Abstract
Effective vaccine‐induced immune responses are particularly essential in older adults who face an increased risk of immunosenescence. However, the complexity and variability of the human immune system make predicting vaccine responsiveness challenging. To address this knowledge gap, our study aimed to characterize immune profiles that are predictive of vaccine responsiveness using “immunotypes” as an innovative approach. We analyzed an extensive set of innate and adaptive immune cell subsets in the whole blood of 307 individuals (aged 25–92) pre‐ and post‐influenza vaccination which we associated with day 28 hemagglutination inhibition (HI) antibody titers. Building on our previous work that stratified individuals into nine immunotypes based on immune cell subsets, we identified two pre‐vaccination immunotypes associated with weak and one showing robust day 28 antibody response. Notably, the weak responders demonstrated HLA‐DR+ T‐cell signatures, while the robust responders displayed a high naïve‐to‐memory T‐cell ratio and percentage of nonclassical monocytes. These specific signatures deepen our understanding of the relationship between the baseline of the immune system and its functional potential. This approach could enhance our ability to identify individuals at risk of immunosenescence. Our findings highlight the potential of pre‐vaccination immunotypes as an innovative tool for informing personalized vaccination strategies and improving health outcomes, particularly for aging populations.
Keywords: aging, immune variation, immunosenescence, influenza vaccination
Our study identifies distinct pre‐vaccination immunotypes linked to responsiveness to the influenza vaccine. These immunotypes exhibit immune subset signatures indicative of baseline immune activation, immune regulation and aging, paving the way for tailored vaccination approaches to ensure the protection of individuals at risk of low vaccine responsiveness.
1. INTRODUCTION
Vaccines are widely recognized as the most effective means of protecting individuals from infectious diseases. However, the variability in effectiveness of influenza vaccines among older adults, ranging from 17%–53%, leaving a significant proportion of the population unprotected (Allen et al., 2020; Goodwin et al., 2006; Osterholm et al., 2012; Pepin et al., 2021; Angela Rose et al., 2020; Sasaki et al., 2011). One potential reason for this variability could be the phenomenon called immunosenescence, an age‐related decline in immune function (Allen et al., 2020; Fulop et al., 2020). Consequently, there is a pressing need to identify biomarkers of immune function at baseline. These biomarkers could help recognize individuals at risk of developing impaired responses to vaccines or pathogens and inform personalized vaccine strategies or treatments (Tsang et al., 2020). To this end, a deeper understanding of vaccine responsiveness and potential predictive biomarkers in aging adults is crucial.
Despite the importance of identifying biomarkers that predict influenza vaccine responses, the number of studies on this topic is limited. Previous studies have described potential pre‐vaccination biomarkers such as CD4+ T memory (Furman et al., 2013; Tomic et al., 2019; Tsang et al., 2014), CD8+ T memory (Carre et al., 2021; Furman et al., 2013; Tomic et al., 2019) and B memory (Tomic et al., 2019; Tsang et al., 2014) cells. Although these subsets are potential predictors of influenza vaccine responses, they exhibit high inter‐individual variation influenced by factors such as age and chronic viral infections like cytomegalovirus (CMV) (Cevirgel et al., 2022). Moreover, the immune system's functionality emerges from a complex network of interactions among various components, not fully represented by individual immune cell subsets, therefore, making single immune subsets poor biomarkers of vaccine responsiveness (Finzer, 2017; Chavali et al., 2008; Forlin et al., 2023; Kaczorowski et al., 2017). With an intent to overcome these limitations, we hypothesized that a combination of immune subsets could provide a more comprehensive representation of the immune network and offers more insightful correlations with immune functionality, for which we use antibody responses to influenza vaccination as a proxy.
To test our hypothesis, we analyzed an extensive number of immune subsets (percentages and absolute numbers) and antibody responses to influenza by assessing hemagglutination inhibition assay (HI) titers pre‐ and post‐ influenza vaccination in 307 individuals (25–92 years old). Baseline individual immune subsets exhibited limited association with day 28 HI titers, whereas stratification of individuals into immunotypes, clusters of individuals that share similar immune cell subset networks irrespective of their age, using an unsupervised approach based on 59 immune subsets did reveal associations with influenza antibody responses (Cevirgel et al., 2022). We identified immunotypes associated with weak or robust antibody responses and biologically interpreted these response patterns. Our findings suggest that pre‐vaccination immunotypes associate with antibody responses to influenza vaccination and contain signatures that improve our understanding of immune aging and post‐vaccination immune subset kinetics. This research could accelerate the translation of knowledge from our fundamental understanding of immunotypes to applications in personalized vaccination strategies and thereby maycontribute to the development of interventions that better protect aging populations.
2. RESULTS
2.1. Characteristics of the study population
To identify potential biomarkers of response to influenza vaccination, we recruited participants from the VITAL cohort study (N = 308, Figure S1) aged 25–92 years old who received the quadrivalent inactivated influenza vaccine (QIV) in autumn 2019 (Figure 1a) (van Baarle et al., 2020). After vaccination, we tracked their cellular and humoral immune responses (Figure 1b). We used previously reported data on immune cell subsets and measured HI titers against the influenza A strains (Cevirgel et al., 2022). We focused on HI titers against the H3N2 (A/Kansas/14/2017) strain, because only 10% of participants were sero‐responders (HI titer ≥40 and a four‐fold increase in HI titer at day 28 compared to baseline) for H1N1 (A/Brisbane/02/2018) (data not shown).
