Abstract
Endometrial cancer (EC) is a common gynecological tumor in females with an increasing incidence over the past few decades. Alcohol consumption has been linked to the occurrence of various cancers; However, epidemiological studies have shown inconsistent associations between alcohol consumption and EC risk. In order to avoid the influence of potential confounding factors and reverse causality in traditional epidemiological studies, we used a method based on genetic principles-Mendelian randomization (MR) analysis to test whether there is a causal relationship between alcohol consumption and EC. MR analysis was conducted using publicly available summary-level data from genome-wide association studies (GWAS). Fifty-seven single nucleotide polymorphisms (SNPs) were extracted as instrumental variables for alcohol exposure from the GWAS and Sequencing Consortium of Alcohol and Nicotine GWAS summary data involving 941,287 participants of European ancestry. SNPs for EC were obtained from the Endometrial Cancer Association Consortium, the Endometrial Cancer Epidemiology Consortium, and the UK Biobank, involving 121,885 European participants. The inverse variance weighted (IVW) method was used as the primary method to estimate the causal effect, and the MR-Egger regression and weighted median method were used as supplementary methods. Sensitivity analyses were conducted using the Mendelian Randomization Pleiotropy RESidual Sum and Outlier global test, MR-Egger intercept test, and leave-one-out analysis to evaluate the impact of pleiotropy on causal estimates. An increase of 1 standard deviation of genetically predicted log-transformed alcoholic drinks per day was associated with a 43% reduction in EC risk [odds ratio (OR) = 0.57, 95% confidence interval (CI) 0.41–0.79, P < 0.001]. Subgroup analysis of EC revealed that alcohol consumption was a protective factor for endometrioid endometrial cancer (EEC) (OR = 0.56, 95% CI 0.38–0.83, P = 0.004) but not for non-endometrioid endometrial cancer (NEC) (OR = 1.36, 95% CI 0.40–4.66, P = 0.626). The MR-Egger regression and weighted median method yielded consistent causal effects with the IVW method. The consistent results of sensitivity analyses indicated the reliability of our causal estimates. Additionally, alcohol consumption was associated with decreased human chorionic gonadotropin (HCG) and insulin-like growth factor 1 (IGF1) levels. This MR study suggests that genetically predicted alcohol consumption is a protective factor for EC, particularly for EEC, and this protective effect may be mediated through the reduction of HCG and IGF1.
Keywords: Alcohol consumption, Mendelian randomization, Endometrial cancer, Causal inference, Protective factors
Subject terms: Cancer epidemiology, Cancer
Introduction
Endometrial cancer (EC) is one of the most common gynecological cancers in women. According to global cancer statistics in 2020, approximately 420,000 new cases of EC are diagnosed each year1. Although the mortality rate of EC is decreasing, the incidence rate of EC has been increasing at a rate of 0.58% per year over the past 30 years2. Among the potential factors contributing to the increased risk of cancer, alcohol consumption has received significant attention. Alcohol consumption is one of the main causes of increased all-cause mortality and is associated with various health conditions3. Globally, approximately 700,000 new cancer cases are attributed to alcohol consumption each year, with women accounting for one-fourth of these cases, indicating a significant burden of female cancer due to alcohol consumption4. The relationship between alcohol consumption and EC has been controversial. A meta-analysis of seven cohort studies showed a J-shaped relationship between alcohol consumption and the risk of EC5. The study found that daily consumption of less than one drink was associated with a decreased risk of EC, while consumption of more than two drinks was associated with an increased risk of EC, but all confidence intervals (CI) included null values. Three other meta-analyses6–8showed no relationship between alcohol consumption and the risk of EC. However, all current studies are observational studies, and therefore, cannot avoid problems such as reverse causation and potential confounding factors, resulting in a low level of evidence. Although randomized controlled trials (RCTs) have a higher level of evidence and can verify causal relationships, these trials are difficult to conduct due to high requirements, strict controls, and ethical considerations. Therefore, there is an urgent need to design well-conducted studies to prove the causal relationship between alcohol consumption and the risk of EC.
Mendelian randomization (MR) uses genetic variations strongly associated with an exposure factor as instrumental variables (IVs) to infer causal relationships between exposure and outcome factors9. According to Mendelian genetics, the random allocation of parental alleles to offspring is equivalent to the randomization process in RCTs, making it less susceptible to traditional confounding factors. In addition, the inheritance of genetic variations from parents satisfies the temporality criterion, which can avoid reverse causality. Therefore, MR studies are considered similar to RCTs but are more cost-effective, and are mainly used to verify causal relationships between exposure factors and outcomes10. Currently, there are many MR studies on alcohol consumption and cancer, such as the finding that alcohol consumption increases the risk of lung cancer in MR analysis11, while drinking with meals can reduce the risk of lung cancer12. Alcohol consumption is a risk factor for colorectal cancer in Asian populations13,14, but not associated with the risk of colorectal cancer in European populations11. Other MR study results also indicate that alcohol consumption is not associated with the risk of breast cancer, ovarian cancer, or bladder cancer11,15,16.
In this study, we used MR to investigate whether there is a causal relationship between genetically predicted alcohol consumption and EC.
Materials and methods
This study is reported strictly according to the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) standard17,18.
