Abstract
Skin aging is characterized by changes in its structural, cellular, and molecular components in both the epidermis and dermis. Dermal aging is distinguished by reduced dermal thickness, increased wrinkles, and a sagging appearance. Due to intrinsic or extrinsic factors, accumulation of excessive reactive oxygen species (ROS) triggers a series of aging events, including imbalanced extracellular matrix (ECM) homeostasis, accumulation of senescent fibroblasts, loss of cell identity, and chronic inflammation mediated by senescence‐associated secretory phenotype (SASP). These events are regulated by signaling pathways, such as nuclear factor erythroid 2‐related factor 2 (Nrf2), mechanistic target of rapamycin (mTOR), transforming growth factor beta (TGF‐β), and insulin‐like growth factor 1 (IGF‐1). Senescent fibroblasts can induce and accelerate age‐related dysfunction of other skin cells and may even cause systemic inflammation. In this review, we summarize the role of dermal fibroblasts in cutaneous aging and inflammation. Moreover, the underlying mechanisms by which dermal fibroblasts influence cutaneous aging and inflammation are also discussed.
Keywords: dermis, ECM, fibroblast, inflammation, senescence
This mini review discusses aging, inflammation, cellular senescence, and their orchestration in cutaneous aging, recognizing fibroblast senescence as central player and inflammation response as key mediator in these processes. We delve into the cascade effects of stress, senescence, SASP, and related signaling in fibroblasts, elucidating how they contribute to the amplification of inflammation and ultimately lead to the appearance and functional alterations observed in aged skin.
Abbreviations
- C/EBPβ
CCAAT/enhancer‐binding protein beta
- CDK
cyclin‐dependent kinases
- CTGF/CCN2
connective tissue growth factor
- ECM
extracellular matrix
- EVs
extracellular vesicles
- GATA4
GATA‐ binding protein 4
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- HO‐1
heme oxygenase 1
- IFNγ
interferon gamma
- IGF‐1
insulin‐like growth factor 1
- JAK‐STAT
Janus kinase‐signal transducer and activator of transcription
- KGF
keratinocytes growth factors
- MAPK
mitogen‐activated protein kinase
- MMPs
matrix metalloproteinases
- mTOR
mechanistic target of rapamycin
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- NQO1
NAD(P)H quinone dehydrogenase 1
- Nrf2
erythroid 2‐related factor 2
- OIS
oncogene‐induced senescence
- ROS
reactive oxygen species
- SAASP
skin aging‐associated protein
- SASP
senescence‐associated secretory phenotype
- SA‐β‐gal
β‐galactosidase
- SOD
superoxide dismutase
- TGF‐β
transforming growth factor‐ beta
- TIMP
tissue inhibitors of MMPs
- TNFα
tumor necrosis factor‐ alpha
- UV
ultraviolet
1. INTRODUCTION
Aging is a complex process resulting in decline in functions of tissues/organs. This event is driven by a variety of intrinsic and extrinsic factors, such as DNA damage accumulation (d’Adda di Fagagna, 2008), shortened telomeres (Olovnikov, 1996), mitochondrial dysfunction (Wiley et al., 2016), and exposure to other environmental stressors (Kammeyer & Luiten, 2015). These factors direct the cells to enter a state of irreversible growth arrest known as senescence (Colavitti & Finkel, 2005; Coppé et al., 2008). Although senescent cells are no longer able to divide, they remain metabolically active and can secrete a mixture of molecules known as senescence‐associated secretory phenotype (SASP) that contribute to inflammation via autocrine and paracrine mechanisms (Rodier et al., 2009).
Skin, primarily comprising the epidermis and the dermis, serves as a physical barrier protecting the body from external insults. The epidermis, a stratified squamous epithelium, is mainly composed of keratinocytes, melanocytes, and Langerhans cells (Liu et al., 2021; Tang et al., 2020). Situated beneath the epidermis, the dermis plays a crucial role in structure and function. It contains an abundant extracellular matrix (ECM) produced by fibroblasts and houses various cell types due to its diverse structures, including vasculature, nerves, sweat glands (Hosseini et al., 2022; Weng et al., 2020) (Figure 1). Constantly exposed to external insults, the skin undergoes significant changes through our lifetime that differentiate the skin of a child from that of an older adult. These changes are caused by a combination of intrinsic aging, also known as chronological aging, and extrinsic aging induced by environmental factors, including air pollution, poor nutrition, smoking, and ultraviolet (UV) light. Both epidermal keratinocyte and dermal fibroblast senescence contribute to the skin aging (Fitsiou et al., 2021; Gruber et al., 2020; Wang et al., 2020). The role of keratinocytes in aging and inflammation has been well summarized by others (Wang et al., 2020). In this review, we focus on the dermal fibroblasts in skin aging and propose the crucial role of dermal fibroblasts in inflammation.
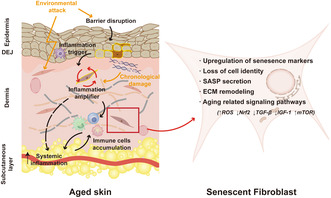
FIGURE 1.
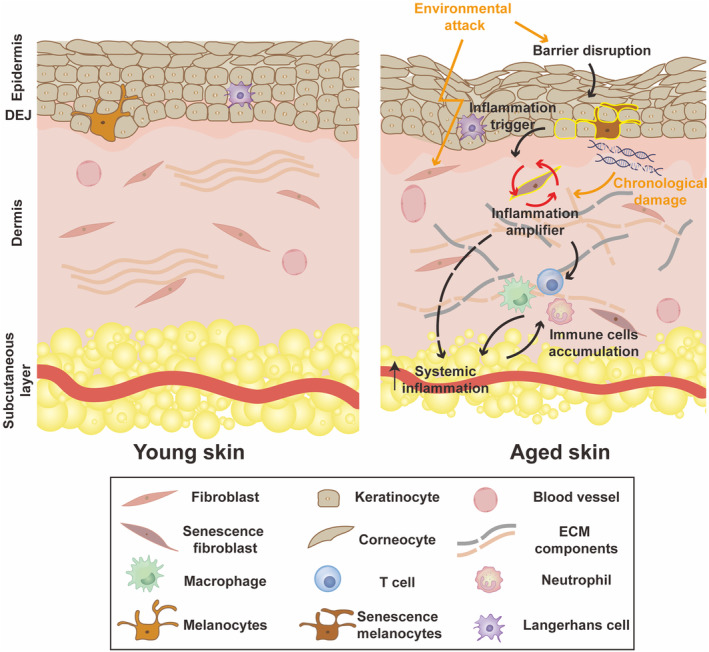
Schematic representation of dermal fibroblasts as a crucial amplifier in skin aging and inflammation. Aged skin is characterized by a dysfunctional epidermal barrier, thinner dermis, accumulated senescent fibroblasts, excessive immune cells, and fragmented ECM components. Chronological changes in cutaneous functions and external stressors induce skin cells such as keratinocytes and melanocytes to initiate skin senescence. Fibroblasts can receive aging signals through paracrine resources, such as neighboring cells like keratinocytes and melanocytes, or through autocrine secretion of SASP induced by intrinsic or extrinsic factors. Senescent fibroblasts play a key role in exacerbating cutaneous aging and amplifying inflammation by producing cytokines, chemokines, and other factors. This attracts and activates immune cells, leading to inflammation not only in the skin but also potentially in other parts of the body.
2. MECHANISMS OF DERMAL AGING
2.1. Excessive ROS, a driving force of dermal aging
Skin aging involves the intricate interplay of various mechanisms and multiple causal processes, such as nuclear DNA damage (García‐Beccaria et al., 2014; Rodier et al., 2009), generation of excessive reactive oxygen species (ROS), and mitochondria dysfunction (Kaneko et al., 2012; Krutmann & Schroeder, 2009; Yang et al., 1995). Among these mechanisms, the oxidative stress theory holds a prominent position, which emphasizes ROS as driving force of aging. ROS, also known as free radicals or oxidants, possess diverse properties and biological functions, ranging from oxidative metabolism to cell signaling (Ray et al., 2012; Sies & Jones, 2020). Under normal physiological conditions, ROS are generated as natural byproducts of cell metabolism. However, as we age, ROS assume a dual role, acting as both primary triggers and critical consequences of skin aging. Intrinsic factors such as mitochondrial dysfunction (Sreedhar et al., 2020), along with external factors like UV radiation (Chaiprasongsuk & Panich, 2022), and other stressors, synergistically augment ROS production and retard ROS removal by antioxidants. Overtime, the accumulation of ROS proceeds to oxidize lipids, nucleic acids, proteins, and organelles, leading to the cell and tissue dysfunction (Lee & Wei, 2001; Rinnerthaler et al., 2015). It is worth noting that a vicious circle exists between oxidative stress and inflammation during aging. ROS serve as signaling molecules that trigger inflammatory responses, and inflammatory cytokines and chemokines in turn generate more ROS and free radicals (Kammeyer & Luiten, 2015; Zinovkin et al., 2022).