FIGURE 1.
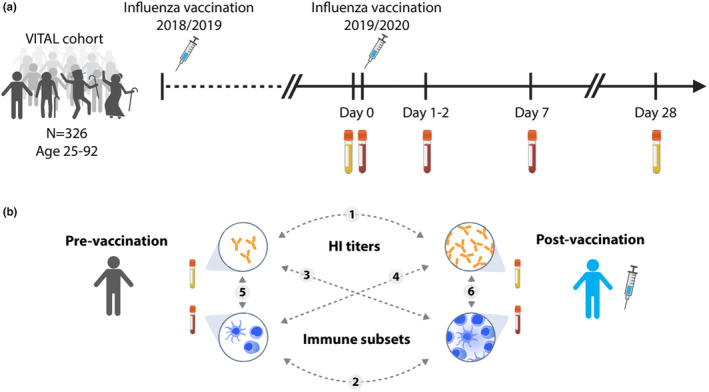
Clinical trial description. (a) Timepoints and sample collections. (b) Overview of relationships between pre−/post‐vaccination hemagglutination inhibition (HI) titers and immune subsets investigated in this study. Dashed lines with numbers describe the relationships investigated in the study.
2.2. Impact of pre‐vaccination influenza antibodies on defining sero‐responders
To investigate antibody responses to influenza virus, we first used the classical sero‐responder definition. Out of 307 individuals, 190 (62%) were characterized as sero‐responders. Before vaccination, an HI titer below the sero‐protection threshold of 40 was observed in 207 out of 307 individuals, which constituted 67% of the total study group (Figure 2a) (Ohmit et al., 2011). At 28 days after vaccination, only 42 out of 307 (13.6%) participants had an HI titer below 40. (Figure 2a).
FIGURE 2.
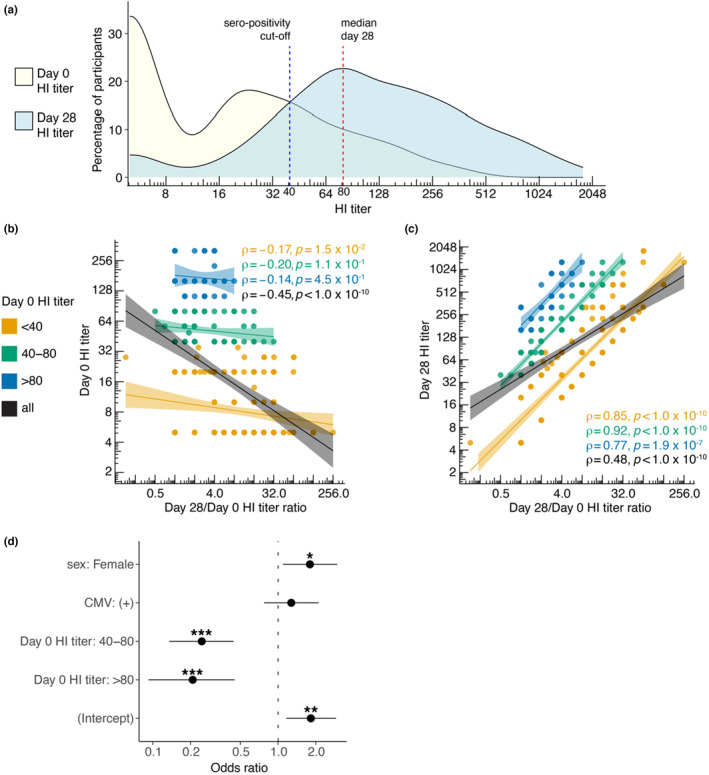
Influence of pre‐vaccination HI titers on post‐vaccination HI titer fold change and sero‐responder categorization. (a) Distribution of participants based on HI titers at day 0 and day 28. Blue dotted line indicates sero‐protection threshold of 40 and red dotted line indicates median post vaccination HI titer. (b) Spearman correlation between day 0 HI titers and day 28/day 0 HI titer ratio. (c) Spearman correlation between day 28 HI titers and day 28/day 0 HI titer ratio. Correlation coefficient rho (ρ) and p values are reported for each pre‐vaccination HI titer group. (d) A logistic regression model demonstrating the odds of becoming a sero‐responder to the influenza vaccination based on sex, CMV and pre‐vaccination HI titers.
Pre‐vaccination HI titers could influence the categorization of individuals as sero‐responders since these have been previously described to correlate with post‐vaccination HI titers (Figure 1b‐circle‐1) (Künzel et al., 1996). We, therefore, stratified individuals into three groups based on their pre‐vaccination HI titers. We used the sero‐protection threshold of 40 and the median value of the post‐vaccination HI titer of 80 to categorize all individuals into three categories: non‐sero‐positive (HI titers <40), low‐sero‐positive individuals (HI titers 40–80), and high‐sero‐positive individuals (HI titers >80). In our data, indeed, pre‐vaccination HI titers were negatively correlated to day 28/ day 0 HI titer fold change (ρ = −0.4, p < 1.0×10−10). Next, we investigated whether pre‐vaccination HI titer categories could reduce the influence of pre‐vaccination titers on the post‐vaccination HI titer fold change. We observed that by using pre‐vaccination HI titer categories, the correlation between pre‐vaccination HI titers and HI titer fold change was diminished (Figure 2b). Specifically, it became non‐significant for the low‐sero‐positive (40–80) and high‐sero‐positive (>80) categories. For the <40 category, the absolute value of the correlation coefficient reduced from 0.45 to 0.17, which was still significant (Figure 2b). Additionally, within the HI titer groups, the correlation between day 28 HI titers and HI titer fold change was stronger compared to the correlation for the whole cohort (ρ = 0.48) since the influence of pre‐vaccination titers was minimized (Figure 2c).
To further dissect other factors that influence the odds of being categorized as a sero‐responder, we performed a logistic regression analysis to assess the influence of sex, CMV seropositivity and pre‐vaccination HI titer groups. The odds of being a sero‐responder in females were 80% higher (p = 2.6×10−2, CI[1.18, 2.96]) than in males. CMV‐seropositivity did not show a significant effect (p = 3.8×10−1). Individuals in pre‐vaccination HI titers of “40–80” and “>80” showed 75% (p = 3.3×10−6, CI[0.14, 0.44]) and 81% (p = 6.1×10−5, CI[0.08, 0.42]) lower odds of being categorized as sero‐responder, respectively compared to “<40” (Figure 2d). Thus, pre‐vaccination HI titers should be taken into account to study antibody responses to influenza vaccine. Therefore, we integrated the pre‐vaccination HI titers groups in our further downstream analyses on vaccine responsiveness to influenza.
2.3. Post‐influenza vaccination immune cell subset kinetics
Humoral responses to vaccination are orchestrated by specific B and T cell subsets in secondary lymphoid organs (Ueno, 2019). Thus far, post‐vaccination changes in numbers of circulating follicular (CXCR5+) CD4+ T cells and plasmablasts (CD19 + CD27 + CD38+) at day 7 were described as some of the best correlates of antibody responses to influenza vaccination (Koutsakos et al., 2019; Ueno, 2019).
Our analyses focused on which immune cell subsets (percentage and absolute number) before (day 0) and after (days 1–2, day 7) vaccination experienced the most significant changes (Dunn's test). Increased cell numbers and percentages of follicular (CXCR5+) CD4+ T naïve (Tn) (CD27 + CD45RO‐) cells and nonclassical monocytes (CD14dimCD16+) and decreased numbers and percentages of CD56dim CD38 + HLA‐DR+ Natural killer (NK) cells were observed as early as 1–2 days post‐vaccination (Figure 3a–c, Table 1). At 7 days post‐vaccination, the increase in follicular CD4+ Tn cells was further amplified (Figure 3a, Table 1), and additionally, significantly higher numbers and percentages of both plasmablasts and activated follicular (CXCR5 + CD38+) CD4+ T cells were identified (Figure 3d,e, Table S1).
FIGURE 3.
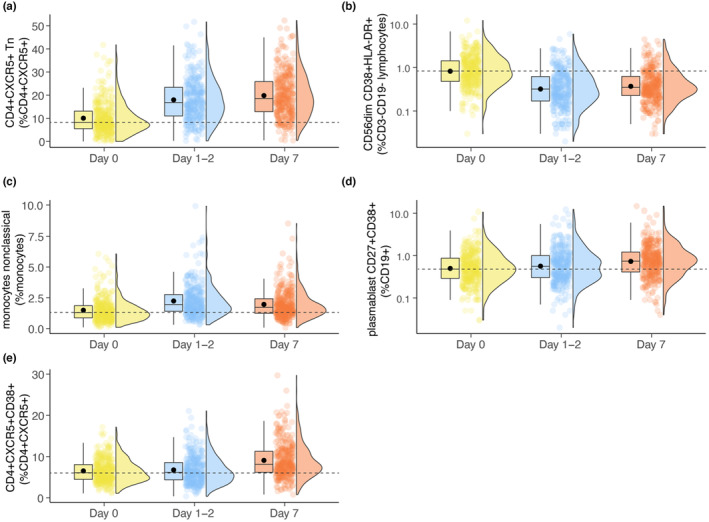
Pre‐ and post‐ influenza vaccination immune subset kinetics with the highest statistical significance. Boxplots depicting mean (dot) and median (line) day 0, day 1–2, and day 7 percentages of (a) follicular (CXCR5+) CD4+ T naïve (Tn, CD27 + CD45RO‐), (b) CD56dimCD38 + HLA‐DR+ Natural killer (NK) (c) nonclassical monocytes (CD14dimCD16+) (d) plasmablasts (CD19 + CD27 + CD38+) and (e) activated (CD38+) follicular (CXCR5+) CD4+ T cells. Dashed line indicates the median value of day 0 for the given immune subset.
TABLE 1.
Immune subsets with the highest significant changes at 1–2 days and 7 days post‐vaccination. Percentages and absolute numbers of top 10 immune subsets are shown.
| Day 1‐2/day 0 immune subsets | |||||||
|---|---|---|---|---|---|---|---|
| Variable (percentage) | p.adj | fc | Variable (absolute number) | p.adj | fc | ||

