Abstract
A new index called the acute-to-chronic (A/C) glycemic ratio has been proposed to better represent the true acute glycemic rise in people with acute disease. However, there has been no previous study investigating the relationship between A/C glycemic ratio and SYNTAX score in patients with diabetic acute coronary syndrome (ACS). The aim of this study is to evaluate the role of A/C glycemic ratio in predicting coronary artery disease severity and SYNTAX score in diabetic patients presenting with ACS. The study included 131 consecutive patients hospitalized for ACS in our hospital, previously diagnosed with diabetes and undergoing percutaneous coronary intervention. The relationship between A/C glycemic ratio and SYNTAX score calculated at the time of admission was determined in univariate and multivariate linear regression analyses. The sample size was divided into three parts (T1, T2, and T3) according to the admission blood glucose (ABG)/estimated average glucose (eAG) ratio. When ABG/eAG and SYNTAX scores were compared, there was no significant difference between the T1 and T2 groups, but a significant increase was found in the T3 group compared with the other two groups (T1: 14.26, T2: 14.77, T3: 24.41; p < 0.001). When multivariate modeling was performed with the two or three most relevant variables (age, estimated glomerular filtration rate [eGFR], and ABG/eAG ratio), the upper tertile of the ABG/eAG variable was correlated with the severity of coronary atherosclerosis and higher SYNTAX score. This study shows that there is a significant relationship between higher ABG/eAG ratio and higher SYNTAX score in diabetic patients presenting with ACS.
Keywords: acute coronary syndrome, diabetes mellitus, HbA1c, acute-to-chronic glycemic ratio, SYNTAX score, coronary artery disease
Acute coronary syndrome (ACS) is a global public health problem. 1 Despite advances in the understanding of mechanisms in ACS, ∼40% of patients who experience a coronary event die within 5 years; the risk of death is five to six times higher in people who experience recurrent events. 2 Revascularization methods have prognostic importance according to the extent of coronary lesions in patients with ACS. Therefore, besides some risk assessment scores that are usually calculated at admission, the SYNTAX score is commonly calculated during coronary angiography to indicate the complexity of coronary lesions. 3 However, estimating the magnitude and severity of coronary involvement at the time of presentation can provide important information for choosing the best therapeutic approach. 4
Elevated plasma glucose levels on admission (acute hyperglycemia) are common in patients admitted to hospital with ACS. 5 6 Acute hyperglycemia has been recognized as an independent predictor of adverse outcomes in both diabetic and nondiabetic patients. 7 8 Acute hyperglycemia causes a prothrombotic state. This modulates the inflammatory response and oxidative stress, causing endothelial dysfunction and impaired microcirculation, 9 10 11 leading to larger infarct size. 12 13 These conditions may explain the association between high plasma glucose and the poor prognosis that accompanies ACS. Patients with acute hyperglycemia typically have a more complex in-hospital clinical course, including a higher incidence of heart failure, cardiogenic shock, and death. 5 6 7 8 9 10 11 12 13 However, admission blood glucose (ABG) may not represent the true acute glycemic status as it is also influenced by chronic glycemic levels, especially in patients with diabetes. Therefore, a new index called the acute-to-chronic (A/C) glycemic ratio or stress hyperglycemia ratio has been proposed to better represent the true acute glycemic rise in people with acute disease. 14 This index is calculated as ABG divided by estimated average glucose (eAG) (ABG/eAG). Recent studies have found that the use of ABG/eAG ratio is a better predictor of poor cardiovascular outcomes in both diabetic and nondiabetic patients with ST-elevation myocardial infarction (STEMI) compared with the use of absolute hyperglycemia. 15 16
There is no previous study in the literature investigating the importance of ABG/eAG ratio in diabetic patients in predicting the prevalence of coronary atherosclerosis in ACS patients with the SYNTAX score. Our aim in this study was to evaluate the role of ABG/eAG ratio in predicting severe atherosclerotic coronary artery involvement in diabetic patients presenting with ACS.
