Abstract
Background
Cardiac injury is frequently reported in COVID-19 patients, the right ventricle (RV) is mostly affected. We systematically evaluated the cardiac function and longitudinal changes in severe COVID-19 acute respiratory distress syndrome (ARDS) admitted to the intensive care unit (ICU) and assessed the impact on survival.
Methods
We prospectively performed comprehensive echocardiographic analysis on mechanically ventilated COVID-19 ARDS patients, using 2D/3D echocardiography. We defined left ventricular (LV) systolic dysfunction as ejection fraction (EF) < 40%, or longitudinal strain (LS) > − 18% and right ventricular (RV) dysfunction if two indices among fractional area change (FAC) < 35%, tricuspid annulus systolic plane excursion (TAPSE) < 1.6 cm, RV EF < 44%, RV–LS > − 20% were present. RV afterload was assessed from pulmonary artery systolic pressure (PASP), PASP/Velocity Time Integral in the right ventricular outflow tract (VTIRVOT) and pulmonary acceleration time (PAcT). TAPSE/PASP assessed the right ventriculoarterial coupling (VACR).
Results
Among 176 patients included, RV dysfunction was common (69%) (RV–EF 41.1 ± 1.3%; RV–FAC 36.6 ± 0.9%, TAPSE 20.4 ± 0.4mm, RV–LS:− 14.4 ± 0.4%), usually accompanied by RV dilatation (RVEDA/LVEDA 0.82 ± 0.02). RV afterload was increased in most of the patients (PASP 33 ± 1.1 mmHg, PAcT 65.3 ± 1.5 ms, PASP/VTIRVOT, 2.29 ± 0.1 mmHg/cm). VACR was 0.8 ± 0.06 mm/mmHg. LV–EF < 40% was present in 21/176 (11.9%); mean LV–EF 57.8 ± 1.1%. LV–LS (− 13.3 ± 0.3%) revealed a silent LV impairment in 87.5%. A mild pericardial effusion was present in 70(38%) patients, more frequently in non-survivors (p < 0.05). Survivors presented significant improvements in respiratory physiology during the 10th ICU-day (PaO2/FiO2, 231.2 ± 11.9 vs 120.2 ± 6.7 mmHg; PaCO2, 43.1 ± 1.2 vs 53.9 ± 1.5 mmHg; respiratory system compliance—CRS, 42.6 ± 2.2 vs 27.8 ± 0.9 ml/cmH2O, all p < 0.0001). Moreover, survivors presented significant decreases in RV afterload (PASP: 36.1 ± 2.4 to 20.1 ± 3 mmHg, p < 0.0001, PASP/VTIRVOT: 2.5 ± 1.4 to 1.1 ± 0.7, p < 0.0001 PAcT: 61 ± 2.5 to 84.7 ± 2.4 ms, p < 0.0001), associated with RV systolic function improvement (RVEF: 36.5 ± 2.9% to 46.6 ± 2.1%, p = 0.001 and RV–LS: − 13.6 ± 0.7% to − 16.7 ± 0.8%, p = 0.001). In addition, RV dilation subsided in survivors (RVEDA/LVEDA: 0.8 ± 0.05 to 0.6 ± 0.03, p = 0.001). Day-10 CRS correlated with RV afterload (PASP/VTIRVOT, r: 0.535, p < 0.0001) and systolic function (RV–LS, 0.345, p = 0.001). LV–LS during the 10th ICU-day, while ΔRV–LS and ΔPASP/RVOTVTI were associated with survival.
Conclusions
COVID-19 improvements in RV function, RV afterload and RV–PA coupling at day 10 were associated with respiratory function and survival.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-024-01241-1.
Keywords: COVID-19, Cardiac function, RV dysfunction, Pulmonary vascular resistance, PEEP, ARDS, Hemodynamics, Strain
Background
Acute cardiac injury is the most common cardiac abnormality in Coronavirus disease 2019 (COVID-19) Acute Respiratory Distress Syndrome (ARDS), mostly defined by cardiac troponin elevation > 99th percentile or basic echocardiographic data [1–5]. The incidence is more common in critically ill patients (around 50%) compared to hospitalized patients in the general wards (20%) [6, 7]. The proposed pathogenetic mechanisms include direct myocardial injury, myocardial oxygen supply–demand imbalance, increased right ventricular (RV) afterload, diffuse endothelialitis and procoagulant activity [8].
The ECHO–COVID study reported that among Intensive Care Units (ICU) patients, mechanically ventilated (MV) or not, 34.5% presented RV and 22% had left ventricular (LV) systolic dysfunction, based on rough visual estimation [9]. A large worldwide survey presenting data through basic echocardiography in COVID-19 patients, indicated that echocardiography may change the management in 33% of the cases [4]. However, more detailed examinations are still rather scarce, especially in MV patients [5, 10, 11]; moreover, longitudinal changes of myocardial damage in MV COVID-19 ARDS patients during the course of ICU stay, have been recently reported in two studies, pointing that different degrees of RV impairment might affect mortality [12, 13]. Considering that the median reported intubation time is 10–14 days [14–17] and that stress cardiomyopathy alleviates by the 7th–10th day after onset [18, 19] we hypothesized that survivors may have improved RV function by that time.
Thus, the aim of the present study is to systematically evaluate the cardiac function and its temporal changes by the 10th ICU day, in intubated COVID-19 ARDS patients using conventional, speckle tracking and three-dimensional (3D) echocardiography and troponin levels. Second, we investigated the possible effects of COVID-19 cardiac function on survival.
Methods
Study population
From 4/2020 to 6/2022, we prospectively evaluated the cardiac function in consecutive MV patients, intubated due to respiratory failure from COVID-19 ARDS, from the University Hospital of Larissa, Greece. This study was approved by the University Hospital of Larissa Ethics Board (Cardiac function in mechanically ventilated COVID-19 ARDS patients, 16965/2020), with a waiver for informed consent, as the assessment of cardiac function was part of the routine care of patients admitted in our ICU. The procedures followed are in accordance with the ethical standards of the local institutional review board and the Helsinki Declaration of 1975. Exclusion criteria were: (1) severe stenosis and/or regurgitation of the aortic valve; (2) pre-existing severe heart failure (< 40%) due to prior myocardial infarction or any other cause; (3) cardiac arrest in the peri-intubation period; (4) known history of pulmonary arterial hypertension with or without right ventricular impairment; (5) moderate/severe known respiratory disease; (6) presence of left bundle branch block (LBBB); and (7) ICU admission due to massive pulmonary embolism (PE) confirmed by computed tomography pulmonary angiography (CTPA). Patients were also excluded if they presented signs of acute cor pulmonale and there was increased suspicion of massive PE and received thrombolysis (8). Poor acoustic window (9). patients with SARS-CoV-2 infection with a cause of ICU admission other than respiratory failure due to ARDS.
Study protocol
All included patients had a full echocardiographic examination performed during the first 48 h of ICU admission. Patients were re-evaluated on the 10 ± 1 ICUday (per-protocol) and whenever necessary. During the echocardiographic examinations, the patients were ventilated according to the patients’ clinical status, respiratory drive, respiratory function.
Measurements
(1) Patient characteristics and comorbidities, (2) Clinical data concerning ventilation/hemodynamics (3) Echocardiography data according to recent guidelines on conducting and reporting critical care echocardiography [20–25].
Echocardiographic parameters: Comprehensive transthoracic echocardiographic examination (System Vivid™ E95, GE Medical Systems, USA-Philips iE33, Philips Medical, USA) was performed to assess RV dimensions and function (2D/3D measurements) and the inferior vena cava (IVC) [21–25]. (Additional file 1).
Left ventricular systolic function was assessed using (1) the Simpson’s method to calculate ejection fraction estimation (2D) and (2) 3D left ventricular volume measurements. Both values 2D and 3D values are reported, as there were missing 3D values in some patients.
Right ventricular dilation was estimated through planimetry at end-diastole from a 4-chamber view quantification comparing the right ventricular end diastolic area (RVEDA) to left ventricular end diastolic area (LVEDA) to calculate their ratio (RVEDA/LVEDA). The RV contractility was estimated through measurements of the RV end-diastolic area (RVEDA) and end-systolic area (RVESA), measured to calculate RV Fractional Area Change (RVFAC % = 100 ×(RVEDA−RVESA)/RVEDA), Tricuspid Annular Plane Systolic Excursion (TAPSE), systolic velocity of the annulus of the tricuspid valve (RV S’) using tissue doppler imaging, while RV isovolumic acceleration (RV IVA) [derived from peak isovolumic velocity (IVV) and acceleration time (AT)] was also assessed. Two-dimensional speckle tracking echocardiography (2D-STE) was used to characterize longitudinal systolic strain [22]. RV longitudinal strain (RV–LS) was measured from the apical four-chamber view and the endocardial border was manually traced delineating a region of interest composed by 6 segments with eventual manual adjustments. Longitudinal strain curves were generated by the software for each RV segment. The RV free wall longitudinal strain (RV–LS) was calculated as the mean of the strain values in the three segments of the RV free wall [21, 25].