Genetic instrumental variables related to alcohol consumption
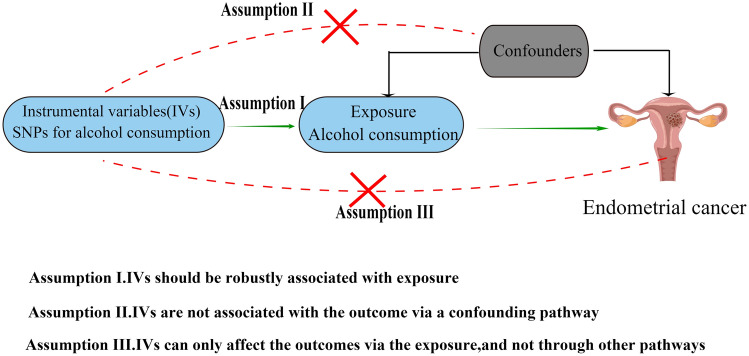
We obtained SNPs related to log-transformed alcoholic consumption from the genome-wide association studies (GWAS) summary data of the GWAS and Sequencing Consortium of Alcohol and Nicotine (GSCAN), which involved 941,287 participants of European ancestry, including 403,939 individuals from the 23andMe dataset and 537,349 from 9 other datasets (excluding UK Biobank)19. The phenotype is based on responses to a single question in the Health Profile survey, "In the last two weeks, how many servings of alcohol did you drink each day? (1 serving equals 12 oz. of beer, 5 oz. of wine, or 1.5 oz. of hard alcohol)" with categorical responses "None" (= 0), "Between 0 and 1" (= 0.5), "1" (= 1), "2" (= 2), "3" (= 3), "4" (= 4), "5 or more" (= 7). The values were then transformed by . As per the MR study design, the IV used to assess the causal relationship between alcohol consumption and EC should satisfy the following assumptions: (1) the IV should be strongly associated with alcohol consumption, (2) the IV should not be correlated with other potential confounding factors, and (3) the IV should not be directly associated with EC or only influence EC through alcohol consumption (Fig. 1). Here, SNPs from the GSCAN GWAS summary data were considered as potential IVs.
Figure 1.
Key assumptions of the Mendelian randomization study.
We obtained 88 independent SNPs as IVs to predict the daily log-transformed alcohol consumption by setting a significant threshold for the strong association between SNPs and alcohol consumption (P < 5 × 10–8) and removing linkage disequilibrium (r2 < 0.001 and distance > 10,000 kb). To satisfy hypothesis III, all selected SNPs were required to have no significant association with EC (P > 5 × 10–5). Additionally, to confirm whether these SNPs were causally related to EC through other phenotypes (i.e., confounding factors), we checked all SNPs by consulting previously published MR studies and using Phenoscanner V2 (http://www.phenoscanner.medschl.cam.ac.uk/). Confounding factors that were already known, including body mass index (BMI)20,21, basal metabolic rate (BMR)22, education level23, and low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C)24, were excluded. Finally, we included 57 SNPs that were strongly associated with EC for further analysis, ensuring the satisfaction of MR hypotheses 2 and 3. To assess weak instrumentality, the F-statistic was calculated using the formula , where R2 represents the proportion of variance in alcohol consumption explained by each SNP, K represents the number of SNPs extracted, and N represents the sample size of the GAWS study that is related to alcohol consumption25. A genetic variant with an F-statistic < 10 is usually considered a weak instrument26. The R2 value was calculated using the following formula: , where EAF is the frequency of the effect allele, Beta is the effect size of the genetic variation on alcohol consumption, and SE is the standard deviation (SD) of the effect size of the genetic variation27 (Supplementary Table S1).
EC in GWAS
Our EC GWAS data obtained EC-associated SNPs, which were provided by the Endometrial Cancer Association Consortium (ECAC), the Epidemiology of Endometrial Cancer Consortium (E2C2), and UK Biobank, comprising 12,906 cases and 108,979 population-matched controls of European ancestry28. EC was further classified by histological type, including 8,758 cases of endometrioid endometrial cancer (EEC) and 1,230 cases of non-endometrioid endometrial cancer (NEC).
There was no overlap between the exposed and outcome samples in this study. For the 57 SNPs associated with alcohol consumption, we obtained summary data from the aforementioned EC GWAS data (Supplementary Table S1).
Statistical analysis
We harmonized the alcohol dataset and the EC dataset by aligning the SNPs' allele directions and removing palindromic and incompatible SNPs. Fixed-effect inverse variance weighted (IVW-FE) meta-analysis was performed for individual SNPs' Wald ratios. The Wald ratio is the causal effect estimate for each SNP, calculated as the Beta in the outcome data divided by the same SNP's Beta in the exposure data. The inverse variance weighted (IVW) method is the primary approach for estimating the overall causal effect of exposure on the outcome, and different IVW models are selected based on the presence or absence of heterogeneity. The IVW method provides the most efficient causal relationship when all SNPs are valid IVs. However, even if one SNP is invalid, the results can still be biased. Therefore, the causal effect is estimated mainly by IVW method, supplemented by MR-Egger regression and weighted median method. The MR-Egger method corrects for pleiotropy and provides a causal effect estimate that is not biased by violating the IV assumption but has lower precision. The weighted median method provides a consistent causal effect estimate even if up to half of the weight comes from invalid SNPs.