Materials and Methods
Data Collection
This is a retrospective and observational study of diabetic patients diagnosed with ACS and treated with percutaneous coronary intervention (PCI) at a tertiary cardiac center. All patients in the period from January 2020 to January 2022 were consecutively included in the study. The following exclusion criteria were applied in this analysis: previously diagnosed hemoglobinopathy, mechanical complications, evidence of acute or chronic infection, active cancer treatment, and missing clinical data for ABG or glycated hemoglobin A1c (HbA1c). Although 200 diabetic ACS patients were planned to be included in the study, the study was completed with 131 patients due to missing data in archival records. During the in-hospital study, all patients were treated according to current ACS guidelines. Basic demographic characteristics such as hypertension, diabetes mellitus (DM), hyperlipidemia, and laboratory and angiographic findings were obtained from the electronic database of our hospital. In addition, all patients in this study were screened from the archival records, and data were collected about their Killip class at admission, preprocedure blood pressure and pulse rate values, electrocardiogram records, and previous medication use.
ABG/eAG ratios at admission were classified into three tertiles and compared in terms of basic clinical information, echocardiographic and angiographic findings. The relationship between the ABG/eAG ratio and the SYNTAX score calculated later in angiography was investigated with appropriate analysis methods.
Laboratory Analysis
In this study, blood samples including hemoglobin levels, white blood cell counts, platelet counts, and blood glucose levels were measured at admission in all patients studied. Hematological parameters were measured as part of an automated complete blood count using Mindray BC 600 hematology analyzers (Mindray Medical, Shenzhen, China). Biochemical measurements were performed using ARCHITECT plus 8000 kits and calibrators (Abbott, Abbott Park, IL). The HbA1c level was determined using a high-performance liquid chromatography analyzer. Total cholesterol and low-density lipoprotein cholesterol were determined after 8 to 12 hours of overnight fasting. Estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal Disease formula (eGFR [mL/min/1.73 m 2 ] = 186 × [creatinine/88.4] − 1.154 × [age] − 0.203 × [0.742 female, 1.210 black]). 17
Coronary Angiography and Percutaneous Coronary Intervention
In all patients, standard coronary angiography was performed via femoral or radial approach using 5 or 6 French Judkins diagnostic catheters (Boston Scientific, MA). For quantitative analysis, all coronary angiograms were digitally recorded (DICOM viewer; MedCom GmbH, Darmstadt, Germany). Immediately after coronary angiography, the infarct-related artery was stented with a drug-eluting or bare-metal stent, or patients with multivessel disease were offered surgical treatment. A certified cardiologist blinded to patient information reassessed the digital angiographic recordings before angioplasty and calculated the SYNTAX score for each patient. The SYNTAX score was calculated using the standard SYNTAX scoring algorithm. 18 19
Definitions
ACS was defined as proposed in the last universal definition of myocardial infarction guideline. 20 In the present study, angiographic no reflow (NR) was defined as a thrombolysis in myocardial infarction (TIMI) flow grade of less than 3 in the absence of coronary spasm or dissection. As suggested in a previous study, mean glucose (eAG) was determined from HbA1c and calculated using the following equation: eAG (mg/dL) = (28.7% × HbA1c) − 46.7. 14 A/C glycemic ratio was calculated as ABG divided by eAG. All patients underwent initial blood glucose measurement at admission, and chronic glucose levels were calculated on the basis of HbA1c. Being on fasting blood glucose monitoring or using oral antidiabetics or insulin that met the American Diabetes Association criteria was accepted as diagnostic criteria for DM. 21
Statistical Analysis
Kolmogorov–Smirnov's or Shapiro–Wilk's tests were used to test the normality of quantitative data. To analyze categorical data, we used a chi-square test (or Fisher's exact test if any expected cell count was <5), and we presented descriptive statistics as number and percentages. We made comparisons between two groups by Mann–Whitney's U test, and we presented descriptive statistics for subgroup comparisons, including median and interquartile range (IQR). Comparisons of parameters among studied groups according to ABG/eAG tertiles were performed by the Kruskal–Wallis' test due to the lack of parametric test assumptions. Bonferroni adjustment Mann–Whitney's U test was used as a post hoc test for multiple comparisons between the groups.