Right ventricular volumes and RV ejection fraction were estimated using three-dimensional echocardiography (3D). A wide-angled, single-beat, high frame rate (Heart Model mode) 3D full-volume images data sets were acquired from the apical four-chamber RV-focused view. Then, RV endocardial surfaces were defined and tracked throughout the cardiac cycle, and a quick minimal manual adjustment was performed in case of unsatisfactory outcomes. Finally, a 3D RV cast, RV volume curves were provided, from which the RV end-diastolic volume (RVEDV), RV end-systolic volume (RVESV), and RVEF were determined. All measures were made offline, using the semi-automated EchoPAC software package.
Right ventricular systolic pressure (RVSP) was estimated from peak tricuspid regurgitation (TR) jet velocity, using the simplified Bernoulli equation. Pulmonary artery systolic pressure (PASP) was estimated from the sum of RVSP plus the central venous pressure. Acute cor pulmonale (ACP) was defined if RVEDA/LVEDA was > 0.6 with presence of paradoxical septal motion.
Pulmonary vascular resistance (PVR) was indirectly estimated through quantification of the PASP (via tricuspid regurgitation velocity), the pulmonary acceleration time (PAcT) of the right ventricular outflow tract (RVOT) Flow velocity Doppler envelop, and the ratio of PASP to the RVOT velocity time (PASP/VTIRVOT) as the ratio integrates PASP and cardiac output and thus better expresses changes in PVRs [26–28]. The presence of a systolic notch on the deceleration part was also reported [28]. Although the systolic notch was reported, it was not considered as an indication of increased PVRs, when calculating RV afterload. Right Ventriculoarterial Coupling, (VACR) was assessed through the Tricuspid Annular Plane Systolic Excursion (TAPSE)/PASP ratio [10, 29].
Definitions for LV/RV impairments are presented in the Additional file 1.
The echocardiographic study was made by one operator (due to the pandemic conditions). Three consecutive cycles (five to ten in case of non-sinus rhythm) were averaged for every parameter. Measurements were assessed by three cardiologists (NK, VV and EZ) and trained doctors [competence in advanced critical care echocardiography (VT)]. Two of these doctors evaluated each measurement (offline using EchoPACK, or the stored videos). In case of > 10% variability, offline re-evaluation was performed with two operators present, to reach agreement.
Statistical analysis
Kolmogorov–Smirnov test was applied to test the variable distribution. Normally distributed variables were expressed as mean ± standard error of means (SEM), while non-normally distributed data were expressed as median (interquartile range); categorical variables were expressed as counts and percentages. The students t test or Wilcoxon rank-sum test were used where appropriate. Paired sample t test was used to compare variables between baseline and follow-up echocardiograms. Categorical variables were compared using the x2 test or Fisher exact test. Unadjusted correlations between TNI, LV/RV function indices, severity scores and respiratory variables were done using Pearson correlation.
Binary logistic regression analysis was used to investigate factors related to survival. Three models were constructed: in the first, baseline values that significantly differed between survivors/non-survivors were entered, in the second model, the significantly different echocardiographic values upon re-evaluation between survivors/non-survivors, and the third included the significantly different changes between survivors/non-survivors.
Reproducibility of echocardiographic measurements were tested on basic RV and LV indices. Interobserver agreement was assessed using interclass correlation coefficients.
Statistical analyses were performed using SPSS version 26.0 (IBM). A value of p < 0.05 (two-tailed) was considered statistically significant.
Results
Patients
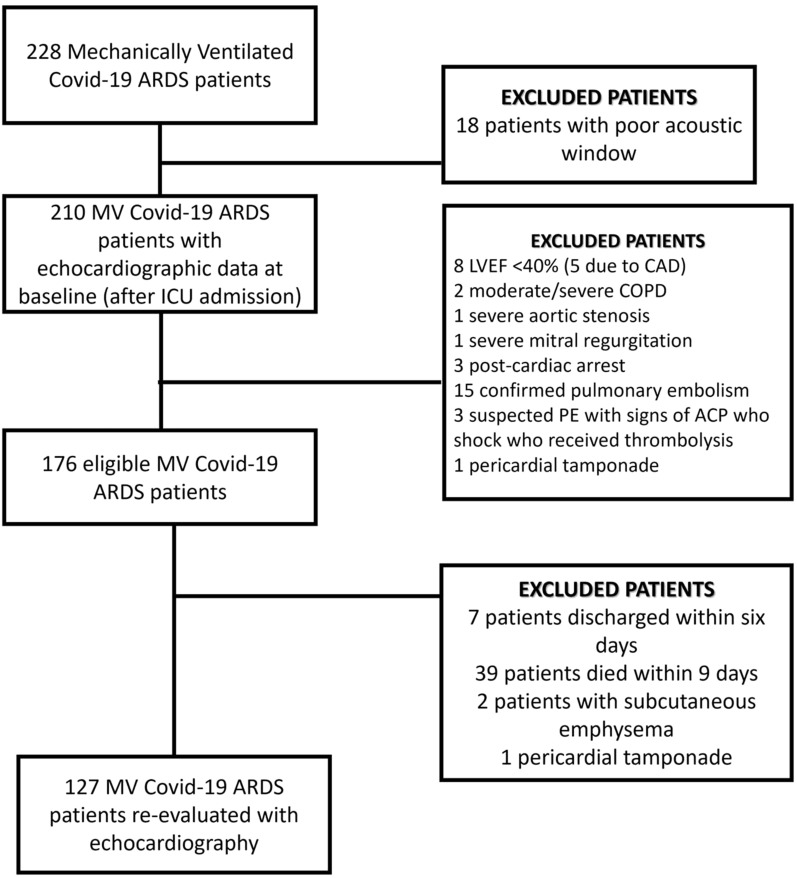
A total of 228 patients with COVID-19 ARDS were admitted, after intubation, in the ICU; 176 patients with echocardiography data upon ICU admission were analyzed (Fig. 1). Demographics, medical history and laboratory date upon ICU admission are presented in Additional file 1: Table S1. The mean age was 67.2 ± 0.8% and 65.8% were male.
Fig. 1.
Flow chart. ACP acute cor pulmonale, ARDS acute respiratory distress syndrome, CAD coronary artery disease, COPD chronic obstructive pulmonary disease, ICU Intensive Care Unit, LBBB left bundle branch block, LVEF left ventricular ejection fraction, MV mechanical ventilation, PE pulmonary embolism;=
Respiratory and hemodynamic variables during initial echocardiography
At the time of the baseline echocardiographic evaluation, all patients were sedated and mechanically ventilated (Volume assist/controlled mode) and no patient presented spontaneous respiratory efforts. In 86.4% of the patients a neuromuscular blocking agent was administered. Variables concerning ventilatory parameters, respiratory variables and mechanics are presented in Table 1 (Additional file 1: Table S1).
Table 1.
Respiratory, hemodynamic variables and respiratory mechanics during the initial echocardiographic evaluation
| Clinical parameters | Data (number of patients with the reported value) |
|---|---|
| Demographics | |
| Age, years | 67.2 ± 0.8 (176) |
| Sex (male), n, % | 121/176 (65.8) |
| APACHE II score | 16.86 ± 0.55 (176) |
| SOFA score | 7.86 ± 0.16 (176) |
| Troponin, ng/ml (0–0.04) | 0.34 ± 0.07 (176) |
| Respiratory variables | |
| Vt, ml/kg, (n) | 6.9 ± 0.1 (176) |
| PEEP, cm H2O | 11.7 ± 0.2 (176) |
| PaO2/FiO2, mm Hg | 94.9 ± 3.8 (176) |
| PaCO2, mm Hg | 53.5 ± 1.6 (176) |
| Hemodynamic Variables | |
| Noradrenaline, μg/kg/min | 0.51 ± 0.07 (176) |
| MAP, mm Hg | 68.53 ± 0.44 (176) |
| HR | 76.07 ± 1.99 (176) |
| CVP, mmHg | 12.60 ± 0.24 (158) |
| ScvO2, % | 69.54 ± 0.52 (156) |
| Lac, mmol/Lt | 3.4 ± 0.5 (148) |
| Respiratory System Mechanics | |
| CRS, ml/cm H2O | 33.8 ± 0.9 (176) |
| Pplat, cmH2O | 25.9 ± 0.4 (176) |
| DP, cmH2O | 14.3 ± 0.3 (176) |
Numbers in parenthesis refer to the actual number of patients in whom the value was measured
APACHE II acute physiology and chronic health evaluation II score, CRS static compliance of the respiratory system, CVP central venous pressure, DP driving pressure, FiO2 fraction of inspired oxygen, HR heart rate, lac lactate, MAP mean arterial pressure, PaCO2 partial pressure of arterial carbon dioxide, PaO2 partial pressure of arterial oxygen, PEEP positive end-expiratory pressure, Pplat plateau pressure, ScvO2 oxygen saturation in venous blood from vena cava, SOFA score sequential organ failure assessment score, Vt tidal volume
One hundred and seventy (96.6%) patients were receiving norepinephrine (mean dose 0.51 ± 0.09 μg/kg/min), while 19 patients (10.4%) were also receiving argipressin (Table 1). Upon admission, 86/176 (48.9%) presented elevated troponin levels [mean troponin 0.67 ± 0.13 ng/ml, in those with abnormal values (> 0.04 ng/ml)]. Age, BMI and sex did not differ between patients with increased and normal troponin levels (Additional file 1: Table S2).
