Abstract
Vertical reading training (VRTr) increases reading speed (RS) significantly in patients with hemianopic field defects (HFD). We ask, how eye movements (EM) contribute to this improvement and whether EM-behavior is affected by the side of HFD. Twenty-one patients, randomly assigned to VRTr or horizontal RTr, trained reading single lines from a screen at home, for 4 weeks. In the clinic, we recorded EM while reading short sentences aloud from a screen before training (T1), directly (T2) and 4 weeks afterwards (T3). RS-screen was correlated with RS during reading printed paragraphs (RS-print) to assess the transfer to everyday life. RS-screen and RS-print correlated positively (horizontal: r > 0.8, vertical: r > 0.9) at all times. Vertical RS did not exceed horizontal RS. We found significant negative correlations of EM-variables and RS-print: in right-HFD with the number of forward saccades (T1: r = − 0.79, T2: r = − 0.94), in left-HFD with the steps during return sweeps (T1: r = − 0.83, T2: r = − 0.56). Training effects remained stable at T3. EM-improvement was specific for the RTr and the side of the HFD: in right-HFD fewer forward saccades after VRTr, in left-HFD fewer steps during return sweeps after HRTr. RTr on a screen transfers to reading printed text in real-life situations.
Trial registration: The study was retrospectively registered in the German Clinical Trials register: DRKS-ID: DRKS00018843, March 13th, 2020.
Subject terms: Translational research, Brain injuries, Stroke, Vision disorders, Brain injuries, Stroke
Introduction
Hemianopic field defects (HFD) can cause severe reading disability. For left-to-right readers, right hemianopia is much more disabling, because they have to perform reading saccades into the scotoma. This was first described by Mauthner 18811 and later confirmed by other authors2–7. A few eye movement studies have shown that patients with right HFD make many small saccades in reading direction and more backward saccades to get through the line5–9. Patients with left HFD have no problem reading through the text on one line, but need additional backward saccades during the return sweep, to find the beginning of the next line6.
The question whether a hemianopic dyslexia occurs depends on the presence and size of a macular sparing (Fig. 1c). The existence of a macular sparing had been discussed controversially over decades, for an overview see10. It was assumed to be a perimetric artefact due to fixational eye movements during perimetry, which cause a shift of the field defect towards the hemianopic side11. We showed in a study based on microperimetry using a Scanning Laser Ophthalmoscope with a 1° test-point grid that macular sparing exists12 and that it is associated with sparing of the occipital pole in lesions of the primary visual cortex.
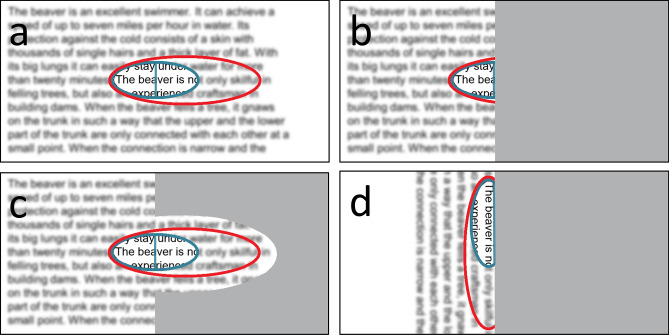
Figure 1.
Macular sparing and reading. (a) The blue oval shows the visual span, or minimum reading visual field, where the letters are seen clearly (2° to the right and left of fixation)27,28. The red oval shows the perceptual span, which can be extended in reading direction up to 5°16. (b) With macular splitting half of the visual span is lost. (c) With macular sparing (> 5°) reading is not impaired. d) Can the seeing visual field at the margin of the scotoma be utilized by vertical reading?
This central seeing area on the hemianopic side is preserved by a double blood supply in patients with hemianopia from stroke (after occlusion of the posterior cerebral artery), if the middle cerebral artery supplies the occipital pole, where the macula is represented. The size of the macular sparing varies dependent on the individual anatomical conditions, but never exceeds 10°10,13. The prevalence of a macular sparing was reported in a retrospective study based on a cohort of 904 HFD patients with 7.3%14. This low percentage might be due to a high portion of patients with traumatic brain injury. On the other hand, one has to consider that perimetric artefacts can mimic sparing due to unstable fixation, and often the visual field is not examined with sufficient precision in clinical settings. The true prevalence is not known and depends on the variable individual anatomical conditions10.
We have previously shown that a macular sparing of > 5° is necessary for unimpaired reading in HFD6,12,15. This confirmed the results about the perceptual span that is necessary for fluent reading in healthy subjects16. McConkie and Rayner found the perceptual span during one fixation asymmetric to the right in left-to-right readers (larger in reading direction)17. We found in patients with left HFD that a perceptual span of > 5° is required also for the return sweep to the next line6. A later study confirmed our finding in simulated hemianopia regarding the word-length effect on reading onset time depending on the size of the “macular sparing”18.
Another feature that should be differentiated from macular sparing, is a “nasotemporal overlap”19 of retinal ganglion cells that leads to a strip of perception of 0.5–1° in the contralateral field along the vertical meridian, but not in the macular area.
The first training studies were about reading moving text4,5,20 and oculomotor training using single words and non-word units8, which was reported to improve reading. There are a few RC-studies on reading training in patients with HFD: Scrolled text improved RS in right HFD21 and search tasks in a line of text increased RS in left HFD more than in right HFD22.
Whereas previous treatment methods focused on oculomotor training, we used a new approach here: We investigated whether patients with HFD can utilize an area of the seeing visual field along the vertical border of the scotoma by turning the text by 90 or 270 deg and then reading in a vertical direction, which can overcome the problem of a limited perceptual span (Fig. 1). Figure 1 shows the conditions of the visual and perceptual span on the text and demonstrates the importance of the perceptual span for undisturbed reading. Studies in healthy subjects on reading in a vertical direction found reading to be much slower than in the horizontal direction, for languages that are usually read horizontally, e.g. English, German, Finnish23–26. This has been attributed to a smaller visual span27,28 in the vertical axis, to a slightly steeper decline of resolution along the vertical meridian29, and to increased crowding28,30. Other studies found that healthy subjects improved only after 10 weeks of training26. A study with healthy subjects, who trained vertical reading text presented as single words (Rapid Serial Visual Presentation, RSVP), showed on average no statistically different reading speed between vertical and horizontal reading speed after 4 days of training, but 5 of the 6 individuals were still faster reading horizontally31. The authors mention a potential of vertical RSVP-training for patients with visual field defects involving the central visual field.
A study on patients with age-related macular degeneration, who had their scotoma shifted in reading direction, showed an improvement of reading speed of text presented as RSVP and rotated by 90°, but this was slower than reading in a horizontal direction32.
We conducted a pilot study with healthy subjects and also found that reading in a vertical direction was slower than in the horizontal direction. Furthermore, the participants reported that they had to concentrate more on the unfamiliar word display than on text comprehension33.
Whereas healthy subjects will not have a perceptual advantage by turning the text, patients with HFD might benefit from reading without being limited by their scotoma. A few studies examined vertical reading in hemianopia and found slower reading speeds34,35. However, none of the previous studies on patients with HFD applied training of vertical reading.
Recently, we performed the first study of training to read vertically oriented text in patients with HFD33,36. The results showed that reading training in vertical text orientation improved reading speed (RS) and quality of life significantly, mainly in patients with right HFD. On the other hand, reading training in horizontal text orientation (from left to right) improved RS significantly, mainly in patients with left HFD. The improved reading speed during vertical reading did not reach the speeds achieved during horizontal reading.
In the previous study36, we focused on the effect of the training on reading speed.
In the current study, we analyze the reading eye movements (EM). Thus, the aim of the current study was to clarify the contribution of EM to the improvement of RS after training in both reading directions. Furthermore, we examined whether the training affects EM behavior specifically in patients with left or right HFD. First results have been reported before37 .
Furthermore, we were interested in detecting pre-existing or newly induced adaptations of EM behavior. For this reason we used a scanning laser ophthalmoscope (SLO) as a supplement to the EM analysis by an infrared eye tracker. The SLO visualized the EM directly on the retina during different tasks12,38,39. Adaptations mean unconscious compensative strategies by the patients – oculomotor and sensory—without training in order to overcome the problem of the reduced perceptual span.
Our previous studies had shown that some patients with hemianopia without macular sparing can use a slightly eccentric (1–1.5°) fixation locus despite normal visual acuity12,38. This adaptive behavior creates a small perceptive area along the vertical midline that can be used for reading. In a previous study we showed12 different fixation patterns dependent on the size of macular sparing: If the sparing was 5°, fixation was central and stable. The smaller the macular sparing (2–4°), the less stable the fixation. In macular splitting (0°) we found either central unstable fixation with frequent saccades towards the hemianopic side or eccentric fixation, both resulting in a shift of the field defect in perimetry towards the hemianopic side.
Another adaptive behavior is the use of hypermetric/predictive saccades to find the next line in left HFD and preview the line in right HFD39–41. For the early stages of HFD, Meienberg and colleagues40 described a staircase pattern of the EM caused by a series of saccadic movements towards a target on the blind side at a distance of ± 10° from the center. The authors call it “safe but slow”. Once the target position had been learned by several trials, the patients performed overshoot saccades to find the target, or predictive saccades, dependent on the target presentation.
This behavior was a short-time adaptation during one session. (One of 3 patients was followed up after 7.5 months. He had changed his performance and made one large saccade that overshot the target). In a later study, Meienberg41 confirmed in 12 patients with right and left HFD that these EM patterns for finding targets (staircase, overshoot, predictive) were independent of the time since onset of the HFD. We conducted an SLO-study39, where we examined dysmetric saccades to a target (± 5° apart from the central fixation cross) and found in principle the same behavior. We showed that dysmetric saccades to targets (5° apart) occurred more frequently in patients without macular sparing (< 4°) than in those with a sparing = > 4°. We did not find a correlation with disease duration (0.2–29 years), which indicates insufficient spontaneous long-term adaptation. The studies mentioned above did not examine the saccadic adaptation behavior during reading. This is why examining these pre-existing spontaneous adaptations and their potential influences on the training effect (and vice versa) were of interest in the current study.