The pathogenic role of accumulated ROS in chronological aging (Papaccio et al., 2022; Poljšak et al., 2012; Tu & Quan, 2016) is supported by a significant increase in ROS levels in aged human fibroblasts in vitro (Kozieł et al., 2011) and in aged rat skin in vivo (Tahara et al., 2001). In addition, a line of evidence also supports the role of excessive ROS in skin photoaging in vitro and in vivo (Jurkiewicz & Buettner, 1996; Li et al., 2018; Masaki et al., 1995; Yasui & Sakurai, 2000). In addition, inhibition of ROS production in these cells reduces the number of cells entering cell‐cycle arrest (Cavinato et al., 2017). The driving force of ROS in skin aging and fibroblast senescence is evident in a mouse model with fibroblast specific superoxide dismutase‐2 (SOD2) deficiency, where mitochondrial superoxide anions accumulate in fibroblasts. Selective SOD2 deficiency results in a severe and accelerated skin aging phenotype, characterized by reduced collagen and dermal thickness, diminished resilience, enhanced DNA damage, and accumulation of senescent fibroblasts (Weyemi et al., 2012). Excessive ROS in fibroblasts can activate several signaling pathways, including mitogen‐activated protein kinase (MAPK), nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB), TGF‐β, and mTOR, leading to the accumulation of senescent fibroblasts, induction of chronic inflammation, and disruption of ECM homeostasis (Ansary et al., 2021; Bang et al., 2021; Chen et al., 2022; Gu et al., 2020).
2.2. Role of senescent dermal fibroblasts in skin aging
Senescence inducers, including stressors (ROS, DNA damage, irradiation), telomere attrition, and mitochondria dysfunction, increase the activity of cyclin‐dependent kinases (CDK) inhibitor proteins, resulting in cell‐cycle arrest. In comparison with young skin (18–29 year), the total number of fibroblasts is reduced by approximately 35% in aged skin (>80 year) (Varani et al., 2000), while the number of senescent fibroblasts is increased with age, as evidenced by a significant increase in p16INK4a positive cells (a senescent cell marker that encodes an inhibitor of CDK4/6) in the dermis of the aged human skin (Ogata et al., 2021; Ressler et al., 2006; Waaijer et al., 2012). The number of p16INK4a positive cells is also correlated with wrinkle formation and elastic morphological changes (Waaijer et al., 2016). Other classical senescence biomarkers, such as p21CIP1, p53, and β‐galactosidase (SA‐β‐gal), are upregulated, and lamin B1 is downregulated in aged or UV‐irradiated fibroblasts (Chen et al., 2008; Dimri et al., 1995; McCart et al., 2017; Ravelojaona et al., 2009; Wang et al., 2017). Like other senescent cells, senescent fibroblasts also experience proliferation arrest, yet remain viable due to their low propensity for apoptosis and inefficient removal by the immune system, causing them to persist in the stroma. An excessive buildup of senescence fibroblasts contributes significantly to skin aging, as these fibroblasts display loss of cell identity (Solé‐Boldo et al., 2020; Salzer et al., 2018; Zou et al., 2021), enhanced release of SASP (Rodier et al., 2009; Waldera‐Lupa et al., 2014), and dysfunction of ECM homeostasis (Treiber et al., 2011; Wlaschek et al., 2021) (Figure 2). Consequently, senescence spreads from cells to cells, fueling the process of dermal aging (da Silva et al., 2019).
FIGURE 2.
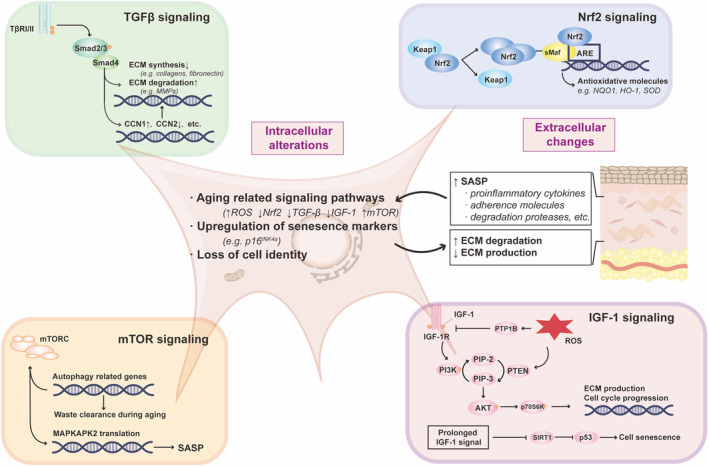
Extracellular and intracellular alterations occur during dermal aging. Intrinsic and extrinsic factors induce alterations in age‐related pathways in dermal fibroblasts, including Nrf2 signaling, TGF‐β signaling, IGF‐1 signaling, and mTOR signaling. Additionally, an upregulation of senescence markers and loss of cell identity are also evident during fibroblast aging. The senescent fibroblasts secrete multiple SASP factors that contribute to inflammatory response and ECM dysregulation.
2.3. Dermal SASP in skin aging
SASP refers to a mixture of molecules (including cytokines, matrix metalloproteinases(MMPs), miRNAs, chemokines, growth factors, and small‐molecule metabolites) released by senescent cells, which have immunoregulatory effects and impact the proliferation and motility of non‐senescent cells. Proteins that are involved in matrix degradation (MMP1, MMP3, MMP10, MMP14, etc.) and proinflammatory processes, such as interleukin‐1β (IL‐1β), IL‐8, IL‐15, interferon gamma (IFNγ) have been found in both skin aging‐associated protein (SAASP) and canonical SASP, suggesting shared senescent traits across different tissue contexts (Waldera Lupa et al., 2015). Moreover, unique expression patterns of proteins related to metabolism and adherence junction interactions were found in SAASP (Waldera Lupa et al., 2015). Epilipomics have revealed the presence of SASP lipids in aged dermal fibroblasts, with lysophosphatidylcholines as a pleiotropic factor that can elicit chemokine release in non‐senescent fibroblasts and interfere with macrophage activity (Narzt et al., 2021).
2.4. Inflammatory factors in SASP bridges inflammatory communication between cells
The inflammatory factors in SASP play a dual role in cellular senescence. In an autocrine manner, they reinforce the senescence and inflammatory state of fibroblast themselves (Acosta et al., 2008; Kumar et al., 1992; Wlaschek et al., 1994). Simultaneously, they can act in a paracrine manner to induce inflammatory response in surrounding cells (Acosta et al., 2013; Ghosh & Capell, 2016; Wlaschek et al., 2021). Senescent human dermal fibroblasts produce extracellular vesicles (EVs) which are less supportive for keratinocyte differentiation and barrier function, but contain higher levels of IL‐6 compared to EVs from young dermal fibroblasts (Choi et al., 2020). SASP cytokines and chemokines, such as IL‐1, IL‐8, and tumor necrosis factor alpha (TNFα), attract immune cells like macrophages, neutrophils, and T cells. SASP secreted by senescent fibroblasts can hinder macrophage‐dependent clearance and potentiate further accumulation of senescent cells (Ogata et al., 2021). Thus, SASP can act as a bridge between fibroblasts and other skin cells during aging. The classical transcription factors controlling SASP secretion include p53, NF‐κB, CCAAT/enhancer‐binding protein beta (C/EBPβ), Janus kinase‐signal transducer and activator of transcription (JAK–STAT), and GATA binding protein 4 (GATA4) (Huggins et al., 2013; Kang et al., 2015; Salminen et al., 2012; Xu et al., 2015). Dermal fibroblasts modulate SASP secretion and senescence phenotypes via signaling pathways, such as Nrf2, mTOR, TGF‐β, and IGF‐1 (Figure 2), which will be discussed later.
2.5. ECM‐modifying enzymes in SASP impair ECM homeostasis
Aged dermis exhibits prominent clinical features, including decreased dermis thickness, reduced resilience, and mechanical force with wrinkled and flabby appearance (Farage et al., 2008). These changes are related to the loss of ECM components during aging, due to a reduced synthesis and enhanced degradation of ECM in aged fibroblasts (Autio et al., 1994; Varani et al., 2006). SASP components like MMPs, which can directly cleavage collagen fibrils, play a significant role in ECM degradation during aging. Previous studies have shown that the levels of MMPs, including MMP1, MMP2, and MMP9, are increased in the aged dermis and cultured fibroblasts derived from aged participants (Qin et al., 2017; Quan et al., 2013). Overexpression of hMMP1 induces aging phenotypes in ex vivo 3D human skin organ culture (Xia et al., 2013) and in vivo mice model (Quan et al., 2023). Additionally, the elevated levels of MMPs are paralleled by a reduction of tissue inhibitors of MMPs (TIMPs) in the aged skin, leading to an imbalance of MMPs/TIMPs and progressive collagen fragmentation (Yokose et al., 2012). TIMP‐1 overexpression protects ECM against degradation and elasticity reduction induced by chronic UVB exposure, while the TIMP‐1 neutralizing antibody acts in an opposite way (Yokose et al., 2012). Besides, fibroblasts of the aged skin decrease the production of ECM, especially the major collagen network components, such as collagen type I and III (Brinckmann et al., 1995; Lovell et al., 1987). This process is primarily a result of the reduction in TGF‐β signaling, which will be discussed in detail later.