Univariate logistic regression was used to investigate the relation between burden of coronary atherosclerosis and confounding parameters in our subjects. After performing univariate analysis, significantly obtained variables (ABG/eAG tertiles, age, and eGFR) were used in multivariate logistic regression analysis. Effects of individual predictors on the extent of atherosclerosis severity were reported by using odds ratio (OR) and 95% confidence interval (CI). As sample size for the extent of atherosclerosis severity modeling must be sufficiently large or the number of predictor variables must be sufficiently conservative for the model to be reliable and accurate, there must be ideally 10 participants having the primary outcome per candidate predictor variables. 22 Therefore, models have been constructed that allow the inclusion of up to two to three parameters associated with the extent of atherosclerosis. Due to the strong association between eGFR and nephropathy, the continuous variable eGFR was included so that statistical power of analysis would not be deteriorated. All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 20. The p -values of less than 0.05 were considered statistically significant, and statistical tests were two sided.
Results
A total of 131 patients with diabetic ACS were included in this study (median [IQR] age, 63 [56–69] years; 49 patients [37.4%] female). NR development was detected in seven patients (5.3%). Of the patients who underwent coronary angiography, 86 (65.6%) were decided for intervention, 38 (29%) for coronary artery bypass graft (CABG), and 7 (5.3%) for medical follow-up. The mean SYNTAX scores (IQR) of the patients included in the study were calculated as 17.9 (10–26). The mean (IQR) admission glucose levels (ABG) were 252.04 (160–316) mg/dL, and the mean ABG/eAG ratio was 1.2 (0.93–1.39).
The sample size was divided into three parts (T1, T2, and T3) according to the ABG/eAG ratio. Table 1 shows the main clinical features and laboratory findings for all patients, divided into ABG/eAG tertiles. The mean age of the patients in the T3 group was higher than the other groups, but no significant difference was found between the other demographic characteristics. The systolic blood pressures, Killip classes, and TIMI risk scores at admission were similar between the groups, but in terms of heart rate, the T3 group had significantly higher heart rates at admission than the other groups. When laboratory investigations were analyzed, HBA1c value was significantly higher in the T2 group. ABG, eAG, and ABG/eAG ratio were significantly higher in the T3 group.
Table 1. The baseline characteristics and laboratory investigations of all patients.
| Demographic features | ABG/eAG tertiles | |||
|---|---|---|---|---|
| T1 (≤0.96); n = 43 | T2 (0.97–1.16); n = 43 | T3 (≥1.17); n = 45 | p -Value | |
| Age, y | 63 (60–68) | 60 (52–65) | 65 (59–71) | 0.046 |
| Male gender | 30 (70) | 30 (70) | 22 (49) | 0.129 |
| Body mass index | 28.02 (25.39–29.40) | 28.41 (26.12–29.37) | 29.09 (25.71–31.56) | 0.773 |
| History | ||||
| Hypertension | 33 (77) | 30 (70) | 35 (75) | 0.645 |
| Hyperlipidemia | 13 (30) | 6 (14) | 12 (27) | 0.174 |
| Coronary artery disease | 12 (28) | 10 23) | 14 (31) | 0.709 |
| Smoking | 26 (60) | 30 (70) | 22 (49) | 0.135 |
| Chronic kidney disease | 6 (14) | 4 (9) | 10 (22) | 0.232 |
| On admission | ||||
| Systolic blood pressure, mm Hg | 135 (127–148) | 141 (130–152) | 137 (120–150) | 0.654 |
| Heart rate | 76 (72–81) | 75 (66–82) | 83 (75–91) | 0.008 |