Main echocardiographic findings
Seventeen patients presented atrial fibrillation (new onset atrial fibrillation in eight) at baseline evaluation; the rest were in sinus rhythm.
RV size/function
Values regarding RV size, RV function, IVC dimensions and distensibility index are presented in Table 2 and Additional file 1: Table S3. RV dilation was present in 151/176 (86%) [mean RV end-diastolic area/LV end-systolic area (RVEDA/LVEDA): 0.8 ± 0.02]. Acute cor pulmonale was present in 35 patients (20%). RVEDA/LVEDA correlated weakly to PaO2/FiO2 (r:− 0.168, p = 0.039) but not to PaCO2 values. RV systolic dysfunction (using at least two of the criteria mentioned in the Additional file 1) was present in 113/176 (64.2%) patients. RV dilation and dysfunction were simultaneously present in 93/173 (54%).
Table 2.
Echocardiographic variables in survivors and non-survivors upon admission and follow-up
| Echocardiographic Variable | COVID-19 ARDS survivors (n = 56)a | COVID-19 ARDS non-survivors (n = 120) | ||||
|---|---|---|---|---|---|---|
| Admission | Day 10 | p value | Admission | Day 10 | p value | |
| Age, years | 65.9 ± 1.5 | 67.4 ± 1.0 | ||||
| Sex, % | 39 (69.6%) | 80 (66.7%) | ||||
| APACHE II score | 16.2 ± 0.9 | 17.3 ± 0.7 | ||||
| SOFA score | 7.5 ± 0.2 | 8.05 ± 0.2 | ||||
| Right ventricle | ||||||
| RVEDA/LVEDA | 0.8 ± 0.05 (49) | 0.6 ± 0.03 (49)# | 0.001 | 0.81 ± 0.03 (78) | 0.89 ± 0.03 (78) | 0.864 |
| ACP | 12 (24.5) | 5 (10.2) | 0.073 | 21 (26.9) | 14 (17.9) | 0.850 |
| RVEDA, cm2 | 22.1 ± 0.6 (49) | 17.8 ± 0.8 (49)* | < 0.0001 | 23 ± 0.8 (78) | 24.4 ± 0.8 (78) | 0.097 |
| RVESA, cm2 | 14.1 ± 0.5 (49) | 10.3 ± 0.5 (49)# | < 0.0001 | 14.5 ± 0.6 (78) | 15.2 ± 0.8 (78) | 0.357 |
| RV FAC, % | 37.4 ± 1.6 (49) | 41.1 ± 1.9 (49) | 0.073 | 35.9 ± 1.6 (78) | 38.9 ± 1.7 (78) | 0.253 |
| RVEDVb, ml | 128.1 ± 13.9 (29)* | 92.8 ± 8.4 (29)* | 0.006 | 110.91 ± 6.02 (44) | 121.3 ± 6.17 (44) | 0.004 |
| RVESVb, ml | 82.4 ± 10.2 (29)* | 52.2 ± 5.3 (29)* | 0.002 | 67.6 ± 3.9 (44) | 73.6 ± 4.4 (44) | 0.127 |
| RVEFb (%) | 36.5 ± 2.9 (29) | 46.6 ± 2.1 (29)* | 0.001 | 38.8 ± 1.5 (44) | 39.1 ± 2 (44) | 0.916 |
| TAPSE, mm | 19.8 ± 0.6 (51) | 21.5 ± 0.5 (51) | 0.008 | 20.3 ± 0.6 (73) | 22 ± 2 (73) | 0.404 |
| RV S’, cm/sec | 16.8 ± 0.8 (53) | 19.9 ± 0.7 (53)# | 0.002 | 15.3 ± 0.6 (74) | 14.1 ± 0.5 (74) | 0.045 |
| RV IVA, m/sec2 | 3.98 ± 0.17 (53) | 4.1 ± 0.17 (53) | 0.318 | 3.66 ± 0.12 (74) | 3.77 ± 0.14 (74) | 0.260 |
| RV–LS, % | − 13.6 ± 0.7 (42) | − 16.7 ± 0.8 (42)# | 0.001 | − 14.3 ± 0.8 (64) | − 12.6 ± 0.5 (64) | 0.017 |
| VTIRVOT, cm | 17 ± 0.7 (41) | 19.4 ± 0.5 (41)# | 0.011 | 16.2 ± 0.5 (60) | 16.1 ± 0.4 (60) | 0.987 |
| PASP, mmHg | 36.1 ± 2.4 (43) | 20.1 ± 3 (43)# | < 0.0001 | 34.9 ± 1.8 (66) | 36.3 ± 1.1 (66) | 0.347 |
| PASP/VTILVOT, mmHg/cm | 2 ± 0.2 (43) | 0.9 ± 0.1 (43)# | < 0.0001 | 2 ± 0.2 (65) | 1.9 ± 0.1 (65) | 0.543 |
| PASP/VTIRVOT, mmHg/cm | 2.5 ± 1.4 (34) | 1.1 ± 0.7 (34)# | < 0.0001 | 2.5 ± 1.5 (57) | 2.4 ± 0.9 (57) | 0.445 |
| Pulmonary AcT, msec | 61 ± 2.5 (41) | 84.7 ± 2.4 (41)# | < 0.0001 | 66.8 ± 2.6 (60) | 62.6 ± 2.3 (60) | 0.096 |
| RVOT notch | 15 (23.4%) | 4 (6.3%) * | 0.033 | 21 (35%) | 13 (21.6%) | 0.321 |
| VACR, mm/mmHg | 0.80 ± 0.1 (43) | 1.5 ± 0.1 (43)# | < 0.0001 | 0.73 ± 0.07 (60) | 0.66 ± 0.08 (60) | 0.497 |
| Left ventricle | ||||||
| LVEDD, mm | 4.66 ± 0.1 (49) | 4.62 ± 0.1 (49) | 0.576 | 4.55 ± 0.06 (78) | 4.53 ± 0.06 (78) | 0.760 |
| IVS, mm | 0.9 ± 0.01 (49) | 0.92 ± 0.01 (78) | ||||
| LVEDA, cm2 | 27.2 ± 1.03 (49) | 27.1 ± 0.8 (49) | 0.917 | 29.2 ± 0.7 (78) | 27.9 ± 0.7 (78) | 0.097 |
| LVESA, cm2 | 17.4 ± 2.2 (49) | 15.9 ± 1.5 (49) | 0.555 | 16.3 ± 1.9 (78) | 12.8 ± 1.2 (78) | 0.357 |
| VTILVOT, cm | 19.9 ± 0.1 (49) | 21.8 ± 0.5 (49)* | 0.005 | 20.2 ± 0.7 (78) | 20.1 ± 0.5 (78) | 0.914 |
| LVEDV, ml (2D) | 81.5 ± 4.5 (49) | 85.9 ± 3.2 (49) | 0.441 | 85.5 ± 4.9 (78) | 80.7 ± 3.1 (78) | 0.361 |
| LVESV, ml (2D) | 30.1 ± 2.5 (49) | 32.1 ± 1.9 (49) | 0.535 | 35.4 ± 3.8 (78) | 32.5 ± 2 (78) | 0.409 |
| EF, % | 62.5 ± 2.6 (49) | 62.2 ± 2.8 (49) | 0.932 | 60.4 ± 2.3 (78) | 59.9 ± 1.7 (78) | 0,869 |
| SV, ml (Simpson’s) | 50.1 ± 3.1 (49) | 53.1 ± 2.7 (49) | 0.600 | 47.6 ± 3.2 (78) | 47.9 ± 2 (78) | 0.754 |
| LVEDV, ml (3D)b | 82.5 ± 3.6 (30) | 84.6 ± 2.8 (30) | 0.553 | 82 ± 3.6 (42) | 85.1 ± 3 (42) | 0.187 |
| LVESV, ml (3D)b | 32.1 ± 1.9 (30) | 34.5 ± 1.7 (30) | 0.266 | 35 ± 2.2 (42) | 35.3 ± 2 (42) | 0.857 |
| SV, ml (3D)b | 50.3 ± 2.5 (30) | 50.1 ± 2.5 (30) | 0.952 | 46.9 ± 2.2 (42) | 49.8 ± 2.1 (42) | 0.221 |
| EF (3D), %b | 61.1 ± 1.5 (30) | 58.6 ± 2.1 (30) | 0.356 | 57.4 ± 1.6 (42) | 58.4 ± 1.6 (42) | 0.869 |
