Keywords: circadian, glucose, lipids, misalignment, shift work
Abstract
Circadian rhythms are endogenous oscillations with approximately a 24-h period that allow organisms to anticipate the change between day and night. Disruptions that desynchronize or misalign circadian rhythms are associated with an increased risk of cardiometabolic disease. This review focuses on the liver circadian clock as relevant to the risk of developing metabolic diseases including nonalcoholic fatty liver disease (NAFLD), insulin resistance, and type 2 diabetes (T2D). Many liver functions exhibit rhythmicity. Approximately 40% of the hepatic transcriptome exhibits 24-h rhythms, along with rhythms in protein levels, posttranslational modification, and various metabolites. The liver circadian clock is critical for maintaining glucose and lipid homeostasis. Most of the attention in the metabolic field has been directed toward diet, exercise, and rather little to modifiable risks due to circadian misalignment or disruption. Therefore, the aim of this review is to systematically analyze the various approaches that study liver circadian pathways, targeting metabolic liver diseases, such as diabetes, nonalcoholic fatty liver disease, using human, rodent, and cell biology models.
NEW & NOTEWORTHY Over the past decade, there has been an increased interest in understanding the intricate relationship between circadian rhythm and liver metabolism. In this review, we have systematically searched the literature to analyze the various experimental approaches utilizing human, rodent, and in vitro cellular approaches to dissect the link between liver circadian rhythms and metabolic disease.
CIRCADIAN SYSTEM
Circadian rhythms are endogenous oscillations with a period of ∼24 h. Such oscillations enable organisms to anticipate changes in the environment, and to tune responses by time of day. In mammals, the master circadian clock is located in the suprachiasmatic nucleus (SCN) in the hypothalamus, which is responsive to light signals transmitted from the retina by direct neural projections. The SCN master clock entrains clocks in peripheral tissues via autonomic and endocrine signals (1). At the molecular level, the clock consists of a transcription-translational feedback loop, with a periodicity of ∼24 h (2). The positive loop consists of core clock genes, circadian locomotor output cycles kaput (CLOCK), and Arnt-like protein (BMAL1) (Fig. 1) (3, 4). There are two negative loops, one is made up of Period 1/2/3(PER) and Cryptochrome 1/2 (CRY) genes (5, 6), and the other by the REVERB/ROR gene products that trans repress or trans activate the core clock gene BMAL1, respectively. This feedback loop operates to regulate a number of physiological processes including heart rate, hormone, and glucose levels (7, 8). Peripheral clocks can also be affected by other Zeitgebers (time cues) such as exercise and feeding. Disruptions that desynchronize or misalign circadian rhythms are linked with cardiometabolic disease (9, 10).
Figure 1.
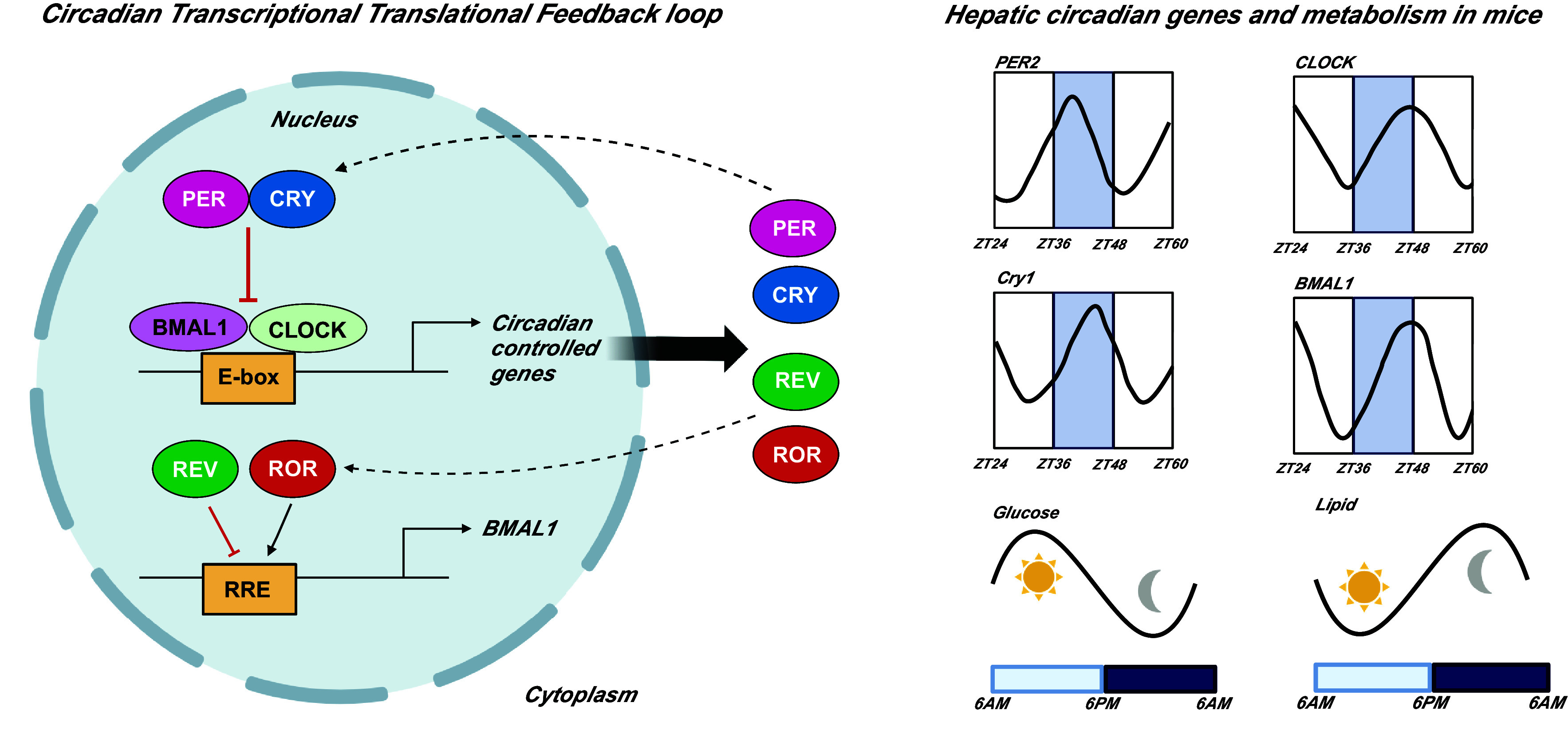
Circadian transcriptional translational feedback loop and hepatic circadian genes and metabolism in mice. Traces for circadian genes adapted from circaDB database. BMAL1, Arnt-like protein; CLOCK, circadian locomotor output cycles kaput; CRY, Cryptochrome 1/2; PER, Period 1/2/3; ZT, Zeitgeber time. Figure created using Biorender.com.
Liver Circadian Biology
In this review, we will focus on the liver circadian clock. Approximately 40% of the hepatic transcriptome oscillates with circadian characteristics (11), and in addition there are 24-h rhythms in protein abundance, posttranslational modification, and many metabolites also oscillate (12). The liver clock responds strongly to the timing of food intake (13–15), and the liver transcriptome is enriched with transcripts involved in cellular adaptation to nutrients (16). Therefore, there is strong evidence of a link between the liver clock machinery, and energy metabolism. Studies in both humans and rodents taken through a circadian challenge (alteration to sleep and feeding behavior) support an association between circadian misalignment and risk factors for, or the prevalence of, energy metabolic diseases including nonalcoholic fatty liver disease (NAFLD), insulin resistance, and type 2 diabetes (T2D).
Circadian regulation of liver glucose signaling.
The liver has a crucial role in regulating glucose homeostasis, and these regulatory circuits are also affected by circadian phase. This allows anticipation and tuned responses to variations in glucose supply and demand during the fed and fasted states (17). In the active phase (day for human, night for mouse), following consumption of a mixed meal, blood glucose rises, and the liver takes up glucose and utilizes it for glycolysis, or stores it as glycogen. In the rest, or fasting, phase the liver adapts to the change in energetic demand by increasing gluconeogenesis and glycogenolysis (18). Key rhythmic factors that play a role in these processes include: glucose transporter 2 (GLUT2), glycogen synthase (GYS), glycogen synthase kinase (GSK3), and the insulin receptor (InsR) (19, 20).
The rhythmic nature of liver glucose metabolism predicts that alterations to the liver clock have implications for glucose homeostasis. For example, genetic disruption of the circadian gene Bmal1 in mice resulted in a reduction in the liver glucose transporter (Glut2), leading to a decrease in postfeeding glucose uptake in mutant mice compared with wild-type mice (20). Cryptochromes (circadian gene) have also been associated with regulation of hepatic gluconeogenesis, affecting G protein-coupled receptor-dependent cAMP accumulation, and activation of cAMP response element-binding protein (CREB) (21). Overexpression of Cry1, specifically in the liver, resulted in lower blood glucose levels and increased insulin sensitivity in diabetic mice (22). Cryptochromes have also been found to serve as promiscuous repressors of nuclear receptor transactivation, notably the fasting-activated glucocorticoid receptor (GR), and so inhibiting fasting induction of phosphoenolpyruvate carboxykinase, a key gluconeogenic enzyme encoding gene (23, 24).
Circadian regulation of liver lipid signaling.
The hepatic circadian clock powerfully regulates lipid metabolism. Studies utilizing Per2, Clock, and REV-ERBα/β knockout models have revealed alterations to the diurnal levels of plasma levels of free fatty acids (also known as nonesterified fatty acids, NEFAs), triglycerides (TG), and cholesterol (25–27); the hepatic circadian machinery has been linked with critical steps of lipid metabolism. Genes involved in triglyceride (TG) synthesis (Gpat2, Agpat1/2, Lipin1/2, and Dgat2) are influenced by the circadian clock leading to a peak and trough of hepatic TG levels (in mice) during the rest and active phases, respectively (28). Hepatic circadian genes also participate in 1) fatty acid synthesis by controlling the expression of Elvol3, Elovl6, Fas (29), 2) regulating β oxidation and ketone body production (30), and 3) determining the expression of key lipid responses nuclear receptors (NRs), liver X receptors (LXRs) peroxisome proliferator-activated receptor (PPAR) (29, 31). The role of the circadian machinery in lipid metabolism has led to it being linked with hepatic steatosis, and has been extensively described elsewhere (32, 33). For a comprehensive overview of circadian genetic mouse studies investigating glucose and lipid signaling, see the study by Kalsbeek et al. (21).
Circadian Experimental Models: Human, Rodent, and Cellular
Most of the attention in the metabolic field has been directed toward diet, exercise, and rather little to modifiable risks due to circadian misalignment or disruption. Circadian misalignment is common in modern industrialized societies where artificial light exposure coupled with work and social demands often leads to environment and behavior running out of alignment with the internal, or circadian time (34). In fact, it is estimated that 20% of the working population is made up of shift workers, 33% of the population sleep less than 6 h per night (35), and 69% experience social jet lag (36). Therefore, developing a deeper understanding of circadian biology and how it relates to the regulation of hepatic glucose and lipid metabolism could have significant implications for identifying novel therapeutic approaches for metabolic disease.
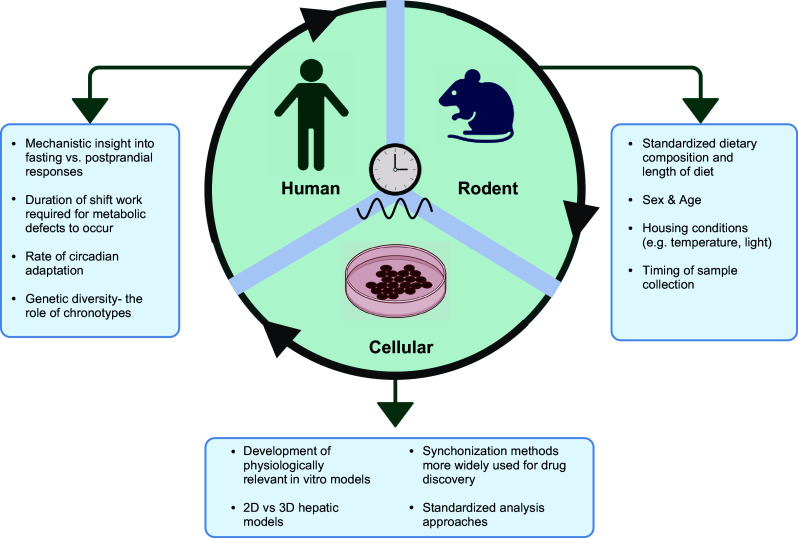
The aim of this review was to reconcile why there are discrepant findings between studies investigating circadian regulation of metabolism. To do this we have brought together information from studies that have utilized human, rodent, and cellular experimental approaches. A comprehensive review of the literature was carried out in April to July 2021 via the electronic database PubMed. The key questions asked were as follows: Human: 1) What are the effects of shiftwork on fasting glucose tolerance and insulin resistance? 2) What are the effects of experimental misalignment protocols in healthy subjects on fasting tolerance and insulin resistance? Rodent (hepatic clock): 1) What are the effects of a diabetic intervention in a rodent model on hepatic regulation of rhythmicity of core clock genes (CLOCK, BMAL1, PER1/2/3, and CRY1/2), glucose, and lipid metabolism-related genes? Hepatic cell biology in vitro systems: Do hepatic cell biology systems (liver slices, primary cells, cell lines) provide a viable experimental approach to assess circadian rhythmicity in diabetes?
A keyword search was conducted by using search terms for humans (night shift, glucose insulin, circadian, and misalignment’), rodents (circadian, circadian rhythm, diurnal, rodent, metabolism, diabetes, and high-fat), and cell biology (circadian rhythm, diurnal, diabetes, and hepatocytes). We choose to focus on markers of metabolic disease, specifically fasted plasma glucose, insulin, and lipids.