3. KEY SIGNALING PATHWAYS INVOLVED IN DERMAL FIBROBLAST SENESCENCE
Several signaling pathways are involved in the dermal aging. First, oxidative stress has long been recognized as a key regulator of aging process. Activation of Nrf2 increases the expression of antioxidation‐related factors, such as heme oxygenase 1 (HO‐1), NAD(P)H quinone dehydrogenase 1 (NQO1), and superoxide dismutase (SOD), to protect against oxidative stress burden and inflammation (Figure 2). Nrf2 activity is reduced during photoaging and chronological aging of human fibroblasts (Kapeta et al., 2010) and murine fibroblasts (Jódar et al., 2011), while silencing of Nrf2 induces premature aging (Kapeta et al., 2010). Accordingly, enhancement of Nrf2 signaling can attenuate aging and inflammation in dermal fibroblasts (Guo et al., 2022; Hseu et al., 2019; Lee et al., 2022; Sklirou et al., 2017). Thus, downregulation of Nrf2 signaling pathway can contribute to dermal aging.
Second, multiple studies indicate the pivotal role of TGF‐β in dermal aging (Figure 2). Physiologically, aged dermis exhibits lower content of ECM (de Bengy et al., 2022; Fisher et al., 1996). TGF‐β signaling can enhance ECM gene expression (collagens, fibronectin, decorin, versican), while inhibiting ECM degradation by downregulation of MMPs and upregulation of TIMPs (Quan & Fisher, 2015; Verrecchia et al., 2001). Either oxidative stress (He et al., 2014) or UV irradiation (Quan et al., 2004) impairs TGF‐β signaling pathway in fibroblasts, resulting in decreased expression of downstream targets, including connective tissue growth factor (CTGF/CCN2) and type I collagen (He et al., 2014; Quan et al., 2004, 2010). Knockdown of TβRII (Quan et al., 2004) or Smad3 (Purohit et al., 2016) impairs collagen synthesis while overexpression of TβRII rescues UV‐induced loss of collagen via activation of TGF‐β signaling. Impaired TGF‐β signaling alters expression levels of CCN1 in dermal fibroblasts of mice, resembling aged skin manifested by wrinkled appearance and disruption of collagen network (Quan et al., 2021). In addition, reduced fibroblast size, a characteristic of dermal fibroblasts in the aged skin, is associated with reduced expression levels of TβRII and diminished ECM production in the aged human skin (Fisher et al., 2016). Hence, abrogated TGF‐β signaling pathway is associated dermal aging.
Moreover, evidence also suggests the involvement of IGF‐1 signaling pathway in dermal aging (Figure 2). IGF‐1 signaling begins with the phosphorylation of IGF‐1Rβ, followed by a series of activation of downstream pathways such as PI3K/AKT/p70S6K or Ras/Raf/MEK/ERK, to regulate cell cycle and protein biosynthesis (Hakuno & Takahashi, 2018; Salminen & Kaarniranta, 2010). IGF‐1 pathway can be deregulated by age‐associated superoxide anion accumulation, leading to limited fibroblast proliferation and collagen deposition (Singh et al., 2015). Transcription factor JunB‐induced inhibition of IGF‐1 promotor activation decreases the levels of IGF‐1 and its downstream PI3K/AKT pathway effectors, resulting in disruption of the metabolic and structural niches of skin stem cells, consequently leading to exacerbation of skin aging (Maity et al., 2021). Aging‐related reduction in circulating IGF‐1 levels and impaired IGF‐1 signaling likely contribute to the atrophy of skin, muscle, and bone in the elderly (Gallagher & LeRoith, 2011). However, long‐term treatment of primary human skin fibroblasts with IGF‐1 induces a premature senescence phenotype (Nagaraj et al., 2022; Tran et al., 2014). Thus, further studies are needed to elucidate the role of IGF‐1 signaling in dermal aging.
Additionally, the contribution of mTOR signaling pathway to dermal aging has been well appreciated because of its regulatory role in cellular metabolism and autophagy (Figure 2). Previous study demonstrated that activation of autophagy promotes the degradation of oxidized metabolites and inhibition of photoaging via inhibition of PI3K/AKT/mTORC1 signaling (Chen et al., 2022; Wang et al., 2019). In contrast, loss of autophagy or upregulation of mTOR signaling contributes to photoaging (Chen et al., 2022; Lim et al., 2020; Wang et al., 2019). Moreover, mTOR can also suppress SASP production via MAPKAPK2 translation in oncogene‐induced senescence (OIS) (Herranz et al., 2015). Treatment with pan‐mTOR inhibitor, AZD8055, can modify the senescence phenotypes in skin fibroblasts (Walters et al., 2016). Similarly, the mTOR inhibitor Rapamycin can decrease the expression p16INK4a and ameliorate SASP secretion in senescent fibroblasts, and lead to visible improvement in aging skin appearance (Chung et al., 2019; Herranz et al., 2015; Laberge et al., 2015). Therefore, dermal aging is linked to activation of mTOR signaling pathway. Inhibition of mTOR signaling pathway can exhibit antiaging benefit.
4. DERMAL FIBROBLASTS AS A POTENTIAL AMPLIFIER TO SKIN AGING AND INFLAMMATION
Chronological changes in cutaneous functions and external stressors induce telomere attrition, ROS accumulation, DNA damage, and mitochondrial dysfunction in dermal fibroblasts, resulting in diverse forms of senescence (Franco et al., 2022). Senescent fibroblasts exhibit irreversible cell‐cycle arrest and release SASP, which distinguishes them from other non‐proliferative cells. SASP, comprised of various components, plays multiple roles in aging. It builds up chronic inflammation through cytokines and chemokines, impairs proliferation by disrupting the release of growth factors, and remodels the ECM through enhanced activation of proteolytic enzymes (Wlaschek et al., 2021). SASP is not only a byproduct of senescent fibroblasts, but it also serves as a messenger to reinforce senescence in both paracrine and autocrine manners (Ghosh & Capell, 2016; Tasdemir & Lowe, 2013; Wlaschek et al., 2021). The cascading effects of stress, senescence, and SASP signaling in fibroblasts might contribute to the development of aging‐associated cutaneous abnormalities, including wrinkles, loss of volume, and elasticity of collagen (Imokawa, 2009; Shuster et al., 1975) impaired wound healing (Mahmoudi et al., 2019; Thanapaul et al., 2022), and might related to the increased risk of inflammatory dermatoses in the elderly (Wang et al., 2020).
The age‐related cutaneous dysfunction could serve as an initiator of inflammation, while the development and sustained inflammation during skin aging is resultant from a coordinated effort among various skin cells, including keratinocytes, fibroblasts, melanocytes, and innate/adaptive immune cells (Figure 1). Recent research has explored the communication between keratinocytes and fibroblasts and has proposed the existence of a positive feedforward loop. Specifically, keratinocyte‐produced IL‐1 appears to play a crucial role in inducing fibroblasts to produce cytokines (IL‐1, IL‐6, IL‐8) and growth factors (keratinocytes growth factors (KGF), granulocyte‐macrophage colony‐stimulating factor (GM‐CSF)). In turn, these factors secreted by fibroblasts regulate the biological functions of keratinocytes, including proliferation, differentiation, and cytokine production (Russo et al., 2020). Additionally, melanocytes are crucial resources of aging signals. Chronological aging and chronic UVR exposure promotes the accumulation of senescent melanocytes, which exhibit disrupted glycolytic metabolism and telomere dysfunction (Park et al., 2023; Victorelli et al., 2019). The accumulated senescent melanocytes produce SASP, which induces paracrine telomere dysfunction transmission in neighboring cells like fibroblasts and keratinocytes through the IP‐10‐CXCR3‐ROS signaling (Victorelli et al., 2019). Thus, while keratinocytes and melanocytes may act as triggers of inflammation, fibroblasts may serve as the amplifiers of inflammation (Figure 1). The SASPs generated by initiators and amplifiers further activate resident immune cells and recruit circulating immune cells to deteriorate inflammation (Fitsiou et al., 2021).