| LV–LS, % | − 13.2 ± 0.8 (41) | − 17.4 ± 0.7 (41)# | < 0.0001 | − 12.5 ± 0.6 (58) | − 11.9 ± 0.5 (58) | 0.298 |
| LV S’, cm/s | 10.5 ± 0.8 (31) | 10.6 ± 0.8 (31) | 0.581 | 10.2 ± 0.6 (65) | 10.4 ± 0.6 (65) | 0.036 |
| Pericardial effusion | 17/56 (30.4%)* | 14/49 (28.6%)* | 1.000 | 53/120 (44.2%) | 39/78 (50%) | 1.000 |
Data are expressed as mean ± standard error of means
A left ventricular late diastolic filling velocity with atrial contraction, ACP, acute cor pulmonale, E, left ventricular early diastolic peak velocity, E’, early diastolic tissue Doppler velocity, EF ejection fraction, LV–LS longitudinal strain of the left ventricle, IVA isovolumic acceleration, IVS interventricular septum, LVEDD left ventricular end diastolic diameter, LVEDV left ventricular end diastolic volume, LVESV left ventricular end systolic volume, LV s’ systolic tissue Doppler velocity measured at the lateral mitral annulus, PASP pulmonary artery systolic pressure, PASP/VTIRVOT pulmonary artery systolic pressure to right ventricular outflow tract velocity time integral ratio, RVEDA/LVEDA right ventricular end diastolic area to left ventricular end diastolic area, RVEDV right ventricular end diastolic volume, RVEF right ventricular ejection fraction, RVESV right ventricular end systolic volume, RVFAC right ventricular fractional area change, RV–LS right ventricular free wall longitudinal strain, RV S’ systolic tissue Doppler velocity measured at the lateral tricuspid annulus, RV SV right ventricular stroke volume, SV stroke volume, TAPSE tricuspid annular plane systolic excursion, VACR right ventricular to pulmonary artery coupling, VTILVOT, left ventricular outflow tract velocity time integral, VTIRVOT, right ventricular outflow tract velocity time integral
aNumbers in parenthesis refer to the actual number of patients that the variable was measured
b3D measurements
Right ventricular longitudinal free wall strain (RV–LS) was severely reduced [RV–LS − 14.3 ± 0.4%. RV–LS > − 20% was present in almost the whole cohort 131/145 (90.3%) patients measured], while RV–LS ≥ − 17 (average value reported in severe COVID-19 patients) was present in 90/145 (62%) [30].
RV afterload
Tricuspid regurgitation could be measured in 150 patients, VTIRVOT in 142 and VTILVOT in 176 patients. Data on PVRs are presented in Table 2 and Additional file 1: Table S3. Increased PVRs were present in 118/150 (78.7%) of the patients using at least one variable from the definition used. PASP > 38 mmHg was present in 44/150 (29.3%) patients, PAcT < 90 ms in 110/142 (77.5%), PASP/VTIRVOT > 2 in 71/128 (55.5%), while a mid-systolic notch in the RVOT pulse wave doppler signal (not included in increased PVR definition) was present in 36/142 (25.4%) patients. PASP/VTIRVOT correlated with TAPSE: − 0.349, p < 0.0001, RV–EF: − 0.277, p = 0.026, PAcT: − 0.296, p = 0.001 and VACR: − 0.523, p < 0.0001.
The right ventriculoarterial coupling (VACR) was impaired (0.8 ± 0.06 mm/mmHg).
Pericardial effusion was present in 70/176 (39.7%) of the patients. In the majority (68/70) the effusion was mild (< 10 mm) (in diastole).
LV function
Mean EF was nearly normal (57.8 ± 1.1%); LV systolic dysfunction (LVEF < 40%) was present in 21/176 (11.9%), while severely decreased (EF < 30%) in 7 patients (4%). None of these patients had any known history of LV cardiomyopathy. However, LV–LS was reduced (− 13.3 ± 0.3%), 127/145 (87.5%) patients presented LV–LS > − 18%, while in 82/145 (56.5%) LV–LS was > − 15.9% (Lower Limit Normal) and > − 14 in 67 (46.2%) (Additional file 1: Table S3).
Echocardiographic time course of COVID-19 cardiac involvement
Echocardiographic re-evaluation upon the 10th ICUday was performed in 127 patients (Fig. 1). Thirty-nine patients had died (day-10 non-survivors). Ten-day non-survivors presented worse respiratory system mechanics and a trend for worse hemodynamics compared to baseline values (Additional file 1: Table S4). Compared to day-10 survivors, they presented higher troponin levels upon ICU admission (0.78 ± 0.19 vs 0.21 ± 0.06 ng/ml, p < 0.0001), higher prevalence of pericardial effusion [22/39 (56.4%) vs 48/137 (35%), p = 0.015] and lower RVFAC (31.7 ± 2% vs 37.7 ± 1%, p = 0.011) (Additional file 1: Table S4).
In the rest 127 patients, RV systolic function (RVFAC: 36.6 ± 0.9 to 39.1 ± 0.1, p = 0.048), and RV afterload (PASP/VTIRVOT: 2.29 ± 1.4 to 1.9 ± 0.1, p < 0.0001 and PAcT: 65.3 ± 1.5 to 72 ± 2.1, p = 0.035), presented significant improvements (Additional file 1: Table S3, Fig. 2). Isovolumic Acceleration of the RV (RV IVA) was normal and did not change upon re-evaluation.
Fig. 2.