HUMAN CIRCADIAN STUDIES
Circadian human studies are difficult to undertake due to technical challenges of controlling for the environment and its powerful timing cues (zeitgebers) of light, and food. Equally, reductionist studies in highly artificial environments can make the practical impact of these studies limited (37). Despite these limitations, human studies provide insight into how alterations to the circadian system can impact upon metabolic health. Here, we review studies investigating metabolic impact (glycemic control) of circadian disruption in both real-world shift workers and in otherwise healthy individuals subjected to misalignment protocols. A total of 120 studies were obtained and 22 original studies were kept after screening abstracts and excluding studies that did not meet the inclusion criteria (see Supplemental Fig. S1). Search results are summarized in Table 1 for shift work studies, and Table 2 for misalignment studies.
Table 1.
Effect of shift work on markers of fasting glucose and insulin and lipid profiles
| References | Study Design | Gender | Age | BMI, kg/m2 | Study Population | Plasma Glucose | Plasma Insulin | Lipid Profile |
|---|---|---|---|---|---|---|---|---|
| Rizza et al. (38) | Cross-sectional study | M (62) F (100) |
44.1–40.3 | 24.2–25.9 | Day workers (DWs) vs. rotating night shift workers (rNWs) | ↔ Fasting glucose (DWs vs. rNWs) | ↔ Fasting insulin (DWs vs. rNWs) ↔ Fasting HOMA-IR (DWs vs. rNWs) |
↔ TG (DWs vs. rNWs) ↔ Total cholesterol (DWs vs. rNWs) ↔ HDL cholesterol (DWs vs. rNWs) ↔ LDL cholesterol (DWs vs. rNWs) |
| Ledda et al. (39) | Cross-sectional study | M (133) F (139) |
39–41 | 21–22 | Healthcare workers (nSWs) vs. healthcare shift workers (SWs) | ↑ Fasting glucose (nSWs vs. SWs) | ↑ Fasting insulin (nSWs vs. SWs) ↑ Fasting HOMA-IR (nSWs vs. SWs) |
Not measured |
| Kiranmala et al. (40) | Cross-sectional study | M (26) F (14) |
29–30 | 23.7–24.4 | Nonshift workers (nSWs) vs. shift workers (SWs) | ↔ Fasting glucose (nSWs vs. SWs) | ↔ Fasting insulin (nSWs vs. SWs) ↔ Fasting HOMA-IR (nSWs vs. SWs) |
↔ TG (nSWs vs. SWs) ↔ Total cholesterol (nSWs vs. SWs) ↔ HDL cholesterol (nSWs vs. SWs) ↔ LDL cholesterol (nSWs vs. SWs) |
| Sharma et al. (41) | Randomized crossover study | M (2) F (10) |
25 ± 1 | 26.9 ± 1.0 | Day workers (DWs) vs. night workers (NWs) Participants assessed after working two consecutive 12 h shifts (either day or night), during their third 12 h shift period for either day or night shift study |
↔ Fasting glucose (DWs vs. NWs) | ↓ Fasting insulin (DWs vs. NWs) | Not measured |
| Schiavo-Cardozo et al. (42) | Cross-sectional study | F (24) | 20–40 | 25–29.9 | Day workers (DWs) vs. night workers (NWs) Day shift—business hours Night shift—7:00 PM to 7:00 AM |
↔ Fasting glucose (DWs vs. NWs) | ↔ Fasting insulin (DWs vs. NWs) ↔ HOMA-IR (DWs vs. NWs) |
↑ TG (DWs vs. NWs) ↔ Total cholesterol (DWs vs. NWs) ↔ HDL cholesterol (DWs vs. NWs) ↔ LDL cholesterol (DWs vs. NWs) |
| Padilha et al. (43) | Cross-sectional study | M (21) | 25–35 | NS | Day workers (DWs), early morning workers (EMWs), night workers (NWs) | Not measured | ↔ HOMA-IR | Not measured |
| Wehrens et al. (44) | Cross-sectional study | NS | 25–45 | 21.5–35.7 | Non shift workers (nSWs) vs. shift workers (SWs) | ↔ Fasting glucose (nSWs vs. SWs) | ↔ Fasting insulin (nSWs vs. SWs) | ↔ TAG (nSWs vs. SWs) ↔ NEFAs (nSWs vs. SWs) |
| Lund et al. (45) | Cross-sectional study | M (10) F (2) |
24–34 | NS | Shift workers: 7-day shifts: 9:00 AM to 5:00 PM (DWs) 7-night shifts: 12:00 AM to 8:00 AM (NWs)Antarctic survey station |
↔ Fasting glucose (DWs vs. NWs) ↑ Postprandial glucose in after 9 days of shift work-improved when switched back to day shifts |
↔ Fasting Insulin (DWs vs. NWs) | ↔ NEFAs (DWs vs. SWs) ↑ Postprandial TAG and NEFA on the second day of night-shift work- remained elevated 2 days following the return to daytime working |
Values in parentheses in Gender column represent number of subjects. HOMA-IR, homeostatic model assessment for insulin resistance; NEFA, nonesterified fatty acids; TAG, triacylglycerol; TG, triglycerides.
Table 2.
Human misalignment studies measuring fasting glucose, insulin, and lipid profiles
| References | Study Design | Gender | Age | BMI, kg/m2 | Misalignment Protocol | Plasma Glucose | Plasma Insulin | Lipid Profile |
|---|---|---|---|---|---|---|---|---|
| Simulated night shift work/Circadian misalignment | ||||||||
| Grant et al. (46) | Control parallel study | 11 (M) | 18–45 | 22–23 | Simulated night work; 1600–1000 Randomly assigned into eating at night (NE) vs. not eating at night (NEN) NE; n = 4 meals; 0700, 1900, 0130 NEN; n = 7 meals; 0700, 0930, 1610, 1900 |
↔ Fasting glucose (NEN vs. NE) Postprandial glucose impaired |
↔ Fasting insulin (NEN vs. NE) Postprandial insulin impaired |
Not measured |
| Morris et al. 2016. (10) | Crossover design | M (3) F (6) |
24–48 | R: 19.3–29.3 | 3 days day 1- sleep/wake cycle inverted by 12 h, followed by staying awake for 16 h Next sleep opportunity- 11:00 AM until 7:00 PM. Sleep/wake protocol maintained until day 3 |
↔ Fasting glucose (aligned vs. misaligned) Postprandial glucose impaired |
↔ Fasting insulin (aligned vs. misaligned) Late phase postprandial insulin 10% higher in circadian misalignment group |
Not measured |
| Morris et al. (47) | Within-participant crossover design | M (8) F (6) |
20–49 | R: 21–29.5 25.4 ± 2.6 |
8-day long protocol: Circadian alignment—participants sleep opportunity occurred between 11:00 PM and 7:00 AM. Circadian misalignment—day 1–3 = 11:00 PM–7:00 AM. day 4—behavioral cycles shifted by 12 h until end of protocol. |
↔ Fasting glucose (aligned vs. misaligned) Postprandial glucose impaired |
↔ Fasting insulin (aligned vs. misaligned) ↔ Fasting ISR (aligned vs. misaligned) ↔ Fasting ISR (AUC) (aligned vs. misaligned) Postprandial insulin impaired |
↑ Fasting FFA levels (misaligned vs. aligned) ↔ FFA (AUC) (misaligned vs. aligned) ↔ TG (AUC) (misaligned vs. aligned) |
| Wefers et al. (48) | Randomized crossover design | M (14) | 22.4 ± 2.8 | 22.3 ± 2.1 | 3-day control protocol and 3.5 day misalignment protocol (12-h rapid shift of cycle). Misalignment participants had matched study procedures to control (e.g., meal time) but 12 h shifted. |
↑ Fasting glucose (misalignment vs. aligned) | ↑ Fasting insulin (misalignment vs. aligned) ↔ Hepatic insulin sensitivity (misalignment vs. aligned) |
↑ Fasting FFA levels (misalignment vs. aligned) ↓ TG (misaligned vs. aligned) ↓ TG (misalignment vs. aligned) |
| Dim light vs. bright light at night | ||||||||
| Albreiki et al. (49) | Randomized two-way crossover study—healthy subjects | M (9) F (8) |
22.3 ± 3.6–22.6 ± 2.2 | 22.9 ± 2.5–22.7 ± 2.2 | Dim light (<5 lux) (DL) vs. bright light (BL) (>500 lux)—between 1800 and 0600 the next day | ↔ Fasting glucose (DL vs. BL) ↑ Postprandial glucose in bright light group |
↔ Fasting insulin (TAUC) (DL vs. BL) | ↑ NEFA in dim light (TAUC) ↔ TAGs (DL vs. BL) |
| Phase advanced/delay misalignment | ||||||||
| Gonnissen et al. (50) | Randomized, single-blinded, crossover design | M (7) F (6) |
24.3 ± 2.5 | 23.6 ± 1.7 | Phase delay: three light-entrained circadian cycles (3 × 21 h—phase advance or 3 × 27 h—phase delay) | Not measured | ↑ Fasting insulin levels (phase delay effect) ↑ HOMA-IR index (phase delay effect) |
Not measured |
| Gonnissen et al. (51) | Randomized single-blinded crossover study | (NS) | 24.3 ± 2.5 | 23.6 ± 1.7 | Three conditions—21, 24, and 27 h cycles. Patients participated in the 24-h control cycle and then 21 and 27 cycle conditions were followed in random order. 24 h—subjects slept for 8 h and awoke for 16 h 21 and 27—subjects stayed time blinded in a respiration chamber during 3 light entrained circadian cycles. |
↔ Fasting glucose (phase advanced) ↑ Fasting glucose (phase delayed) |
↑ Fasting Insulin (phase advanced) ↔ Fasting Insulin (phase delay) |
Not measured |
| Wehrens et al. (44) | NS | 25–45 | 21.5–35.7 | Phase delay for 4 days: Awake for 30.5 h followed by a 4-h recovery nap and a recovery sleep |
↔ Fasting glucose (baseline vs. sleep deprivation) | ↔ Fasting Insulin (baseline vs. sleep deprivation) | ↑ Fasting FFA levels (baseline vs. sleep deprivation) ↓ TG (baseline vs. sleep deprivation) |
|
| Sleep fragmentation | ||||||||
| Stamatakis et al. (52) | M (9) F (2) |
18–29 | 24.3 ± 0.9 | Sleep was experimentally fragmented for 2 nights using auditory and mechanical stimuli | ↓ Glucose effectiveness (post-fragmentation vs. pre-fragmentation) | ↓ Insulin sensitivity (IVGTT) (post-fragmentation vs. pre-fragmentation) | Not measured | |
| Sleep restriction | ||||||||
| Eckel et al. (53) | Crossover counterbalanced design. | F (8) M (8) |
22.4 ± 4.8 | 22.9 ± 2.4 | Restricted sleep schedule: Simulated 5-day work week of 5 h per night sleep |
Not measured | ↓ Insulin sensitivity(OGTT and IVGTT) | Not measured |
| Sleep restriction with different light exposures | ||||||||
| Gil-Lozano et al. (54) | Randomized study design. | M (8) | 21.1 ± 0.9 | 23.9 ± 1 | T1—normal light-dark cycle with regular sleep T2—sleep deprivation in the dark T3—sleep deprivation with nocturnal light exposure T4—sleep deprivation with nocturnal filtered light exposure |
Not measured | ↓ Fasting insulin (5 h postmeal) (regular sleep vs. sleep deprived 6:00 AM) | Not measured |
| Sleep restriction plus phase delay | ||||||||
| Buxton et al Shea. (55) | F (10) M (11) |
23 ± 2 = young 60 ± 5 = older |
(NS) | Spent >5 wk in controlled laboratory conditions. Initial baseline segment of optimal sleep and 3 wk of sleep restriction (5.6 h per 24 h) combined with circadian disruption (recurring 28 h days). | ↔ Fasting glucose (baseline vs. sleep deprivation) ↑ Fasting glucose AUC (baseline vs. sleep deprivation) |
↓ Fasting insulin ↓ Fasting insulin AUC (baseline vs. sleep deprivation) |
Not measured | |
| Sleep restriction plus circadian misalignment vs. sleep restriction | ||||||||
| Leproult et al. (56) | Parallel group design | F (7) M (19) |
Mean—22.5 | Alignment group—23.1 ± 2.4 Misalignment—22.2 ± 2.5 |
Circadian alignment: 3 inpatient days with 10 h bedtimes followed by 8 inpatient days of sleep restriction to 5 h with fixed nocturnal bedtimes Circadian misalignment: sleep delayed by 8.5 h with sleep restriction to 5 h |
↓ Glucose (circadian alignment vs. misalignment) | ↑ Insulin (circadian alignment vs. misalignment) | Not measured |
Values in parentheses in Gender column represent number of subjects. AUC, area under the curve; FFA, free fatty acid; HOMA-IR, homeostatic model assessment for insulin resistance; ISR, insulin secretion rate; IVGTT, intravenous glucose tolerance test; NEFA, nonesterified fatty acids; OGTT, oral glucose tolerance test; TAG, triacylglycerol; TAUC, time area under curve; TG, triglycerides.
Metabolic Effects of Shift Work in Humans
Shift work is the most prevalent cause of circadian misalignment, with up to 20% of workers engaged in working shifts, which has been associated with a 10%–40% increased risk of developing T2D (57). Although shift work is associated with other at-risk behaviors including smoking, dietary choices, and sleep deprivation, even after controlling for these factors there is an increased risk of obesity (58, 59). A study comparing healthcare, nonshift workers (HCNSWs) and healthcare shift workers (HCSWs) younger than 50 yr of age with a body mass index (BMI) less than 24.9 kg/m2 reported an increase in fasting blood glucose concentration in HCSW compared with HCNSWs (39) (see Table 1). In contrast, another study reported that young (29–30 yr), healthy individuals who had a relatively short duration of shift work (1 yr, 1–2 night shift duties/week) showed no change in fasting plasma glucose and insulin concentrations (40). It has been suggested a minimum of 5 years of shift work is needed for the effects on lipids and glucose metabolism to manifest fully (60, 61). However, other studies have reported that subjects who had worked shift work for at least 5 years showed no difference in plasma fasting glucose, insulin, TG, and nonesterified fatty acids (NEFAs) concentrations when compared with individuals who were not shift workers (44). Therefore, the duration of time it takes for shift work to have a negative impact on plasma markers of glucose, insulin, and lipids in healthy individuals remains uncertain, and it is plausible that effects would be seen earlier in individuals defined as metabolically unhealthy (e.g., increased BMI and hepatic steatosis).