|
CD56dim CD38 + HLA‐DR+ | 3.94416E‐31 | 0.40 |

|
CD56dim CD38 + HLA‐DR+ | 9.28937E‐31 | 0.41 |

|
CD4+ CXCR5+ Tn | 5.36118E‐31 | 1.92 | Monocytes nonclassical | 1.66234E‐24 | 1.64 | |
| CD4+ CXCR5+ Tcm | 2.30582E‐23 | 0.88 |

|
CD4+ CXCR5+ Tn | 1.1087E‐23 | 1.97 | |

|
CD56dim CD95 + HLA‐DR‐ | 2.39897E‐21 | 0.45 |

|
Monocytes nonclassical HLA‐DR+ | 3.35792E‐20 | 1.63 |
| Monocytes nonclassical | 5.34936E‐19 | 1.52 | CD56dim CD95 + HLA‐DR‐ | 5.54292E‐16 | 0.46 | ||
| Monocytes classical | 1.43581E‐18 | 0.95 |

|
CD8+ Tcm CD95 + HLA‐DR‐ | 5.19481E‐15 | 0.66 | |
| CD56dim CD95‐HLA‐DR‐ | 8.37833E‐16 | 1.02 |

|
CD56dim CD95 + HLA‐DR+ | 2.65594E‐12 | 0.53 | |
| CD56dim CD95 + HLA‐DR+ | 4.16043E‐14 | 0.55 |

|
CD8+ CXCR5 + CD95+ | 4.9424E‐12 | 0.55 | |

|
CD4 + CXCR5 + CD95+ | 4.53831E‐14 | 0.84 | CD8+ CD95 + HLA‐DR‐ | 8.07561E‐12 | 0.69 | |
| CD8+ CD95 + HLA‐DR‐ | 8.23721E‐14 | 0.73 | CD8+ CD95+ | 2.44069E‐11 | 0.70 | ||
| Day 7/Day 0 immune subsets | |||||||
|---|---|---|---|---|---|---|---|
| Variable (percentage) | p.adj | fc | Variable (absolute number) | p.adj | fc | ||

|
CD4+ CXCR5+ Tn | 2.84189E‐41 | 2.17 |

|
CD4+ CXCR5+ Tn | 2.91996E‐42 | 2.86 |
| CD4+ CXCR5+ Tcm | 3.7126E‐31 | 0.87 |

|
CD56dim CD38 + HLA‐DR+ | 4.25727E‐29 | 0.45 | |

|
CD56dim CD38 + HLA‐DR+ | 3.09541E‐29 | 0.47 |

|
CD4+ CXCR5 + CD38+ | 2.66323E‐27 | 1.79 |
| CD56dim CD95 + HLA‐DR‐ | 2.47147E‐20 | 0.49 | CD4+ CXCR5 + CD38 + HLA‐DR‐ | 1.36465E‐25 | 1.73 | ||

|
CD4+ CXCR5 + CD38‐HLA‐DR‐ | 4.66271E‐19 | 0.97 | CD4+ CXCR5 + HLA‐DR+ | 2.0469E‐21 | 2.15 | |

|
CD56dim CD95‐HLA‐DR‐ | 2.04788E‐17 | 1.02 | CD4+ CXCR5+ Tem | 6.46852E‐18 | 1.78 | |