Recent evidence from scRNAseq of chronological aging and photoaging in humans and mice highlight fibroblasts as a major responder in age‐related inflammation. For instance, Lin et al. demonstrated that UV‐irradiated mouse skin mainly induces inflammatory responses in fibroblasts (Lin et al., 2022). The transcriptomic atlas suggests that fibroblasts exhibit the highest level of aging‐related transcriptional variability among all the identified skin cell types (Zou et al., 2021). These observations align with the defective ECM and thinned dermis observed in aged human skin. Therefore, it is important to focus on regulating the amplifier of inflammation in fibroblasts to control inflammation, restore ECM homeostasis, and maintain a youthful and healthy appearance. However, further research is required to validate the link between dermal fibroblast function and inflammation.
5. CONCLUDING REMARKS
As we are aging, everyone will eventually face a problem of skin aging. Fibroblast senescence is attributed to alterations in multiple signaling pathways, including Nrf2, TGF‐β, IGF‐1, and mTOR. Dermal fibroblast senescence can induce and exacerbate cutaneous inflammation, while sustained cutaneous inflammation can lead to chronic systemic inflammation, that is, inflammaging (Franco et al., 2022; Pilkington et al., 2021). Thus, dermal fibroblast senescence can potentially and negatively impact overall health of human being. Therefore, attenuation of skin aging, including fibroblast senescence, can benefit, at least, some health conditions in the elderly. Because multiple mechanisms can contribute to fibroblast senescence, development of ideal approaches to prevent/treat dermal aging is still a challenge although applications of antioxidants show some benefits (Boo, 2022; Lee et al., 2022; Lephart, 2016). Additionally, further studies are needed to delineate the link between fibroblast senescence and inflammaging.
AUTHOR CONTRIBUTIONS
Jing Zhang: Conception and design, manuscript writing, figure design. Haoyue Yu: Conception and design, manuscript writing, figure design. Mao‐Qiang Man: Manuscript revision. Lizhi Hu: Conception and design, financial support, manuscript revision, final approval of manuscript.
FUNDING INFORMATION
This research was funded by National Natural Science Foundation of China, grant number NSFC 81972962 and 82273563 to L.H.
CONFLICT OF INTEREST STATEMENT
All authors declared no potential conflict of interest.
ACKNOWLEDGMENTS
None.
Zhang, J. , Yu, H. , Man, M.‐Q. , & Hu, L. (2024). Aging in the dermis: Fibroblast senescence and its significance. Aging Cell, 23, e14054. 10.1111/acel.14054
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Acosta, J. C. , Banito, A. , Wuestefeld, T. , Georgilis, A. , Janich, P. , Morton, J. P. , Athineos, D. , Kang, T.‐W. , Lasitschka, F. , Andrulis, M. , Pascual, G. , Morris, K. J. , Khan, S. , Jin, H. , Dharmalingam, G. , Snijders, A. P. , Carroll, T. , Capper, D. , Pritchard, C. , … Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 15, 978–990. 10.1038/ncb2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta, J. C. , O’Loghlen, A. , Banito, A. , Guijarro, M. V. , Augert, A. , Raguz, S. , Fumagalli, M. , Costa, M. D. , Brown, C. , Popov, N. , Takatsu, Y. , Melamed, J. , Fagagna, F. , d’Adda di Bernard, D. , Hernando, E. , & Gil, J. (2008). Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell, 133, 1006–1018. 10.1016/j.cell.2008.03.038 [DOI] [PubMed] [Google Scholar]
- Ansary, T. M. , Hossain, M. R. , Kamiya, K. , Komine, M. , & Ohtsuki, M. (2021). Inflammatory molecules associated with ultraviolet radiation‐mediated skin aging. International Journal of Molecular Sciences, 22, 3974. 10.3390/ijms22083974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autio, P. , Risteli, J. , Haukipuro, K. , Risteli, L. , & Oikarinen, A. (1994). Collagen synthesis in human skin in vivo: Modulation by aging, ultraviolet B irradiation and localization. Photodermatology, Photoimmunology & Photomedicine, 10, 212–216. [PubMed] [Google Scholar]
- Bang, E. , Kim, D. H. , & Chung, H. Y. (2021). Protease‐activated receptor 2 induces ROS‐mediated inflammation through Akt‐mediated NF‐κB and FoxO6 modulation during skin photoaging. Redox Biology, 44, 102022. 10.1016/j.redox.2021.102022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boo, Y. C. (2022). Ascorbic acid (vitamin C) as a cosmeceutical to increase dermal collagen for skin antiaging purposes: Emerging combination therapies. Antioxidants, 11, 1663. 10.3390/antiox11091663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinckmann, J. , Açil, Y. , Wolff, H. H. , & Müller, P. K. (1995). Collagen synthesis in (sun‐) aged human skin and in fibroblasts derived from sun‐exposed and sun‐protected body sites. Journal of Photochemistry and Photobiology. B, 27, 33–38. 10.1016/1011-1344(94)07051-o [DOI] [PubMed] [Google Scholar]
- Cavinato, M. , Koziel, R. , Romani, N. , Weinmüllner, R. , Jenewein, B. , Hermann, M. , Dubrac, S. , Ratzinger, G. , Grillari, J. , Schmuth, M. , & Jansen‐Dürr, P. (2017). UVB‐induced senescence of human dermal fibroblasts involves impairment of proteasome and enhanced autophagic activity. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72, 632–639. 10.1093/gerona/glw150 [DOI] [PubMed] [Google Scholar]
- Chaiprasongsuk, A. , & Panich, U. (2022). Role of phytochemicals in skin photoprotection via regulation of Nrf2. Frontiers in Pharmacology, 13, 823881. 10.3389/fphar.2022.823881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Zhang, H. , Yang, Y. , Zhang, S. , Wang, J. , Zhang, D. , & Yu, H. (2022). Metformin attenuates UVA‐induced skin photoaging by suppressing mitophagy and the PI3K/AKT/mTOR pathway. International Journal of Molecular Sciences, 23, 6960. 10.3390/ijms23136960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Kang, J. , Xia, J. , Li, Y. , Yang, B. , Chen, B. , Sun, W. , Song, X. , Xiang, W. , Wang, X. , Wang, F. , Wan, Y. , & Bi, Z. (2008). p53‐related apoptosis resistance and tumor suppression activity in UVB‐induced premature senescent human skin fibroblasts. International Journal of Molecular Medicine, 21, 645–653. [PubMed] [Google Scholar]
- Choi, E.‐J. , Kil, I. S. , & Cho, E.‐G. (2020). Extracellular vesicles derived from senescent fibroblasts attenuate the dermal effect on keratinocyte differentiation. International Journal of Molecular Sciences, 21, 1022. 10.3390/ijms21031022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, C. L. , Lawrence, I. , Hoffman, M. , Elgindi, D. , Nadhan, K. , Potnis, M. , Jin, A. , Sershon, C. , Binnebose, R. , Lorenzini, A. , & Sell, C. (2019). Topical rapamycin reduces markers of senescence and aging in human skin: An exploratory, prospective, randomized trial. GeroScience, 41, 861–869. 10.1007/s11357-019-00113-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavitti, R. , & Finkel, T. (2005). Reactive oxygen species as mediators of cellular senescence. IUBMB Life, 57, 277–281. 10.1080/15216540500091890 [DOI] [PubMed] [Google Scholar]
- Coppé, J.‐P. , Patil, C. K. , Rodier, F. , Sun, Y. , Muñoz, D. P. , Goldstein, J. , Nelson, P. S. , Desprez, P.‐Y. , & Campisi, J. (2008). Senescence‐associated secretory phenotypes reveal cell‐nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biology, 6, 2853–2868. 10.1371/journal.pbio.0060301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Adda di Fagagna, F. (2008). Living on a break: Cellular senescence as a DNA‐damage response. Nature Reviews. Cancer, 8, 512–522. 10.1038/nrc2440 [DOI] [PubMed] [Google Scholar]
- da Silva, P. F. L. , Ogrodnik, M. , Kucheryavenko, O. , Glibert, J. , Miwa, S. , Cameron, K. , Ishaq, A. , Saretzki, G. , Nagaraja‐Grellscheid, S. , Nelson, G. , & von Zglinicki, T. (2019). The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell, 18, e12848. 10.1111/acel.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bengy, A.‐F. , Lamartine, J. , Sigaudo‐Roussel, D. , & Fromy, B. (2022). Newborn and elderly skin: Two fragile skins at higher risk of pressure injury. Biological Reviews, 97, 874–895. 10.1111/brv.12827 [DOI] [PubMed] [Google Scholar]
