risk Information System
- NOAEL
no-observed-adverse-effect level
- PDIR
physiological daily inhalation rate
- EPA
Environmental Protection Agency
- AEGL
acute exposure guideline levels
- WHO
World Health Organization
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemrestox.2c00110.
Experimental details for the identification of multiple addition products) and additional NMR data and information for vaping and spiking experiments (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.chemrestox.2c00110
The authors declare no competing financial interest.
Contributor Information
Paul J. Kerber, Department of Chemistry, Portland State University, Portland, Oregon 97207-0751, United States
Anna K. Duell, Department of Chemistry, Portland State University, Portland, Oregon 97207-0751, United States
Marley Powers, Department of Chemistry, Portland State University, Portland, Oregon 97207-0751, United States.
Robert M. Strongin, Department of Chemistry, Portland State University, Portland, Oregon 97207-0751, United States
David H. Peyton, Department of Chemistry, Portland State University, Portland, Oregon 97207-0751, United States
REFERENCES
- (1).Wang TW; Neff LJ; Park-Lee E; Ren C; Cullen KA; King BA E-cigarette Use Among Middle and High School Students - United States, 2020. MMWR Morb. Mortal. Wkly. Rep 2020, 69, 1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kosmider L; Sobczak A; Fik M; Knysak J; Zaciera M; Kurek J; Goniewicz ML Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob. Res 2014, 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Rickard BP; Ho H; Tiley JB; Jaspers I; Brouwer KLR E-Cigarette Flavoring Chemicals Induce Cytotoxicity in HepG2 Cells. ACS Omega 2021, 6, 6708–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Olmedo P; Goessler W; Tanda S; Grau-Perez M; Jarmul S; Aherrera A; Chen R; Hilpert M; Cohen JE; Navas-Acien A; et al. Metal Concentrations in e-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ. Health Perspect 2018, 126, 027010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Jensen RP; Strongin RM; Peyton DH Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci. Rep 2017, 7, 42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Li Y; Burns AE; Tran LN; Abellar KA; Poindexter M; Li X; Madl AK; Pinkerton KE; Nguyen TB Impact of e-Liquid Composition, Coil Temperature, and Puff Topography on the Aerosol Chemistry of Electronic Cigarettes. Chem. Res. Toxicol 2021, 34, 1640–1654. [DOI] [PubMed] [Google Scholar]
- (7).Qu Y; Kim KH; Szulejko JE The effect of flavor content in e-liquids on e-cigarette emissions of carbonyl compounds. Environ. Res 2018, 166, 324–333. [DOI] [PubMed] [Google Scholar]
- (8).Salam S; Saliba NA; Shihadeh A; Eissenberg T; El-Hellani A Flavor-Toxicant Correlation in E-cigarettes: A Meta-Analysis. Chem. Res. Toxicol 2020, 33, 2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Gillman IG; Pennington ASC; Humphries KE; Oldham MJ Determining the impact of flavored e-liquids on aldehyde production during Vaping. Regul. Toxicol. Pharmacol 2020, 112, 104588. [DOI] [PubMed] [Google Scholar]
- (10).Khlystov A; Samburova V Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ. Sci. Technol 2016, 50, 13080–13085. [DOI] [PubMed] [Google Scholar]
- (11).Vreeke S; Peyton DH; Strongin RM Triacetin Enhances Levels of Acrolein, Formaldehyde Hemiacetals, and Acetaldehyde in Electronic Cigarette Aerosols. ACS Omega 2018, 3, 7165–7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Duell AK; McWhirter KJ; Korzun T; Strongin RM; Peyton DH Sucralose-Enhanced Degradation of Electronic Cigarette Liquids during Vaping. Chem. Res. Toxicol 2019, 32, 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kim SA; Smith S; Beauchamp C; Song Y; Chiang M; Giuseppetti A; Frukhtbeyn S; Shaffer I; Wilhide J; Routkevitch D; et al. Cariogenic potential of sweet flavors in electronic-cigarette liquids. PLoS One 2018, 13, No. e0203717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Behar RZ; Luo W; McWhirter KJ; Pankow JF; Talbot P Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep 2018, 8, 8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Behar RZ; Davis B; Wang Y; Bahl V; Lin S; Talbot P Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. Vitro 2014, 28, 198–208. [DOI] [PubMed] [Google Scholar]
- (16).Piechowski JM; Bagatto B Cardiovascular function during early development is suppressed by cinnamon flavored, nicotine-free, electronic cigarette vapor. Birth Defects Res. 2021, 113, 1215–1223. [DOI] [PubMed] [Google Scholar]