As considering the length of time individuals have been engaged in shift work is an important experimental variable when designing shift worker studies, one study presented in Table 2 provided insight into the metabolic derangement at different time points after shift work (45). They assessed individuals at three time points: 1) during the daytime of the normal working week; 2) within 2 wk of commencing the night shift; and 3) day 16, during the daytime on the second day of a return to day working. Although no data were shown, the authors stated that fasting and postprandial plasma glucose, insulin, and NEFAs concentrations were unchanged between day workers and shift workers. They also reported that after working 9 days of shift work there was an increase in postprandial, but not fasting, glucose concentrations, which improved when the subjects switched back to day shifts. In contrast, postprandial TG and NEFA increased on the second day of the night shift work and remain elevated 2 days following the return to daytime working. The observed differences in the responses of plasma glucose and lipids after shift work may be due the dynamics of plasma glucose versus lipids. After food consumption, plasma glucose peaks at 15–30 min after food consumption and returns to baseline after ∼1 h. Whereas, dietary fat has a more prolonged postprandial excursion than glucose (6–8 h in healthy individuals). Dietary lipids are transported in chylomicrons that deliver TG to various tissues, for example, adipose tissue for storage, muscle, and heart for immediate use (62–64). The observed differences in the responses of plasma glucose and lipids after shiftwork may be due to the dynamics of plasma glucose versus lipids. The duration of shift work that results in changes in fasted plasma markers that reflect increased risk of metabolic disease remains unclear. Five years of shift work is considered to be the period of time where the metabolic effects fully manifest but this is yet to be clearly demonstrated (60, 61). Moreover, it is plausible that the duration required to see metabolic effects is dependent/influenced by other factors including an individual’s phenotype (e.g., obesity, T2D, and dyslipidemia), lifestyle factors (e.g., diet and exercise), and gender and race. Current on-going studies will provide longitudinal data that may provide further insight. Another area that requires clarity is how long it takes shift workers to revert back to normal metabolic health, once they stop shift work. Although some have attempted to address this question (65, 66), it would be of interest to study individuals on a longer-term basis (5 year’s plus) to determine how switching between shift work and nonshift work affects metabolic health, and ultimately determine the rate of circadian adaptation (ability to adapt between day and night shifts) [reviewed extensively elsewhere (67)]. One way in which longitudinal information can be achieved without invasive protocols is the use of wearable devices, that can capture heart rate, sleep, and physical exertion throughout the day and night. Although not identified in the systematic search used in this review, studies that have used such technologies have provided valuable insight into shift workers both during their working shifts and their off days providing comprehensive overview of the effects of shift work (68). The advancement of artificial intelligence methodology has also provided further value to the use of wearable devices in human circadian studies (69).
Altogether the studies reviewed indicate that there is much work to be done to improve experimental methods to characterize the relationship between shift work and metabolic disease. The studies included in this review are in healthy individuals (defined by BMI) and thus the findings cannot be fully extrapolated to populations with preexisting metabolic disease who may have abnormal fasting lipid and glucose parameters at baseline. It is plausible that more notable and subtle changes occur in the postprandial phase before a change in fasting concentrations. An alternative explanation may be that healthy individuals metabolically adapt to changes in working hours (such as seen in shift work) through compensatory mechanisms and it is only when circadian patterns are misaligned that more notable effects are evident. Studies that have investigated the effects of shift work in patients with type 2 diabetes have provided evidence that night-shift work is associated with poorer glycemic control (57, 70–72).
Metabolic Effects of Misalignment Studies in Humans
Circadian misalignment studies involve manipulating timing of sleep, food intake, and light to shift the circadian clock and allow for the assessment of acute response to manipulations. Table 2 summarizes the variety of experimental approaches used for circadian misalignment. From the studies reviewed here that have described the misalignment protocol used as “simulated night shift work,” one study (1/4; 25%) reported a change in fasting glucose and insulin (Table 2) (48). Utilizing a rapid shift protocol, misalignment resulted in a significant increase in fasting plasma glucose, circulating NEFA, and skeletal muscle insulin resistance compared with control conditions (48). The difference in observations from other studies could be explained by the rapid shift protocol that was used. Several studies reviewed in Table 2 (10, 46, 47, 49) also report impaired postprandial glucose and insulin responses under misaligned conditions. Similar to the shift worker studies it may be that fasting responses are more resilient to the effects of alterations to the body clock compared with postprandial responses. Why alterations to the body clock impair postprandial responses to fasting are difficult to explain in current studies but may be due to individual’s phenotype and the length of time they have been engaged in shift work. In addition, measuring postprandial responses can be challenging as perturbations to the system (i.e., food) protocols can be varied and it can be challenging to utilize mixed nutritional challenges. Therefore, fasting measures are often used as a standardized protocol for metabolic studies.
Although not included in the studies reviewed in Table 2, studies that have utilized plasma proteomics have provided interesting insight into the effects of misalignment on the proteome landscape. It has been found that simulated nightshift work has rapid and wide-reaching impacts on 24-h time-of-day protein patterns. It has been reported that overall, 62 proteins had altered 24-h average levels, and 76 had altered 24-h time of day patterns with 11 proteins overlapping between these two categories, resulting in 127 total proteins being altered during circadian misalignment (73). Plasma metabolomics is another technique that has yielded information on the effects of shift work on key metabolic pathways, tryptophan, glutathione, alanine, glycine, and serine metabolism (74). Adoption of techniques such as plasma metabolomics can provide a detailed overview of how the human plasma proteome is altered due to alterations to the body clock. Ultimately, this level of information will allow for greater precision and knowledge focused on the timing of assessing omics health biomarkers. And all of which will lead to improved understanding of healthy physiology and disease processes associated with shift work.
When exploring the role of biological rhythms in human participants, it is challenging to clearly separate the adverse metabolic implications of misalignment from those derived from disrupted sleep. A consistent finding from studies (52–54) that have manipulated sleep as the misalignment protocol (sleep fragmentation and sleep restriction) is reports of reduced insulin sensitivity (Table 2). These findings alongside the studies where sleep has not necessarily been restricted but moved to another time of day, point to the importance of sleep as an independent risk factor (away from just shifts to the body clock) to metabolic health. This is a key consideration when interpreting the findings of shift worker studies or misalignment protocols designed to mimic shift work conditions. Often studies are completed in controlled experimental settings where the study individuals may still be achieving good quality and quantity of sleep, just at a different time of day, and thus not experiencing the consequences of sleep deprivation. Whereas outside of experimental settings, those working shifts may find it difficult to sleep during the day and therefore sleep deprivation is a likely risk factor for metabolic disease in these individuals. In fact, large UK population-based studies have shown that in 277,000 workers, shift workers report less sleep per 24 h/day than nonshift workers, alongside poorer sleep quality (75). Indeed, one of human studies listed in Table 2 combined circadian misalignment with partial sleep loss and reported reduced metabolic rates and increased plasma glucose concentrations, predisposing these individuals to an increased risk of obesity and diabetes (55).
A key consideration that is now emerging and can help to explain the large variation in tolerance to shift work and represents a “natural experiment” through which to dissect the impact of misalignment is by considering chronotype. Chronotype results from complex gene-environment interactions, which may go some way to determining the metabolic consequences of night shift work. Chronotype is the predisposition to be a morning or evening person and has been reported to have an impact on the metabolic effects of shift work (76). Carriers of a PER2 missense variant have a longer circadian period measured in controlled experimental conditions (77), and tend to have a late chronotype. It has been suggested that late chronotypes (that is individuals who prefer to wake and sleep later in the day) suffer less from the negative effects of circadian and sleep disruption when working night shifts, as compared with early chronotypes (78, 79). Studies investigating the effect of chronotype in nurses have provided further evidence, as those who reported as intermediate chronotype, but not late chronotype, showed an increased risk of T2D with longer durations of night shift work (79). Therefore, taking into consideration chronotype may help in better identifying acute and chronic health effects of shift work and allow for individually tailored prevention strategies.
Taken together, misalignment studies have provided insight into how alterations to the body clock can result in increased risk of metabolic consequences, in particular postprandial glucose and insulin appear to have a stronger response to alterations to the body clock than fasting responses.
Lessons from Human Circadian Studies So Far
Shift work and misalignment studies have provided insight into how alterations to the body clock can result in metabolic consequences in healthy individuals (80). The studies reviewed here have focused on fasting plasma glucose, insulin, and lipid parameters. Further expanding experimental techniques such as the use of isotope tracers in human circadian studies to measure gluconeogenesis and de novo lipogenesis will provide detailed mechanistic insight to link circadian mechanisms with metabolic liver disease (81, 82). A further key point to note from the studies reviewed in Tables 1 and 2 and simulated shift work studies not reviewed here is that the majority are conducted predominately in male participants. Sexual dimorphism in many biological processes and diseases is now well established (83, 84). Recent studies are providing evidence of a link between sexual dimorphism and circadian molecular machinery in humans (85). In addition, although outside the scope of this paper, the role of race in metabolic circadian oscillations in humans is an area that requires further investigation. For a comprehensive overview of the role of race, sex, and age in circadian metabolism see the work by Zhang et al. (86).
There remain many unanswered questions in relation to the metabolic effects of shift work and circadian misalignment, most likely due to the heterogeneous nature of shift work schedules and misalignment protocols used. The current limitations and methodological considerations in human shift worker studies are reviewed elsewhere (87, 88). One potential interesting area that requires further exploration beyond just profiling the metabolic effects of shift work is strategies to realign the body clock that could be utilized by shift workers. Time-restricted feeding, defined as restricting food intake to 8–12 h each day (89), has been shown in human studies to reduce body weight (90), and improvements in glucose and LDL and HDL cholesterol (91, 92).
Human circadian studies have furthered our understanding of how alterations to the body clock, through shift work or misalignment, can impact upon metabolism to potentially increase the risk of metabolic diseases including T2D. However, due to the limitations associated with human studies evidence of the role the specific endocrine organs play in metabolic disturbances remains to be fully elucidated. Rodent studies can go some way to addressing this knowledge gap and will be the focus of the next section.
RODENT CIRCADIAN STUDIES
To better assess how circadian regulation of metabolism relates to metabolic disturbances, mouse models of obesity have been instrumental. Animal models help bridge the gap between human and in vitro studies allowing for organ-specific changes to be assessed, and the possibility of genetic and dietary, and activity-related manipulations. For this systematic search, we chose to focus on the liver due to the key metabolic role it plays in regulation of glucose and lipid metabolism. A total of 168 studies were obtained and 12 original studies were kept after screening abstracts and excluding studies that did not meet the inclusion criteria (see Supplemental Fig. S2). Search results are summarized in Table 3.
Table 3.
