Abstract
Peptidoglycan (PG), a component of the bacterial cell wall, has various immunomodulating activities, including the capacity to induce delayed-type hypersensitivity reactions to antigens administered in Freund’s adjuvant. We report that PG induces interleukin-12 (IL-12) mRNA production and IL-12 secretion by mouse macrophages. The capacity of PG to induce IL-12 production, like its previously reported immunomodulating activities, was dependent on the structure of its peptide subunit. PG from Bacillus megaterium and Staphylococcus aureus induced IL-12 production, whereas PG from Micrococcus luteus and Corynebacterium poinsettiae did not. The ability of most bacterial PGs to induce IL-12 production suggests that they play an important role in triggering host defense mechanisms against bacterial infections.
Peptidoglycan (PG) is a polymer present in the cell wall of almost all bacterial species. It is composed of glycan chains cross-linked by short peptides. PG is responsible for cell wall rigidity. PG possesses various immunomodulating activities (9) and therefore probably plays an important role in triggering host responses to bacterial infections.
The monomeric subunit of PG, usually a disaccharide-tetrapeptide, also possesses immunoadjuvant properties (21); the smallest structure with these activities is N-acetyl-muramyl-l-alanyl-d-isoglutamine (muramyl-dipeptide) (1). The structure of the peptide portion of PG can vary among gram-positive species (25). Some PGs, such as those of Micrococcus luteus and Corynebacterium poinsettiae, are devoid of immunomodulating properties (10, 11, 15).
The mechanism of action of PG has been the subject of many studies. PG is a mitogen (6, 11) and a polyclonal activator (8) of B cells and induces macrophages to secrete various cytokines, including interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (13, 27, 31, 32). Although much less toxic, PG shares many properties with bacterial lipopolysaccharide (LPS). Both molecules are known to interact with the macrophage receptor CD14 (12). One of the most striking properties of PG is its ability to substitute for mycobacteria in complete Freund’s adjuvant and to induce a delayed-type hypersensitivity reaction to antigens present in the water-in-oil emulsion (22). Delayed-type hypersensitivity is classified as a typical Th1 response (19), and IL-12 has been shown to be of critical importance for the induction of this type of response (14, 16, 28).
The aim of this study was to determine the capacity of PGs with various structures to induce IL-12 production by macrophages.
MATERIALS AND METHODS
PGs.
The bacterial species used were Bacillus megaterium ATCC 14581, Staphylococcus aureus Copenhagen, M. luteus A270 (Institut Pasteur), and C. poinsettiae NCPP 846. PGs were purified as previously described (11). Briefly, bacteria were disrupted by sonication, and cell walls were collected by differential centrifugation and digested with trypsin (0.5 mg/ml) and RNase (5 μg/ml) in 0.05 M phosphate buffer (pH 7.8) at 37°C for 16 h. The residue was treated with 1% sodium dodecyl sulfate in a boiling water bath for 10 min and then centrifuged. The pellet was washed thoroughly with distilled water. Teichoic acid was extracted with 10% trichloroacetic acid at 4°C for 3 days. The insoluble residue was washed three times in distilled water and lyophilized.
The chemical composition of the peptide moiety of each preparation was checked by amino acid analysis. The expected ratio of amino acids present in the various PGs was found, and only traces of other amino acids were detected. Before use, PGs were resuspended by brief sonication in Hanks’ balanced salt solution and placed for 3 min in a boiling water bath. All PG preparations were tested for LPS contamination in the Limulus amoebocyte lysate assay (Kinetic-QCL; Bio-Whittaker, Emerainville, France). In all preparations, LPS activity was less than 2 endotoxin units per mg of PG.
PG lysozyme digestion.
A sample of PG from B. megaterium (1 mg/ml) was treated for 3 min in a boiling water bath and incubated overnight at 37°C, in sterile conditions, with 40 μg of egg white lysozyme (Sigma Chemical Co., L’Isle d’Abeau Chesnes, France) per ml in 0.15 M phosphate buffer (pH 6.2).
Macrophages.
