Abstract
Introduction
For acute myeloid leukemia (AML), prognosis is particularly poor in patients harboring FMS-like tyrosine kinase 3 (FLT3) gene mutations, though routine screening for these mutations at diagnosis has been shown to be insufficient. The understanding of the impact of FLT3 mutations on treatment decisions is limited.
Methods
In this retrospective, observational study, we investigated the key epidemiological characteristics, treatment patterns and responses among adult patients with newly diagnosed (ND) AML in China, who initiated treatment from January 1, 2015, to December 31, 2019, or progressed to relapsed/refractory (R/R) AML by December 31, 2020.
Results
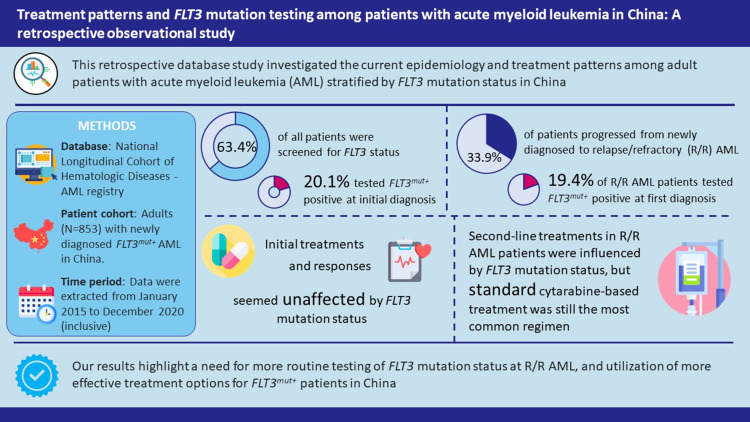
Of the 853 ND AML patients included, 63.4% were screened for FLT3 status, and 20.1% tested positive (FLT3MUT) at initial diagnosis. Of 289 patients who progressed to R/R AML during the study period, 24.9% were screened at the diagnosis of R/R AML, and 19.4% tested positive; 20.5% of screened patients changed FLT3 status at first diagnosis of R/R AML. Initial treatment regimens or treatment responses did not seem to differ in patients with ND AML by FLT3 mutation status. In patients with R/R AML, there was an apparent difference in second-line treatment choices by FLT3 mutation status; however, the number of FLT3-mutated patients were limited to demonstrate any meaningful distinction. FLT3-mutated R/R AML was associated with shorter relapse time.
Conclusion
Study findings showed that there was a lack of routine testing for FLT3 mutations at first diagnosis of R/R AML, and initial treatment decisions did not differ by FLT3 mutation status. Given the clinical burden of FLT3MUT, likelihood of FLT3 status changes, and emerging FLT3 inhibitors, further routine FLT3 screening is needed to optimize treatment of R/R AML.
Keywords: acute myeloid leukemia, epidemiology, real-world, retrospective study
Graphical Abstract
Introduction
Acute myeloid leukemia (AML) is the predominant form of leukemia in adults, both globally and in China.1–3 It is characterized by the rapid proliferation of undifferentiated myeloblasts and a poor prognosis, particularly for older individuals, with most patients relapsing after initial treatment.4,5 For patients with newly diagnosed (ND) AML, initial treatment comprises induction chemotherapy to reduce leukemic cell numbers to below detectable levels, followed by consolidation therapy to eliminate any remaining leukemic cells and reduce the risk of relapse.6,7
Allogeneic hematopoietic stem-cell transplantation (allo-HSCT) is indicated for use either as first-line therapy in patients achieving remission following chemotherapy, for whom the estimated probability of relapse without allo-HSCT would be >35–40%, or as second-line treatment following relapse after initial chemotherapy.8 However, relapse after allo-HSCT is relatively common (25–55%), with survival rates after 4 years ranging between 20% and 35%.9 As an alternative therapeutic pathway, several key genetic mutations have been shown to be closely associated with the pathogenesis of AML and poorer prognoses in AML patients,10,11 with their inhibition linked to improved clinical outcomes in relapsed/refractory (R/R) AML patients.12 Recent advances in next-generation sequencing technology have enhanced our understanding of these mutations underlying AML and the genomic heterogeneity between patients,5 providing an opportunity for more personalized, less intensive therapies and the development of agents that directly target the affected pathways.
Approximately 30% of patients with AML harbor mutations in the FMS-like tyrosine kinase 3 (FLT3) gene;10 one of the most common types of FLT3 mutations is an internal tandem duplication (FLT3-ITD), which may be present in 25% of patients.13 A recent study of 171 Chinese patients reported the incidence of FLT3-ITD to be 18.1% and found it to be associated with a significantly reduced rate of complete remission (CR).14 FLT3-ITD, as well as the less common point mutations within the activation loops of the FLT3 tyrosine kinase domain (FLT3-TKD), are associated with an increased risk of relapse and reduced survival rates.15,16 It is therefore recommended that patients with ND AML are screened for FLT3 mutations, so that they can receive targeted treatment with FLT3 inhibitors.17 While clinical trials for next-generation FLT3 inhibitors are ongoing, two FLT3 inhibitors, midostaurin and gilteritinib, have been approved by the US Food and Drug Administration (FDA), for ND AML and R/R AML, respectively.5 At the time of this study, only gilteritinib has received regulatory approval in China (in 2021).18 Both midostaurin and gilteritinib have been shown to significantly increase CR rate and overall survival compared to treatment with standard chemotherapy.19,20
Despite the emergence and ongoing development of FLT3 inhibitors, and recommendations from clinical guidelines, the occurrence of routine screening for FLT3 mutations in patients with AML is still limited.21 Although previous studies across multiple countries have noted that FLT3MUT patients were treated more aggressively than wild-type FLT3 patients,22 further trends in treatment pattern by FLT3 mutation status were not identified.
In this real-world, single-center study, we investigated epidemiologic characteristics, treatment patterns, clinical outcomes in patients with AML in China. We also sought to determine the prevalence of FLT3 mutation testing and stratify outcomes by FLT3 mutation status.
Materials and Methods
Study Design and Patients
This was a retrospective, longitudinal, observational cohort study based on historical data from the National Longitudinal Cohort of Hematologic Diseases (NICHE)-AML registry, of the Institute of Hematology and Blood Diseases Hospital (IHBDH), the leading hospital for the China Alliance for Blood Diseases. The study included patient data recorded from January 1, 2015, to December 31, 2020. No a priori hypotheses were tested.
All eligible patients were aged ≥18 years at the time of initial diagnosis and initiated on treatment for ND AML between January 1, 2015, and December 31, 2019; of these patients, those who subsequently progressed to R/R AML (from the initial treatment for ND AML) during this period (plus 1 year of follow-up to December 31, 2020) were identified. Patients were split by disease progression status (ND and R/R AML), whether FLT3 mutation testing was performed, and, if so, the result of the test (wild-type FLT3 [FLT3WT] and mutated FLT3 [FLT3MUT]).
