Abstract
I/St mice, previously characterized as susceptible to Mycobacterium tuberculosis H37Rv, were given 103 or 105 CFU intravenously. At two time points postinoculation, the cell suspensions that resulted from enzymatic digestion of lungs were enumerated and further characterized phenotypically and functionally. Regarding the T-cell populations recovered at 2 and 5 weeks postinfection, two main results were obtained: (i) the population of CD44− CD45RB+ cells disappeared within 2 weeks postinfection, while the number of CD44+ CD45RB−/low cells slowly increased between weeks 2 and 5; (ii) when cocultured with irradiated syngeneic splenocytes, these lung T cells proliferated in the presence of H37Rv sonicate. Using H37Rv sonicate and irradiated syngeneic splenocytes to reactivate lung T cells, we selected five CD3+ CD4+ CD8− T-cell clones. In addition to the H37Rv sonicate, the five clones react to both a short-term culture filtrate and an affinity-purified 15- to 18-kDa mycobacterial molecule as assessed by the proliferative assay. However, there was a clear difference between T-cell clones with respect to cytokine (gamma interferon [IFN-γ] and interleukin-4 [IL-4] and IL-10) profiles: besides one Th1-like (IFN-γ+ IL-4−) clone and one Th0-like (IFN-γ+ IL-4+ IL-10+) clone, three clones produced predominantly IL-10, with only marginal or no IL-4 and IFN-γ responses. Inhibition of mycobacterial growth by macrophages in the presence of T cells was studied in a coculture in vitro system. It was found that the capacity to enhance antimycobacterial activity of macrophages fully correlated with INF-γ production by individual T-cell clones following genetically restricted recognition of infected macrophages. The possible functional significance of cytokine diversity among T-cell clones is discussed.
Extensive studies of experimental Mycobacterium tuberculosis infection in the mouse model have revealed significant differences in the susceptibility of inbred mouse strains to infection (for reviews, see references 8 and 29). Mechanisms providing resistance versus susceptibility remain largely unknown, despite a general consensus that the CD4+ T cells, which activate the effector response of infected macrophages, play a pivotal role in protection (33). To date, conclusions about T-cell involvement in antimycobacterial protection are based almost exclusively on experiments in which T cells from spleens and lymph nodes from infected, vaccinated animals or the peripheral blood of tuberculosis patients were used. Much less is known, however, concerning regulation of antimycobacterial responses in the lung, the anatomical site where tuberculosis predominantly develops. Given that alveolar and interstitial lung macrophages readily suppress T-lymphocyte proliferation (20, 21, 27, 28, 39, 41) and that progression of tuberculosis strongly augments the suppressive activity of these cells (3), assessment of T-cell responsiveness against lung infection in situ is not an easy task.
We have shown previously that the susceptibility to M. tuberculosis H37Rv, measured by the mean survival time (MST) following a high-dose intravenous (i.v.) challenge, is controlled by, in addition to other genes, the Tbc-1 autosomal locus (4, 30). The Tbc-1s recessive allele expressed in the I/St mouse strain, unlike its dominant Tbc-1r counterpart present in the A/Sn mouse strain, determines a high level of susceptibility to the lethal disease triggered by M. tuberculosis. When I/St and A/Sn mice were challenged i.v. with freshly thawed, unfiltered mycobacteria at a dose of 5 × 105 CFU/mouse, the MST of I/St mice was three times shorter than that of resistant A/Sn mice (21.1 ± 0.6 and 62.7 ± 2.5 days, respectively) (4, 31). Later it was shown that (i) at least 95% of CFU in the bacterial suspension used were formed by clumps of mycobacteria, and this resulted in a severe underestimation of the challenge dose; (ii) filtered mycobacteria should be injected with a dose exceeding 6 × 106 to 8 × 106 CFU/mouse to cause mortality with comparable MST; and (iii) the bacterial load in the lungs of I/St mice exceeded 5 × 107/organ as early as 2 weeks following challenge (our unpublished observations).
In this report, we describe the specific response of T cells recovered from enzymatically disrupted lungs of susceptible I/St mice following infection, as well as the establishment of T-cell clones from this source. The acquisition of specific proliferative responsiveness to mycobacterial antigens by lung T cells during tuberculosis infection was accompanied by an increase of CD44+ CD45RB−/low cells and the disappearance of the CD44− CD45RB+ population. Mycobacterial sonicate- and short-term culture filtrate (ST-CF)-reactive T-cell clones were developed from I/St lungs, albeit at a low rate. These CD3+ CD4+ CD8− T-cell clones differed in their cytokine profiles. It was found also that the capacity of these T-cell clones to promote inhibition of mycobacterial growth by macrophages in vitro correlates with gamma interferon (IFN-γ) secretion in an antigen-specific and major histocompatibility complex (MHC)-restricted manner.
MATERIALS AND METHODS
Animals.
I/St mice were bred at the Animal Facilities of the Central Institute for Tuberculosis (Moscow, Russia). Female mice 2 to 4 months of age were infected and used as a source of lung T cells and splenic antigen-presenting cells (APCs).
Infection.
Mice were infected i.v. with 103 or 105 CFU of M. tuberculosis H37Rv from the collection of the Central Institute for Tuberculosis, Moscow, Russia. Following 3 weeks of growth on Löwenstein-Jensen medium at 37°C, mycobacteria were suspended in sterile saline containing 0.05% Tween 20 and 0.1% bovine serum albumin (BSA; Sigma, St. Louis, Mo.), washed, aliquoted (10 mg of initially obtained semidry bacterial mass in 1 ml) and stored at −80°C. To obtain the log-phase bacteria for challenge, 50 μl from freshly thawed aliquots was added to 5 ml of Dubos broth (Difco, Detroit, Mich.) supplemented with 0.5% BSA (Sigma) and incubated for 1 week at 37°C. Then 0.5 ml of mycobacterial suspension was diluted in 20 ml of fresh warm Dubos-BSA medium and further cultured for 1 week. The resulting suspension was filtered through a sterile 4-μm-pore-size filter to remove clumps. To estimate the CFU content in filtrate, 1 μl from each 1:10 serial dilution was plated onto Dubos agar (Difco), and the total number of microcolonies in the spot was calculated 3 days later under an inverted microscope. The bulk of filtered culture was stored at 4°C, and it was found that no change in the CFU content occurred during this period. Before being injected into mice, the suspension was centrifuged (4°C, 3,000 × g, 20 min), and mycobacteria were resuspended in sterile saline. This preparation contains log-phase mycobacteria only, and unlike nonfiltered preparations, the CFU count precisely reflects the number of mycobacteria in suspension.