The mouse macrophage cell line J774 was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, 50 μg of gentamicin per ml, and 100 U of penicillin per ml. A cell suspension (5 × 105 in 1.5 ml) was plated on 40-mm-diameter tissue culture dishes and allowed to adhere for 2 h.
The macrophage monolayers were then treated with various concentrations of LPS (LPS from Salmonella typhimurium; Difco Laboratories, Detroit, Mich.) and PGs and incubated for 24 h at 37°C with 5% CO2. Culture supernatants were harvested for IL-12 assay. Cells were harvested at various times for RNA extraction.
RT-PCR.
Total RNA was isolated from macrophages by a single-step method with the Extract-all reagent (Eurobio, Les Ulis, France) as recommended by the manufacturer, and cDNA was synthesized in a volume of 20 μl as follows. Ten microliters of RNA preparation was mixed with 1 μl of oligo(dT) (Pharmacia, Uppsala, Sweden) and heated for 5 min at 70°C. A mixture of 4 μl of reverse transcriptase (RT) buffer, 2 μl of 0.1 M dithiothreitol, 1 μl of Superscript RNase H− RT (Life Technologies, Cergy-Pontoise, France), 50 μM deoxynucleoside triphosphates (Pharmacia), and 1 μl of RNAguard (Pharmacia) was added; the mixture was incubated for 90 min at 38°C and heated for 5 min at 95°C.
PCR amplifications were performed in a total volume of 50 μl. The PCR mixture consisted of 3.5 μl of PCR buffer (100 mM Tris-HCl [pH 9], 15 mM MgCl2, 500 mM KCl, 1% Triton X-100, 0.1% gelatin), 50 μmol of each deoxynucleoside triphosphate, 2.5 μl of glycerol, and 2.5 U of Taq DNA polymerase (ATGC, Noisy le Grand, France) per reaction. Five microliters of 10-fold-diluted cDNA and 10 pmol of each primer were then added. The following oligonucleotides were used as described previously (23): for β-actin, 5′-GATCCACATCTGCTGGAAGGT-3′ and 5′-GGTGACGAGGCCCAGAGCAAG-3′ (nucleotides 242 to 1151); for IL-12 p40, 5′-GACCCTGCCCATTGAACTGGC-3′ and 5′-CAACGTTGCATCCTAGGATCG-3′ (nucleotides 639 to 1034). The reaction mixtures were overlaid with 100 μl of paraffin oil and incubated for 5 min at 95°C. A total of 29 cycles for β-actin and 33 cycles for IL-12 p40 (95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, with an additional final step of 10 min) were run in a Crocodile II thermal cycler (Appligene, Pleasanton, Calif.). PCR products were then separated in 1.5% agarose gel by electrophoresis and visualized by ethidium bromide staining.
IL-12 assay.
IL-12 was quantified in culture supernatants by using a sandwich enzyme-linked immunosorbent assay (ELISA) (Genzyme, Cambridge, Mass.) according to the manufacturer’s instructions. This assay is specific for both free and heterodimer-associated p40 chains, and its detection limit is 10 pg/ml.
RESULTS
Induction of IL-12 mRNA by PG.
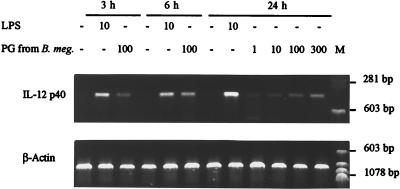
PG from B. megaterium induced the production of IL-12 mRNA by J774 cells. IL-12 mRNA was detected as early as 3 h after the addition of PG (Fig. 1). Only a weak signal was obtained with 1 μg/ml, and the signal intensity increased with the PG concentration. No signal was detected in the negative control (unstimulated J774 cells), whereas IL-12 mRNA was strongly induced by LPS.
FIG. 1.