We hypothesize that the improvement of reading speed in either text orientation will be influenced by specific changes in EM patterns dependent on the side of the HFD. We also anticipate that training to read single lines of text on a screen will to some extent transfer to reading printed text in a real-life situation.
Methods
Study design
Patients were randomly assigned to either of two training groups: Group V (n = 11) to train reading in vertical text orientation, and group H (n = 10) to train reading in horizontal text orientation. The horizontal training was supposed to be a placebo training and served as a control.
Examinations were performed before training (T1), directly after end of training (T2) and after four weeks without training (follow-up, T3).
Patients
We screened a large sample of 862 patients with visual field defects due to brain damage of different pathologies. Due to the strict inclusion and exclusion criteria (see below), only 36 of these 862 patients with HFD could be recruited.
Of these, 15 had to be dropped, for a variety of reasons: They either did not show up after training (n = 5), had insufficient training intensity (n = 3), aphasia (n = 2), suspected alexia (n = 2), developmental dyslexia (n = 1), AMD (n = 1), or for personal reasons (n = 1).
Finally, 21 patients with hemianopia (left, n = 11; right, n = 10, one of them with upper right quadrantanopia) participated in the study. The examinations were conducted between 07 March, 2016 and 20 August, 2018.
The detailed clinical data at baseline are shown in Table 1. There was no difference between the groups regarding age, disease duration, side of the HFD, size of macular sparing, reading speed of printed paragraphs, or cognitive ability.
Table 1.
Demographic and clinical data at baseline.
| Group V | Group H | ∆ | |
|---|---|---|---|
| n | 11 | 10 | |
| Age [years] | 58.88 (12; 40.25–80.17) | 65.95(8.3; 57.25–81.17) | p > 0.05 |
| Cause | |||
| Stroke | 4 | 6 | |
| Hemorrhage | 1 | 2 | |
| Tumor | 3 | 2 | |
| Trauma | 2 | 0 | |
| Duration of disease [years] | 5.1 (7.75; 0.5–26.00) | 5.9 (6.72; 1.00–19.00) | p > 0.05 |
| HFD right | 6 | 4 | |
| HFD left | 5 | 6 | |
| Macular sparing [°] | 1 (1.55; 0–5) | 0.9 (1.2; 0–4) | p > 0.05 |
| RS-print at baseline (IReST) [wpm] | 95.57 (36.26; 31–144) | 102 (32.27; 45–146) | p > 0.05 |
| MoCA | 24.55 (3.39; 20–30) | 23.70 (3.34; 19–29) | p > 0.05 |
RS-print reading speed of printed paragraphs at first visit, wpm words per minute, MoCA Score in the Montreal Cognitive Assessment Test.
Means (standard deviation; min–max).
All patients had a macular sparing of ≤ 5 degrees, which was an inclusion criterion (see below). Macular splitting was found in 10 patients, a sparing of 1° in 7 patients, of 2° in 2 patients, of 4° and 5° in one patient each.
Inclusion criteria:
HFD since ≥ 6 months to exclude interference with spontaneous recovery
macular sparing of ≤ 5 degrees to include only patients with limited perceptual span
normal or nearly normal cognitive ability (assessed by the Montreal cognitive assessment (MoCA), (MoCa scale = > 18)42
visual acuity of at least 0.6 (0.2 LogMAR)
Reading speed < 150 words per minute
German as native language
Ability to consent
Exclusion criteria:
Additional visual field defects
Any eye disease, except mild cataract
Spatial neglect (assessed by line bisection test)
Lack of interest in reading
Insufficient co-operation
Aphasia, alexia
Reading impairment of other origin
These strict inclusion and exclusion criteria were chosen to include only patients without macular sparing (≤ 5°), who have a reduced perceptual span, and to make the final group as homogeneous as possible, which also made recruitment very difficult.
Patient examinations
Clinical examinations at baseline
Clinical neuro-ophthalmologic and orthoptic examinations were performed in all patients, including perimetry (30°), tests of best-corrected visual acuity (far/near), binocular status, motility, and eye morphology.
Microperimetry was performed to assess the size of macular sparing by semi-automated, custom-designed software for an SLO with a test grid of 1 degree, performed monocularly on both eyes.
Specific examinations
An overview of all procedures and measurements of variables is shown in Table 2.
Table 2.
Overview on the procedures, measurements of variables and correlations at T1, T2, T3.
| Reading speeds (analyzed according to reading direction, group and side) | RS-print-H: paragraphs |
| RS-print-V: paragraphs | |
| RS-screen-H: sentences | |
| RS-screen-V: sentences | |
| Correlations of RS | RS-print-H versus RS-screen-H |
| RS-print-V versus RS-screen-V | |
| EM variables (horizontal reading, analyzed according to side of HFD) | Side of HFD |
| Number of forward saccades per word | |
| Number of backward saccades per word | |
| Number of additional steps during the return sweep | |
| Fixation duration | |
| Correlations between EM variables during horizontal reading and RS-print- H | |
| SLO: adaptations | Fixation locus (central/eccentric) |
| During fixation of a cross | |
| During reading | |
| Dysmetric saccades | |
RS-print-H Reading speed during horizontal reading of printed paragraphs, RS-print-V Reading speed during vertical reading of printed paragraphs, RS-screen-H Reading speed during horizontal reading of sentences on the screen, RS-screen-V Reading speed during vertical reading of sentences on the screen, HFD hemianopic field defect, EM eye movements.
Eye movements during reading short sentences on a screen
Eye movements (EM) were recorded by an infrared limbus tracker (JAZZ-novo, Ober Consulting, Poznan, Poland) with a sampling rate of 1000Hz and a spatial resolution of 0.1°. EM were recorded while short, standardized sentences43,44 were read aloud from a screen at three different times: Before training (T1), directly after training (T2), and 4 weeks after the end of training (T3).
Texts used in the examinations at T1, T2, T3 (Fig. 2)
Figure 2.
Text material. The left graphs show examples of the short sentences (Radner) displayed for reading on the screen during EM recording. The right graphs show examples of the IReST paragraphs for reading printed text, which were presented as single lines. Text material was presented horizontally or vertically, rotated in counterclockwise (short text left) or clockwise direction (paragraph right) – depending on the patient’s preference. All 21 patients read the texts in horizontal orientation at all times. Only the patients with vertical training read the texts also in vertical direction.
Short, standardized sentences (Radner43: 14 words, 3 lines each; set in 12pt Times New Roman) were presented on the screen during eye tracking. Two sentences were shown together in order to provide 6 lines of text with 5 line-return sweeps. For reading print, one standardized text paragraph (IReST, set in 1.2 M Times New Roman)45,46 (130 words, 15 lines) was presented line by line, either in horizonal or in vertical orientation. We use the terms “orientation” for the text (0°, 270° or 90°) and the term “direction” for the reading process during training and during examinations in the clinic. Both types of text were presented in horizontal orientation to all patients, in vertical orientation only to the patients in the vertical training group V. A new, but linguistically equivalent, text was used for each examination to avoid repetition, which prevented memorizing the content.
Outcome variables
Reading speed (RS)
Reading speed during reading sentences from a screen during eye tracking (RS-screen):
Two short Radner sentences on a screen were read aloud in the clinic during eye tracking, either in horizontal (RS-screen-H) or in vertical text orientation (RS-screen-V) at the three time points. It was calculated as reading time (measured by the eye tracking software) divided by the number of read words (2*14 words) multiplied by 60.
In contrast, in our previous paper36 we assessed reading speed during the training at the computer (cRS) at home, which was measured by the training software. Furthermore, we measured reading speed during reading printed paragraphs aloud (RS-print) at the three time points in the clinic. RS-print was the main outcome variable and showed significant improvement after training, which was specific for each training group and HFD-side. Furthermore, there was a high positive correlation between RS-print and cRS. This showed that reading performance after training on a screen can be applied to everyday reading of printed text, because it transferred to everyday life.
Therefore, we use RS-print, which was already determined in our previous paper in the same cohort of patients, in the current study again to test its correlation with RS-screen, which could indicate whether training effects of the examined EM variables tested in the current paradigm transfer to everyday life. To derive RS-print in words per minute (wpm), the reading time for each paragraph was measured by stopwatch and was then divided by the number of correctly read words and multiplied by 60.
EM variables
We analysed the number of forward saccades per word, the number of backward saccades per word and the number of additional steps during the return sweep to reach the beginning of the next line and fixation duration.
Definition of eye movement variables
Forward and backward saccades: A saccade was defined as a fast eye movement of minimal amplitude of 0.5 deg (the width of the letter “n”) preceded or followed by a fixation of at least 100 ms.
Additional steps during the return sweep: Since patients with left HFD have difficulties finding the beginning of the next line, we examined their behavior during the return sweep to the beginning of the next line: We counted the additional steps during the return sweep that were performed until the patient started to read the text on the new line. These additional steps are hypometric search saccades, in contrast to reading saccades (as defined above). The return sweep itself was not included, because it occurs at the end of each line.
Fixation duration was used as a measure of processing time. Individual mean fixation duration per word was calculated as
A fixation was defined as a period without eye movements of = > 0.5° that was followed or preceded by a saccade and was at least 100 ms long, which neglects the saccade durations. The number of fixations was calculated as the sum of forward and backward saccades.
The eye movement recordings during reading vertical lines of text allowed analyzing RS-screen-V (see above). However, they were not of sufficient quality for detailed analysis of the EM variables, because our eye tracker is less accurate in the vertical direction.
Therefore, we calculated the correlations between the eye movement variables that were analyzed during horizontal reading with the reading speed for printed text in horizontal text orientation (RS-print-H).