CFU counts.
Two and five weeks following infection, serial dilutions of 0.2-ml samples of digested lung tissue in sterile saline were plated on Dubos agar medium (Difco). Eighteen days later, M. tuberculosis H37Rv counts were estimated.
Lung cell suspensions.
Suspensions of interstitial lung cells were obtained as described by Holt et al. (21), with modifications. Mice were anesthetized with an overdose of barbiturate, and blood vessels were washed out with 0.02% EDTA-Hanks balanced salt solution (HBSS) until the tissue turned milk white. Repeated bronchoalveolar lavage with EDTA-HBSS via cannulated trachea was performed to remove alveolar cells. Lung tissue sliced into 1- to 2-mm3 pieces was incubated in RPMI 1640 containing 5% fetal calf serum, antibiotics, 10 mM HEPES (all components from HyClone, Carlington, The Netherlands), collagenase (150 U/ml), and DNase (50 U/ml) (Sigma). A volume of 15 ml was used to digest lungs from three mice representing the experimental group. After 1.5 h of incubation at 37°C, single-cell suspensions were obtained by vigorous pipetting. Cells were washed three times and resuspended in complete culture medium (RPMI 1640 supplemented with 5% fetal calf serum, 10 mM HEPES, 2 mM l-glutamine, 1% nonessential amino acids, pyruvate, 5 × 10−5 M 2-mercaptoethanol, and antibiotics [all components from HyClone]). The lung cells obtained (hereafter referred to as unseparated lung cells) were further enriched in T lymphocytes. For this purpose, plastic-adherent cells were removed by incubating cells on petri dishes (Costar, Badhoevedorp, The Netherlands) for 1 h at 37°C. Nonadherent cells were further depleted of nylon wool-adherent cells by passage through a 10-ml syringe column containing 0.7 g of nylon wool (Fenwall, San Francisco, Calif.). After incubation for 1 h at 37°C, cells were eluted with warm culture medium and used in the assays. Viability of cells, as determined by trypan blue exclusion, was more than 93%.
Cloning of lung T cells.
Lung cell suspension enriched in T lymphocytes was obtained 5 weeks following infection of mice with 105 CFU. Cells were cultured at 106 cells/well with splenic APCs, gamma irradiated at 12 Gy from a 60Co source (0.7 × 106 cells/well), and H37Rv sonicate (10 μg/ml) in wells of 24-well plates in complete culture medium (see above). Three days later, live cells enriched in blasts were isolated by centrifugation on Lympholyte M gradient (Cedarlane Laboratories, Hornby, Ontario, Canada) at 2,500 × g for 20 min at 23°C. Cells were cloned by limiting dilution in the wells of flat-bottom 96-well plates in the presence of fresh APCs (4 × 105 cells/well) and H37Rv sonicate (6 μg/ml) at 500, 1,500, and 4,500 cells/well (our preliminary experiments indicated inefficiency of lung T-cell cloning at lower concentrations). Cultural medium was supplemented with 15% conditioned medium (40-h supernatant from concanavalin A-activated murine splenocyte cultures, absorbed with α-methylmannoside (10 mg/ml; Sigma) as a source of cytokines. On day 14, all wells were restimulated with fresh medium containing 10% conditioned medium, APCs (2 × 104/well), and H37Rv sonicate (6 μg/ml). The wells with growing clones were then determined under an inverted microscope, and positive wells were restimulated in situ or split into new wells every 10 to 14 days.
Proliferative response.
A total of 105 T-enriched lung cells or 4 × 104 T-cell clones were cocultured with 3 × 105 APCs in a well of a 96-well flat-bottom plate (Costar) at 37°C under 5% CO2. Cells were stimulated with either H37Rv sonicate (2), ST-CF (kindly provided by P. Andersen, Statens Seruminstitut, Copenhagen Denmark), or 15- to 18-kDa antigen (each at 10 μg/ml). The latter was affinity purified from H37Rv sonicate by using immunoglobulin G2b monoclonal antibodies (MAbs) produced by S4C1G4 hybridoma developed from lymph nodes of BALB/c mice following repeated immunizations with H37Rv sonicate in incomplete Freund’s adjuvant. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting have shown that S4C1G4 MAbs specifically bind the 16-kDa protein as well as two additional minor (presumably modified) 15- and 18-kDa components. This antigen is described in detail by Avdienko et al. (5).
All cultures were performed in triplicate, and nonstimulated wells served as controls. Cultures were pulsed with 0.5 μCi of [3H]thymidine for the last 18 h of the 48- to 72-h incubation. The label uptake was measured in a liquid scintillation counter (Wallac, Turku, Finland) after the well’s contents were transferred onto fiberglass filters by using a semiautomatic cell harvester (Scatron, Oslo, Norway).
T-lymphocyte immunophenotyping by flow cytometry.
A total of 2 × 105 to 5 × 105 cells from the indicated source were washed twice in phosphate-buffered saline containing 0.01% NaN3 and 0.5% BSA and incubated for 30 min at 4°C in the same buffer containing 20% normal mouse serum to block nonspecific antibody binding. After an additional wash, cells were single or double stained with directly conjugated antibodies (30 min, 4°C). MAbs PE (phycoerythrin)-anti-CD3 (clone 29B), FITC (fluorescein isothiocyanate)-anti-CD4 (clone H129.19), and PE-anti-CD8a (clone 53-6.7) were obtained from Sigma; MAbs FITC-anti-Mac-3 (clone M3/84), FITC-anti-TCRαβ (T-cell receptor alpha and beta chains) (clone H57-597), PE-anti-CD44 (clone IM7), and FITC-anti-CD45RB (clone 16A) were obtained from PharMingen (San Diego, Calif.). Stained cells were washed twice, fixed with 1% paraformaldehyde, and analyzed by flow cytometry.
An EPICS ELITE flow cytometer (Coulter Corporation, Miami, Fla.) equipped with a CYONICS argon laser (Uniphase, San Jose, Calif.) (excitation at 488 nm; 15-mW power; barrier filters at 488BK, 550DL, 525BP, 625DL, and 575BP) was used throughout the study. At least 104 cells of each sample were analyzed, and the data were processed with MultiGraph software (Coulter). Controls for unstained cells were analyzed at each time point.
Cytokine assays.