|
CD4+ CXCR5 + CD38+ | 1.53147E‐16 | 1.33 | CD4+ CD95‐HLA‐DR+ | 3.9182E‐17 | 1.69 | |

|
Monocytes classical HLA‐DR+ | 2.66911E‐15 | 0.74 |

|
CD56dim CD95 + HLA‐DR‐ | 6.38607E‐17 | 0.49 |

|
CD4+ Tcm CD95‐HLA‐DR+ | 1.20994E‐14 | 1.62 |

|
CD4+ CXCR5 + CD38‐HLA‐DR+ | 4.3846E‐16 | 1.99 |
| CD4+ CXCR5 + CD95+ | 1.47547E‐14 | 0.85 |

|
CD56dim CD95 + HLA‐DR+ | 7.30719E‐14 | 0.50 | |
Note: Kruskal–Wallis test followed by Dunn's test is used.Benjamini–Hochberg adjusted p‐values (p.adj) and fold changes (fc) are reported. Kruskal–Wallis test followed by Dunn's test is used Benjamini–Hochberg adjusted p‐values (p.adj) and fold changes (fc) are reported.
Note:  , adaptive immune subsets;
, adaptive immune subsets;  , innate immune subsets.
, innate immune subsets.
Considering the correlation between pre‐vaccination antibody titers and post‐vaccination antibody fold change, we determined whether the changes in immune subset numbers and percentages were also associated with pre‐vaccination values. The correlations between the pre‐ and post‐vaccination immune subsets revealed negative correlation coefficients ranging from −0.2 to −0.8 with a mean of −0.4 (Figure 1b‐circle‐2, Table S2). Interestingly, these post‐vaccination changes in immune subsets were not significantly associated with pre‐vaccination HI titer groups (linear regression, adj.p > 0.05) (Figure 1b‐circle‐3, Table S3).
2.4. Weak and robust vaccine responder profiles revealed by immunotypes
We conducted regression analyses to identify pre‐vaccination immune cell subsets that may be associated with the day 28 influenza antibody response (Figure 1b‐circle‐4). We factored in potential confounding variables such as sex, CMV‐seropositivity, and pre‐vaccination HI titer groups into our models.
At baseline, percentages of monocytes, IgD + CD27 + CD95+ memory B cells and CD8+ T effector memory cells (Tem) were positively associated with day 28 HI titers, whereas, numbers of follicular HLA‐DR + CD4+ and follicular CD38 + CD4+ T‐cells showed a negative association (p < 0.05). Nevertheless, these correlations were marked by low coefficients and not significant after multiple test corrections (Benjamini‐Hochberg, p.adj >0.05), indicating weak associations with antibody responses (Table S4).
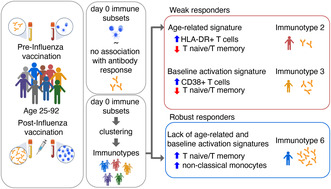
We hypothesized that the combination of immune subset variables representing different aspects of the immune network would associate better with vaccine responsiveness than individual immune subset variables. Previously we had stratified individuals from the same cohort into nine distinct immunotypes, in an unsupervised fashion, based on similarities among 59 immune cell subsets, including T cells, B cells, NK cells, monocytes, and granulocytes (Cevirgel et al., 2022). In our current analysis, we examined how these previously identified immunotypes associate with pre−/post‐vaccination HI titers and sero‐responder profiles (Figure 1b‐circle‐5). Pre‐vaccination HI titers remained similar across immunotypes (Kruskal‐Wallis, p = 0.55) (Figure 4a). At day 28, immunotype‐2 showed the lowest HI titers. In contrast, immunotype‐6 showed the highest ‐HI titers at day 28 and the highest day 28/day0 fold change in HI titer compared to other immunotypes (Figure 4b,c). Additionally, after subcategorizing sero‐responders based on day 28 HI titers of 40–80 or > 80 as low‐sero‐responder and high‐sero‐responder, respectively, individuals with immunotype‐6 showed the highest whereas immunotype‐2 showed the lowest percentage of high‐sero‐responders, although not statistically significant (chi‐squared test, high‐sero‐responders, p = 4.0×10−1) (Figure 4d).
FIGURE 4.
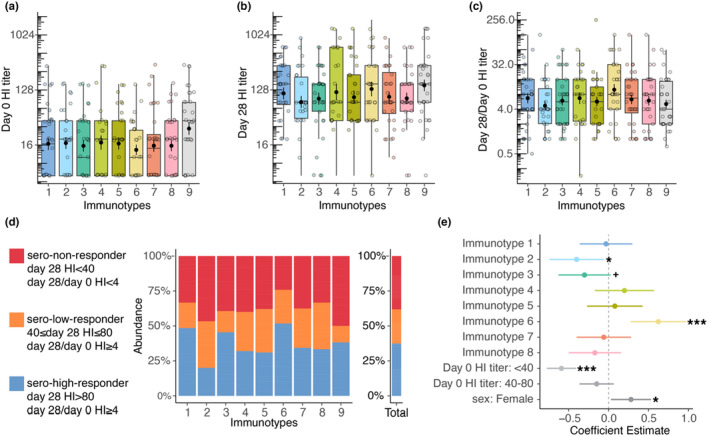
Identification of weak and robust vaccine responders through immunotypes. (a) Day 0 HI titers, (b) Day 28 HI titers, and (c) Day 28/day 0 HI titer ratio in immunotypes. (d) Comparative distributions of non‐, low‐, and high‐sero‐responder groups within different immunotypes and across the total cohort. (e) Regression models of day 28 HI titers for immunotypes after correcting for pre‐vaccination HI titers and sex.
To confirm our findings above, we performed regression analyses of the day 28 HI titer, using immunotypes while adjusting variables that were significant for sero‐responder categorization, namely sex and pre‐vaccination HI titer groups. Among the nine immunotypes, immunotype‐2 and immunotype‐3 showed negative coefficients (p = 2.2×10−2 and p = 6.9×10−2, respectively), whereas immunotype‐6 exhibited a significant positive coefficient (p = 4.9×10−4) for day 28 HI titers (Figure 4e). These analyses further highlight the variation in antibody responses to influenza vaccination among individuals with different pre‐vaccination immunotypes.
2.5. Baseline and post‐vaccination differences between weak and robust responder immunotypes
To elucidate the immune characteristics of immunotypes associated with weak and robust antibody responses, we compared their pre‐ and post‐vaccination immune subset composition. Immunotype‐6, which is associated with a robust antibody response, exhibited significantly higher T naïve (Tn) to T memory (Tm) ratios for both CD4+ and CD8+ cells and nonclassical monocytes at baseline, compared to the rest of the immunotypes (Figure 5a). These T cell subset ratios were previously described as aging‐related immune phenotype markers (Ramasubramanian et al., 2022) that were associated with biological age and chronological age, respectively. At baseline, immune subset composition of immunotype‐2 (associated with a weak antibody response), was dominated by increased percentages of HLA‐DR expressing CD8+, CD4+ and follicular CD4+ T cell subsets as previously reported (Cevirgel et al., 2022) (Figure 5a). In contrast, at baseline, immunotype‐3 (associated with a weak antibody response) showed significantly higher Tn/Tm CD4+ and CD8+ ratio and percentages of CD38+ CD4+ Tregs, CD38+ CD4+ and CXCR5 + CD38+ CD4+ T cells compared to rest of the immunotypes (Figure 5a) which suggested an activated immune environment. In addition, both immunotypes‐2 and ‐3, compared to the rest of the immunotypes, shared a cellular composition characterized by lower percentages of nonclassical monocytes and CD95‐HLA‐DR‐ B cells at baseline (Figure 5a).
FIGURE 5.
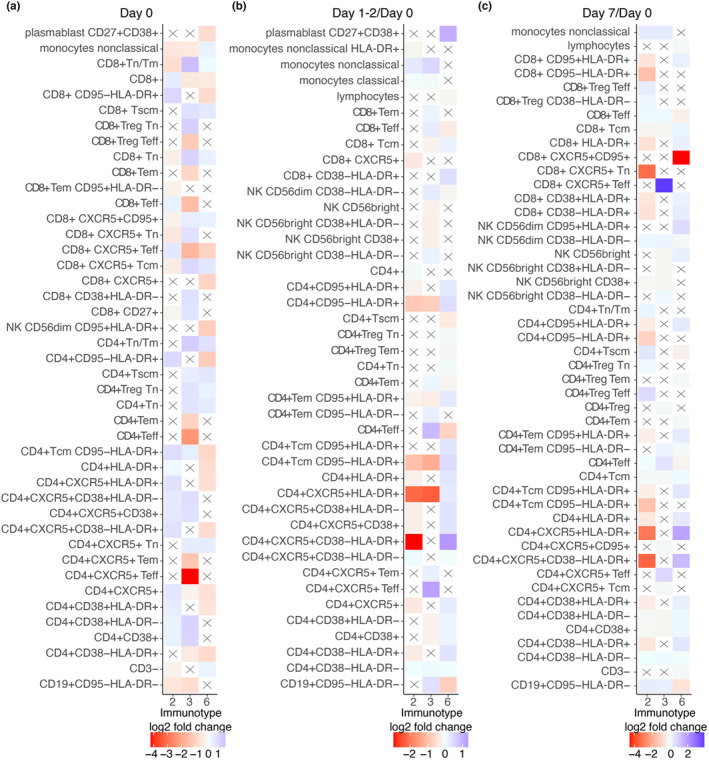
Pre‐ and post‐vaccination cellular features of weak and robust responder immunotypes. Immune subset differences at (a) baseline, (b) 1–2 days and (c) 7 days after vaccination for weak responder immunotypes 2 and 3, and robust responder immunotype 6, each compared to the rest of the immunotypes. Only the top 10 immune subsets with the highest and the lowest fold changes, which are also statistically significant (adj.p ≤ 0.05), are shown for each immunotype. An “X” symbol indicates a non‐significant comparison.
At 1–2 days post‐vaccination, the most predominant immune subset change for individuals classified in immunotype‐6 compared to rest of the immunotypes was a rapid increase in percentage of CD38‐HLA‐DR+ follicular CD4+ T cells (Figure 5b). In contrast, both immunotype‐2 and ‐3 did not show a similar early increase in percentage of HLA‐DR+ follicular CD4+ T cells compared to the rest of the immunotypes (Figure 5b). Unexpectedly, as early as 1–2 days post‐vaccination, we observed an increased percentage of plasmablasts for individuals in immunotype‐6 (Figure 4b).
At day 7, the most prominently increased immune subsets in immunotype‐6 compared to the rest of the immunotypes were percentages of HLA‐DR+ follicular CD4+ T cells and other HLA‐DR+ T cell subsets (Figure 5c). Percentages of activated follicular CD4+ T cells and plasmablasts were also significantly increased in immunotype‐6 (Table S5). Both immunotype‐2/3 showed a significant increase in percentages of T effector (Teff) cell subsets at day 7 compared to the rest of the immunotypes. For immunotype‐3, the highest increase was observed in percentage of follicular CD8+ Teff cells, whereas for immunotype‐2, CD4+ Teff Tregs showed the highest increase (Figure 5c). Remarkably, in immunotype‐6, percentage of CD95+ circulating follicular CD8+ T cells showed a significant decrease.