- Dimri, G. P. , Lee, X. , Basile, G. , Acosta, M. , Scott, G. , Roskelley, C. , Medrano, E. E. , Linskens, M. , Rubelj, I. , & Pereira‐Smith, O. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America, 92, 9363–9367. 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage, M. A. , Miller, K. W. , Elsner, P. , & Maibach, H. I. (2008). Intrinsic and extrinsic factors in skin ageing: A review. International Journal of Cosmetic Science, 30, 87–95. 10.1111/j.1468-2494.2007.00415.x [DOI] [PubMed] [Google Scholar]
- Fisher, G. J. , Datta, S. C. , Talwar, H. S. , Wang, Z. Q. , Varani, J. , Kang, S. , & Voorhees, J. J. (1996). Molecular basis of sun‐induced premature skin ageing and retinoid antagonism. Nature, 379, 335–339. 10.1038/379335a0 [DOI] [PubMed] [Google Scholar]
- Fisher, G. J. , Shao, Y. , He, T. , Qin, Z. , Perry, D. , Voorhees, J. J. , & Quan, T. (2016). Reduction of fibroblast size/mechanical force down‐regulates TGF‐β type II receptor: Implications for human skin aging. Aging Cell, 15, 67–76. 10.1111/acel.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsiou, E. , Pulido, T. , Campisi, J. , Alimirah, F. , & Demaria, M. (2021). Cellular senescence and the senescence‐associated secretory phenotype as drivers of skin photoaging. Journal of Investigative Dermatology, 141, 1119–1126. 10.1016/j.jid.2020.09.031 [DOI] [PubMed] [Google Scholar]
- Franco, A. C. , Aveleira, C. , & Cavadas, C. (2022). Skin senescence: Mechanisms and impact on whole‐body aging. Trends in Molecular Medicine, 28, 97–109. 10.1016/j.molmed.2021.12.003 [DOI] [PubMed] [Google Scholar]
- Gallagher, E. J. , & LeRoith, D. (2011). Is growth hormone resistance/IGF‐1 reduction good for you? Cell Metabolism, 13, 355–356. 10.1016/j.cmet.2011.03.003 [DOI] [PubMed] [Google Scholar]
- García‐Beccaria, M. , Martínez, P. , Flores, J. M. , & Blasco, M. A. (2014). In vivo role of checkpoint kinase 2 in signaling telomere dysfunction. Aging Cell, 13, 810–816. 10.1111/acel.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, K. , & Capell, B. C. (2016). The senescence‐associated secretory phenotype: Critical effector in skin cancer and aging. The Journal of Investigative Dermatology, 136, 2133–2139. 10.1016/j.jid.2016.06.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, F. , Kremslehner, C. , Eckhart, L. , & Tschachler, E. (2020). Cell aging and cellular senescence in skin aging — Recent advances in fibroblast and keratinocyte biology. Experimental Gerontology, 130, 110780. 10.1016/j.exger.2019.110780 [DOI] [PubMed] [Google Scholar]
- Gu, Y. , Han, J. , Jiang, C. , & Zhang, Y. (2020). Biomarkers, oxidative stress and autophagy in skin aging. Ageing Research Reviews, 59, 101036. 10.1016/j.arr.2020.101036 [DOI] [PubMed] [Google Scholar]
- Guo, K. , Liu, R. , Jing, R. , Wang, L. , Li, X. , Zhang, K. , Fu, M. , Ye, J. , Hu, Z. , Zhao, W. , & Xu, N. (2022). Cryptotanshinone protects skin cells from ultraviolet radiation‐induced photoaging via its antioxidant effect and by reducing mitochondrial dysfunction and inhibiting apoptosis. Frontiers in Pharmacology, 13, 1036013. 10.3389/fphar.2022.1036013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakuno, F. , & Takahashi, S.‐I. (2018). IGF1 receptor signaling pathways. Journal of Molecular Endocrinology, 61, T69–T86. 10.1530/JME-17-0311 [DOI] [PubMed] [Google Scholar]
- He, T. , Quan, T. , Shao, Y. , Voorhees, J. J. , & Fisher, G. J. (2014). Oxidative exposure impairs TGF‐β pathway via reduction of type II receptor and SMAD3 in human skin fibroblasts. Age, 36, 9623. 10.1007/s11357-014-9623-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz, N. , Gallage, S. , Mellone, M. , Wuestefeld, T. , Klotz, S. , Hanley, C. J. , Raguz, S. , Acosta, J. C. , Innes, A. J. , Banito, A. , Georgilis, A. , Montoya, A. , Wolter, K. , Dharmalingam, G. , Faull, P. , Carroll, T. , Martínez‐Barbera, J. P. , Cutillas, P. , Reisinger, F. , … Gil, J. (2015). mTOR regulates MAPKAPK2 translation to control the senescence‐associated secretory phenotype. Nature Cell Biology, 17, 1205–1217. 10.1038/ncb3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini, M. , Koehler, K. R. , & Shafiee, A. (2022). Biofabrication of human skin with its appendages. Advanced Healthcare Materials, 11, e2201626. 10.1002/adhm.202201626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hseu, Y.‐C. , Chang, C.‐T. , Gowrisankar, Y. V. , Chen, X.‐Z. , Lin, H.‐C. , Yen, H.‐R. , & Yang, H.‐L. (2019). Zerumbone exhibits antiphotoaging and Dermatoprotective properties in ultraviolet A‐irradiated human skin fibroblast cells via the activation of Nrf2/ARE defensive pathway. Oxidative Medicine and Cellular Longevity, 2019, 4098674. 10.1155/2019/4098674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins, C. J. , Malik, R. , Lee, S. , Salotti, J. , Thomas, S. , Martin, N. , Quiñones, O. A. , Alvord, W. G. , Olanich, M. E. , Keller, J. R. , & Johnson, P. F. (2013). C/EBPγ suppresses senescence and inflammatory gene expression by heterodimerizing with C/EBPβ. Molecular and Cellular Biology, 33, 3242–3258. 10.1128/MCB.01674-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa, G. (2009). Mechanism of UVB‐induced wrinkling of the skin: Paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. The Journal of Investigative Dermatology. Symposium Proceedings, 14, 36–43. 10.1038/jidsymp.2009.11 [DOI] [PubMed] [Google Scholar]
- Jódar, L. , Mercken, E. M. , Ariza, J. , Younts, C. , González‐Reyes, J. A. , Alcaín, F. J. , Burón, I. , de Cabo, R. , & Villalba, J. M. (2011). Genetic deletion of Nrf2 promotes immortalization and decreases life span of murine embryonic fibroblasts. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 66, 247–256. 10.1093/gerona/glq181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz, B. A. , & Buettner, G. R. (1996). EPR detection of free radicals in UV‐irradiated skin: Mouse versus human. Photochemistry and Photobiology, 64, 918–922. 10.1111/j.1751-1097.1996.tb01856.x [DOI] [PubMed] [Google Scholar]
- Kammeyer, A. , & Luiten, R. M. (2015). Oxidation events and skin aging. Ageing Research Reviews, 21, 16–29. 10.1016/j.arr.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Kaneko, N. , Vierkoetter, A. , Kraemer, U. , Sugiri, D. , Matsui, M. , Yamamoto, A. , Krutmann, J. , & Morita, A. (2012). Mitochondrial common deletion mutation and extrinsic skin ageing in German and Japanese women. Experimental Dermatology, 21(Suppl 1), 26–30. 10.1111/j.1600-0625.2012.01499.x [DOI] [PubMed] [Google Scholar]
- Kang, C. , Xu, Q. , Martin, T. D. , Li, M. Z. , Demaria, M. , Aron, L. , Lu, T. , Yankner, B. A. , Campisi, J. , & Elledge, S. J. (2015). The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science, 349, aaa5612. 10.1126/science.aaa5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeta, S. , Chondrogianni, N. , & Gonos, E. S. (2010). Nuclear erythroid factor 2‐mediated proteasome activation delays senescence in human fibroblasts. The Journal of Biological Chemistry, 285, 8171–8184. 10.1074/jbc.M109.031575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozieł, R. , Greussing, R. , Maier, A. B. , Declercq, L. , & Jansen‐Dürr, P. (2011). Functional interplay between mitochondrial and proteasome activity in skin aging. The Journal of Investigative Dermatology, 131, 594–603. 10.1038/jid.2010.383 [DOI] [PubMed] [Google Scholar]