Eligible patients also had at least 1 year of follow-up at the data cut (December 31, 2020); however, patients with less than 1 year of follow-up were eligible if the reason for loss of follow-up was death. Patients were excluded if they had acute promyelocytic leukemia or were participating in clinical trials.
The study was conducted in accordance with the Declaration of Helsinki and International Conference of Harmonisation guidelines. The study was approved by the institutional review board committee of the IHBDH. The review board committee confirmed that informed consent was not needed from participants, and all data were anonymized prior to the current study.
Treatment
This was a non-interventional, real-world registry study and patients did not receive treatment as part of the study. However, historical medical data extracted from the NICHE-AML registry also included information regarding treatments received by patients, and the corresponding treatment responses.
Endpoints
The primary objectives were to investigate the key epidemiological characteristics among patients with AML in China and to understand the treatment patterns and responses among patients stratified by ND, R/R, and FLT3 mutation status. Specifically, the primary endpoints were as follows: cross-sectional distribution of patients with ND AML, relapsed AML, and refractory AML (patients who were classified as having refractory AML at any point during the study) at the time of data cut (December 31, 2020); rate of progression from ND to R/R AML during study period; proportion of patients who received FLT3 mutation testing at initial diagnosis and at first diagnosis of R/R disease; and prevalence of patients harboring FLT3 mutations among all tested patients.
Other primary endpoints were the first-line and R/R treatment patterns and responses, including distribution of therapies being used; use of stem-cell transplantation; and the proportion of patients achieving a CR or CR with incomplete hematologic recovery (CRi).
Secondary and exploratory objectives included descriptions of patient baseline demographics and disease characteristics at initial diagnosis.
Statistical Analysis
Continuous variables were calculated as mean with standard deviation (SD) and median with interquartile range (IQR); categorical variables were calculated as counts and proportions. Summary descriptive statistics collected were the length of follow-up and the number of follow-up visits per person for AML treatment at the IHBDH.
The progression rate from ND to relapsed and refractory AML was determined from the available data, with the total number of patients with ND AML forming the denominator. Data were calculated for the following six study groups constructed according to R/R status and FLT3 mutation status: (1) FLT3WT at initial diagnosis; (2) FLT3MUT at initial diagnosis; (3) unknown FLT3 mutation status at initial diagnosis; (4) FLT3WT at R/R disease; (5) FLT3MUT at R/R disease; and (6) unknown FLT3 mutation status at R/R disease.
All measurements were summarized descriptively, and no statistical comparisons were made across study groups. All analyses were conducted using a complete case analysis approach and patients with missing values in relation to patient and disease characteristics were omitted from the corresponding statistic. Regarding treatment information, ongoing treatments without complete data for treatment response and number of treatment cycles were also omitted; the absence of treatment name, dosage, and administration date were considered an indicator that a patient did not receive treatment at the corresponding line.
Due to the nature of the NICHE-AML registry database, all patients included in the current study were initially classed as ND and a subset of patients progressed to R/R during the follow-up period. The index date for patients with ND AML was defined as the initiation date of the first therapy; for patients with R/R disease after the initial treatment, the index date was the date of confirmation of R/R AML. To maximize the achievable sample size in the study groups, a patient could contribute to multiple study groups, eg, patients with ND AML who progressed to R/R AML during follow-up would contribute to both the ND and R/R study groups.
Results
Patient and Disease Characteristics
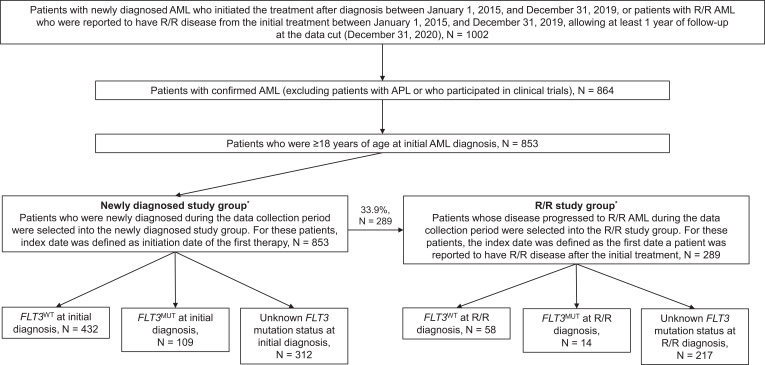
A total of 1002 patients were identified from the NICHE-AML registry with ND AML and who initiated treatment or had progressed to R/R AML at treatment initiation during the study period. Of these, 853 patients were eligible and were enrolled in the study (Figure 1). In total, 541 (63.4%) patients received FLT3 mutation testing at initial AML diagnosis: 432 patients were diagnosed with FLT3WT, 109 with FLT3MUT, and 312 with unknown FLT3 mutation status at enrolment (Table 1a).
Figure 1.
Flow of patients through the study. *All patients had newly diagnosed AML at entry to the NICHE-AML historical database.
Abbreviations: AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase-3; MUT, mutated; R/R, relapsed/refractory; WT, wild type.
Table 1.