Enzyme-linked immunosorbent assays (ELISAs) were used to assay interleukin-4 (IL-4), IL-5, IL-10, and IFN-γ in 48-h T-cell clone culture supernatants. The following capture and detecting (biotinylated) MAbs specific for mouse cytokines were purchased from PharMingen: for IFN-γ, clones R4-6A2 and XMG1.2 (sensitivity, 312 pg/ml); for IL-4, clones 11B11 and BVD6-24G2 (sensitivity, 62 pg/ml); for IL-5, clones TRFK5 and TRFK4 (sensitivity, 24 pg/ml); for IL-10, clones JES5-2A5 and JES5-16E3 (sensitivity, 312 pg/ml). ELISAs were performed as instructed by the manufacturer. A standard curve for each assay was generated with known concentrations of mouse recombinant IL-4 (rIL-4; PharMingen), rIL-5 (PharMingen), rIL-10 (Sigma), and rIFN-γ (Genzyme, Boston, Mass.).
Inhibition of mycobacterial growth in vitro.
Mice were injected intraperitoneally with 3% peptone in saline (Sigma). Five days later, peritoneal exudate cells were eluted from peritoneal cavities with heparin (10 U/ml)-containing HBSS and washed twice; 15 × 106 peritoneal exudate cells in 3 ml of antibiotic-free cultural medium (see above) were incubated for 1 h on 60-mm diameter petri dishes (Costar) at 37°C. After removal of nonadherent cells, adherent cells were detached from plastic by incubation in 2 mM EDTA–phosphate-buffered saline for 30 min at room temperature, with subsequent pipetting and washing. Then 6 × 104 thus-prepared macrophages (>90% nonspecific esterase-positive cells) were put in a well of a flat-bottom 96-well plate (Costar) and loaded with 12 × 104 live mycobacteria. Preliminary experiments showed that at least 90% of initially added mycobacteria were macrophage associated within the first 6 h of incubation, as measured by CFU counts in culture supernatants and cell lysates. Thus, T cells (6 × 104 cells/well) or various dilutions of their supernatants were added to the cultures without washing off unattached mycobacteria. T-cell clones were isolated on Lympholyte M gradient (Cedarlane Laboratories) and washed three times in antibiotic-free medium. T-cell-free cultures of mycobacterium-loaded macrophages, supplemented and not supplemented with murine rIFN-γ (100 U/ml; Pharmingen), served as positive and negative controls, respectively. Viability of macrophages loaded with mycobacteria exceeded 90% within the first 24 h of culture, as measured by neutral red uptake. Multiplication of mycobacteria was assessed by [3H]uracil uptake (counts per minute) as described previously (34, 38). Briefly, [3H]uracil (1 μCi/well; Isotop, St. Petersburg, Russia) was added for the last 18 h of the 96-h incubation. Cultures were terminated by passage through fiberglass filters for subsequent liquid scintillation counting. Before filtration, macrophages were disrupted by freezing and thawing.
Statistical analysis.
The statistical significance of differences was estimated by Student’s t test and the Wilcoxon U test. P < 0.05 was considered statistically significant.
RESULTS
Infection.
Preliminary experiments showed that two experimental conditions are requisite to obtain mycobacterium-specific lung T cells retaining proliferative capacity: (i) induction of a relatively mild, rather than acute, infection which is accompanied by less severe immune suppression; and (ii) elimination of the majority of macrophages present in the lung cell population (3). In an attempt to establish a milder infection and thus obtain reactive lung T cells for in vitro tests, we infected I/St mice with two doses, 105 and 103 CFU/mouse, of filtered, single-cell, log-phase mycobacteria. As expected, the mycobacterial loads {means for six to nine mice ± standard error of the mean in lungs at 2 and 5 weeks postinfection were higher in mice challenged with 105 CFU [(3.2 ± 0.5) × 104 and (3.8 ± 0.3) × 106 CFU, respectively] than in mice challenged with 103 CFU [(2.0 ± 0.6) × 103 and (6.7 ± 1.2) × 104 CFU, respectively]}. As these results show, the mycobacterial population increased markedly between weeks 2 and 5 postinfection. An analogous challenge of A/Sn mice resulted in a 10- to 20-fold-lower mycobacterial load in the lungs (data not shown), suggesting that I/St mice continue to express the susceptible phenotype when infected with low doses of M. tuberculosis. The low-dose challenge caused mortality in I/St mice on week 10 (105 CFU) or month 5 (103 CFU), indicating a relatively slow development of the pathological process. A/Sn mice infected with 105 CFU did not die within the 5-month period of observation, again confirming their resistance to infection.
Composition of lung cell suspensions.
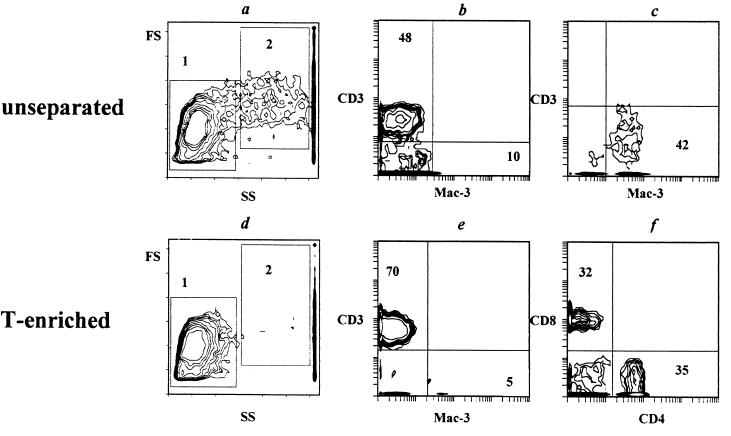
Single-cell suspensions from enzymatically disrupted lungs of infected and naive mice were obtained and assessed with respect to T-cell and macrophage content. Results of a representative flow cytometry experiment performed week 5 following infection with 105 CFU are shown in Fig. 1. The nonseparated lung cell population included at least two distinct subpopulations (Fig. 1a): (i) relatively homogeneous lymphocyte-size cells (zone 1, 46% of cells) and (ii) a heterogeneous population of larger cells of variable size (zone 2, 31% of cells). When these cells were double stained with anti-CD3 and anti-Mac-3 antibodies, it was found, as expected, that T lymphocytes reside predominantly within zone 1 (Fig. 1b), whereas macrophages reside within zone 2 (Fig. 1c). When lung cell suspensions were enriched in T cells by plastic and nylon wool adherence, the vast majority of cells disappeared from the macrophage-size zone, whereas the lymphocyte-size zone remained stable (Fig. 1d) and almost free from macrophages (Fig. 1e). A similar lymphocyte-macrophage distribution was observed when cells derived from naive mice, as well as from other groups of infected mice, were analyzed (data not shown).
FIG. 1.