Hepatic circadian, glucose and lipid genes in dietary rodent models of obesity
| References | Species | Dietary Intervention | Phenotype | Effect of Diabetic Intervention on Expression of Hepatic Circadian Genes across Time | Effect of Diabetic Intervention on Expression of Liver Glucose Metabolism Genes across Time | Effect of Diabetic Intervention on Expression of Lipid Metabolism Genes across Time |
|---|---|---|---|---|---|---|
| (changes in blood glucose, body weight) | *arrow indicates change from control sample | *arrow indicates change from control sample | *arrow indicates change from control sample | |||
| Dietary models | ||||||
| Oosterman et al. (93) | Rat (Wistar, male) | 5 wk—Saturated fat (beef tallow) and 30% sucrose water | ↑ WAT ↑ Hepatocytes with fat ↑ Plasma insulin |
↔ Per2 (ZT0–ZT24) ↔ Bmal1 (ZT0–ZT24) ↔ Cry1 (ZT0–ZT24) ↔ Rev-erbα (ZT0–ZT24) |
↑ Pgc1α at ZT12 ↑ Glucokinase at ZT12 *No statistics provided |
↑ Acc at ZT24 ↔ Fas *No statistics provided |
| Woodie et al. (94) | Mouse (C5Bl/6N, male) | 16 wk HFD (40% fat) |
↑ Body weight↑ GTT ↑ Blood glucose (ZT5) ↑ Serum insulin (ZT13, ZT17, and ZT21) |
↓ Bmal1 (ZT17 and ZT21) ↓ Rev-erbα (ZT9 and ZT13) ↔ Clock ↔ Per2 ↔ Cry1 |
Not measured | Not measured |
| Quagliarini et al. (95) | Mouse (C57BL/6J, male) | 12 wk HFD (58% fat with sucrose) | ↑ Body weight ↑ Fat mass ↓ Lean mass |
↔ Cry1 (ZT0–ZT20) ↔ Cry2 (ZT0–ZT20) ↔ Per1 (ZT0–ZT20) ↔ Per2 (ZT0–ZT20) ↔ Bmal1 (ZT0–ZT20) ↔ Rorα (ZT0–ZT20) ↔ Rorγ (ZT0–ZT20) ↔ Rev-erbα (ZT0–ZT20) ↔ Rev-erbβ (ZT0–ZT20) |
RNA-Seq pathway annotation: ↓ glucose metabolism (ZT0, ZT4, ZT8) |
RNA-Seq pathway annotation: ↑ Metabolism of lipids and lipoproteins (ZT0–ZT20) ↑ Fatty acid, triacylglycerol, and ketone body metabolism (ZT0–ZT20) |
| Toledo et al. (96) | Mouse (C57BL/6J) | 8 wk HFD (60% fat) | ↑ Blood glucose | ↓ Cry1 (3:00 AM, 7:00 AM) ↓ Bmal1 (7:00 AM, 11:00 AM) ↑ Clock (3:00 AM) ↔ Per1 (7:00–3:00 AM) |
↑ G6p (7:00 PM) ↑ Pck1 (11:00 AM, 3:00 PM, 7:00 PM) ↑ Fbp1 (7:00 PM) |
Not measured |
| Guan et al. (97) | Mouse (C57BL/6J, male) | 12 wk HFD (60% fat) | Not profiled | ↔ Rev-erbα ↔ Bmal1 ↔ Rory ↔ Cry1 ↔ Per2 |
Not measured | ↑ Fasn (enhanced rhythmicity) ↑ Acaca (enhanced rhythmicity) ↑ Acly (enhanced rhythmicity) ↑ Elovl5 (enhanced rhythmicity) |
| Prasai et al. (98) | Mouse (C57BL/6J, male) | 10 wk HFD (60% fat) | ↑ Body weight ↑ Plasma insulin (2:00 PM and 8:00 PM) |
↔ Bmal1 ↔ Per2 |
↑ Pepck at 2 pm | ↔ Ppar |
| Eckel-Mahan et al. (29) | Mouse (C57/BL6J, male) | 10 wk HFD (60% fat) | ↑ Body weight ↑ Liver weight ↑ Blood glucose |
↑ Cry1 at ZT16 ↑ Per2 (ZT4 and ZT8) ↓ Per2 (ZT16) ↓ Bmal1 (ZT4) ↓ Rev-erbα at ZT8 ↔ Clock at ZT0-ZT24 |
↑ Glucokinase (ZT0, ZT4, ZT8, ZT12, ZT20) | ↑ Pparα (ZT0–ZT20) |
| Matsumoto et al. (99) | Mouse (C57/BL6J, male) | 7 days HFD (37% fat) | Not profiled | ↓ Rev-erbα at ZT3 | Srebp1 phase advance | ↔ Dbp |
| Sutton et al. (100) | Mouse (C57BL/6J, male) | 12 wk HFD (60% fat) | ↔ Body weight ↔ Body composition |
↔ Bmal1 ↔ Per2 ↔ Rev-erbα |
Not measured | Not measured |
| Hsieh et al. (101) | Mouse (C57BL/6, male) | 11 mo HFD (45% fat) | ↑ Body weight ↑ Blood glucose ↑ Plasma cholesterol ↑ Serum insulin |
↑ Per1 at ZT9 ↑ Per2 at ZT9 ↑ Per3 at ZT9 ↑ Cry1 at ZT9 ↑ Cry2 at ZT9 ↑ Bmal1 at ZT3 and ZT9 ↔ Clock at ZT3 and ZT9 |
↑ Pgc1α at ZT3 and ZT9 ↔ Pgc1β at ZT3 and ZT9 ↑ Pepck at ZT3 and ZT9 ↑ Pdk4 at ZT3 and ZT9 |
↑ Dbp at ZT3 |
| Yanagihara et al. (102) | Mouse (C57/BL6J, female) | 8 wk HFD (40%) | ↑ Body weight ↑ Blood glucose ↑ Total cholesterol ↑ High-density lipoprotein-cholesterol ↑ Triglyceride |
↑ BMAL1 at ZT18 ↔ Clock ↔ Per1 ↔ Per2 ↔ Cry1 ↔ Cry2 |
Not measured | ↔ Dbp |
| Kohsaka et al. (103) | Mouse (C57/BL6J, male) | 6 wk HFD (60%) | ↓ Clock at ZT4 and ZT8 ↓ Bmal1 at ZT4 and ZT8 ↓ Per2 at ZT16 and ZT20 |
↑ SREBP-1c at ZT4 and ZT8 (Fig. 4D) | ↑ Acc at ZT0-ZT24 ↑ Fas at ZT0, −4, −8, −12, −16, −20 ↑ Fabp1 at ZT0–ZT24 |
BMAL1, Arnt-like protein; CLOCK, circadian locomotor output cycles kaput; CRY, Cryptochrome 1/2; GTT, glucose tolerance test; HFD, high-fat diet; PER, Period 1/2/3; ZT, Zeitgeber time.
One of the key questions in the circadian metabolic field is whether alterations to the liver clock precede metabolic disease or does metabolic disease cause alterations to the liver circadian clock. It is well established that the circadian clock exerts remarkable control of metabolism, but also that the information about metabolic state is transmitted back to the circadian clock. This creates a cross talk between metabolic and circadian genes, which in human studies can be difficult to entangle. Therefore, mouse models provide an experimental platform to explore metabolic cross talk and find answers to unanswered questions regarding circadian control of metabolism. As the focus of this review is on liver metabolism, we choose to focus on changes in circadian, glucose and lipid metabolic genes in rodent dietary models of obesity. We acknowledge that gene expression is not necessarily reflective of protein or functional metabolic changes but for comparison of findings across papers this experimental marker was chosen.
Hepatic Circadian Transcriptional Changes in Rodent Dietary Models of Obesity
Out of the studies looking at circadian transcriptional changes in response to diet-induced obesity included in this review, five (5/12; 42%) reported no change to circadian gene expression across a 24-h period, despite those studies reporting obesity-related phenotypes (increased body weight and glucose intolerance). This provides some evidence that metabolic disturbances can precede defects in the transcriptional control of the circadian clock, however seven (7/12; 58%) studies reported altered expression of at least one of the circadian genes. Interestingly, there appears to be no specific diet duration or composition that causes changes in circadian gene expression, as a wide range of diet duration (5–16 wk) and macronutrient composition (37%–60% fat) have been utilized. One study that reported no change in circadian gene expression across time in a rat model of obesity [saturated fat (beef tallow) and 30% sucrose water] observed that when food intake was shifted to the light phase in rats, which is typically the fed phase, there was a 12-h shift in circadian genes (93). The negative metabolic effects of shifting food intake to the fasted period of the day are a consistent finding in the rodent literature (14, 104). This is in-line with the human studies referenced in human circadian studies that report the beneficial metabolic effects of time-restricted feeding. These observations suggest that a diet rich in fat and sugar can shift the clock to the wrong time, but that also feeding time can further exacerbate the negative impact of a high-caloric diet on metabolic health.
Bmal1 and Clock.
Of the studies that reported changes in circadian gene expression in diet-induced obese rodent models (7/12; 58%) (93, 95, 97, 98, 100), there were varying changes in what circadian genes are either up- or downregulated (see Table 3). Bmal1 and Clock genes are the two genes measured most often (7/7 for Bmal1; 6/7 for Clock). Interestingly, Bmal1 was reported to change in more studies than Clock, with one study reporting increased expression of Bmal1 at Zeitgeber time (ZT)3 and ZT9 (101) and another study, ZT18 (102), and four studies reporting downregulated expression at ZT4, ZT18, ZT17, and ZT21 (29, 94, 96, 103). Whereas, four studies reported no change in Clock gene expression (29, 94, 101, 102), one with upregulation at ZT15 (96), and one with downregulation at ZT4 and ZT8 (103). The one study that reported upregulation of Bmal1 gene expression was undertaken in female mice (102), whereas all other studies showing no effect was in male mice, suggesting sexual dimorphism in circadian gene nutrient response. Similar to human studies there is an underrepresentation of female animals in circadian-based rodent studies. Sexual dimorphism exists in rodent models in relation to rate of weight gain, metabolic parameters and insulin sensitivity, and expression of key circadian genes. Female rodents have been reported to have the ability to adapt their circadian behavior more rapidly than males (105–107). This would indicate that females may be more protected than males from the negative effects of circadian disruption, such as weight gain. Therefore, the importance of sex differences in chronobiology should not be underestimated.
One explanation for why there is no clear finding on what happens to Bmal1 and Clock gene expression in a rodent model challenged with a high-fat diet is reports that show a nutritional challenge disrupts the Bmal1 Clock chromatin recruitment to Nampt resulting in reduced NAD+ (29). Indicating that how the circadian machinery interacts with metabolic end points during a metabolic challenge is more than just alterations to circadian gene expression.
Per2 and Cry1/2.
Period (Per2) and Cryptochrome genes make up the negative part of the circadian molecular machinery. Out of the studies reviewed here, Per2 and Cry1/2 were not measured in all studies. Per2 was measured in five studies (5/12; 42%), with two reporting upregulation of Per2 at ZT4, ZT8, and ZT9, and a further two studies reporting downregulation at ZT16 and ZT20. Cry1 was measured in five studies (5/12; 42%), with two reporting no change, two showing upregulation at ZT9 and ZT16, and one downregulation at ZT15 and ZT19. In addition to gene expression, it has been reported that protein levels of CRY1 are downregulated at ZT12 in both nucleus and cytosolic fractions, which does not match the gene expression data, indicating the importance of matching up gene expression data with protein measurements (102). Cry1 has been reported to exert metabolic affects independent of changes in their own gene expression. AMPK has been shown to rhythmically regulate CRY1 stability in mouse livers (108). Fasting glucose and insulin responses are typically impaired in mouse models of obesity, which can lead to inhibition of AMPK, resulting in the inability of AMPK to stabilize CRY1, and consequently metabolic effects on the circadian clock. Indeed, Cry1 has been linked with regulation of hepatic gluconeogenesis, providing further links between cryptochromes and glucose homeostasis (22).
In contrast to Cry1, Cry2 was relatively underreported in the studies presented in Table 3. One study reported no change in Cry2 (102) with a second study reporting upregulation at ZT9 (101). The difference in findings between the two studies may be due to diet duration as the study reporting upregulation of Cry2 fed mice a high-fat diet for 11 mo, and the study with no change fed mice for 8 wk. These findings indicate that Cry2 is altered at the later stages of metabolic disease; however more studies are required to confirm this. The lack of studies reporting on Cry2 expression is surprising given that human genome-wide association studies have reported Cry2 single-nucleotide polymorphisms (SNPs) to be associated with fasting glucose and insulin-related traits (109), and more recent reports showing a significant correlation between hepatic Cry2 gene expression and hepatic triglyceride content (110). To gain a comprehensive understanding the role of Cry2 in metabolic disease, more studies are required.
REV-ERBs and RORα/γ.
The nuclear receptor Reverbα was reported in two studies, and RORα/γ was not reported in any of the studies (see Table 3). The two studies that included observations in Reverbα, reported downregulation at ZT3 and ZT8. Interestingly, one of the studies reported changes after just 7 days of high-fat diet (37% saturated fat). Nuclear receptors are lipid sensors and act as bridge between lipid signaling molecules and transcription responses, and thus are highly responsive to lipid challenges. REV-ERBα has been reported to be a repressor of lipogenic gene expression during the light phase of the diurnal cycle (ZT10) (111). More recent studies have built on the role of REV-ERBα in hepatic lipogenesis and reported that under basal conditions REV-ERBα does not suppress hepatic lipogenesis, but only when metabolically challenged, for example high-fat diet or mistimed feeding (112). Therefore, as the studies reviewed in Table 3 are from mice challenged with a high-fat diet, the observed increase in REV-ERBα indicates REV-ERBα is no longer able to repress lipogenic gene expression to the same extent as control animals and may play an integral role in increased adiposity in high-fat diet mice. The lack of reports identified in this search on RORα/γ is surprising given the established links between ROR and metabolic liver disease. RORα is decreased in liver biopsies taken from patients with NAFLD, and liver-specific RORα knockout mouse models exhibit exacerbated weight gain and insulin resistance. This is mediated by enhanced transcriptional activity of PPARγ resulting in uncontrolled lipogenesis. Although RORα/γ observations are not included in the papers identified in this search there are a number of papers that have reported on nutritional challenges to ROR and observed that RORγ is increased with high-sugar feeding at ZT16 but decreased with high-fat feeding at ZT16 and ZT20 (113). Surprisingly, RORα was unchanged in both the high-sugar fed and high-fat mice, which is similar to other reports in rats fed a high-sucrose diet (114), however these data are in contrast to the observations in human liver biopsies taken from patients with NAFLD and nonalcoholic steatohepatitis (NASH) (115–117). The discrepancy between findings in RORα gene expression in rodent and human studies not only indicates that is more required to clarify the role or RORα in metabolic liver disease, but also that rodent studies may not always represent the ideal model for mimicking human metabolic disease.
From the studies reviewed here, there is no consistent finding of disruption to the circadian genes in a high-fat diet mouse model of obesity. These findings suggest that a diet-induced obesity does not necessarily remodel the circadian clock molecular machinery but may disrupt the rhythmicity of metabolic pathways as reviewed in the next section.
Hepatic Glucose and Lipid Metabolic Gene Changes in Rodent Dietary Models of Obesity
Glucose transcriptional factors and genes were reported in 8 of 12 studies, with five studies observing upregulation of Pgc1α, Gck, G6p, Pck1, Fbp1, and Pdk4, and one study reporting downregulation of glucose metabolic genes at ZT0, ZT4, and ZT8 (95). Out of these studies there appears to be no trend in the direction of the circadian genes. For example, Gck was upregulated in two studies, with one study showed no change (93), whereas another reported an upregulation of Cry1 and Per2, and downregulation of ZT4, and REV-ERBα at ZT8 (29). The divergence in findings may be due to species as one study was in rats and the other in mice and different diet compositions and feeding time between the two studies. Gck is an interesting gene from a circadian perspective as it is rhythmic throughout the day, peaking during the feeding period in rodents. Recent reports have shown that administrating Gck activator to obese Zucker rats at the start of the feeding period is more beneficial in improving glycemic control and markers of hepatic inflammation, than dosing animals at the start of the fasted period, or continuously (118). How these findings link in with the circadian clock machinery was not explored in this study, but the findings do highlight the therapeutic value of considering time of day for drug administration. How drugs can be repurposed from a chronotherapy perspective is reviewed elsewhere (119). In addition to Gck, G6p, and Pck1, two key genes involved in hepatic gluconeogenesis were elevated in the mouse model of obesity. One study reported that G6p was elevated at 7:00 PM, and Pck1 at 11:00 AM, 3:00 PM, and 7:00 PM (96). Elevated expression of these key genes is not surprising as it is well established that in rodent metabolic disease models there is excessive hepatic gluconeogenesis resulting in increased glucose production and elevated blood glucose levels (120, 121). In addition, G6p is involved in lipogenesis as it encodes the rate-limiting enzyme that mediates the reaction step of NADPH production in the pentose phosphate pathway. Therefore, identifying optimal day of time to target these genes could be beneficial in reducing excessive gluconeogenesis and lipogenesis. Small molecule compounds that target various circadian genes have shown beneficial effect on glucose homeostasis in mouse models of obesity. Studies utilizing a CRY stabilizer (KL001) in isolated mouse hepatocytes repress glucagon-dependent induction of Pck1 and G6p (122). In addition, in vivo studies utilizing a ROR agonist, nobiletin, have reported blood glucose lowering effects in rodent models of obesity, but mechanisms of ROR regulation of gluconeogenesis are yet to be determined (123).