Demographics and Disease Characteristics of Patients with (a) ND and (b) R/R AML in the NICHE-AML Registry
| (a) | ||||
| Characteristic | All ND AML Patients N = 853 | FLT3WT N = 432 | FLT3MUT N = 109 | Unknown FLT3 Mutation Statusa N = 312 |
| Follow-up duration, days | 573.4 (475.9) | 494.8 (364.2) | 399.8 (328.1) | 742.9 (592.6) |
| Age at diagnosis, y | 42.5 (12.8) | 43.1 (12.8) | 44.0 (12.5) | 41.3 (12.8) |
| Male, n (%) | 448 (52.5) | 244 (56.5) | 51 (46.8) | 153 (49.0) |
| Known mutation status at diagnosis, n (%) | 541 (63.4) | 432 (100) | 109 (100) | - |
| FLT3 | 109 (20.1) | 0 | 109 (100) | - |
| NPM1 | 109 (20.1) | 66 (15.3) | 43 (39.4) | - |
| CEBPA | 103 (19.0) | 89 (20.6) | 14 (12.8) | - |
| IDH1 | 38 (7.0) | 32 (7.4) | 6 (5.5) | - |
| Other acquired mutation | 533 (98.5) | 428 (99.1) | 105 (96.3) | - |
| Cytogenetic status, n (%) | 832 (97.5) | 421 (97.5) | 105 (96.3) | 306 (98.1) |
| Abnormal karyotype | 416 (50.0) | 235 (55.8) | 42 (40.0) | 139 (45.4) |
| FAB classification,23 n (%) | 650 (76.2) | 345 (79.9) | 77 (70.6) | 228 (73.1) |
| M0 | 3 (0.5) | 2 (0.6) | 0 | 1 (0.4) |
| M1 | 6 (0.9) | 2 (0.6) | 1 (1.3) | 3 (1.3) |
| M2 | 253 (38.9) | 158 (45.8) | 20 (26.0) | 75 (32.9) |
| M4 | 113 (17.4) | 54 (15.7) | 12 (15.6) | 47 (20.6) |
| M5 | 265 (40.8) | 126 (36.5) | 44 (57.1) | 95 (41.7) |
| M6 | 6 (0.9) | 1 (0.3) | 0 | 5 (2.2) |
| M7 | 1 (0.2) | 0 | 0 | 1 (0.4) |
| Type of AML | ||||
| De novo | 849 (99.5) | 428 (99.1) | 109 (100) | 312 (100) |
| Secondary | 4 (0.5) | 4 (0.9) | 0 | 0 |
| Risk stratification7 | 746 (87.5) | 427 (98.8) | 108 (99.1) | 211 (67.6) |
| Favorable | 283 (37.9) | 200 (46.8) | 82 (75.9) | 1 (0.5) |
| Intermediate | 423 (56.7) | 227 (53.2) | 26 (24.1) | 170 (80.6) |
| Adverse | 40 (5.4) | 0 | 0 | 40 (19.0) |
| (b) | ||||
| Characteristic | All R/R AML Patients N = 289 | FLT3WT N = 58 | FLT3MUT N = 14 | Unknown FLT3 Mutation Statusb N = 217 |
| Follow-up duration, days | 562.7 (438.3) | 606.2 (371.7) | 479.2 (261.3) | 556.4 (463.2) |
| Age at diagnosis, y | 43.6 (13.5) | 43.5 (13.1) | 44.4 (15.4) | 43.6 (13.6) |
| Male, n (%) | 154 (53.3) | 32 (55.2) | 9 (64.3) | 113 (52.1) |
| Known mutation status at diagnosis, n (%) | 183 (63.3) | 45 (77.6) | 9 (64.3) | 129 (59.4) |
| FLT3 | 29 (15.8) | 3 (6.7) | 4 (44.4) | 22 (17.1) |
| NPM1 | 30 (16.4) | 8 (17.8) | 3 (33.3) | 19 (14.7) |
| CEBPA | 45 (24.6) | 20 (44.4) | 1 (11.1) | 24 (18.6) |
| IDH1 | 15 (8.2) | 2 (4.4) | 2 (22.2) | 11 (8.5) |
| Other acquired mutation | 182 (99.5) | 44 (97.8) | 9 (100) | 129 (100) |
| Cytogenetic status, n (%) | 285 (98.6) | 56 (96.6) | 14 (100) | 215 (99.1) |
| Abnormal karyotype | 135 (47.4) | 21 (37.5) | 2 (14.3) | 112 (52.1) |
| FAB classification,23 n (%) | 229 (79.2) | 56 (96.6) | 11 (78.6) | 162 (74.7) |
| M0 | 0 | 0 | 0 | 0 |
| M1 | 2 (0.9) | 2 (3.6) | 0 | 0 |
| M2 | 87 (38.0) | 30 (53.6) | 1 (9.1) | 56 (34.6) |
| M4 | 34 (14.8) | 5 (8.9) | 2 (18.2) | 27 (16.7) |
| M5 | 103 (45.0) | 19 (33.9) | 7 (63.6) | 77 (47.5) |
| M6 | 2 (0.9) | 0 | 1 (9.1) | 1 (0.6) |
| M7 | 0 | 0 | 0 | 0 |
| Type of AML | ||||
| De novo | 287 (99.3) | 58 (100) | 14 (100) | 215 (99.1) |
| Secondary | 2 (0.7) | 0 | 0 | 2 (0.9) |
| Risk stratification7c | 254 (87.9) | 55 (94.8) | 14 (100) | 185 (85.3) |
| Favorable | 88 (34.6) | 31 (56.4) | 5 (35.7) | 52 (28.1) |
| Intermediate | 152 (59.8) | 23 (41.8) | 9 (64.3) | 120 (64.9) |
| Adverse | 14 (5.5) | 1 (1.8) | 0 | 13 (7.0) |
Notes: Data are mean (SD), unless otherwise stated. aGenetic testing results were not available for patients with unknown FLT3 mutation status at initial AML diagnosis; bGenetic testing results at initial diagnosis were summarized among patients with relevant information at initial diagnosis; cEach patient was classified into only one risk category.
Abbreviations: AML, acute myeloid leukemia; CEBPA, CCAAT enhancer binding protein alpha; FAB, French-American-British; FLT3, FMS-like tyrosine kinase-3; IDH, isocitrate dehydrogenase; MUT, mutated; ND, newly diagnosed; NPM1, nucleophosmin 1; R/R, relapsed or refractory; SD standard deviation, WT, wild-type, y, years.
During the study period, 289 (33.9%) patients progressed from ND to R/R AML, including 234 (27.4%) who relapsed after initial treatment and 55 (6.4%) who were refractory to initial treatment (Tables 1b and 2). Seventy-two (24.9%) patients with R/R AML received FLT3 mutation testing at first diagnosis of R/R AML, of whom 58 had FLT3WT and 14 had FLT3MUT disease; the FLT3 mutation status was unknown for the remaining 217 patients with R/R AML (Table 2).
Table 2.
Prevalence, Method, and Outcome of FLT3 Mutation Testing for Patients with AML
| Key Epidemiologic Characteristic, n (%) | All ND AML Patients N = 853 | All R/R AML Patients N = 289 |
|---|---|---|
| Progression from ND to R/R AML | 289 (33.9) | – |
| Relapsed after initial treatment | 234 (27.4) | – |
| Refractory to initial treatment | 55 (6.4) | – |
| FLT3 mutation testing | ||
| At initial AML diagnosis | 541 (63.4) | – |
| At first R/R diagnosis | – | 72 (24.9) |
| NGS testing method | 541 (100) | 72 (100) |
| Other testing method | 0 | 0 |
| FLT3MUT positivity among tested patientsa | ||
| At initial AML diagnosis | 109 (20.1) | – |
| FLT3-ITD mutation | 53 (48.6) | – |
| FLT3-TKD mutation | 32 (29.4) | – |
| Unspecified FLT3 mutation | 26 (23.9) | – |
| At first R/R AML diagnosis | – | 14 (19.4) |
| FLT3-ITD mutation | – | 9 (64.3) |
| FLT3-TKD mutation | – | 6 (42.9) |
| Unspecified FLT3 mutation | – | 1 (7.1) |
| FLT3 testing at both initial AML and first R/R diagnoses | 54 (18.7) | |
| Lost FLT3 mutation at first R/R | – | 3 (5.6) |
| Gained FLT3 mutation at first R/R | – | 5 (9.3) |
| FLT3-ITD vs FLT3-TKD mutation switch at first R/R | – | 3 (5.6) |
| From FLT3-ITD to FLT3-TKD | – | 1 (33.3) |
| From FLT3-TKD to FLT3-ITD | – | 2 (66.7) |
Notes: aPatients could harbor both FLT3-ITD and FLT3-TKD mutations, therefore, the percentages of each FLT3 mutation type may not add up to 100%.
