Dear Editor:
Granulomatous rosacea (GR) was considered as a variant of rosacea apart from four subtypes: erythematotelangiectatic (ET), papulopustular (PP), ocular, and phymatous1. However, granuloma was also found in ET or PP form of rosacea, so GR was considered as a histologic variant rather than a distinct clinical subtype2. The updated classification unified the subtypes to encompass wide range of phenotypic combinations, focusing on the subclinical histological inflammation3. In other words, GR could still be interpreted as a histologic variant, featured by distinctive granulomatous inflammation indicating the underlying chronic and severe tissue reaction3,4.
Lupus miliaris disseminatus faciei (LMDF) manifests as multiple discrete yellow-brown, dome-shaped papules on the centrofacial area5. The etiology of tuberculosis was refuted, suggesting the immune reaction to the antigens from injured hair follicles or Demodex folliculorum 6. Histologic hallmark is epithelioid granuloma surrounding caseation necrosis, which can be obscure in early or late stage7.
Differential diagnosis of them is important because of the differences in therapeutic responsiveness and prognosis8. GR was known to be improved by tetracycline, but LMDF was known to show relatively favorable response on corticosteroid. GR tends to show chronic progress but LMDF can resolve spontaneously within 2 years, accompanying pitted scars. Overlapping clinical manifestations and histology between them can easily lead to misdiagnosis5,6,8. Therefore, this study investigated the perceptional pattern to reflect real-world dilemma in diagnosis, correlating the clinical appearance and histopathology.
Patients were selected who received biopsy on suspicion of GR or LMDF in the Busan Paik and Haeundae Paik Hospital between 1995 and 2020. Five dermatologists reviewed all photographs without clinical or histopathological information. Any training or opinion exchange was not given to minimize the confirmation bias.
The subjects were classified into five groups: ‘GR,’ ‘LMDF,’ ‘Indeterminate GR (I-GR),’ ‘Indeterminate LMDF (I-LMDF),’ or ‘Others.’ If 4–5 physicians had impressions of GR or LMDF, the subjects were assorted into ‘GR’ or ‘LMDF’ groups respectively. If 2–3 physicians did, the subjects were included in ‘I-GR’ or ‘I-LMDF’ groups respectively. The subjects were classified into ‘Others’ if none or 1 impression of GR or LMDF was given. This study was approved by Inje University Institutional Review Board (No.: 2021-04-039).
A total of 62 case were enrolled and their clinico-histopathologic features were described in Table 1. The ‘GR’ group showed significantly higher female proportion than ‘LMDF’ group (1:3.5 vs. 1:0.8), with a higher mean age (51.7 vs. 44.4). Vascular symptom and history of aggravation on external stimuli were significantly more common in ‘GR’ group than ‘LMDF’ group (77.7% vs. 23%, 61.1% vs. 15.3%). Other clinical impressions were folliculitis, acne vulgaris, contact dermatitis, seborrheic dermatitis, ET rosacea, and PP rosacea.
Table 1. The clinical and histopathologic features of the subjects.