Induction by B. megaterium PG of IL-12 p40 mRNA in the J774 macrophage cell line. Macrophages were incubated with the indicated concentrations (micrograms per milliliter) of LPS or PG from B. megaterium. Unstimulated macrophages were used as a control. Total RNA was extracted at the indicated times, reverse transcribed, and amplified by PCR with primers specific for IL-12 p40 and β-actin. Amplified products were submitted to agarose gel electrophoresis and stained with ethidium bromide. M, molecular size markers.
Correlation between PG peptide subunit structure and IL-12 mRNA induction.
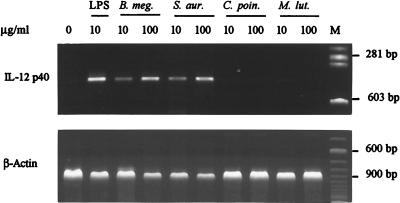
The peptides subunit of the PGs used to stimulate J774 cells are shown in Table 1. The PGs from B. megaterium and S. aureus, which are known to possess immunomodulating activities, induced IL-12 mRNA synthesis (Fig. 2), whereas PGs from M. luteus and C. poinsettiae, which are devoid of immunomodulating activities, did not.
TABLE 1.
Peptide subunits of PGs
| Organism | Peptide sequence |
|---|---|
| B. megaterium | l-Ala-d-Glu −γ m-A2pm-d-Alaa |
| S. aureus | l-Ala-d-Glu-(NH2) −γl-Lys-d-Ala |
| M. luteus | l-Ala-d-Glu-(Gly) −γl-Lys-d-Ala |
| C. poinsettiae | Gly-d-Glu −γl-Hsr-d-Alab |
m-A2pm, meso-diaminopimelic acid.
Hsr, homoserine.
FIG. 2.
Correlation between PG structure and the ability to induce IL-12 p40 mRNA. Macrophages were incubated for 24 h with the indicated concentrations of LPS or with PG from B. megaterium (B. meg.), S. aureus (S. aur.), C. poinsettiae (C. poin.), and M. luteus (M. lut.). IL-12 p40 and β-actin mRNAs were amplified as described in the legend to Fig. 1. Results are representative of three experiments. M, molecular size markers.
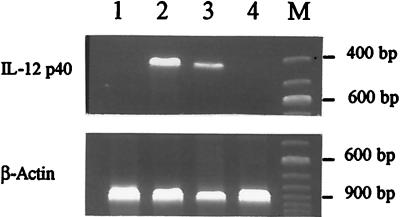
Lysozyme digestion of PG from B. megaterium strongly reduced its activity, showing that its polymeric structure is necessary to induce IL-12 mRNA (Fig. 3).
FIG. 3.
Effect of lysozyme digestion on the capacity of PG from B. megaterium to induce IL-12 mRNA production in J774 cells. RT-PCR was carried out after 24 h of incubation. Lanes: 1, control; 2, LPS (10 μg/ml); 3, PG from B. megaterium (100 μg/ml); 4, PG from B. megaterium (100 μg/ml after lysozyme digestion). M, molecular size markers.
Induction of IL-12 secretion by PG.
To determine whether IL-12 was secreted by J774 cells, culture supernatants were harvested after 24 h of exposure to PG, and IL-12 was quantified by ELISA. PGs from B. megaterium and S. aureus, which induce IL-12 mRNA, also induced IL-12 secretion. PGs from C. poinsettiae and M. luteus were inactive. PG from B. megaterium had no activity after lysozyme digestion (Table 2).
TABLE 2.
Induction of IL-12 release by PGs
| Stimulant added | Concn (μg/ml) | IL-12 (pg/ml)a
|
|
|---|---|---|---|
| Expt 1 | Expt 2 | ||
| None (control) | <10 | <10 | |
| PG from B. megaterium | 100 | 289 ± 19 | 183 ± 26 |
| PG from B. megaterium + lysozyme | 100 | 13 ± 7 | 24 ± 20 |
| PG from S. aureus | 100 | 206 ± 8 | 140 ± 8 |
| PG from C. poinsettiae | 100 | 10 ± 3 | <10 |
| PG from M. luteus | 100 | 13 ± 2 | <10 |
| LPS | 1 | 2,920 ± 828 | 5,033 ± 360 |
Supernatants of J774 cells were harvested after 24 h of incubation, and the IL-12 concentration was determined by ELISA. Results are means of three determinations ± standard deviations.