In this study, in order to ensure the reliability of MR results, we used a variety of sensitivity analysis methods. Firstly, we use the Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) method to detect and eliminate the horizontal multiple effects (outliers) of SNP in order to reduce the bias of causality29. Secondly, we use Cochran Q test to evaluate the consistency of each SNP estimate. If there is no significant heterogeneity, IVW-FE method is used; if there is significant heterogeneity, random effect IVW method (IVW-MRE) is used. Thirdly, we use MR-Egger intercept test to detect the existence of horizontal multiplicity. If horizontal multiplicity is found, the IVW method may not be suitable, so we use the weighted median method as the main analysis method30. MR-Egger intercept test and MR-PRESSO method can also help us to test whether the hypothesis of MR II and III is true. Finally, "Leave-one-out" analysis was used to assess the robustness of MR estimates by eliminating a different SNP in each iteration to quantify the causal influence of outlying SNPs and to ensure that deleting SNPs did not affect the MR estimates. Statistical power calculations were performed using an online tool (available at https://shiny.cnsgenomics.com/mRnd/ )31.
In addition, to investigate the potential mechanisms underlying the association between alcohol consumption and EC, we employed a MR approach to determine whether alcohol consumption affects established risk factors for EC. The mechanism by which alcohol consumption affects the risk of EC is unclear, it may be related to changes in hormone levels. Experimental study found that estrogen levels spike sharply for a short period of time after women drink alcohol32,33. A meta-analysis34 involving 200,000 women also showed that alcohol consumption is associated with increased concentrations of sex hormones [including estradiol (E2), estrone, androsterone, etc.], and EC is one of the most common hormone-dependent cancers. Therefore, we mainly studied several common hormone-related risk factors for EC, including E235, human chorionic gonadotropin (HCG)36, insulin-like growth factor 1 (IGF1), sex hormone-binding globulin (SHBG)35, Total testosterone (TT) and membrane-associated progesterone receptor component 2 (PGRMC2)36. These GWAS summary data are available at the MRC IEU Open GWAS repository (https://gwas.mrcieu.ac.uk/) (Supplementary Table S2). In order to explore whether alcohol consumption mediates the risk of EC by affecting hormone levels, we continue to use 57 SNP from MR analysis of alcohol consumption and EC as tool variables for alcohol consumption, and perform MR analysis with hormone levels as the outcome. A series of sensitivity analyses were carried out to ensure the robustness of the results.
All MR analyses were performed using the “TwoSampleMR” and “MRPRESSO”packages in version 4.2.2 of R software.
Results
Causal relationship between alcohol consumption and EC
We ultimately included 57 alcohol-related SNPs in the analysis. The F-statistic for each individual instrumental variable and the overall F-statistic were both greater than 10, indicating no bias due to weak instruments in the study (Supplementary Table S1). With an odds ratio (OR) of 0.57 and a precision of 88% for EC, we obtained an unbiased causal estimate.
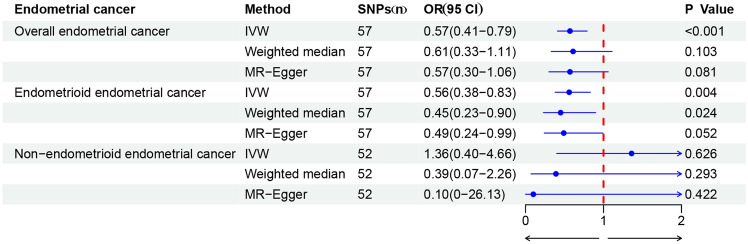
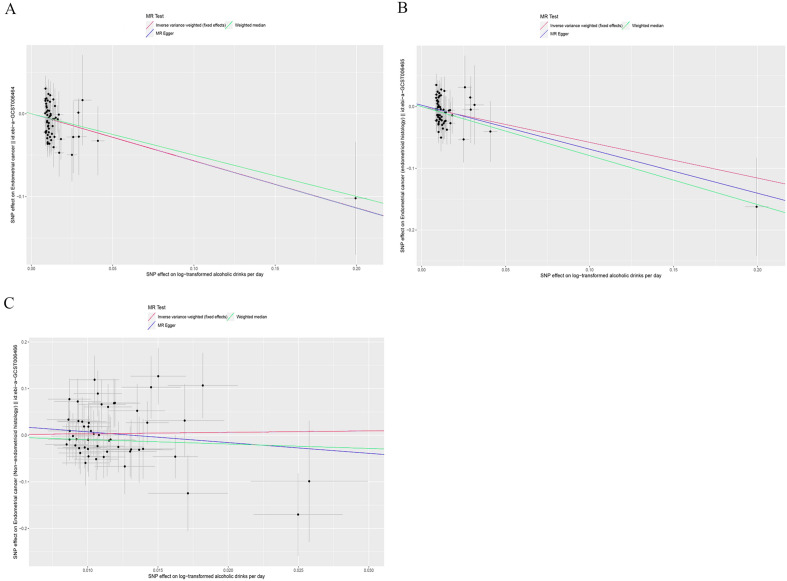
MR analysis showed that an increase of 1 SD in the natural log-transformed alcohol consumption per day was associated with a 43% reduction in EC risk (OR = 0.57, 95% CI 0.41–0.79, P < 0.001). The Weighted median method (OR = 0.61, 95% CI 0.33–1.11, P = 0.103) and the MR-Egger regression method (OR = 0.57, 95% CI 0.30–1.06, P = 0.081) also demonstrated consistent protective effects of alcohol consumption on EC (Fig. 2). The causal estimates for each SNP from the three MR methods are shown in Fig. 3 scatter plots.