In contrast to the studies reporting increased expression of glucose metabolic genes, one study (95) reported a decrease (glucose metabolic genes based on RNA-Seq pathway annotation) at ZT0, ZT4, and ZT8. It is not clear why this finding is different to other studies included in Table 3 as the duration and composition of diet were not drastically different to other studies, and animals had increased body weight and fat mass. Interestingly, lipid metabolism genes were upregulated in this study that is similar to reports in the other studies included in Table 3. Diet-induced obesity was reported in one study to increase the rhythmicity of genes involved in de novo lipogenesis (Fasn, Acaca, Acly, Elovl5), and also the rate of hepatic fatty acid synthesis as measured by tracing deuterated water into newly synthesized fatty acids (97). This study also showed increased rhythmicity in fatty acid oxidation genes (Acox1, Aldh3a2, Acca1, Hadh), and that this process was upregulated at ZT10 relative to ZT22. This study went on to determine that targeting PPARα with PPAR agonists at the peak of its expression (ZT8) was more beneficial in reducing lipid metabolic genes and serum TG that using the agonist at the trough of PPARα ZT20. Thus, providing further evidence of how considering time of day for administration is a key consideration when determining the efficacy of drugs targeting metabolic pathways.
What Have We Learned from Rodent Circadian Experimental Studies?
Rodent studies provide an experimental model to look at organ-specific circadian effects in a much more tightly controlled environment compared with human studies. It is apparent from the studies reviewed here that there is no consistent finding as to whether liver circadian genes are upregulated, downregulated, or unchanged in a high-fat diet rodent model. The inconsistency in findings may reflect the utilization of different dietary fat composition and duration the animals were exposed to the diet. It is also important to consider how housing temperature and light-dark cycles may affect findings. There may be different light-dark schedules between laboratories and animal facilities, and potentially unintentional light disruption at night will cause profound disruption to circadian rhythm and thus affect experimental results. In addition, stress-induced housing circumstances such as limited nesting opportunities and lack of socialization will cause an increase in stress hormones (cortisol) that will in turn disrupt the circadian clock and may affect glucose metabolism.
Despite some of the limitations of animal studies outlined here, they do allow for in vivo testing of small molecule compounds that target circadian genes to determine how they can potentially be therapeutically beneficial (123–127). Although not reviewed here, genetic mouse models where circadian genes have been knocked out have also been crucial in dissecting out the link between the circadian clock and metabolism, and are extensively reviewed (21). Rodent studies have vastly expanded our understanding of how circadian biology links with metabolism and allowed for further exploration than is possible in human studies. However, there remains a large gap in translating animal research into the clinic, possibly due to differential metabolic pathways and drug responses between animal and models. These disparities may be even more pronounced in the circadian field as rodents are nocturnal compared with humans that are diurnal. Therefore, there is a great drive to develop human centric cellular models that better recapitulate human physiology.
CELLULAR CIRCADIAN EXPERIMENTAL SYSTEMS
The use of cell biology in vitro systems in the circadian field has expanded over the past decade due to the high throughput nature and the ability for experimental conditions to be manipulated. Cell models provide an environment to test the effect of varying nutrient challenges to the hepatic circadian clock, drug screening, and genetic manipulations. However, there is debate over whether in vitro cell biology models retain circadian rhythmic properties, leading to on-going challenges to improve in vitro hepatic circadian cell biology models. To follow on from the rodent studies reviewed in the previous section, we chose to focus on studies using hepatic in vitro cell biology systems. A total of 42 studies were obtained and eight original studies were kept after screening abstracts and excluding studies that did not meet the inclusion criteria (see Supplemental Fig. S3). Search results are summarized in Table 3.
Liver Slices, Primary Mouse Hepatocytes, Cell Lines, and Organoids
The utilization of organ slices for circadian measurements has expanded over the past 20 years with the development of the PER2::LUC reporter mouse that contains a PERIOD2:LUCIFERASE fusion protein that can be used as a real-time reporter of circadian dynamics (128). The first reports of the use of liver slices taken from PER2::LUC mouse showed self-sustained oscillations in luminescence from the mPER2::LUC fusion protein for a 20-day period (128). A reduction in amplitude was evident at day 5 of recording, but a discernable circadian rhythm of luminescence in liver was maintained until day 20. The use of liver slices from PER2::LUC reporter mice has expanded over the past decade, however, only one study was identified in our search (129). In addition to liver slices taken from PER2::LUC mice, a number of studies included in Table 4 and elsewhere in the literature have isolated primary hepatocytes from the PER2::LUC mouse strain and completed bioluminescence recordings. The advantage of the bioluminescent approach is that it allows for cells to be prepared and cultured in tissue culture plates that can then be run on a luminescence plate reader for multiple days. However, the read out only provides information on Per2 activity and although circadian information can be inferred from this, it only provides data on one circadian gene. Other approaches include the use primary mouse hepatocytes and cell lines where samples are taken for gene expression or protein at numerous time points throughout 24–48 h, as shown in Table 4.
Table 4.
Cell biology in vitro hepatic systems measuring circadian rhythmicity
| References | Cell/Tissue Type | Circadian Experimental Measure | Synchronization Method (Dose, Treatment Time) | ZT Time Period Used (Data shown in paper) | Analysis Approach |
|---|---|---|---|---|---|
| Tissue slices | |||||
| Wible et al. (129) | Liver slices from Nrf2-NULL PER2::LUC mouse (MYM and YMK strains) |
Bioluminescence of PER2-drive luciferase expression | Dexamethasone (200 nM) | 0–5 days | Data recordings excluded if bioluminescence trace did not fit a sine with >80% goodness of fit to the baseline |
| Primary cells | |||||
| Guan et al. (130) | Hepatocytes isolated from HepDKO mice | mRNA (BMAL1, Clock, Cry1, Cry2, REV-ERBα, REV-ERBβ) | None | 0, 4, 8, 12, 16, 20, 24 | JKT cycle |
| Marbach-Breitruck et al. (131) | Hepatocytes isolated from PER2::LUC mice |
Bioluminescence of PER2::luciferase expression | None | 0–180 h | Chronostar software |
| Jacobi et al. (132) | Cultured hepatocytes isolated from LBmal1KO mice | Bioenergetic assay at ZT6 and ZT18 | Not stated | 6, 18 h | Not stated |
| Liu et al. (133) | Cultured hepatocytes from LLPARDKO mice | Western blot (BMAL1) | Dexamethasone (100 nM, 1 h) | 5–35 h | Not stated |
| Wang et al. (28) | Cultured hepatocytes isolated from Lgr4m/m mice | Western blot (BMALl1) | 50% horse serum (2 h) | 0, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 h | Not stated for Bmal1 (JKT analysis applied for other genes of interest) |
| Torra et al. (134) | Cultured hepatocytes isolated from Sprague–Dawley rats | Western blot (REV-ERBα) | Dexamethasone (6 h) | 10, 16, 22, 28, 34, 40, 46, 52 | Mann–Whitney test comparing levels at the lowest time point (22 h) |
| Cell lines | |||||
| Dufour et al. (135) | HepG2 | mRNA (BMAL1, CLOCK, CRY1, CRY2, Per1, REV-ERBα, REV-ERBβ) | 50% horse serum (2 h) | 0, 4, 8, 12, 16, 20, 24 | Students paired t test at each time point |
| Tracey et al. (136) | HepG2 | mRNA (Nr1dp1) | 50% horse serum (2 h) | 1, 6, 11, 16, 21, 26 | Not stated |
| Lin et al. (137) | HL-7702 hepatocytes (immortalized human cell) | Western blot (PER2, REV-ERBα,) | Dexamethasone (100 nM, 2 h) | 24, 28, 32, 36, 40, 44, 48 h | Not stated |
| Wible et al. (129) | MMH-D3 hepatocytes | Bioluminescence of PER2::luciferase expression Western blot (PER2) |
Dexamethasone (200 nM) | 0, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 h | Data recordings excluded if bioluminescence trace did not fit a sine with >80% goodness of fit to the baseline |
| Organoids | |||||
| Leone et al. (138) | Hepanoids isolated from C57BL/6 mice | Western blot (PER2, Bmal1) | 50% horse serum | 8, 16, 24, 32, 40, 48 h | Not stated |
BMAL1, Arnt-like protein; CLOCK, circadian locomotor output cycles kaput; CRY, Cryptochrome 1/2; PER, Period 1/2/3; ZT, Zeitgeber time.
In addition to the use of liver slices and primary mouse hepatocytes, cell lines are often used for circadian studies as they can provide a human hepatic model that can be high throughput. Studies identified here using the established cell lines, HepG2 and HL-7702 hepatocytes, report gene expression and protein as outputs across 24- to 48-h period. In addition, one study used a mouse hepatic cell line, MMH-D3 hepatocytes transduced with Per2 lentiviral luciferase reporter (129). Recent advances in the hepatocyte field are the development of three-dimensional (3-D) models that resemble the structure of in vivo tissue, cell-cell and cell-matrix interactions, and provide an in vivo-like biophysical environment. One study was identified that isolated hepatocytes from C57BL/mice and cultured them into hepanoids (3-D cultures of liver cells) and took samples for protein measurements across a 48-h period (138). The use of 3-D liver cell culture is expanding due to the promise that they offer a more physiological environment compared with two-dimensional (2-D) cultures; how they can be used for circadian based studies remains to be determined. The limited amount of studies using in vitro hepatic models for circadian research from our search indicates that there a number of complexities associated with using these models.
In vitro entrainment.
Cells in culture for a prolonged period of time can result in desynchronized circadian rhythms. This desynchrony can be restored experimentally with “Zeitgebers,” which are exogenously administered stimuli, for example, serum starvation or dexamethasone for 1–2 h. Common methods used for in vitro synchronization of circadian rhythms have been extensively reviewed (139). Table 4 shows the varying synchronization methods used for the hepatic in vitro cell biology systems identified in this review. Methods used to synchronize the circadian clock in culture may have undesired effects on the in vitro stimulation, in fact, even standard procedures, such as splitting cells or refreshing culture medium can act as synchronizers. Despite this, adding synchronization to a cell culture-based system may improve the physiological relevance of appropriate in vitro methods by reflecting more closely on the biological in vivo situation. It has been reported in human cell line models (HME1, M13SV1, Caco-2, HCT116) that synchronization in vitro can improve cellular response sensitivity in response to chemicals (140), indicating its importance for drug discovery studies.
Analysis approaches.
Analysis approach needs consideration when interpreting findings from studies using cell biology models for circadian studies. As circadian measurements are made across multiple times points, often spanning 24 to 72 h, data can be noisy, thus statistical modeling, and curve fitting become an important component for accurate interpretation of rhythmic properties. An example of a rhythmic trace and the parameters that can be measured from this are presented in Fig. 2. To analyze this data regression method are often utilized, where the circadian process is represented by a sinusoidal equation (see Fig. 2). Numerous approaches have been previously used to measure rhythms including those based on autocorrelation (141), curve-fitting (142), and Fourier analysis (143). More recent developments in the field have been the introduction of JKT_CYCLE, which is a nonparametric statistical algorithm that can identify and characterize cycling variables in a large dataset (144). The JKT_CYCLE algorithm is available as a computationally efficient R script. Interestingly, only one study identified in this review stated using the JKT_cycle software. There has also been the development of an online platform, Biological Data Repository (BioDare) that allows for data storage, data sharing, and processing and analysis, without the need of R scripts (145).
Figure 2.
Potential directions for circadian experimental models exploring the role of circadian disruption in metabolism. Figure created using Biorender.com.
Moving Forward with Hepatic In Vitro Cell Biology Circadian Models
An ongoing challenge in circadian biology research is the improvement of current in vitro systems. Current in vitro model systems that have screened small molecule compounds targeting various circadian genes use U2OS PER2:LUC cells and mouse embryonic fibroblasts (MEFS) isolated from PER2::LUC reporter mice (146–148). Both cell models oscillate with high amplitude in culture and thus provide a high throughput platform for testing circadian small molecule compounds; however, neither cell models are hepatocytes. Advancements in imaging techniques such as Circa-SCOPE can provide high-throughput single-cell imaging through time (149), however are yet to be reported to have been used in hepatic cell systems. Therefore, improvement of in vitro hepatic culture systems would allow for more organ- and disease-specific phenotyping, drug screening, and ultimately improve research reproducibility. In vivo animal circadian experiments are a huge undertaking for research teams to capture samples at various time points throughout the 24 h cycle. Even if the animal light-dark cycle is manipulated these studies will still often require sample collection during the night. They also require a large number of animals that highlights how the development of advanced in vitro hepatic circadian models will better comply with the 3R’s (refinement, reproducibility, and reduction).