- Krutmann, J. , & Schroeder, P. (2009). Role of mitochondria in photoaging of human skin: The defective powerhouse model. The Journal of Investigative Dermatology. Symposium Proceedings, 14, 44–49. 10.1038/jidsymp.2009.1 [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Millis, A. J. , & Baglioni, C. (1992). Expression of interleukin 1‐inducible genes and production of interleukin 1 by aging human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America, 89, 4683–4687. 10.1073/pnas.89.10.4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge, R.‐M. , Sun, Y. , Orjalo, A. V. , Patil, C. K. , Freund, A. , Zhou, L. , Curran, S. C. , Davalos, A. R. , Wilson‐Edell, K. A. , Liu, S. , Limbad, C. , Demaria, M. , Li, P. , Hubbard, G. B. , Ikeno, Y. , Javors, M. , Desprez, P.‐Y. , Benz, C. C. , Kapahi, P. , … Campisi, J. (2015). MTOR regulates the pro‐tumorigenic senescence‐associated secretory phenotype by promoting IL1A translation. Nature Cell Biology, 17, 1049–1061. 10.1038/ncb3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. C. , & Wei, Y. H. (2001). Mitochondrial alterations, cellular response to oxidative stress and defective degradation of proteins in aging. Biogerontology, 2, 231–244. 10.1023/a:1013270512172 [DOI] [PubMed] [Google Scholar]
- Lee, J.‐J. , Ng, S.‐C. , Hsu, J.‐Y. , Liu, H. , Chen, C.‐J. , Huang, C.‐Y. , & Kuo, W.‐W. (2022). Galangin reverses H2O2‐induced dermal fibroblast senescence via SIRT1‐PGC‐1α/Nrf2 signaling. International Journal of Molecular Sciences, 23, 1387. 10.3390/ijms23031387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart, E. D. (2016). Skin aging and oxidative stress: Equol's anti‐aging effects via biochemical and molecular mechanisms. Ageing Research Reviews, 31, 36–54. 10.1016/j.arr.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Li, Y.‐F. , Ouyang, S.‐H. , Tu, L.‐F. , Wang, X. , Yuan, W.‐L. , Wang, G.‐E. , Wu, Y.‐P. , Duan, W.‐J. , Yu, H.‐M. , Fang, Z.‐Z. , Kurihara, H. , Zhang, Y. , & He, R.‐R. (2018). Caffeine protects skin from oxidative stress‐induced senescence through the activation of autophagy. Theranostics, 8, 5713–5730. 10.7150/thno.28778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, G.‐E. , Park, J. E. , Cho, Y. H. , Lim, D. S. , Kim, A.‐J. , Moh, S. H. , Lee, J. H. , & Lee, J. S. (2020). Alpha‐neoendorphin can reduce UVB‐induced skin photoaging by activating cellular autophagy. Archives of Biochemistry and Biophysics, 689, 108437. 10.1016/j.abb.2020.108437 [DOI] [PubMed] [Google Scholar]
- Lin, Y. , Cao, Z. , Lyu, T. , Kong, T. , Zhang, Q. , Wu, K. , Wang, Y. , & Zheng, J. (2022). Single‐cell RNA‐seq of UVB‐radiated skin reveals landscape of photoaging‐related inflammation and protection by vitamin D. Gene, 831, 146563. 10.1016/j.gene.2022.146563 [DOI] [PubMed] [Google Scholar]
- Liu, X. , Zhu, R. , Luo, Y. , Wang, S. , Zhao, Y. , Qiu, Z. , Zhang, Y. , Liu, X. , Yao, X. , Li, X. , & Li, W. (2021). Distinct human Langerhans cell subsets orchestrate reciprocal functions and require different developmental regulation. Immunity, 54, 2305–2320.e11. 10.1016/j.immuni.2021.08.012 [DOI] [PubMed] [Google Scholar]
- Lovell, C. R. , Smolenski, K. A. , Duance, V. C. , Light, N. D. , Young, S. , & Dyson, M. (1987). Type I and III collagen content and fibre distribution in normal human skin during ageing. The British Journal of Dermatology, 117, 419–428. 10.1111/j.1365-2133.1987.tb04921.x [DOI] [PubMed] [Google Scholar]
- Mahmoudi, S. , Mancini, E. , Xu, L. , Moore, A. , Jahanbani, F. , Hebestreit, K. , Srinivasan, R. , Li, X. , Devarajan, K. , Prélot, L. , Ang, C. E. , Shibuya, Y. , Benayoun, B. A. , Chang, A. L. S. , Wernig, M. , Wysocka, J. , Longaker, M. T. , Snyder, M. P. , & Brunet, A. (2019). Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature, 574, 553–558. 10.1038/s41586-019-1658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity, P. , Singh, K. , Krug, L. , Koroma, A. , Hainzl, A. , Bloch, W. , Kochanek, S. , Wlaschek, M. , Schorpp‐Kistner, M. , Angel, P. , Ignatius, A. , Geiger, H. , & Scharffetter‐Kochanek, K. (2021). Persistent JunB activation in fibroblasts disrupts stem cell niche interactions enforcing skin aging. Cell Reports, 36, 109634. 10.1016/j.celrep.2021.109634 [DOI] [PubMed] [Google Scholar]
- Masaki, H. , Atsumi, T. , & Sakurai, H. (1995). Detection of hydrogen peroxide and hydroxyl radicals in murine skin fibroblasts under UVB irradiation. Biochemical and Biophysical Research Communications, 206, 474–479. 10.1006/bbrc.1995.1067 [DOI] [PubMed] [Google Scholar]
- McCart, E. A. , Thangapazham, R. L. , Lombardini, E. D. , Mog, S. R. , Panganiban, R. A. M. , Dickson, K. M. , Mansur, R. A. , Nagy, V. , Kim, S.‐Y. , Selwyn, R. , Landauer, M. R. , Darling, T. N. , & Day, R. M. (2017). Accelerated senescence in skin in a murine model of radiation‐induced multi‐organ injury. Journal of Radiation Research, 58, 636–646. 10.1093/jrr/rrx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj, K. , Sarfstein, R. , Laron, Z. , & Werner, H. (2022). Long‐term IGF1 stimulation leads to cellular senescence via functional interaction with the thioredoxin‐interacting protein, TXNIP. Cell, 11, 3260. 10.3390/cells11203260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narzt, M.‐S. , Pils, V. , Kremslehner, C. , Nagelreiter, I.‐M. , Schosserer, M. , Bessonova, E. , Bayer, A. , Reifschneider, R. , Terlecki‐Zaniewicz, L. , Waidhofer‐Söllner, P. , Mildner, M. , Tschachler, E. , Cavinato, M. , Wedel, S. , Jansen‐Dürr, P. , Nanic, L. , Rubelj, I. , El‐Ghalbzouri, A. , Zoratto, S. , … Lämmermann, I. (2021). Epilipidomics of senescent dermal fibroblasts identify Lysophosphatidylcholines as pleiotropic senescence‐associated secretory phenotype (SASP) factors. The Journal of Investigative Dermatology, 141, 993–1006.e15. 10.1016/j.jid.2020.11.020 [DOI] [PubMed] [Google Scholar]
- Ogata, Y. , Yamada, T. , Hasegawa, S. , Sanada, A. , Iwata, Y. , Arima, M. , Nakata, S. , Sugiura, K. , & Akamatsu, H. (2021). SASP‐induced macrophage dysfunction may contribute to accelerated senescent fibroblast accumulation in the dermis. Experimental Dermatology, 30, 84–91. 10.1111/exd.14205 [DOI] [PubMed] [Google Scholar]
- Olovnikov, A. M. (1996). Telomeres, telomerase, and aging: Origin of the theory. Experimental Gerontology, 31, 443–448. 10.1016/0531-5565(96)00005-8 [DOI] [PubMed] [Google Scholar]
- Papaccio, F. , Arino, A. D. , Caputo, S. , & Bellei, B. (2022). Focus on the contribution of oxidative stress in skin aging. Antioxidants (Basel), 11(6), 1121. 10.3390/antiox11061121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Y. J. , Kim, J. C. , Kim, Y. , Kim, Y. H. , Park, S. S. , Muther, C. , Tessier, A. , Lee, G. , Gendronneau, G. , Forestier, S. , Ben‐Khalifa, Y. , Park, T. J. , & Kang, H. Y. (2023). Senescent melanocytes driven by glycolytic changes are characterized by melanosome transport dysfunction. Theranostics, 13, 3914–3924. 10.7150/thno.84912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington, S. M. , Bulfone‐Paus, S. , Griffiths, C. E. M. , & Watson, R. E. B. (2021). Inflammaging and the skin. The Journal of Investigative Dermatology, 141, 1087–1095. 10.1016/j.jid.2020.11.006 [DOI] [PubMed] [Google Scholar]
- Poljšak, B. , Dahmane, R. G. , & Godić, A. (2012). Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerologica Alpina, Pannonica et Adriatica, 21, 33–36. [PubMed] [Google Scholar]