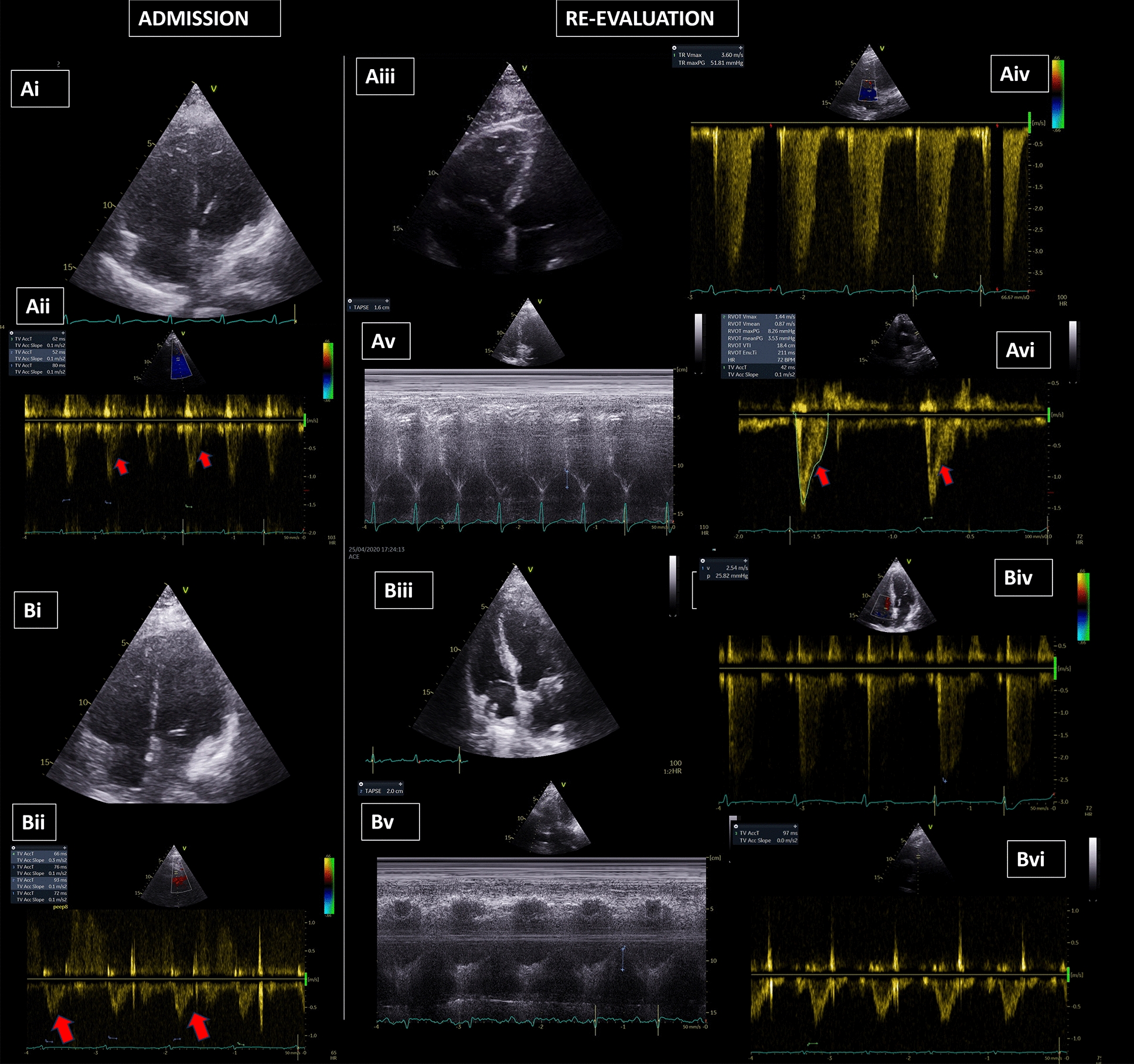
Echocardiographic findings in patients with COVID-19 ARDS. A Non-survivor patient. (Αi) non-survivor with RV dilation on ICU admission; (Aii) PAcT: 64.6 ms Red arrows indicate the early systolic notch in the ascending part of the RVOT envelope. Please not the triangular spahe of the RVOT envelope indicating increased PVRs. (Aiii) RV dilatation upon re-evaluation; (Aiv) TRV: 3.6 m/sec ≥ PASP = 51.81 mmHg + (CVP: 13 mmHg) = 64.25 mmHg (Av) TAPSE: 1.6 cm (re-evaluation); (Avi) PAcT: 42 ms Red arrows indicate the early systolic notch in the ascending part of the RVOT envelope. Please not the triangular spahe of the RVOT envelope indicating increased PVRs. B Survivor patient. (Bi) survivor with RV dilatation upon ICU admission; (Bii) PAcT: 76.75 ms Red arrows indicate the early systolic notch in the ascending part of the RVOT envelope. Please not the triangular spahe of the RVOT envelope indicating increased PVRs, (Biii) normal size of the RV upon re-evaluation; (Biv) TRV: 2.54 m/sec ≥ PASP = 25.82 mmHg + (CVP: 8 mmHg) = 33.82 mmHg (Bv) TAPSE: 2 cm; (Bvi) PAcT: 95 ms, Please not the normal parabolic shape of the RVOT envelope in indicating normal PVRs. ARDS acute respiratory distress syndrome, CVP central venous pressure, COVID-19 coronavirus disease 2019, PASP pulmonary artery systolic pressure, PVR pulmonary vascular resistance, PAcT pulmonary acceleration time, RV right ventricle, TAPSE tricuspid annular plane systolic excursion, TRV tricuspid regurgitation velocity
Troponin
Upon admission, 86/176 (48.9%) presented elevated troponin levels [mean troponin 0.67 ± 0.13 ng/ml, in those with abnormal values (> 0.04 ng/ml)]. Age, BMI and sex did not differ between patients with increased and normal troponin levels (Additional file 1: Table S2).
The RV was more dilated (RVEDA/LVEDA: 0.85 ± 0.04 vs 0.79 ± 0.04, p = 0.037) in patients with higher troponin. Troponin levels were not correlated with RVEF or RV–LS. On the contrary, troponin presented a positive correlation to the presence of pericardial effusion (r:0.293, p < 0.0001).
Outcome
Patients were followed up until ICU discharge. The survival rate in the present cohort was 34.8%. Oxygenation did not differ (PaO2/FiO2: 86.3 ± 5.2 vs 95.9 ± 4.7 mmHg, p = 0.196), but survivors had better lung mechanics (Pplat: 23.9 ± 0.5 vs 27.3 ± 0.4 cmH2O, p < 0.0001, driving pressure (DP): 12.3 ± 0.4 vs 15.4 ± 0.5 cmH2O, p < 0.0001, respiratory system compliance (CRS): 38.7 ± 1.5 vs 31 ± 1.1 ml/cmH2O, p < 0.0001) (Additional file 1: Table S5) upon admission. Survivors also presented lower troponin levels at baseline (0.05 ± 0.01 vs 0.49 ± 0.1 ng/ml, p = 0.001).
Respiratory variables upon re-evaluation were significantly different in the two subgroups (survivors vs non-survivors) (Table 3). Survivors presented significant improvements in PaO2/FiO2, 231.2 ± 11.9 vs 120.2 ± 6.7 mmHg; PaCO2, 43.1 ± 1.2 vs 53.9 ± 1.5 mmHg; respiratory system compliance—CRS, 42.6 ± 2.2 vs 27.8 ± 0.9 ml/cmH2O, all p < 0.0001. Echocardiographic variables in survivors/non-survivors are presented in Table 2. Baseline echocardiographic data did not differ between survivors/non-survivors, apart from a higher incidence of pericardial effusion in non-survivors (30.4% vs 44.2%, p = 0.019). In survivors only, a decrease in RV afterload was noted (PASP: 36.1 ± 2.4 to 20.1 ± 3 mmHg, p < 0.0001, PASP/VTIRVOT: 2.5 ± 1.4 to 1.1 ± 0.7, p < 0.0001, PAcT: 61 ± 2.5 to 84.7 ± 2.4 ms, p < 0.0001), associated with RV systolic function improvement (RVEF: 36.5 ± 2.9% to 46.6 ± 2.1%, p = 0.001 and RV–LS: − 13.6 ± 0.7% to − 16.7 ± 0.8%, p = 0.001) In addition, RV dilation subsided in survivors (RVEDA/LVEDA: 0.8 ± 0.05 to 0.6 ± 0.03, p = 0.001). Right ventriculoarterial coupling improved significantly in survivors only (VACR: 0.8 ± 0.1 to 1.5 ± 0.1, p < 0.0001), while in non survivors further worsened (VACR: 0.73 ± 0.07 to 0.66 ± 0.08, p < 0.0001). Survivors presented significant improvements in LV systolic function as depicted by LV–LS (− 17.4 ± 0.7% vs − 13.2 ± 0.8%, p < 0.0001) compared to non-survivors (− 11.9 ± 0.5% vs − 12.5 ± 0.76%, p = 0.298). Respiratory variables upon re-evaluation correlated with RV function and afterload (Additional file 1).
Table 3.
Respiratory variables between survivors and non-survivors upon echocardiographic re-evaluation
| Survivors | Non-survivors | p value | |
|---|---|---|---|
| Mode (volume control/pressure support) | 16/33 | 75/3 | < 0.0001 |
| PEEP, cmH2O | 7.9 ± 0.4 | 11.1 ± 0.4 | < 0.0001 |
| Driving pressure, cmH2O | 11 ± 0.4 | 15 ± 0.3 | < 0.0001 |
| CRS, ml/cmH2O | 42.6 ± 2.2 | 27.8 ± 0.9 | < 0.0001 |
| PaO2/FiO2, mmHg | 231.2 ± 11.9 | 120.2 ± 6.7 | < 0.0001 |
| PaCO2, mmHg | 43.1 ± 1.2 | 53.9 ± 1.5 | < 0.0001 |
| pH | 7.43 ± 0.01 | 7.26 ± 0.01 | < 0.0001 |
| Noradrenaline, μcg/kg/min | 0.06 ± 0.02 | 0.46 ± 0.05 | < 0.0001 |
CRS respiratory system compliance, PaCO2 partial carbon dioxide pressure, PaO2/FiO2 partial oxygen pressure/fraction of inspired oxygen, PEEP positive end expiratory pressure
Three multivariable regression models were constructed to identify values associated with mortality. In the first, baseline values that significantly differed between survivors/non-survivors were entered and revealed that only CRS (OR 0.842 95%CI 0.721–0.982, p = 0.028) and Pplateau (OR 1.425 95%CI 1.024–1.982, p = 0.036) were independent factors. In the second model, the significantly different echocardiographic values upon re-evaluation were tested, revealing D-10 LV–LS (OR 1.881 95%CI 1.105–3.203, p = 0.020), while in the third model ΔPASP/VTIRVOT (OR 78.269 95%CI 2.578–2376.236, p = 0.021) and ΔRV–LS (OR 0.032 95%CI 0.001–0.908, p = 0.044) were independently associated with mortality (Additional file 1: Table S6).