Abbreviations: AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase-3; ITD, internal tandem duplication; MUT, mutated; ND, newly diagnosed; NGS, next generation sequencing; R/R, relapsed or refractory; TKD, tyrosine kinase domain.
By study end, the cross-sectional distribution of patients with ND, relapsed, and refractory (at any point during the study) AML was 66.1% (564/853), 25.4% (217/853), and 8.4% (72/853), respectively.
FLT3 Mutation Testing
The proportion of ND AML patients tested for FLT3 mutation status increased year-on-year, from 29% in 2016 to 87% in 2017, 89% in 2018, and 96% in 2019. A similar pattern of increased FLT3 mutation testing was observed for patients with R/R AML, although the overall proportion tested remained lower than for ND AML (3% in 2016, 19% in 2017, 16% in 2018, and 53% in 2019).
FLT3 Mutations
Among patients with any FLT3MUT at initial diagnosis, the proportions with FLT3-ITD, FLT3-TKD, and unspecified FLT3 mutations were 48.6%, 29.4%, and 23.9%, respectively. The corresponding proportions among patients with any FLT3MUT at first diagnosis of R/R disease were 64.3%, 42.9%, and 7.1% (Table 2).
Among patients who received FLT3 testing at both initial diagnosis and first diagnosis of R/R AML, 5.6% and 9.3% of patients lost and gained FLT3MUT at first diagnosis of R/R AML, respectively. Additionally, 5.6% of patients switched from FLT3-ITD to FLT3-TKD or vice versa at first diagnosis of R/R AML.
Treatment Patterns and Responses
Newly Diagnosed and Relapsed/Refractory AML
The most common regimen (70.6%; 602/853) for first-line induction therapy in patients with ND AML was cytarabine plus daunorubicin. Following first-line induction therapy, 87.1% (743/853) of patients received consolidation therapy, most commonly high-dose cytarabine (63.1%; 469/743) (Table 3a). On average, patients received one treatment cycle per induction regimen and one to three treatment cycles per consolidation regimen. For patients with R/R AML, induction therapy with cytarabine plus daunorubicin was the most common regimen (73.7%; 213/289), while high-dose cytarabine comprised the most common consolidation therapy (58.3%; 134/230) (Table 3b).
Table 3.
(a) First-Line Treatments Recorded for Patients with ND AML and (b) Treatments Recorded Prior to First Diagnosis of R/R AML in Patients with R/R AML
| (a) | ||||||||
| Therapy | All Patients with ND AML (N = 853) | FLT3WT at Initial Diagnosis (N = 432) | FLT3MUT at Initial Diagnosis (N = 109) | Unknown FLT3 Mutation Status (N = 312) | ||||
| Patients,a n (%) | Mean (SD) Ara-C Dose (mg/m2), Cycles | Patients, n (%) | Mean (SD) Ara-C Dose (mg/m2), Cycles | Patients, n (%) | Mean (SD) Ara-C Dose (mg/m2), Cycles | Patients, n (%) | Mean (SD) Ara-C Dose (mg/m2), Cycles | |
| Induction | 853 (100) | 432 (100) | 109 (100) | 312 (100) | ||||
| DAb | 602 (70.6) | 166.1 (289.5), 1.0 (0.1) | 341 (78.9) | 154.3 (232.3), 1.0 (0.0) | 91 (83.5) | 147.8 (222.4), 1.0 (0.1) | 170 (54.5) | 199.3 (401.5), 1.0 (0.0) |
| IAc | 133 (15.6) | 118.8 (16.5), 1.1 (0.3) | 92 (21.3) | 117.9 (15.7), 1.1 (0.3) | 26 (23.9) | 119.9 (18.6), 1.1 (0.4) | 15 (4.8) | 122.8 (18.1), 1.0 (0.0) |
| HAD | 126 (14.8) | 233.1 (362.9), 1.0 (0.2) | 4 (0.9) | 120.7 (8.6), 1.0 (0.0) | 2 (1.8) | 119.3 (3.1), 1.0 (0.0) | 120 (38.5) | 238.7 (370.9), 1.0 (0.2) |
| MA | 61 (7.2) | 107.4 (22.8), 1.0 (0.0) | 30 (6.9) | 106.6 (25.6), 1.0 (0.0) | 5 (4.6) | 126.2 (10.3), 1.0 (0.0) | 26 (8.3) | 104.7 (19.7), 1.0 (0.0) |
| Otherd | 140 (16.4) | – | 83 (19.2) | – | 25 (22.9) | – | 40 (12.8) | – |
| Consolidation | 743 (87.1) | 371 (85.9) | 97 (89.0) | 275 (88.1) | ||||
| HiDAC | 469 (63.1) | 6004.0 (323.3), 2.6 (0.6) | 245 (66.0) | 6021.1 (322.7), 2.6 (0.7) | 62 (63.9) | 6042.8 (235.0), 2.6 (0.7) | 162 (58.9) | 5964.4 (348.5), 2.7 (0.6) |
| MA | 236 (31.8) | 495.4 (979.4), 1.9 (0.8) | 78 (21.0) | 140.5 (303.3), 1.4 (0.6) | 14 (14.4) | 229.9 (445.7), 1.4 (0.5) | 144 (52.4) | 659.5 (1134.4), 2.1 (0.9) |
| DA | 216 (29.1) | 582.8 (1083.4), 1.6 (0.5) | 92 (24.8) | 138.1 (250.6), 1.8 (0.5) | 20 (20.6) | 119.0 (15.1), 1.7 (0.5) | 104 (37.8) | 1131.6 (1416.8), 1.5 (0.5) |
| MiDAC | 124 (16.7) | 2346.1 (799.6), 1.9 (0.5) | 71 (19.1) | 2314.8 (811.5), 2.0 (0.6) | 13 (13.4) | 2395.0 (805.6), 1.8 (0.4) | 40 (14.5) | 2386.9 (785.5), 1.9 (0.4) |
| HA | 108 (14.5) | 112.9 (36.2), 1.7 (0.6) | 52 (14.0) | 113.1 (48.8), 1.7 (0.5) | 7 (7.2) | 119.3 (10.6), 1.7 (0.5) | 49 (17.8) | 111.8 (19.8), 1.8 (0.6) |