2.6. Nonactivated follicular CD4+ T cell increase exhibits superior post‐vaccination association with influenza antibody responses
We showed that individual immune subset variables at pre‐vaccination failed to associate with influenza antibody responses, whereas specific immunotypes did associate with weak or robust vaccine responders. Next, we investigated the associations between post‐vaccination immune subset kinetics and day 28 HI titers in regression models (Figure 1b‐circle‐6), while adjusting for factors such as sex, CMV‐seropositivity and pre‐vaccination HI titers (Table S4, Figure 6a).
FIGURE 6.
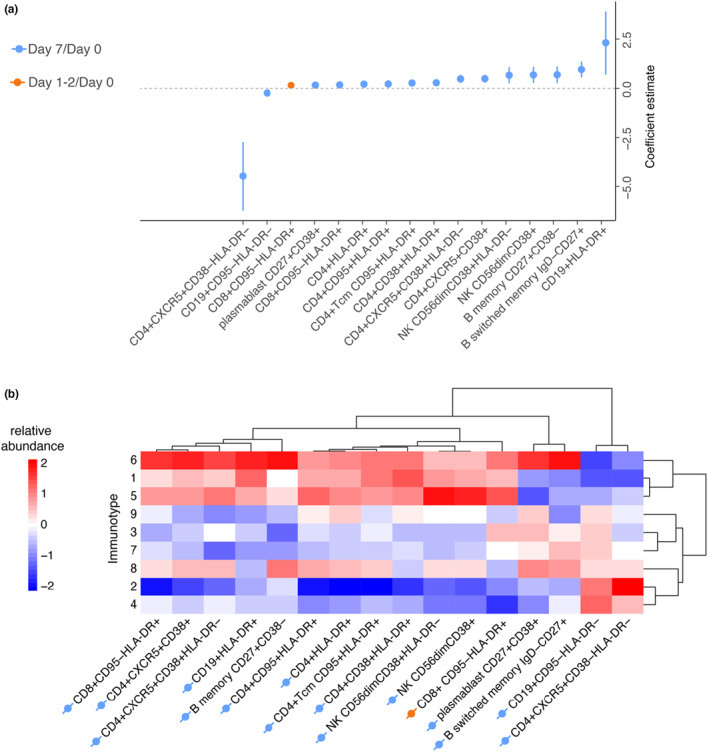
An increase in the percentage of nonactivated follicular CD4+ T cells at day 7 is the strongest associate of antibody response. (a) Regression models of day 28 HI titers using pre‐vaccination immune subsets and post‐vaccination immune subsets kinetics, corrected for sex, CMV‐seropositivity, and pre‐vaccination HI titers. Percentages of the immune subset that are significant after false discovery rate correction are shown. Blue and orange indicate fold changes of day 7/day 0 and day 1‐2/day 0 immune subsets, respectively. (b) A heatmap of post‐vaccination fold changes in immune subsets significantly associated with antibody response is shown for each immunotype. Dendrograms are calculated based on correlation distance.
An increase in percentage of nonactivated (CD38‐HLA‐DR‐) follicular (CXCR5+) CD4+ T cells at day 7 compared to day 0 showed a stronger but negative association (larger absolute coefficient value) with antibody responses (adj.p = 1.1×10−4) than activated (CD38+) follicular CD4+ T cell (adj.p = 2.0×10−4) and plasmablasts (adj.p = 1.4×10−2) (Figure 6a). To validate our findings, we independently applied Ridge and random forest regression analyses to the same dataset. In both models, an increase in percentage of nonactivated follicular CD4+ T cells demonstrated the strongest variable importance to post‐vaccination antibody responses (Figure S2a–b).
Next, we hypothesized that the increase or decrease of specific post‐vaccination immune cell subsets we identified in our regression models would be linked to the responder profile of immunotypes. Indeed, immunotype‐6, which is associated with robust antibody response, had higher fold changes of subsets that are positively associated with antibody responses. In contrast, immunotype‐2 and ‐3, which are associated with weak antibody response, had decreased fold changes for those immune subsets (Figure 6b). Thus, specific immunotypes not only associate with antibody responses but also larger increases in cell subsets associated with antibody responses.
3. DISCUSSION
Improving vaccine effectiveness is crucial, especially considering older adults who may be more susceptible to immune dysfunction and suboptimal vaccine responses. Identifying those at higher risk of immune dysfunction is of paramount importance, as this would enable us to devise personalized vaccination strategies and possibly implement alternative therapeutic approaches for their protection (Tsang et al., 2020). In this study, we aimed to determine immune subset profiles of at‐risk individuals by examining the links between pre‐ and post‐vaccination immune subsets and influenza antibody titers. We showed that specific pre‐vaccination immunotypes, based on 59 immune subsets representing distinct immune profiles, were associated with weak (immunotype‐2 & 3) and robust (immunotype‐6) responsiveness to influenza vaccination (Cevirgel et al., 2022).
Predicting post‐vaccination humoral and cellular responses based on pre‐vaccination numbers of immune cell subsets remains challenging for two main reasons. Firstly, substantial inter‐individual immune variation makes it difficult to identify biomarkers (Brodin & Davis, 2017; Cevirgel et al., 2022; Liston et al., 2021). Secondly, the relationship between pre‐vaccination immune profiles and the functional capabilities of these immune profiles is yet to be fully explored. Although several studies investigated the associates of influenza vaccination using pre‐ and post‐vaccination immune cell subsets, these studies focused on individual immune subsets as predictors of vaccination responses and did not explain the immune profiles of weak responders at pre−/post‐vaccination (Furman et al., 2013; Lakshmikanth et al., 2020; Tsang et al., 2014). We argue that such previously identified immune subsets provide incomplete insight into vaccine responsiveness since immune functionality is an emergent property of the intricate immune network, and hence, cannot be entirely attributed to individual immune cell subsets (Finzer, 2017; Chavali et al., 2008; Forlin et al., 2023). We hypothesized that a broader perspective, focusing on the composition of immune subsets, could provide a more holistic representation of the immune landscape and functionality than individual immune subsets.
Our findings endorse this hypothesis as weak response‐associated immunotype‐2 showed immune signatures of aging‐related immune subsets including a lower Tn/Tm CD4+ and CD8+ T cell ratio, immune regulation associated subsets marked by increased HLA‐DR expression in CD4+ and CD8+ T cells (Arruvito et al., 2014; Baecher‐Allan et al., 2006; Machicote et al., 2018) and lower nonclassical monocytes which are associated with vascular homeostasis (Narasimhan et al., 2019) Moreover, immunotype‐2 exhibited this perturbated immune network and lower immune stability despite being younger on average than immunotype‐6 (robust response‐associated), with a median age of 55 and 69 respectively. This suggests that, aside from chronological age, the composition of the immune network also holds substantial significance in vaccine responsiveness. Our analysis also highlighted the importance of regulation/activation markers at pre‐vaccination state of the immune system. Immunotype‐3, which is a weak response‐associated immunotype, did not exhibit aging‐related immune signatures. It did however show an activated immune landscape at pre‐vaccination, characterized by the percentage of CD38+ cells in total CD4+ T cells, follicular CD4+ and regulatory CD4+ T cell subsets. An activated immune environment with increased activated regulatory cells could hamper the development of potent immune responses which may explain the weaker response we observed in immunotype‐3 (Rocamora‐Reverte et al., 2021). On the other hand, immunotype‐6 (robust response associated), despite being older, showed a higher Tn/Tm CD4+ and CD8+ T cell ratio and nonclassical monocytes, and a lower HLA‐DR+ CD4+ and CD8+ T cells, and lack of baseline activated T cells, the opposite phenotype of weak responders.
The conventional sero‐responder categorization based on day 0 and day 28 HI titers could result in overlooking individuals who may not meet the standard criteria for responders due to a negative correlation between pre‐vaccination HI titers and day 28/ day 0 HI titer fold change (Avey et al., 2020). This limitation further complicates the identification of at‐risk individuals. Changes in post‐vaccination immune subsets could be important additional variables to study impaired vaccine responses and aid identification of at‐risk individuals. Because unlike day 28 HI titers, post‐vaccination immune subset changes were not associated with pre‐vaccination HI titers. We observed that HLA‐DR+ follicular CD4+ T cells increase as early as 1–2 days after vaccination whereas CD38+ follicular CD4+ T cells peak 7 days after vaccination. Lack of HLA‐DR and CD38 expression and accumulation of these nonactivated (CD38‐ HLA‐DR‐) circulating follicular (CXCR5+) CD4+ T cells at day 7 were a superior associate of antibody response compared to previously known correlates such as CD38+ follicular CD4+ T cells and plasmablasts. We postulate that these nonactivated follicular cells could be either “cell activation failures” or “actively suppressed.” Further in‐depth phenotyping of nonactivated follicular CD4+ T cells may reveal their heterogeneity and markers of inhibition/modulation which could help to explain these mechanisms.