Cellular composition of unseparated (a to c) and T-enriched (d to f) cell suspensions prepared from lungs of mice infected with 105 CFU of H37Rv (5 weeks postinfection). (a and d) size-structure analysis; (b, e, and f) antibody double staining of cells from lymphocyte-size zone 1; (c) F antibody double staining of cells from macrophage-size zone 2. Numbers in panels b, c, e, and f represent the percentages of each subset, and the minimum contour represents three events. Note that cell separation procedures led to a marked elimination of cells from zone 2 (compare panels d and a). Three independent experiments gave similar results. Analogous results were obtained with lung cells recovered from naive mice.
Surface phenotype of lung T cells.
The yield of CD3+, TCRαβ+, CD4+, and CD8+ cells was assessed by flow cytometry of T-enriched suspensions (see the footnote to Table 1). Between weeks 2 and 5 following challenge with 103 CFU/mouse, the lung T-cell content was not greater than that in naive mice (Table 1). On the contrary, a marked accumulation of bulk CD3+, TCRαβ+, and individual CD4+ and CD8+ T-cell populations was registered following challenge with 105 CFU (two- to threefold difference from values for naive mice; P < 0.05). Notably, the initially elevated T-cell content remained stable throughout this period, indicating an early onset of chronic inflammation. The CD4/CD8 ratio in the CD3-positive population was close to 1:1 (Fig. 1f).
TABLE 1.
Cell yield from lungs of I/St mice before and after challengea
| Infection dose (CFU) | Cell yield (106/mouse)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 2 wk postinfection
|
5 wk postinfection
|
|||||||
| Total | CD3+ | CD4+ | CD8+ | Total | CD3+ | CD4+ | CD8+ | |
| 0 (control) | 1.2 ± 0.2 | 0.8 ± 0.2 | 0.5 ± 0.1 | 0.3 ± 0.1 | ||||
| 103 | 1.8 ± 0.3 | 1.0 ± 0.2 | 0.6 ± 0.1 | 0.4 ± 0.1 | 1.8 ± 0.4 | 1.2 ± 0.4 | 0.7 ± 0.2 | 0.6 ± 0.2 |
| 105 | 5.0 ± 1.4 | 2.8 ± 0.6 | 1.4 ± 0.5 | 1.0 ± 0.2 | 4.5 ± 1.8 | 2.6 ± 0.3 | 1.5 ± 0.3 | 1.2 ± 0.2 |
Cells were obtained by enzymatic disruption from lungs of three mice in each group, pooled, enriched for T cells by sequential plastic and nylon wool passages, and counted (total). Percentages of CD3+, CD4+, and CD8+ cells were estimated by flow cytometry, and the yield of corresponding T-cell subsets was calculated. The yield of TCRαβ+ cells (not shown) never differed from that of CD3+ cells by more than 2%. Values are means from three independent experiments ± standard errors. Values which differ significantly from those for naive mice are in bold (P < 0.05 as determined by Student’s t test and Wilcoxon’s U criterion).
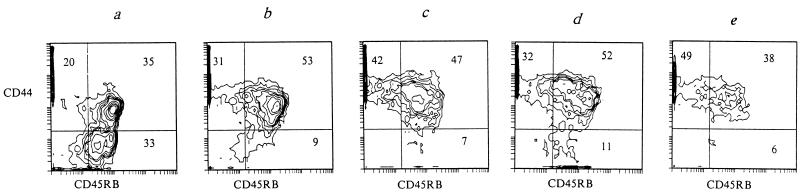
Comparing the expression of CD45RB (the surface marker of naive/resting T cells) and CD44 (the marker of T-cell activation) antigens in naive and infected mice, we found that both challenging doses were sufficient for T-cell activation. Indeed, in lungs of naive I/St mice, CD44− CD45RB+ cells, which are usually referred to as naive/resting cells (7, 9, 11, 24, 25), were readily detected and represented about one-third of the entire T-cell population (Fig. 2a). On the other hand, as early as 2 weeks following challenge with either dose of mycobacteria, these cells were almost undetectable. Instead, the proportions of both CD44+ CD45RB+ and CD44+ CD45RB−/low populations increased (Fig. 2b and d). Progression of the infection to the 5-week point was accompanied by a further increase of the CD44+ CD45RB−/low cell population and a slow decrease of the CD44+ CD45RB+ cell population (Fig. 2c and e).
FIG. 2.
The ratio of CD44low CD45RBhigh to CD44high CD45RBlow changes over the course of M. tuberculosis infection in naive mice (a), mice infected with 103 M. tuberculosis CFU (b and c), and mice infected with 105 CFU (d and e). Lung cells were isolated 2 (b and d) and 5 (c and e) weeks following challenge. Numbers represent the percentages in each quadrant. Two independent experiments gave similar results.
Lung T cells are reactive to H37Rv sonicate as assessed by proliferation.
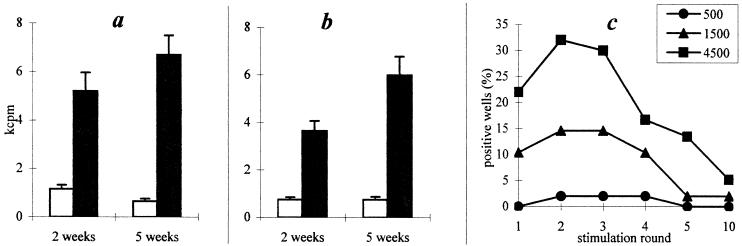
Flow cytometry data are in agreement with the results of proliferative assays. Thus, the antigen-specific proliferative response of lung T cells recovered from mice infected with either dose of M. tuberculosis was registered as early as 2 weeks following challenge and remained stable for at least 5 weeks (Fig. 3a and b), indicating that in tuberculosis-susceptible I/St mice, challenges of both 103 and 105 CFU/mouse induce the activation of mycobacterium-specific T lymphocytes in lungs.
FIG. 3.
Antigen-specific proliferative response of T-enriched lung cells to M. tuberculosis sonicate (a and b) and efficacy of their cloning (c). I/St mice were infected with either 103 (a) or 105 (b) CFU; 2 and 5 weeks later, proliferation was assessed as [3H]thymidine uptake (expressed as mean ± standard deviation of triplicate determinations). Open bars, medium; solid bars, H37Rv sonicate. Results of one representative experiment of three are displayed. (c) The key indicates initial numbers of blast cells per well (see text for details).
T-cell clones from infected lungs.
To study in more detail the reactivity of lung T cells to mycobacterial antigens, we have established a panel of mycobacterium-specific T-cell clones from lung tissue. Of the 30 initially spotted T-cell clones, 14 survived the fifth stimulation cycle (Fig. 3c). Although nine clones ceased proliferation between the 6th and 10th stimulation cycles, five clones returned to stable growth. These five clones, which expressed the CD3+ TCRαβ+ CD4+ CD8− phenotype, were characterized with respect to antigen specificity, cytokine production, and modulation of antimycobacterial function of infected macrophages.