| Ara-C+HDAC | 67 (9.0) | 5851.6 (526.5), 2.0 (0.7) | 41 (11.1) | 5921.0 (404.8), 1.8 (0.7) | 8 (8.2) | 6044.8 (149.9), 1.9 (0.8) | 18 (6.5) | 5651.0 (729.2), 2.2 (0.7) |
| Decitabine | 62 (8.3) | -, 2.5 (1.5) | 33 (8.9) | -, 2.5 (1.6) | 5 (5.2) | -, 3.4 (1.7) | 24 (8.7) | -, 2.1 (1.0) |
| IA | 62 (8.1) | 183.8 (325.9), 1.1 (0.2) | 47 (12.7) | 167.1 (266.5), 1.0 (0.2) | 8 (8.2) | 116.5 (18.5), 1.0 (0.0) | 7 (2.5) | 351.3 (663.1), 1.2 (0.5) |
| AA | 60 (8.1) | 105.8 (45.3), 1.5 (0.5) | 34 (9.2) | 113.1 (52.5), 1.5 (0.5) | 6 (6.2) | 115.8 (23.1), 1.7 (0.5) | 20 (7.3) | 90.1 (32.7), 1.5 (0.5) |
| Otherd | 234 (31.5) | - | 117 (31.5) | - | 20 (20.6) | - | 75 (27.3) | - |
| HSCT | ||||||||
| Allogeneic | 62 (7.3) | - | 32 (7.4) | - | 12 (11.0) | - | 18 (5.8) | - |
| Autologous | 1 (0.1) | - | 1 (0.2) | - | 0 | - | 0 | - |
| (b) | ||||||||
| Therapy | All Patients with R/R AML (N = 289) | FLT3WT at R/R Diagnosis (N = 58) | FLT3MUT at R/R Diagnosis (N = 14) | Unknown FLT3 Mutation Status (N = 217) | ||||
| Patients,a n (%) | Mean (SD) Ara-C Dose (mg/m2), Cycles | Patients, n (%) | Mean (SD) Ara-C Dose (mg/m2), Cycles | Patients, n (%) | Mean (SD) Ara-C Dose (mg/m2), Cycles | Patients, n (%) | Mean (SD) Ara-C Dose (mg/m2), Cycles | |
| Induction | 289 (100) | 58 (100) | 14 (100) | 217 (100) | ||||
| DAb | 213 (73.7) | 182 (355.3), 1.0 (0.0) | 48 (82.8) | 201.8 (332.5), 1.0 (0.0) | 10 (71.4) | 121.1 (8.1), 1.0 (0.0) | 155 (71.4) | 180.6 (373.8), 1.0 (0.0) |
| IAc | 50 (17.3) | 116.6 (15.3), 1.2 (0.4) | 7 (12.1) | 112.0 (6.0), 1.2 (0.5) | 2 (14.3) | 121.7 (8.0), 1.0 (0.0) | 41 (18.9) | 117.2 (16.6), 1.2 (0.4) |
| HAD | 43 (14.9) | 199.3 (308.8), 1.0 (0.2) | 3 (5.2) | 104.4 (8.2), 1.0 (0.0) | 2 (14.3) | 111.0 (9.7), 1.0 (0.0) | 38 (17.5) | 208.7 (323.2), 1.1 (0.2) |
| MA | 30 (10.4) | 109.8 (20.1), 1.0 (0.0) | 6 (10.3) | 110.9 (17.9), 1.0 (0.0) | 1 (7.1) | 104.1 (-), 1.0 (-) | 23 (10.6) | 109.7 (21.5), 1.0 (0.0) |
| D-AA | 16 (5.5) | 48.5 (16.7), 1.1 (0.3) | 0 | - | 0 | - | 16 (7.4) | 48.5 (16.7), 1.1 (0.3) |
| Otherd | 33 (11.4) | - | 4 (6.9) | - | 1 (7.1) | - | 26 (12.0) | - |
| Consolidation | 230 (79.6) | 55 (94.8) | 12 (85.7) | 163 (75.1) | ||||
| HiDAC | 134 (58.3) | 5979.1 (325.1), 2.7 (0.6) | 35 (63.6) | 5980.4 (315.4), 2.7 (0.6) | 8 (66.7) | 5985.1 (301.4), 2.8 (0.4) | 91 (55.8) | 5977.9 (332.5), 2.6 (0.7) |
| MA | 80 (34.8) | 528.0 (1015.6), 1.8 (0.8) | 14 (25.5) | 273.6 (662.1), 1.7 (0.9) | 4 (33.3) | 112.6 (9.2), 1.4 (0.5) | 62 (38.0) | 592.0 (1079.9), 1.8 (0.7) |
| DAb | 68 (29.6) | 655.5 (1146.5), 1.6 (0.6) | 15 (27.3) | 250.3 (589.8), 1.6 (0.5) | 0 | - | 53 (32.5) | 768.1 (1238.0), 1.6 (0.6) |
| MiDAC | 43 (18.7) | 2175.4 (724.6), 1.9 (0.5) | 10 (18.2) | 1961.5 (172.2), 2.1 (0.4) | 1 (8.3) | 4165.8 (-), 2.0 (-) | 32 (19.6) | 2185.4 (759.5), 1.8 (0.5) |
| HA | 34 (14.8) | 121.8 (37.7), 1.7 (0.6) | 9 (16.4) | 148.5 (50.7), 1.9 (0.3) | 1 (8.3) | 117.8 (-), 2.0 (-) | 24 (14.7) | 111.0 (26.0), 1.6 (0.6) |
| Decitabine | 20 (8.7) | -, 1.9 (1.1) | 3 (5.5) | -, 1.0 (0.0) | 1 (8.3) | -, 3.0 (-) | 16 (9.8) | -, 1.8 (1.1) |
| Ara-C+HDAC | 18 (7.8) | 5915.5 (397.5), 1.8 (0.6) | 2 (3.6) | 5613.8 (284.9), 1.0 (0.0) | 1 (8.3) | 6095.8 (-), 1.0 (-) | 15 (9.2) | 5932.4 (406.3), 1.9 (0.6) |
| AA | 17 (7.4) | 106.3 (52.8), 1.6 (0.5) | 3 (5.5) | 175.4 (49.4), 1.8 (0.4) | 0 | - | 14 (8.6) | 88.1 (36.7), 1.5 (0.5) |
| IAc | 14 (6.1) | 135.5 (61.1), 1.0 (0.0) | 4 (7.3) | 187.5 (50.1), 1.0 (0.0) | 0 | - | 10 (6.1) | 114.7 (53.7), 1.0 (0.0) |
| Otherd | 60 (26.1) | - | 11 (20.0) | - | 1 (8.3) | - | 34 (20.9) | - |
| HSCT | - | |||||||
| Allogeneic | 12 (4.2) | - | 1 (1.7) | - | 2 (14.3) | - | 9 (4.1) | - |
| Autologous | 0 | - | 0 | - | 0 | - | 0 | - |
Notes: aTreatment information was summarized for each regimen and categorized by therapy type. This column summarizes the number of patients who ever received a specific treatment regimen. A patient receiving multiple treatment regimens under one treatment type would be counted for each received treatment regimen; therefore, the percentages under each treatment type may not add up to 100%; bDA regimen included DA 7+3, DA 5+2, and other DA. DA 7+3 refers to cytarabine daily for 7 days and daunorubicin daily for 3 days. DA 5+2 refers to cytarabine daily for 5 days and daunorubicin daily for 2 days; cIA regimen included IA 7+3, IA 5+2, and other IA. IA 7+3 refers to cytarabine daily for 7 days and idarubicin daily for 3 days. IA 5+2 refers to cytarabine daily for 5 days and idarubicin daily for 2 days; dOther refers to a combined group of individual regimens used by <5% of patients.