Figure 2.
MR estimates from different methods of assessing the causal effect of alcohol consumption on EC.
Figure 3.
Scatter plots for Mendelian randomization (MR) analyses of the causal relationship between alcohol consumption and EC. (A) alcohol consumption-EC. (B) alcohol consumption-EEC. (C) alcohol consumption-NEC.
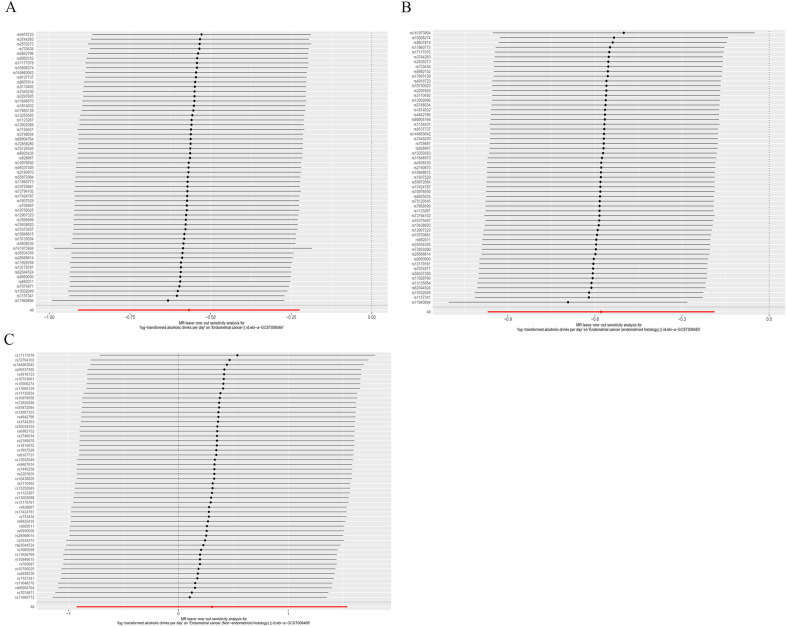
In sensitivity analysis (Table 1), the MR-PRESSO global test (P = 0.366) indicated the absence of outlier SNPs, and the MR-Egger intercept had a value of 3.75E−05 (P = 0.993), which suggested no evidence of significant pleiotropy in our causal estimates, meeting the assumptions 2 and 3 of MR. Our IVW method, Cochrane's Q statistic, was 59.97 (P = 0.334), indicating a low heterogeneity and reliable causal effect in our study. Finally, the leave-one-out sensitivity analysis also indicated that no outlier SNP affected the overall causal effect estimation (Fig. 4).
Table 1.
Sensitivity analysis of alcohol consumption causally linked to endometrial cancer.
| Endometrial cancer | MR-PRESSO test | Heterogeneity test | Horizontal pleiotropy | |||
|---|---|---|---|---|---|---|
| Global test P value | Q-statistics | P value | Egger-intercept | se | P value | |
| Overall endometrial cancer | 0.366 | 59.97 | 0.334 | 3.75E-05 | 0.0045 | 0.993 |
| Endometrioid endometrial cancer | 0.615 | 51.25 | 0.655 | 0.0023 | 0.0051 | 0.654 |
| Non-endometrioid endometrial cancer | 0.482 | 50.86 | 0.479 | 0.0302 | 0.0320 | 0.351 |
Figure 4.
MR leave-one-out sensitivity analyses of the causal relationship between alcohol consumption and EC. (A) alcohol consumption-EC. (B) alcohol consumption-EEC. (C) alcohol consumption-NEC.
In the analysis of EC subtypes, the causal effect was only found in EEC (Fig. 2). It was observed that alcohol consumption was still a protective factor in EEC (OR = 0.56, 95% CI 0.38–0.83, P = 0.004), but was not associated with NEC (OR = 1.36, 95% CI 0.40–4.66, P = 0.626). Moreover, the Weighted median method and MR-Egger regression method also produced consistent results. The causal estimates for each SNP in the three MR methods for each subtype are shown in Fig. 3. In addition, a series of sensitivity analyses, such as the MR-Egger regression intercept test, MR-PRESSO, Cochrane's Q test (Table 1), and leave-one-out analysis (Fig. 4), demonstrated the robustness of the causal relationships in each subtype.
Causal relationship between alcohol consumption and EC risk factors
MR analysis of the causal relationship between alcohol consumption and other risk factors for EC shows that alcohol consumption is a risk factor positively associated with E2 increase and a protective factor negatively associated with HCG and IGF1 decrease (Table 2). Although horizontal pleiotropy was present in the MR analysis of alcohol consumption and E2, the weighted median method provided consistent causal estimates even in the presence of horizontal pleiotropy. However, alcohol consumption has no causal relationship with SHBG, TT and PGRMC2. Sensitivity analysis of alcohol consumption and hormone MR is shown in Supplementary Table S3.
Table 2.