Procedures (see also Table 2 above)
Reading speed for sentences read off the screen (RS-screen) during eye tracking
Two short sentences (Radner) were presented on the screen horizontally and vertically. The six lines were displayed together for assessment of the EM during 5 return sweeps per trial.
Different sentences were used for each visit and each task.
In group V, the individually preferred reading orientation of the vertical line was determined during the baseline examination. The two line orientations were from bottom to top with an angle of rotation of 90 deg in the counterclockwise direction (for reading upward), or from top to bottom with an angle of rotation of 90° in the clockwise direction (for reading downward; see Fig. 2, above). Nine of the 11 patients chose the direction from top to bottom.
The patients read aloud, which allows determining the reading time, the mistakes made, and assessing fluency and comprehension. The disadvantage is the higher noise of the EM recordings due to mechanical artifacts.
Reading speed for printed paragraphs (RS-print)
During reading printed paragraphs on a chart (IReST), upcoming text lines beyond the actually read line were covered by a sheet of paper by the examiner – corresponding to the text presentation during the training. Furthermore, covering the upcoming lines is a common habit of patients in everyday life, which we recommend in clinical practice, because it makes orientation on the page easier. Patients who had trained reading single horizontal lines of text, read the test paragraphs only in horizontal orientation at the three visits. Patients who had trained reading single lines in vertical orientation, read the paragraphs in vertical and horizontal orientation at the three visits. For each visit and each task, different IReST paragraphs were used, all of which belonged to the same performance category46.
Training
Single lines of text were presented in either horizontal (group H) or vertical (group V) orientation on a computer screen. The patients could choose texts from different categories which were provided by the software, or they could download any text from the Internet. The training software transformed the text into single lines in the required orientation. The text was then displayed line by line, centered on the screen, either horizontally or vertically from top to bottom, or vice versa. The patients went to the next line pressing a button (right arrow key or space bar), and they could also move backwards line-by-line by pressing the left arrow key. The patients trained at home for 30 min, twice a day, for 5 days a week, for 4 weeks. The training intensity was calculated and documented by the software.
Fixation and reading assessed by scanning laser ophthalmoscope (SLO)
We used an SLO (Rodenstock 101, Ottobrunn, Germany) to visualize the exact fixation locus on the stimulus. The SLO has a spatial resolution of 0.1° and a temporal resolution of 50 half- frames per second. The SLO shows the image of the retina and the stimulus simultaneously, which shows the position of the fovea relative to the stimulus without calibration. We used this advantage of the SLO-method to perform a descriptive analysis by visualizing the retinal fixation locus during different tasks and to detect pre-existing or potentially new adaptations, such as eccentric fixation and predictive saccades, which are immediately visible when the patient’s fovea lands outside the line of text. Note that an infrared eye tracker cannot show this process as directly as an SLO. The reason is that the SLO shows absolute time of both, the fixation locus on the text and the beginning of vocalization, which can be monitored by the soundtrack.
Since the SLO-examination can be performed only monocularly, we always examined the dominant eye, which was determined by a pin-hole test47.
The fixation locus – central or eccentric—was assessed:
During fixation of a cross (20 s)
During reading a short sentence of 3 lines, 14 words, using the standardized Radner sentences (Times New Roman, size 1.6 M and 2.5 M). In the SLO, we used only one Radner sentence per screen in order to create a well centered text field in the SLO image to prevent shadowing artifacts that could be caused by temporary misalignment of the eye relative to the instrument.
We observed the landing positions of the fovea within and outside the text to detect potential eccentric fixations and/or dysmetric saccades.
Dysmetric saccades
Note that the problem of the limited perceptual span during reading is only present in horizontal reading due to the vertical splitting of the visual field in HFD. Hypometric saccades to find the next line in left HFD or finding the line end in right HFD are time-consuming39,41. The HFD patient could overcome the lack of text preview by developing hypermetric (overshooting) and, in the best of cases, predictive saccades during horizontal reading. Therefore, we aimed to examine whether such adaptations would actually happen during horizontal reading in patients with HFD.
Statistics
Data were analyzed using IBM SPSS statistics (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). For the mostly not normally distributed EM variables we applied non-parametric tests: Friedmann-test, post-hoc Wilcoxon signed rank test without Bonferroni correction, because it is a closed test procedure48. We applied parametric tests for the correlations (Pearson correlation coefficient r), if the variables were normally distributed. In the other cases, we calculated the Spearman correlation coefficient rho.
The required level of significance α for all tests was set at 0.05 (two-sided). Unless otherwise stated, the Shapiro–Wilk normality test and graphical Q–Q plots were used to determine the shape of the distributions. The data were analyzed by training group (V vs. H) and by the side of the HFD (left vs. right). The statistical values are shown in Tables 3 and 4.
Table 3.
Statistical data: Reading Speed values and correlations.
| RS | T1 | T2 | T3 | Tests |
|---|---|---|---|---|
| Median (IQR) | ||||
| RS-screen horizontal [wpm] | ||||
| All patients (n = 19;17;17) |
100.6 (67.98–121.74) |
110.53 (92.11–126.79) |
100.6 (71.10–135.49) |
ft(T1–T2–T3): χ2(2) = 4.980, p = .083 |
| Group H (n = 8;8;7) |
113.19 (79.10–126.57) |
123.19 (93.63–130.02) |
134.4 (78.50–136.59) |
ft(T1–T2–T3): χ2(2) = 2.800, p = .247 |
| Group V (n = 11;9;10) |
81.95 (52.50–114.29) |
108.39 (91.87–124.07) |
87.8 (63.06–121.90) |
ft(T1–T2–T3): χ2(2) = 4.710, p = .095 |
| HFD left (n = 9;10;9) |
110.53 (91.21–129.42) |
123.19 (93.25–138.31) |
100.6 (65.27–141.98) |
ft(T1–T2–T3): χ2(2) = 2.516, p = .284 |
| HFD right (n = 10;7;8) |
77.98 (50.09–116.15) |
105 (89.36–120.86) |
97.85 (75.00–129.77) |
ft(T1–T2–T3): χ2(2) = 2.800, p = .247 |
| RS-print-H horizontal [wpm] | ||||
| All patients, n = 21 |
112 (66.75–124.00) |
125 (87.25–143.40) |
123 (73.80–138.50) |
ft(T1-T2-T3): χ2(2) = 8.667, p = .013 |
| w(T1–T2): Z = − 0.762, p = .014; w(T1-T3): Z = − 0.810, p = .009; w(T2–T3): Z = − 0.48, p = .877 | ||||
| Group V, n = 11 |
99 (63.00–128.00) |
99.28 (77.00–143.00) |
115 (73.60–131.40) |
ft(T1–T2–T3): χ2(2) = 1.273, p = .529 |
| Group H, n = 10 |
112.5 (67.38–123.00) |
127.4 (104.00–144.78) |
124.5 (71.50–144.56) |
ft(T1–T2–T3): χ2(2) = 9.800, p = .007 |
| w(T1–T2): Z = − 1.10, p = .014; w(T1–T3): Z = − 1.30, p = .004; w(T2–T3): Z = − 0.200, p = .655 | ||||
| HFD left, n = 11 |
113 (80.00–120.00) |
129.8 (99.28–144.00) |
126 (82.50–141.00) |
ft(T1–T2–T3): χ2(2) = 7.818, p = .020 |
| w(T1–T2): Z = − 1.182, p = .006; w(T1–T3): Z = − 0.727, p = .088; w(T2–T3): Z = 0.455, p = .286 | ||||
| HFD right, n = 10 |
104.5 (47.78–129.00) |
109.75 (57.23–143.20) |
119 (59.00–130.35) |
ft(T1–T2–T3): χ2(2) = 4.200, p = .122 |
| RS-screen vertical [wpm]) | ||||
| All patients of group V (n = 11) |
74.67 (47.95–96.49) |
100 (45.41–111.26) |
92.31 (47.59–100.00) |
ft(T1–T2–T3): χ2(2) = 7.800, p = .020 w(T1–T2): Z = − 1.200, p = .007; w(T1–T3): Z = − .900, p = .044; w(T2–T3): Z = .300, p = .502 |
| HFD left (n = 4;5,5) |
73.46 (55.67–96.10) |
71.19 (50.57–115.63) |
73.68 (42.93–101.12) |
ft(T1–T2–T3): χ2(2) = 1.500, p = .472 |
| HFD right (n = 6;6;6) |
75.67 (28.09–96.80) |
102.83 (38.87–107.93) |
93.88 (50.07–100.61) |
ft(T1–T2–T3): χ2(2) = 7.000, p = .030 w(T1–T2): Z = − 1.500, p = .009; w(T1–T3): Z = − 1.000, p = .083; w(T2–T3): Z = − .500, p = .386 |
| RS-print-V vertical [wpm] | Friedmann-Test, Post-hoc Wilcoxon signed rank test | |||
| All patients of group V, n = 11 |
90 (44.56–112.00) |
104 (56.50–116.40) |
104 (58.20–111.00) |
ft(T1–T2–T3): χ2(2) = 14.000, p < .001 w(T1–T2): Z = − 1.318, p = .002; w(T1–T3): Z = − 1.409, p < .001; w(T2-T3): Z = − 0.091, p = .831 |
| HFD left, n = 5 |
70 (43.03–116.00) |
77 (57.51–130.70) |
73 (58.80–121.20) |
ft(T1–T2–T3): χ2(2) = 6.400, p = .041 w(T1–T2): Z = − 1.600, p = .011; w(T1–T3): Z = − 0.800, p = .206; w(T2-T3): Z = 0.800, p = .618 |
| HFD right, n = 6 |
93.85 (46.66–102.04) |
104.5 (47.44–110.25) |
104.7 (49.21–116.25) |
ft(T1–T2–T3): χ2(2) = 11.565, p = .003 w(T1–T2): Z = − 1.083, p < .001; w(T1–T3): Z = − 1.917, p = .149; w(T2-T3): Z = − 0.833, p = .180 |
| Correlations | ||||
| RS-print – RS-screen (both vertical) | Pearson's r | Pearson's r | Pearson's r | |
| All patients | .933 (n = 10, p < .001) | .946 (n = 11, p < .001) | .911 (n = 11, p < .001) | |
| HFD left | .908 (n = 4, p = .092) | .921 (n = 5, p = .026) | .883 (n = 5, p = .047) | |
| HFD right | .981 (n = 6, p < .001) | .976 (n = 6, p = .026) | .935 (n = 6, p = .006) | |
| RS-print-H – RS-screen-H | Pearson's r | Pearson's r | Pearson's r | |
| All patients | .909 (n = 21, p < .001) | .845 (n = 17, p < .001) | .772 (n = 17, p < .001) | |
| HFD left | .916 (n = 9, p < .001) | .881 (n = 10, p < .001) | .704 (n = 9, p = .034) | |
| HFD right | .945 (n = 10, p < .001) | .927 (n = 7, p = .003) | .868 (n = 8, p = .005) | |
All procedures and measurements of analyzed data.