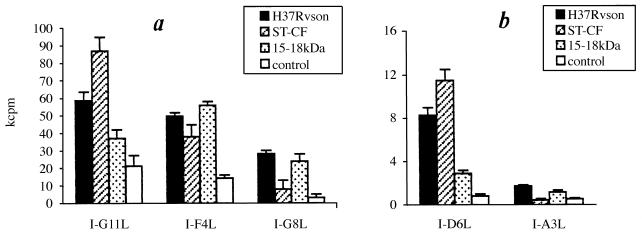
Antigen specificity was assessed by proliferation in the presence of three mycobacterial preparations: (i) H37Rv sonicate, i.e., a bulk mycobacterial antigenic substance; (ii) ST-CF, i.e., a mixture of products secreted by live mycobacteria; and (iii) 15- to 18-kDa affinity-purified mycobacterial antigen, which is recognized by the majority of purified protein derivative-specific T-cell clones of several H-2 haplotypes (our unpublished observation). As shown in Fig. 4, all clones were H37Rv sonicate reactive, although up to a 30-fold difference in [3H]thymidine uptake between individual clones was registered (Fig. 4). Both ST-CF and 15- to 18-kDa antigens stimulated proliferation, albeit with different levels of efficacy, of all clones except I-A3L. The latter, however, responded very weakly even to sonicate, and we never succeeded in its broad expansion.
FIG. 4.
Specificity of proliferative response of individual lung T-cell clones to indicated mycobacterial antigens. T-cell clones (4 × 104) were cocultured with 105 APCs with or without the indicated antigens; 60 h later, [3H]thymidine was added. Proliferation was assessed as [3H]thymidine uptake (expressed as mean ± standard deviation of triplicate determinations). Results of one representative experiment of three are shown (less than 15% differences were seen between individual experiments). For convenience, the results for individual clones are separated into panels a (strong response) and b (weak response).
Cytokine production by T-cell clones.
The production of Th1 versus Th2 cytokines by individual T-cell clones was assessed in culture supernatants following 48 h of incubation in the presence or absence of H37Rv sonicate (Table 2). On the basis of IFN-γ-positive, IL-4-negative, and weak IL-5 responses, we consider clone I-F4L as belonging to the Th1-like subset. Clone I-G11L secreted both Th1- and Th2-type cytokines in an antigen-specific manner, indicating its Th0 nature. Classification of clones I-G8L and I-D6L remains unclear, since low to negative IFN-γ and IL-4 production was combined with prominent IL-10 synthesis. Finally, clone I-A3L probably belongs to the Th2-like subset (IL-4 and IL-10, but not IFN-γ, production). Thus, distinct CD4-positive mycobacterium-specific T-cell subsets were activated in lungs during the course of infection.
TABLE 2.
Cytokine production by lung T-cell clones
| Clone | Concn (pg/ml)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ
|
IL-4
|
IL-5
|
IL-10
|
|||||
| Ag | Control | Ag | Control | Ag | Control | Ag | Control | |
| I-G11L | 10,300 | 0 | 272 | 0 | 195 | 0 | 1,850 | 0 |
| I-F4L | 20,200 | 5,000 | 0 | 0 | 35 | 0 | 0 | 0 |
| I-G8L | 4,900 | 1,100 | 0 | 0 | 0 | 0 | 1,850 | 1,570 |
| I-A3L | 0 | ND | 290 | ND | 0 | ND | 587 | ND |
| I-D6L | 0 | 0 | 0 | 0 | 0 | 0 | 792 | 0 |
T cells (6 × 105) were cocultured for 48 h with 106 irradiated splenic APCs with (Ag) or without (Control) antigen (10 μg of H37Rv sonicate per ml). Cytokine contents in two independently obtained sets of supernatants of each clone were measured by ELISA in triplicate (standard deviation of <10% among triplicate determinations). Positive results obtained in two independent experiments differed less than 25%. 0, concentration lower than the sensitivity of the ELISA. ND, not determined.
Lung T-cell clones modify antimycobacterial activity of peritoneal macrophages.
It is widely accepted that T cells participate in antimycobacterial defense by activating the effector functions of infected macrophages, primarily via IFN-γ production (13, 33, 36). To determine whether this is true for lung T cells specific to mycobacterial antigens, we assessed whether the clones described above were capable of enhancing the antimycobacterial activity of macrophages in vitro. For this purpose, T-cell clones were cocultured with either syngeneic or allogeneic peritoneal macrophages loaded with live M. tuberculosis H37Rv, and mycobacterial growth was assessed by [3H]uracil incorporation (Fig. 5a).
FIG. 5.
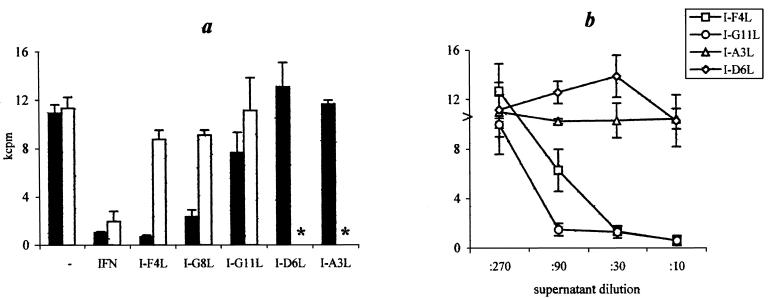
Stimulation of macrophage antimycobacterial activity by lung T-cell clones and their supernatants. Peritoneal macrophages (6 × 104/well) were loaded with 12 × 104 live mycobacteria, and either 6 × 104 T-cell clones (a) or serial dilutions of their cultural supernatants (b) (see the footnote to Table 2) were added to cultures. Following 96 h of incubation, activity of mycobacterial growth was measured as [3H]uracil uptake (mean ± standard deviation). (a) Solid bars, syngeneic I/St system; open bars, allogeneic system (B6 macrophages cocultured with I/St T cells); asterisk, not tested. (b) Arrowhead, no T-cell clone supernatant was added. The results of one of three representative experiments are shown.
Macrophages alone effected approximately 25 to 30% restriction of mycobacterial growth, and exogenous IFN-γ-activated macrophages (positive control) inhibited mycobacterial growth almost completely (90%). In the syngeneic system, T-cell clones I-F4L and I-G8L had similar effects (80 to 96% mycobacterial growth inhibition). The Th0-like clone I-G11L was much less potent (30 to 40% of inhibition), and T-cell clones I-A3L and I-D6L, which produced no IFN-γ, were unable to inhibit mycobacterial growth. Macrophage activation by T-cell clones in our coculture system was, as expected, genetically restricted: I/St T cells did not increase the antimycobacterial activity of allogeneic B6 macrophages.