Based on the post‐vaccination changes in immune subsets that were associated with antibody responses, we observed that immunotypes 1, 5, and 6 clustered more closely together on dendrograms based on correlation distances, compared to other immunotypes, suggesting a similar pattern in post‐vaccination immune subset kinetics. Notably, these immunotypes exhibited a similar pattern on correlation distance based dendogram on their pre‐vaccination immune subsets as well, which we reported previously (Cevirgel et al., 2022). This supports our view that the baseline state of the immune network is associated with its responsiveness. However, among the three closely related immunotypes only immunotype‐6 demonstrated a statistically significant difference in achieving higher day 28 antibody titers in our models. Furthermore, although immunotypes 1, 5, and 6 have similar trends in post‐vaccination kinetics compared to the rest of the immunotypes, only immunotype 6 showed the highest post‐vaccination kinetics for the immune subsets that were associated with antibody response. Lack of this post‐vaccination kinetics in immunotypes 1 and 5 could explain why these were not detected as phenotypes of robust responders.
Our study is not without limitations. While immunotypes‐2/−3–6 display significant differences in antibody responses, there remains considerable variation in their antibody responses within these phenotypes. Since immunotypes contain individuals from various age groups these individuals are likely to have encountered different influenza strains throughout their lives (Cevirgel et al., 2022). This leads to a phenomenon known as immune imprinting or original antigenic sin where immune memory of a pathogen's initial strain could limit immune system's ability to respond to a new strain could be a factor in this variation (Turner et al., 2020). Moreover, our findings on influenza vaccination may not translate into other types of vaccines or cohorts. Future research should investigate whether baseline immune profiles associate with vaccine responsiveness in different older populations and vaccine platforms. In our study, although cellular senescence and exhaustion related markers such as CD57, PD‐1, CTLA‐4, were not included, these markers would be highly relevant to investigate in relation to immunotypes.
In conclusion, our study emphasizes that the composition of pre‐vaccination immune subsets, or “immunotypes,” may serve as a superior indicator of the functional capacity of the immune response to vaccination, as compared to individual immune cell subsets. This approach could potentially identify individuals at risk of suboptimal vaccine response, thereby guiding the development of more targeted and personalized vaccination strategies. In a broader context, these insights hold promise not only for evaluating vaccine responsiveness but also for understanding immune function in general, which could pave the way to a more profound comprehension of immune health.
4. METHODS AND MATERIAL
4.1. Cohort description and sampling
The Vaccines and InfecTious diseases in the Aging popuLation (VITAL) is a cohort started in 2019 in the Netherlands as described previously (Cevirgel et al., 2022; van Baarle et al., 2020). In short, VITAL contains young, middle‐aged and community‐dwelling older adults (N = 326) (aged 25–98 years old) who were vaccinated with the previous year's seasonal influenza vaccine in 2018–2019. In 2019–2020, these individuals were vaccinated with the seasonal quadrivalent inactivated subunit influenza vaccine (QIV); Influvac Tetra (Abbott Biologicals B.V. The Netherlands) which contained neuraminidase and hemagglutinin from the following viral strains: A/Brisbane/02/2018, IVR‐190(H1N1); A/Kansas/14/2017, NYMC X‐327 (H3N2); B/Maryland/15/2016, NYMC BX‐69A (B/Victoria/2/87 lineage); and B/Phuket/3073, wildtype (B/Yamagata/16/88 lineage) (Abbott Biologicals B.V. The Netherlands). For immune phenotyping whole blood samples (day 0, day 1–2, day 7) and for HI assay serum samples (day 0, day 28) were collected (Figure 1a). Individuals were excluded when they use or used immune‐modulatory drugs or have a disease that make them immunocompromised, including recipient of an organ‐ or bone marrow transplant, used high‐dose of daily corticosteroids or received chemotherapy in the last 3 years. In addition, individuals were excluded when they had a history of allergic reaction to vaccine components and factors that may interfere with blood collection, including known anemia (hemoglobin 8.5 mmol/L for men, 7.5 mmol/L for women) and known or suspected coagulation disorder, or known infection with immunodeficiency virus and/or hepatitis B and/or C virus. The overall health status of the VITAL cohort is relatively high as these individuals are community‐dwelling adults and relatively non‐frail (Rockwood frailty, mean = 0.15, 95% CI 0.14–0.16).
Ethical approval was obtained through the Medical Research Ethics Committee Utrecht (NL69701.041.19, EudraCT: 2019–000836‐24). All participants provided written informed consent and all procedures were performed with Good Clinical Practice and in accordance with the principles of the Declaration of Helsinki.
4.2. Flow cytometry immune phenotyping
Flow cytometry and immune subset gating was performed as recently described (Cevirgel et al., 2022). In short, whole blood samples before (day 0), and after vaccination (day 1–2, day 7) were stained with anti‐human fluorophore‐conjugated antibodies and acquired on a 4‐lasr LSRII Fortressa X20 flow cytometer (BD Biosciences). Absolute number of cells was calculated from event counts of TruCOUNT beads (BD Biosciences). Both percentage and absolute numbers of immune subsets were studied since the former describes the relative abundance of a subset in the parent population whereas the latter represents the changes in total immune cell counts.
4.3. Detection of baseline anti‐influenza virus antibodies
To investigate antibody responses to QIV, antibodies against the H3N2 (A/Kansas/14/2017) strain were measured at Viroclinics (Rotterdam, the Netherlands) using the HI assay according to the standard methods of the World Health Organization (WHO) as explained in (ECDC, 2017; Huber et al., 2019; Luytjes et al., 2012). In short, a dilution series of serum samples was incubated with four Hemagglutinin Units (HAU) influenza virus for 20 min and thereafter incubated for 30 min with 0.25% turkey erythrocytes. Subsequently, the antibody titer (geometric mean titer) was determined as the reciprocal of the highest dilution of serum that prevents complete hemagglutination wells.
4.4. CMV seropositivity
Immunoglobulin G antibodies against CMV were quantified in serum by a multiplex immunoassay developed in‐house and CMV‐seropositivity thresholds were adapted from a previous study, as recently described (Cevirgel et al., 2022).
4.5. Statistical modeling
Generalized linear model with binomial distribution (GLM) was built from sex, CMV‐seropositivity and pre‐vaccination HI titer groups to evaluate the effects on sero‐responder categorization. The GLM's Logit estimates were converted into Odds Ratio values through exponential transformation. For day 28 HI titer regression models, which is count data, negative binomial distribution showed a good fit to antibody data. Therefore, Generalized Additive Models for Location Scale and Shape (GAMLSS) function from “gamlss” R package which allowed negative binomial distribution were used (Stasinopoulos & Rigby, 2007). For immunotype and day 0 HI titer categories sum coding was used to compare the mean of a dependent variable for a given level to the overall mean of the dependent variable (Schad et al., 2020).
4.6. Clustering analyses
At pre‐vaccination, individuals were clustered into immunotypes as recently described (Cevirgel et al., 2022). In short, pairwise spearman correlation matrix of individuals based on 59 baseline immune subsets was calculated. The number of clusters (immunotypes) was decided based on Gap statistics and the data was clustered using k‐means.
4.7. Statistical analysis