| Antiplatelet use | 27 (63) | 14 (33) | 20 (44) | 0.018 |
| B-blocker use | 20 (47) | 9 (21) | 17 (38) | 0.041 |
| ACE inhibitor use | 34 (79) | 27 (63) | 36 (80) | 0.121 |
| Statin use | 9 (21) | 4 (9) | 11 (24) | 0.160 |
| OAD use | 41 (95) | 41 (95) | 45 (100) | 0.340 |
| Insulin use | 17 (40) | 12 (28) | 14 (31) | 0.494 |
| Laboratory assessment | ||||
| HbA1c, % | 8.45 (6.9–10.2 | 9.80 (8–11.4) | 8.79 (7.2–10.4 | 0.019 |
| ABG, mg/dL | 166 (133–196) | 253 (192–310) | 333 (267–390) | <0.001 |
| eAG, mg/dL | 195.7 (150–245) | 234.7 (183–280) | 205.5 (160–252) | 0.019 |
| ABG/eAG ratio | 0.84 (0.76–0.93) | 1.07 (1.04–1.12) | 1.66 (1.37–1.95) | <0.001 |
| Hemoglobin, g/dL | 13.3 (11.7–14.6) | 13.9 (13.3–15) | 13.3 (11.8–14.6) | 0.239 |
| Peak troponin I, ng/dL | 166 (27–1247) | 110 (29–921) | 199 (34–846) | 0.613 |
| Total cholesterol, mg/dL | 181.9 (146–193) | 202.9 (170–238) | 195 (154–226) | 0.280 |
| LDL cholesterol, mg/dL | 108.1 (79–126) | 128 (91–145) | 119.9 (80–143) | 0.163 |
| eGFR, mL/min/1.73 m 2 | 84 (71–98) | 91 (83–107) | 78 (58–95) | 0.055 |
Abbreviations: ABG, admission blood glucose; ACE, angiotensin-converting enzyme; eAG, estimated average glucose; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; LDL, low-density lipoprotein; OAD, oral antidiabetic.
Note: Data are presented as median (interquartile range) or number (percentage) of patients.
Table 2 shows the angiographic parameters of the patients according to ABG/eAG tertiles. Angiographic parameters revealed that three-vessel disease was much more common in the T3 group when the number of diseased vessels was analyzed. SYNTAX score and the number of patients transferred to cardiovascular surgery for bypass were significantly higher in the T3 group. When SYNTAX scores were closely analyzed, there was no significant difference between the T1 and T2 groups, but there was a significant increase in the T3 group compared with the other two groups (T1: 14.26, T2: 14.77, T3: 24.41; p < 0.001). When the infarct-related artery was evaluated, lesion length was significantly greater in the T3 group compared with the other groups ( p : 0.004), and the proportion of patients undergoing drug-eluting stent implantation was significantly lower in the T3 group compared with the other groups ( p < 0.001). A total of two patients developed in-hospital cardiac arrest, both of whom had high SYNTAX scores.
Table 2. Angiographic data of all patients.
| Angiographic parameters | ABG/eAG tertiles | ||||
|---|---|---|---|---|---|
| T1 (≤0.96); n = 43 | T2 (0.97–1.16); n = 43 | T3 (≥1.17); n = 45 | p -Value | ||
| Number of diseased vessels | 0 | 2 (5) | 3 (7) | 0 | 0.006 |
| 1 | 15 (35) | 12 (28) | 4 (9) | ||
| 2 | 14 (33) | 11 (26) | 11 (24) | ||
| 3 | 12 (28) | 17 (39) | 30 (67) | ||
| SYNTAX score | 14.26 (7–17) | 14.77 (7–21) | 24.41 (17–32) | <0.001 | |
| Angiography result | PCI | 38 (88) | 29 (67) | 19 (42) | <0.001 |
| CABG | 3 (7) | 9 (21) | 26 (58) | ||
| Medical follow-up | 2 (5) | 5 (12) | 0 | ||
| IRA | |||||
| Diameter, mm | 26 (16–38) | 23.2 (15–28) | 31.4 (24–38) | 0.004 | |
| DES implantation | 36 (84) | 29 (67) | 19 (42) | <0.001 | |
| Stent length, mm | 35 (20–48) | 28 (20–30) | 36 (23–48) | 0.549 | |
| No reflow | |||||
| Patients | 2 (5) | 2 (5) | 3 (7) | 0.888 | |
Abbreviations: ABG, admission blood glucose; eAG, estimated average glucose; eGFR, estimated glomerular filtration rate; CABG, coronary artery bypass graft; DES, drug-eluting stent; IRA, infarct-related artery; PCI, percutaneous coronary intervention.