We have further tested whether augmentation of bacteriostatic activity depends on direct contact between T cells and macrophages or whether production of soluble factors by T cells following antigen-specific activation is sufficient. As shown in Fig. 5b, antibiotic-free supernatants of clones I-F4L and I-G11L, collected after 48 h stimulation with APCs and the antigen, promoted mycobacterial growth inhibition by macrophages in a dose-dependent manner. In contrast, no such activity was provided by the supernatants of I-D6L and I-A3L, the clones which did not produce IFN-γ. Unlike in the coculture system, bacteriostatic activity of IFN-γ-containing supernatants was readily demonstrated when B6 instead of I/St macrophages were loaded with mycobacteria, indicating that the genetically restricted phase of the response was the activation of T cells for cytokine production. Taken together, these results suggest that the MHC-restricted recognition of mycobacterial antigens triggers lung T-cell clones to produce factors, IFN-γ in the first instance, which promote the antimycobacterial function of macrophages in an MHC-unrestricted manner.
DISCUSSION
Immunological mechanisms which operate in mycobacterium-infected lungs remain largely obscure. Here we describe the parameters of local T-cell immune responses which develop in the lungs of tuberculosis-susceptible I/St mice during the relatively slow progression of the infection. We are aware that tuberculous infection in mice initiated by i.v. challenge differs in many aspects not only from human disease but also from experimental infection initiated by the pulmonary route. Despite the progressive accumulation of virulent mycobacteria in lungs after i.v. challenge, it is difficult to compare data obtained in pulmonary and hematogenous models. Extra lung pathology may modulate the course of disease, although after i.v. challenge mycobacterial content in the spleen and liver tends to stabilize after initially peaking (12, 32). However, we presume that a low-dose challenge of genetically susceptible mice mimics, at least partly, pulmonary tuberculosis. Indeed, it is widely accepted that only a small proportion (genetically susceptible individuals?) of the tuberculosis-infected human population develops the disease and that the vast majority of cases are mild and chronic (10, 26). Thus, we infected mice with either 103 or 105 M. tuberculosis CFU, since both doses do not cause mortality in I/St mice, at least within 2 months.
Several lines of evidence indicate that alveolar macrophages induce a state of T-cell unresponsiveness (3, 20, 41). Thus, we removed plastic- and nylon wool-adherent cells from lung cell suspensions to study the functional activity of T cells. Despite the almost complete elimination of macrophages (not more than 5% of Mac-3+ cells remained), the content of CD3+ TCRαβ+ cells in T-enriched suspensions remained relatively low (55 to 70%), possibly due to contamination with nonadherent epithelial cells. On the other hand, Strickland et al. (40) have recently shown that in the rat model, T-cell activation is accompanied by a temporary decrease in TCR complex expression and that among CD5+ lung T cells, only about 60% express CD3 and TCR molecules. A marked lung tissue infiltration with CD3+, CD4+, and CD8+ cells was registered following infection of mice with 105, but not 103, CFU. The yield of T cells remained stable between weeks 2 and 5 following infection, suggesting a chronic type of inflammation.
Infection caused an accumulation of the primed, antigen-experienced T cells in the lungs. An abundant population of CD44− CD45R+ naive/resting lung T cells, characteristic of naive mice, disappeared as early as 2 weeks following infection. The CD44+ CD45RB−/low population accumulated in lungs as the infection progressed (Fig. 2). These results are in agreement with data of Griffin and Orme (15), who described an increase in CD44 expression along with variable CD45RB expression on CD4+ splenocytes of tuberculosis-infected B6 mice. Besides accumulation of cells with an activated CD44+ CD45RB−/low phenotype, we found a mycobacterium-specific proliferative response of T-enriched lung cells (Fig. 3). Thus, a chronic course of infection in susceptible I/St mice was accompanied by lung tissue infiltration and local accumulation of mycobacterium-specific T cells which, however, were unable to heal the infection. We are now studying how the degree of lung tissue infiltration, the cellular composition of lung infiltrate, and the T-cell reactivity are changed during the course of tuberculosis infection in genetically resistant mice. Preliminary results indicate that more CD8+ T cells are accumulated in the lungs of A/Sn than of I/St mice. Further studies are needed to clarify whether this provides a higher resistance to the infection in the former strain.
For a better understanding of the mechanisms of mycobacterium-induced responses in the site of infection, T-cell clones generated from lung-infiltrating cells could be a valuable tool. However, their establishment and maintenance are complicated. Holt et al. (22) and Strickland et al. (42) have pointed to a low frequency of lung T-cell clonal growth, even though in their experiments cells were stimulated in vitro with mitogens. We also observed an exceptionally low efficacy of T-cell cloning from lungs following intratracheal immunization of mice with Pseudomonas aeruginosa (23). This could be due to a peculiar physiological state of lung T cells: there is evidence that a major proportion of these cells are locked in G0/G1 phase of the cycle (40) and that lung T cells more readily undergo apoptosis than T cells from peripheral blood (19). In the present study, we were able to expand only 5 of 30 initially growing T-cell clones from lungs of I/St mice challenged with 105 M. tuberculosis CFU (Fig. 3c). To our knowledge, this is the first successful generation of antigen-specific T-cell clones from interstitial lung tissue following infection. We are aware that the results obtained apply to only a narrow panel of highly selected lung T-cell clones. However, a marked diversity among clones with respect to intensity of response and cytokine profiles gives some idea about possible variants of T-cell response which coexist in tuberculosis-infected lungs.
The antigen used for T-cell clone development was the M. tuberculosis H37Rv ultrasound sonicate. T-cell clones readily responded also to ST-CF. This finding is in agreement with the hypothesis that the mycobacterial antigens which provoke strong and broad T-cell reactivity in mice and humans reside in the fraction of secreted proteins (1, 6). Interestingly, 15- to 18-kDa mycobacterial antigen, affinity purified from H37Rv sonicate (5), was recognized by all five T-cell clones derived from I/St (H-2j) mice (Fig. 4). Taking into account that the majority of T-cell clones developed from the lymph nodes of B10.D2 (H-2d) and B6 (H-2b) mice following immunization with purified protein derivative also recognize this antigen (data not shown), its further study as a potential vaccine candidate with promiscuous T-cell reactivity is warranted.