Statistical analyses were performed using R (v4.2.2) and Rstudio (v2022.12.0 + 353). The sero‐responder categorization based on pre‐vaccination HI titers, sex, and CMV was implemented via the General Linear Models (GLM) from stats package (v4.2.2). Day 28 HI titer regression models were built using the GAMLSS package (v5.4.12). Heatmaps were generated with the pheatmap library (v1.0.12), and rstatix package (v0.7.2) was used for additional statistical analyses. For group comparisons of immune subsets at day 0, day 1–2, and day 7, the Kruskal–Wallis test was employed, followed by Dunn's test when p‐values were found to be significant. P values were adjusted for multiple comparisons using the Benjamini–Hochberg correction and reported as “adj.p”. Statistical significance is indicated in tables as follows: + p < 0.1 *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
AUTHOR CONTRIBUTIONS
Alper Cevirgel, Sudarshan A. Shetty, Nynke Rots, Anne‐Marie Buisman, and Debbie van Baarle conceptualized the study. Alper Cevirgel, Sudarshan A. Shetty performed methodology. Alper Cevirgel, Sudarshan A. Shetty, Martijn Vos, Nening M. Nanlohy LB, Josine van Beek and Nynke Rots performed the study. Alper Cevirgel and Sudarshan A. Shetty visualized the data. Nynke Rots, Anne‐Marie Buisman, and Debbie van Baarle were involved in funding acquisition. Lisa Beckers, Elske Bijvank, Josine van Beek, and Debbie van Baarle were involved in project administration. Sudarshan A. Shetty, Anne‐Marie Buisman, and Debbie van Baarle supervised the study. Alper Cevirgel wrote the original draft. Alper Cevirgel, Sudarshan A. Shetty, Anne‐Marie Buisman, Debbie van Baarle are involved in writing—reviewing and editing.
FUNDING INFORMATION
The VITAL project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No. 806776 and the Dutch Ministry of Health, Welfare and Sport. The JU receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA‐members.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Supporting information
Figure S1: Flowchart of data analyses.
Figure S2: Percentage of nonactivated follicular CD4+ T cell increase at day 7 is the most important associate of antibody responses in ridge & random forest regression models. (a) Coefficients (Ridge regression) and (b) variable importance (random forest regression) of day 28 HI titer regression analyses. Percentages of significant immune subsets (adj.p < 0.05) that associated with antibody responses are used in both regression analyses.
Table S1: Differences in immune subsets between days 0 and 1–2, and between days 0 and 7.
Table S2: Correlations between pre‐ and post‐vaccination immune subsets.
Table S3: Regression models investigating the association of post‐vaccination immune subset kinetics with pre‐vaccination HI titer groups, sex, and CMV status.
Table S4: Regression models analyzing the influence of immune subsets (measured on days 0, 1–2, and 7) on day 28 antibody titers, accounting for pre‐vaccination HI titer groups, sex, and CMV status.
Table S5: Immune subset comparisons between immunotypes 2–3‐6 and rest of the immunotypes for day 0, day 1–2 and day 7.
ACKNOWLEDGMENTS
We thank Markus Viljanen for day 28 antibody response modeling, Marieke van Heiden for reading and giving feedback on the manuscript and Megan Barnes, Lysanne Bakker, Silvia Cohen, Shirley Man, Ilse Akkerman, Ruben Wiegmans for their help in handling the clinical samples. Human and vial icons in Figure 1 are created using BioRender.com.
Cevirgel, A. , Shetty, S. A. , Vos, M. , Nanlohy, N. M. , Beckers, L. , Bijvank, E. , Rots, N. , van Beek, J. , Buisman, A.‐M. , & van Baarle, D. (2024). Pre‐vaccination immunotypes reveal weak and robust antibody responders to influenza vaccination. Aging Cell, 23, e14048. 10.1111/acel.14048
DATA AVAILABILITY STATEMENT
Research data are not shared since the primary endpoints are not yet published. The codes used in the manuscript are available from GitHub (https://github.com/alpercevirgel/Immunotype‐Influenza‐Response)
REFERENCES
- Allen, J. C. , Toapanta, F. R. , Chen, W. H. , & Tennant, S. M. (2020). Understanding immunosenescence and its impact on vaccination of older adults. Vaccine, 38, 8264–8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruvito, L. , Payaslian, F. , Baz, P. , Podhorzer, A. , Billordo, A. , Pandolfi, J. , Semeniuk, G. B. , Arribalzaga, E. B. , & Fainboim, L. (2014). Identification and clinical relevance of naturally occurring human CD8+HLA‐DR+ regulatory T cells. Journal of Immunology, 193, 4469–4476. [DOI] [PubMed] [Google Scholar]
- Avey, S. , Mohanty, S. , Chawla, D. G. , Meng, H. , Bandaranayake, T. , Ueda, I. , Zapata, H. J. , Park, K. , Blevins, T. P. , Tsang, S. , Belshe, R. B. , Kaech, S. M. , Shaw, A. C. , & Kleinstein, S. H. (2020). Seasonal variability and shared molecular signatures of inactivated influenza vaccination in young and older adults. Journal of Immunology, 204, 1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher‐Allan, C. , Wolf, E. , & Hafler, D. A. (2006). MHC class II expression identifies functionally distinct human regulatory T cells. Journal of Immunology, 176, 4622–4631. [DOI] [PubMed] [Google Scholar]
- Brodin, P. , & Davis, M. M. (2017). Human immune system variation. Nature Reviews Immunology, 17, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre, C. , Wong, G. , Narang, V. , Tan, C. T. Y. , Chong, J. , Chin, H. X. , Xu, W. , Lu, Y. , Chua, M. , Poidinger, M. , Tambyah, P. A. , Nyunt, M. S. Z. , Ng, T. P. , Larocque, D. , Hessler, C. , Bosco, N. , Quemeneur, L. , & Larbi, A. (2021). Endoplasmic reticulum stress response and bile acid signatures associate with multi‐strain seroresponsiveness during elderly influenza vaccination. iScience, 24, 102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevirgel, A. , Shetty, S. , Vos, M. , Nanlohy, N. , Beckers, L. , Bijvank, E. , Rots, N. , Van, J. B. , Buisman, A. , & Van, D. B. (2022). Identification of aging‐associated immunotypes and immune stability as indicators of post‐vaccination immune activation. Aging Cell, 21, e13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavali, A. K. , Gianchandani, E. P. , Tung, K. S. K. , Lawrence, M. B. , Peirce, S. M. , & Papin, J. A. (2008). Characterizing emergent properties of immunological systems with multi‐cellular rule‐based computational modeling. Trends in Immunology, 29, 589–599. [DOI] [PubMed] [Google Scholar]
- ECDC . (2017). Immunity following influenza disease and administration of influenza vaccines. Available at: https://www.ecdc.europa.eu/en/seasonal‐influenza/prevention‐and‐control/vaccines/immunity [Accessed October 16, 2023].
- Finzer, P. (2017). How we become ill: Investigating emergent properties of biological systems could help to better understand the pathology of diseases. EMBO Reports, 18, 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlin, R. , James, A. , & Brodin, P. (2023). Making human immune systems more interpretable through systems immunology. Trends in Immunology., 44, 577–584. [DOI] [PubMed] [Google Scholar]
- Fulop, T. , Larbi, A. , Hirokawa, K. , Cohen, A. A. , & Witkowski, J. M. (2020). Immunosenescence is both functional adaptive and dysfunctional maladaptive. Seminars in Immunopathology, 42, 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman, D. , Jojic, V. , Kidd, B. A. , Shen‐Orr, S. S. , Price, J. V. , Jarrell, J. A. , Tse, T. , Huang, H. , Lund, P. , Maecker, H. T. , Utz, P. J. , Dekker, C. L. , Koller, D. , & Davis, M. M. (2013). Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Molecular Systems Biology, 9, 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, K. , Viboud, C. , & Simonsen, L. (2006). Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine, 24, 1159–1169. [DOI] [PubMed] [Google Scholar]