Note: Data are presented as median (interquartile range) or number (percentage) of patients.
When we compare those with SYNTAX scores of 32 and below with those with SYNTAX scores above 32 in Table 3 , we see that age is significantly higher in the group with SYNTAX scores above 32 ( p < 0.001). When we divided the ABG/eAG ratio into groups, the number of patients included in the T3 section was significantly higher in the group with SYNTAX above 32 ( p : 0.02). In addition, the presence of nephropathy was found to be higher in the group with a SYNTAX score above 32.
Table 3. Comparison of demographic data according to SYNTAX score.
| Total N = 131 |
SYNTAX (0–32) N = 116 |
SYNTAX >32 N = 15 |
p -Value | |
|---|---|---|---|---|
| Age | 63 (56–69) | 63 (53–68) | 69 (65–73) | 0.001 |
| Female gender | 49 (37) | 40 (35) | 9 (60) | 0.08 |
| HL | 31 (24) | 29 (25) | 2 (13) | 0.52 |
| HT | 98 (75) | 85 (73) | 13 (87) | 0.35 |
| ABG/eAG tertiles | ||||
| T1 | 43 (33) | 41 (35) | 2 (13) | 0.02 |
| T2 | 43 (33) | 40 (34) | 3 (20) | |
| T3 | 45 (34) | 35 (30) | 10 (67) | |
| Diabetic nephropathy | 20 (15) | 15 (13) | 5 (33) | 0.04 |
| eGFR | 90 (71–100) | 92 (76–102) | 62 (53–79) | 0.001 |
| Hemoglobin | 13.6 (12.3–14.7) | 13.8 (12.5–14.8) | 12.1 (10.8–13.5) | 0.009 |
| LDL | 108 (88–138) | 106 (87–135) | 114 (111–153) | 0.16 |
| İnsulin use | 43 (33) | 36 (31) | 7 (47) | 0.22 |
| Statin use | 24 (18) | 22 (19) | 2 (13) | 0.59 |
| No reflow | 7 (5) | 5 (4) | 2 (13) | 0.18 |
Abbreviations: ABG, admission blood glucose; eAG, estimated average glucose; eGFR, estimated glomerular filtration rate; HL, hyperlipidemia; HT, hypertension; LDL, low-density lipoprotein.
Note: Data are presented as median (interquartile range) or number (percentage) of patients.
Including tertiles of the ABG/eAG, 10 confounding factors that are related to severity of coronary atherosclerosis by means of SYNTAX scores were analyzed with univariate models ( Fig. 1A ). When multivariate modeling was performed with the two or three most relevant variables (age, eGFR, and ABG/eAG ratio), the upper tertile of the ABG/eAG variable was correlated with the severity of coronary atherosclerosis and higher SYNTAX score. In a full model, T3 tertile group has not lost its statistical significance after multivariate analysis (OR: 5.05; 95% CI: 1.00–25.72 p < 0.05) ( Fig. 1B ).
Fig. 1.