The diversity in cytokine profiles of individual lung T-cell clones was impressive. In a panel consisting of five T-cell clones only in addition to the Th1-like clone I-F4L and the Th0-like clone I-G11L, there were three clones with a less clear cytokine spectrum (Table 2). These T-cell clones demonstrated strong IL-10 production, either exclusively (I-D6L) or accompanied by a moderate secretion of IFN-γ (I-G8L) or IL-4 (I-A3L). The proportion of IFN-γ- and IL-4/IL-10-secreting T-cell clones in our study corresponds well to the data of Hernandez-Pando et al. (17) for polyclonal cytokine production by lung T cells. In their experiments, at the chronic stage of tuberculous infection (30 to 120 days, i.e., a time point very close to that at which T-cell clones were generated in our study), the cytokine profile corresponded to a Th0-like balance. It was suggested that such mixed Th1-Th2 activity favors immunological lesions and tissue necrosis by tumor necrosis factor alpha (18, 35).
Recently Groux et al. (16) have proposed an immunoregulatory role of IL-10-producing T cells which are activated at the site of inflammation. In their mouse transgenic model of inflammatory bowel disease, they obtained a panel of ovalbumin-specific CD4+ T-cell clones which resemble three unusual clones from our panel in that they (i) produced large quantities of IL-10 and variable amounts of other cytokines and (ii) proliferated weakly upon antigenic stimulation. These cells, which the authors called T regulatory cells 1, were able to suppress the antigen-specific immune response of other cells and thus down-regulate the pathological consequences in vivo. Probably, a similar T-cell subset plays an important down-regulatory role in tuberculosis-affected lungs. The need for mechanisms which support the balance between microbicidal and histopathological responses and thus prevent compromising of the lung function is obvious.
There is little doubt that specific CD4+ T cells carry out their antimycobacterial functions by augmenting effector activity of macrophages, particularly through IFN-γ production. The pivotal protective role of IFN-γ was directly demonstrated in vivo by a dramatic increase of susceptibility to mycobacteria of mice with a genetically disrupted IFN-γ structural gene (14). In vitro, there was an increase of bactericidal capacity of mycobacterium-loaded macrophages after treatment with rIFN-γ and CD4+ T-cell supernatants. The effect was abrogated by adding IFN-γ-specific MAbs to cultures (13, 36, 37). We have extended these observations by showing that the capacity of CD4+ T cells from tuberculosis-infected lung tissue to combat infection depends greatly on their ability to produce IFN-γ in response to mycobacteria. As shown in Fig. 5, all three IFN-γ-producing T-cell clones, as well as their supernatants, inhibited mycobacterial growth in macrophage cultures (interestingly, the Th1-like clone was the most and the Th0-like clone was the least potent inhibitor in the coculture system), whereas IFN-γ-negative clones were nonprotective. These results partly resemble those of Silva et al. (37), who showed that IFN-γ-containing supernatants of T-cell clones specific to Mycobacterium leprae antigen hsp65 inhibited M. tuberculosis growth in bone marrow-derived murine macrophages. Surprisingly, in a coculture system similar to ours, these authors did not find a definite correlation between IFN-γ positivity and antimycobacterial potency of T-cell clones. Nevertheless, their hypothesis that inhibition of mycobacterial growth is due to T-cell cytotoxicity against infected macrophages rather than to IFN-γ production is not definitely proved. First, the antimycobacterial activity of the only noncytotoxic, IFN-γ-producing T-cell clone was not studied in their work. Second, the efficient inhibition of mycobacterial growth by T-cell clone supernatants observed in our two systems indicates that cell-to-cell contact is not required. Third, many parameters of immune responsiveness to a heat shock protein of mycobacteria, the molecule variants of which are readily produced by the host cells, could well differ from the response to mycobacterium-specific components.
Thus, we believe that IFN-γ production by CD4+ T cells should still be considered an important mechanism of antimycobacterial defense which, as shown in this work, operates directly in the site of tuberculosis infection. However, mycobacterium-specific T cells producing several cytokine combinations, not necessarily along with IFN-γ, are readily activated in the infected lung and could play an important role in the protective and/or pathogenic response during the course of disease.
ACKNOWLEDGMENTS
We are grateful to P. Andersen for providing ST-CF and V. Avdienko for providing 15- to 18-kDa antigen.
This work was supported in part by INTAS grant 94-1966 and by the WHO Global Program for Vaccine Development.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apt A S, Avdienko V G, Nikonenko B V, Moroz A M, Skamene E. Distinct H-2 complex control of mortality, and immune responses to tuberculosis infection in virgin and BCG-vaccinated mice. Clin Exp Immunol. 1993;94:322–329. doi: 10.1111/j.1365-2249.1993.tb03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apt A S, Kramnik I B, Moroz A M. Regulation of T cell proliferative responses by cells from solid lung tissue of M. tuberculosis-infected mice. Immunology. 1991;73:73–179. [PMC free article] [PubMed] [Google Scholar]
- 4.Apt A S, Nikonenko B V, Moroz A M, Averbakh M M. Genetic analysis of factors determining susceptibility to tuberculosis. Bull Exp Biol Med. 1982;12:83–85. . (In Russian.) [PubMed] [Google Scholar]
- 5.Avdienko, V. G., S. Y. Kondrashov, I. V. Shabunin, and V. I. Litvinov. Two distinct antigens from Mycobacterium tuberculosis and their use for detection of specific antibodies in sera of TB patients by ELISA. Probl. Tuberc., in press.