| Variables | Total | GR | I-GR | I-LMDF | LMDF | Others | |
|---|---|---|---|---|---|---|---|
| No. | 62 | 18 | 10 | 8 | 13 | 13 | |
| Age (mean ± SD) | 47.3±13.8 | 51.7±11.9 | 46.9±14.7 | 47.9±15.6 | 44.4±13.0 | 44.2±15.4 | |
| Sex (male:female) | 1:1.3 | 1:3.5 | 1:0.6 | 1:3.0 | 1:0.8 | 1:0.8 | |
| Vascular symptom | 53.2% | 77.7%* | 60.0% | 50.0% | 23.0% | 46.1% | |
| Aggravation on external stimuli | 40.3% | 61.1%* | 40.0% | 37.5% | 15.3% | 38.4% | |
| Lesion distribution | |||||||
| Eyelid | 45.1% | 27.7% | 50.0% | 75.0%* | 84.6%* | 7.7% | |
| Forehead | 77.4% | 83.3% | 70.0% | 75.0% | 92.3% | 61.5% | |
| Nose | 70.9% | 83.3% | 90.0% | 50.0% | 100.0% | 23.0% | |
| Perioral area | 64.5% | 61.1% | 50.0% | 87.6% | 76.9% | 53.9% | |
| Cheek | 85.4% | 100.0% | 80.0% | 62.5% | 92.3% | 76.9% | |
| Chin | 72.5% | 88.9% | 50.0% | 75.0% | 69.2% | 69.2% | |
| Extrafacial area | 8.1% | 11.1% | 0.0% | 12.5% | 7.7% | 8.3% | |
| Lesion appearance | |||||||
| Background erythema | 59.6% | 88.9%* | 60.0% | 37.5% | 46.2% | 46.2% | |
| Telangiectasia | 58.0% | 93.3%* | 90.0%* | 50.0% | 30.8% | 30.8% | |
| Deep-seated papule | 32.2% | 22.2% | 10.0% | 50.0% | 76.9%* | 7.7% | |
| Pustule | 16.1% | 33.3% | 0.0% | 0.0% | 23.0% | 7.7% | |
| Histopathology | |||||||
| Caseation necrosis | 17.7% | 5.6% | 10.0% | 37.5%* | 38.5%* | 7.7% | |
| Epithelioid granuloma | 59.6% | 55.6% | 60.0% | 75.0% | 84.6% | 30.8% | |
| Capillary dilatation | 83.8% | 88.9% | 90.0% | 87.5% | 84.6% | 69.2% | |
| Solar elastosis | 53.2% | 61.1% | 70.0% | 50.0% | 46.2% | 38.5% | |
| Demodex folliculorum | 14.5% | 27.8% | 10.0% | 12.5% | 7.7% | 7.7% | |
GR: granulomatous rosacea, I-GR: indeterminate granulomatous rosacea, I-LMDF: indeterminate lupus miliaris disseminatus faciei, LMDF: lupus miliaris disseminatus faciei, SD: standard deviation.
*p<0.05.
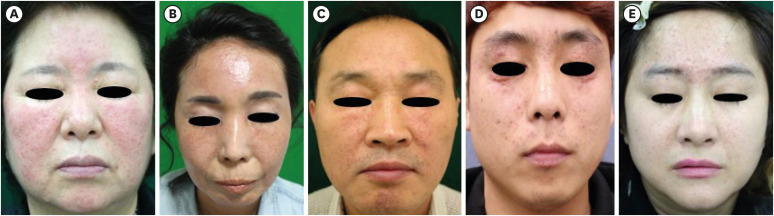
Representative clinical pictures were shown in Fig. 1. Eyelid involvement was significant in ‘LMDF’ and ‘I-LMDF’ groups (84.6%, 75%). It gradually increased from ‘GR’ (27.7%) to ‘LMDF’ group (84.6%). The ‘LMDF’ group showed significantly higher incidence of deep-seated papules (76.9%), however background erythema (88.9%) and telangiectasia (93.3%) were significant in ‘GR’ group. Telangiectasia gradually decreased from ‘GR’ (93.3%) to ‘LMDF’ group (30.8%). Unlike previous studies, extrafacial involvement was not significantly different between ‘GR’ group (11.1%) and ‘LMDF’ group (7.7%), which might be attributed to the small subject number in this study.
Fig. 1. The representative clinical pictures of each group. (A) Granulomatous rosacea. (B) Indeterminate granulomatous rosacea. (C) Indeterminate lupus miliaris disseminatus faciei. (D) Lupus miliaris disseminatus faciei. (E) Others.