- Purohit, T. , He, T. , Qin, Z. , Li, T. , Fisher, G. J. , Yan, Y. , Voorhees, J. J. , & Quan, T. (2016). Smad3‐dependent regulation of type I collagen in human dermal fibroblasts: Impact on human skin connective tissue aging. Journal of Dermatological Science, 83, 80–83. 10.1016/j.jdermsci.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Qin, Z. , Balimunkwe, R. M. , & Quan, T. (2017). Age‐related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. The British Journal of Dermatology, 177, 1337–1348. 10.1111/bjd.15379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, T. , & Fisher, G. J. (2015). Role of age‐associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini‐review. Gerontology, 61, 427–434. 10.1159/000371708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, T. , He, T. , Kang, S. , Voorhees, J. J. , & Fisher, G. J. (2004). Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor‐beta type II receptor/Smad signaling. The American Journal of Pathology, 165, 741–751. 10.1016/s0002-9440(10)63337-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, T. , Little, E. , Quan, H. , Qin, Z. , Voorhees, J. J. , & Fisher, G. J. (2013). Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. The Journal of Investigative Dermatology, 133, 1362–1366. 10.1038/jid.2012.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, T. , Shao, Y. , He, T. , Voorhees, J. J. , & Fisher, G. J. (2010). Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. The Journal of Investigative Dermatology, 130, 415–424. 10.1038/jid.2009.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, T. , Xia, W. , He, T. , Calderone, K. , Bou‐Gharios, G. , Voorhees, J. J. , Dlugosz, A. A. , & Fisher, G. J. (2023). Matrix Metalloproteinase‐1 expression in fibroblasts accelerates dermal aging and promotes papilloma development in mouse skin. The Journal of Investigative Dermatology, 143, 1700–1707.e1. 10.1016/j.jid.2023.02.028 [DOI] [PubMed] [Google Scholar]
- Quan, T. , Xiang, Y. , Liu, Y. , Qin, Z. , Yang, Y. , Bou‐Gharios, G. , Voorhees, J. J. , Dlugosz, A. A. , & Fisher, G. J. (2021). Dermal fibroblast CCN1 expression in mice recapitulates human skin dermal aging. The Journal of Investigative Dermatology, 141, 1007–1016. 10.1016/j.jid.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelojaona, V. , Robert, A. M. , & Robert, L. (2009). Expression of senescence‐associated β‐galactosidase (SA‐β‐gal) by human skin fibroblasts, effect of advanced glycation end‐products and fucose or rhamnose‐rich polysaccharides. Archives of Gerontology and Geriatrics, 48, 151–154. 10.1016/j.archger.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Ray, P. D. , Huang, B.‐W. , & Tsuji, Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling, 24, 981–990. 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler, S. , Bartkova, J. , Niederegger, H. , Bartek, J. , Scharffetter‐Kochanek, K. , Jansen‐Dürr, P. , & Wlaschek, M. (2006). p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell, 5, 379–389. 10.1111/j.1474-9726.2006.00231.x [DOI] [PubMed] [Google Scholar]
- Rinnerthaler, M. , Bischof, J. , Streubel, M. K. , Trost, A. , & Richter, K. (2015). Oxidative stress in aging human skin. Biomolecules, 5, 545–589. 10.3390/biom5020545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier, F. , Coppé, J.‐P. , Patil, C. K. , Hoeijmakers, W. A. M. , Muñoz, D. P. , Raza, S. R. , Freund, A. , Campeau, E. , Davalos, A. R. , & Campisi, J. (2009). Persistent DNA damage signaling triggers senescence‐associated inflammatory cytokine secretion. Nature Cell Biology, 11, 973–979. 10.1038/ncb1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, B. , Brembilla, N. C. , & Chizzolini, C. (2020). Interplay between keratinocytes and fibroblasts: A systematic review providing a new angle for understanding skin fibrotic disorders. Frontiers in Immunology, 11, 648. 10.3389/fimmu.2020.00648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, A. , & Kaarniranta, K. (2010). Insulin/IGF‐1 paradox of aging: Regulation via AKT/IKK/NF‐kappaB signaling. Cellular Signalling, 22, 573–577. 10.1016/j.cellsig.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Salminen, A. , Kauppinen, A. , & Kaarniranta, K. (2012). Emerging role of NF‐κB signaling in the induction of senescence‐associated secretory phenotype (SASP). Cellular Signalling, 24, 835–845. 10.1016/j.cellsig.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Salzer, M. C. , Lafzi, A. , Berenguer‐Llergo, A. , Youssif, C. , Castellanos, A. , Solanas, G. , Peixoto, F. O. , Stephan‐Otto Attolini, C. , Prats, N. , Aguilera, M. , Martín‐Caballero, J. , Heyn, H. , & Benitah, S. A. (2018). Identity noise and Adipogenic traits characterize dermal fibroblast aging. Cell, 175, 1575–1590.e22. 10.1016/j.cell.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Shuster, S. , Black, M. M. , & McVitie, E. (1975). The influence of age and sex on skin thickness, skin collagen and density. The British Journal of Dermatology, 93, 639–643. 10.1111/j.1365-2133.1975.tb05113.x [DOI] [PubMed] [Google Scholar]
- Sies, H. , & Jones, D. P. (2020). Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nature Reviews. Molecular Cell Biology, 21, 363–383. 10.1038/s41580-020-0230-3 [DOI] [PubMed] [Google Scholar]
- Singh, K. , Maity, P. , Krug, L. , Meyer, P. , Treiber, N. , Lucas, T. , Basu, A. , Kochanek, S. , Wlaschek, M. , Geiger, H. , & Scharffetter‐Kochanek, K. (2015). Superoxide anion radicals induce IGF‐1 resistance through concomitant activation of PTP1B and PTEN. EMBO Molecular Medicine, 7, 59–77. 10.15252/emmm.201404082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklirou, A. D. , Gaboriaud‐Kolar, N. , Papassideri, I. , Skaltsounis, A.‐L. , & Trougakos, I. P. (2017). 6‐bromo‐indirubin‐3’‐oxime (6BIO), a glycogen synthase kinase‐3β inhibitor, activates cytoprotective cellular modules and suppresses cellular senescence‐mediated biomolecular damage in human fibroblasts. Scientific Reports, 7, 11713. 10.1038/s41598-017-11662-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé‐Boldo, L. , Raddatz, G. , Schütz, S. , Mallm, J.‐P. , Rippe, K. , Lonsdorf, A. S. , Rodríguez‐Paredes, M. , & Lyko, F. (2020). Single‐cell transcriptomes of the human skin reveal age‐related loss of fibroblast priming. Communications Biology, 3, 188. 10.1038/s42003-020-0922-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedhar, A. , Aguilera‐Aguirre, L. , & Singh, K. K. (2020). Mitochondria in skin health, aging, and disease. Cell Death & Disease, 11, 444. 10.1038/s41419-020-2649-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara, S. , Matsuo, M. , & Kaneko, T. (2001). Age‐related changes in oxidative damage to lipids and DNA in rat skin. Mechanisms of Ageing and Development, 122, 415–426. 10.1016/s0047-6374(00)00257-8 [DOI] [PubMed] [Google Scholar]
- Tang, J. , Fewings, E. , Chang, D. , Zeng, H. , Liu, S. , Jorapur, A. , Belote, R. L. , McNeal, A. S. , Tan, T. M. , Yeh, I. , Arron, S. T. , Judson‐Torres, R. L. , Bastian, B. C. , & Shain, A. H. (2020). The genomic landscapes of individual melanocytes from human skin. Nature, 586, 600–605. 10.1038/s41586-020-2785-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir, N. , & Lowe, S. W. (2013). Senescent cells spread the word: Non‐cell autonomous propagation of cellular senescence. The EMBO Journal, 32, 1975–1976. 10.1038/emboj.2013.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanapaul, R. J. R. S. , Shvedova, M. , Shin, G. H. , Crouch, J. , & Roh, D. S. (2022). Elevated skin senescence in young mice causes delayed wound healing. GeroScience, 44, 1871. 10.1007/s11357-022-00551-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, D. , Bergholz, J. , Zhang, H. , He, H. , Wang, Y. , Zhang, Y. , Li, Q. , Kirkland, J. L. , & Xiao, Z.‐X. (2014). Insulin‐like growth factor‐1 regulates the SIRT1‐p53 pathway in cellular senescence. Aging Cell, 13, 669–678. 10.1111/acel.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber, N. , Maity, P. , Singh, K. , Kohn, M. , Keist, A. F. , Ferchiu, F. , Sante, L. , Frese, S. , Bloch, W. , Kreppel, F. , Kochanek, S. , Sindrilaru, A. , Iben, S. , Högel, J. , Ohnmacht, M. , Claes, L. E. , Ignatius, A. , Chung, J. H. , Lee, M. J. , … Scharffetter‐Kochanek, K. (2011). Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell, 10, 239–254. 10.1111/j.1474-9726.2010.00658.x [DOI] [PubMed] [Google Scholar]
- Tu, Y. , & Quan, T. (2016). Oxidative stress and human skin connective tissue aging. Cosmetics, 3, 28. 10.3390/cosmetics3030028 [DOI] [Google Scholar]