Abbreviations: AA, cytarabine and aclarubicin; AML, acute myeloid leukemia; Ara-C, cytarabine; Ara-C+HDAC, cytarabine and histone deacetylase; DA, daunorubicin and cytarabine; D-AA, decitabine, cytarabine, and aclarubicin; FLT3, FMS-like tyrosine kinase-3; HA, homoharringtonine and cytarabine; HAD, homoharringtonine, cytarabine, and daunorubicin; HSCT, hematopoietic stem-cell transplantation; HiDAC, high-dose cytarabine; IA, idarubicin and cytarabine; MA, cytarabine and mitoxantrone; MiDAC, mid-dose cytarabine; MUT, mutated; ND, newly diagnosed; SD, standard deviation; WT, wild type.
Following first-line induction, 88.0% (751/853) of patients with ND AML achieved CR or CRi; 91.8% (682/743) maintained CR/CRi following consolidation therapy. Among patients with ND AML who received HSCT (7.4%, 63/853), 85.7% (54/63) achieved CR/CRi (Table 4a). Among all patients with ND AML who received first-line treatment, 27.4% (234/853) relapsed following CR/CRi.
Table 4.
First-Line Treatment Response Among (a) Patients with Newly Diagnosed AML and (b) All Patients with R/R AML, and Those with R/R AML and FLT3WT, FLT3MUT, and Unknown FLT3 Mutation Status
| (a) | |||||
| Therapy | All newly Diagnosed Patients (N = 853) | ||||
| Patients Who Received Treatment(s), n (%) | Treatment Response, n (%) | Relapsed, n (%) | Refractory, n (%) | ||
| CR/CRi Not Yet Achieved/Observed | Ever Achieved CR/CRi | ||||
| Induction therapy | 853 (100) | 102 (12.0) | 751 (88.0) | 234 (27.4) | 55 (6.4) |
| Consolidation therapy | 743 (87.1) | 61 (8.2) | 682 (91.8) | ||
| HSCT | 63 (7.4) | 9 (14.3) | 54 (85.7) | ||
| (b) | |||||
| Therapy | All R/R diagnosed patients (N = 289) | ||||
| Patients who received treatment(s), n (%) | Treatment response, n (%) | Time from treatment initiation to first relapse after initial treatment (days), mean (SD) [median (IQR)] | Time from first documented CR/CRi to first relapse after initial treatment (days), mean (SD) [median (IQR)] | ||
| CR/CRi not yet achieved/observed | Ever achieved CR/CRi | ||||
| Induction therapy | 289 (100) | 59 (20.4) | 230 (79.6) | 358.0 (241.5) [306.5 (254.2)] | 314.3 (242.6) [252.0 (268.0)] |
| Consolidation therapy | 230 (79.6) | 29 (12.6) | 201 (87.4) | ||
| HSCT | 12 (4.2) | 4 (33.3) | 8 (66.7) | ||
| Therapy | FLT3WT (N = 58) | ||||
| Patients who received treatment(s), n (%) | Treatment response, n (%) | Time from treatment initiation to first relapse after initial treatment (days), mean (SD), [median (IQR)] | Time from the first documented CR/CRi to the first relapse after the initial treatment (days), mean (SD) [median (IQR)] | ||
| CR/CRi not yet achieved/observed | Ever achieved CR/CRi | ||||
| Induction therapy | 58 (100) | 3 (5.2) | 55 (94.8) | 366.9 (220.3) [331.5 (235.8)] | 321.8 (220.1) [291.0 (212.8)] |
| Consolidation therapy | 55 (94.8) | 4 (7.3) | 51 (92.7) | ||
| HSCT | 1 (1.7) | 0 (0) | 1 (100) | ||
| Therapy | FLT3MUT at R/R (N = 14) | ||||
| Patients who received treatment(s), n (%) | Treatment response, n (%) | Time from treatment initiation to first relapse after initial treatment (days), mean (SD), [median (IQR)] | Time from first documented CR/CRi to first relapse after initial treatment (days), mean (SD), [median (IQR)] | ||
| CR/CRi not yet achieved/observed | Ever achieved CR/CRi | ||||
| Induction therapy | 14 (100) | 2 (14.3) | 12 (85.7) | 320.0 (172.5) [294.0 (123.8)] | 271.0 (174.3) [242.0 (126.0)] |
| Consolidation therapy | 12 (85.7) | 1 (8.3) | 11 (91.7) | ||
| HSCT | 2 (14.3) | 2 (100) | 0 (0) | ||
| Therapy | Unknown FLT3 mutation status at R/R (N = 217) | ||||
| Patients who received treatment(s), n (%) | Treatment response, n (%) | Time from treatment initiation to first relapse after initial treatment (days), mean (SD), [median (IQR)] | Time from first documented CR/CRi to first relapse after initial treatment (days), mean (SD), [median (IQR)] | ||
| CR/CRi not yet achieved/observed | Ever achieved CR/CRi | ||||
| Induction therapy | 217 (100) | 54 (24.9) | 163 (75.1) | 357.8 (252.7) [293.0 (283.0)] | 314.9 (254.3) [235.0 (292.0)] |
| Consolidation therapy | 163 (75.1) | 24 (14.7) | 139 (85.3) | ||
| HSCT | 9 (4.1) | 2 (22.2) | 7 (77.9) | ||
Abbreviations: AML, acute myeloid leukemia; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; HSCT, hematopoietic stem-cell transplantation; FLT3, FMS-like tyrosine kinase-3; HSCT, hematopoietic stem-cell transplantation; MUT, mutated; R/R, relapsed/refractory; SD, standard deviation; WT, wild type.
Overall, patients with R/R AML received a median of two lines of therapy, with 82.0% (237/289) patients receiving second-line therapy (Table S1). The most common second-line regimens in the R/R AML setting were cytarabine plus mitoxantrone (24.5%; 58/237), decitabine, cytarabine plus aclarubicin (22.8%; 54/237), cytarabine plus aclarubicin (16.9%; 40/237), daunorubicin plus cytarabine (13.5%; 32/237), homoharringtonine plus cytarabine (12.2%; 29/237), decitabine (11.4%; 27/237) and fludarabine, cytarabine plus granulocyte-colony stimulating factor (11.0%; 26/237). Third- and fourth-line therapy were received by, respectively, 41 (14.2%) and 15 (5.2%) patients in the R/R AML setting.
For patients who relapsed during the study period after achieving CR/CRi with first-line therapy, median (IQR) times from treatment initiation and first documented CR/CRi to the first relapse were 306.5 (254.2) and 252.0 (268.0) days, respectively (Table 4b). Approximately half (48.9%; 116/237) of patients with R/R AML achieved CR/CRi following second-line therapy, and 26.8% (11/41) and 26.7% (4/15) achieved CR/CRi following third- and fourth-line therapy.