- Huber, S. K. R. , Hendriks, M. , Jacobi, R. , van de Kassteele, J. , van de Kassteele, J. , Mandersloot‐Oskam, J. C. , van Boxtel, R. A. J. , Wensing, A. M. J. , Wensing, A. M. J. , Rots, N. Y. , Luytjes, W. , Luytjes, W. , & van Beek, J. (2019). Immunogenicity of influenza vaccines: Evidence for differential effect of secondary vaccination on humoral and cellular immunity. Frontiers in Immunology, 9, 3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski, K. J. , Shekhar, K. , Nkulikiyimfura, D. , Dekker, C. L. , Maecker, H. T. , Davis, M. M. , Chakraborty, A. K. , & Brodin, P. (2017). Continuous immunotypes describe human immune variation and predict diverse responses. Proceedings of the National Academy of Sciences of the United States of America., 114, E6097–E6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsakos, M. , Nguyen, T. H. O. , & Kedzierska, K. (2019). With a little help from T follicular helper friends: Humoral immunity to influenza vaccination. Journal of Immunology., 202, 360–367. [DOI] [PubMed] [Google Scholar]
- Künzel, W. , Künzel, W. , Glathe, H. , Engelmann, H. , & Van Hoecke, C. (1996). Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine, 14, 1108–1110. [DOI] [PubMed] [Google Scholar]
- Lakshmikanth, T. , Muhammad, S. A. , Olin, A. , Chen, Y. , Mikeš, J. , Fagerberg, L. , Gummesson, A. , Bergström, G. , Uhlén, M. , & Brodin, P. (2020). Human immune system variation during 1 year. In Human immune system variation during 1 year. Cell Reports. [DOI] [PubMed] [Google Scholar]
- Liston, A. , Humblet‐Baron, S. , Duffy, D. , & Goris, A. (2021). Human immune diversity: From evolution to modernity. Nature Immunology, 22, 1479–1489. [DOI] [PubMed] [Google Scholar]
- Luytjes, W. , Luytjes, W. , Luytjes, W. , Enouf, V. , Enouf, V. , Schipper, M. , Schipper, M. , Schipper, M. , Gijzen, K. , Liu, W. M. , van der Lubben, M. , Meijer, A. , Meijer, A. , van der Werf, S. , & Soethout, E. C. (2012). HI responses induced by seasonal influenza vaccination are associated with clinical protection and with seroprotection against non‐homologous strains. Vaccine, 30, 5262–5269. [DOI] [PubMed] [Google Scholar]
- Machicote, A. , Belén, S. , Baz, P. , Billordo, L. A. , & Fainboim, L. (2018). Human CD8+HLA‐DR+ regulatory T cells, similarly to classical CD4+Foxp3+ cells, suppress immune responses via PD‐1/PD‐L1 Axis. Frontiers in Immunology, 9, 2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan, P. B. , Narasimhan, P. B. , Marcovecchio, P. , Hamers, A. A. J. , & Hedrick, C. C. (2019). Nonclassical monocytes in health and disease. Annual Review of Immunology, 37, 439–456. [DOI] [PubMed] [Google Scholar]
- Ohmit, S. E. , Petrie, J. G. , Cross, R. T. , Johnson, E. , & Monto, A. S. (2011). Influenza Hemagglutination‐inhibition antibody titer as a correlate of vaccine‐induced protection. The Journal of Infectious Diseases, 204, 1879–1885. [DOI] [PubMed] [Google Scholar]
- Osterholm, M. T. , Kelley, N. S. , Sommer, A. , & Belongia, E. A. (2012). Efficacy and effectiveness of influenza vaccines: A systematic review and meta‐analysis. Lancet Infectious Diseases, 12, 36–44. [DOI] [PubMed] [Google Scholar]
- Pepin, S. , Nicolas, J.‐F. , Nicolas, J.‐F. , Szymański, H. , Leroux‐Roels, I. , Schaum, T. , Bonten, M. J. M. , Icardi, G. , Shrestha, A. , & Tabar, C. (2021). Immunogenicity and safety of a quadrivalent high‐dose inactivated influenza vaccine compared with a standard‐dose quadrivalent influenza vaccine in healthy people aged 60 years or older: A randomized phase III trial. Human Vaccines & Immunotherapeutics, 17, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubramanian, R. , Meier, H. C. S. , Vivek, S. , Klopack, E. , Crimmins, E. M. , Faul, J. , Nikolich‐Žugich, J. , & Thyagarajan, B. (2022). Evaluation of T‐cell aging‐related immune phenotypes in the context of biological aging and multimorbidity in the health and retirement study. Immunity & Ageing : I & A, 19(1), 33. 10.1186/s12979-022-00290-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocamora‐Reverte, L. , Melzer, F. L. , Würzner, R. , & Weinberger, B. (2021). The complex role of regulatory T cells in immunity and aging. Frontiers in Immunology, 11, 616949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, A. , Rose, A. M. C. , Kissling, E. , Emborg, H.‐D. , Larrauri, A. , McMenamin, J. , Pozo, F. , Trebbien, R. , Trebbien, R. , Mazagatos, C. , Whitaker, H. , Whitaker, H. , & Valenciano, M. (2020). Interim 2019/20 influenza vaccine effectiveness: Six European studies, September 2019 to January 2020. Eurosurveillance, 25, 2000153.32183932 [Google Scholar]
- Sasaki, S. , Sullivan, M. , Narváez, C. F. , Holmes, T. H. , Furman, D. , Zheng, N. Y. , Nishtala, M. , Nishtala, M. , Wrammert, J. , Smith, K. , Smith, K. J. , James, J. A. , Dekker, C. L. , Davis, M. M. , Wilson, P. C. , Greenberg, H. B. , & He, X.‐S. (2011). Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine‐specific antibodies. Journal of Clinical Investigation, 121, 3109–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad, D. J. , Vasishth, S. , Hohenstein, S. , & Kliegl, R. (2020). How to capitalize on a priori contrasts in linear (mixed) models: A tutorial. Journal of Memory and Language, 110, 104038. [Google Scholar]
- Stasinopoulos, D. M. , & Rigby, R. A. (2007). Generalized additive models for location scale and shape (GAMLSS) in R. Journal of Statistical Software, 23, 1–46. [Google Scholar]
- Tomic, A. , Tomic, I. , Rosenberg‐Hasson, Y. , Dekker, C. L. , Maecker, H. T. , & Davis, M. M. (2019). SIMON, an automated machine learning system, reveals immune signatures of influenza vaccine responses. Journal of Immunology, 203, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, J. S. , Dobaño, C. , Van‐Damme, P. , Moncunill, G. , Marchant, A. , Othman, R. B. , Sadarangani, M. , Koff, W. C. , & Kollmann, T. R. (2020). Improving vaccine‐induced immunity: Can baseline predict outcome? Trends in Immunology, 41, 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, J. S. , Schwartzberg, P. L. , Kotliarov, Y. , Biancotto, A. , Xie, Z. , Germain, R. N. , Wang, E. , Olnes, M. J. , Narayanan, M. , Golding, H. , Moir, S. , Dickler, H. B. , Perl, S. , & Cheung, F. (2014). Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell, 157, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, J. S. , Zhou, J. Q. , Han, J. C. , Han, J. , Schmitz, A. J. , Rizk, A. A. , Alsoussi, W. B. , Lei, T. , Amor, M. , McIntire, K. M. , Meade, P. , Strohmeier, S. , Brent, R. I. , Richey, S. T. , Haile, A. , Yang, Y. R. , Klebert, M. K. , Suessen, T. , Teefey, S. A. , … Ellebedy, A. H. (2020). Human germinal centres engage memory and naive B cells after influenza vaccination. Nature, 586, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, H. (2019). Tfh cell response in influenza vaccines in humans: What is visible and what is invisible. Current Opinion in Immunology, 59, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baarle, D. , Bollaerts, K. , Giudice, G. D. , Lockhart, S. , Luxemburger, C. , Postma, M. J. , Timen, A. , & Standaert, B. (2020). Preventing infectious diseases for healthy ageing: The VITAL public‐private partnership project. Vaccine, 38(37), 5896–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Flowchart of data analyses.
Figure S2: Percentage of nonactivated follicular CD4+ T cell increase at day 7 is the most important associate of antibody responses in ridge & random forest regression models. (a) Coefficients (Ridge regression) and (b) variable importance (random forest regression) of day 28 HI titer regression analyses. Percentages of significant immune subsets (adj.p < 0.05) that associated with antibody responses are used in both regression analyses.
Table S1: Differences in immune subsets between days 0 and 1–2, and between days 0 and 7.
Table S2: Correlations between pre‐ and post‐vaccination immune subsets.
Table S3: Regression models investigating the association of post‐vaccination immune subset kinetics with pre‐vaccination HI titer groups, sex, and CMV status.
Table S4: Regression models analyzing the influence of immune subsets (measured on days 0, 1–2, and 7) on day 28 antibody titers, accounting for pre‐vaccination HI titer groups, sex, and CMV status.
Table S5: Immune subset comparisons between immunotypes 2–3‐6 and rest of the immunotypes for day 0, day 1–2 and day 7.
Data Availability Statement
Research data are not shared since the primary endpoints are not yet published. The codes used in the manuscript are available from GitHub (https://github.com/alpercevirgel/Immunotype‐Influenza‐Response)