One area of in vitro development that promises to deliver conditions that better represent the physiological environment is 3-D culture systems, often referred to as organoids. These 3-D structures can be maintained over long periods of time (>1 wk) and allow for complex structures similar to in vivo liver settings as they are able to maintain genetic sustainability. Models such as this can provide high-throughput cell biology for disease modeling, biobanking, drug screening, and toxicity, which go way beyond the maximum capacity of mouse models (150). Studies that have compared primary human hepatocytes (PHH) in 2-D versus 3-D have provided evidence to show that 3-D cultured PHH have higher levels of proteins involved in drug absorption, distribution, metabolism, and excretion, and that the proteome is much more stable compared with 2-D cultured PHH (151). A more extensive review of hepatic 3-D models is provided in Refs. 152 and 153. Organoid models also provide a platform for coculture of different liver cell types (hepatocytes, immune cells, stromal cells, and fibroblasts). This could be a key advancement in the circadian cell biology field as recent reports have shown that Kupffer cells are also influenced by the circadian clock and feeding cues (130). In addition to organoids, hepatocytes cultured in a collagen gel sandwich configuration have been shown to express tight gap junctions and maintain hepatocyte-specific functions (albumin and urea secretion) for several weeks and show robust rhythms compared with hepatocytes culture in 2-D (154). Thus, providing a hepatic cell model that can be used for studies challenging cells with nutritional challenges and drug responses over a period longer than 7 days. In addition, reports using 3-D mammary epithelial cells have provided insight into how stiffening of the mechano-environment can impact circadian function (155). Liver fibrosis is often observed in patients with late-stage NASH (cirrhosis) and thus developing in vitro models that can mimic cell stiffening will be a key advancement in the development of disease-relevant cell biology models (156). Although, the development of 3-D culture systems has significantly advanced over the past decade there are still several limitations including heterogeneity of the size of the organoids, structural and functional issues, and the use of Matrigel that can limit experimental capabilities (150). Despite this, organoid technology does promise to potentially bridge the gap between basic science in vitro work and translational human studies, and thus provide accurate models that can be better predictive tools for chronotherapy and chronopharmacology studies.
CONCLUSIONS
Over the past few decades, the tools for studying the mechanisms underlying circadian timekeeping and its role in hepatic metabolic regulation have been constantly refined. Challenges remain to bridge the gap between human and rodent circadian studies and improvement in human in vitro models can go some way to providing experimental platforms that can help in generating novel knowledge and therapeutics relating to circadian impact on human health and disease. In addition, although not extensively reviewed here, omics technology such as metabolomics, proteomics, single-cell, and spatial omics have been well adopted in circadian rodent studies (157–159) and are now being used in human and in vitro studies. The utilization of omics technologies will provide far-reaching implications for our understanding of circadian disruption in liver metabolic disease. Further understanding of circadian metabolism is key for better-determining responses to drugs for chronotherapy but can also have important implications for problems such as drug addiction (160) and thus wider societal issues.
The aim of this review was to explore the reasons behind the inconsistent outcomes observed in studies examining the influence of circadian rhythms on metabolism. We have highlighted that background science is not secure, and there is a need for better-designed groups in human and rodent experimental studies, and experimental design to be detailed to adhere to frameworks such as ARRIVE guidelines.
SUPPLEMENTAL DATA
Supplemental Figs. S1, S2, and S3: https://doi.org/10.6084/m9.figshare.21750020.
GRANTS
This work was supported by a Novo Nordisk Postdoctoral Fellowship run in partnership with the University of Oxford (to L.J.D); the British Heart Foundation Fellowship FS/15/56/31645 and FS/SBSRF/21/31013 (to L.H.); Wellcome Trust Clinical Research Training Fellowship Ref. 102176/B/13/Z (to T.M.); DWR MRC Programme Grant MR/P023576/1; and Wellcome Trust 107849/Z/15/Z.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.J.D. conceived and designed research; D.K. performed experiments; L.J.D. analyzed data; L.J.D. prepared figures; L.J.D. drafted manuscript; L.J.D., T.M., L.H., and D.W.R. edited and revised manuscript; L.J.D., D.K., T.M., L.H., and D.W.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Linacre College; University of Oxford for the Sir Paul Nurse Junior Research Fellowship (nonstipendiary) to L.J.D.
REFERENCES
- 1. Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937, 1998. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 2. Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15 Spec No 2: R271–R277, 2006. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 3. Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280: 1564–1569, 1998. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 4. King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell 89: 641–653, 1997. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193–205, 1999. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 6. Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105: 683–694, 2001. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 7. Zhang L, Jain MK. Circadian regulation of cardiac metabolism. J Clin Invest 131, 2021. doi: 10.1172/JCI148276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev 93: 107–135, 2013. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mason IC, Qian J, Adler GK, Scheer F. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia 63: 462–472, 2020. doi: 10.1007/s00125-019-05059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab 101: 1066–1074, 2016. doi: 10.1210/jc.2015-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Downton P, Sanna F, Maidstone R, Poolman TM, Hayter EA, Dickson SH, Ciccone NA, Early JO, Adamson A, Spiller DG, Simpkins DA, Baxter M, Fischer R, Rattray M, Loudon ASI, Gibbs JE, Bechtold DA, Ray DW. Chronic inflammatory arthritis drives systemic changes in circadian energy metabolism. Proc Natl Acad Sci USA 119: e2112781119, 2022. doi: 10.1073/pnas.2112781119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338: 349–354, 2012. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 14. Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA 106: 21453–21458, 2009. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenwell BJ, Trott AJ, Beytebiere JR, Pao S, Bosley A, Beach E, Finegan P, Hernandez C, Menet JS. Rhythmic food intake drives rhythmic gene expression more potently than the hepatic circadian clock in mice. Cell Rep 27: 649–657.e5, 2019. doi: 10.1016/j.celrep.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 16. Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab 29: 303–319.e4, 2019. doi: 10.1016/j.cmet.2018.x08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aviram R, Manella G, Asher G. The liver by day and by night. J Hepatol 74: 1240–1242, 2021. doi: 10.1016/j.jhep.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 18. Rui L. Energy metabolism in the liver. Compr Physiol 4: 177–197, 2014. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol 20: 227–241, 2019. doi: 10.1038/s41580-018-0096-9. [DOI] [PubMed] [Google Scholar]
- 20. Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105: 15172–15177, 2008. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalsbeek A, la Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab 3: 372–383, 2014. doi: 10.1016/j.molmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 16: 1152–1156, 2010. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reinke H, Asher G. Circadian clock control of liver metabolic functions. Gastroenterology 150: 574–580, 2016. doi: 10.1053/j.gastro.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 24. Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480: 552–556, 2011. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab 12: 509–520, 2010. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev 26: 657–667, 2012. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang J, Mauvoisin D, Martin E, Atger F, Galindo AN, Dayon L, Sizzano F, Palini A, Kussmann M, Waridel P, Quadroni M, Dulić V, Naef F, Gachon F. Nuclear proteomics uncovers diurnal regulatory landscapes in mouse liver. Cell Metab 25: 102–117, 2017. doi: 10.1016/j.cmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, Dilag-Penilla SA, Dyar KA, Baldi P, Sassone-Corsi P. Reprogramming of the circadian clock by nutritional challenge. Cell 155: 1464–1478, 2013. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chavan R, Feillet C, Costa SS, Delorme JE, Okabe T, Ripperger JA, Albrecht U. Liver-derived ketone bodies are necessary for food anticipation. Nat Commun 7: 10580, 2016. doi: 10.1038/ncomms10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell 126: 801–810, 2006. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 32. Gnocchi D, Pedrelli M, Hurt-Camejo E, Parini P. Lipids around the clock: focus on circadian rhythms and lipid metabolism. Biology (Basel) 4: 104–132, 2015. doi: 10.3390/biology4010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saran AR, Dave S, Zarrinpar A. Circadian rhythms in the pathogenesis and treatment of fatty liver disease. Gastroenterology 158: 1948–1966.e1, 2020. doi: 10.1053/j.gastro.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106: 4453–4458, 2009. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sheehan CM, Frochen SE, Walsemann KM, Ailshire JA. Are U.S. adults reporting less sleep?: findings from sleep duration trends in the National Health Interview Survey, 2004–2017. Sleep 42: zsy221, 2019. doi: 10.1093/sleep/zsy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol 22: 939–943, 2012. [Erratum in Curr Biol 23: 737, 2013]. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 37. Lewis P, Oster H, Korf HW, Foster RG, Erren TC. Food as a circadian time cue—evidence from human studies. Nat Rev Endocrinol 16: 213–223, 2020. doi: 10.1038/s41574-020-0318-z. [DOI] [PubMed] [Google Scholar]
- 38. Rizza S, Longo S, Piciucchi G, Romanello D, Mavilio M, Montagna M, Coppeta L, Martelli E, Magrini A, Federici M. Carotid intimal medial thickness in rotating night shift is related to IL1β/IL6 axis. Nutr Metab Cardiovasc Dis 30: 1826–1832, 2020. doi: 10.1016/j.numecd.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 39. Ledda C, Cinà D, Matera S, Mucci N, Bracci M, Rapisarda V. High HOMA-IR index in healthcare shift workers. Medicina (Kaunas, Lithuania) 55: 186, 2019. doi: 10.3390/medicina55050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kiranmala K, Aslam M, Mishra BK, Jhamb R, Madhu SV. Association of postprandial triglyceride responses with insulin resistance among rotational night shift healthcare workers. Exp Physiol 104: 819–825, 2019. doi: 10.1113/EP087514. [DOI] [PubMed] [Google Scholar]
- 41. Sharma A, Laurenti MC, Dalla Man C, Varghese RT, Cobelli C, Rizza RA, Matveyenko A, Vella A. Glucose metabolism during rotational shift-work in healthcare workers. Diabetologia 60: 1483–1490, 2017. doi: 10.1007/s00125-017-4317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schiavo-Cardozo D, Lima MM, Pareja JC, Geloneze B. Appetite-regulating hormones from the upper gut: disrupted control of xenin and ghrelin in night workers. Clin Endocrinol (Oxf) 79: 807–811, 2013. doi: 10.1111/cen.12114. [DOI] [PubMed] [Google Scholar]
- 43. Padilha HG, Crispim CA, Zimberg IZ, Folkard S, Tufik S, de Mello MT. Metabolic responses on the early shift. Chronobiol Int 27: 1080–1092, 2010. doi: 10.3109/07420528.2010.489883. [DOI] [PubMed] [Google Scholar]
- 44. Wehrens SM, Hampton SM, Finn RE, Skene DJ. Effect of total sleep deprivation on postprandial metabolic and insulin responses in shift workers and non-shift workers. J Endocrinol 206: 205–215, 2010. doi: 10.1677/JOE-10-0077. [DOI] [PubMed] [Google Scholar]
- 45. Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol 171: 557–564, 2001. doi: 10.1677/joe.0.1710557. [DOI] [PubMed] [Google Scholar]
- 46. Grant CL, Coates AM, Dorrian J, Kennaway DJ, Wittert GA, Heilbronn LK, Pajcin M, Della Vedova C, Gupta CC, Banks S. Timing of food intake during simulated night shift impacts glucose metabolism: a controlled study. Chronobiol Int 34: 1003–1013, 2017. doi: 10.1080/07420528.2017.1335318. [DOI] [PubMed] [Google Scholar]
- 47. Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FA. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA 112: E2225–2234, 2015. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wefers J, van Moorsel D, Hansen J, Connell NJ, Havekes B, Hoeks J, van Marken Lichtenbelt WD, Duez H, Phielix E, Kalsbeek A, Boekschoten MV, Hooiveld GJ, Hesselink MKC, Kersten S, Staels B, Scheer F, Schrauwen P. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci USA 115: 7789–7794, 2018. doi: 10.1073/pnas.1722295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Albreiki MS, Middleton B, Hampton SM. A single night light exposure acutely alters hormonal and metabolic responses in healthy participants. Endocr Connect 6: 100–110, 2017. doi: 10.1530/EC-16-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gonnissen HK, Mazuy C, Rutters F, Martens EA, Adam TC, Westerterp-Plantenga MS. Sleep architecture when sleeping at an unusual circadian time and associations with insulin sensitivity. PLoS One 8: e72877, 2013. doi: 10.1371/journal.pone.0072877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gonnissen HK, Rutters F, Mazuy C, Martens EA, Adam TC, Westerterp-Plantenga MS. Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr 96: 689–697, 2012. doi: 10.3945/ajcn.112.037192. [DOI] [PubMed] [Google Scholar]