V vertical training group, H horizontal training group; wpm words per minute; HFD hemianopic field defect; IQR interquartile range; ft Friedman test; w post hoc Wilcoxon signed-rank test; Z test-statistics (Wilcoxion signed rank test), no Bonferroni correction (closed testing procedure), n number of patients, p significance value.
The values of RS-print are from our previous paper36.
Reading speed of printed paragraphs (IReST), in horizontal (RS-print-H) and vertical (RS-print-V) direction and reading speed of short sentences on the screen during eye tracking (Radner) in horizontal (RS-screen-H) and vertical (RS-screen-V) direction. Correlations between RS-print and RS-screen.
Table 4.
Statistical data. EM-Variables.
| EM-Variables | T1 | T2 | T3 | Tests |
|---|---|---|---|---|
| Median (IQR) | ||||
| Forward saccades | ||||
| HFD left (n = 9;10;9) | 45,231 | 41,275 | 41,640 | ft(T1–T2–T3): χ2(2) = 3.161, p = .206 |
| (0.90–1.30) | (1.05–1.38) | (0.95–1.30) | ||
| HFD right (n = 10;7;8) | 16,803 | 43,101 | 45,292 | ft(T1–T2–T3): χ2(2) = 0.737, p = .692 |
| (1.07–2.55) | (1.14–1.50) | (1.10–1.50) | ||
| Backward saccades | ||||
| HFD left (n = 9;10;9) | 0.61 | 0.60 | 0.71 | ft(T1–T2–T3): χ2(2) = 2.467, p = .291 |
| (0.46–0.87) | (0.49–0.74) | (0.45–1.16) | ||
| HFD right (n = 10;7;8) | 0.77 | 0.50 | 0.63 | ft(T1–T2–T3): χ2(2) = 4.562, p = .104 |
| (0.43–1.08) | (0.39–0.75) | (0.50–0.87) | ||
| Additional steps during return sweep | ||||
| HFD left (n = 9;10;9) | 45,047 | 1.00 | 42,736 | ft(T1–T2–T3): χ2(2) = 4.067, p = .131 |
| (0.67–2.00) | (0.33–1.42 ) | (0.67–1.83) | ||
| HFD right (n = 10;7;8) | 0.63 | 0.50 | 0.33 | ft(T1–T2–T3): χ2(2) = 0.105, p = .949 |
| (0.42–0.88) | (1.67–0.67) | (1.67–1.08) | ||
| Fixation duration | ||||
| HFD left (n = 9;10;9) | 0.30 | 0.27 | 0.30 | ft(T1–T2–T3): χ2(2) = 6.750, p = .034 |
| (0.27–0.35) | (0.25–0.32) | (0.29–0.37) | w(T1–T2): Z = 1.125, p = .024; | |
| w(T1–T3): Z = 0.000, p = 1.000; | ||||
| w(T2–T3): Z = − 1.125, p = .024 | ||||
| HFD right (n = 10;7;8) | 0.34 | 0.34 | 0.33 | ft(T1–T2–T3): χ2(2) = 0.000, p = 1.000 |
| (0.31–0.37) | (0.30–0.36) | (0.29–0.37) | ||
| Correlations | ||||
| RS-print-H – forward saccades | Pearson's r | Pearson's r | Pearson's r | |
| HFD left | − .459 (n = 9, p = .214) | − .581 (n = 10, p = .101) | − .461 (n = 9, p = .211) | |
| HFD right | − .786 (n = 10, p = .007) | − .949 (n = 7, p = .001) | − .591 (n = 8, p = .123) | |
| RS-print-H – backward saccades | Spearman-Rho | Spearman-Rho | Pearson's r | |
| HFD left | Rho = − .678 (n = 9, p = .045) | Rho = − .806 (n = 10, p = .005) | − .555 (n = 9, p = .121) | |
| HFD right | Rho = − .912 (n = 10, p < .001) | Rho = − .865 (n = 7, p = .012) | − .770 (n = 8, p = .025) | |
| RS-print-H – additional steps | ||||
| During return sweep | Pearson's r/Spearman-Rho | Pearson's r/Spearman-Rho | Pearson's r/Spearman-Rho | |
| HFD left | r = − .833 (n = 9, p = .005) | Rho = − .561 (n = 10, p = .092) | r = − .729 (n = 9, p = .026) | |
| HFD right | Rho = − .738 (n = 10, p = .015) | r = − .727 (n = 7, p = .064) | Rho = − .405 (n = 8, p = .319) | |
| RS-print-H – fixation duration | Pearson's r | Pearson's r | Pearson's r | |
| HFD left | − .551 (n = 9, p = .124) | − .635 (n = 10, p = .048) | − .596 (n = 9, p = .090) | |
| HFD right | − .679 (n = 10, p = .031) | − .607 (n = 7, p = .149) | − .570 (n = 8, p = .140) | |
V vertical training group, H horizontal training group; HFD hemianopic field defect; IQR interquartile range; ft Friedman test; w post hoc Wilcoxon signed-rank test; Z test-statistics (Wilcoxon signed rank test), n number of cases, p significance value. Correlations correlations between reading speed of printed text (RS-print) horizontally with the EM variables; n number of patients. Spearman Rho Spearman’s rank correlation coefficient (Spearman's ρ), Pearson r Pearson product-moment correlation coefficient.
In cases where the data were non-normally distributed, Spearman’s Rho was calculated, otherwise Pearson’s r.
Ethical approval and consent to participate
We confirm that all experiments were performed in accordance with relevant guidelines and regulations. The project was approved by the ethics committee of the medical faculty of the University of Tübingen, Germany (Reference number: 066/2016B02) and written informed consent was obtained directly from the patients. This study was conducted in agreement with the tenets of the declaration of Helsinki. The study was registered in the German Clinical Trials register (DRKS-ID: DRKS00018843, March 13th, 2020).
Results
To enhance readability, the text only conveys the rounded medians and the significant p-values, while all other statistics are shown in Tables 3 and 4.
Reading speed while reading off the screen during eye tracking (RS-screen)
Horizontal reading
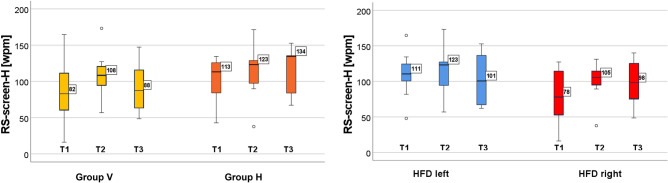
RS-screen-H improved in both groups and sides, but the improvement did not reach statistical significance:
For the entire cohort, RS-screen-H improved from T1 to T2 (medians 101–111 wpm.
If the cohort was separated by training group, RS-screen-H increased from T1–T2 in both training groups (group H: 113–123 wpm; group V: 82–108 wpm; Fig. 3 left). In group H, RS remained stable or even increased further at T3. If they were separated by the side of the HDF, both sub-groups of patients showed an improvement (left: 111–123 wpm, right: 78–105 wpm; Fig. 3 right). (Fig. 3 and Table 3).
Figure 3.
Reading speed during reading in horizontal direction on the screen during eye tracking (RS-screen). Increase of RS-screen from T1 to T2 in both groups (left graph) and in left and right HFD (right graph), however not reaching significance.
Vertical reading
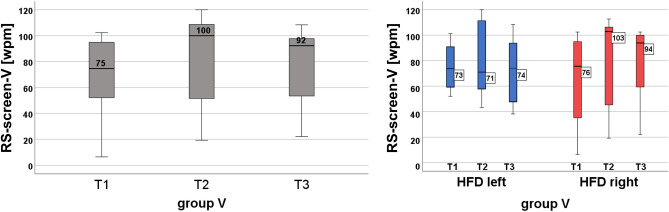
The entire group V improved RS-screen-V significantly from T1 to T2 (medians 75–100 wpm, p = 0.007) and from T1 to T3 (medians 75–92, p = 0.044). The effect remained stable at T3 (see Fig. 4).
Figure 4.
Reading speed during reading in vertical direction on the screen during eye tracking (RS-screen). Significant improvement in the entire group V (left graph) from T1 to T2 and from T1 to T3. Separated by the side of the HFD (right graph): no change in left HFD, significant improvement in right HFD.
Separated according to the side of the HDF, there was no change in those with a left HFD (medians 73–71-74 wpm), but in those with a right HFD, RS-screen-V improved significantly from T1 to T2 (medians 76–103 wpm, p = 0.009). The effect remained stable at T3. This shows the specific effect of vertical training on vertical reading speed.
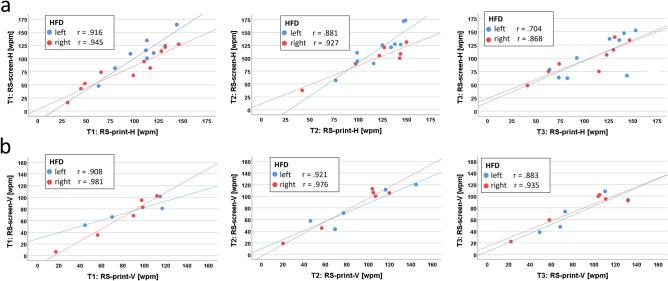
Correlation between RS-screen and RS-print (Fig. 5 and Table 3)
Figure 5.