Causal effect from alcohol consumption to endometrial cancer risk factors.
| Outcomes | GWAS ID | Number of SNPs | Method | Causal effect | 95% CI | P value |
|---|---|---|---|---|---|---|
| Estradiol | ebi-a-GCST90012105 | 55 | Weighted median | 1.03 | 1.00–1.06 | 0.034 |
| Human chorionic gonadotropin | prot-a-688 | 57 | IVW(MRE) | 0.55 | 0.32–0.95 | 0.031 |
| Insulin-like growth factor 1 | ukb-d-30770_irnt | 45 | IVW(MRE) | 0.88 | 0.80–0.98 | 0.017 |
| Sex hormone-binding globulin levels | ebi-a-GCST90012111 | 57 | IVW(FE) | 1.02 | 0.99–1.04 | 0.207 |
| Total Testosterone | ieu-b-4864 | 47 | IVW(MRE) | 1.08 | 0.99–1.17 | 0.680 |
| Membrane-associated progesterone receptor component 2 | prot-a-2260 | 57 | IVW(MRE) | 0.77 | 0.45–1.30 | 0.327 |
Discussion
The results of this MR analysis indicate a strong causal relationship between genetically predicted alcohol consumption and EC risk. Specifically, an increase of one SD in log-transformed alcohol consumption per day was associated with a 43% lower risk of EC. Subgroup analyses showed that genetically predicted alcohol consumption had a protective effect only in the EEC subtype of EC, while the protective effect disappeared for NEC. In addition, we found that the protective effect of alcohol consumption on EC may be related to a decrease in HCG and IGF1.
Alcohol consumption is a biologically plausible cancer-promoting factor. Firstly, the ethanol metabolite acetaldehyde is recognized as a carcinogen, which can cause cancer in humans by binding to cellular proteins and DNA37. Secondly, alcohol consumption significantly increases postmenopausal women's estrogen levels38, which may increase the risk of estrogen-dependent cancers. Estrogen has long been considered a factor in the development of EC. However, there has been controversy about the relationship between alcohol consumption and EC risk, with numerous studies attempting to prove their relationship. Only a small amount of evidence suggests that alcohol consumption may increase the risk of EC. An Italian retrospective study found a positive correlation between alcohol consumption and EC risk39. Another prospective cohort study involving 41,574 participants of multiple races found that daily alcohol consumption of ≥ 2 drinks increased postmenopausal EC risk (RR = 2.01, 95CI%: 1.3–3.11) compared to not alcohol consumption40. Conversely, more evidence suggests a weak negative or no correlation between alcohol consumption and EC risk. A retrospective study in Japan found a negative correlation between alcohol consumption and EC risk in non-flushing women after drinking (P trend = 0.001)41. Conversely, this protective effect of alcohol consumption disappeared in patients who experienced flushing after drinking, which may be related to insufficient acetaldehyde dehydrogenase leading to an increase in acetaldehyde levels. A retrospective study in the United States also indicated a negative correlation between alcohol consumption and EC risk in obese women42. The NIH-AARP Diet and Health Study found a significant negative correlation between alcohol consumption and EC risk in women with a BMI ≥ 25 kg/m2 in a prospective cohort study involving 114,414 participants (P = 0.04)43. Another retrospective study found a significant negative correlation between moderate alcohol consumption and young (< 50 years old) women's EC risk44. A prospective study involving 68,067 participants from nurse health research showed that moderate alcohol consumption was associated with a 22% reduced risk of EC compared with non-drinkers45. Although prospective studies have less selection and recall bias than retrospective studies, they are still subject to bias due to potential confounding factors. For example, all studies did not adjust for dietary fiber intake, which has been reported to modify the association between alcohol consumption and estrogen-dependent cancers46. Additionally, we found that the protective effect of alcohol on EC was related to pathological type, and previous studies did not stratify by pathological type, which may also be a source of bias in the results. Researchers should be cautious when interpreting the results, as genetic variation has long-term effects on exposure levels, and the estimated values of MR studies are often larger than those of RCTs. Therefore, the causal estimates obtained from MR studies should not be directly interpreted as the direct effects of intervention in practice. Furthermore, although the study indicates that alcohol consumption can reduce the risk of EC, it can also lead to a series of other health problems.
The effect of alcohol on tumors may involve a variety of intermediate phenotypes. Although breast cancer and ovarian cancer are estrogen-dependent cancers, MR studies have not confirmed that alcohol consumption is associated with their risk11,15. This may suggest that alcohol consumption may activate some protective factors that counteract the effects of estrogen on these hormone-dependent tumors. E2 is a known risk factor for EC, which has been confirmed by the MR study47. Our study also found that alcohol consumption does increase E2 levels and increase the risk of EC, which is consistent with previous studies34,38,48. However, we also found that alcohol consumption can reduce HCG and IGF1 levels, this may be a relevant factor in reducing the risk of EC. HCG is related to endometrial proliferation and malignant tumor49,50. A cohort study of 677,247 women showed that increased HCG levels were significantly associated with EC (HR = 1.98, 95% CI 1.33–2.95)51. IGF1 receptors are widely distributed in endometrium52. IGF1 expression and signal transduction play an important role in the proliferation, secretion and menstrual cycle changes of premenopausal endometrium. IGF1 is also involved in the occurrence and development of EC53,54 and is related to the prognosis of EC52. In addition, IGF1 is also a downstream molecule of E2 and participates in the proliferation of EC cells55. There is evidence that alcohol consumption can lead to a decrease in IGF1 levels48,56, and our MR study also confirmed this.