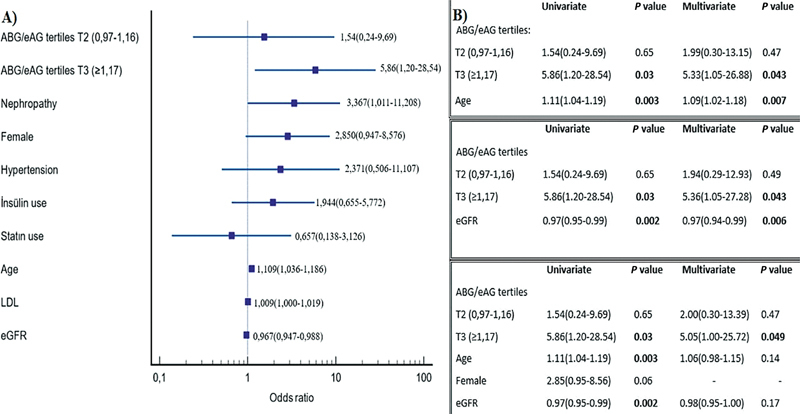
Statistical model of our study group. ( A ) The relative significance of each parameter showing relation with severity of SYNTAX score. ( B ) Association of related parameters with coronary atherosclerotic burden by univariate and multivariate models in our study group. ABG, admission blood glucose; eAG, estimated average glucose; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein.
Discussion
The most important result we obtained in our study is that the ABG/eAG ratio, which is a new index and has been used frequently recently, is positively correlated with coronary artery disease severity and SYNTAX score in ACS patients with a previous diagnosis of DM. A high ABG/eAG ratio at admission was an independent predictor of a high SYNTAX score, even after adjustment for covariates. To the best of our knowledge, our study is the first to show a significant association between ABG/eAG ratio and SYNTAX score in these patients.
Current guidelines recommend an early invasive strategy in patients presenting with ACS, especially if high-risk criteria are present. 23 The judgment of the severity of coronary lesions by coronary angiography is made by calculating the SYNTAX score. According to the SYNTAX score, the severity of coronary lesions is determined, and PCI or CABG is decided accordingly. Patients with a high SYNTAX score (especially ≥33) are prioritized for CABG, whereas patients with a low SYNTAX score (especially ≤22) are prioritized for PCI.
It is well known that chronic hyperglycemia can further accelerate the development of endothelial dysfunction and the pathological process of atherosclerosis, leading to diffuse coronary artery lesions that cause further atherosclerotic vascular changes and worse clinical outcomes. 24 Type 2 DM (T2DM) leads to advanced atherosclerotic vascular changes through endothelial dysfunction, dyslipidemia, formation of advanced glucose end products, endothelial glucose accumulation, increased oxidative stress, and low-grade inflammatory responses. 25 On the other hand, stress hyperglycemia, that is, the A/C glycemic ratio (ABG/eAG), may aggravate T2DM by impairing the timely access of glucose or insulin to target tissues and may cause plaque vulnerability in addition to severe coronary artery stenosis. This underlying mechanism leads to worse clinical outcomes. 26
Acute hyperglycemia in patients presenting with ACS is a physiologic response to stress hormones such as cortisol or epinephrine due to activation of the sympathetic system. Experimental studies have shown that the sudden increase in blood glucose during acute myocardial injury can cause a prothrombotic state and endothelial dysfunction, as well as increase free radical formation, leading to cell and tissue damage and thus causing a larger infarct size. 27 28 Consistent with these in vitro studies, observational clinical trials have found that the ABG level in STEMI is a strong predictor of worse survival rates and increased risk of major adverse cardiac events (MACEs) such as acute heart failure and NR, especially in patients without prior diabetes. 29 30 However, as ABG only represents the stress response to acute myocardial injury and is associated with increased release of stress hormones, it may not be a true reflection of acute glucose levels, especially in diabetic ACS patients.
A recently described index, the ABG/eAG ratio, combines both acute and chronic glucose levels. 14 Therefore, it can be considered as an indicator of true acute glycemic elevation in critically ill patients, including ACS patients. In a previous prospective study of 1,553 ACS patients, Marenzi et al 15 found that the ABG/eAG ratio was a better predictor of in-hospital MACEs and mortality than admission glucose levels alone. Furthermore, Gao et al 16 investigated the effect of ABG/eAG ratio on in-hospital morbidity and mortality in STEMI patients undergoing primary percutaneous intervention. Based on their study findings, they concluded that ABG/eAG provides more meaningful in-hospital prognostic information than ABG alone, especially in diabetic STEMI patients undergoing primary PCI. However, the association of ABG/eAG ratio with SYNTAX score and coronary artery disease severity in diabetic ACS patients has been unknown until now. In this study, when we examined the ABG/eAG ratio by dividing it into three different tertiles, we found that the SYNTAX score of patients with high tertiles was significantly higher and the importance of the ABG/eAG ratio in predicting diffuse disease with a SYNTAX score above 32 was determined independently of other variables.