Correlation between RS-print and RS-screen. (a) in horizontal text orientation, (b) in vertical text orientation. High positive correlation between RS-print and RS-screen in both orientations and at all three time points. Regression lines added.
Horizontal reading
For the entire cohort, there was a high positive correlation between RS-print-H and RS-screen-H (T1: r = 0.909, p < 0.001; T2: r = 0.845, p < 0.001; T3: r = 0.772, p < 0.001).
Separated according to the side of the HFD, there was a high positive correlation between RS-print-H and RS-screen-H for left and right HFD patients at all 3 time points (left HFD: T1: r = 0.916, p < 0.001; T2: r = 0.881, p < 0.001; T3: r = 0.704, p = 0.034 ; right HFD: T1: r = 0.945, p < 0.001; T2: r = 0.927, p = 0.003, T3: r = 0.868, p = 0.005) (Fig. 5a).
Vertical reading
For the entire group V, there was a high positive correlation between RS-print-V and RS-screen-V at all 3 time points (T1: r = 0.933, p < 0.001; T2: r = 0.946, p < 0.001; T3: r = 0.911, p < 0.001) – see Fig. 5b. The high correlation coefficients were statistically significant for left HFD at T2 and T3 (T1: r = 0.981, p = 0.92; T2: r = 0.921, p = 0.026; T3: r = 0.883, p = 0.047) and for right HFD at all 3 time points (T1: r = 0.981, p < 0.001; T2: r = 0.976, p = 0.026; T3: r = 0.935, p = 0.006).
These high positive correlations between reading speeds of printed text and on the screen in horizontal and vertical reading at all 3 time points showed the excellent comparability of the results. This enabled us to focus on the correlations between RS-print and the specific EM variables in the following analysis. We chose RS-print, because this is the measure of the transfer to daily life. As the EM variables could only be analyzed for horizontal reading, the horizontal reading speed of printed text (RS-print-H) was used for the calculations of correlations.
The typical reading problem is different in left and right HFD. For patients with a left HFD, the problem is to find the next line. Those with a right HFD have to make many small saccades in reading direction. Therefore, the training effect on the EM variables is also expected to be different, and we will focus on the results dependent on the side of the HFD (below).
Specific EM variables
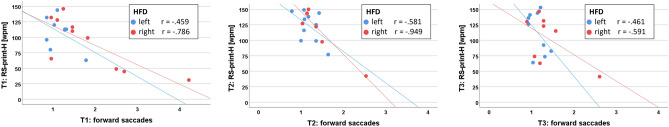
Forward saccades per word (Fig. 6 and Table 4)
Figure 6.
Correlation between RS-print and number of forward saccades per word. High negative correlation between forward saccades and RS-print for right HFD at T1 and T2. Right HFD-patients start with a higher number compared with the left HFD-patients. No correlation for left HFD. This shows that in right HFD the improvement of RS is caused by the decrease of the number of forward saccades per word. Regression lines were added.
The mean word length of the Radner sentences was 5 letters (range: 2–11). In left HFD, there was no change between the time points (1.11–1.13–1.14 saccades/word), but in right HFD, the number of forward saccades decreased from 1.46 (T1) to 1.18 (T2) – without reaching statistical significance (Table 4).
However, the correlation between forward saccades and RS-print-H showed high negative correlations for right HFD at T1 (r = − 0.786, p = 0.007) and T2 (r = − 0.949, p = 0.001). Right HFD-patients start with a higher number of forward saccades compared with the left HFD-patients (Fig. 6 and Table 4). There was only a moderate negative correlation for left HFD (T1: r = − 0.459; T2: r = − 0.581, T3: r = − 0.461), without statistical significance.
This shows that in patients with a right HFD, the improvement of RS is caused by the decrease of the number of forward saccades per word.
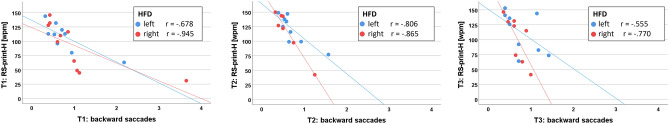
Backward saccades per word (Fig. 7)
Figure 7.
Correlation between RS-print and number of backward saccades per word. High negative correlation of backward saccades with RS-print at T1 and T2, stronger for right HFD. The decrease of the number of backward saccades influences the RS-improvement in all patients, but more in right HFD-patients, who start with a higher number of backward saccades. Regression lines were added.
There was no change from T1 to T2 in patients with left HFD (0.61–0.6), but a decrease in those with right HFD (from 0.77 to 0.5), without reaching statistical significance (see Table 4). However, the negative correlation of backward saccades with RS-print-H was high at T1 and T2, and stronger for patients with right HFD (left HFD: T1: rho = − 0.678, p = 0.045; T2: rho = -0.806, p = 0.005; T3: r = 0.555, p = 0.121; right HFD: T1: rho = − 0.912, p < 0.001; T2: rho = − 0.865, p = 0.012, T3: r = 0.770, p = 0.025) (Fig. 7 and Table 4).
The decrease of the number of backward saccades thus influenced the improvement of RS in all patients, but more in those with right HFD, who start with a higher number of backward saccades (Fig. 7 and Table 4).
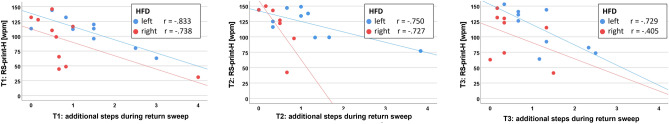
Additional steps during the return sweep (Fig. 8)
Figure 8.
Correlation between RS-print and number of additional saccadic steps during the return sweep. High negative correlation for left HFD, no correlation for right HFD. This shows that in left HFD the improvement of RS is caused by the decrease of the number of additional backward saccades during the return sweep. Regression lines were added.
Additional steps during the return sweep decreased from T1 to T2, which was more pronounced in cases with left (1.5–1.0) than in those with right HFD (0.63–0.50) but did not reach statistical significance (Table 4). However, the correlations between the number of additional steps during the return sweep and RS-print-H showed a high negative correlation for patients with left HFD at T1 and T3 (T1: rho = − 0.833, p = 0.005; T2: r = − 0.561, p = 0.092; T3: r = − 0.729, p = 0.026), and a negative correlation in right HFD only at T1 (T1: rho = − 0.738, p = 0.015; T2: r = − 0.727, p = 0.064; T3: rho = − 0.405, p = 0.319) (Fig. 8 and Table 4).
In cases with left HFD, the improvement of RS is caused by the reduced number of additional steps during the return sweep.
Fixation duration
The fixation duration decreased statistically significantly during training, but only slightly, in patients with left HFD (from 0.30 ms at T1 to 0.27 ms at T2. p = .024), and it increases again from T2 to T3 (0.30 ms). There was a moderate negative correlation between fixation duration and RS-print -H in patients with left HFD at T2 (r = − 0.635, p = 0.048). In right HFD, there was no change of fixation duration (T1: 0.34, T2: 0.34, T3: 0.33 ms) and only a moderate correlation with RS-print-H at T1 (r = − 679, p = 0.031), see Table 4. We conclude that fixation duration contributes to the improvement of RS only slightly and only in patients with a left HFD.
Training intensity
The required training time was 2 × 30 min per day, 5 days per week, for 4 weeks (1200 min = 20h). The cumulative results showed a median training time for all patients of 1236.45 min (IQR 872.2–1417.4, range 715.48 – 1963.1 min) (103%). The median training time per day was 62 min, this means that there was on average good compliance.
Fixation and reading assessed by SLO
Fixation locus while fixating a cross for 20 s
Our focus was to examine whether the retinal fixation locus was the fovea (central, as in healthy subjects and in patients with HFD with macular sparing) or outside the fovea (paracentral, eccentric), which is of significance for reading (see above), and whether it changed during the training.
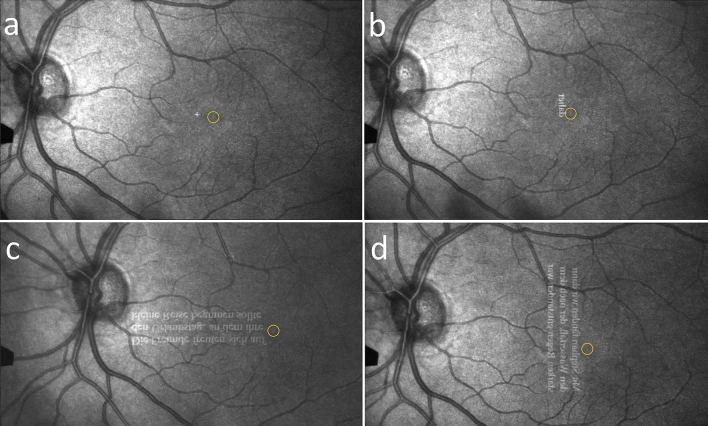
At T1, ten patients fixated centrally with occasional short saccades of 1–2° towards the blind hemifield; 10 patients changed between central and eccentric fixation (by 1°), i.e. they had not established an eccentric preferred retinal locus while one patient had adopted eccentric fixation (by 1–1.5°), shown in Fig. 9a.
Figure 9.
SLO images of a right HFD patient’s retina with eccentric fixation. (a) eccentric fixation of a cross, (b) reading a single word in vertical direction. By using a slightly eccentric retinal locus she creates a small perceptual area along the vertical midline, thus placing the line optimally in the seeing visual hemifield. (c) reading a short sentence in horizontal orientation. The text appears upside down for the examiner, but upright for the patient. The image shows an example of the foveola (yellow circle) landing outside the end of the line. This is a favorable spontaneous adaptive behavior that indicates that the benefit by shifting the text into the seeing hemifield existed before the training. It can be caused by a hypermetric saccade and eccentric fixation during reading. (d) reading a short sentence in vertical direction. The lines are not limited by the scotoma. The patient reads the first line (right) with her eccentric fixation locus and shifts the line into her seeing hemifield.