Advantages and limitations
Our study has several important advantages. Firstly, we conducted the first MR study to investigate the causal relationship between alcohol consumption and EC, which is a major advantage as it simulates an RCT and avoids reverse causation and potential confounding factors in observational studies. Secondly, we utilized Phenoscanner V2 and reviewed the literature to exclude SNPs with pleiotropic effects, meeting the three assumptions for MR analysis as much as possible. Additionally, given the large sample sizes in these studies and the robust alcohol-related instrumental variables (F statistics > 10), our study has sufficient statistical power (88%) to detect robust and precise causal effect estimates.
However, there are also some limitations. Firstly, our study used SNPs as alternative indicators for alcohol consumption, rather than directly studying the amount and frequency of alcohol consumption, which may have some measurement errors. Besides, SNPs as a phenotype for alcohol consumption may be influenced by gene environment interactions, which may lead to biased results. Secondly, because the study aggregated different types of alcoholic beverages, the effects of certain components, such as the flavonoids in red wine, were ignored. Therefore, it was not possible to distinguish whether different types of alcoholic beverages and drinking patterns have differential causal effects. Finally, since the study population consisted of European individuals, it may not be generalizable to other populations.
Conclusion
The results of this MR study support a negative causal relationship between genetically predicted alcohol consumption and EC, which was only found in EEC, providing higher-level evidence for the long-standing association between alcohol consumption and EC risk. However, more evidence is still needed to support this conclusion.
Supplementary Information
Abbreviations
- EC
Endomerial cancer
- MR
Mendelian randomization
- GWAS
Genome-wide association studies
- SNPs
Single nucleotide polymorphisms
- GSCAN
GWAS and Sequencing Consortium of Alcohol and Nicotine
- IVW
Inverse variance weighted
- MR-PRESSO
Mendelian Randomization Pleiotropy RESidual Sum and Outlier
- SD
Standard deviation
- OR
Odds ratio
- CI
Confidence interval
- EEC
Endometrioid endometrial cancer
- NEC
Non-endometrioid endometrial cancer
- E2
Estradiol
- HCG
Human chorionic gonadotropin
- IGF1
Insulin-like growth factor 1
- SHBG
Sex hormone-binding globulin
- TT
Total testosterone
- PGRMC2
Progesterone receptor component 2
- RCTs
Randomized controlled trials
- IVs
Instrumental variables
- BMI
Body mass index
- BMR
Basal metabolic rate
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- ECAC
Endometrial Cancer Association Consortium
- E2C2
Epidemiology of Endometrial Cancer Consortium
- IVW-FE
Fixed-effect inverse variance weighted
- IVW-MRE
Random effects IVW method
Author contributions
The research was designed by P.N.. The data analysis and drafting of the manuscript were completed by J.Y. and X.Q.. J.Y. and X.Q. made significant contributions to the content and data modification in the process of revision. A.Z. and F.J. provided technical support. H.C., J.Z. and L.Y. collected the data. All authors contributed to data interpretation, reviewed and approved the final version manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. P.N. had final responsibility for the decision to submit for publication.
Data availability
Data used in the present study are all publicly available. GWAS summary data for alcohol consumption (Supplementary Table S4) were available in (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6358542/) and the remaining GWAS summary data (Supplementary Table S2 provides all GWAS ID) were obtained from the MRC IEU OpenGWAS repository (https://gwas.mrcieu.ac.uk/).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jie Yang and Xiang Qu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-53926-z.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Gong TT, Liu FH, et al. Global, regional, and national burden of endometrial cancer, 1990–2017: Results from the global burden of disease study, 2017. Front. Oncol. 2019;9:1440. doi: 10.3389/fonc.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BO, Berdzuli N, Ilbawi A, et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health. 2023;8(1):e6–e7. doi: 10.1016/S2468-2667(22)00317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rumgay H, Shield K, Charvat H, et al. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021;22(8):1071–1080. doi: 10.1016/S1470-2045(21)00279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friberg E, Orsini N, Mantzoros CS, et al. Alcohol intake and endometrial cancer risk: A meta-analysis of prospective studies. Br. J. Cancer. 2010;103(1):127–131. doi: 10.1038/sj.bjc.6605698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Q, Xu L, Zhou B, et al. Alcohol consumption and the risk of endometrial cancer: A meta-analysis. Asia Pac. J. Clin. Nutr. 2011;20(1):125–133. [PubMed] [Google Scholar]
- 7.Turati F, Gallus S, Tavani A, et al. Alcohol and endometrial cancer risk: A case-control study and a meta-analysis. Cancer Causes Control. 2010;21(8):1285–1296. doi: 10.1007/s10552-010-9556-z. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Guo P, Li H, et al. Does alcohol consumption modify the risk of endometrial cancer? A dose-response meta-analysis of prospective studies. Arch. Gynecol. Obstet. 2017;295(2):467–479. doi: 10.1007/s00404-016-4263-y. [DOI] [PubMed] [Google Scholar]