In addition, the presence of a negative correlation between eGFR levels and SYNTAX score in our study is consistent with similar studies in the literature. Ekici et al 31 investigated the relationship between eGFR and SYNTAX score in patients with stable coronary artery disease and found a significant negative correlation. Cay et al 32 investigated the relationship between coronary atherosclerosis and decreased renal function and found a significant association.
With these results, it will be possible to make an opinion about the high SYNTAX score by looking at the ABG/eAG ratio at the bedside in patients hospitalized from the emergency department with ACS. Thus, we will have a chance to plan earlier coronary intervention in patients with a high SYNTAX score. However, due to the design of the study, further prospective and large-scale studies are required to confirm our findings and study conclusions.
Limitations
Some limitations of our study should be noted before interpreting the results. Our study is a single-center retrospective study with a limited sample size. Furthermore, the ABG/eAG ratio was only measured at patient presentation, and information on serial changes in the ABG/eAG ratio was not available. Since the study included diabetic ACS patients who underwent early CAG, our results may not be generalizable to all ACS patients.
Conclusion
This study shows a significant association between higher ABG/eAG ratio and higher SYNTAX score in patients with DM presenting with ACS. Moreover, a high ABG/eAG ratio was an independent predictor of a high SYNTAX score, even after adjustment for relevant variables. Thus, with the ABG/eAG ratio available at the bedside, patients with DM presenting with ACS have the opportunity to gain insight into the SYNTAX score and to plan an earlier invasive strategy.
Footnotes
Conflict of Interest None declared.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015 Lancet 2016388(10053):1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen J L, Mahaffey K W, Becker R C et al. Coordinated series of studies to evaluate characteristics and mechanisms of acute coronary syndromes in high-risk patients randomly assigned to enoxaparin or unfractionated heparin: design and rationale of the SYNERGY Library. Am Heart J. 2004;148(02):269–276. doi: 10.1016/j.ahj.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Kaya A, Kurt M, Tanboga I H et al. Relation of neutrophil to lymphocyte ratio with the presence and severity of stable coronary artery disease. Clin Appl Thromb Hemost. 2014;20(05):473–477. doi: 10.1177/1076029612473517. [DOI] [PubMed] [Google Scholar]
- 4.Maleki M, Tajlil A, Separham A et al. Association of neutrophil to lymphocyte ratio (NLR) with angiographic SYNTAX score in patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) J Cardiovasc Thorac Res. 2021;13(03):216–221. doi: 10.34172/jcvtr.2021.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oswald G A, Corcoran S, Yudkin J S.Prevalence and risks of hyperglycaemia and undiagnosed diabetes in patients with acute myocardial infarction Lancet 19841(8389):1264–1267. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara M, Kagawa E, Inoue I et al. Impact of admission hyperglycemia and diabetes mellitus on short- and long-term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol. 2007;99(12):1674–1679. doi: 10.1016/j.amjcard.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Eitel I, Hintze S, de Waha S et al. Prognostic impact of hyperglycemia in nondiabetic and diabetic patients with ST-elevation myocardial infarction: insights from contrast-enhanced magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5(06):708–718. doi: 10.1161/CIRCIMAGING.112.974998. [DOI] [PubMed] [Google Scholar]
- 8.Planer D, Witzenbichler B, Guagliumi G et al. Impact of hyperglycemia in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: the HORIZONS-AMI trial. Int J Cardiol. 2013;167(06):2572–2579. doi: 10.1016/j.ijcard.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Stranders I, Diamant M, van Gelder R E et al. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004;164(09):982–988. doi: 10.1001/archinte.164.9.982. [DOI] [PubMed] [Google Scholar]