- 52. Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 137: 95–101, 2010. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eckel RH, Depner CM, Perreault L, Markwald RR, Smith MR, McHill AW, Higgins J, Melanson EL, Wright KP Jr.. Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Curr Biol 25: 3004–3010, 2015. doi: 10.1016/j.cub.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 54. Gil-Lozano M, Hunter PM, Behan LA, Gladanac B, Casper RF, Brubaker PL. Short-term sleep deprivation with nocturnal light exposure alters time-dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am J Physiol Endocrinol Metab 310: E41–E50, 2016. doi: 10.1152/ajpendo.00298.2015. [DOI] [PubMed] [Google Scholar]
- 55. Buxton OM, Cain SW, O'Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4: 129ra43, 2012. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63: 1860–1869, 2014. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vetter C, Dashti HS, Lane JM, Anderson SG, Schernhammer ES, Rutter MK, Saxena R, Scheer F. Night shift work, genetic risk, and type 2 diabetes in the UK Biobank. Diabetes Care 41: 762–769, 2018. doi: 10.2337/dc17-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barbadoro P, Santarelli L, Croce N, Bracci M, Vincitorio D, Prospero E, Minelli A. Rotating shift-work as an independent risk factor for overweight Italian workers: a cross-sectional study. PloS one 8: e63289, 2013. doi: 10.1371/journal.pone.0063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Di Lorenzo L, De Pergola G, Zocchetti C, L'Abbate N, Basso A, Pannacciulli N, Cignarelli M, Giorgino R, Soleo L. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord 27: 1353–1358, 2003. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 60. De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol 38: 848–854, 2009. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- 61. Gu F, Han J, Laden F, Pan A, Caporaso NE, Stampfer MJ, Kawachi I, Rexrode KM, Willett WC, Hankinson SE, Speizer FE, Schernhammer ES. Total and cause-specific mortality of U.S. nurses working rotating night shifts. Am J Prev Med 48: 241–252, 2015. doi: 10.1016/j.amepre.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. D'Aquila T, Hung YH, Carreiro A, Buhman KK. Recent discoveries on absorption of dietary fat: presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim Biophys Acta 1861: 730–747, 2016. doi: 10.1016/j.bbalip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJ, Gilbert M, Karpe F, Frayn KN. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 56: 168–176, 2007. doi: 10.2337/db06-0822. [DOI] [PubMed] [Google Scholar]
- 64. Ebenbichler CF, Kirchmair R, Egger C, Patsch JR. Postprandial state and atherosclerosis. Curr Opin Lipidol 6: 286–290, 1995. doi: 10.1097/00041433-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 65. Torquati L, Mielke GI, Brown WJ, Kolbe-Alexander T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand J Work Environ Health 44: 229–238, 2018. doi: 10.5271/sjweh.3700. [DOI] [PubMed] [Google Scholar]
- 66. Sookoian S, Gemma C, Fernández Gianotti T, Burgueño A, Alvarez A, González CD, Pirola CJ. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med 261: 285–292, 2007. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 67. Boivin DB, Boudreau P, Kosmadopoulos A. Disturbance of the circadian system in shift work and its health impact. J Biol Rhythms 37: 3–28, 2022. doi: 10.1177/07487304211064218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Feng T, Booth BM, Baldwin-Rodríguez B, Osorno F, Narayanan S. A multimodal analysis of physical activity, sleep, and work shift in nurses with wearable sensor data. Sci Rep 11: 8693, 2021. doi: 10.1038/s41598-021-87029-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Y, Cordina-Duverger E, Komarzynski S, Attari AM, Huang Q, Aristizabal G, Faraut B, Léger D, Adam R, Guénel P, Brettschneider JA, Finkenstädt BF, Lévi F. Digital circadian and sleep health in individual hospital shift workers: a cross sectional telemonitoring study. EBioMedicine 81: 104121, 2022. doi: 10.1016/j.ebiom.2022.104121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Manodpitipong A, Saetung S, Nimitphong H, Siwasaranond N, Wongphan T, Sornsiriwong C, Luckanajantachote P, Mangjit P, Keesukphan P, Crowley SJ, Hood MM, Reutrakul S. Night-shift work is associated with poorer glycaemic control in patients with type 2 diabetes. J Sleep Res 26: 764–772, 2017. doi: 10.1111/jsr.12554. [DOI] [PubMed] [Google Scholar]
- 71. Reutrakul S, Punjabi NM, Van Cauter E. Impact of sleep and circadian disturbances on glucose metabolism and type 2 diabetes. In: Diabetes in America, edited by Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE.. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, 2018. [PubMed] [Google Scholar]
- 72. Axelsson J, Puttonen S. Night shift work increases the risk for type 2 diabetes. Evid Based Med 17: 193–194, 2012. doi: 10.1136/ebmed-2012-100649. [DOI] [PubMed] [Google Scholar]
- 73. Depner CM, Melanson EL, McHill AW, Wright KP Jr.. Mistimed food intake and sleep alters 24-hour time-of-day patterns of the human plasma proteome. Proc Natl Acad Sci USA 115: E5390–E5399, 2018. doi: 10.1073/pnas.1714813115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Harding BN, Skene DJ, Espinosa A, Middleton B, Castaño-Vinyals G, Papantoniou K, Navarrete JM, Such P, Torrejón A, Kogevinas M, Baker MG. Metabolic profiling of night shift work—The HORMONIT study. Chronobiol Int 39: 1508–1516, 2022. doi: 10.1080/07420528.2022.2131562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wyse CA, Celis Morales CA, Graham N, Fan Y, Ward J, Curtis AM, Mackay D, Smith DJ, Bailey MES, Biello S, Gill JMR, Pell JP. Adverse metabolic and mental health outcomes associated with shiftwork in a population-based study of 277,168 workers in UK biobank. Ann Med 49: 411–420, 2017. doi: 10.1080/07853890.2017.1292045. [DOI] [PubMed] [Google Scholar]
- 76. Lane JM, Qian J, Mignot E, Redline S, Scheer F, Saxena R. Genetics of circadian rhythms and sleep in human health and disease. Nat Rev Genet 24: 4–20, 2023. doi: 10.1038/s41576-022-00519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chang AM, Duffy JF, Buxton OM, Lane JM, Aeschbach D, Anderson C, Bjonnes AC, Cain SW, Cohen DA, Frayling TM, Gooley JJ, Jones SE, Klerman EB, Lockley SW, Munch M, Rajaratnam SMW, Rueger M, Rutter MK, Santhi N, Scheuermaier K, Van Reen E, Weedon MN, Czeisler CA, Scheer F, Saxena R. Chronotype genetic variant in PER2 is associated with intrinsic circadian period in humans. Sci Rep 9: 5350, 2019. doi: 10.1038/s41598-019-41712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Juda M, Vetter C, Roenneberg T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythms 28: 141–151, 2013. doi: 10.1177/0748730412475042. [DOI] [PubMed] [Google Scholar]
- 79. Vetter C, Devore EE, Ramin CA, Speizer FE, Willett WC, Schernhammer ES. Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care 38: 1707–1713, 2015. doi: 10.2337/dc15-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Proper KI, van de Langenberg D, Rodenburg W, Vermeulen RCH, van der Beek AJ, van Steeg H, van Kerkhof LWM. The relationship between shift work and metabolic risk factors: a systematic review of longitudinal studies. Am J Prev Med 50: e147–e157, 2016. doi: 10.1016/j.amepre.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 81. Rabinowitz M, Wetherill GW, Kopple JD. Studies of human lead metabolism by use of stable isotope tracers. Environ Health Perspect 7: 145–153, 1974. doi: 10.1289/ehp.747145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim IY, Suh SH, Lee IK, Wolfe RR. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp Mol Med 48: e203, 2016. doi: 10.1038/emm.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Qian J, Morris CJ, Caputo R, Wang W, Garaulet M, Scheer F. Sex differences in the circadian misalignment effects on energy regulation. Proc Natl Acad Sci USA 116: 23806–23812, 2019. doi: 10.1073/pnas.1914003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Oliva M, Muñoz-Aguirre M, Kim-Hellmuth S, Wucher V, Gewirtz ADH, Cotter DJ, et al. . The impact of sex on gene expression across human tissues. Science 369: eaba3066, 2020. doi: 10.1126/science.aba3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Talamanca L, Gobet C, Naef F. Sex-dimorphic and age-dependent organization of 24-hour gene expression rhythms in humans. Science 379: 478–483, 2023. doi: 10.1126/science.add0846. [DOI] [PubMed] [Google Scholar]
- 86. Zhang C, Tait C, Minacapelli CD, Bhurwal A, Gupta K, Amin R, Rustgi VK. The role of race, sex, and age in circadian disruption and metabolic disorders. Gastro Hep Adv 1: 471–479, 2022. doi: 10.1016/j.gastha.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Knutsson A. Methodological aspects of shift-work research. Chronobiol Int 21: 1037–1047, 2004. doi: 10.1081/cbi-200038525. [DOI] [PubMed] [Google Scholar]
- 88. Härmä M, Gustavsson P, Kolstad HA. Shift work and cardiovascular disease—do the new studies add to our knowledge? Scand J Work Environ Health 44: 225–228, 2018. doi: 10.5271/sjweh.3727. [DOI] [PubMed] [Google Scholar]
- 89. Melkani GC, Panda S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J Physiol 595: 3691–3700, 2017. doi: 10.1113/JP273094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. LeCheminant JD, Christenson E, Bailey BW, Tucker LA. Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br J Nutr 110: 2108–2113, 2013. doi: 10.1017/S0007114513001359. [DOI] [PubMed] [Google Scholar]
- 91. Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Rumpler WV, Baer DJ, Egan J, Mattson MP. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 56: 1729–1734, 2007. doi: 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Mattson MP. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr 85: 981–988, 2007. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Oosterman JE, Koekkoek LL, Foppen E, Unmehopa UA, Eggels L, Verheij J, Fliers E, la Fleur SE, Kalsbeek A. Synergistic effect of feeding time and diet on hepatic steatosis and gene expression in male Wistar rats. Obesity (Silver Spring) 28, Suppl 1: S81–S92, 2020. doi: 10.1002/oby.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Woodie LN, Johnson RM, Ahmed B, Fowler S, Haynes W, Carmona B, Reed M, Suppiramaniam V, Greene MW. Western diet-induced obesity disrupts the diurnal rhythmicity of hippocampal core clock gene expression in a mouse model. Brain Behav Immun 88: 815–825, 2020. doi: 10.1016/j.bbi.2020.05.053. [DOI] [PubMed] [Google Scholar]
- 95. Quagliarini F, Mir AA, Balazs K, Wierer M, Dyar KA, Jouffe C, Makris K, Hawe J, Heinig M, Filipp FV, Barish GD, Uhlenhaut NH. Cistromic reprogramming of the diurnal glucocorticoid hormone response by high-fat diet. Mol Cell 76: 531–545.e5, 2019. doi: 10.1016/j.molcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Toledo M, Batista-Gonzalez A, Merheb E, Aoun ML, Tarabra E, Feng D, Sarparanta J, Merlo P, Botrè F, Schwartz GJ, Pessin JE, Singh R. Autophagy regulates the liver clock and glucose metabolism by degrading CRY1. Cell Metab 28: 268–281.e4, 2018. doi: 10.1016/j.cmet.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Guan D, Xiong Y, Borck PC, Jang C, Doulias PT, Papazyan R, Fang B, Jiang C, Zhang Y, Briggs ER, Hu W, Steger D, Ischiropoulos H, Rabinowitz JD, Lazar MA. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Cell 174: 831–842.e12, 2018. doi: 10.1016/j.cell.2018.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Prasai MJ, Mughal RS, Wheatcroft SB, Kearney MT, Grant PJ, Scott EM. Diurnal variation in vascular and metabolic function in diet-induced obesity: divergence of insulin resistance and loss of clock rhythm. Diabetes 62: 1981–1989, 2013. doi: 10.2337/db11-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Matsumoto E, Ishihara A, Tamai S, Nemoto A, Iwase K, Hiwasa T, Shibata S, Takiguchi M. Time of day and nutrients in feeding govern daily expression rhythms of the gene for sterol regulatory element-binding protein (SREBP)-1 in the mouse liver. J Biol Chem 285: 33028–33036, 2010. doi: 10.1074/jbc.M109.089391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sutton GM, Centanni AV, Butler AA. Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity. Endocrinology 151: 1570–1580, 2010. doi: 10.1210/en.2009-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hsieh MC, Yang SC, Tseng HL, Hwang LL, Chen CT, Shieh KR. Abnormal expressions of circadian-clock and circadian clock-controlled genes in the livers and kidneys of long-term, high-fat-diet-treated mice. Int J Obes (Lond) 34: 227–239, 2010. doi: 10.1038/ijo.2009.228. [DOI] [PubMed] [Google Scholar]
- 102. Yanagihara H, Ando H, Hayashi Y, Obi Y, Fujimura A. High-fat feeding exerts minimal effects on rhythmic mRNA expression of clock genes in mouse peripheral tissues. Chronobiol Int 23: 905–914, 2006. doi: 10.1080/07420520600827103. [DOI] [PubMed] [Google Scholar]
- 103. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6: 414–421, 2007. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 104. Glad CA-M, Kitchen EEJ, Russ GC, Harris SM, Davies JS, Gevers EF, Gabrielsson BG, Wells T. Reverse feeding suppresses the activity of the GH axis in rats and induces a preobesogenic state. Endocrinology 152: 869–882, 2011. doi: 10.1210/en.2010-0713. [DOI] [PubMed] [Google Scholar]
- 105. Anderson ST, Meng H, Brooks TG, Tang SY, Lordan R, Sengupta A, Nayak S, Mřela A, Sarantopoulou D, Lahens NF, Weljie A, Grant GR, Bushman FD, FitzGerald GA. Sexual dimorphism in the response to chronic circadian misalignment on a high-fat diet. Sci Transl Med 15: eabo2022, 2023. doi: 10.1126/scitranslmed.abo2022. [DOI] [PubMed] [Google Scholar]