- 9.Lawlor DA, Harbord RM, Sterne JA, et al. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 10.Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res. Synth. Methods. 2019;10(4):486–496. doi: 10.1002/jrsm.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson SC, Carter P, Kar S, et al. Smoking, alcohol consumption, and cancer: A mendelian randomisation study in UK Biobank and international genetic consortia participants. PLoS Med. 2020;17(7):e1003178. doi: 10.1371/journal.pmed.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Hu Q, Wang J, et al. Habitual consumption of alcohol with meals and lung cancer: A Mendelian randomization study. Ann. Transl. Med. 2021;9(3):263. doi: 10.21037/atm-20-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Y, Huang J, Wong MCS. Associations of alcohol and coffee with colorectal cancer risk in East Asian populations: A Mendelian randomization study. Eur J Nutr. 2023;62(2):749–756. doi: 10.1007/s00394-022-03002-x. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Ye D, Zhou W, et al. Alcohol consumption and colorectal cancer risk: A mendelian randomization study. Front Genet. 2022;13:967229. doi: 10.3389/fgene.2022.967229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Jiang X, Niu Z. Alcohol consumption and risk of breast and ovarian cancer: A Mendelian randomization study. Cancer Genet. 2020;245:35–41. doi: 10.1016/j.cancergen.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Xiong J, Yang L, Deng YQ, et al. The causal association between smoking, alcohol consumption and risk of bladder cancer: A univariable and multivariable Mendelian randomization study. Int J Cancer. 2022;151(12):2136–2143. doi: 10.1002/ijc.34228. [DOI] [PubMed] [Google Scholar]
- 17.Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: The STROBE-MR statement. Jama. 2021;326(16):1614–1621. doi: 10.1001/jama.2021.18236. [DOI] [PubMed] [Google Scholar]
- 18.Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. Bmj. 2021;375:n2233. doi: 10.1136/bmj.n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 2019;51(2):237–244. doi: 10.1038/s41588-018-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed M, Mulugeta A, Lee SH, et al. Adiposity and cancer: A Mendelian randomization analysis in the UK biobank. Int. J. Obes. 2021;45(12):2657–2665. doi: 10.1038/s41366-021-00942-y. [DOI] [PubMed] [Google Scholar]
- 21.Painter JN, O'Mara TA, Marquart L, et al. Genetic risk score Mendelian randomization shows that obesity measured as body mass index, but not waist: Hip ratio, is causal for endometrial cancer. Cancer Epidemiol. Biomarkers Prev. 2016;25(11):1503–1510. doi: 10.1158/1055-9965.EPI-16-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Qiu J, Meng F, et al. Insight into the causality between basal metabolic rate and endometrial and ovarian cancers: Analysis utilizing systematic Mendelian randomization and genetic association data from over 331,000 UK biobank participants. Eur. J. Clin. Invest. 2023;53(6):e13971. doi: 10.1111/eci.13971. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Wang R, Chen C, et al. Educational attainment and endometrial cancer: A Mendelian randomization study. Front. Genet. 2022;13:993731. doi: 10.3389/fgene.2022.993731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kho PF, Amant F, Annibali D, et al. Mendelian randomization analyses suggest a role for cholesterol in the development of endometrial cancer. Int. J. Cancer. 2021;148(2):307–319. doi: 10.1002/ijc.33206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011;40(3):740–752. doi: 10.1093/ije/dyq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017;26(5):2333–2355. doi: 10.1177/0962280215597579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shim H, Chasman DI, Smith JD, et al. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PLoS One. 2015;10(4):e0120758. doi: 10.1371/journal.pone.0120758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Mara TA, Glubb DM, Amant F, et al. Identification of nine new susceptibility loci for endometrial cancer. Nat. Commun. 2018;9(1):3166. doi: 10.1038/s41467-018-05427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbanck M, Chen CY, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2013;42(5):1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsburg ES, Mello NK, Mendelson JH, et al. Effects of alcohol ingestion on estrogens in postmenopausal women. Jama. 1996;276(21):1747–1751. doi: 10.1001/jama.1996.03540210055034. [DOI] [PubMed] [Google Scholar]
- 33.Mendelson JH, Lukas SE, Mello NK, et al. Acute alcohol effects on plasma estradiol levels in women. Psychopharmacology. 1988;94(4):464–467. doi: 10.1007/BF00212838. [DOI] [PubMed] [Google Scholar]
- 34.Tin ST, Smith-Byrne K, Ferrari P, et al. Alcohol intake and endogenous sex hormones in women: Meta-analysis of cohort studies and Mendelian randomization. Res. Sq. 2023 doi: 10.21203/rs.3.rs-3249588/v1. [DOI] [PubMed] [Google Scholar]