At T2, only 4/21 patients changed their behavior: Two patients changed their fixation locus between central and eccentric less often, i.e. they showed more stable fixation than at T1. They improved their RS markedly (one patient by 27 wpm, another one by 44.8 wpm). One patient changed from central to eccentric fixation, one patient vice versa—both without improvement of RS. Therefore, the training did not have a general effect on fixation of a cross.
Fixation locus during text reading
Twenty patients fixated the texts centrally in either text orientation. One patient with right HFD in the vertical training group had already adopted eccentric fixation before training and gained a 1–1.5° perceptive area along the vertical midline, which was especially beneficial in vertical reading (Fig. 9b, d). She improved RS by the vertical reading training markedly, i.e. by 17 wpm from T1 to T2 and by 21 wpm from T1 to T3 (90/107/111 wpm). During horizontal reading, RS did not change (128/127/130 wpm), but was higher than in vertical reading. This patient’s disease duration was 26 years, so that she was already adapted to using an eccentric fixation locus during horizontal reading. This example shows that vertical reading in combination with using an eccentric retinal locus provides improved sensory conditions for reading.
Fovea landing (dysmetric saccades) during text reading
The aim of this analysis was to describe reading strategies by observing where the patients’ fovea landed on the horizontal text in order to identify their reading problem by hypometric saccades at the line beginning (in left HFD) or line end (in right HFD), as well as potential adaptive behaviors by hypermetric and predictive saccades.
At T1, we analyzed the pre-existing pattern of dysmetric saccades at the line beginning for left HFD and at the line end for right HFD.
During reading 3 lines of text horizontally, the patients showed the typical eye movement patterns dependent on the side of the HFD.
Patients with left HFD (n = 11)
At T1, eight out of 11 patients did not show adaptive behavior: The patients often missed the beginning of a word within the line and the beginning of the next line. During the return sweep we observed different behaviors: In some cases, the patient`s fovea landed near to the beginning of the next line, which was followed by several backward steps until the beginning was found. In other cases, the patient´s fovea landed within the 2nd or 3rd word of the next line, the patient made 1 or 2 forward and backward saccades, then realized that this was not the correct continuation of the context, and then made another return sweep to the beginning of the line. These hypometric saccades are ineffective and time-consuming. We saw in 3 patients the fovea landing just left of the first letter by a predictive saccade39,41, probably supported by eccentric fixation. This adaptation provides a preview benefit for finding the beginning of the next line.
At T2; here, we examined whether the patients changed their behavior of dysmetric saccades. Four out of 6 patients of the horizontal training group and 2 out of 5 in the vertical training group had reduced their dysmetric saccade behavior or enhanced their overshooting or predictive strategy. The improved landing positions were not associated with the increase of RS-print-H, which shows that the improvement of RS-print-H during horizontal reading was primarily due to the improved EM by less additional steps during the return sweep by the horizontal training.
Patients with right HFD (n = 10)
A1 T1: Four out of 10 patients did not show an adaptive behavior: The patients had to go through the line with many short forward and backward saccades. At T1, four patients landed within the last word of the line. Six patients had adopted an adaptive behavior, when the fovea landed outside the end of the line. An example is shown in Fig. 9c.
At T2: All 4 patients in the horizontal training group improved their dysmetric or predictive behavior. Of the 6 patients in the vertical training group, only one patient improved the predictive saccades at the line end during horizontal reading, five did not show any change of their pre-existing behavior. In all patients with right HFD, there was no association with the increase of RS-print-H in horizontal reading. It shows that the increase of RS-print in right HFD is caused by the vertical reading training. For the patients having trained reading in vertical text orientation, no improvement of RS-print-H in horizontal direction is to be expected.
Summary of the results
The training effect was specific for the reading direction: Vertical training improved RS-screen and RS-print only for vertical reading, horizontal training improved RS-screen and RS-print only for horizontal reading. All RS-improvements were statistically significant, except for RS-screen horizontal. The effects of the EM variables after training depended on the side of the HFD: In patients with right HFD, the improvement of RS-print was caused by the decrease of the number of forward saccades per word and to a lesser degree by a lower number of backward saccades. In cases with left HFD, the improvement of RS-print was primarily caused by the decrease of the number of additional saccadic steps during the return sweep, which takes less time. A lower number of backward saccades and a shorter fixation duration further contributed to the improvement.
However, RS during vertical reading did not reach the same level as during horizontal reading.
Transfer of the training benefit to daily life is indicated by the stable effect at T3 and the high positive correlation between RS-screen and RS-print despite having trained on a screen. The majority of the patients showed an adaptive behavior of various degrees before the training. At T2, 8 of 10 patients of group H and 2 of 10 patients of group V showed a slightly more advanced adaptation.
Discussion
Previous studies on healthy persons with simulated hemianopia18 and studies on patients with hemianopia, but without training34,35, assumed a potential benefit by vertical reading training, although reading was slower in vertical text orientation. Whereas rotating the text will not be of advantage to individuals without visual field defect, patients with HFD can benefit from the improved sensory input, because their perceptual span will not be limited by the scotoma.
In the current study, we found statistically significant improvement of reading speed in the trained reading direction. This means that the training had a specific effect. Even though the improvement of RS-screen-H did not reach statistical significance, the increase of RS-screen-H of = > 10 wpm is considered clinically relevant.
In addition, the side of the HFD played an important role: Patients with right HFD benefited from vertical reading training, in contrast to patients with left HFD, who improved their reading speed during horizontal training. This agrees with the findings by DeJong and coworkers that in left HFD, the text rotation was not helpful, whereas in right HFD the smallest speed reduction occurred with 90° clockwise rotated texts and without previous training34. In contrast, patients with right HFD are more disabled, because their scotoma is in reading direction and thus, they need to make many small saccades to get through the line1–7. It is conceivable that they can benefit most if the limitation of their perceptual span is abolished by reading along the vertical midline and placing the text line in the seeing hemifield. In contrast, patients with left HFD have a problem finding the beginning of the next line, which is evidenced by making additional saccadic steps during the return sweep5–7,9. RS-screen was highly correlated with RS-print in vertical and horizontal text orientation. As RS-print is a measure of the transfer to daily life, we looked for correlations between the EM variables and RS-print.
The EM variables improved dependent on the side of the HFD:
In right HFD, RS improved only in the vertical training group and was primarily caused by the decrease of the number of forward saccades, and less by a decrease of the number of backward saccades. This beneficial effect concerns the primary difficulty of getting through the line in patients with right HFD.
In left HFD, RS improved only in the horizontal training group. This is primarily caused by the decreased number of additional steps during the return sweep that affects the primary difficulty to find the beginning of the next line. The decrease of fixation duration further contributes to the improvement.
We used the SLO to visualize the fovea landing at the line beginning in left HFD and at the line end in right HFD and looked for potential adaptations before and after the training. The fixation locus (central or eccentric) was not influenced by the training. However, eccentric reading along the vertical midline is especially beneficial, because it allows shifting the line to be read into the seeing hemifield.
Hypermetric and predictive saccades occur when the fovea lands outside the beginning of the line in patients with left HFD and outside the line end in patients with right HFD. This is a favorable spontaneous adaption from which the patient benefits and which may have existed already before the training. The majority of our patients showed such adaptions of various degrees before the training. After the training, eight of 10 patients of group H and 2 of 10 of group V showed a slightly more advanced adaptation. This could have been supported by horizontal training. It is conceivable that such adaptations could be supported by specific oculomotor training8. It is possible that the vertical training had no effect, because the line beginnings and ends are always visible here. Figure 9 c shows an example for the fovea landing outside the end of the line. This is a favorable spontaneous adaptive behavior that indicates that the benefit by shifting the text into the seeing hemifield existed before the training. In this patient, this can be caused by both: hypermetric saccadic strategy and eccentric fixation.
Meienberg41 and our previous study39 found that these spontaneous adaptations are not correlated with the disease duration, which indicates insufficient spontaneous long-term adaptation and demonstrates the demand for specific reading training.
RS during vertical reading did not reach the same level as during horizontal reading. This could have several reasons:
1) In alphabetic languages, which are usually read horizontally, the perceptual span during reading was found to extend farther in the reading direction. This was found for reading from left to right17,49, such as English and all other European languages, as well as for languages that are read from right to left, such as Hebrew50, Arabic51 and Urdu49. This shows that the script of a language and the learned reading direction influence the size of the perceptual span in order to create a preview benefit to determine the size of the next saccade.
2) Life-long experience with reading in a horizontal direction in alphabetic scripts not only trains the horizontal reading eye movements, but also the recognition of word shape. Vertical reading saccades and rotated word shapes are unfamiliar and challenging. Even more difficult is the presentation of alphabetic words as “marquee”, where the single letters are written one below the other, which loses the word shape completely and slows reading considerably23,25,52.
However, in logographic scripts – such as Japanese and Chinese - the words can be written in both directions without changing their shape - as they keep the same orientation. Each Chinese character represents a word or part of a composed word.
It is interesting that in readers who are used to read in both directions - as in Chinese and Japanese - the difference between horizontal and vertical reading performance disappears53. However, event-related potentials have shown that the recognition of Chinese characters was delayed in occipito–temporal sites if the orientation of the characters was changed54. In a recent study, we found that the meaning of a Chinese character is primarily processed visually55. Therefore, changing the character orientation interferes with the logographic nature of the character.
Mongolian script, which is alphabetic, is an exception in that it is conventionally written vertically (from top to bottom, with line progression from left to right), but it can also be read horizontally (from left to right). Horizontal text orientation can be achieved by rotating the vertical text by 90° counterclockwise (opposite to what we did here). This way, the orthographic units (letters, words) are always aligned with the reading direction. A study by Su et al.56 found the perceptual span to be similar in both reading directions, which again demonstrates the flexibility of the perceptual span.