Treatment Regimens and Patterns by FLT3 Mutation Status
Initial treatment regimens recorded for patients with ND AML did not appear to be influenced by FLT3 mutation status: 78.9% (341/432) and 83.5% (91/109) of patients with FLT3WT and FLT3MUT received cytarabine plus daunorubicin induction therapy, respectively (Table 3a). However, differences in treatment patterns for patients with ND AML were noted between those with known and unknown FLT3 mutation status: specifically, a higher proportion of patients with confirmed FLT3MUT received cytarabine plus daunorubicin induction therapy than those with an unknown FLT3 mutation status (83.5% [91/109] vs 54.5% [170/312]; Table 3a). For patients with ND AML, there were no notable differences in treatment responses between patients with FLT3MUT or FLT3WT AML, or for patients with unknown FLT3 mutation status at initial diagnosis.
Following R/R AML diagnosis, there were apparent differences in the choice of second-line treatment regimens according to FLT3 mutation status; however, the number of patients with FLT3MUT AML included was low (n = 14), and it is therefore unclear whether these differences are meaningful. Among patients with R/R AML who relapsed after achieving CR/CRi with first-line therapy, those with FLT3MUT AML had a shorter median (IQR) time from treatment initiation to first relapse than patients with FLT3WT AML (294.0 [123.8] vs 331.5 [235.8] days) and shorter median (IQR) time from CR/CRi to first relapse (242.0 [126.0] vs 291.0 [212.8] days) (Table 4b).
Discussion
This study presents a real-world investigation of the current epidemiology and treatment patterns among adult patients with AML stratified by FLT3 mutation status in China. In our analysis of patient records from the NICHE-AML registry of the IHBDH in China, approximately one-third of patients progressed from ND to R/R AML during the study period, and the prevalence of FLT3MUT was approximately 20% among both patients with ND AML and those with R/R AML. The presence of FLT3MUT did not appear to influence the choice of initial treatment selected for patients in this registry, with DA and HiDAC being the mainstay induction and consolidation regimens, respectively, consistent with clinical practice guidelines in China for AML.6 For patients with R/R AML, there was an apparent difference in the second-line treatment regimens for patients with FLT3WT and FLT3MUT, although the most common regimens in both cases were still cytarabine-based, which are standard in this setting.24,25
In the NICHE-AML registry, testing for FLT3 mutation status increased from 2016 to 2019. These findings reflect the recommendations included in AML clinical practice guidelines in China, which have suggested routine testing for FLT3 mutation at the time of disease diagnosis since 2017.26 The increased occurrence of FLT3 mutation testing could also reflect the availability of FLT3-inhibitor agents. Approximately half (48.6%) of the patients with FLT3MUT at ND AML diagnosis harbored a FLT3-ITD mutation, while this percentage increased to 64.3% for patients who progressed to R/R AML; the corresponding proportions for those with FLT3-TKD were 29.4% in ND AML and 42.9% in R/R AML. However, it should be noted that the type of FLT3 mutation was unspecified for almost one-quarter (23.9%) of patients at ND diagnosis. The proportions of ND AML patients with either FLT3-ITD or FLT3-TKD in the NICHE-AML registry were both higher than anticipated from the published literature.13,14 In addition, there appears to be mounting evidence in favor of repeated mutational testing at diagnosis and at each relapse, since the emergence and type of mutation can greatly influence outcomes and, therefore, the choice of treatment.27 Our results further support the need for repeated testing, as we observed that at first diagnosis of R/R AML, 5.6% and 9.3% of patients lost and gained FLT3MUT, respectively, with a further 5.6% of patients switching between FLT3 mutations.
We observed differences in the clinical outcomes of patients with AML depending on FLT3 mutation status. In particular, FLT3MUT carriers had a shorter time to relapse than those with FLT3WT, from both initial diagnosis and CR/CRi; this is consistent with previous studies that have reported worse clinical outcomes for patients with a FLT3 mutation, particularly FLT3-ITD.11
A key strength of this study is the information system at IHBDH, one of the largest hematology centers in China that provides the highest level of care to patients with AML, which has been established for more than 20 years and includes comprehensive data; from this, the NICHE-AML dataset provided an adequate sample size of 853 patients with median follow-up of 425 days. While the registry comprises patients from IHBDH, a single hospital in Northern China, patients are referred there from other regions, thus the cohort covers a large geographical area.
Nevertheless, our study has a number of limitations, some of which are inherent to retrospective studies, which should be considered when interpreting the data. Guidelines for the diagnosis of AML in China in 2017 have included testing for FLT3-ITD mutations;26 however, FLT3 testing may have had limited availability for patients who initiated on treatment between 2015 and 2017. The mean age of patients at initial diagnosis (42.5 years) was younger than the median age of diagnosis in US populations,28 and all patients received intensive treatment; as older patients may have different disease trajectories and/or could only receive supportive care, the generalizability of these findings to AML patients may be limited outside of China. The study variables available for analysis were restricted to those recorded in the registry, and some relevant data, such as reasons for treatment discontinuation or switching, were not included and could not be retrieved retrospectively. The data are limited to patients with R/R AML owing to the small sample size; as such, these results should be interpreted with caution. Due to the observational nature of the study, no formal hypothesis testing was performed and all analyses were descriptive. The NICHE-AML registry comprises only passive follow-up data relating to hospital visits to IHBDH and lacks systematic follow-up data for survival, disease progression and longitudinal treatment patterns, and treatments received at other hospitals. It should be noted that a thorough review was also performed to exclude patients with long gaps between visits or a short follow-up period; however, this approach might introduce bias, as patients’ adherence may be associated with certain characteristics such as socioeconomic status and age. Genetic/molecular marker testing has formed part of the standard of care at IHBDH since 2017; therefore, the proportion of patients who underwent FLT3 mutation testing in the present study may be higher than that in general practice in China, as testing occurs routinely only in larger hematological centers. Despite this, the proportion of patients with known FLT3 mutation status at first diagnosis of R/R AML was still relatively low, at approximately one quarter, and observations relating to this population should thus be considered provisional. While we observed that FLT3 testing increased during the study period, by 2019 only approximately half of patients were being tested at diagnosis with R/R AML, versus essentially all patients being tested at ND AML.
Conclusion
In conclusion, we found that one-fifth of patients tested harbored an FLT3 mutation and that FLT3MUT was associated with poorer clinical outcomes. However, we also observed that many patients with AML were not tested for FLT3 mutations, particularly at first diagnosis of R/R AML. Furthermore, treatment regimens were not significantly influenced by FLT3 status; this may be attributed to the fact that the first FLT3 inhibitor, gilteritinib, only received regulatory approval in China in 2021.18 Taken together, our results highlight a need for more routine testing of FLT3 mutation status at R/R AML, and utilization of more targeted treatment options for those patients possessing FLT3 mutations (eg, FLT3 inhibitor agents) in China.
Acknowledgments
The study was sponsored by Astellas Pharma Singapore Pte. Ltd. Medical writing support was provided by Rhian Harper Owen, PhD, from Lumanity, who assisted in drafting the manuscript under the direction of the authors and provided editorial support throughout its development. Editorial support was funded by Astellas Pharma, Inc.