- (17).Clapp PW; Pawlak EA; Lackey JT; Keating JE; Reeber SL; Glish GL; Jaspers I Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol 2017, 313, L278–L292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Clapp PW; Lavrich KS; van Heusden CA; Lazarowski ER; Carson JL; Jaspers I Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol 2019, 316, L470–L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wavreil FDM; Heggland SJ Cinnamon-flavored electronic cigarette liquids and aerosols induce oxidative stress in human osteoblast-like MG-63 cells. Toxicol. Rep 2020, 7, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kosmider L; Sobczak A; Prokopowicz A; Kurek J; Zaciera M; Knysak J; Smith D; Goniewicz ML Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax 2016, 71, 376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bitzer ZT; Goel R; Reilly SM; Elias RJ; Silakov A; Foulds J; Muscat J; Richie JP Jr. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radical Biol. Med 2018, 120, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Talih S; Salman R; El-Hage R; Karam E; Karaoghlanian N; El-Hellani A; Saliba N; Eissenberg T; Shihadeh A Might limiting liquid nicotine concentration result in more toxic electronic cigarette aerosols? Tobac. Control 2021, 30, 348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Baker AN; Bakke AJ; Branstetter SA; Hayes JE Harsh and Sweet Sensations Predict Acute Liking of Electronic Cigarettes, but Flavor Does Not Affect Acute Nicotine Intake: A Pilot Laboratory Study in Men. Nicotine Tob. Res 2021, 23, 687–693. [DOI] [PubMed] [Google Scholar]
- (24).Duell AK; Pankow JF; Peyton DH Nicotine in tobacco product aerosols: ‘It’s deja vu all over again. Tobac. Control 2020, 29, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).CORESTA. Routine Analytical Machine for E-Cigarette Aerosol Generation and Collection - Definitions and Standard Conditions. 2018. https://www.coresta.org/routine-analyticalmachine-e-cigarette-aerosol-generation-and-collection-definitionsand-standard (accessed Mar 22, 2018).
- (26).Duell AK; Pankow JF; Gillette SM; Peyton DH Boiling points of the propylene glycol + glycerol system at 1 atmosphere pressure: 188.6-292 degrees C without and with added water or nicotine. Chem. Eng. Commun 2018, 205, 1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Duell AK; Pankow JF; Peyton DH Free-Base Nicotine Determination in Electronic Cigarette Liquids by 1H NMR Spectroscopy. Chem. Res. Toxicol 2018, 31, 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Jensen RP; Luo W; Pankow JF; Strongin RM; Peyton DH Hidden formaldehyde in e-cigarette aerosols. N. Engl. J. Med 2015, 372, 392–394. [DOI] [PubMed] [Google Scholar]
- (29).Erythropel HC; Jabba SV; DeWinter TM; Mendizabal M; Anastas PT; Jordt SE; Zimmerman JB Formation of flavorant-propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob. Res 2019, 21, 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Pankow JF; Kim K; Luo W; McWhirter KJ Gas/Particle Partitioning Constants of Nicotine, Selected Toxicants, and Flavor Chemicals in Solutions of 50/50 Propylene Glycol/Glycerol As Used in Electronic Cigarettes. Chem. Res. Toxicol 2018, 31, 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Salamanca JC; Munhenzva I; Escobedo JO; Jensen RP; Shaw A; Campbell R; Luo W; Peyton DH; Strongin RM Formaldehyde Hemiacetal Sampling, Recovery, and Quantification from Electronic Cigarette Aerosols. Sci. Rep 2017, 7, 11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Salamanca JC; Meehan-Atrash J; Vreeke S; Escobedo JO; Peyton DH; Strongin RM E-cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non-averse to users. Sci. Rep 2018, 8, 7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Chen JY; Canchola A; Lin YH Carbonyl Composition and Electrophilicity in Vaping Emissions of Flavored and Unflavored E-LiquidsNLM PubMed-not-MEDLINE. Toxics 2021, 9, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Friedman M; Kozukue N; Harden LA Cinnamaldehyde Content in Foods Determined by Gas Chromatography–Mass Spectrometry. J. Agric. Food Chem 2000, 48, 5702–5709. [DOI] [PubMed] [Google Scholar]
- (35).Yu C; Li Y-L; Liang M; Dai S-Y; Ma L; Li W-G; Lai F; Liu X-M Characteristics and hazards of the cinnamaldehyde oxidation process. RSC Adv. 2020, 10, 19124–19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Son Y; Mishin V; Laskin JD; Mainelis G; Wackowski OA; Delnevo C; Schwander S; Khlystov A; Samburova V; Meng Q Hydroxyl Radicals in E-Cigarette Vapor and E-Vapor Oxidative Potentials under Different Vaping Patterns. Chem. Res. Toxicol 2019, 32, 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Bhagwat SV; Vijayasarathy C; Raza H; Mullick J; Avadhani NG Preferential effects of nicotine and 4-(N-methyl- N-nitrosamino)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase a4-4 induction and increased oxidative stress in the rat brain. Biochem. Pharmacol 1998, 56, 831–839. [DOI] [PubMed] [Google Scholar]