Furthermore, the influence of the experienced reading direction was examined in experiments using different tasks: In a product choice task, it has been reported that in Japanese readers` reading direction primed activating the corresponding direction of visual information processing57. Another study examined the counting direction in a cross-cultural study and showed that the horizontal and vertical counting direction was strongly influenced by the reading direction during recent exposure and longstanding experience58. These results show the significance of an individual`s experience for priming a preferred direction in different tasks.
3) A shorter visual span, where letters are seen clearly, i.e. a lower resolution was reported for the vertical meridian compared with the horizontal one27–29. Yu et al.25 examined vertical reading using Rapid Serial Visual Presentation (RSVP) to eliminate reading eye movements as well as short sentences on flashcards. RSVP showed a higher reading speed than flashcard presentation. They found that the slower reading speed in the vertical conditions was caused by the shorter visual span and not by the vertical reading eye movements.
The visual span is defined by the retinal resolution based on the cone density, whereas the perceptual span can be increased in reading direction by parafoveal information processing, where the letters are no longer seen completely clearly. The perceptual span is a dynamic feature during reading eye movements16 and is flexible and can be trained, as described above.
Some patients with long-standing HFD, who had probably developed spontaneous adaptive strategies, mentioned that they would have welcomed training sooner after onset of their disorder.
The following points can be seen as weaknesses of the current study:
The sample size was not big enough to form further subgroups.
EM variables could be analyzed only for horizontal reading, since the recordings for vertical reading were not of sufficient quality due to technical reasons.
Previous studies on vertical reading with healthy subjects did not show a difference in reading speed, if the text was rotated clockwise or counterclockwise25,52. Our patients of group V could choose their preferred rotation of the vertical text before the training. All but two chose to read from top to bottom (both had right HFD) during the training. It might have been better, if all patients with right HFD had read from top to bottom and those with left HFD from bottom to top, because the next line would always appear in the seeing hemifield.
Conclusions
Based on our data and the studies mentioned above, we believe that the slower vertical reading speed in our study is caused by having to make unfamiliar vertical reading saccades and by the unfamiliar rotated word shapes. It is conceivable that longer and earlier training could overcome or reduce these difficulties by increasing the perceptual span in vertical reading direction and by getting used to rotated word shapes. Future studies should investigate, whether the training effect can thus be enhanced.
The data confirmed our hypotheses by showing that reading in either text orientation was improved specifically by the training, and that the effects on the EM pattern depended on the side of the HFD.
Transfer of the training benefit to daily life is indicated by the stable effect at T3 and the fact that the patients could apply their improved reading performance to reading printed paragraphs despite having trained reading single lines of text on a screen. Thus, we could show that training to read text on a screen transferred to reading printed text in a real-life situation.
Acknowledgements
We thank Manfred MacKeben, PhD, The Smith Kettlewell Eye Research Institute, San Francisco, USA, for fruitful discussions and editorial support. We acknowledge support from the Open Access Publication Fund of the University of Tübingen.
Abbreviations
- AMD
Age-related macular degeneration
- cRS
Reading speed measured during the training at the computer
- EM
Eye movements
- Group H
Training group to train reading in horizontal text orientation
- Group V
Training group to train reading in vertical text orientation
- HFD
Hemianopic field defects
- IQR
Interquartile range
- IReST
The International Reading Speed Texts; standardized text paragraphs
- LogMAR
Logarithm of the Minimum Angle of Resolution
- MoCA
Montreal cognitive assessment
- RS
Reading speed
- RS-print
Reading speed measured while reading printed standardized paragraphs
- RS-print-H
Reading speed measured while reading printed standardized paragraphs in horizontal text orientation
- RS-print-V
Reading speed measured while reading printed standardized paragraphs in vertical text orientation
- RS-screen
Reading speed measured while reading short sentences on a screen
- RS-screen-H
Reading speed measured while reading short sentences on a screen in horizontal text orientation
- RS-screen-V
Reading speed measured while reading short sentences on a screen in vertical text orientation
- RSVP
Rapid Serial Visual Presentation
- SLO
Scanning Laser Ophthalmoscope
- T1
Time before training
- T2
Time directly after the training
- T3
Time 4 weeks after end of training
- wpm
Words per minute
Author contributions
S.T.K.: Study design, data collection, analyses, manuscript writing and drafting. S.K.G., P.K.: participated in the study design, data collection, analysis, manuscript writing and drafting. H.O.K.: Participated in the study design. P.M.: participated in the data analysis. A.C.H.: participated in the data collection. All authors reviewed and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported by the Tistou and Charlotte Kerstan Foundation and by the Lechler Foundation.
Data availability
The datasets and raw data which were collected and analysed during this study can be shared and are available from the corresponding author upon reasonable request. For privacy and data protection reasons, it is not possible to make this data publicly available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mauthner L. Gehirn und Auge [Brain and Eye] Bergmann; 1881. pp. 1881–370. [Google Scholar]
- 2.Wilbrand H. Über die makulär-hemianopische Lesestörung und die v. Monakowsche Projektion der Makula auf die Sehsphäre [On the macularhemianopic reading disorder and the v. Monakowian projection of the macula on the visual sphere] Klin Monatsbl Augenheilkd. 1907;45:1–39. [Google Scholar]
- 3.Mackensen G. Die Untersuchung der Lesefähigkeit als klinische Funktionsprüfung [Examination of reading ability as a clinical functional test] Fortschr Augenheilkd. 1962;12:344–379. [Google Scholar]
- 4.Zihl J, Krischer C, Meißen R. Die hemianopische Lesestörung und ihre Behandlung. Der Nervenarzt. 1984;55(6):317–323. [PubMed] [Google Scholar]
- 5.Zihl J. Eye movement patterns in hemianopic dyslexia. Brain J. Neurol. 1995;118(Pt 4):891–912. doi: 10.1093/brain/118.4.891. [DOI] [PubMed] [Google Scholar]
- 6.Trauzettel-Klosinski S, Brendler K. Eye movements in reading with hemianopic field defects: The significance of clinical parameters. Graefes Arch. Clin. Exp. Ophthalmol. 1998;236(2):91–102. doi: 10.1007/s004170050048. [DOI] [PubMed] [Google Scholar]
- 7.McDonald SA, Spitsyna G, Shillcock RC, Wise RJ, Leff AP. Patients with hemianopic alexia adopt an inefficient eye movement strategy when reading text. Brain J. Neurol. 2006;129(Pt 1):158–167. doi: 10.1093/brain/awh678. [DOI] [PubMed] [Google Scholar]
- 8.Schuett S, Heywood CA, Kentridge RW, Zihl J. Rehabilitation of hemianopic dyslexia: Are words necessary for re-learning oculomotor control? Brain J. Neurol. 2008;131(12):3156–3168. doi: 10.1093/brain/awn285. [DOI] [PubMed] [Google Scholar]
- 9.Ciuffreda KJ, Han Y, Kapoor N, Ficarra AP. Oculomotor rehabilitation for reading in acquired brain injury. Neuro Rehabil. 2006;21(1):9–21. [PubMed] [Google Scholar]
- 10.Horton JC, Economides JR, Adams DL. The mechanism of macular sparing. Ann. Rev. Vis. Sci. 2021;7(1):155–179. doi: 10.1146/annurev-vision-100119-125406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bischoff P, Lang J, Huber A. Macular sparing as a perimetric artefact. Am. J. Ophthalmol. 1995;119:72–80. doi: 10.1016/S0002-9394(14)73816-4. [DOI] [PubMed] [Google Scholar]
- 12.Trauzettel-Klosinski S, Reinhard J. The vertical field border in hemianopia and its significance for fixation and reading. Invest. Ophthalmol. Vis. Sci. 1998;39(11):2177–2186. [PubMed] [Google Scholar]
- 13.Horton JC, Fahle M, Mulder T, Trauzettel-Klosinski S. Adaptation, perceptual learning, and plasticity of brain functions. Graefe's Arch. Clin. Exp. Ophthalmol. 2017;255(3):435–447. doi: 10.1007/s00417-016-3580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Homonymous hemianopias. Neurology. 2006;66(6):906. doi: 10.1212/01.wnl.0000203913.12088.93. [DOI] [PubMed] [Google Scholar]
- 15.Trauzettel-Klosinski S. Rehabilitation for visual disorders. J. Neuro Ophthalmol. 2010;30(1):47899. doi: 10.1097/WNO.0b013e3181ce7e8f. [DOI] [PubMed] [Google Scholar]
- 16.McConkie GW, Rayner K. The span of the effective stimulus during a fixation in reading. Percep. Psychophys. 1975;17(6):578–586. doi: 10.3758/BF03203972. [DOI] [Google Scholar]
- 17.McConkie GW, Rayner K. Asymmetry of the perceptual span in reading. Bull. Psychon. Soc. 1976;8(5):365–368. doi: 10.3758/BF03335168. [DOI] [Google Scholar]
- 18.Rubino C, Yeung SC, Barton JJS. The impact of central sparing on the word-length effect in hemianopia. Cogn. Neuropsychol. 2016;33(7–8):353–361. doi: 10.1080/02643294.2016.1232707. [DOI] [PubMed] [Google Scholar]