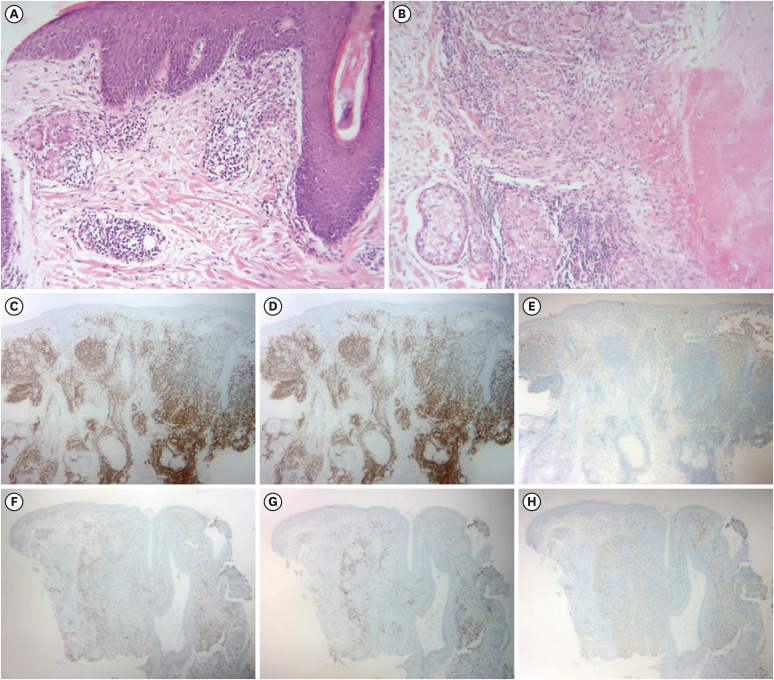
Representative histological images were shown in Fig. 2. Caseation necrosis was significant in ‘LMDF’ and ‘I-LMDF’ groups (38.5%, 37.5%). The proportion of epithelioid granuloma gradually increased from ‘GR’ (55.6%) to ‘LMDF’ group (84.6%). In contrast, capillary dilatation, solar elastosis, and D. folliculorum were more common in ‘GR’ group, albeit statistically insignificant. CD3 (pan T-cell marker) and CD8 (cytotoxic T-cell marker) were positive in both ‘GR’ and ‘LMDF’ groups, however CD68 (macrophage marker) stain was weaker in the former. Although both diseases have pathophysiology of the T-cell-attracting chemokines and subsequent cytokine cascades, macrophage activity might be more markedly upregulated in the LMDF as indicated in the previous report9.
Fig. 2. Histopathology. (A) Granulomatous rosacea showed dermal epithelioid granuloma, solar elastosis, capillary dilatation, and Demodex folliculorum (H&E; ×100). (B) Lupus miliaris disseminatus faciei showed caseation necrosis and adjacent epithelioid granuloma (H&E; ×100). (C-E) Infiltrated cells of granulomatous rosacea were positive on CD3 and CD8 (T cell markers), but weakly positive on CD68 (macrophage marker). (CD3 ×40, CD8 ×40, CD68 ×40) (F-H) Infiltrated cells of lupus miliaris disseminatus faciei were positive on CD3, CD8, and CD68. (CD3 ×40, CD8 ×40, CD68 ×40).
H&E: hematoxylin and eosin.
Previously known features of LMDF distinctive from GR were the predilection of young men, eyelid and perioral involvement, absence of background erythema or vascular symptoms, less reactivity to external stimuli, favorable response to corticosteroid, and self-limited course with scarring5,6,8. Histologically, LMDF shows larger-sized granuloma and central necrosis with less capillary dilatation, solar elastosis, or D. folliculorum than GR5,6.
In this study, ‘GR’ group showed significantly higher proportion of female, background erythema, and telangiectasia, while ‘LMDF’ group showed significant eyelid involvement and deep-seated papules. In other words, physician’s perceptual pattern was focused on sex, erythema, telangiectasia, eyelid invasion, and deep-seated papules to get impression.
Interestingly, clinical classification well predicted the histological features. ‘LMDF’ group showed significant caseation necrosis, while ‘GR’ group showed slightly more capillary dilatation, solar elastosis, and D. folliculorum. Prevalent deep-seated papules of ‘LMDF’ group might indicate the histological granuloma with caseation necrosis. Significant telangiectasia of ‘GR’ group might indicate the histological capillary dilatation, while background erythema might reflect the histological photoaging manifested as solar elastosis, and Demodex-related inflammation with vasodilation.
The proportion of caseation necrosis in ‘LMDF’ group was consistent with the previous studies (20%–43%)7. The absence of caseation necrosis could be attributed to the biopsy of early or late lesion, and distinction with GR was challening in those cases7. The proportion of caseation necrosis in ‘GR’ group was also consistent with previous studies (10%), and consideration of other information, including vascular symptoms or therapeutic response, was required to rule out LMDF8.