Interobserver variability
In case of disagreement in measurements (> 10% variability) re-evaluation was performed with all operators present, to reach agreement. Interobserver agreement (in the captured echocardiographic measurements) was high [interclass correlation coefficients for different indices were: RV FAC: 0.955, TAPSE: 0.960, RV–LS: 0.957, RV EF: 0.919, PASP: 0.966, VTIRVOT: 0.988, VTILVOT: 0.942, LV EF(2D): 0.979; LV EF(3D): 0.934]. Bland and Altman plots are presented in Additional file 1: Table S7.
Discussion
To our knowledge, this is the first study to comprehensively evaluate the temporal course of cardiac impairment with echocardiography in a large cohort of mechanically ventilated COVID-19 ARDS patients and investigate its implications in survival. COVID-19. The results indicate the presence of significant RV dilation and systolic dysfunction, accompanied by increased pulmonary vascular resistances. Although baseline echocardiographic data did not differ between survivors and non-survivors, the right ventricular size, function and afterload improved only in survivors after a 10-day period, and so did both LV and the RV longitudinal strain. In fact, the improvements in RV afterload were associated with RV systolic function improvements and survival. Finally, the respiratory system function upon re-evaluation correlated with RV function and afterload.
RV function–RV afterload
During the pandemic, the RV has been extensively identified as the most frequent cardiovascular target in COVID-19 [7, 8, 30]. To be more strict, we defined RV systolic dysfunction when there were at least two indices indicating systolic impairment. Both RV dilation and dysfunction were present in 56% of the patients. RV–LS was severely impaired (− 14.4%) and RV–LS > − 20% was present in 91% of the patients, corroborating previously reported data in small cohorts of mixed (ICU and non-ICU) COVID-19 patients [31–33]. Even using more strict criteria (RV–LS > − 17% as the reference value, reported in COVID-19 patients [30]) impaired strain was observed in 62% of the patients. Current data in COVID-19 report a greater RV strain impairment in the more severe patients. RV–LS has been reported to have higher values (more impaired) in ARDS than non-ARDS patients (− 21.3% vs. − 24.6%), in ICU than non-ICU patients (− 17.5% vs − 19.8%) and in non-survivors than survivors (− 14% vs − 19%) [30, 34–36]. However, the MV settings and respiratory system mechanics are not reported, thus, one cannot conclude on the effects of MV on the observed results. RV involvement in COVID-19 ARDS seems to be related to the increased afterload due to COVID-19-induced microthrombosis, lung mechanics’ impairment and mechanical ventilator settings, which may have additional contribution, as we have recently shown [37]. Surprisingly, RV IVA, a load independent variable indicating RV systolic dysfunction, was normal during the two timepoints of measurements, supporting the role of increased RV afterload in RV dysfunction.
In our cohort, baseline echocardiographic values concerning the RV did not differ between survivors and non-survivors. On the contrary, re-evaluation during the first 10 days of ICU stay revealed a reversible impairment of RV dilation, systolic dysfunction and afterload in survivors. Moreover, ΔPASP/VTIRVOT marker of RV afterload, was associated with survival. This might reflect the improvement in the RV afterload burden, resulting from improvements in ARDS, respiratory system mechanics and vascular obstruction, thus decreasing the amplitude of positive pressure (PEEP) requirements, as indicated in the respiratory variable differences between survivors and non-survivors upon re-evaluation. Right ventricular failure development during the course of ICU stay was associated with worsening in respiratory system physiology (oxygenation, ventilatory ratio and driving pressure) in a recent study presenting echocardiographic data in COVID-19 ARDS patients, further supporting the importance of RV afterload in the myocardial performance of the RV [13]. Right ventricular failure was ultimately associated with mortality [13]. On the other hand, direct myocardial inflammation attenuation cannot be excluded. Moreover, we cannot conclude whether the lack of RV improvement in non-survivors might also present a marker of septic cardiomyopathy presenting upon the 10th ICUday, as sepsis was more frequent in this subset of patients. RV dysfunction is a common finding in early sepsis [37].
Right ventricular–arterial coupling (VACR)
The present study depicts the uncoupling between RV contractility and afterload in MV COVID-19 ARDS patients. In our cohort, ventriculoarterial uncoupling was equally impaired in survivors vs non-survivors. Only in survivors did VACR improve, accompanying possibly the improvements in RV systolic function and the decrease in RV afterload, while in non-survivors it further deteriorated (Table 2). Early and pronounced RV–PA uncoupling has been recently reported in COVID-19 ARDS patients; survivors presented a TAPSE/PASP of 0.89 ± 0.29 vs 0.51 ± 0.22 mm/mmHg found in non-survivors; again the information on lung mechanics is missing [10]. Under this perspective, we highlight the interplay between respiratory system function, RV function and afterload. Herein, we show that the careful evaluation of RV myocardial performance in relation to RV afterload, affected by respiratory physiology and underlined by a means of measuring RV–PA coupling (herein assessed through TAPSE/PASP) is of great significance to assess patient outcomes.
LV function
Using conventional echocardiographic measurements, LV function was within normal levels in most patients; EF < 40% was present in 11.9% of the patients. In a multicenter study across European ICUs, using conventional echocardiography, LV systolic dysfunction was found in 22% of the patients (69% mechanically ventilated), 30% of whom had a previous history of cardiomyopathy [9]. In our study, we excluded patients with pre-existing left ventricular dysfunction, so that the findings could more clearly depict the impact of COVID-19 in cardiac function. In accordance with our results, Doyen et al. found that among 30 MV COVID-19 ARDS patients, LV systolic dysfunction was present in 13% [38].
Interestingly, speckle tracking echocardiography revealed a “silent” impairment in 87.5% of the intubated COVID-19 patients, with a mean LV–LS of − 13.3 ± 0.3%. Various studies have focused on LV–LS in COVID-19 patients, ranging between − 17.9% and (− 18.4%), but included patients of different illness severity, usually spontaneously breathing and only a minority included MV patients [35, 39]. In our study, including the most severe ARDS patients, with a mean PaO2/FiO2 of 94.9 mmHg under MV, with the majority (91.5%) requiring vasopressors, LV–LS was lower and probably reflected the true myocardial dysfunction, not revealed with LVEF, a load dependent parameter. This discrepancy between LVEF and LV–LS was recently pointed in a cohort of mixed severity COVID-19 patients [35]. Janus et al. reported a mean LV–LS of − 11.8% in 31 patients, yet there are no data concerning pneumonia severity and oxygenation impairment [40]. To our knowledge, our study is the first to report results on strain imaging in a homogenous population of MV COVID-19 ARDS patients. Moreover, contradicting previous findings reporting the ability of LV–LS to predict survival in mixed severity cohorts [36, 40], we found that only LV–LS upon re-evaluation, along with PASP/LVOTVTI, were independently associated with mortality in MV patients.
Troponin
Troponin levels have been used to indicate myocardial inflammation in COVID-19 patients. [41]. Yet, TNI was measured when clinically indicated, thus, the correlation of TNI to the presence of RV/LV dysfunction might encounter a selection bias. Subsequent scarce echocardiographic data have reported conflicting results concerning the correlation to cardiac function impairment [32, 42, 43]. In our study, troponin was increased in 47.3% of the patients. TNI levels were higher in patients with greater RV dilation but, troponin did not correlate to RV dysfunction indices. Jansson et all found that acute myocardial injury, occurred in 82% ICU COVID-19 ARDS patients, yet troponin did not correlate with RV/LV impairment [43, 44]. Similarly, Karagodin et al., in the global WASE COVID-19 study, found that troponin was increased in 35% of the patients included, contrasting the overall good RV and LV function [36]. Indeed, increased troponin in critical illness and sepsis is multifactorial and may not result from direct myocardial necrosis; [45, 46]. Thus, troponin levels might serve as a primary marker of illness severity and second, reflect direct myocardial damage.
Moreover, we found an increased incidence of pericardial effusion supporting previous data [38]. Pericardial effusion presents a direct sign of cardiac involvement, although not warranting intervention, in the majority of the patients. It may also indicate severity of infection, as a higher incidence has been reported in ICU vs non-ICU patients (23.2% vs 16.3%) [36]. Pericardial effusions and higher troponin were more frequently found in non-survivors, indicating probably that they suffered a direct myocardial COVID-19 impairment. Troponin levels and pericarditis might indicate disease severity, not depicted by usual scores (SOFA, APACHE II).