- 19.Reinhard J, Trauzettel-Klosinski S. Nasotemporal overlap of retinal ganglion cells in humans: A functional study. Investig. Ophthalmol. Vis. Sci. 2003;44(4):1568–1572. doi: 10.1167/iovs.02-0313. [DOI] [PubMed] [Google Scholar]
- 20.Kerkhoff G, Münßinger U, Eberle-strauss G, Stögerer E. Rehabilitation of hemianopic alexia in patients with postgeniculate visual field disorders. Neuropsychol. Rehabil. 1992;2(1):21–42. doi: 10.1080/09602019208401393. [DOI] [Google Scholar]
- 21.Spitzyna GA, Wise RJS, McDonald SA, Plant GT, Kidd D, Crewes H, et al. Optokinetic therapy improves text reading in patients with hemianopic alexia. Neurology. 2007;68(22):1922. doi: 10.1212/01.wnl.0000264002.30134.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aimola L, Lane AR, Smith DT, Kerkhoff G, Ford GA, Schenk T. Efficacy and feasibility of home-based training for individuals with homonymous visual field defects. Neurorehabilitation Neural Repair. 2013;28(3):207–218. doi: 10.1177/1545968313503219. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt D, Ullrich D, Rossner R. Horizontal and vertical reading: A comparative investigation of eye movements. Ger. J. Ophthalmol. 1993;2(4–5):251–255. [PubMed] [Google Scholar]
- 24.Laarni J, Simola J, Kojo I, Risto N. Reading vertical text from a computer screen. Behav. Inf. Technol. 2004;23(2):75–82. doi: 10.1080/01449290310001648260. [DOI] [Google Scholar]
- 25.Yu D, Park H, Gerold D, Legge GE. Comparing reading speed for horizontal and vertical English text. J. Vis. 2010;10(2):21. doi: 10.1167/10.2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahlén E, Hills CS, Hanif HM, Rubino C, Barton JJS. Learning to read upside-down: A study of perceptual expertise and its acquisition. Exp. Brain Res. 2014;232(3):1025–1036. doi: 10.1007/s00221-013-3813-9. [DOI] [PubMed] [Google Scholar]
- 27.Aulhorn E. Über die Fixationsbreite und Fixationsfrequenz beim Lesen gerichteter Konturen. Pflüger's Archiv für die gesamte Physiologie des Menschen und der Tiere. 1953;257:318–328. doi: 10.1007/BF00363531. [DOI] [PubMed] [Google Scholar]
- 28.Yu D, Legge GE, Wagoner G, Chung STL. Sensory factors limiting horizontal and vertical visual span for letter recognition. J. Vis. 2014;14(9):466112. doi: 10.1167/14.9.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wertheim T. Peripheral visual acuity Wertheim. Am. J. Optom. Physiol. Opt. 1980;57(12):915–924. doi: 10.1097/00006324-198012000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Pelli DG, Tillman KA, Freeman J, Su M, Berger TD, Majaj NJ. Crowding and eccentricity determine reading rate. J. Vis. 2007;7(2):20. doi: 10.1167/7.2.20. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian A, Legge GE, Wagoner GH, Yu D. Learning to read vertical text in peripheral vision. Optomet. Vis. Sci. 2014;91(9):79966. doi: 10.1097/OPX.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calabrèse A, Liu T, Legge GE. Does vertical reading help people with macular degeneration: An exploratory study. PLOS ONE. 2017;12(1):e0170743. doi: 10.1371/journal.pone.0170743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabisch P. Lesen in vertikaler Textorientierung bei homonymer Hemianopsie – eine Trainingsstudie [Reading in vertical text orientation in homonymous hemianopia—A training study] [Doctoral thesis] (Medical Faculty, University of Tuebingen, 2023).
- 34.de Jong D, Kaufmann-Ezra S, Meichtry JR, von Arx S, Cazzoli D, Gutbrod K, et al. The influence of reading direction on hemianopic reading disorders. J. Clin. Exp. Neuropsychol. 2016;38(10):1077–1083. doi: 10.1080/13803395.2016.1189884. [DOI] [PubMed] [Google Scholar]
- 35.Hepworth L, Rowe F, Waterman H. VeRSE: Vertical reading strategy efficacy for homonymous hemianopia after stroke: A feasibility study. Br. Ir. Orthopt. J. 2019;15(1):28–35. doi: 10.22599/bioj.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuester-Gruber S, Kabisch P, Cordey A, Karnath HO, Trauzettel-Klosinski S. Training of vertical versus horizontal reading in patients with hemianopia—A randomized and controlled study. Graefe's Arch. Clin. Exp. Ophthalmol. 2021;259(3):745–757. doi: 10.1007/s00417-020-04952-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trauzettel-Klosinski S, Kabisch P, Karnath H-O, Kuester-Gruber S. Eye movements during vertical and horizontal reading training in patients with hemianopia—A randomized and controlled study. Investig. Ophthalmol. Vis. Sci. 2023;64(8):4083. [Google Scholar]
- 38.Trauzettel-klosinski S. Eccentric fixation with hemianopic field defects: A valuable strategy to improve reading ability and an indication of cortical plasticity. Neuro Ophthalmol. 1997;18(3):117–131. doi: 10.3109/01658109709044129. [DOI] [Google Scholar]
- 39.Reinhard JI, Damm I, Ivanov IV, Trauzettel-Klosinski S. Eye movements during saccadic and fixation tasks in patients with homonymous hemianopia. J. Neuroophthalmol. 2014;34(4):354–361. doi: 10.1097/WNO.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 40.Meienberg O, Zangemeister WH, Rosenberg M, Hoyt WF, Stark L. Saccadic eye movement strategies in patients with homonymous hemianopia. Ann. Neurol. 1981;9(6):537–544. doi: 10.1002/ana.410090605. [DOI] [PubMed] [Google Scholar]
- 41.Meienberg O. Eye movement patterns in homonymous hemianopsia and visual hemineglect. Criteria of oculographic delineation based on 19 cases and their diagnostic significance. Klinische Monatsblatter fur Augenheilkunde. 1988;192(2):108–12. doi: 10.1055/s-2008-1050084. [DOI] [PubMed] [Google Scholar]
- 42.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 43.Radner W, Willinger U, Obermayer W, Mudrich C, Velikay-Parel M, Eisenwort B. A new reading chart for simultaneous determination of reading vision and reading speed. Klin Monbl Augenheilkd. 1998;213(3):174–181. doi: 10.1055/s-2008-1034969. [DOI] [PubMed] [Google Scholar]
- 44.Radner W, Obermayer W, Richter-Mueksch S, Willinger U, Velikay-Parel M, Eisenwort B. The validity and reliability of short German sentences for measuring reading speed. Graefe's Arch. Clin. Exp. Ophthalmol. 2002;240(6):461–467. doi: 10.1007/s00417-002-0443-5. [DOI] [PubMed] [Google Scholar]
- 45.Hahn GA, Penka D, Gehrlich C, Messias A, Weismann M, Hyvärinen L, et al. New standardised texts for assessing reading performance in four European languages. Brit. J. Ophthalmol. 2006;90(4):480. doi: 10.1136/bjo.2005.087379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trauzettel-Klosinski S, Dietz K, the IReST Study Group Standardized assessment of reading performance: The new international standardized reading texts IReST. Investig. Ophthalmol. Vis. Sci. 2012;53(9):5452–5461. doi: 10.1167/iovs.11-8284. [DOI] [PubMed] [Google Scholar]
- 47.Walls GL. A theory of ocular dominance. AMA Arch. Ophthalmol. 1951;45(4):387–412. doi: 10.1001/archopht.1951.01700010395005. [DOI] [PubMed] [Google Scholar]
- 48.Hochberg Y, Tamhane AC. Multiple Comparison Procedures. John Wiley & Sons, Inc; 1987. [Google Scholar]
- 49.Paterson KB, McGowan VA, White SJ, Malik S, Abedipour L, Jordan TR. Reading direction and the central perceptual span in urdu and english. PLOS ONE. 2014;9(2):e88358. doi: 10.1371/journal.pone.0088358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollatsek A, Bolozky S, Well AD, Rayner K. Asymmetries in the perceptual span for Israeli readers. Brain Language. 1981;14(1):174–180. doi: 10.1016/0093-934X(81)90073-0. [DOI] [PubMed] [Google Scholar]
- 51.Jordan TR, Almabruk AA, Gadalla EA, McGowan VA, White SJ, Abedipour L, et al. Reading direction and the central perceptual span: Evidence from Arabic and English. Psychon. Bull. Rev. 2014;21(2):505–511. doi: 10.3758/s13423-013-0510-4. [DOI] [PubMed] [Google Scholar]
- 52.Byrne MD. Reading vertical text: Rotated VS. marquee. Proc. Hum. Factors Ergon. Soc. Ann. Meeting. 2002;46(17):1633–5. doi: 10.1177/154193120204601722. [DOI] [Google Scholar]
- 53.Osaka N, Oda K. Effective visual field size necessary for vertical reading during Japanese text processing. Bull. Psychon. Soc. 1991;29(4):345–347. doi: 10.3758/BF03333939. [DOI] [Google Scholar]
- 54.Zhang Y, Yuan J, Bao B, Zhang Q. The recognition potential and rotated Chinese characters. Brain Res. 2008;1233:98–105. doi: 10.1016/j.brainres.2008.07.080. [DOI] [PubMed] [Google Scholar]
- 55.Kuester-Gruber S, Faisst T, Schick V, Righetti G, Braun C, Cordey-Henke A, et al. Is learning a logographic script easier than reading an alphabetic script for German children with dyslexia? PLOS ONE. 2023;18(2):e0282200. doi: 10.1371/journal.pone.0282200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su J, Yin G, Bai X, Yan G, Kurtev S, Warrington KL, et al. Flexibility in the perceptual span during reading: Evidence from Mongolian. Atten. Percep. Psychophys. 2020;82(4):1566–1572. doi: 10.3758/s13414-019-01960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ariga A. Reading habits contribute to the effects of display direction on product choice. PLOS ONE. 2018;13(12):e0209837. doi: 10.1371/journal.pone.0209837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Göbel SM. Up or down? Reading direction influences vertical counting direction in the horizontal plane—A cross-cultural comparison. Front. Psychol. 2015;6:4566. doi: 10.3389/fpsyg.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and raw data which were collected and analysed during this study can be shared and are available from the corresponding author upon reasonable request. For privacy and data protection reasons, it is not possible to make this data publicly available.