- 106. Kettner NM, Mayo SA, Hua J, Lee C, Moore DD, Fu L. Circadian dysfunction induces leptin resistance in mice. Cell Metab 22: 448–459, 2015. doi: 10.1016/j.cmet.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhu L, Zou F, Yang Y, Xu P, Saito K, Othrell Hinton A Jr, Yan X, Ding H, Wu Q, Fukuda M, Sun Z, Tong Q, Xu Y. Estrogens prevent metabolic dysfunctions induced by circadian disruptions in female mice. Endocrinology 156: 2114–2123, 2015. doi: 10.1210/en.2014-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326: 437–440, 2009. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. . New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42: 105–116, 2010. [Erratum in Nat Genet 42: 464, 2010]. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Machicao F, Peter A, Machann J, Königsrainer I, Böhm A, Lutz SZ, Heni M, Fritsche A, Schick F, Königsrainer A, Stefan N, Häring HU, Staiger H. Glucose-raising polymorphisms in the human clock gene cryptochrome 2 (CRY2) affect hepatic lipid content. PLoS One 11: e0145563, 2016. doi: 10.1371/journal.pone.0145563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 331: 1315–1319, 2011. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hunter AL, Pelekanou CE, Adamson A, Downton P, Barron NJ, Cornfield T, Poolman TM, Humphreys N, Cunningham PS, Hodson L, Loudon ASI, Iqbal M, Bechtold DA, Ray DW. Nuclear receptor REVERBα is a state-dependent regulator of liver energy metabolism. Proc Natl Acad Sci USA 117: 25869–25879, 2020. doi: 10.1073/pnas.2005330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li D, Ikaga R, Ogawa H, Yamazaki T. Different expressions of clock genes in fatty liver induced by high-sucrose and high-fat diets. Chronobiol Int 38: 762–778, 2021. doi: 10.1080/07420528.2021.1889579. [DOI] [PubMed] [Google Scholar]
- 114. Sun S, Hanzawa F, Kim D, Umeki M, Nakajima S, Sakai K, Ikeda S, Mochizuki S, Oda H. Circadian rhythm-dependent induction of hepatic lipogenic gene expression in rats fed a high-sucrose diet. J Biol Chem 294: 15206–15217, 2019. doi: 10.1074/jbc.RA119.010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Starmann J, Fälth M, Spindelböck W, Lanz KL, Lackner C, Zatloukal K, Trauner M, Sültmann H. Gene expression profiling unravels cancer-related hepatic molecular signatures in steatohepatitis but not in steatosis. PLoS One 7: e46584, 2012. doi: 10.1371/journal.pone.0046584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Suppli MP, Rigbolt KTG, Veidal SS, Heebøll S, Eriksen PL, Demant M, Bagger JI, Nielsen JC, Oró D, Thrane SW, Lund A, Strandberg C, Kønig MJ, Vilsbøll T, Vrang N, Thomsen KL, Grønbæk H, Jelsing J, Hansen HH, Knop FK. Hepatic transcriptome signatures in patients with varying degrees of nonalcoholic fatty liver disease compared with healthy normal-weight individuals. Am J Physiol Gastrointest Liver Physiol 316: G462–G472, 2019. doi: 10.1152/ajpgi.00358.2018. [DOI] [PubMed] [Google Scholar]
- 117. Jouffe C, Weger BD, Martin E, Atger F, Weger M, Gobet C, Ramnath D, Charpagne A, Morin-Rivron D, Powell EE, Sweet MJ, Masoodi M, Uhlenhaut NH, Gachon F. Disruption of the circadian clock component BMAL1 elicits an endocrine adaption impacting on insulin sensitivity and liver disease. Proc Natl Acad Sci USA 119: e2200083119, 2022. doi: 10.1073/pnas.2200083119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kroon T, Hagstedt T, Alexandersson I, Ferm A, Petersson M, Maurer S, Zarrouki B, Wallenius K, Oakes ND, Boucher J. Chronotherapy with a glucokinase activator profoundly improves metabolism in obese Zucker rats. Sci Transl Med 14: eabh1316, 2022. doi: 10.1126/scitranslmed.abh1316. [DOI] [PubMed] [Google Scholar]
- 119. Marjot T, Ray DW, Tomlinson JW. Is it time for chronopharmacology in NASH? J Hepatol 76: 1215–1224, 2022. doi: 10.1016/j.jhep.2021.12.039. [DOI] [PubMed] [Google Scholar]
- 120. Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413: 131–138, 2001. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 121. Andrikopoulos S, Proietto J. The biochemical basis of increased hepatic glucose production in a mouse model of type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 38: 1389–1396, 1995. doi: 10.1007/BF00400598. [DOI] [PubMed] [Google Scholar]
- 122. Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA, Doyle FJ 3rd, Schultz PG, Kay SA. Identification of small molecule activators of cryptochrome. Science 337: 1094–1097, 2012. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, Yoo SH, Chen Z. The small molecule Nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab 23: 610–621, 2016. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nohara K, Mallampalli V, Nemkov T, Wirianto M, Yang J, Ye Y, Sun Y, Han L, Esser KA, Mileykovskaya E, D'Alessandro A, Green CB, Takahashi JS, Dowhan W, Yoo SH, Chen Z. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat Commun 10: 3923, 2019. doi: 10.1038/s41467-019-11926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nohara K, Nemkov T, D'Alessandro A, Yoo SH, Chen Z. Coordinate regulation of cholesterol and bile acid metabolism by the clock modifier nobiletin in metabolically challenged old mice. Int J Mol Sci 20: 4281, 2019. doi: 10.3390/ijms20174281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Reitz CJ, Alibhai FJ, Khatua TN, Rasouli M, Bridle BW, Burris TP, Martino TA. SR9009 administered for one day after myocardial ischemia-reperfusion prevents heart failure in mice by targeting the cardiac inflammasome. Commun Biol 2: 353, 2019. doi: 10.1038/s42003-019-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mia S, Kane MS, Latimer MN, Reitz CJ, Sonkar R, Benavides GA, Smith SR, Frank SJ, Martino TA, Zhang J, Darley-Usmar VM, Young ME. Differential effects of REV-ERBα/β agonism on cardiac gene expression, metabolism, and contractile function in a mouse model of circadian disruption. Am J Physiol Heart Circ Physiol 318: H1487–H1508, 2020. doi: 10.1152/ajpheart.00709.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wible RS, Ramanathan C, Sutter CH, Olesen KM, Kensler TW, Liu AC, Sutter TR. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. eLife 7: e31656, 2018. doi: 10.7554/eLife.31656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Guan D, Xiong Y, Trinh TM, Xiao Y, Hu W, Jiang C, Dierickx P, Jang C, Rabinowitz JD, Lazar MA. The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science 369: 1388–1394, 2020. doi: 10.1126/science.aba8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Marbach-Breitrück E, Matz-Soja M, Abraham U, Schmidt-Heck W, Sales S, Rennert C, Kern M, Aleithe S, Spormann L, Thiel C, Gerlini R, Arnold K, Klöting N, Guthke R, Rozman D, Teperino R, Shevchenko A, Kramer A, Gebhardt R. Tick-tock hedgehog-mutual crosstalk with liver circadian clock promotes liver steatosis. J Hepatol 70: 1192–1202, 2019. doi: 10.1016/j.jhep.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 132. Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, Unluturk U, Li X, Kong X, Hyde AL, Gangl MR, Mair WB, Lee CH. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab 22: 709–720, 2015. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Liu S, Brown JD, Stanya KJ, Homan E, Leidl M, Inouye K, Bhargava P, Gangl MR, Dai L, Hatano B, Hotamisligil GS, Saghatelian A, Plutzky J, Lee CH. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature 502: 550–554, 2013. doi: 10.1038/nature12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Torra IP, Tsibulsky V, Delaunay F, Saladin R, Laudet V, Fruchart J-C, Kosykh V, Staels B. Circadian and glucocorticoid regulation of Rev-erbα expression in liver. Endocrinology 141: 3799–3806, 2000. doi: 10.1210/endo.141.10.7708. [DOI] [PubMed] [Google Scholar]
- 135. Dufour CR, Levasseur MP, Pham NH, Eichner LJ, Wilson BJ, Charest-Marcotte A, Duguay D, Poirier-Héon JF, Cermakian N, Giguère V. Genomic convergence among ERRα, PROX1, and BMAL1 in the control of metabolic clock outputs. PLoS Genet 7: e1002143, 2011. doi: 10.1371/journal.pgen.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tracey CJ, Pan X, Catterson JH, Harmar AJ, Hussain MM, Hartley PS. Diurnal expression of the thrombopoietin gene is regulated by CLOCK. J Thromb Haemost 10: 662–669, 2012. doi: 10.1111/j.1538-7836.2012.04643.x. [DOI] [PubMed] [Google Scholar]
- 137. Lin R, Mo Y, Zha H, Qu Z, Xie P, Zhu ZJ, Xu Y, Xiong Y, Guan KL. CLOCK acetylates ASS1 to drive circadian rhythm of ureagenesis. Mol Cell 68: 198–209.e6, 2017. doi: 10.1016/j.molcel.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 138. Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17: 681–689, 2015. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xie Y, Tang Q, Chen G, Xie M, Yu S, Zhao J, Chen L. New insights into the circadian rhythm and its related diseases. Front Physiol 10: 682, 2019. doi: 10.3389/fphys.2019.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ndikung J, Storm D, Violet N, Kramer A, Schönfelder G, Ertych N, Oelgeschläger M. Restoring circadian synchrony in vitro facilitates physiological responses to environmental chemicals. Environ Int 134: 105265, 2020. doi: 10.1016/j.envint.2019.105265. [DOI] [PubMed] [Google Scholar]
- 141. Levine JD, Funes P, Dowse HB, Hall JC. Signal analysis of behavioral and molecular cycles. BMC Neurosci 3: 1, 2002. doi: 10.1186/1471-2202-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Straume M. DNA microarray time series analysis: automated statistical assessment of circadian rhythms in gene expression patterning. Methods Enzymol 383: 149–166, 2004. doi: 10.1016/S0076-6879(04)83007-6. [DOI] [PubMed] [Google Scholar]
- 143. Wichert S, Fokianos K, Strimmer K. Identifying periodically expressed transcripts in microarray time series data. Bioinformatics 20: 5–20, 2004. doi: 10.1093/bioinformatics/btg364. [DOI] [PubMed] [Google Scholar]
- 144. Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms 25: 372–380, 2010. doi: 10.1177/0748730410379711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zielinski T, Moore AM, Troup E, Halliday KJ, Millar AJ. Strengths and limitations of period estimation methods for circadian data. PLoS One 9: e96462, 2014. doi: 10.1371/journal.pone.0096462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chen Z, Yoo SH, Park YS, Kim KH, Wei S, Buhr E, Ye ZY, Pan HL, Takahashi JS. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci USA 109: 101–106, 2012. doi: 10.1073/pnas.1118034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Miller S, Aikawa Y, Sugiyama A, Nagai Y, Hara A, Oshima T, Amaike K, Kay SA, Itami K, Hirota T. An isoform-selective modulator of cryptochrome 1 regulates circadian rhythms in mammals. Cell Chem Biol 27: 1192–1198.e5, 2020. doi: 10.1016/j.chembiol.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 148. Kolarski D, Miller S, Oshima T, Nagai Y, Aoki Y, Kobauri P, Srivastava A, Sugiyama A, Amaike K, Sato A, Tama F, Szymanski W, Feringa BL, Itami K, Hirota T. Photopharmacological manipulation of mammalian CRY1 for regulation of the circadian clock. J Am Chem Soc 143: 2078–2087, 2021. doi: 10.1021/jacs.0c12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Manella G, Aizik D, Aviram R, Golik M, Asher G. Circa-SCOPE: high-throughput live single-cell imaging method for analysis of circadian clock resetting. Nat Commun 12: 5903, 2021. doi: 10.1038/s41467-021-26210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hu W, Lazar MA. Modelling metabolic diseases and drug response using stem cells and organoids. Nat Rev Endocrinol 18: 744–759, 2022. doi: 10.1038/s41574-022-00733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bell CC, Dankers ACA, Lauschke VM, Sison-Young R, Jenkins R, Rowe C, Goldring CE, Park K, Regan SL, Walker T, Schofield C, Baze A, Foster AJ, Williams DP, van de Ven AWM, Jacobs F, Houdt JV, Lähteenmäki T, Snoeys J, Juhila S, Richert L, Ingelman-Sundberg M. Comparison of hepatic 2D sandwich cultures and 3D spheroids for long-term toxicity applications: a multicenter study. Toxicol Sci 162: 655–666, 2018. doi: 10.1093/toxsci/kfx289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Harrison SP, Baumgarten SF, Verma R, Lunov O, Dejneka A, Sullivan GJ. Liver organoids: recent developments, limitations and potential. Front Med (Lausanne) 8: 574047, 2021. doi: 10.3389/fmed.2021.574047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lee J, Mun SJ, Shin Y, Lee S, Son MJ. Advances in liver organoids: model systems for liver disease. Arch Pharm Res 45: 390–400, 2022. doi: 10.1007/s12272-022-01390-6. [DOI] [PubMed] [Google Scholar]
- 154. Guenthner CJ, Luitje ME, Pyle LA, Molyneux PC, Yu JK, Li AS, Leise TL, Harrington ME. Circadian rhythms of Per2::Luc in individual primary mouse hepatocytes and cultures. PLoS One 9: e87573, 2014. doi: 10.1371/journal.pone.0087573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yang N, Williams J, Pekovic-Vaughan V, Wang P, Olabi S, McConnell J, Gossan N, Hughes A, Cheung J, Streuli CH, Meng QJ. Cellular mechano-environment regulates the mammary circadian clock. Nat Commun 8: 14287, 2017. doi: 10.1038/ncomms14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, Kassir R, Singhal R, Mahawar K, Ramnarain D. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord 22: 63, 2022. doi: 10.1186/s12902-022-00980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Deota S, Lin T, Chaix A, Williams A, Le H, Calligaro H, Ramasamy R, Huang L, Panda S. Diurnal transcriptome landscape of a multi-tissue response to time-restricted feeding in mammals. Cell Metab 35: 150–165.e4, 2023. doi: 10.1016/j.cmet.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sato S, Dyar KA, Treebak JT, Jepsen SL, Ehrlich AM, Ashcroft SP, Trost K, Kunzke T, Prade VM, Small L, Basse AL, Schönke M, Chen S, Samad M, Baldi P, Barrès R, Walch A, Moritz T, Holst JJ, Lutter D, Zierath JR, Sassone-Corsi P. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab 34: 329–345.e328, 2022. doi: 10.1016/j.cmet.2021.12.016. [DOI] [PubMed] [Google Scholar]
- 159. Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenta D, Jastroch M, Schneider S, de Mateo S, Cervantes M, Abbondante S, Tognini P, Orozco-Solis R, Kinouchi K, Wang C, Swerdloff R, Nadeef S, Masri S, Magistretti P, Orlando V, Borrelli E, Uhlenhaut NH, Baldi P, Adamski J, Tschöp MH, Eckel-Mahan K, Sassone-Corsi P. Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 174: 1571–1585.e11, 2018. doi: 10.1016/j.cell.2018.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cervantes M, Lewis RG, Della-Fazia MA, Borrelli E, Sassone-Corsi P. Dopamine D2 receptor signaling in the brain modulates circadian liver metabolomic profiles. Proc Natl Acad Sci USA 119: e2117113119, 2022. doi: 10.1073/pnas.2117113119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1, S2, and S3: https://doi.org/10.6084/m9.figshare.21750020.