D. folliculorum was more common in ‘GR’ group than ‘LMDF’ group (27.8% vs. 7.7%), suggesting closer relationship with the former. Although several reports considered D. folliculorum to induce LMDF through delayed hypersensitivity reaction, this study might reflect the well-established strong relationship of D. folliculorum with rosacea through toll-like receptor 2, cathelicidin, and activation of macrophage and CD8-positive cytotoxic T cell10.
This study has limitations in that the subject number was small and the subjects were not initially confirmed as GR or LMDF. Therefore, other diseases such as folliculitis or acne vulgaris would have acted as confounding factors. In addition, this study could not evaluate the prognostic factors of GR and LMDF because of several missing data about therapeutic progress, not unified treatment regimen, and variable follow-up period. It would be helpful to perform prospective study with standardized management protocol with objective method for evaluation of therapeutic response.
This study clearly showed the perceptional pattern in evaluating granulomatous facial dermatoses. In other words, this study implicated the risk of misdiagnosis of GR or LMDF based merely on clinical appearance or histopathology. To avoid the diagnostic pitfalls, comprehensive information, including vascular symptoms, aggravating factors, and treatment progress, would be necessary.
Footnotes
FUNDING SOURCE: None.
CONFLICTS OF INTEREST: The authors have nothing to disclose.
DATA SHARING STATEMENT: All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Sánchez JL, Berlingeri-Ramos AC, Dueño DV. Granulomatous rosacea. Am J Dermatopathol. 2008;30:6–9. doi: 10.1097/DAD.0b013e31815bc191. [DOI] [PubMed] [Google Scholar]
- 2.Aroni K, Tsagroni E, Lazaris AC, Patsouris E, Agapitos E. Rosacea: a clinicopathological approach. Dermatology. 2004;209:177–182. doi: 10.1159/000079886. [DOI] [PubMed] [Google Scholar]
- 3.Gallo RL, Granstein RD, Kang S, Mannis M, Steinhoff M, Tan J, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148–155. doi: 10.1016/j.jaad.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 4.van Zuuren EJ, Arents BW, van der Linden MM, Vermeulen S, Fedorowicz Z, Tan J. Rosacea: new concepts in classification and treatment. Am J Clin Dermatol. 2021;22:457–465. doi: 10.1007/s40257-021-00595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Scheur MR, van der Waal RI, Starink TM. Lupus miliaris disseminatus faciei: a distinctive rosacea-like syndrome and not a granulomatous form of rosacea. Dermatology. 2003;206:120–123. doi: 10.1159/000068457. [DOI] [PubMed] [Google Scholar]
- 6.Chougule A, Chatterjee D, Yadav R, Sethi S, De D, Saikia UN. Granulomatous rosacea versus lupus miliaris disseminatus faciei-2 faces of facial granulomatous disorder: a clinicohistological and molecular study. Am J Dermatopathol. 2018;40:819–823. doi: 10.1097/DAD.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 7.el Darouti M, Zaher H. Lupus miliaris disseminatus faciei--pathologic study of early, fully developed, and late lesions. Int J Dermatol. 1993;32:508–511. doi: 10.1111/j.1365-4362.1993.tb02835.x. [DOI] [PubMed] [Google Scholar]
- 8.Seo JI, Shin MK. Lupus miliaris disseminatus faciei versus granulomatous rosacea: a case report. Case Rep Dermatol. 2021;13:321–329. doi: 10.1159/000517209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexanian C, Liakos W, Toussi A, Kao J, Cheng MY, Wang EA, et al. Immune profiling of lupus miliaris disseminatus faciei and successful management with anti-tumour necrosis factor therapy. Clin Exp Dermatol. 2021;46:910–914. doi: 10.1111/ced.14684. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y, Wu LX, Zhang JH, Zhou N, Luan XL. Demodex-induced Lupus miliaris disseminatus faciei: a case report. Medicine (Baltimore) 2020;99:e21112. doi: 10.1097/MD.0000000000021112. [DOI] [PMC free article] [PubMed] [Google Scholar]