Funding Statement
The study was sponsored by Astellas Pharma Singapore Pte. Ltd. Medical writing support and editorial support were funded by Astellas Pharma, Inc.
Abbreviations
Allo-HSCT, allogeneic hematopoietic; AML, acute myeloid leukemia; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; FDA, US Food and Drug Administration; FLT3, FMS-like tyrosine kinase 3; FLT3-ITD, FMS-like tyrosine kinase 3 internal tandem duplication; FLT3MUT, FMS-like tyrosine kinase 3 mutated; FLT3-TKD, FMS-like tyrosine kinase 3 tyrosine kinase domain; FLT3WT, FMS-like tyrosine kinase 3 wild-type; IHBDH, Institute of Hematology and Blood Diseases Hospital; IQR, interquartile range; ND, newly diagnosed; NICHE, National Longitudinal Cohort of Hematologic Diseases; R/R, relapsed/refractory; SD, standard deviation.
Data Sharing Statement
Researchers may request access to anonymized participant-level data, trial-level data and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki and International Conference of Harmonisation guidelines. The study was approved by the institutional review board committee of the IHBDH. The review board committee confirmed that informed consent was not needed from participants, and all data were anonymized prior to the current study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Li-Jen Cheng and Prabhuram Krishnan are employees of Astellas Pharma Singapore Pte. Ltd. Christopher H. Young is an employee of Astellas Pharma US Inc. Jia Zhong and Eric Q. Wu are employees of Analysis Group, Inc., an HEOR CRO company contracted by Astellas to undertake analysis. Jianxiang Wang participated in an advisory board for AbbVie. The authors report no other conflicts of interest in this work.
References
- 1.Chen B-A, Huang Z-H, Zhang X-P, et al. An epidemiological investigation of leukemia incidence between 2003 and 2007 in Nanjing, China. J Hematol Oncol. 2010;3:21. doi: 10.1186/1756-8722-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yi M, Li A, Zhou L, Chu Q, Song Y, Wu K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017. J Hematol Oncol. 2020;13(1):72. doi: 10.1186/s13045-020-00908-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y, Shi O, Zeng Q, et al. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp Hematol Oncol. 2020;9:14. doi: 10.1186/s40164-020-00170-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thol F, Ganser A. Treatment of relapsed acute myeloid leukemia. Curr Treat Options Oncol. 2020;21(8):66. doi: 10.1007/s11864-020-00765-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newell LF, Cook RJ. Advances in acute myeloid leukemia. BMJ. 2021;375:n2026. doi: 10.1136/bmj.n2026 [DOI] [PubMed] [Google Scholar]
- 6.Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guidelines for the diagnosis and treatment of relapsed/refractory acute myelogenous leukemia (2021). Zhonghua Xue Ye Xue Za Zhi. 2021;42(8):624–627. doi: 10.3760/cma.j.issn.0253-2727.2021.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guidelines for the diagnosis and treatment of adult acute myeloid leukemia (not APL) (2021). Zhonghua Xue Ye Xue Za Zhi. 2021;42(8):617–623. doi: 10.3760/cma.j.issn.0253-2727.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. doi: 10.1182/blood.2022016867 [DOI] [PubMed] [Google Scholar]
- 9.Thol F, Heuser M. Treatment for relapsed/refractory acute myeloid leukemia. HemaSphere. 2021;5(6):e572. doi: 10.1097/HS9.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy VE, Smith CC. FLT3 mutations in acute myeloid leukemia: key concepts and emerging controversies. Front Oncol. 2020;10:612880. doi: 10.3389/fonc.2020.612880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perl AE, Hosono N, Montesinos P, et al. Clinical outcomes in patients with relapsed/refractory FLT3-mutated acute myeloid leukemia treated with gilteritinib who received prior midostaurin or sorafenib. Blood Cancer J. 2022;12(5):84. doi: 10.1038/s41408-022-00677-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitika, Wei J, Hui A-M. Role of biomarkers in FLT3 AML. Cancers. 2022;14(5):1164. doi: 10.3390/cancers14051164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R-Q, Chen C-J, Jing Y, et al. Characteristics and prognostic significance of genetic mutations in acute myeloid leukemia based on a targeted next-generation sequencing technique. Cancer Med. 2020;9(22):8457–8467. doi: 10.1002/cam4.3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–2784. doi: 10.1182/blood-2007-08-109090 [DOI] [PubMed] [Google Scholar]
- 16.Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters—an analysis of 3082 patients. Blood. 2008;111(5):2527–2537. doi: 10.1182/blood-2007-05-091215 [DOI] [PubMed] [Google Scholar]
- 17.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Astellas Pharma Inc. Astellas’ XOSPATA® (gilteritinib) receives conditional approval by china’s national medical products administration for relapsed or refractory acute myeloid leukemia with a FLT3 mutation. Available from: https://newsroom.astellas.us/2021-02-03-Astellas-XOSPATA-R-gilteritinib-Receives-Conditional-Approval-by-Chinas-National-Medical-Products-Administration-for-Relapsed-or-Refractory-Acute-Myeloid-Leukemia-with-A-FLT3-Mutation. Accessed September 22, 2022.
- 19.Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740. doi: 10.1056/NEJMoa1902688 [DOI] [PubMed] [Google Scholar]
- 20.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464. doi: 10.1056/NEJMoa1614359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312. doi: 10.1038/s41375-018-0357-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin JD, Yang H, Song Y, Kinrich D, Shah MV, Bui CN. Treatment patterns and healthcare resource utilization in patients with FLT3-mutated and wild-type acute myeloid leukemia: a medical chart study. Eur J Haematol. 2019;102(4):341–350. doi: 10.1111/ejh.13205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD, Cassileth PA, Head DR, et al. Report of the national cancer institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8(5):813–819. doi: 10.1200/JCO.1990.8.5.813 [DOI] [PubMed] [Google Scholar]
- 24.Koenig K, Mims A. Relapsed or primary refractory AML: moving past MEC and FLAG-ida. Curr Opin Hematol. 2020;27(2):108–114. doi: 10.1097/MOH.0000000000000561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Lv -T-T, Zhou X-F, Huang Y, Liu -D-D, Yuan G-L. Efficacy of common salvage chemotherapy regimens in patients with refractory or relapsed acute myeloid leukemia: a retrospective cohort study. Medicine. 2018;97(39):e12102. doi: 10.1097/MD.0000000000012102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of adult acute myeloid leukemia (not APL) (2017). Zhonghua Xue Ye Xue Za Zhi. 2017;38(3):177–182. doi: 10.3760/cma.j.issn.0253-2727.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daver N, Venugopal S, Ravandi F. FLT3 mutated acute myeloid leukemia: 2021 treatment algorithm. Blood Cancer J. 2021;11(5):104. doi: 10.1038/s41408-021-00495-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acute myeloid leukemia — cancer stat facts. Available from: https://seer.cancer.gov/statfacts/html/amyl.html. Accessed June 29, 2023.