- 6.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budd R C, Cerottini J, Horvath C, Bron C, Pedrazzini T, MacDonald R C. Distinction of origin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 8.Buschman E, Apt A S, Nikonenko B V, Moroz A M, Averbakh M M, Skamene E. Genetics aspects of innate resistance and acquired immunity to mycobacteria in inbred mice. Springer Semin Immunopathol. 1988;10:319–333. doi: 10.1007/BF02053844. [DOI] [PubMed] [Google Scholar]
- 9.Butterfuld K, Fathman C G, Budd R C. A subset of memory CD4+ helper T lymphocytes identified by expression of Pgp-1. J Exp Med. 1989;169:1461–1466. doi: 10.1084/jem.169.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dannenberg A M., Jr . Immunology of tuberculosis and leprosy: a symposium. Bethesda, Md: US-Japan Cooperative Medical Sciences Program; 1983. Pathogenesis of tuberculosis: native and acquired resistance in animals and humans; pp. 344–354. [Google Scholar]
- 11.Dianzani U, Luqman M, Rojo J, Yagi J, Baron J L, Woods A, Janeway C, Bottomly K. Molecular associations on the T cell surface correlate with immunological memory. Eur J Immunol. 1990;20:2249–2257. doi: 10.1002/eji.1830201014. [DOI] [PubMed] [Google Scholar]
- 12.Dunn P L, North R J. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in lungs, induce lung pathology, and cause mortality in mice. Infect Immun. 1995;63:3428–3437. doi: 10.1128/iai.63.9.3428-3437.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flesch I, Kaufmann S H E. Mycobacterial growth inhibition by interferon-γ-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987;138:4408–4413. [PubMed] [Google Scholar]
- 14.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B A. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin J P, Orme I M. Evolution of CD4+ T-cell subsets following infection of naive and memory immune mice with Mycobacterium tuberculosis. Infect Immun. 1994;62:1683–1690. doi: 10.1128/iai.62.5.1683-1690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, De Vries J E, Roncarolo M G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Pando R, Orozco E H, Sampieri A, Pavon L, Velasquillo C, Larriva-Sahol J, Alcocer J M, Madrid M V. Correlation between the kinetics of Th-1/Th-2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997;65:3317–3327. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herry I, Bonay M, Bouchonnet F, Schuller M P, Lecossier D, Tazi A, Lynch D H, Hance A J. Extensive apoptosis of lung T-lymphocytes maintained in vitro. Am J Respir Cell Mol Biol. 1996;15:339–347. doi: 10.1165/ajrcmb.15.3.8810637. [DOI] [PubMed] [Google Scholar]
- 20.Holt P G. Downregulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol. 1986;63:261–270. [PMC free article] [PubMed] [Google Scholar]
- 21.Holt P G, Degebrodt A, O’Leary C, Krska K, Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985;62:586–593. [PMC free article] [PubMed] [Google Scholar]
- 22.Holt P G, Kees U R, Shon-Hegrad M A, Rose A, Ford J, Bilyk J, Bilyk N, Bowman R, Robinson B W S. Limiting-dilution analysis of T cells extracted from solid human lung tissue: comparison of precursor frequencies for proliferative responses and lymphokine production between lung and blood T cells from individual donors. Immunology. 1988;64:649–654. [PMC free article] [PubMed] [Google Scholar]
- 23.Kondratieva, T. K., N. V. Kobets, S. V. Khaidukov, V. V. Yeremeev, I. V. Lyadova, A. S. Apt, M. F. Tam, and M. M. Stevenson. Characterisation of T-cell clones derived from lymph nodes and lungs of Pseudomonas aeruginosa-susceptible and resistant mouse strains. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 24.Lee W T, Vitetta E S. The differential expression of homing and adhesion molecules on virgin and memory T cells in the mouse. Cell Immunol. 1991;132:215–222. doi: 10.1016/0008-8749(91)90020-c. [DOI] [PubMed] [Google Scholar]
- 25.Lee W T, Yin X-M, Vitetta E S. Functional and ontogenetic analysis of murine CD45Rhi and CD45Rlo CD4+ T cells. J Immunol. 1990;144:3288–3295. [PubMed] [Google Scholar]
- 26.Lenzini L, Rottoli P, Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977;27:230–237. [PMC free article] [PubMed] [Google Scholar]
- 27.Mbawuike I N, Herscowitz H B. Role of activation in alveolar macrophage-mediated suppression of the plaque-forming cell response. Infect Immun. 1988;56:577–582. doi: 10.1128/iai.56.3.577-581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCombs C C, Michalski J P, Westerfield B T, Light R W. Human alveolar macrophages suppress the proliferative response of peripheral blood lymphocytes. Chest. 1982;82:266–271. doi: 10.1378/chest.82.3.266. [DOI] [PubMed] [Google Scholar]
- 29.McLeod R, Buschman E, Arbuckle L D, Skamene E. Immunogenetics in the analysis of resistance to intracellular pathogens. Curr Opin Immunol. 1995;7:539–552. doi: 10.1016/0952-7915(95)80100-6. [DOI] [PubMed] [Google Scholar]
- 30.Nikonenko B V, Apt A S, Mezhlumova M B, Avdienko V G, Yeremeev V V, Moroz A M. Influence of the mouse Bcg, Tbc-1 and xid genes on resistance and immune responses to tuberculosis infection and efficacy of BCG vaccination. Clin Exp Immunol. 1996;104:37–43. doi: 10.1046/j.1365-2249.1996.d01-643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikonenko B V, Apt A S, Moroz A M, Averbakh M M. Genetic analysis of susceptibility of mice to H37Rv tuberculosis infection: sensitivity versus relative resistance. In: Skamene E, editor. Genetic control of host resistance to infection and malignancy. New York, N.Y: Alan R. Liss, Inc.; 1985. pp. 291–298. [Google Scholar]
- 32.North R J, Izzo A A. Mycobacterial virulence. Virulent strains of Mycobacterium tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177:1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 34.Rook G A W, Champion B R, Steele J, Varey A M, Stanford J L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985;59:414–420. [PMC free article] [PubMed] [Google Scholar]
- 35.Rook G A W, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–284. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 36.Rook G A W, Steele J, Ainsworth M, Champion B R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986;59:333–338. [PMC free article] [PubMed] [Google Scholar]
- 37.Silva C L, Silva M F, Pietro R C L R, Lowrie D B. Characterization of T cells that confer a high degree of protective immunity against tuberculosis in mice after vaccination with tumor cells expressing mycobacterial hsp65. Infect Immun. 1996;64:2400–2407. doi: 10.1128/iai.64.7.2400-2407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stach J L, Gros P, Forget A, Skamene E. Phenotypic expression of genetically controlled natural resistance to Mycobacterium bovis (BCG) J Immunol. 1984;132:883–892. [PubMed] [Google Scholar]
- 39.Stevenson M M, Kondratieva T K, Apt A S, Tam M F, Skamene E. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin Exp Immunol. 1995;99:98–105. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strickland D, Kees U R, Holt P G. Regulation of T-cell activation in the lung: isolated lung T cells exhibit surface phenotypic characteristics of recent activation including down-modulated T-cell receptors, but are locked into the G0/G1 phase of the cell cycle. Immunology. 1996;87:242–249. doi: 10.1046/j.1365-2567.1996.460541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strickland D, Kees U R, Holt P G. Regulation of T-cell activation in the lung: alveolar macrophages induce reversible T-cell anergy in vitro associated with inhibition of interleukin-2 receptor signal transduction. Immunology. 1996;87:250–258. doi: 10.1046/j.1365-2567.1996.459542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strickland D, Thepen T, Kees U R, Kraal G, Holt P G. Regulation of T-cell function in lung tissue by pulmonary alveolar macrophages. Immunology. 1993;80:266–272. [PMC free article] [PubMed] [Google Scholar]