DISCUSSION
IL-12 is a heterodimeric cytokine produced in response to infection by various pathogens (3, 5, 23). In vitro, both live and killed bacteria induce IL-12 production by macrophages (4, 5, 7, 14, 17, 26). Among the bacterial components able to induce IL-12 production, LPS, present in gram-negative bacteria, is highly effective (7, 26). Heat shock proteins and double-stranded RNA have also been reported to induce IL-12 synthesis (26).
We found that PG also triggered IL-12 production. PG activity was not related to LPS contamination. To minimize the risk of LPS contamination, PGs were extracted from gram-positive species. Only traces of LPS activity were found in the PG preparations with the Limulus amoebocyte lysate assay. Moreover, the capacity to induce IL-12 secretion was lost after lysozyme treatment, showing that the biological activity depended on the polymeric structure of PG and not on LPS contamination. The capacity of PG to induce the production of other cytokines is also reduced by lysozyme treatment (27, 31).
In most PGs, the first two amino acids of the peptide subunit are l-Ala-d-Glu. These PGs were previously found to exhibit immunomodulating activities (10, 11, 31, 32), and here we show that they induce IL-12 production. The PGs from M. luteus and C. poinsettiae, which are devoid of immunomodulating activities (10, 11, 15, 31, 32), failed to induce IL-12 production. The peptide subunit sequences of these PGs exhibit unusual features. In M. luteus, the α-carboxyl group of the glutaminyl residue is linked to a glycyl residue. In C. poinsettiae PG, the first amino acid is glycine instead of l-alanine. Moreover, the α-carboxyl group of the glutaminyl residue is involved in interpeptide bridge formation. The key role of the first two amino acids of the peptide subunit has been confirmed in studies of synthetic PG derivatives (1).
IL-12 is a key cytokine in the host response to bacterial infection (2). Macrophage IL-12 production is necessary to induce natural killer cells to synthesize gamma interferon (28, 29), which plays a major role in innate resistance to infection, by activating macrophage bactericidal functions.
IL-12 also plays an important role in the regulation of adaptive immune responses, by favoring the differentiation of CD4+ T cells toward the Th1 pathway (28). IL-12 participates in the induction of delayed-type hypersensitivity (16) and contact sensitivity (20), both of which are mediated by Th1 cells. Th1 cells are also involved in resistance to intracellular pathogens by the production of gamma interferon. The role of IL-12 in resistance to various bacterial infections is shown by the detrimental effect of anti-IL-12 monoclonal antibody administration (3, 5, 18, 24, 30, 33). By inducing IL-12 production, PG both triggers innate immunity and regulates adaptive immunity. It is thus likely that PG plays a major role in triggering host responses to bacteria, especially gram-positive species, which lack LPS.
ACKNOWLEDGMENT
We thank Delphine Verjat (Pharmacie Centrale des Hôpitaux, Assistance Publique de Paris) for performing the Limulus amoebocyte lysate assay.