- 10.Worthley M I, Holmes A S, Willoughby S R et al. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007;49(03):304–310. doi: 10.1016/j.jacc.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 11.Williams S B, Goldfine A B, Timimi F K et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97(17):1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 12.Krinsley J S, Egi M, Kiss A et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care. 2013;17(02):R37. doi: 10.1186/cc12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egi M, Bellomo R, Stachowski E et al. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39(01):105–111. doi: 10.1097/CCM.0b013e3181feb5ea. [DOI] [PubMed] [Google Scholar]
- 14.Roberts G W, Quinn S J, Valentine N et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. 2015;100(12):4490–4497. doi: 10.1210/jc.2015-2660. [DOI] [PubMed] [Google Scholar]
- 15.Marenzi G, Cosentino N, Milazzo V et al. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Diabetes Care. 2018;41(04):847–853. doi: 10.2337/dc17-1732. [DOI] [PubMed] [Google Scholar]
- 16.Gao S, Liu Q, Ding X, Chen H, Zhao X, Li H. Predictive value of the acute-to-chronic glycemic ratio for in-hospital outcomes in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Angiology. 2020;71(01):38–47. doi: 10.1177/0003319719875632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modification of Diet in Renal Disease Study Group . Levey A S, Bosch J P, Lewis J B, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(06):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Sianos G, Morel M A, Kappetein A P et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(02):219–227. [PubMed] [Google Scholar]
- 19.SYNTAX Investigators . Serruys P W, Morice M C, Kappetein A P et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 20.ESC Scientific Document Group . Thygesen K, Alpert J S, Jaffe A S et al. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2019;40(03):237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41 01:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 22.Harrell F E. Heidelberg: Springer; 2015. Regression Modeling Strategies with Applications to linear Models, Logistic Regression and Survival Analysis; pp. 25–572. [Google Scholar]
- 23.ESC Scientific Document Group . Collet J-P, Thiele H, Barbato E et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P K, Agarwal S, Ellis S G et al. Association of glycemic control with mortality in patients with diabetes mellitus undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7(04):503–509. doi: 10.1161/CIRCINTERVENTIONS.113.001107. [DOI] [PubMed] [Google Scholar]
- 25.Domingueti C P, Dusse L M, Carvalho Md, de Sousa L P, Gomes K B, Fernandes A P. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30(04):738–745. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Shen Y, Liu Y H et al. Impact of glycemic control on the association of endothelial dysfunction and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(01):64. doi: 10.1186/s12933-021-01257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monnier L, Mas E, Ginet C et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 28.Baranyai T, Nagy C T, Koncsos G et al. Acute hyperglycemia abolishes cardioprotection by remote ischemic perconditioning. Cardiovasc Diabetol. 2015;14:151. doi: 10.1186/s12933-015-0313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.David R B, Almeida E D, Cruz L V et al. Diabetes mellitus and glucose as predictors of mortality in primary coronary percutaneous intervention. Arq Bras Cardiol. 2014;103(04):323–330. doi: 10.5935/abc.20140130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J W, Zhou Y J, Cao S J, Yang Q, Yang S W, Nie B. Impact of stress hyperglycemia on in-hospital stent thrombosis and prognosis in nondiabetic patients with ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24(05):352–356. doi: 10.1097/MCA.0b013e328361a942. [DOI] [PubMed] [Google Scholar]
- 31.Ekici B, Tanındı A, Sayın I. Effects of glomerular filtration rate on the severity of coronary heart disease. Turk Kardiyol Dern Ars. 2016;44(02):123–129. doi: 10.5543/tkda.2015.48323. [DOI] [PubMed] [Google Scholar]
- 32.Cay S, Metin F, Korkmaz S. Association of renal functional impairment and the severity of coronary artery disease. Anadolu Kardiyol Derg. 2007;7(01):44–48. [PubMed] [Google Scholar]