- 35.Ruth KS, Day FR, Tyrrell J, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020;26(2):252–258. doi: 10.1038/s41591-020-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun BB, Maranville JC, Peters JE, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73–79. doi: 10.1038/s41586-018-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Secretan B, Straif K, Baan R, et al. A review of human carcinogens–Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033–1034. doi: 10.1016/S1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 38.Rinaldi S, Peeters PH, Bezemer ID, et al. Relationship of alcohol intake and sex steroid concentrations in blood in pre- and post-menopausal women: The European Prospective Investigation into Cancer and Nutrition. Cancer Causes Control. 2006;17(8):1033–1043. doi: 10.1007/s10552-006-0041-7. [DOI] [PubMed] [Google Scholar]
- 39.Parazzini F, La Vecchia C, D'Avanzo B, et al. Alcohol and endometrial cancer risk: Findings from an Italian case-control study. Nutr. Cancer. 1995;23(1):55–62. doi: 10.1080/01635589509514361. [DOI] [PubMed] [Google Scholar]
- 40.Setiawan VW, Monroe KR, Goodman MT, et al. Alcohol consumption and endometrial cancer risk: The multiethnic cohort. Int. J. Cancer. 2008;122(3):634–638. doi: 10.1002/ijc.23072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosono S, Matsuo K, Kajiyama H, et al. Reduced risk of endometrial cancer from alcohol drinking in Japanese. Cancer Sci. 2008;99(6):1195–1201. doi: 10.1111/j.1349-7006.2008.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webster LA, Weiss NS. Alcoholic beverage consumption and the risk of endometrial cancer. Cancer and Steroid Hormone Study Group. Int. J. Epidemiol. 1989;18(4):786–791. doi: 10.1093/ije/18.4.786. [DOI] [PubMed] [Google Scholar]
- 43.Yang HP, Gierach GL, Danforth KN, et al. Alcohol and endometrial cancer risk in the NIH-AARP diet and health study. Int J Cancer. 2011;128(12):2953–2961. doi: 10.1002/ijc.25623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swanson CA, Wilbanks GD, Twiggs LB, et al. Moderate alcohol consumption and the risk of endometrial cancer. Epidemiology. 1993;4(6):530–536. doi: 10.1097/00001648-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Je Y, De Vivo I, Giovannucci E. Long-term alcohol intake and risk of endometrial cancer in the Nurses' Health Study, 1980–2010. Br. J. Cancer. 2014;111(1):186–194. doi: 10.1038/bjc.2014.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhim AS, Fassier P, Latino-Martel P, et al. Prospective association between alcohol intake and hormone-dependent cancer risk: modulation by dietary fiber intake. Am. J. Clin. Nutr. 2015;102(1):182–189. doi: 10.3945/ajcn.114.098418. [DOI] [PubMed] [Google Scholar]
- 47.Thompson DJ, O'Mara TA, Glubb DM, et al. CYP19A1 fine-mapping and Mendelian randomization: Estradiol is causal for endometrial cancer. Endocr. Relat. Cancer. 2016;23(2):77–91. doi: 10.1530/ERC-15-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tin Tin S, Key TJ, Reeves GK. Alcohol intake and endogenous hormones in pre- and postmenopausal women: Findings from the UK Biobank. Cancer Epidemiol. Biomarkers Prev. 2021;30(12):2294–2301. doi: 10.1158/1055-9965.EPI-21-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konishi I, Koshiyama M, Mandai M, et al. Increased expression of LH/hCG receptors in endometrial hyperplasia and carcinoma in anovulatory women. Gynecol. Oncol. 1997;65(2):273–280. doi: 10.1006/gyno.1997.4656. [DOI] [PubMed] [Google Scholar]
- 50.Arcangeli A, Noci I, Fortunato A, et al. The LH/hCG axis in endometrial cancer: A new target in the treatment of recurrent or metastatic disease. Obstet. Gynecol. Int. 2010;2010:48. doi: 10.1155/2010/486164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park AL, Huang T, Meschino WS, et al. Prenatal biochemical screening and a woman's long-term risk of cancer: A population-based cohort study. JNCI Cancer Spectr. 2020;4(1):pkz077. doi: 10.1093/jncics/pkz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Y, Shi L, Chen P, et al. Identification of six candidate genes for endometrial carcinoma by bioinformatics analysis. World J. Surg. Oncol. 2020;18(1):161. doi: 10.1186/s12957-020-01920-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayabe T, Tsutsumi O, Sakai H, et al. Increased circulating levels of insulin-like growth factor-I and decreased circulating levels of insulin-like growth factor binding protein-1 in postmenopausal women with endometrial cancer. Endocr. J. 1997;44(3):419–424. doi: 10.1507/endocrj.44.419. [DOI] [PubMed] [Google Scholar]
- 54.Shibel R, Sarfstein R, Nagaraj K, et al. The olfactory receptor gene product, OR5H2, modulates endometrial cancer cells proliferation via interaction with the IGF1 signaling pathway. Cells. 2021;10:6. doi: 10.3390/cells10061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kashima H, Shiozawa T, Miyamoto T, et al. Autocrine stimulation of IGF1 in estrogen-induced growth of endometrial carcinoma cells: Involvement of the mitogen-activated protein kinase pathway followed by up-regulation of cyclin D1 and cyclin E. Endocr. Relat. Cancer. 2009;16(1):113–122. doi: 10.1677/ERC-08-0117. [DOI] [PubMed] [Google Scholar]
- 56.Lavigne JA, Baer DJ, Wimbrow HH, et al. Effects of alcohol on insulin-like growth factor I and insulin-like growth factor binding protein 3 in postmenopausal women. Am. J. Clin. Nutr. 2005;81(2):503–507. doi: 10.1093/ajcn.81.2.503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the present study are all publicly available. GWAS summary data for alcohol consumption (Supplementary Table S4) were available in (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6358542/) and the remaining GWAS summary data (Supplementary Table S2 provides all GWAS ID) were obtained from the MRC IEU OpenGWAS repository (https://gwas.mrcieu.ac.uk/).