- (38).Linert W; Bridge MH; Huber M; Bjugstad KB; Grossman S; Arendash GW In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson’s and Alzheimer’s diseases. Biochim. Biophys. Acta 1999, 1454, 143–152. [DOI] [PubMed] [Google Scholar]
- (39).Erythropel HC; Davis LM; de Winter TM; Jordt SE; Anastas PT; O’Malley SS; Krishnan-Sarin S; Zimmerman JB Flavorant-Solvent Reaction Products and Menthol in JUUL E-Cigarettes and Aerosol. Am. J. Prev. Med 2019, 57, 425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Jabba SV; Diaz AN; Erythropel HC; Zimmerman JB; Jordt S-E Chemical Adducts of Reactive Flavor Aldehydes Formed in E-Cigarette Liquids Are Cytotoxic and Inhibit Mitochondrial Function in Respiratory Epithelial Cells. Nicotine Tob. Res 2020, 22, S25–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).El-Hellani A; Salman R; El-Hage R; Talih S; Malek N; Baalbaki R; Karaoghlanian N; Nakkash R; Shihadeh A; Saliba NA Nicotine and Carbonyl Emissions From Popular Electronic Cigarette Products: Correlation to Liquid Composition and Design Characteristics. Nicotine Tob. Res 2018, 20, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Reilly SM; Bitzer ZT; Goel R; Trushin N; Richie JP Free Radical, Carbonyl, and Nicotine Levels Produced by Juul Electronic Cigarettes. Nicotine Tob. Res 2019, 21, 1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Pankow JF; Kim K; McWhirter KJ; Luo W; Escobedo JO; Strongin RM; Duell AK; Peyton DH Benzene formation in electronic cigarettes. PLoS One 2017, 12, No. e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Li L; Van De Vijver R; Sribala G; Weng J; Van Geem K Pyrolysis Study of Cinnamaldehyde Model Compound with Analytical Py-GC×GC-FID/TOF-MS. Chem. Eng. Trans 2020, 80, 79–84. [Google Scholar]
- (45).Assessment, U. E. N. C. for E. Toluene CASRN 108-88-3 | IRIS|US EPA, ORD. https://iris.epa.gov/ChemicalLanding/&substance_nmbr=118.UnitedStatesEnvironmentalProtectionAgency (accessed April 19, 2022).
- (46).Exposure Factors Handbook (2011. Edition). https://19january2017snapshot.epa.gov/expobox/exposure-factorshandbook-2011-edition_.html. United States Environmental Protection Agency; (accessed Mar 11, 2022). [Google Scholar]
- (47).Kosmider L; Jackson A; Leigh N; O’Connor R; Goniewicz ML Circadian Puffing Behavior and Topography Among E-cigarette UsersNLM PubMed-not-MEDLINE. Tobac. Regul. Sci 2018, 4, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Styrene Results - AEGL Program. https://www.epa.gov/aegl/styrene-results-aegl-program. United States Environmental Protection Agency; (accessed Mar 11, 2022). [Google Scholar]
- (49).IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. In Styrene, Styrene-7,8-oxide, and Quinoline. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Styrene-Styrene-7-8-oxide-And-Quinoline-2019, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, 2019. [Google Scholar]
- (50).(a) Goniewicz ML; Knysak J; Gawron M; Kosmider L; Sobczak A; Kurek J; Prokopowicz A; Jablonska-Czapla M; Rosik-Dulewska C; Havel C; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobac. Control 2014, 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim Y-H; Kim K-H A novel method to quantify the emission and conversion of VOCs in the smoking of electronic cigarettes. Sci. Rep 2015, 5, 16383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Exposure to benzene: a major public health concern. https://www.who.int/publications/i/item/WHO-CED-PHE-EPE-19.4.2. The World Health Organization, (accessed Mar 11, 2022). [Google Scholar]
- (52).Namysl S; Pelucchi M; Pratali Maffei L; Herbinet O; Stagni A; Faravelli T; Battin-Leclerc F Experimental and modeling study of benzaldehyde oxidation. Combust. Flame 2020, 211, 124–132. [Google Scholar]
- (53).Son Y; Weisel C; Wackowski O; Schwander S; Delnevo C; Meng Q The Impact of Device Settings, Use Patterns, and Flavorings on Carbonyl Emissions from Electronic Cigarettes. Int. J. Environ. Res. Publ. Health 2020, 17, 5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Fix BV; OConnor RJ; Goniewicz ML; Leigh NL; Cummings M; Hitchman SC; Fong GT; El Nahas G; Hammond D; McNeill A; et al. Characterisation of vaping liquids used in vaping devices across four countries: results from an analysis of selected vaping liquids reported by users in the 2016 ITC Four Country Smoking and Vaping Survey. Tobac. Control 2021, DOI: 10.1136/tobaccocontrol-2020-056338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Tierney PA; Karpinski CD; Brown JE; Luo W; Pankow JF Flavour chemicals in electronic cigarette fluids. Tobac. Control 2016, 25, e10–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