The study’s monocentric character is a certain limitation. Yet, a large number of consecutive intubated patients with severe ARDS underwent a comprehensive echocardiographic evaluation on ICU admission, while echocardiographic data on the time course of cardiac function are also presented. The selection of the re-evaluation timepoint, although seems arbitrary at first sight, was based on the median intubation time of 10–14 days reported in large-scale observational studies [14–17]. Moreover, the re-evaluation echocardiographic study was performed under different conditions as many patients were receiving less sedation and were on a spontaneous mode; thus, we do not discuss on IVC distensibility differences between the two timepoints. The cohort presented increased mortality (68.2%); the patients were admitted only after intubation, thus we included a cohort with a rather increased illness severity. In fact, disease severity scores were higher in our cohort compared to other studies reporting mortality rates between 35% and 50.6% [14–17]. Among the most severe patients, the mean case fatality rate has been found around 45% in patients receiving invasive mechanical ventilation, while a case fatality rate exceeding 78%, has also been noted, depending on the timing of intubation, time with respiratory distress or other regional disparities [17, 22, 27, 47]. Many patients were intubated “late”, a factor found to impact lung mechanics and survival [48], while the immunomodulatory treatments that the patients received might have also affected the outcome, but this issue is beyond the scope of the present study. Finally, the exact interobserver variability cannot be calculated for every measurement, as per protocol, we re-evaluated the measurements with > 10% variability with all operators present to reach agreement. Moreover, only one operator at a time performed each echocardiographic study, due to the pandemic’s nature.
Conclusion
The study confirms that the myocardial function, especially the right ventricle, is affected in MV COVID-19 ARDS patients. COVID-19 improvements in RV function, RV afterload and RV–PA coupling at day 10 were associated with respiratory function improvements and survival. Further multicentric study is needed to confirm these findings and assess the therapeutic and prognostic impact of serial comprehensive echocardiography.
Supplementary Information
Additional file 1: Table S1. Demographics in the whole cohort. Table S2. Demographic data and respiratory variables in COVID-19 ARDS patients according to the presence of increased troponin levels. Table S3. Echocardiographic variables in the whole cohort upon ICU admission and upon the 10th ICU day. Table S4. Clinical data and echocardiographic variables of initial evaluation between 10-day survivors and non-survivors. Table S5. Baseline characteristics and outcome between survivors and non-survivors. Table S6. Univariate and multivariate regression models to identify predictors of survival. Table S7. Bland and Aldman scatter plots to estimate interobserver variability for different RV measurements. Table S8. Clinical characteristics and echocardiographic variables in patients stratified according to the troponin value upon admission. Echocardiographic data upon re-evaluation on the 10th ICU day.
Acknowledgements
None.
Abbreviations
- AcT
Acceleration time
- ANOVA
Analysis of variance
- ARDS
Acute respiratory distress syndrome
- AUC
Area under the curve
- BMI
Body mass index
- CI
Confidence interval
- CVP
Central venous pressure
- COVID-19
Coronavirus disease 2019
- CRS
Static compliance of the respiratory system
- ΔIVC
Respiratory variability in Inferior Vena Cava diameter [(IVCmax–IVCmin)/IVCmin]
- DP
Driving pressure
- E
Left ventricular early diastolic peak velocity
- E’
Early diastolic tissue Doppler velocity
- EF
Ejection fraction
- FiO2
Fraction of inspired oxygen
- ICU
Intensive Care Unit
- IVC
Inferior vena cava
- lac
Lactate
- LBBB
Left bundle brunch block
- LV
Left ventricle
- LVOT
Left ventricular outflow tract
- LV–LS
Longitudinal strain of the left ventricle
- LV S’
Systolic tissue Doppler velocity measured at the lateral mitral annulus
- MAP
Mean arterial pressure
- MV
Mechanical ventilation
- PaCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- PASP
Pulmonary artery systolic pressure
- PASP/VTILVOT
Pulmonary artery systolic pressure to left ventricular outflow tract velocity time integral ratio
- PASP/VTIRVOT
Pulmonary artery systolic pressure to right ventricular outflow tract velocity time integral ratio
- PEEP
Positive end expiratory pressure
- Ppl
Pleural pressure
- Pplat
Plateau pressure
- PPV
Pulse pressure variation
- PVR
Pulmonary vascular resistance
- RV
Right ventricle
- ROC
Receiver operating characteristics
- RVEDA/LVEDA
Right ventricular end diastolic area to left ventricular end diastolic area
- RVEDV
Right ventricular end diastolic volume
- RVESV
Right ventricular end systolic volume
- RVEF
Right ventricular ejection fraction
- RVFAC
Right ventricular fractional area change
- RVOT
Right ventricular outflow tract
- RV–LS
Right ventricular longitudinal strain
- RV S’
Systolic tissue Doppler velocity measured at the lateral tricuspid annulus
- SEM
Standard error of means
- ScvO2
Oxygen saturation in venous blood from vena cava
- SV
Stroke volume
- TAPSE
Tricuspid annular plane systolic excursion
- VACR
Ventriculoarterial coupling of the right ventricle to the pulmonary artery
- Vt
Tidal volume
- VTILVOT
Left ventricular outflow tract velocity time integral
- VTIRVOT
Right ventricular outflow tract velocity time integral
- SARS-COV-2
Severe acute respiratory syndrome coronavirus 2
Author contributions
VT, GEZ and EZ designed the study, VT, GEZ, NK, VV, PZ, KP collected the data; VT, JP, DM performed the statistical analyses; VT, GEZ and EZ interpreted the results and drafted the manuscript; all other authors contributed substantially to data collection and draft revision. EZ had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of data analyses. All authors read and approved the final manuscript.
Funding
The study did not receive any funds.
Availability of data and materials
The data sets will be available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the University Hospital of Larissa Ethics Board (Cardiac function in mechanically ventilated COVID-19 ARDS patients, 16965/2020), with a waiver for informed consent, as the assessment of cardiac function was part of the routine care of patients admitted in our ICU.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests related to the present study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coromilas EJ, Kochav S, Goldenthal I, et al. Worldwide survey of COVID-19-associated arrhythmias. Circ Arrhythm Electrophysiol. 2021;14(3):e009458. doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Shu H, Liu H, et al. The peak levels of highly sensitive troponin I predicts in-hospital mortality in COVID-19 patients with cardiac injury: a retrospective study. Eur Heart J Acute Cardiovasc Care. 2021;10(1):6–15. doi: 10.1093/ehjacc/zuaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Task Force for The Management of COVID-19 of The European Society of Cardiology European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 1-epidemiology, pathophysiology, and diagnosis. Eur Heart J. 2021 doi: 10.1093/eurheartj/ehab696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taieb P, Szekely Y, Lupu L, et al. Risk prediction in patients with COVID-19 based on haemodynamic assessment of left and right ventricular function. Eur Heart J Cardiovasc Imaging. 2021;22(11):1241–1254. doi: 10.1093/ehjci/jeab169. [DOI] [PubMed] [Google Scholar]
- 6.Gawałko M, Kapłon-Cieślicka A, Hohl M, et al. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30:100631. doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng MP, Cau A, Lee TC, Angiotensin Receptor Blocker Coronavirus Study (ARBs) CORONA I et al. Acute cardiac injury in coronavirus disease 2019 and other viral infections—a systematic review and meta-analysis. Crit Care Med. 2021;49(9):1558–1566. doi: 10.1097/CCM.0000000000005026. [DOI] [PubMed] [Google Scholar]
- 8.Helms J, Combes A, Aissaoui N. Cardiac injury in COVID-19. Intensive Care Med. 2022;2022(48):111–113. doi: 10.1007/s00134-021-06555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S, Vignon P, Mekontso-Dessap A, ECHO-COVID Research Group et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: a multi-national observational study (the ECHO-COVID study) Intensive Care Med. 2022;48(6):667–678. doi: 10.1007/s00134-022-06685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Alto M, Marra AM, Severino S, et al. Right ventricular-arterial uncoupling independently predicts survival in COVID-19 ARDS. Crit Care. 2020;24(1):670. doi: 10.1186/s13054-020-03385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagate F, Masi P, d'Humières T, et al. Advanced echocardiographic phenotyping of critically ill patients with coronavirus-19 sepsis: a prospective cohort study. J Intensive Care. 2021;9(1):12. doi: 10.1186/s40560-020-00516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Vieillard-Baron A, Evrard B, Prat G, Chew MS, Balik M, Clau-Terré F, De Backer D, Mekontso Dessap A, Orde S, Morelli A, Sanfilippo F, Charron C, Vignon P, ECHO-COVID Study Group Echocardiography phenotypes of right ventricular involvement in COVID-19 ARDS patients and ICU mortality: post-hoc (exploratory) analysis of repeated data from the ECHO-COVID study. Intensive Care Med. 2023;49(8):946–956. doi: 10.1007/s00134-023-07147-z. [DOI] [PubMed] [Google Scholar]