REFERENCES
- 1.Adam A, Petit J-F, Lefrancier P, Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- 2.Biron C A, Gazzinelli R T. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 3.Bohn E, Autenrieth I B. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-γ production in NK cells and CD4+ T cells. J Immunol. 1996;156:1458–1468. [PubMed] [Google Scholar]
- 4.Chong C, Bost K L, Clemets J D. Differential production of interleukin-12 mRNA by murine macrophages in response to viable or killed Salmonella spp. Infect Immun. 1996;64:1154–1160. doi: 10.1128/iai.64.4.1154-1160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 6.Damais C, Bona C, Chedid L, Fleck J, Nauciel C, Martin J P. Mitogenic effect of bacterial peptidoglycans possessing adjuvant activity. J Immunol. 1975;115:268–271. [PubMed] [Google Scholar]
- 7.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziarski R. Polyclonal activation of immunoglobulin secretion in B lymphocytes induced by staphylococcal peptidoglycan. J Immunol. 1980;125:2478–2483. [PubMed] [Google Scholar]
- 9.Dziarski R. Effects of peptidoglycan on the cellular components of the immune system. In: Seidl P H, Schleifer K H, editors. Biological properties of peptidoglycan. Berlin, Germany: Walter de Gruyter; 1986. pp. 229–247. [Google Scholar]
- 10.Goguel A-F, Lespinats G, Nauciel C. Peptidoglycans extracted from gram-positive bacteria: expression of antitumor activity according to peptide structure and route of injection. J Natl Cancer Inst. 1982;68:657–663. [PubMed] [Google Scholar]
- 11.Guenounou M, Goguel A-F, Nauciel C. Study of adjuvant and mitogenic activities of bacterial peptidoglycans with different structures. Ann Immunol (Paris) 1982;133D:3–13. [PubMed] [Google Scholar]
- 12.Gupta D, Kirkland T N, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–23316. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- 13.Heumann D, Barras C, Severin A, Glauser M P, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 15.Kotani S, Watanabe Y, Kinoshita F, Kato K, Schleifer K H, Perkins H R. Inabilities as an immunoadjuvant of cell walls of the group B peptidoglycan types and those of arthrobacters. Biken J. 1977;20:1–4. [PubMed] [Google Scholar]
- 16.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C-Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 17.Mahon B P, Ryan M S, Griffin F, Mills K H G. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect Immun. 1996;64:5295–5301. doi: 10.1128/iai.64.12.5295-5301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastroeni P, Harrison J A, Chabalgoity J A, Hormaeche C E. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect Immun. 1996;64:189–196. doi: 10.1128/iai.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 20.Müller G, Saloga J, Germann T, Schuler G, Knop J, Enk A H. IL-12 as mediator and adjuvant for the induction of contact sensitivity in vivo. J Immunol. 1995;155:4661–4668. [PubMed] [Google Scholar]
- 21.Nauciel C, Fleck J, Mock M, Martin J P. Activité adjuvante de fractions monomériques de peptidoglycanes bactériens dans l’hypersensibilité de type retardé. C R Acad Sci Ser D. 1973;277:2841–2844. [PubMed] [Google Scholar]
- 22.Nauciel C, Fleck J, Martin J P, Mock M, Nguyen-Huy H. Adjuvant activity of bacterial peptidoglycans on the production of delayed hypersensitivity and on antibody response. Eur J Immunol. 1974;4:352–356. doi: 10.1002/eji.1830040509. [DOI] [PubMed] [Google Scholar]
- 23.Pie S, Truffa-Bachi P, Pla M, Nauciel C. Th1 response in Salmonella typhimurium-infected mice with a high or low rate of bacterial clearance. Infect Immun. 1997;65:4509–4514. doi: 10.1128/iai.65.11.4509-4514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders B M, Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance of mice to Mycobacterium avium complex infection. Infect Immun. 1995;63:4011–4015. doi: 10.1128/iai.63.10.4011-4015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleifer K H, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skeen M J, Miller M A, Shinnick T M, Ziegler H K. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 27.Timmerman C P, Mattsson E, Martinez-Martinez L, de Graaf L, van Strijp J A G, Verbrugh H A, Verhoef J, Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 29.Tripp C S, Wolf S F, Unanue E R. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripp C S, Gately M K, Hakimi J, Ling P, Unanue E R. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-γ. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 31.Vacheron F, Guenounou M, Nauciel C. Induction of interleukin 1 secretion by adjuvant-active peptidoglycans. Infect Immun. 1983;42:1049–1054. doi: 10.1128/iai.42.3.1049-1054.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vacheron F, Guenounou M, Zinbi H, Nauciel C. Release of a cytotoxic factor by macrophages stimulated with adjuvant-active peptidoglycans. J Natl Cancer Inst. 1986;77:549–553. [PubMed] [Google Scholar]
- 33.Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infect Immun. 1995;63:1387–1390. doi: 10.1128/iai.63.4.1387-1390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]