- Varani, J. , Dame, M. K. , Rittie, L. , Fligiel, S. E. G. , Kang, S. , Fisher, G. J. , & Voorhees, J. J. (2006). Decreased collagen production in chronologically aged skin: Roles of age‐dependent alteration in fibroblast function and defective mechanical stimulation. The American Journal of Pathology, 168, 1861–1868. 10.2353/ajpath.2006.051302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani, J. , Warner, R. L. , Gharaee‐Kermani, M. , Phan, S. H. , Kang, S. , Chung, J. H. , Wang, Z. Q. , Datta, S. C. , Fisher, G. J. , & Voorhees, J. J. (2000). Vitamin A antagonizes decreased cell growth and elevated collagen‐degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. The Journal of Investigative Dermatology, 114, 480–486. 10.1046/j.1523-1747.2000.00902.x [DOI] [PubMed] [Google Scholar]
- Verrecchia, F. , Chu, M.‐L. , & Mauviel, A. (2001). Identification of novel TGF‐β/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach*. Journal of Biological Chemistry, 276, 17058–17062. 10.1074/jbc.M100754200 [DOI] [PubMed] [Google Scholar]
- Victorelli, S. , Lagnado, A. , Halim, J. , Moore, W. , Talbot, D. , Barrett, K. , Chapman, J. , Birch, J. , Ogrodnik, M. , Meves, A. , Pawlikowski, J. S. , Jurk, D. , Adams, P. D. , van Heemst, D. , Beekman, M. , Slagboom, P. E. , Gunn, D. A. , & Passos, J. F. (2019). Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. The EMBO Journal, 38, e101982. 10.15252/embj.2019101982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaijer, M. E. C. , Gunn, D. A. , Adams, P. D. , Pawlikowski, J. S. , Griffiths, C. E. M. , van Heemst, D. , Slagboom, P. E. , Westendorp, R. G. J. , & Maier, A. B. (2016). P16INK4a positive cells in human skin are indicative of local elastic fiber morphology, facial wrinkling, and perceived age. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71, 1022–1028. 10.1093/gerona/glv114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaijer, M. E. C. , Parish, W. E. , Strongitharm, B. H. , van Heemst, D. , Slagboom, P. E. , de Craen, A. J. M. , Sedivy, J. M. , Westendorp, R. G. J. , Gunn, D. A. , & Maier, A. B. (2012). The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell, 11, 722–725. 10.1111/j.1474-9726.2012.00837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldera Lupa, D. M. , Kalfalah, F. , Safferling, K. , Boukamp, P. , Poschmann, G. , Volpi, E. , Götz‐Rösch, C. , Bernerd, F. , Haag, L. , Huebenthal, U. , Fritsche, E. , Boege, F. , Grabe, N. , Tigges, J. , Stühler, K. , & Krutmann, J. (2015). Characterization of skin aging–associated secreted proteins (SAASP) produced by dermal fibroblasts isolated from intrinsically aged human skin. Journal of Investigative Dermatology, 135, 1954–1968. 10.1038/jid.2015.120 [DOI] [PubMed] [Google Scholar]
- Waldera‐Lupa, D. M. , Kalfalah, F. , Florea, A.‐M. , Sass, S. , Kruse, F. , Rieder, V. , Tigges, J. , Fritsche, E. , Krutmann, J. , Busch, H. , Boerries, M. , Meyer, H. E. , Boege, F. , Theis, F. , Reifenberger, G. , & Stühler, K. (2014). Proteome‐wide analysis reveals an age‐associated cellular phenotype of in situ aged human fibroblasts. Aging, 6, 856–878. 10.18632/aging.100698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, H. E. , Deneka‐Hannemann, S. , & Cox, L. S. (2016). Reversal of phenotypes of cellular senescence by pan‐mTOR inhibition. Aging (Albany NY), 8, 231–244. 10.18632/aging.100872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A. S. , Ong, P. F. , Chojnowski, A. , Clavel, C. , & Dreesen, O. (2017). Loss of Lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Scientific Reports, 7, 15678. 10.1038/s41598-017-15901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Charareh, P. , Lei, X. , & Zhong, J. L. (2019). Autophagy: Multiple mechanisms to protect skin from ultraviolet radiation‐driven photoaging. Oxidative Medicine and Cellular Longevity, 2019, 8135985. 10.1155/2019/8135985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Man, M.‐Q. , Li, T. , Elias, P. M. , & Mauro, T. M. (2020). Aging‐associated alterations in epidermal function and their clinical significance. Aging, 12, 5551–5565. 10.18632/aging.102946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, T. , Wu, P. , Zhang, W. , Zheng, Y. , Li, Q. , Jin, R. , Chen, H. , You, C. , Guo, S. , Han, C. , & Wang, X. (2020). Regeneration of skin appendages and nerves: Current status and further challenges. Journal of Translational Medicine, 18, 53. 10.1186/s12967-020-02248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyemi, U. , Parekh, P. R. , Redon, C. E. , & Bonner, W. M. (2012). SOD2 deficiency promotes aging phenotypes in mouse skin. Aging (Albany NY), 4, 116–118. 10.18632/aging.100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley, C. D. , Velarde, M. C. , Lecot, P. , Liu, S. , Sarnoski, E. A. , Freund, A. , Shirakawa, K. , Lim, H. W. , Davis, S. S. , Ramanathan, A. , Gerencser, A. A. , Verdin, E. , & Campisi, J. (2016). Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabolism, 23, 303–314. 10.1016/j.cmet.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlaschek, M. , Heinen, G. , Poswig, A. , Schwarz, A. , Krieg, T. , & Scharffetter‐Kochanek, K. (1994). UVA‐induced autocrine stimulation of fibroblast‐derived collagenase/MMP‐1 by interrelated loops of interleukin‐1 and interleukin‐6. Photochemistry and Photobiology, 59, 550–556. 10.1111/j.1751-1097.1994.tb02982.x [DOI] [PubMed] [Google Scholar]
- Wlaschek, M. , Maity, P. , Makrantonaki, E. , & Scharffetter‐Kochanek, K. (2021). Connective tissue and fibroblast senescence in skin aging. The Journal of Investigative Dermatology, 141, 985–992. 10.1016/j.jid.2020.11.010 [DOI] [PubMed] [Google Scholar]
- Xia, W. , Hammerberg, C. , Li, Y. , He, T. , Quan, T. , Voorhees, J. J. , & Fisher, G. J. (2013). Expression of catalytically active matrix metalloproteinase‐1 in dermal fibroblasts induces collagen fragmentation and functional alterations that resemble aged human skin. Aging Cell, 12, 661–671. 10.1111/acel.12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Tchkonia, T. , Ding, H. , Ogrodnik, M. , Lubbers, E. R. , Pirtskhalava, T. , White, T. A. , Johnson, K. O. , Stout, M. B. , Mezera, V. , Giorgadze, N. , Jensen, M. D. , LeBrasseur, N. K. , & Kirkland, J. L. (2015). JAK inhibition alleviates the cellular senescence‐associated secretory phenotype and frailty in old age. Proceedings of the National Academy of Sciences of the United States of America, 112, E6301–E6310. 10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. H. , Lee, H. C. , & Wei, Y. H. (1995). Photoageing‐associated mitochondrial DNA length mutations in human skin. Archives of Dermatological Research, 287, 641–648. 10.1007/BF00371736 [DOI] [PubMed] [Google Scholar]
- Yasui, H. , & Sakurai, H. (2000). Chemiluminescent detection and imaging of reactive oxygen species in live mouse skin exposed to UVA. Biochemical and Biophysical Research Communications, 269, 131–136. 10.1006/bbrc.2000.2254 [DOI] [PubMed] [Google Scholar]
- Yokose, U. , Hachiya, A. , Sriwiriyanont, P. , Fujimura, T. , Visscher, M. O. , Kitzmiller, W. J. , Bello, A. , Tsuboi, R. , Kitahara, T. , Kobinger, G. P. , & Takema, Y. (2012). The endogenous protease inhibitor TIMP‐1 mediates protection and recovery from cutaneous photodamage. The Journal of Investigative Dermatology, 132, 2800–2809. 10.1038/jid.2012.204 [DOI] [PubMed] [Google Scholar]
- Zinovkin, R. A. , Kondratenko, N. D. , & Zinovkina, L. A. (2022). Does Nrf2 play a role of a master regulator of mammalian aging? Biochemistry (Mosc), 87, 1465–1476. 10.1134/S0006297922120045 [DOI] [PubMed] [Google Scholar]
- Zou, Z. , Long, X. , Zhao, Q. , Zheng, Y. , Song, M. , Ma, S. , Jing, Y. , Wang, S. , He, Y. , Esteban, C. R. , Yu, N. , Huang, J. , Chan, P. , Chen, T. , Izpisua Belmonte, J. C. , Zhang, W. , Qu, J. , & Liu, G.‐H. (2021). A single‐cell transcriptomic atlas of human skin aging. Developmental Cell, 56, 383–397.e8. 10.1016/j.devcel.2020.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