- 13.Evrard B, Goudelin M, Giraudeau B, François B, Vignon P. Right ventricular failure is strongly associated with mortality in patients with moderate-to-severe COVID-19-related ARDS and appears related to respiratory worsening. Intensive Care Med. 2022;48(6):765–767. doi: 10.1007/s00134-022-06730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, Klauber J, Janssens U, Marx G, Weber-Carstens S, Kluge S, Pfeifer M, Grabenhenrich L, Welte T, Busse R. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernández M, Gea A, Arruti E, Aldecoa C, Martínez-Pallí G, Martínez-González MA, Slutsky AS, Villar J. COVID-19 Spanish ICU Network. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46(12):2200–2211. 10.1007/s00134-020-06192-2. Erratum in: Intensive Care Med. 2020 Dec 2; PMID: 32728965; PMCID: PMC7387884. [DOI] [PMC free article] [PubMed]
- 16.Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, Dongelmans DA, Hollmann MW, Horn J, Vlaar APJ, Schultz MJ, Neto AS, Paulus F, PRoVENT-COVID Collaborative Group Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9(2):139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estenssoro E, Loudet CI, Ríos FG, Kanoore Edul VS, Plotnikow G, Andrian M, Romero I, Piezny D, Bezzi M, Mandich V, Groer C, Torres S, Orlandi C, Rubatto Birri PN, Valenti MF, Cunto E, Sáenz MG, Tiribelli N, Aphalo V, Reina R, Dubin A, SATI-COVID-19 Study Group Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. 2021;9(9):989–998. doi: 10.1016/S2213-2600(21)00229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beesley SJ, Weber G, Sarge T, Nikravan S, Grissom CK, Lanspa MJ, Shahul S, Brown SM. Septic cardiomyopathy. Crit Care Med. 2018;46(4):625–634. doi: 10.1097/CCM.0000000000002851. [DOI] [PubMed] [Google Scholar]
- 19.Lanspa MJ, Cirulis MM, Wiley BM, et al. Right ventricular dysfunction in early sepsis and septic shock. Chest. 2021;159(3):1055–1063. doi: 10.1016/j.chest.2020.09.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanfilippo F, Huang S, Herpain A, et al. The PRICES statement: an ESICM expert consensus on methodology for conducting and reporting critical care echocardiography research studies. Intensive Care Med. 2021;47(1):1–13. doi: 10.1007/s00134-020-06262-5. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 22.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto T, Dulgheru R, Bernard A, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18(8):833–840. doi: 10.1093/ehjci/jex140. [DOI] [PubMed] [Google Scholar]
- 24.Barbier C, Loubières Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- 25.Takahama H, McCully RB, Frantz RP, et al. Unraveling the RV ejection Doppler envelope: insight into pulmonary artery hemodynamics and disease severity. JACC Cardiovasc Imaging. 2017;10(10PtB):1268–1277. doi: 10.1016/j.jcmg.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Abbas AE, Fortuin FD, Schiller NB, et al. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41(6):1021–1027. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 27.Roule V, Labombarda F, Pellissier A, et al. Echocardiographic assessment of pulmonary vascular resistance in pulmonary arterial hypertension. Cardiovasc Ultrasound. 2010;8:21. doi: 10.1186/1476-7120-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opotowsky AR, Clair M, Afilalo J, et al. A simple echocardiographic method to estimate pulmonary vascular resistance. Am J Cardiol. 2013;112(6):873–882. doi: 10.1016/j.amjcard.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tello K, Wan J, Dalmer A, et al. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging. 2019;12(9):e009047. doi: 10.1161/CIRCIMAGING.119.009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleakley C, Singh S, Garfield B, et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int J Cardiol. 2021;327:251–258. doi: 10.1016/j.ijcard.2020.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janus SE, Hajjari J, Karnib M, et al. Prognostic value of left ventricular global longitudinal strain in COVID-19. Am J Cardiol. 2020;131:134–136. doi: 10.1016/j.amjcard.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassen MCH, Skaarup KG, Lind JN, et al. Echocardiographic abnormalities and predictors of mortality in hospitalized COVID-19 patients: the ECHOVID-19 study. ESC Heart Fail. 2020;7(6):4189–4197. doi: 10.1002/ehf2.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bursi F, Santangelo G, Sansalone D, et al. Prognostic utility of quantitative offline 2D-echocardiography in hospitalized patients with COVID-19 disease. Echocardiography. 2020;37(12):2029–2039. doi: 10.1111/echo.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13(11):2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karagodin I, Singulane CC, Descamps T, WASE-COVID Investigators et al. Ventricular changes in patients with acute COVID-19 infection: follow-up of the world alliance societies of echocardiography (WASE-COVID) study. J Am Soc Echocardiogr. 2022;35(3):295–304. doi: 10.1016/j.echo.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karagodin I, Carvalho Singulane C, Woodward GM, WASE-COVID Investigators et al. Echocardiographic correlates of in-hospital death in patients with acute COVID-19 infection: the world alliance societies of echocardiography (WASE-COVID) study. J Am Soc Echocardiogr. 2021;34(8):819–830. doi: 10.1016/j.echo.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsolaki V, Zakynthinos GE, Papanikolaou J, et al. PEEP de-escalation in COVID-19-induced acute respiratory distress syndrome unloads the right ventricle improving hemodynamics and oxygenation. Am J Respir Crit Care Med. 2023;208(2):205–208. doi: 10.1164/rccm.202301-0154LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doyen D, Dupland P, Morand L, et al. Characteristics of cardiac injury in critically ill patients with coronavirus disease 2019. Chest. 2021;159(5):1974–1985. doi: 10.1016/j.chest.2020.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Heuvel FMA, Vos JL, Koop Y, et al. Cardiac function in relation to myocardial injury in hospitalized patients with COVID-19. Neth Heart J. 2020;28(7–8):410–417. doi: 10.1007/s12471-020-01458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baycan OF, Barman HA, Atici A, et al. Evaluation of biventricular function in patients with COVID-19 using speckle tracking echocardiography. Int J Cardiovasc Imaging. 2021;37(1):135–144. doi: 10.1007/s10554-020-01968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Rothschild E, Baruch G, Peri Y, Arbel Y, Topilsky Y. Spectrum of cardiac manifestations in COVID-19: A systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bieber S, Kraechan A, Hellmuth JC, et al. Left and right ventricular dysfunction in patients with COVID-19-associated myocardial injury. Infection. 2021;49(3):491–500. doi: 10.1007/s15010-020-01572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thygesen K, Alpert JS, Jaffe AS, Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 44.Jansson S, Blixt PJ, Didriksson H, et al. Incidence of acute myocardial injury and its association with left and right ventricular systolic dysfunction in critically ill COVID-19 patients. Ann Intensive Care. 2022;12(1):56. doi: 10.1186/s13613-022-01030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia MA, Rucci JM, Thai KK, et al. Association between troponin I levels during sepsis and post sepsis cardiovascular complications. Am J Respir Crit Care Med. 2021;204(5):557–565. doi: 10.1164/rccm.202103-0613OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Røsjø H, Varpula M, Hagve TA, Karlsson S, Ruokonen E, Pettilä V, Omland T, FINNSEPSIS Study Group Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med. 2011;37(1):77–85. doi: 10.1007/s00134-010-2051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsolaki V, Makris D, Zakynthinos E. COVID-19 mortality differences: patient-related data and intensive care unit load are prerequisites. Ann Am Thorac Soc. 2022;19(9):1622–1623. doi: 10.1513/AnnalsATS.202203-230LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsolaki VS, Zakynthinos GE, Mantzarlis KD, et al. Driving pressure in COVID-19 acute respiratory distress syndrome is associated with respiratory distress duration before intubation. Am J Respir Crit Care Med. 2021;204(4):478–481. doi: 10.1164/rccm.202101-0234LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Demographics in the whole cohort. Table S2. Demographic data and respiratory variables in COVID-19 ARDS patients according to the presence of increased troponin levels. Table S3. Echocardiographic variables in the whole cohort upon ICU admission and upon the 10th ICU day. Table S4. Clinical data and echocardiographic variables of initial evaluation between 10-day survivors and non-survivors. Table S5. Baseline characteristics and outcome between survivors and non-survivors. Table S6. Univariate and multivariate regression models to identify predictors of survival. Table S7. Bland and Aldman scatter plots to estimate interobserver variability for different RV measurements. Table S8. Clinical characteristics and echocardiographic variables in patients stratified according to the troponin value upon admission. Echocardiographic data upon re-evaluation on the 10th ICU day.
Data Availability Statement
The data sets will be available from the corresponding author upon reasonable request.